1. Introduction
Globally, the prevalence of chronic pain is high at 10% to 50% of the adult population.18,23,40,57,63,64 Chronic pain not only impairs the quality of life of patients and their caregivers40,75 but also has a large socioeconomic cost comprising direct healthcare costs and indirect costs due to reduced workforce participation.10,25
Chronic pain is often difficult to alleviate adequately with clinically used analgesic and/or adjuvant agents because of lack of efficacy and/or dose-limiting side effects.123 Thus, there is a large unmet medical need for novel, efficacious, and well-tolerated nonopioid analgesics for the relief of chronic pain.123 This unmet need is a powerful driver of research on chronic pain mechanisms as well as on novel analgesics discovery.114 In the past 3 decades, numerous receptors, ion channels, and enzymes have been identified at multiple levels of the somatosensory nervous system in neurons and/or nonneuronal cells as potential targets for novel analgesics discovery. Based on the huge collective effort to date to reveal chronic pain mechanisms, a reasonable prediction 20 years ago would have been that by 2022, multiple, novel highly efficacious nonopioid analgesics would have been added to the armamentarium for front-line clinicians to prescribe. That this is not the case prompts the question “Why not?” Reasons in common with other therapeutic areas include toxicity and/or poor tolerability, poor pharmaceutical properties, and unsuitable pharmacokinetics of the clinical candidate. However, the major impediment to successful preclinical to clinical research translation in the novel nonopioid analgesics field is lack of efficacy in early phase clinical trials.28
2. Root cause analysis of failed clinical trials of novel nonopioid analgesics
Factors contributing to poor efficacy in clinical trials of investigational nonopioid analgesics for the relief of chronic pain include incomplete knowledge of the underlying pain mechanisms, inappropriate analgesic drug target selection, insufficient drug target engagement at clinically tolerable therapeutic drug concentrations, use of rodent pain models that do not adequately recapitulate the pathobiology of the chronic pain conditions that will be targeted in human clinical trials and in the clinical setting, pain behaviours in rodent models that do not adequately model ongoing pain in patients, between-sex differences, lack of robust and validated pain biomarkers, and problems with clinical trial design and execution including patient selection (Fig. 1). These factors are addressed in the next few sections of this review.
Figure 1.
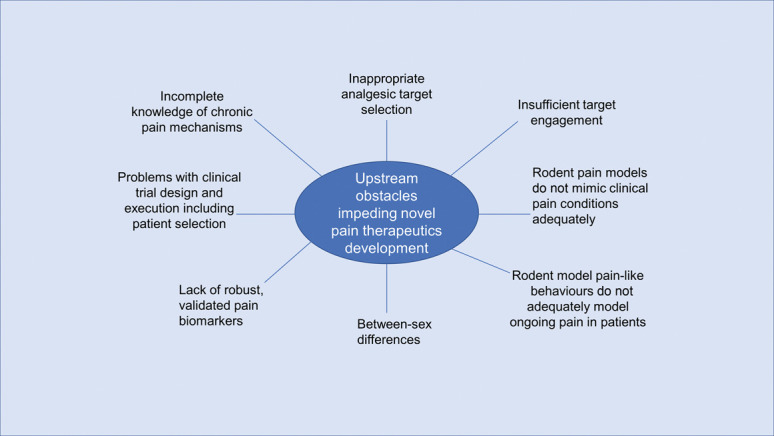
Schematic diagram of obstacles impeding the translation of promising preclinical efficacy data to successful clinical trials in patients suffering from various chronic pain conditions.
2.1. Analgesic drug target—which to choose?
According to the mouse pain gene and human pain gene databases curated by Jeff Mogil85 and the Pain Research Forum78 (https://www.painresearchforum.org/resources/pain-gene-resource; accessed March 16, 2022), respectively, there are currently 430 mouse and 94 human genes implicated in the pathobiology of pain. However, the precise nature of how these pain genes and their encoded targets interact in the pathophysiology of individual chronic pain conditions in patients is unclear. It is thus unsurprising that modulation of a single target receptor, enzyme, or ion channel to evoke promising pain relief in rodent models of chronic pain has in most instances not translated to significant pain relief relative to placebo in early phase clinical trials.121,124 Examples of “failed” novel analgesics include neurokinin-1 receptor antagonists,99 chemokine receptor-2 antagonists,44 fatty acid amide hydrolase inhibitors,38 Nav1.7 inhibitors,49 and p38 mitogen-activated protein kinase (MAPK) inhibitors,76 to name but a few.121 The overall poor preclinical to clinical translation of novel analgesics for relief of chronic pain suggested that between-species differences in biology between humans and rodents may have been underestimated.65 However, this notion is countered by the fact that pain-related behaviours are phylogenetically ancient and widespread.66
2.2. Drug–target engagement at tolerable doses
Once a target is selected and lead molecules are synthesized that modulate the target, another factor to consider is how much target engagement is necessary to alleviate chronic pain and how much is needed to safely block a pain response without blocking “protective” pronociceptive signalling in response to acute insults such as major trauma or surgery. It is plausible that clinical trial “failures” may have been underpinned, at least in part, by doses that were too low to ensure adequate target engagement to block pathological pronociceptive signalling and alleviate chronic pain.49
2.3. Discovery approaches for novel therapeutics to alleviate chronic pain
There are 2 overarching approaches for discovery of novel nonopioid analgesics for alleviating chronic pain in humans. The first is to discover molecules that cross the blood–brain barrier and enter the brain and spinal cord to reduce central sensitization and/or augment descending inhibitory neurotransmission, with the net result being relief of chronic pain. The second is to discover agents that are peripherally restricted and so excluded from the brain and spinal cord to avoid central nervous system side effects. The latter modulate targets in peripheral components of the somatosensory nervous system to reduce pronociceptive input into the spinal dorsal horn, thereby leading to indirect attenuation of central sensitization that underpins the development and maintenance of various chronic pain conditions over time.116
2.4. Predictive validity of rodent pain models
Another factor potentially contributing to poor preclinical to clinical research translation in the novel pain therapeutics field is that the animal models used to assess efficacy may inadequately recapitulate the pain pathobiology in patient populations recruited into early phase clinical trials.124 For example, preclinical efficacy may have been assessed in mice or rats exhibiting pain-like behaviour because of a mechanically induced peripheral nerve injury, whereas clinical trials may have been conducted in patients with painful diabetic neuropathy as a consequence of a prolonged period of poorly controlled type 2 diabetes. To address this issue, multiple research groups, including my own, have invested considerable resources in establishing optimised rodent pain models with back-translated pharmacological data, with the aim to more closely mimic individual chronic pain conditions in humans.11 Examples include rat models of breast cancer–induced bone pain95 and prostate cancer–induced bone pain,39,69 rat models of antiretroviral toxic neuropathy (ATN)53,117 and HIV-associated neuropathy,118 the chronic phase of the monoidoacetate rat model of knee osteoarthritis,34 a rat model of mechanical low back pain,73,79 a mouse model of chronic low back pain,62 mechanical nerve injury,43 an EAE mouse model of central neuropathic pain,45,46,48 a genetic model of painful diabetic neuropathy in the type 2 diabetic Zucker diabetic fatty rat,24,77,93 as well as models of chemotherapy-induced peripheral neuropathy in both rats32,33,60 and mice.41,92,110 However, it is too early to draw conclusions on the effectiveness of this strategy to identify novel analgesic drug leads that will be more likely to achieve clinical trial success.
2.5. Pain behavioural end points
Identification of pain-like end points other than evoked reflexive behaviours has attracted research attention aimed at better mimicking spontaneous ongoing pain in patients. To this end, burrowing,4,72,90,100,119 gait analysis,34,111,122 facial grimacing,20,56,67 and electroencephalography52,58 have been proposed as nonevoked surrogate measures of pain in rodents. However, for translational success, reliable pain biomarkers as translatable end points from the preclinical to the clinical research setting are needed and this is addressed in the next section.
2.6. Pain biomarkers
The dearth of robust, validated pain biomarkers in animal models that have utility for use in human clinical studies has hindered mechanistically driven development of novel pain therapeutics to alleviate chronic pain.86,114 Despite 3 decades of effort, the extent to which individual chronic pain conditions are underpinned by shared mechanisms remains unclear.86 In 2017, a task force concluded that quantitative sensory testing, skin biopsy, and neuroimaging have potential applicability as diagnostic, prognostic, predictive, and pharmacodynamic biomarkers.107 Other techniques proposed to have pain biomarker potential include microneurography, biochemical (eg, nerve growth factor [NGF], cytokines, and chemokines) and genetic signatures, patient-derived neurons, electroencephalography/magnetoencephalography,114 exosomes,17 and hyperspectral autofluorescence imaging.29 However, acceptance of “chronic pain biomarkers” as valid and reliable by international regulatory agencies such as the U.S. Food and Drug Administration and the European Medicines Agency (EMA) will be conditional on their demonstrated utility in clinical trials of analgesic drug candidates in patients recruited into these trials.86,114 As yet, there are no chronic pain biomarkers that have reached this level of validation.
2.7. Challenges in the conduct of novel analgesic clinical trials
Estimates are that only 57% of novel analgesic candidates tested in phase 3 clinical trials progress to regulatory approval.28 This implies that many novel investigational analgesics with phase 2 clinical trial data sufficiently promising to progress to phase 3 trials do not go on to produce positive phase 3 results.28 Reasons for this high level of attrition in late-stage clinical development include (1) false positive phase 2 trial results; (2) incorrect dosage selection based on phase 2 trial data; (3) phase 2 and phase 3 clinical trial designs had features compromising assay sensitivity, eg, entry criteria that were too heterogeneous or inappropriate outcome measures; (4) insufficient increase in sample size between phase 2 and phase 3 to allow for potentially increased heterogeneity in the target population in larger phase 3 trials; and (5) low-quality execution of the phase 2 or phase 3 trials.28 To address these issues, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials has made a concerted effort to develop and publish recommendations on the design of phase 2 and phase 3 clinical trials in patients with chronic pain to optimise clinical trial validity, assay sensitivity, and clinical trial execution.21,22,27,28
2.8. Navigating obstacles impeding novel pain therapeutics development
From the foregoing, there are multiple obstacles impeding success in the development of novel nonopioid pain therapeutics that are efficacious, safe, and well-tolerated for relief of chronic pain in patients. The high degree of difficulty in achieving success has led several large pharmaceutical companies to exit the novel pain therapeutics field because it is “too hard.” Nevertheless, others in industry and academia continue to persist despite the long (15+ years) and arduous path to success.
3. Angiotensin II type 2 receptor antagonists for alleviating peripheral neuropathic pain
As noted in preceding sections, most novel nonopioid analgesics with promising preclinical pain relief data have not translated to successful proof-of-concept (POC) phase 2a clinical trials in patients.121 An exception is EMA401, a highly selective, orally active, peripherally restricted, small molecule, angiotensin II type 2 (AT2) receptor antagonist.87 The preclinical and clinical development of EMA401 as a novel, first-in-class, nonopioid analgesic for the relief of peripheral neuropathic pain71,88,106 is elaborated in the remaining sections of this review.
3.1. Classical renin–angiotensin system
Angiotensin II (Ang II) is the major bioactive peptide of the classical renin–angiotensin system (Fig. 2) that was first discovered >120 years ago.112 Angiotensin II is formed by a multistep enzymatic process commencing with formation of the inactive precursor peptide, angiotensinogen in the liver, which is secreted into the bloodstream for transport to the kidney.113 Angiotensinogen is cleaved by the enzyme, renin, which is secreted by the juxtaglomerular apparatus in the kidney, to form the inactive decapeptide, angiotensin I.113 This in turn is cleaved predominantly by the enzyme, angiotensin-converting enzyme (ACE), to the biologically active octapeptide, Ang II.55,113 Angiotensin II binds with nM affinity at both the angiotensin II type 1 (AT1) receptor and the AT2 receptor.19 Both receptors are members of the superfamily of seven-transmembrane domain G-protein-coupled receptors (GPCRs), but their sequence homology is only 34%19 and they have tissue-specific expression and functional effects.81 The well-known pressor effect of Ang II is mediated by the AT1 receptor in the cardiovascular system, and this led to the development of AT1 receptor blockers and ACE inhibitors for the treatment of hypertension.55 By contrast, AT2 receptor function was long regarded as enigmatic with the Ang II–AT2 receptor signalling axis thought to antagonize some, but not all, of the cellular actions of Ang II–AT1 receptor signalling.55,91
Figure 2.
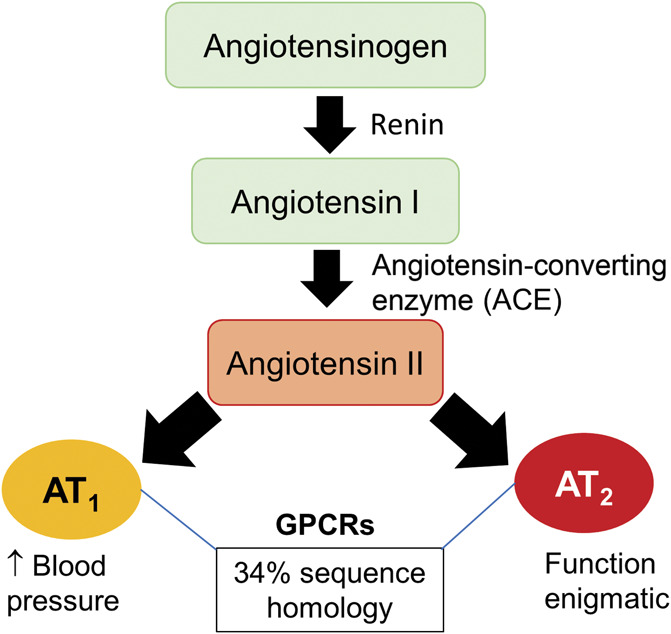
Schematic diagram of the classical renin–angiotensin system.
3.2. Angiotensin II type 2 receptor: crystal structure and biochemical pharmacology
It was not until 2017 that the crystal structure of the AT2 receptor was first described128 which confirmed its enigmatic nature.91 Unlike most GPCRs, helix 8 of the AT2 receptor was not in the canonical position parallel to the membrane pointing outside of the TM7 bundle.128 Instead, helix 8 adopted an unusual conformation by flipping over and interacting with the intracellular ends of transmembrane (TM) 3, TM5, and TM6.128 Consistent with earlier work by others,16,115 neither Ang II nor its agonist analogue, [Sar1-Ile8]-Ang II, induced G-protein or β-arrestin signal transduction at the AT2 receptor.6 This may be due to the increased polarity of intracellular loop 1 to impede engagement of classical GPCR effectors such as G proteins or β arrestins.16 Another possible explanation is that the AT2 receptor requires additional effectors for activity.36 This notion is supported by earlier work whereby Ang II signalling via the AT2 receptor induced neurite outgrowth and morphological differentiation of cultured neuroblastoma x glioma hybrid NG108-15 cells of neuronal origin.15,84 Angiotensin II signalling was underpinned at least in part by increased nitric oxide (NO) production,15 inhibition of p21ras, and sustained activation (phosphorylation) of p44/p42 MAPK, an absolute mediator of neurite outgrowth.26,84 In addition, Ang II/AT2 receptor signalling induced sustained activation of p44/p42 MAPK (also called ERK1/2) and the corresponding neurite outgrowth was mediated by phosphorylation of the high-affinity NGF receptor, TrkA.84
3.3. Nitric oxide has a pathobiological role in neuropathic pain
Augmented NO production due to persistent activation of the N-methyl-d-aspartate-NO synthase-nitric oxide signalling cascade at multiple levels of the somatosensory nervous system has long been implicated in the pathobiology of neuropathic pain.108,129 This led to multiple drug discovery programs aimed at producing neuronal and/or inducible NO synthase inhibitors for relief of neuropathic pain (reviewed by Mukherjee and colleagues).68 As an alternative strategy, I hypothesized that AT2 receptor antagonism may reduce elevated NO generation in neuropathic pain and so evoke pain relief. I pitched this idea to the inaugural “Trailblazer Challenge Ideas Competition” held towards the end of 2003 by UniQuest, the technology transfer company of The University of Queensland (UQ). I was awarded an AU$8000 prize which I gave to my UQ chemistry collaborator (Professor Craig Williams) to synthesize for me a batch of PD123,319, an AT2 receptor antagonist used as a tool compound in the cardiovascular field. About a year later, my laboratory tested the pain relief efficacy of PD123,319 (which I renamed EMA200) in a widely used rat model of neuropathic pain. The positive animal efficacy data led to the filing of a provisional patent that was published 18 months later.101 A UQ spin-out company, Spinifex Pharmaceuticals, was formed in mid-2005 to commercialize this discovery with initial investment capital of AU$3.25m. In the ensuing year, my research team generated efficacy data for multiple AT2 receptor antagonists in 2 rat models of peripheral neuropathic pain for patent enablement, together with in vitro radioligand binding data and pharmacokinetic (PK) data in rats. Research on the mechanism of action was also initiated. An overview of our work, extended by others in more recent years, is outlined below.
3.4. Angiotensin II type 2 receptor antagonists: nonclinical data
3.4.1. In vitro binding assays
The radioligand binding data (Table 1) for the small-molecule AT2 receptor antagonists of interest, namely, EMA200 (also called PD123,319), and its structural analogues, EMA300 (also called PD121,981) and EMA400 (also called PD126,055) (Fig. 3), showed they have >1000-fold binding selectivity at the cloned rat AT2 receptor relative to the cloned rat AT1 receptor.103 Of these, EMA400 had the highest AT2 relative to AT1 receptor binding selectivity at >30,000.103 As EMA400 is a racemic mixture of the S- and R-enantiomers (EMA401 and EMA402, respectively), these were assessed individually, and the S-enantiomer (EMA401) was found to have the highest binding specificity at both rat and human AT2 receptors (>10,000).103 In more recent work by others, the nM binding affinity of EMA401 at the AT2 receptor was confirmed along with the binding affinities of a new series of EMA401 analogues.30
Table 1.
Radioligand binding affinity and angiotensin II type 2/AT1 binding selectivity at cloned rat and human angiotensin II type 2 and AT1 receptors (adapted from Smith and colleagues with permission103).
| Cloned rat receptors* | AT2R | AT1R | Binding affinity selectivity AT2R/AT1R | ||
|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||
| Ligand | KD (nM) | KD (nM) | |||
| Angiotensin II | 1.6 | 1.0 | 14.9 | 1.1 | 9.3 |
| IC50 (nM) | IC50 (µM) | ||||
| EMA200 | 71.7 | 14.5 | 210.5 | 121.5 | ∼3000 |
| EMA300 | 46.5 | 3.3 | 49.9 | 10.2 | >1000 |
| EMA400 | 75.2 | 10.0 | 2918 | 1270 | >30,000 |
| EMA401 | 39.5 | 5.2 | 408 | 335 | >10,000 |
| 2630 | 12 | ||||
| EMA402 | 804 | 85.4 | 106 | ||
| Cloned human receptors | |||||
| Ligand | IC50 (nM) | IC50 (nM) | |||
| Saralasin## | 0.19 | 0.48 | 0.40 | ||
| Mean IC50 (nM) | Mean IC50 (µM) | ||||
| EMA401# | 39 | n.c. | >10,000 but n.c. | ||
| EMA402# | 1100 | 59 | 53.6 | ||
| Compound 1530 | 25 | 9 | >10 | >400 | |
n=3 to 4 separate experiments performed in duplicate except for EMA402 (R-enantiomer of EMA400), where n = 2 separate experiments each performed in duplicate.
For the human receptors, n = 1# or 2## performed in duplicate.
AT2R, angiotensin II type 2 receptor; AT1R, angiotensin II type 1 receptor; SEM, standard error of the mean; n.c., not calculable.
Figure 3.
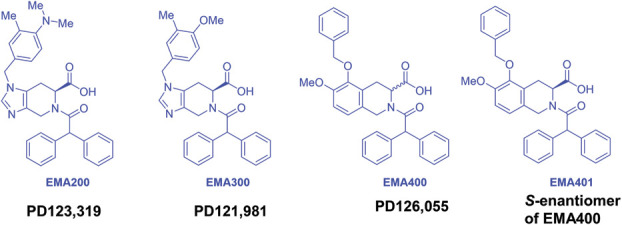
Chemical structures of the small molecule angiotensin II type 2 receptor antagonists—EMA200, EMA300, EMA400, and EMA401.
3.4.2. In vivo pain relief in rodent models of chronic pain
Highly selective, small molecule AT2 receptor antagonists alleviated pain-like behaviour in multiple rodent pain models, and these data are briefly described in the following paragraphs and summarized in Table 2.
Table 2.
Selective, small molecule, angiotensin II type 2 recptor antagonists alleviate pain-like behavior in rodent models of neuropathic and chronic inflammatory pain.
| Pain type | Rodent model and pain-like behavioral end points | AT2R antagonist(s) evaluated and dosing regimen | ED50 (95%CI) (mg/kg) | Ref | ||
|---|---|---|---|---|---|---|
| EMA200 | EMA300 | EMA400/EMA401 or analogue | ||||
| Neuropathic | CCI of the sciatic nerve in the rat; mechanical allodynia | Single i.p. bolus doses; EMA200 1-10 mg/kg; EMA300 1-10 mg/kg; EMA400 0.003-0.03 mg/kg | 3.22 (2.02-5.14) | 0.78 (0.08-7.68) | 0.013 (0.008-0.021) | 103 |
| Single oral dose; EMA401 (30 mg/kg) | NA | NA |

|
30 | ||
| Single oral dose; compound 15 (EMA401 analogue) | NA | NA |

|
30 | ||
| ddC-induced ATN in the rat; mechanical allodynia (single doses); mechanical hyperalgesia (repeat doses) | Single i.p. bolus doses; EMA200 at 0.3–10 mg/kg | 3.2 (1.43-7.0) | NA | NA | 105 | |
| Twice-daily i.p. doses; EMA300 at 1, 10, and 30 mg/kg for 3 days | NA |

|
NA | 105 | ||
| SNI of sciatic nerve in mice; mechanical and cold allodynia | Single i.p. dose; EMA200 at 10 mg/kg |

|
NA | NA | ||
| Single perisciatic dose of EMA200 (30 nmol) |

|
NA | NA | 96 | ||
| Single intrathecal dose of EMA200 (30 nmol) | Inactive | NA | NA | 96 | ||
| Acute paclitaxel neuropathic pain in mice; mechanical and cold allodynia | Single i.v. doses as prevention protocol; i.v. EMA200 at 20 mg/kg |

|
NA |

|
123 | |
| Oral EMA401 at 10 ng/kg | ||||||
| Inflammatory | Unilateral i.pl. FCA-induced inflammatory pain in the rat; mechanical and thermal hyperalgesia | Single i.p. dose of EMA200 at 10 mg/kg on day 7; chronic i.p. dosing of EMA200 at 5 mg/kg/d for 7 consecutive days as a prevention protocol |

|
NA | NA | 97 |

|
||||||
| Unilateral i.pl. FCA-induced inflammatory pain in the rat | Single i.p. dose of EMA200 at 10 mg/kg on day 2, day 4, or day 7 | X | 96 | |||
| FCA-induced monoarthritic pain in the rat knee joint | Single i.p. bolus doses of EMA300 and EMA400 at 0.1-10 mg/kg. | NA |

|

|
102 | |
| FCA-induced vestibulodynia in the rat; perivaginal mechanical allodynia | Chronic i.p. doses of EMA200 at 5 mg/kg/d) for 7 consecutive days as a prevention protocol |

|
NA | NA | 14 | |
| Inflammatory and neuropathic | Prostate cancer–induced bone pain in the rat; mechanical allodynia and thermal hyperalgesia in ipsilateral and contralateral hind paws | Single i.v. doses of EMA200 at 0.3–10 mg/kg | 0.8 (0.61-1.03) ipsi; 1.8 (1.2-2.8) contra; | NA | NA | 70 |
| 3.9 (3.0-5.2) ipsi; 5.5 (3.9-7.7) contra | ||||||
| Acute pain | i.pl. or intrathecal Ang II in noninjured mice; mechanical allodynia | Single i.pl. dose of EMA200 (10 pmol) |

|
NA | NA | 97 |
| Single intrathecal dose of EMA200 (10 pmol) | X | |||||
Ang II, angiotensin II; AT2, angiotensin II type 2; ATN, antiretroviral neuropathy; CCI, chronic constriction injury; CI, confidence interval; contra, contralateral; ddC, dideoxycytidine; FCA, Freund complete adjuvant; i.p., intraperitoneal; ipsi, ipsilateral; i.v., intravenous; NA, not assessed; SNI, spared nerve injury.
3.4.2.1. Neuropathic pain
Single intraperitoneal (i.p.) bolus doses of each of EMA200, EMA300, and EMA400 evoked dose-dependent relief of mechanical allodynia in the ipsilateral hind paws of rats with a chronic constriction injury (CCI) of the sciatic nerve, a widely used rat model of peripheral neuropathic pain.103 The mean ED50 values were 3.22, 0.78, and 0.013 mg/kg for EMA200, EMA300, and EMA400, respectively (Table 2), and at the doses tested, there were no discernible side effects in these animals.103 As the antiallodynic efficacy of EMA300 was abolished in CCI mice null for the AT2 receptor with intermediate effects in the hemizygotes, the AT2 receptor was confirmed as the target.104 More recently, oral (p.o.) EMA401 (10 mg/kg) given as a single dose or by once-daily dosing for 7 days in CCI rats according to an intervention protocol alleviated thermal hyperalgesia in the ipsilateral hind paws (Table 2).52 In addition, in CCI rats (Table 2), single doses of EMA401 (30 mg/kg p.o.) alleviated mechanical allodynia in the ipsilateral hind paws with a potency similar to that of an orally active 6-cyano-substituted benzoxazole analogue of EMA401 (compound 15) and orally administered pregabalin.30
The efficacies of single i.p. doses of EMA200 (Smith Laboratory, Australia) and twice-daily i.p. dosing of EMA300 for 3 consecutive days (Rice Lab, United Kingdom) were assessed in a rat model of ATN induced by dideoxycytidine (ddC). In brief, single doses of EMA200 evoked dose-dependent antiallodynia in the bilateral hind paws with an ED50 of 3.2 mg/kg (Table 2),105 mirroring the ED50 for EMA200 in CCI rats.103 Twice-daily dosing of EMA300 at 30 mg/kg (but not 1 or 10 mg/kg) for 3 days alleviated mechanical hyperalgesia in the bilateral hind paws (Table 2).105 The antihyperalgesic potency of EMA300 did not differ from that of i.p. gabapentin 30 mg/kg dosed twice daily for 3 days, and importantly, these effects were fully reversed by 1 week after dosing cessation.105
In the spared sciatic nerve injury (SNI) mouse model of peripheral neuropathic pain, single doses of EMA200 (10 mg/kg i.p.) alleviated mechanical and cold allodynia in the ipsilateral hind paws (Table 2) that did not differ between males and females.96 This dose of EMA200 also evoked pain relief in SNI mice when assessed using 2 nonevoked pain behavioural assays, namely, the mechanical conflict-avoidance test and the cold preference/avoidance test.98 These findings are aligned with the antiallodynia evoked by EMA200 in both CCI rodents103,104 and a rat model of ATN.105 As perisciatic application of EMA200 (30 nmol) evoked antiallodynia in SNI mice but intrathecal EMA200 (30 nmol) did not (Table 2), this implicated a peripheral mechanism of action.96 In SNI mice, the AT1 receptor blocker, losartan (10 mg/kg i.p.), lacked efficacy which excluded a role for AT1 receptor signalling in evoking allodynia in SNI mice.96 Importantly, EMA200-evoked antiallodynia in SNI mice was independent of any hemodynamic changes because systemic dosing did not influence blood pressure in contrast to the AT1 receptor blocker, losartan.96
In a mouse model of acute paclitaxel-induced neuropathic pain, pretreatment with intravenous (i.v.) EMA200 (20 mg/kg) or oral EMA401 (10 mg/kg) prevented the development of mechanical and cold allodynia (Table 2) that otherwise developed in the hind paws of saline pretreated mice.127 On the contrary, pretreatment with either ACE inhibitors or the AT1 receptor blocker, losartan, lacked antiallodynic efficacy127 mimicking the lack of losartan efficacy in SNI mice.96
3.4.2.2. Mixed neuropathic and chronic inflammatory pain
Prostate cancer–induced bone pain (PCIBP) is underpinned by both inflammatory and neuropathic mechanisms69 and so EMA200 pain relief efficacy was assessed in a rat model of PCIBP. In brief, single i.v. doses of EMA200 evoked dose-dependent relief of mechanical allodynia and thermal hyperalgesia in the bilateral hind paws (Table 2).70 The ED50s for alleviating mechanical allodynia and thermal hyperalgesia in the ipsilateral hind paws were 0.8 and 3.9 mg/kg, respectively, and the corresponding ED50s for the contralateral hind paws were 1.8 and 5.5 mg/kg, respectively (Table 2).70
3.4.2.3. Chronic inflammatory pain
In the Freund complete adjuvant (FCA)-induced rat model of chronic inflammatory pain in 1 hind paw, a 7-day infusion of EMA200 (5 mg/kg/day i.p.) given as a prevention protocol blocked the development of mechanical and thermal hyperalgesia in the ipsilateral hind paws (Table 2) that otherwise developed in FCA rats given a 7-day saline infusion.13 In addition, for FCA rats with fully developed hyperalgesia in the ipsilateral hind paws, a single dose of EMA200 (10 mg/kg i.p.) on day 7 fully alleviated thermal hyperalgesia with a partial effect on mechanical hyperalgesia (Table 2).13 However, contrary results were reported in FCA mice because single doses of EMA200 (10 mg/kg i.p.) administered on day 2, day 4, or day 7 of the model lacked efficacy (Table 2), as did the AT1 receptor blocker, losartan (10 mg/kg i.p).96
Vestibulodynia is a chronic inflammatory pain condition characterized by perivaginal mechanical allodynia, hyperinnervation, and abundant inflammatory cell infiltration.14 In an FCA-induced rat model of this condition, a chronic 7-day infusion of EMA200 (5 mg/kg/d i.p.) given as a prevention protocol attenuated mechanical allodynia in the vestibular tissue by day 3 and this effect was maintained until at least day 6 of the infusion (Table 2).14
3.4.2.4. Noninjured mice
In noninjured male and female mice, intraplantar (i.pl.) Ang II evoked dose-dependent mechanical allodynia, but not heat hyperalgesia, in the ipsilateral hind paws.97 As mechanical allodynia was attenuated by i.pl. EMA200 (10 pmol) (Table 2) but not i.pl. losartan (10 pmol) and it was abolished in AT2 receptor knockout (KO) but not AT1 receptor KO mice, a key pathobiological role for the AT2 receptor was implicated.97 Observations that intrathecal Ang II did not induce hind paw hypersensitivity in noninjured mice affirmed a peripheral mechanism.97
3.5. Angiotensin II type 2 receptor antagonists in rodent neuropathic pain models: Ex vivo mechanistic insights
3.5.1. Angiotensin II
Angiotensin II immunofluorescence (IF) expression levels are elevated in the lumbar dorsal root ganglia (DRG) from rat and mouse models of peripheral neuropathic pain as well as in a rat model of mixed neuropathic/inflammatory pain. In brief, for CCI rats with fully developed mechanical allodynia in the ipsilateral hind paws, Ang II IF levels were increased ∼2-fold to 5-fold in the ipsilateral lumbar DRG relative to the respective levels in the sham controls.47,104 Angiotensin II IF was colocalized with that for a subset of neurons (NeuN), as well as CD3+ T cells and glial fibrillary acidic protein (GFAP) as a marker of satellite glial cell activation.47 In the ipsilateral lumbar DRG of vehicle-treated CCI rats, there was an ∼10-fold increase in the number of Ang II-expressing CD3+ T cells and an ∼5-fold increase in the total number of T cells relative to the respective levels in the sham group.47 At the time of peak effect (1 hour postdose) of EMA300 in CCI rats, the otherwise augmented ipsilateral lumbar DRG levels of Ang II IF were reduced to match the respective levels in the sham controls.47,104 In particular, the number of Ang II-expressing CD3+ T cells and total CD3+ T cells were reduced to match the corresponding numbers in the sham group,47 thus implicating a mechanism whereby AT2 receptor antagonism blocks autocrine and paracrine activation of Ang II/ AT2 receptor-expressing CD3+ T cells in the ipsilateral lumbar DRG.35 This notion is supported by earlier work where Ang II-dependent influx of CD3+ T cells into the rat kidney was inhibited by an AT2 receptor antagonist.120 In the ipsilateral lumbar DRG of these CCI rats, the increased GFAP expression was not attenuated by a single dose of EMA300.47 A role for augmented Ang II signalling via the AT2 receptor to increase GFAP expression is supported by work from others whereby a 7-day infusion of a subpressor dosing regimen of Ang II potentiated hind paw hypersensitivity in CCI rats which was accompanied by satellite glial cell activation in the ipsilateral lumbar DRG of the same animals.82 Interestingly, expression levels of individual NGF isoforms (pro-NGF and mature NGF) were significantly reduced in the ipsilateral lumbar DRG of CCI rats to match the corresponding sham group, when assessed by western blot.47 At the time of peak effect of a single dose of EMA300 in these animals, the otherwise reduced mature NGF expression levels were fully restored to match the corresponding levels in the sham controls.47 As NGF is also expressed by satellite glial cells, future work directed at defining temporal changes in NGF expression patterns in both neuronal and nonneuronal cells in the lumbar DRG in multiple peripheral neuropathic pain conditions is warranted to gain detailed insight into the interplay between augmented AT2-mediated and NGF-mediated pronociceptive signalling.47
For PCIBP rats exhibiting bilateral hind paw hypersensitivity, there were similar findings in the lumbar DRG whereby Ang II IF levels were increased in both the ipsilateral (6.5-7.0-fold) and the contralateral (3.5-4.0-fold) DRG, relative to the respective levels for the sham controls.70 Similarly, in SNI mice, Ang II levels quantified using a quantitative enzyme-linked immunosorbent assay were markedly elevated (8-10-fold) in the ipsilateral, but not the contralateral, sciatic nerve or in sciatic nerves from the sham controls.96 A peripheral mechanism is implicated because Ang II levels were unchanged in the spinal cords of both SNI and sham-operated mice.96
3.6. Angiotensin II type 2 receptor
There are conflicting reports on AT2 receptor expression by DRG neurons. Immunohistology with an Abcam antibody (ab19134) showed that AT2 receptor IF levels did not differ between the ipsilateral lumbar DRG from CCI and sham rats.47,104 This was mirrored in PCIBP rats and confirmed using quantitative real-time polymerase chain reaction that showed that the total lumbar DRG levels of AT2 receptor mRNA in PCIBP rats did not differ from that of the sham group.70 Because of questions raised on the specificity of commercially available AT2 receptor antibodies,31 Agtr2GFP reporter mice were used to gain more insight. Somewhat unexpectedly, the GFP signal was undetectable in the DRG and the superficial layers of the spinal dorsal horn in these mice.96 This was unlikely due to low GFP signal intensity as a subset of NF200+ fibres (but not CGRP+ fibres) were GFP-stained in the sciatic nerves, as were NF200+ and NeuN+ somata in deeper laminae of the spinal dorsal horn and the ventral horn.96 In Agtr2GFP SNI mice, the GFP signal was undetectable in DRG neurons or microglia and/or MΦs but there was a large and sustained infiltration of MΦs to the site of nerve injury in both sexes, but not in the spared sural nerve fibres.96 In SNI Agtr2GFP mice, overlap of the macrophage marker, F4/80, with the GFP signal suggested that MΦs at the site of nerve injury express the AT2 receptor, whereas the lack of a GFP signal in the spinal cord indicated that microglia do not.96
Macrophage Fas-induced apoptosis (MaFIA)/SNI mice were used by 2 groups independently96,125,126 to assess a pathobiological role for peripheral MΦs in SNI-induced hind paw hypersensitivity. Two different protocols were used to deplete peripheral MΦs in MaFIA/SNI mice, and this may underpin the differing conclusions drawn as outlined below.96,125,126
Angiotensin II type 2 receptor–expressing peripheral MΦs in MaFIA/SNI mice were depleted by Shepherd and colleagues96 over a 5-day period, and this was accompanied by progressive attenuation of allodynia in the ipsilateral hind paws. This was reversible because allodynia redeveloped in a temporal manner in the ipsilateral hind paws on cessation of peripheral MΦ depletion, and this was accompanied by repopulation of the injured sciatic nerves with infiltrating MΦs.96 The depletion protocol did not reduce the expected increase in MΦ and/or microglia density in the spinal dorsal horn of MaFIA/SNI mice which led to the conclusion that peripheral MΦs have a critical role in the development of allodynia in these animals.96 In the depleted mice, there was a 50% reduction in blood monocyte and/or MΦs, which was accompanied by a near-complete loss of infiltrating MΦs at the site of nerve injury.96 By contrast, Yu and colleagues125,126 used a milder macrophage depletion protocol in MaFIA/SNI mice to implicate an expansion of resident DRG MΦs rather than MΦs at the nerve injury site as being critical for initiation and maintenance of allodynia in the ipsilateral hind paws of MaFIA/SNI mice (Fig. 4). In support of this latter notion, selective removal of MΦs at the nerve injury site had no impact on the pain phenotype.125,126
Figure 4.
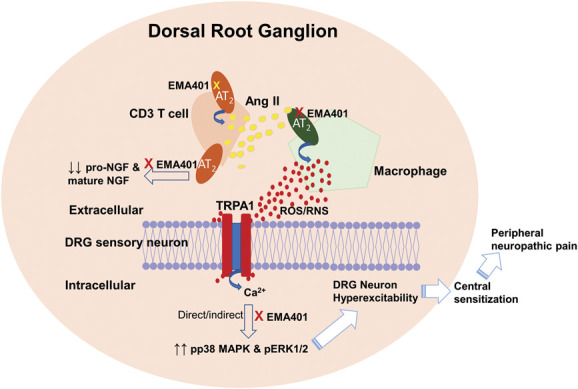
Schematic diagram of augmented Ang II from CD3+ T cells and possibly other cells signalling through the AT2 receptor on peripheral macrophages to generate reactive oxygen species and/or reactive nitrogen species (ROS and/or RNS) that transactivates the TRPA1 receptor on DRG neurons resulting in neuronal hyperexcitability, a hallmark of peripheral neuropathic pain. Augmented Ang II signalling by the AT2 receptor also induces activation of p38 MAPK and pERK1/2 that is reduced by a single bolus dose of small molecule AT2 receptor antagonists at the time of peak effect to match the corresponding levels in the sham controls. Augmented Ang II signalling via the AT2 receptor also reduces levels of pro-NGF and mature NGF in the ipsilateral lumbar DRG with levels of mature NGF restored to match the corresponding levels in the sham controls at the time of peak effect of an AT2 receptor antagonist. The precise interplay between TRPA1 receptor activation, increased expression levels of pp38 MAPK and pERK1/2, and reduced expression levels of pro-NGF and mature NGF in the ipsilateral lumbar DRG in rodent pain models is currently unclear and remains to be defined. Ang II, angiotensin II; AT2, angiotensin II type 2; DRG, dorsal root ganglia; MAPK, mitogen-activated protein kinase; NGF, nerve growth factor.
A critical role for peripheral MΦs in the development of hind paw hypersensitivity in SNI mice was further confirmed using radiation to deplete bone marrow haematopoietic progenitor cells in Agtr2-wild-type (WT) mice, which was followed by transplantation with haematopoietic progenitor cells from either Agtr2-KO or Agtr2-WT donors.96 Eight weeks later, an SNI was induced, and 5 days later, allodynia was present in the ipsilateral hind paws of mice that had received the haematopoietic progenitors from Agtr2-WT mice.96 By contrast, allodynia did not develop in those that had received haematopoietic progenitors from Agtr2-KO mice.96
3.6.1. Angiotensin II–angiotensin II type 2 receptor signalling and activated mitogen-activated protein kinases in the dorsal root ganglia of rodent pain models
As noted in section 3.2, Ang II signalling via the AT2 receptor induces neurite outgrowth in cultured cells of neuronal origin with this effect mediated by persistent activation of ERK1/2.26 Notably, there are high expression levels of activated (phosphorylated) p38 MAPK and ERK1/2 in painful neuromas in humans9 and experimental neuromas in rats,83 which develop because of abnormal sprouting of peripheral nerve fibres. Of particular interest, pp38 MAPK and/or pERK1/2 can phosphorylate multiple voltage-gated ion channels, including Nav1.7,83,109 Nav1.8,37 Cav2.2 calcium channels,61 and TRPV1,42 all of which are expressed by DRG neurons and are implicated in the pathobiology of neuropathic pain. Based on these data, we assessed the extent to which the antiallodynic effects of small molecule AT2 receptor antagonists were underpinned by inhibition of p38 MAPK and ERK1/2 activation in the lumbar DRG from CCI and PCIBP rats exhibiting hind paw hypersensitivity. In brief, augmented levels of pp38 MAPK and pERK1/2 in the ipsilateral lumbar DRG of CCI rats were attenuated at the time of peak effect of EMA300 (1 hour postdose) to match the respective levels in the sham controls.47,104 Similarly, in PCIBP rats, EMA200 at the time of peak effect attenuated the otherwise elevated levels of pp38 MAPK and pERK1/2 in the lumbar DRG to match the respective levels in the sham group.70 These ex vivo findings are aligned with in vitro work by Anand and colleagues3 who showed that exposure of cultured rat DRG neurons to Ang II or compound 21 (AT2 receptor agonist) increased the formation of pp38 MAPK and pERK1/2 and this was attenuated by coexposure of neurons to EMA401.3 Together, these data implicate a role for augmented Ang II signalling via the AT2 receptor that leads to activation (phosphorylation) of p38 MAPK and ERK1/2 in the ipsilateral lumbar DRG, which contribute to the pathobiology of peripheral neuropathic pain47,104 and mixed neuropathic/inflammatory pain.70
3.6.2. Angiotensin II/angiotensin II type 2 receptor signalling and TRPA1 in mice
Allodynia evoked by i.pl. Ang II injection into the ipsilateral hind paws of mice is mediated by the TRPA1 and not the TRPV1 or TRPV4 receptor because this allodynia was abolished in TRPA1-receptor KO, but not TRPV1 or TRPV4 KO mice.97 This notion was affirmed in SNI mice as a TRPA1 antagonist (A967079), but not a TRPV1 antagonist (AMG9810), attenuated allodynia in the ipsilateral hind paws.97 In a rat model of visceral pain, TRPA1 activation increased expression levels of pp38 MAPK and pERK1/2 in DRG neurons.50,51 The extent to which augmented Ang II signalling via the AT2 receptor enhances signalling through the TRPA1 receptor in DRG neurons to increase expression levels of pp38 MAPK and pERK1/2 in the lumbar DRG of rodent models of neuropathic pain remains to be investigated.
3.7. Tissue renin–angiotensin system in peripheral components of the somatosensory nervous system
The aforementioned ex vivo data implicate the presence of a tissue renin–angiotensin system in peripheral components of the somatosensory nervous system, and this is addressed in the 2 sections below.
3.7.1. Dorsal root ganglion neurons
As noted in section 3.5.2, data on AT2 receptor expression by DRG neurons are conflicting. In adult rat DRG, quantitative real-time polymerase chain reaction showed that mRNAs for multiple renin–angiotensin system components including the AT2 receptor, angiotensinogen, renin, ACE, and cathepsin D were present.12,70,80 In situ hybridization confirmed the cytoplasmic presence of angiotensinogen mRNA in rat DRG neurons.80 Semiquantitative RT-PCR showed that exposure of female postnatal day 15 (P15) cultured DRG neurons to an inflammatory soup for 24 hours increased AT2 receptor mRNA expression.7 In both rat and human DRG, intracellular Ang II staining in neurons and their processes was colocalized with pronociceptive neuropeptides (substance P and calcitonin gene-related peptide) in some neurons.7,80 In human DRG extracts, high-performance liquid chromatography and radioimmunoassay confirmed the presence of angiotensin peptides.80 In human nerve tissue extracts, Ang II was the major angiotensin peptide because Ang III levels were below the lower limit of detection of the enzyme-linked immunosorbent assay.3 Using immunohistology with a goat AT2 receptor antibody (sc-48452), AT2 receptor IF was colocalised with that of both Ang II and the transient receptor potential cation channel subfamily V member 1 (TRPV1) in a large proportion of cultured small-diameter adult human DRG neurons.2,3 Using the same antibody with cultured P15 primary female rat DRG neurons showed that AT2 receptor IF was colocalized with isolectin B4, a marker of nonpeptidergic neurons.7 Angiotensin II type 2 receptors on cultured adult rat DRG neurons were functional as exposure of these neurons to either Ang II or an AT2 receptor agonist (compound 21) increased neurite outgrowth that was attenuated by the AT2 receptor antagonist, EMA401, without producing neurotoxicity.2,3 There were similar findings for AT2 receptor-mediated neurite outgrowth for P15 female rat DRG neurons.7
On the contrary, Agtr2 mRNA in mouse DRG neurons was undetectable using RT-PCR and high-throughput RNAseq in agreement with a lack of detectable GFP in DRG sections from Agtr2GFP reporter mice and insignificant expression of AGTR2 mRNA by next-generation deep sequencing of total RNA from human DRG.97 Thus, caution is required when interpreting AT2 receptor IF data due to questions raised on the specificity of commercially available AT2 receptor antibodies31; additional investigation is warranted.
3.7.2. Peripheral macrophages
A role for AT2 receptor-expressing peritoneal MΦs in the pathogenesis of peripheral neuropathic pain is supported by high-throughput mRNA expression data in the National Center for Biotechnology Information-Gene Expression Omnibus database whereby mouse and human MΦs express Agtr2 and AGTR2 mRNA, respectively.97 Angiotensin II type 2 receptors on mouse peritoneal MΦs are functional as their in vitro stimulation with Ang II or an AT2 receptor agonist (CGP42112A) induced ERK1/2 phosphorylation that was sensitive to EMA200, but not losartan, and it was abolished in MΦs from AT2 receptor KO, but not AT1 receptor KO mice.97
3.8. Angiotensin II/angiotensin II type 2 receptor signalling induces ROS/RNS formation by peritoneal macrophages, but not dorsal root ganglion neurons
The presence of functional AT2 receptors on mouse peritoneal MΦs was confirmed by live cell imaging.97 In brief, their exposure to either Ang II or CGP42112A induced time-dependent and concentration-dependent generation of reactive oxygen species/reactive nitrogen species (ROS and/or RNS) that did not differ between the sexes.97 ROS and/or RNS formation was sensitive to EMA200, abolished in MΦs from AT2-KO mice, and attenuated by the free radical scavenger, N-acetylcysteine.97 Primary cultures of mouse and human DRG neurons did not seem to express functional AT2 receptors as their Ang II exposure did not elicit ROS/RNS generation.97 However, contrary findings were reported by Anand et al.2,3 such that Ang II-induced neurite outgrowth by cultured adult rat DRG neurons was inhibited by EMA401 without producing neurotoxicity.2,3 These differences require additional investigation.
3.8.1. Angiotensin II type 2 receptor and transactivation of TRPA1
Functional AT2 receptors on MΦs have a critical role in MΦ to DRG neuron transactivation of neuronal TRPA1 on DRG neurons as Ang II exposure induced a sustained increase in intracellular calcium signalling in cocultures of AT2 receptor KO mouse DRG neurons with WT mouse peritoneal MΦ, but not the converse.97 Similar findings were produced in cocultures of human DRG neurons and a human monocyte and/or MΦ cell line.97
Taken together, the in vitro data implicate a critical role for augmented Ang II-induced activation of the AT2 receptor on peripheral MΦs to increase ROS and/or RNS generation which transactivates TRPA1 on DRG neurons to induce neuronal hyperexcitability and central sensitization in the spinal dorsal horn, a process that underpins the pathobiology of peripheral neuropathic pain.97 These effects were all attenuated by AT2 receptor antagonists in vivo resulting in pain relief in rodent neuropathic pain models (Table 2) and analgesia in a POC clinical trial in patients with postherpetic neuralgia, a type of peripheral neuropathic pain that is often intractable.87
4. Angiotensin II type 2 receptor antagonists: preclinical pharmacokinetics
It is essential to assess the PK and oral bioavailability of novel analgesics from drug discovery to inform selection of drug candidates that will have sufficient systemic exposure over a suitably long period to enable optimal target engagement without evoking dose-limiting toxicity. Hence, the preclinical pharmacokinetics of each of EMA200, EMA300 (sodium salt), EMA400, and EMA401 (sodium salt) were assessed in naive rats after bolus dosing by the i.v. and oral routes.103 The PK parameters derived from the plasma concentration vs time curve data for each molecule of interest are summarized in Table 3 for between-analogue comparison.103 In brief, the dose-normalized systemic exposure of oral EMA401 was 20 to 40-fold greater than that for EMA200 and EMA300 (Table 3). The volume of distribution (Vd) of EMA401 at 3.7 L/kg was an order of magnitude lower than that for EMA200 and EMA300 (Table 3), consistent with the fact that EMA401 does not cross the blood–brain barrier.30,52,71 The plasma clearance of EMA401 at 1.1 L/h/kg was 9-fold and 6-fold lower than that for EMA200 and EMA300, respectively, and the oral bioavailability of EMA400 and EMA401 was ∼30%, which is acceptable, whereas that for EMA200 and EMA300 at <10% was not (Table 3).
Table 3.
Summary of mean pharmacokinetic parameters and oral bioavailability of EMA200, EMA300, EMA400, and EMA401 (S-enantiomer of EMA400) in adult male SD rats (adapted from Smith and colleagues103 with permission).
| Parameter | EMA200 | EMA300 (sodium salt) | EMA400 | EMA401 (sodium salt) |
|---|---|---|---|---|
| t½ (h) | 3.6 | 8.2 | 5.9 | 2.3 |
| Vd (L/kg) | 47.3 | 76.8 | 6.0 | 3.7 |
| Cl (L/h/kg) | 9.3 | 6.1 | 0.71 | 1.1 |
| Oral Tmax (h) | 0.23 | 0.36 | 0.33 | 2.0 |
| Dose-normalized oral Cmax (kg/mL) | 7.3 | 6.8 | 130.4 | 88.5 |
| Dose-normalized oral AUC0-∞ (hour.kg/mL) | 6.5* | 13.9 | 398.6 | 301 |
| F (%) | 5.9 | 7.1 | 28 | 33.2 |
AUC0-∞ = area under the plasma concentration vs time curve from time zero extrapolated to infinity; Cl = systemic clearance; Cmax = peak plasma concentration after oral dosing; F (%) = oral bioavailability; t½ = terminal elimination half-life; Tmax = time of Cmax after oral dosing; Vd = volume of distribution; *value based on AUC0-t, where t is the last measurable plasma concentration.
Overall, the antiallodynic potency rank order of EMA400 > EMA300 > EMA200 in male CCI rats is consistent with the corresponding dose-normalized systemic exposure rank order in rats.103 The suitability of EMA401 as the drug candidate for progression to formal development was supported by its good potency, its >10,000-fold binding selectivity over the AT1 receptor, and its favourable PK.103 The poor ability of EMA401 to cross the blood–brain barrier30,52,103 suggested that central nervous system side effects may be minimal in human clinical trials. In vitro metabolism of EMA401 in hepatocytes from mouse, rat, dog, monkey, and human showed that it had relatively low metabolic stability and that glucuronidation was a major metabolic pathway.74
4.1. Toxicity and safety studies
After completing an Investigational New Drug (IND)-enabling toxicology and safety pharmacology program of EMA401 in animals, oral EMA401 (sodium salt) was progressed by Spinifex Pharmaceuticals into phase 1 clinical trials in healthy young and healthy older volunteers according to single ascending dose and multiple ascending dose paradigms. The respective maximum tolerated doses were 2000 mg (single dose) and 800 mg twice daily.106
5. Early phase clinical trials
Based on the favourable safety, tolerability, and PK properties of oral EMA401 in healthy human subjects, it was progressed by Spinifex into a Phase 2a POC clinical trial in patients with postherpetic neuralgia (PHN), a type of peripheral neuropathic pain that is notoriously difficult to treat.87 This was the first randomized controlled trial reported in any disease where the therapeutic potential of AT2 receptor antagonism was shown.88
5.1. Proof-of-concept clinical trial of EMA401 in patients with postherpetic neuralgia
This POC trial (ACTRN12611000822987) was designed as a randomized, double-blind, parallel-group study to assess the efficacy, safety, and PK of twice-daily oral doses (100 mg) of either EMA401 or placebo in 183 informed, consenting patients with PHN of at least 6-month duration.87 EMA401 and placebo were administered for a 4-week period in accordance with recommendations for POC study designs for novel analgesics.27 This trial was conducted at 29 study sites across 6 countries with 92 patients randomized to EMA401 and 91 patients randomized to placebo.87 Randomization was according to a centralised randomization schedule and blocked by site to increase assay sensitivity.22,28 There were 87 and 84 completers in the EMA401 and placebo groups, respectively.87 The primary efficacy end point was change in mean pain intensity between baseline and the final (fourth) dosing week measured using an 11-point numerical rating scale.87 Encouragingly, EMA401 provided superior relief of PHN relative to placebo at the end of 28 days of treatment with significant differences between the 2 groups evident by day 21 of treatment (Fig. 5).87 Importantly, there were no serious adverse events in the EMA401 group.87 Based on these data, the number needed to treat to obtain ≥50% pain relief for EMA401 was estimated at 6.7, which was comparable to pregabalin 300 mg/day (5.2), pregabalin 600 mg/day (4.0), gabapentin ≥ 1200 mg/day (7.7), and topical capsaicin 8% (7.1).106 In 2015, Spinifex Pharmaceuticals was acquired by Novartis.
Figure 5.
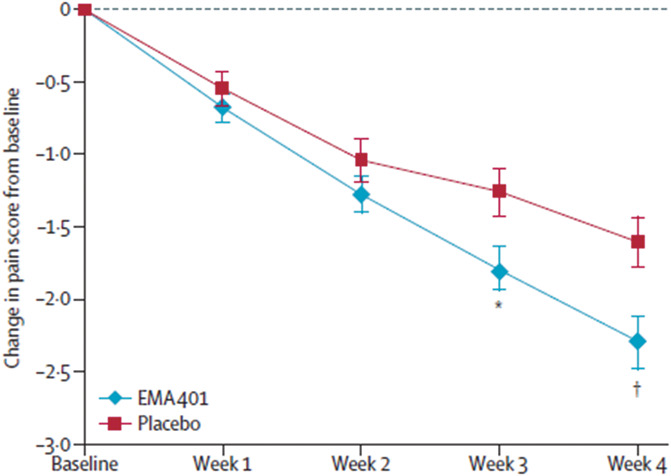
In a randomized, double-blind, parallel group, placebo-controlled, POC clinical trial in patients with postherpetic neuralgia (PHN) of at least 6-month duration, twice-daily dosing with oral EMA401 (sodium salt) at 100 mg for 4 weeks evoked superior relief of PHN relative to placebo at the end of 28 days of treatment with significant differences between the 2 groups evident by day 21 of treatment. Reproduced from Ref. 87, with permission from Elsevier.
5.2. Phase 2 dose-ranging clinical trials of EMA401
The positive POC phase 2a clinical trial results in PHN and the minimal treatment emergent adverse effects evoked by EMA401 administered at 100 mg twice daily for 4 weeks justified the initiation by Novartis of two 13-week, dose-ranging, efficacy, and safety phase 2b clinical trials of oral EMA401 in patients with PHN (NCT03094195) and painful diabetic neuropathy (PDN; NCT03297294). Unfortunately, these 2 trials were terminated prematurely in 2019 because of unexpected adverse histopathologic findings in the livers of cynomolgus monkeys in a 39-week preclinical toxicity study.89 In both phase 2b trials, the mean reduction in the pain score was numerically in favour of EMA401 dosed at 100 mg twice daily at the end of week 12. However, no firm conclusions could be drawn because of the premature termination and that the 300-mg twice-daily dosing regimen was not assessed.89 Importantly, for patients dosed with oral EMA401 at 25 or 100 mg twice daily for up to 13 weeks, EMA401 was well-tolerated and there were no changes in liver biochemical parameters during or after these trials were ceased.89 A possible mechanism for the unexpected hepatotoxicity in cynomolgus monkeys may involve acyl migration of EMA401 glucuronide to produce products with potential to form covalent adducts with liver proteins that may act as a hapten to the immune system, but this remains to be determined.
6. Conclusions
Discovery and development of novel nonopioid analgesics for the relief of chronic pain is a high-risk, arduous, and lengthy process (>15 years), with many obstacles to be navigated (Fig. 1). In the past 3 decades, an enormous collective global effort has been directed at enhancing knowledge on chronic pain mechanisms and/or identification of potential pain biomarkers to facilitate preclinical to clinical research translation, and this is ongoing. My research team and others have invested considerable resources aimed at improving the extent to which rodent pain models mimic individual chronic pain conditions experienced by patients. Use of these optimised models has the potential to also assist with identification of mechanisms in common between various chronic pain subtypes. In the clinical trial arena, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trial consortium has contributed in a major way to the development and dissemination of recommendations on the design of phase 2 and phase 3 clinical trials of novel analgesic agents aimed at optimising clinical trial validity, assay sensitivity, and clinical trial execution.21,22,27,28 These are all essential steps for navigating obstacles that impede the translation of promising preclinical efficacy data of novel pain therapeutics to positive clinical trial outcomes. A target with positive POC clinical trial data is the AT2 receptor.87 However, phase 2b trials of the clinical candidate, EMA401, were terminated prematurely because of unexpected hepatotoxicity in cynomolgus monkeys in a 39-week toxicity study.89 Nevertheless, in work by others, there are second generation small-molecule AT2 receptor antagonists in development for the relief of peripheral neuropathic pain.
Conflict of interest statement
M.T. Smith is named inventor on The University of Queensland (UQ) patents for the use of AT2 receptor antagonists in neuropathic pain and chronic inflammatory pain. This discovery was commercialized by the UQ spin-out company, Spinifex Pharmaceuticals Pty Ltd that was formed in 2005. Spinifex was acquired by Novartis in 2015, and clinical development was terminated by Novartis in 2019 because of unexpected hepatotoxicity in a long-term animal toxicity study. M.T. Smith is undertaking contract research in collaboration with Boehringer Ingelheim Pharma GmbH & Co. KG and with Argenica Pharmaceuticals.
Acknowledgements
CIPDD infrastructure is supported by the Queensland Government Smart State Research Facilities Programme. CIPDD is also supported by the Therapeutic Innovation Australia (TIA). TIA is supported by the Australian Government through the National Collaborative Research Infrastructure Strategy (NCRIS) programme.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Al-Massri KF, Ahmed LA, El-Abhar HS. Pregabalin and lacosamide ameliorate paclitaxel-induced peripheral neuropathy via inhibition of JAK/STAT signaling pathway and Notch-1 receptor. Neurochem Int 2018;120:164–71. [DOI] [PubMed] [Google Scholar]
- [2].Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P. Angiotensin II type 2 receptor (AT(2) R) localization and antagonist mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons. Eur J Pain 2013;17:1012–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anand U, Yiangou Y, Sinisi M, Fox M, MacQuillan A, Quick T, Korchev YE, Bountra C, McCarthy T, Anand P. Mechanisms underlying clinical efficacy of angiotensin II type 2 receptor (AT2R) antagonist EMA401 in neuropathic pain: clinical tissue and in vitro studies. Mol Pain 2015;11;38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice ASC. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain 2012;16:485–95. [DOI] [PubMed] [Google Scholar]
- [5].Asada H, Horita S, Hirata K, Shiroishi M, Shiimura Y, Iwanari H, Hamakubo T, Shimamura T, Nomura N, Kusano-Arai O, Uemura T, Suno C, Kobayashi T, Iwata S. Crystal structure of the human angiotensin II type 2 receptor bound to an angiotensin II analog. Nat Struct Biol 2018;25:570–76. [DOI] [PubMed] [Google Scholar]
- [6].Asada H, Inoue A, Kadji FMN, Hirata K, Shiimura Y, Im D, Shimamura T, Nomura N, Iwanari H, Hamakubo T, Kusano-Arai O, Hisano H, Uemura T, Suno C, Aoki J, Iwata S. Structure 2020;28:418–25. [DOI] [PubMed] [Google Scholar]
- [7].Benitez SG, Seltzer AM, Messina DN, Foscolo MR, Patterson SI, Acosta CG. Cutaneous inflammation differentially regulates the expression and function of angiotensin-II types 1 and 2 receptors in rat primary sensory neurons. J Neurochem 2020;152:675–96. [DOI] [PubMed] [Google Scholar]
- [8].Bharate SS. Recent developments in pharmaceutical salts: FDA approvals from 2015 to 2019. Drug Discov Today 2021;26:384–98. [DOI] [PubMed] [Google Scholar]
- [9].Black JA, Nikolajsen L, Kroner K, Jensen TS, Waxman SG. Multiple sodium channel isoforms and mitogen-activated protein kinases are present in painful human neuromas. Ann Neurol 2008;64:644–53. [DOI] [PubMed] [Google Scholar]
- [10].Breivik H, Eisenberg E, O'Brien T, OPENMinds. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013;1229:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caudle RM, Smith MT, Romero-Sandoval EA. Editorial: verification of animal pain models by reverse translation. Front Pharmacol 2021;12:778880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chakrabarty A, Blacklock A, Svojanovsky S, Smith PG. Estrogen elicits dorsal root ganglion axon sprouting via a renin-angiotensin system. Endocrinol 2008;149:3452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chakrabarty A, Liao Z, Smith PG. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory pain. J Pain 2013;14:1053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chakrabarty A, Liao Z, Mu Y, Smith PG. Inflammatory renin-angiotensin system disruption attenuates sensory hyperinnervation and mechanical hypersensitivity in a rat model of provoked vestibulodynia. J Pain 2018;19:264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cote F, Laflamme L, Payet MD, Gallo-Payet N. Nitric oxide, a new second messenger involved in the action of angiotensin II on neuronal differentiation of NG108-15 cells. Endocr Res 1998;24:403–07. [DOI] [PubMed] [Google Scholar]
- [16].Connolly A, Holleran BJ, Simard E, Baillargeon J-P, Lavigne P, Leduc R. Interplay between intracellular loop 1 and helix VIII of the angiotensin II type 2 receptor controls its activation. Biochem Pharmacol 2019;168:330–38. [DOI] [PubMed] [Google Scholar]
- [17].D'Agnelli S, Gerra MC, Bignami E, Arendt-Nielsen L. Exosomes as a new pain biomarker opportunity. Mol Pain 2020;16:1744806920957800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults. MMWR Morb Mortal Wkly Rep 2018;67:1001–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Deraet M, Rihakova L, Boucard A, Perodin J, Sauve S, Mathiew AP, Guillemette G, Leduc R, Lavigne P, Escher E. Angiotensin II is bound to both receptors AT1 and AT2, parallel to the transmembrane domains and in an extended form. Can J Physiol Pharmacol 2002;80:418–25. [DOI] [PubMed] [Google Scholar]
- [20].De Rantere D, Schuster CJ, Reimer JN, Pang DSJ. The relationship between the Rat Grimace Scale and mechanical hypersensitivity testing in three experimental pain models. Eur J Pain 2016;20:417–26. [DOI] [PubMed] [Google Scholar]
- [21].Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, Chappell A, Chartier K, Cleeland CS, Costello A, Cowan P, Dimitrova R, Ellenberg S, Farrar JT, French JA, Gilron I, Hertz S, Jadad AR, Jay GW, Kalliomaki J, Katz NP, Kerns RD, Manning DC, Mcdermott MP, Mcgrath PJ, Narayana A, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Reeve BB, Rhodes T, Sampaio C, Simpson DM, Stauffer JW, Stucki G, Tobias J, White RE, Witter J. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. PAIN 2010;149:177–93. [DOI] [PubMed] [Google Scholar]
- [22].Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, Mcdermott MP, Munera C, Porter L, Rauschkolb C, Rice AS, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. PAIN 2012;153:1148–58. [DOI] [PubMed] [Google Scholar]
- [23].Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open 2016;6:e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garcia-Perez E, Schonberger T, Sumalla M, Stierstorfer B, Sola R, Doods H, Serra J, Gorodetskaya N. Behavioural, morphological and electrophysiological assessment of the effects of type 3 diabetes mellitus on large and small nerve fibres in Zucker diabetic fatty, Zucker lean and Wistar rats. Eur J Pain 2018;22:1457–72. [DOI] [PubMed] [Google Scholar]
- [25].Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2022;13:715–24. [DOI] [PubMed] [Google Scholar]
- [26].Gendron L, Laflamme L, Rivard N, Asselin C, Payet MD, Gallo-Payet N. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol Endocrinol 1999;13:1615–26. [DOI] [PubMed] [Google Scholar]
- [27].Gewandter JS, Dworkin RH, Turk DC, Mcdermott MP, Baron R, Gastonguay MR, Gilron I, Katz NP, Mehta C, Raja SN, Senn S, Taylor C, Cowan P, Desjardins P, Dimitrova R, Dionne R, Farrar JT, Hewitt DJ, Iyengar S, Jay GW, Kalso E, Kerns RD, Leff R, Leong M, Petersen KL, Ravina BM, Rauschkolb C, Rice AS, Rowbotham MC, Sampaio C, Sindrup SH, Stauffer JW, Steigerwald I, Stewart J, Tobias J, Treede RD, Wallace M, White RE. Research designs for proof-of-concept chronic pain clinical trials: IMMPACT recommendations. PAIN 2014;155:1683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gewandter JS, Dworkin RH, Turk DC, Devine EG, Hewitt D, Jensen MP, Katz NP, Kirkwood AA, Malamut R, Markman JD, Vrijens B, Burke L, Campbell JN, Carr DB, Conaghan PG, Cowan P, Doyle MK, Edwards RR, Evans SR, Farrar JT, Freeman R, Gilron I, Juge D, Kerns RD, Kopecky EA, McDermott MP, Niebler G, Patel KV, Rauck R, Rice ASC, Rowbotham M, Sessler NE, Simon LS, Singla N, Skljarevski V, Tockarshewsky T, Vanhove GF, Wasan AD, Witter J. Improving study conduct and data quality in clinical trials of chronic pain treatments: IMMPACT recommendations. J Pain 2020;21:931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gosnell ME, Staikopoulos V, Anwer AG, Mahbub SB, Hutchinson MR, Mustafa S, Goldys EM. Autofluorescent imprint of chronic constriction nerve injury identified by deep learning. Neurobiol Dis 2021;160:105528. [DOI] [PubMed] [Google Scholar]
- [30].Guo Y, Huang X, Liao W, Meng L, Xu D, Ye C, Chen L, Hu T. Discovery and optimization of highly potent and selective AT2R antagonists to relieve peripheral neuropathic pain. ACS Omega 2021;6:15412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hafko R, Villapol S, Nostramo R, Symes A, Sabban EL, Inagami T, Saavedra JM. Commercially available angiotensin II At2 receptor antibodies are nonspecific. Plos One 2013;8:e69234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Han FY, Wyse BD, Smith MT. Optimization and pharmacological characterization of a refined cisplatin-induced rat model of peripheral neuropathic pain. Behav Pharmacol 2014;25:732–40. [DOI] [PubMed] [Google Scholar]
- [33].Han FY, Kuo A, Nicholson JR, Corradini L, Smith MT. Comparative analgesic efficacy of pregabalin administered according to either a prevention protocol or an intervention protocol in rats with cisplatin-induced peripheral neuropathy. Clin Exp Pharmacol Physiol 2018;45:1067–75. [DOI] [PubMed] [Google Scholar]
- [34].Han FY, Brockman DA, Nicholson JR, Corradini L, Smith MT. Gait analysis as a robust pain behavioural endpoint in the chronic phase of the monoiodoacetate-induced knee joint pain in the rat. Behav Pharmacol 2022;33:23–31. [DOI] [PubMed] [Google Scholar]
- [35].Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 2009;296:R208–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Horiuchi M, Iwanami J, Mogi M. Regulation of angiotensin II receptors beyond the classical pathway. Clin Sci 2012;123:193–203. [DOI] [PubMed] [Google Scholar]
- [37].Hudmon A, Choi JS, Tyrrell L, Black JA, Rush AM, Waxman SG, Dib-Hajj SD. Phosphorylation of sodium channel Na(v)1.8 by p38 mitogen-activated protein kinase increases current density in dorsal root ganglion neurons. J Neurosci 2008;28:3190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. PAIN 2012;153:1837–46. [DOI] [PubMed] [Google Scholar]
- [39].Imam MZ, Kuo A, Nicholson JR, Corradini L, Smith MT. Assessment of the anti-allodynic efficacy of a glycine transporter 2 inhibitor relative to pregabalin and duloxetine in a rat model of prostate cancer-induced bone pain. Pharmacol Rep 2020;72:1418–25. [DOI] [PubMed] [Google Scholar]
- [40].Inoue S, Kobayashi F, Nishihara M, Young-Chang PA, Ikemoto T, Kawai T, Inoue M, Hasegawa T, Ushida T. Chronic pain in the Japanese community - prevalence, characteristics and impact on quality of life. PLOS one 2015;10:e0129262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ito S, Tajima K, Nogawa M, Inoue N, Kyoi T, Takahashi Y, Sasagawa T, Nakamura A, Kotera T, Ueda M, Yamashita Y, Banno K. Etodolac, a cyclooxygenase-2 inhibitor, attenuates paclitaxel-induced peripheral neuropathy in a mouse model of mechanical allodynia. J Pharmacol Exp Ther 2012;342:53–60. [DOI] [PubMed] [Google Scholar]
- [42].Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002;36:57–68. [DOI] [PubMed] [Google Scholar]
- [43].Joshi SK, Hernandez G, Mikusa JP, Zhu CZ, Zhong C, Salyers A, Wismer CT, Chandran P, Decker MW, Honore P. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience 2006;143:587–96. [DOI] [PubMed] [Google Scholar]
- [44].Kalliomaki J, Attal N, Jonzon B, Bach FW, Huizar K, Ratcliffe S, Eriksson B, Janecki M, Danilov A, Bouhassira D. AZD2423 PTN Study Group. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. PAIN 2013;154:761–67. [DOI] [PubMed] [Google Scholar]
- [45].Khan N, Woodruff TM, Smith MT. Establishment and characterization of an optimized mouse model of multiple sclerosis-induced neuropathic pain using behavioural, pharmacologic, histologic and immunohistochemical methods. Pharmacol Biochem Behav 2014;136:13–27. [DOI] [PubMed] [Google Scholar]
- [46].Khan N, Gordon R, Woodruff TM, Smith MT. Antiallodynic effects of alpha lipoic acid in an optimized RR-EAE mouse model of MS-neuropathic pain are accompanied by attenuation of upregulated BDNF-TrkB-ERK signaling in the dorsal horn of the spinal cord. Pharma Res Per 2015;3:e00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Khan N, Muralidharan A, Smith MT. Attenuation of the infiltration of angiotensin II expressing CD3+ T-cells and the modulation of nerve growth factor in lumbar dorsal root ganglia – a possible mechanism underpinning analgesia produced by EMA300, an angiotensin II Type 2 (AT2) receptor antagonist. Front Mol Neurosci 2017;10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khan N, Kuo A, Brockman DA, Cooper MA, Smith MT. Pharmacological inhibition of the NLRP3 inflammasome as a potential target for multiple sclerosis induced central neuropathic pain. Inflammopharmacology 2018;26:77–86. [DOI] [PubMed] [Google Scholar]
- [49].Kingwell K. Nav1.7 withholds its pain potential. Nat Rev Drug Discov 2019;18:321–23. [DOI] [PubMed] [Google Scholar]
- [50].Kondo T, Obata K, Miyoshi K, Sakurai J, Tanaka J, Miwa H, Noguchi. Transient receptor potential A1 mediates gastric distention-induced visceral pain in rats. Gut 2009;58:1342–52. [DOI] [PubMed] [Google Scholar]
- [51].Kondo T, Sakurai J, Miwa H, Noguchi K. Activation of p38 MAPK through transient receptor potential A1 in a rat model of gastric distension-induced visceral pain. Neuroreport 2013;24:68–72. [DOI] [PubMed] [Google Scholar]
- [52].Koyama S, LeBlanc BW, Smith KA, Roach C, Levitt J, Edhi MM, Michishita M, Komatsu T, Mashita O, Tanikawa A, Yoshikawa S, Saab CY. An electroencephalography bioassay for preclinical testing of analgesic efficacy. Sci Rep 2018;8:16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kuo A, Nicholson JR, Corradini L, Smith MT. Establishment and characterisation of a stavudine (d4T)-induced rat model of antiretroviral toxic neuropathy (ATN) using behavioural and pharmacological methods. Inflammopharmacol 2019;27:387–96. [DOI] [PubMed] [Google Scholar]
- [54].Kuo A, Corradini L, Nicholson JR, Smith MT. Assessment of the anti-allodynic and anti-hyperalgesic efficacy of a glycine transporter 2 inhibitor relative to pregabalin, duloxetine and indomethacin in a rat model of cisplatin-induced peripheral neuropathy. Biomolecules 2021;11:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Laghlam D, Jozwiak M, Nguyen LS. Renin-angiotensin-aldosterone system and immunomodulation: a state-of-the-art review. Cells 2021;10:1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenverg AMJM, Ferrari MD, Craig KD, Mogil JS. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 2010;7:447–9. [DOI] [PubMed] [Google Scholar]
- [57].Leadley RM, Armstrong N, Lee YC, Allen A, Kleijnen J. Chronic diseases in the European Union: the prevalence and health cost implications of chronic Pain. J Pain Palliat Care Pharmacother 2012;26:310–25. [DOI] [PubMed] [Google Scholar]
- [58].LeBlanc BW, Bowary PM, Chao YC, Lii TR, Saab CY. Electroencephalographic signatures of pain and analgesia in rats. PAIN 2016;157:2330–40. [DOI] [PubMed] [Google Scholar]
- [59].Leung VSY, Benoit-Biancamamo M-O, Pang DSJ. Performance of behavioral assays: the Rat Grimace Scale, burrowing activity and a composite behavior score to identify visceral pain in an acute and chronic colitis model. Pain Rep 2019;4:e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ling B, Coudore F, Decalonne L, Eschalier A, Authier N. Comparative antiallodynic activity of morphine, pregabalin and lidocaine in a rat model of neuropathic pain produced by one oxaliplatin injection. Neuropharmacology 2008;55:724–8. [DOI] [PubMed] [Google Scholar]
- [61].Martin SW, Butcher AJ, Berrow NS, Richards MW, Paddon RE, Turner DJ, Dolphin AC, Sihra TS, Fitzgerald EM. Phosphorylation sites on calcium channel alpha1 and beta subunits regulate ERK-dependent modulation of neuronal N-type calcium channels. Cell Calcium 2006;39:275–92. [DOI] [PubMed] [Google Scholar]
- [62].Millecamps M, Tajerian M, Sage EH, Stone LS. Behavioral signs of chronic back pain in the SPARC-null mouse. Spine (Phila Pa 1976) 2011;36:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Miller A, Sanderson K, Bruno R, Breslin N, Neil AL. The prevalence of pain and analgesia use in the Australian population: findings from the 2011 to 2012 Australian National Health Survey. Pharmacoepidemiol Drug Saf 2017;26:1403–10. [DOI] [PubMed] [Google Scholar]
- [64].Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth 2019;123:e273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci 2009;10:283–94. [DOI] [PubMed] [Google Scholar]
- [66].Mogil JS. The translatability of pain across species. Philos Trans R Soc Lond B Biol Sci 2019;374:20190286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mogil JS, Pang DS, Dutra GGS, Chambers CT. The development and use of facial grimace scales for pain measurement in animals. Neurosci Biobehav Rev 2020;116:480–93. [DOI] [PubMed] [Google Scholar]
- [68].Mukherjee P, Cinelli MA, Kang S, Silverman RB. Development of nitric oxide synthase inhibitors for neurodegeneration and neuropathic pain. Chem Soc Rev 2014;43:6814–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Muralidharan A, Wyse BD, Smith MT. Optimization and characterization of a rat model of prostate cancer-induced bone pain using behavioral, pharmacological, radiological, histological and immunohistochemical methods. Pharmacol Biochem Behav 2013;106:33–46. [DOI] [PubMed] [Google Scholar]
- [70].Muralidharan A, Wyse BD, Smith MT. Analgesic efficacy and mode of action of a selective small molecule angiotensin II type 2 receptor antagonist in a rat model of prostate cancer-induced bone pain. Pain Med 2014;15:93–110. [DOI] [PubMed] [Google Scholar]
- [71].Muralidharan A, Smith MT. Targeting angiotensin II type 2 receptor pathways to treat neuropathic pain and inflammatory pain. Expert Opin Ther Targets 2015;19:25–35. [DOI] [PubMed] [Google Scholar]
- [72].Muralidharan A, Kuo A, Jacob M, Lourdesamy JS, Soares LM, De Carvalho P, Nicholson JR, Corradini L, Smith MT. Comparison of burrowing and stimuli-evoked pain behaviors as end-points in rat models of inflammatory pain and peripheral neuropathic pain. Front Behav Neurosci 2016;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Muralidharan A, Park TWS, Mackie JT, Gimenez LGS, Kuo A, Nicholson JR, Corradini L, Smith MT. Establishment and characterization of a novel rat model of mechanical low back pain using behavioral, pharmacologic and histologic methods. Front Pharmacol 2017;8:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Murgasova R, Carreras ET, Suetterlin-Hachmann M, da Silva Torrao LR, Kitterman M, Alexandra V, Fredenhagen A. Non-clinical characterization of the disposition of EMA401, a novel small molecule angiotensin II type 2 (AT2R) antagonist. Biopharm Drug Dispos 2020;41:166–83. [DOI] [PubMed] [Google Scholar]
- [75].Ojeda B, Salazar A, Duenas M, Torres LM, Mico JA, Failde I. The impact of chronic pain: the perspective of patients, relatives, and caregivers. Fam Syst Health 2014;32:399–407. [DOI] [PubMed] [Google Scholar]
- [76].Ostenfeld T, Krishen A, Lai RY, Bullman J, Baines AJ, Green J, Anand P, Kelly M. Analgesic efficacy and safety of the novel p38 MAP kinase inhibitor, losmapimod, in patients with neuropathic pain following peripheral nerve injury: a double-blind, placebo-controlled study. Eur J Pain 2013;17:844–57. [DOI] [PubMed] [Google Scholar]
- [77].Otto KJ, Wyse BD, Cabot PJ, Smith MT. Longitudinal study of painful diabetic neuropathy in the Zucker diabetic fatty rat model of type 2 diabetes: impaired basal G-protein activity appears to underpin marked morphine hyposensitivity at 6 months. Pain Med 2011;12:437–50. [DOI] [PubMed] [Google Scholar]
- [78].Pain research forum. Available at: https://www.painresearchforum.org/resources/pain-gene-resource. Accessed March 16, 2022. [Google Scholar]
- [79].Park TSW, Khan N, Kuo A, Nicholson JR, Corradini L, Smith MT. Characterisation of a rat model of mechanical low back pain at an advanced stage using immunohistochemical methods. Clin Exp Pharmacol Physiol 2021;48:96–106. [DOI] [PubMed] [Google Scholar]
- [80].Patil J, Schwab A, Nussberger J, Schaffner T, Saavedra JM, Imboden H. Intraneuronal angiotensinergic system in rat and human dorsal root ganglia. Regul Pept 2010;162:90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 2006;86:747–803. [DOI] [PubMed] [Google Scholar]
- [82].Pavel J, Oroszova Z, Hricova L, Lukacova N. Effect of subpressor dose of angiotensin II on pain-related behavior in relation with neuronal injury and activation of satellite glial cells in the rat dorsal root ganglia. Cell Mol Neurobiol 2013;33:681–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Persson AK, Gasser A, Black JA, Waxman SG. Nav1.7 accumulates and co-localizes with phosphorylated ERK1/2 within transected axons in early experimental neuromas. Exp Neurol 2011;230:273–9. [DOI] [PubMed] [Google Scholar]
- [84].Plouffe B, Guimond M-O, Beaudry H, Gallo-Payet N. Role of tyrosine kinase receptors in angiotensin II AT2 receptor signaling: involvement in neurite outgrowth and in p42/p44mapk activation in NG108-15 cells. Endocrinol 2006;147:4646–54. [DOI] [PubMed] [Google Scholar]
- [85].re3data.org: Pain Genes Database; editing status 2021-01-12; re3data.org - Registry of Research Data Repositories. Available at: 10.17616/R3WP95. Accessed March 16, 2022. [DOI]
- [86].Reckziegel D, Vachon-Presseau E, Petre B, Schnitzer TJ, Baliki MN, Apkarian AV. Deconstructing biomarkers for chronic pain: context- and hypothesis-dependent biomarker types in relation to chronic pain. PAIN 2019;160(Suppl 1):S37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Rice ASC, Dworkin RH, McCarthy TD, Anand P, Bountra C, McCloud PI, Hill J, Cutter G, Kitson G, Desem N, Raff M; for the EMA401-003 study group. EMA401, an orall administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial. Lancet 2014;383:1637–47. [DOI] [PubMed] [Google Scholar]
- [88].Rice AS, Smith MT. Angiotensin II type 2-receptor: new clinically validated target in the treatment of neuropathic pain. Clin Pharmacol Ther 2015;97:128–30. [DOI] [PubMed] [Google Scholar]
- [89].Rice ASC, Dworkin RH, Finnerup NB, Attal N, Anand P, Freeman R, Piaia A, Callegari F, Doerr C, Mondal S, Narayanan N, Ecochard L, Flossbach Y, Pandhi S. Efficacy and safety of EMA401 in peripheral neuropathic pain: results of two randomised, double-blind, phase 2 studies in patients with postherpetic neuralgia and painful diabetic neuropathy. PAIN 2021;162:2578–89. [DOI] [PubMed] [Google Scholar]
- [90].Rutten K, Gould SA, Bryden L, Doods H, Christoph T, Pekcec A. Standard analgesics reverse burrowing deficits in a rat CCI model of neuropathic pain, but not in models of type 1 and type 2 diabetes-induced neuropathic pain. Behav Brain Res 2018;350:129–38. [DOI] [PubMed] [Google Scholar]
- [91].Sadybekov A, Katritch V. Breaking the enigma code of angiotensin II type 2 receptor signaling. Structure 2020;28:390–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Salat K, Furgala A, Malikowska-Racia. Searching for analgesic drug candidates alleviating oxaliplatin-induced cold hypersensitivity in mice. Chem Biol Drug Des 2019;93:1061–72. [DOI] [PubMed] [Google Scholar]
- [93].Schuelert N, Gorodetskaya N, Just S, Doods H, Corradini L. Electrophysiological characterization of spinal neurons in different models of diabetes type 1- and type2-induced neuropathy in rats. Neuroscience 2015;291:146–54. [DOI] [PubMed] [Google Scholar]
- [94].Seto Y, Takase M, Tsuji Y, To H. Pregabalin reduces cisplatin-induced mechanical allodynia in rats. J Pharmacol Sci 2017;134:175–80. [DOI] [PubMed] [Google Scholar]
- [95].Shenoy P, Kuo A, Vetter I, Smith MT. Optimization and in vivo profiling of a refined rat model of walker 256 breast cancer cell-induced bone pain using behavioral, radiological, histological, immunohistochemical and pharmacological methods. Front Pharmacol 2017;8:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shepherd AJ, Micke AD, Golden JP, Mack MR, Halavi CM, de Kloet AD, Samineni VK, Kim BS, Krause EG, Gereau RW, IV, Mohapatra DP. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci USA 2018;115:E8057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shepherd AJ, Copits BA, Mickle AD, Karlsson P, Kadunganattil S, Haroutounian S, Tadinada SM, de Kloet AD, Valtcheva MV, McIlvried LA, Sheahan TD, Jain S, Ray PR, Usachev YN, Dussor G, Krause EG, Price TJ, Gereau RW, IV, Mohapatra DP. Angiotensin II triggers peripheral macrophage-to-sensory neuron redox crosstalk to elicit pain. J Neurosci 2018b;38:7032–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Shepherd AJ, Mohapatra DP. Pain hypersensitivities associated with experimental neuropathy in mice by angiotensin II type-2 receptor antagonism. Anesth Analg 2019;128:e84–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Sindrup SH, Graf A, Sfikas N. The NK1-receptor antagonist TKA731 in painful diabetic neuropathy: a randomised, controlled trial. Eur J Pain 2006;10:567–71. [DOI] [PubMed] [Google Scholar]
- [100].Sliepen SHJ, Diaz-Delcastillo M, Korioth J, Olsen RB, Appel CK, Christoph T, Heegaard A-M, Rutten K. Cancer-induced bone pain impairs burrowing behaviour in mouse and rat. In Vivo 2019;33:1125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Smith MT, Wyse B. Methods of treatment or prophylaxis (of neuropathic conditions) PCT/AU2005/001975, 2006. [Google Scholar]
- [102].Smith MT. Methods of treatment or prophylaxis of inflammatory pain PCT/AU2007/00339, 2007. World Intellectual Property Organisation, International Publication Number: WO/2007/106938. [Google Scholar]
- [103].Smith MT, Wyse BD, Edwards SR. Small molecule angiotensin II type 2 receptor (AT2R) antagonists as novel analgesics for neuropathic pain: comparative pharmacokinetics, radioligand binding and efficacy in rats. Pain Med 2013a;106:33–46. [DOI] [PubMed] [Google Scholar]
- [104].Smith MT, Woodruff Wyse BD, Muralidharan A, Walther T. A small molecule angiotensin II type 2 receptor (AT2R) antagonist produces analgesia in a rat model of neuropathic pain by inhibition of p38 mitogen activated protein kinase (MAPK) and p44/p42 MAPK activation in the dorsal root ganglia. Pain Med 2013b;14:1557–68. [DOI] [PubMed] [Google Scholar]
- [105].Smith MT, Lau T, Wallace VCJ, Wyse BD, Rice ASC. Analgesic efficacy of small-molecule angiotensin II type 2 receptor antagonists in a rat model of antiretroviral toxic polyneuropathy. Behav Pharmacol 2014;25:137–46. [DOI] [PubMed] [Google Scholar]
- [106].Smith MT, Anand P, Rice ASC. Selective small molecule angiotensin II type 2 receptor antagonists for neuropathic pain: preclinical and clinical studies. PAIN 2016;157(suppl l):S33–41. [DOI] [PubMed] [Google Scholar]
- [107].Smith SM, Dworkin RH, Turk DC, Baron R, Polydefkis M, Tracey I, Borsook D, Edwards RR, Harris RE, Wager TD, Arendt-Nielsen L, Burke LB, Carr DB, Chappell A, Farrar JT, Freeman R, Gilron I, Goli V, Haeussler J, Jensen T, Katz NP, Kent J, Kopecky EA, Lee DA, Maixner W, Markman JD, McArthur JC, McDermott MP, Parvathenani L, Raja SN, Rappaport BA, Rice ASC, Rowbotham MC, Tobias JK, Wasan AD, Witter J. The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain 2017;18:757–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].South SM, Smith MT. Analgesic tolerance to opioids. Pain Clin Updates 2001;9:1–4. [Google Scholar]
- [109].Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrel L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. J Neurosci 2010;30:1637–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Starobova H, Mueller A, Deuis JR, Carter DA, Vetter I. Inflammatory and neuropathic gene expression signatures of chemotherapy-induced neuropathy induced by vincristine, cisplatin, and oxaliplatin in C57BL/6J mice. J Pain 2020;21:182–94. [DOI] [PubMed] [Google Scholar]
- [111].Tappe-Theodor A, King T, Morgan MM. Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci Biobehav Rev 2019;100:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tigerstedt R, Bergman PQ. Niere und Kreislauf1. Skandinavisches Archiv Für Physiologie 1898;8:223–71. [Google Scholar]
- [113].Timmermans PB, Wong PC, Chiu AT, Herblin WF, Smith RD. New perspectives in angiotensin system control. J Hum Hypertens 1993;7(suppl 2):S19–31. [PubMed] [Google Scholar]
- [114].Tracey I, Woolf CJ, Andrews NA. Composite pain biomarker signatures for objective assessment and effective treatment. Neuron 2019;101:783–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Turu G, Szidonya L, Gáborik Z, Buday L, Spät A, Clark AJL, Hunyady L. Differential beta-arrestin binding of AT1 and AT2 angiotensin receptors. FEBS Lett 2006;580:41–5. [DOI] [PubMed] [Google Scholar]
- [116].Viswanath O, Urits I, Burns J, Charipova K, Gress K, McNally A, Urman RD, Welschmeyer A, Berger AA, Kassem H, Sanchez MG, Kaye AD, Eubanks TN, Cornett EM, Ngo AL. Central neuropathic mechanisms in pain signaling pathways: current evidence and recommendations. Adv Ther 2020;37:1946–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Wallace VCJ, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice ASC. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain 2007a;130:2688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. PAIN 2007b;133:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wodarski R, Delaney A, Ultenius C, Morland R, Andrews N, Baastrup C, Bryden LA, Caspani O, Christoph T, Gardiner MJ, Huang W, Kennedy JD, Koyama S, Li D, Ligocki M, Lindsten A, Machin I, Pekcec A, Robens A, Rotariu SM, Vo S, Segerdahl M, Stenfors C, Svensson CI, Treede RD, Uto K, Yamamoto K, Rutten K, Rice AS. Cross-centre replication of suppressed burrowing behaviour as an ethologically relevant pain outcome measure in the rat: a prospective multicentre study. PAIN 2016;157:2350–65. [DOI] [PubMed] [Google Scholar]
- [120].Wolf G, Ziyadeh FN, Thaiss F, Tomaszewski J, Caron RJ, Wenzel U, Zahner G, Helmchen U, Stahl RA. Angiotensin II stimulates expression of the chemokine RANTES in rat glomerular endothelial cells. Role of the angiotensin type 2 receptor. J Clin Invest 1997;100:1047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Woolf CJ. Capturing novel non-opioid targets. Biol Psychiatry 2020;87:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Xu Y, Tian NX, Bai QY, Chen Q, Sun XH, Wang Y. Gait assessment of pain and analgesics: comparison of the DigiGait™ and CatWalk™ gait imaging systems. Neurosci Bull 2019;35:401–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Yekkirala AS, Robertson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 2017;16:545–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Yezierski RP, Hansson P. Inflammatory and neuropathic pain from bench to bedside: what went wrong?. J Pain 2018;19:571–88. [DOI] [PubMed] [Google Scholar]
- [125].Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI. Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 2020;11:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Yu X, Basbaum A, Guan Z. Contribution of colony-stimulating factor 1 to neuropathic pain. Pain Rep 2021;6:e883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zanata GC, Pinto LG, da Silva NR, Lopes AHP, de Oliveira FFB, Schivo IRS, Cunha FQ, McNaughton P, Cunha TM, Silva RL. Blockade of bradykinin receptors or angiotensin II type 2 receptor prevents paclitaxel-associated acute pain syndrome in mice. Eur J Pain 2021;25:189–98. [DOI] [PubMed] [Google Scholar]
- [128].Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, Sadybekov A, Zamlynny B, Rudd MT, Hollenstein K, Tolstikova A, White TA, Hunter MS, Weierstall U, Liu W, Babaoglu K, Moore EL, Katz RD, Shipman JM, Garcia-Calvo M, Sharma S, Sheth P, Soisson SM, Stevens RC, Katrich V, Cherezov V. Structural basis for selectivity and diversity in angiotensin II receptors. Nature 2017;544:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Zimmerman M. Pathobiology of neuropathic pain. Eur J Pharmacol 2001;429:23–37. [DOI] [PubMed] [Google Scholar]


