Abstract
In the northeastern U.S., Borrelia burgdorferi sensu stricto, the agent of Lyme disease, is maintained between vertebrate hosts and subadult deer ticks (the northern clade of Ixodes scapularis, formerly known as Ixodes dammini). Theoretical arguments suggest that the force of transmission would be greatest when infected nymphal ticks focus their bites on the same host as the uninfected larvae. Stage-specific differences in host utilization would reduce the force of transmission, but to date such differences remain understudied. We determined the host utilization differences of larval and nymphal deer ticks using bloodmeal analysis of host-seeking nymphs and adults collected from 5 field sites in New England. Matched cohorts of ticks (nymphs=506, adults=451), i.e. ticks that had fed during the same summer season, were used to control for yearly host population variations. Infection status of all ticks was determined by real time PCR. Nymphal deer ticks were more likely to have fed on birds and sciurids (13% vs 3%, and 41% vs 9%, respectively p<0.001) and larvae were more likely to have fed on shrews (26% vs 3%, p<0.001). Similarly, ticks that had fed on a mouse or a shrew as larvae were likely to become infected (OR= 3.195, 95% CI [1.9, 5.1] and OR=2.5[1.6,3.8] respectively), and they were positively associated with infection prevalence at our sites. However, very few nymphs fed on shrews, and they were not associated with infection, raising the question of how uninfected shrews acquire infection each year. Sciurids did not appear to contribute to the enzootic cycle at our sites, which may be due to the low numbers of larvae that fed on them. Sciurid-fed ticks of either stage were not associated with infection. Both stages of ticks were less likely to be infected if they had fed on deer (OR=0.08 [0.02.0.3] and OR=0.4 [0.2,0.7] tested as nymphs and adults, respectively) and thus deer likely served to reduce the force of transmission at our sites. Site-specific analysis of differential host utilization by subadult deer ticks may contribute to appropriate targeting of interventions and thereby promote reducing risk of acquiring Lyme disease and the other deer tick-transmitted infections.
Keywords: Ixodes, Borrelia, Bloodmeal, Tick, Hosts
1. Introduction
In the northeastern United States, the enzootic cycle of Borrelia burgdorferi sensu stricto, the agent of Lyme disease, depends on spirochetes circulating between mammalian reservoir hosts and the subadult stages of the northern clade of Ixodes scapularis, previously known as Ixodes dammini, referred to hereafter as the deer tick. Nymphal ticks seek hosts in the early summer months, in May and June, and may transmit infection during feeding. The infected hosts then serve to infect larval ticks that emerge later in the summer, with peak numbers in August and September. Larvae that acquire spirochetes become infected nymphs the following year which can then transmit the infection to naive hosts during their bloodmeal. The adult stage of the tick does not contribute directly to the cycle because they feed primarily on deer, which have poor reservoir competence. They are, however, the reproductive host for the tick and their feeding success largely determines tick abundance.
Maintenance of the enzootic cycle depends on Borrelia-infected nymphs, which are also the main source of infection for humans. The density of infected nymphs or entomological inoculation rate (DIN or EIR) are used as estimators for the intensity of enzootic transmission or human risk (Mather et al., 1996; Spielman, 1999). EIR is calculated by multiplying the nymphal tick abundance by the prevalence of infection. It is highly variable in time and space and depends on complex interactions between the spirochete, the tick vector, their hosts, as well as abiotic factors such as weather. The basic reproduction number for B. burgdorferi s.s. depends on the hosts that provide bloodmeals for subadult deer ticks. Theoretical arguments (Spielman et al., 1984) suggest that the force of enzootic transmission is greatest when infected nymphs focus their bites on the same hosts as those that feed larvae. Enzootic transmission would be reduced when the hosts for the larvae and nymphs differ. The contribution of less reservoir competent hosts such as deer (Telford III et al., 1988) to feeding subadult deer ticks would further reduce the force of transmission, which has been characterized as zooprophylaxis (Spielman et al., 1984) or the dilution effect (LoGiudice et al., 2003). Analyzing host utilization patterns for deer ticks is the basis for understanding EIR and ultimately provides the data required to develop, implement, and evaluate interventions that seek to target the source of infection.
Differential host utilization was recognized in the earliest studies on the biology of ticks (Hooker et al., 1912) and comprises preferences for specific hosts, in addition to their capacity to provide an adequate bloodmeal and thereby promote tick development. Host capacity, i.e. the ability to serve as a host, may be dynamic: partial or incomplete feeding may occur due to grooming behavior or because of immune reactions of the host due to repeated infestation (Davidar et al., 1989; Keesing et al., 2009; Shaw et al., 2003). Deer ticks are often characterized as generalist feeders because they have been recorded from many kinds of animals. However, the deer tick and closely related species appear to have clear host preferences (Egyed, 2017; James and Oliver, 1990; Slowik and Lane, 2009). Adult deer ticks infest deer and other mid- to large-sized animals but never rodents or birds (Spielman et al., 1979), whereas the subadults are considered to be generalists in their host utilization. Interestingly, we compared host bloodmeal remnants in field-collected nymphal deer ticks and demonstrated that larvae had preferentially fed on mice as opposed to deer, but Amblyomma americanum nymphs collected from the same site had fed preferentially on deer as larvae and never on rodents (Goethert and Telford III, 2022a). Finally, host utilization is also determined by host availability (Van Oosten et al., 2016), which depends on the local diversity of animals, in addition to their distribution and abundance within habitat where ticks are found. Host utilization, then, is the result of a complex and dynamic mix of host availability, host preference, and host infestation tolerance.
The potential impact of differences in host utilization on the force of transmission has not been directly analyzed. Models for the B. burgdorferi s.s. transmission cycle have tended to incorporate only the different host use of adult and subadult ticks (Occhibove et al., 2021; Ostfeld et al., 2018; Ratti et al., 2021). The potential differences in host utilization between larvae and nymphs have been largely overlooked, even though field studies have demonstrated significant differences in the infestation of likely reservoir hosts such mice, voles, and chipmunks (Main et al., 1982; Matuschka et al., 1991; Schmidt et al., 1999). Unfortunately, studies relying on trapping animals have issues with sampling bias, as not all species are equally likely to be trapped. Furthermore, infestation should not be considered evidence that ticks would successfully feed to repletion and molt the next stage (Keesing et al., 2009). Direct measurement of feeding patterns of larval and nymphal ticks may be accomplished by bloodmeal identification in host seeking nymphs and adults, respectively. Accordingly, we determined whether host utilization differs between nymphal and larval deer ticks within 5 sites in New England using our retrotransposon based bloodmeal identification assays. In addition, we determined the infection status of the ticks to directly measure the relative contribution of each host species to B. burgdorferi s.s.- infected nymphs at our field sites.
2. Methods
Tick collections:
Host seeking deer ticks were collected from vegetation and leaf litter over a 4-year period. Collections comprised Nantucket Island and 4 sites in Rhode Island; EF, MB, Trust and Crew all of which have been described previously (Goethert et al., 2021; Spielman et al., 1981). All sites have mixed successional forests with greenbriar, poison ivy and bittersweet understory. Nantucket has a less diverse mammalian fauna; in particular, it lacks carnivores, mustelids, and most sciurids. To control as much as possible for yearly variations in host availability, we collected matched cohorts of questing nymphs and adults, i.e. ticks that had fed on hosts (as larvae and nymphs, respectively) during the same summer season (Fig. 1). In our field sites, deer ticks have a 2-year life cycle. Larvae feed during the late summer and emerge as nymphs early the next summer, almost a full year later. Nymphs feed in the early summer and emerge as adults in the fall only 4 months later. To examine ticks that had fed during the same season, adults collected in the fall were matched with nymphs that were collected the next summer (Fig. 1). In 2019–2020, ticks from Rhode Island were held at 9 °C for a month before processing. All others were frozen at −20 °C immediately after collection.
Fig. 1. Strategy for collecting matched cohorts of ticks.

Larvae and nymphs that had fed during the same summer season (boxes) were collected as questing ticks after they had molted to the next stage (circles). For example, adults (green circle) collected during Fall 2020 derive from nymphs that had fed during the summer of the same year (green box). Larvae that fed the same season do not emerge as nymphs until the next summer. Thus, nymphs that were collected during summer 2021 (green circle) derive from larvae that had fed during the summer 2020 (green box). Similarly, larvae and nymphs that fed during the summer of 2021 (blue box) were collected as nymphs in the summer of 2022 (blue circle) and the adults in the fall of 2021 (blue circle).
DNA extraction/pathogen testing:
Ticks were processed and tested for B. burgdorferi s.s. as previously described (Goethert et al., 2021). Briefly, ticks were surface sterilized with 5% bleach solution before being placed into individual tubes. They were then crushed using a heat-sealed p1000 pipette tip and extracted using 50 µl of QuickExtract for DNA (Lucigen Corporation, Middleton, WI) following the manufacturer’s instructions. Real time PCR was conducted to assess infection status with B. burgdorferi s.s. using previously published primers (Tokarz et al., 2017).
Bloodmeal analysis:
Bloodmeal analysis was conducted using real time PCR targeting mammalian retrotransposons. A detailed protocol for our bloodmeal identification assay has been published (Goethert, 2021). The ticks in this study were tested for: mouse, vole, rabbit, bird, shrew, squirrel/chipmunk, deer, opossum and skunk/raccoon. Note that the primers targeting squirrel/chipmunk also amplify other Sciuridae. The opossum and skunk/raccoon primers (Table 1) were developed in 2020 and were tested for sensitivity and specificity as previously described (Goethert et al., 2021). Nantucket is not known to be populated by these animals; so initially the ticks from there were not tested with these primers. In 2020, only ticks that had tested negative with all other primers were tested for opossums and skunk/raccoons. In 2021, these primers were incorporated into our regular testing, and all ticks were tested with them. Reactions were multiplexed as follows: multiplex 1= mouse, vole, rabbit; multiplex 2= skunk/raccoon, shrew, deer; and multiplex 3= bird, squirrel, opossum.
Table 1.
Newly designed primers and probes for deer tick bloodmeal identification of opossums and skunks/raccoons.
| Host | Primers and Probes | Fluorophore | Sequence | Sensitivitya | Specificity |
|---|---|---|---|---|---|
|
| |||||
| Opossum | Opo-1-48F | gaggtcctrg gttcaaatct | 10−8 ng/μl | Didelphidae | |
| Opo-1-148R-TG | aaggcagaag agcagtaagg | ||||
| Opo-1-148R-CA | aaggcagaag agtggtaagg | ||||
| Opo-1-85P | HEX | agctgtgtga ccctgggca | |||
| Skunk/Raccoon | Skrac-7F | ctgccttyrg ctcaggt | 10−6 ng/μl | Mephitidae | |
| RacSINE-129R | atcacaagta ggcagagagg | 10−7 ng/μl | Procyon | ||
| RacSINE-33P | HEX | agggtcctgg gatcgagcc | |||
Sensitivity is measured as the last dilution to test positive.
Data analysis:
Graphs and linear regression analysis were conducted using Prism. Prevalence estimates and odds ratios were calculated using online Statpages (https://statpages.info/ctab2x2.html, https://statpages.info/confint.html). Only significant p-values are reported. If ticks tested positive for multiple bloodmeal hosts and there was a difference between the Cts >6 (representative of 2 orders of magnitude), the host with the lower Ct was determined to be the host upon which the tick actually fed. Otherwise, the tick is counted as having fed on both hosts.
Data availablity:
The data from this study have been deposited in the OSF data repository and can be found at https://osf.io/bsnp8/?view_only=411a923027de4afbb63eb3f3cc7aa9c8.
3. Results
During 4 years, we collected 2 matched cohorts of ticks that had fed during the same summer from each field site in the study, for a total of 451 adults matched with 506 nymphs (Table 2). We conducted bloodmeal identification on adult ticks to determine what they had fed on as nymphs, and on nymphal ticks to determine what they had fed on as larvae. We were able to successfully identify bloodmeal host from 76% of the adult ticks tested but only 68% of the nymphs (Fig. 2). The frequency of mouse or deer bloodmeals did not differ for adults and nymphs, 15 and 20% of the ticks respectively, or for the that of rabbits, which fed about 5% overall. However, significantly more adult ticks had fed on birds as nymphs than did nymphs that had fed as larvae (13% vs 3% p<0.001). This demonstrates that more nymphs utilized birds as a host than larvae. The greatest differences detected were for shrews and squirrels/chipmunks. Larvae were more likely to have fed on shrews (tested as nymphs) than nymphs (tested as adults), 26% vs 3% p<0.001. We conclude that larvae often feed on shrews, but nymphs do not. In contrast, squirrels/chipmunks were more likely to be identified as the bloodmeal host in adults than in nymphs (41% vs 9% p<0.001), indicating that nymphal ticks had often fed on squirrels/chipmunks, but larvae did not. For our 4 mainland sites, birds rarely had fed more than 5% of the ticks. In contrast, on Nantucket, birds accounted for 13% of the nymphal bloodmeal hosts and 34% of the adults (Figure S1).
Table 2. Matched cohorts of deer ticks that had fed during the same summer season collected for the study.
See Fig. 1 for interpretations of when each host seeking tick had fed during the previous life stage.
| Location | Field Site | Stage-Year collected | Stage-Year fed | No. |
|---|---|---|---|---|
|
| ||||
| Nantucket | Field Station | Adults-2019 | Nymphs-2019 | 47 |
| Nymphs-2020 | Larvae-2019 | 85 | ||
| Field Station | Adults-2021 | Nymphs-2021 | 43 | |
| Nymphs-2022 | Larvae-2021 | 41 | ||
| Rhode Island | MB | Adults-2020 | Nymphs-2020 | 40 |
| Nymphs-2021 | Larvae-2020 | 47 | ||
| MB | Adults-2021 | Nymphs-2021 | 48 | |
| Nymphs-2022 | Larvae-2021 | 47 | ||
| Crew | Adults-2020 | Nymphs-2020 | 43 | |
| Nymphs-2021 | Larvae-2020 | 49 | ||
| Crew | Adults-2021 | Nymphs-2021 | 46 | |
| Nymphs-2022 | Larvae-2021 | 47 | ||
| EF | Adults-2020 | Nymphs-2020 | 44 | |
| Nymphs-2021 | Larvae-2020 | 48 | ||
| EF | Adults-2021 | Nymphs-2021 | 46 | |
| Nymphs-2022 | Larvae-2021 | 47 | ||
| Trust | Adults-2020 | Nymphs-2020 | 43 | |
| Nymphs-2021 | Larvae-2020 | 48 | ||
| Trust | Adults-2021 | Nymphs-2021 | 46 | |
| Nymphs-2022 | Larvae-2021 | 47 | ||
| Total Adults | 451 | |||
| Total Nymphs | 506 | |||
Fig. 2. Bloodmeal analysis of matched cohorts of adult and nymphal deer ticks.

We present the proportion with 95% confidence intervals of bloodmeal host detected in nymphs (the host that the ticks had fed on as a larvae, light gray) and detected in adults (the host that the ticks had fed on as nymphs, black). Estimates that are significantly different are marked. *p<0.05. sqrl=squirrel or other Sciuridae.
In our mainland sites, none of the ticks tested positive for skunk/raccoon, and only 2 ticks (1 nymph and 1 adult) tested positive for opossum. Similarly, only 3 ticks (2 nymphs and 1 adult) tested positive for vole. Therefore, these hosts are not included in subsequent analyses. Multiple bloodmeal hosts were detected rarely, less than 5% for most field sites (Figure S1). The proportion of nymphal ticks infected by B. burgdorferi s.s. was highly variable by field site but also by year. It ranged from 6% of the nymphs from Rhode Island site MB in 2021 to 85% of the adults from Rhode Island site EF that same year (Table S1). Sequential years at the same field site were often highly variable. The largest difference was at the Rhode Island-MB site: 6% of the nymphs collected in 2021 and for the next summer 38% were infected (Table S1). Bloodmeal analysis showed that most of the infected nymphal ticks had fed as larvae on mice and shrews (35% and 40% respectively), but 10% had fed on squirrels/chipmunks (Fig. 3A). Rabbits, birds and deer each were responsible for fewer than 5% of the infected ticks. Bloodmeal host was unknown for 20% of these ticks; this is similar to the rate that we see overall for nymphs in this study. Odds ratios were calculated to estimate the likelihood that an infected nymph had fed on each host as a larva. Having fed on a mouse or a shrew was significantly associated with becoming infected with spirochetes (OR= 3.195, 95% CI [1.9, 5.1] for mice, and OR=2.5[1.6,3.8] for shrews (Fig. 3C)). Rabbits, birds and squirrels/chipmunks were not associated with infection in nymphs. However, having fed on either a deer or unknown was negatively associated (OR=0.08 [0.02.0.3] for deer, and OR=0.4 [0.2,0.6] for unknown). The contribution of each host to the B. burgdorferi s.s. enzootic cycle was unique to each field site (Figure S2). For example, on Nantucket, shrews appeared to be the most important host with over half of the infected nymphs having evidence of feeding on them. Mice, on the other hand, were equally important at most of the mainland sites. Birds were only associated with infection at the Rhode Island-Trust site.
Fig. 3. Bloodmeal analysis of ticks testing positive for B. burgdorferi s.s.
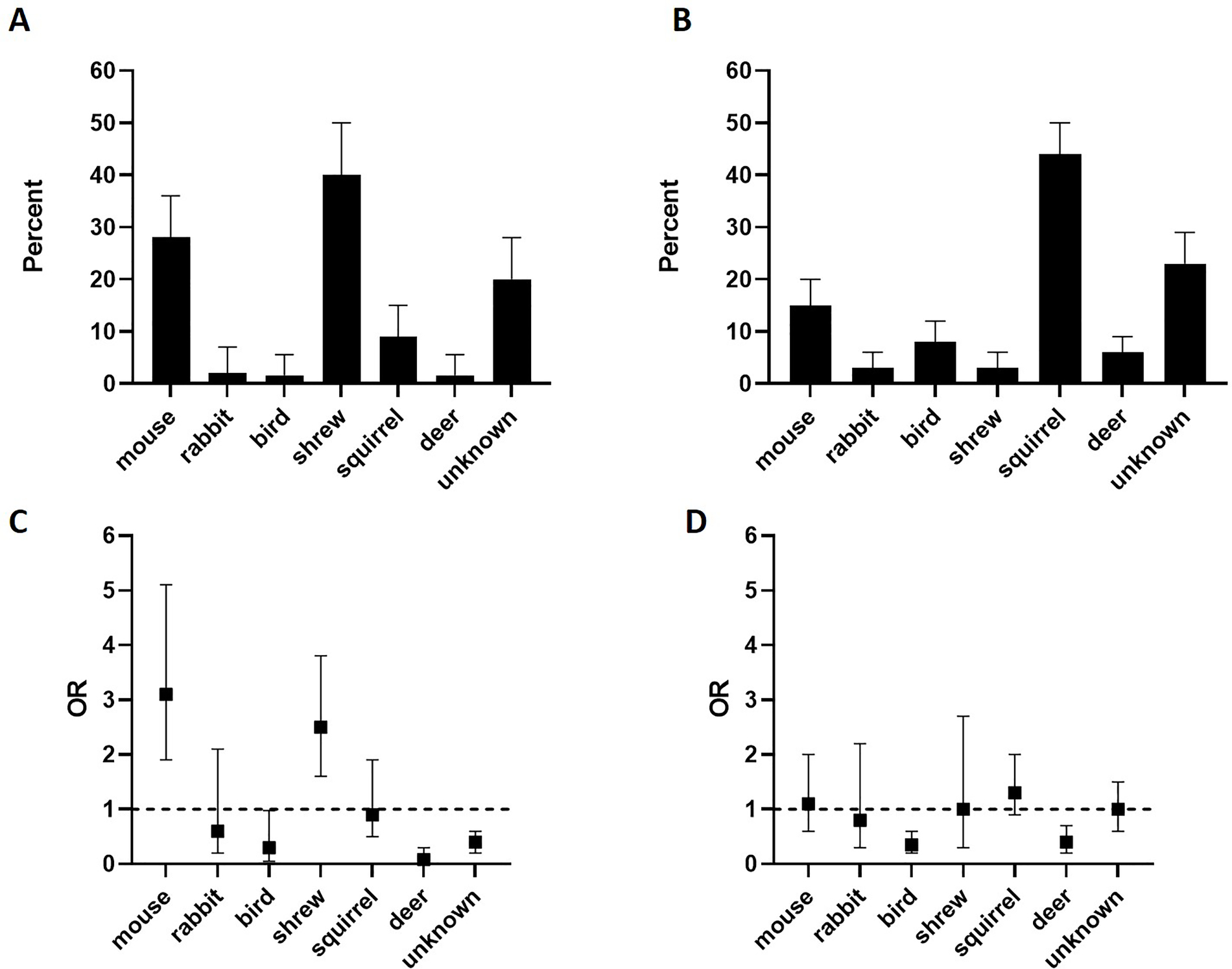
Top: the proportion of ticks (nymphs= A, adults=B) having fed on each host with 95% confidence intervals. Bottom: the likelihood that an infected tick (nymphs-C, adults-D) had fed on each host. Odds ratios are shown as point estimates with a 95% confidence interval. A line is drawn at OR=1 which equals no association. Note that squirrel includes gray squirrels, chipmunks and other Sciuridae as our primers cannot distinguish between them.
Host identification for adult ticks infected with B. burgdorferi s.s. (Fig. 3B) largely reflected the distribution of bloodmeal hosts of all the adults (Fig. 2). Most had fed on squirrel/chipmunk (44%) as nymphs (Fig. 3B), but this did not lead to a significant association with infected ticks (OR=1.3 [0.9, 2.0] Fig. 3D). Infected adults had also fed on mice (15%) and birds (8%), but less so on rabbits, deer and shrews; each were found in fewer than 10% of the infected adult ticks. None of the hosts had a significant association with infection in adults, but feeding on either bird or deer was significantly associated with not being infected (OR=0.35[0.2,0.6] for bird and OR=0.4 [0.2,0.7] for deer). We were unable to identify bloodmeal host in 23% of the infected ticks, which is similar to the overall estimate of unknowns in adult ticks, suggesting that the failure to identify a host bloodmeal is not related to infection status.
To determine whether a host significantly contributed to the enzootic cycle of B. burgdorferi s.s., we examined the relationship between the prevalence of infection in nymphal ticks at our field sites (commonly used as a proxy for the intensity of transmission) and the proportion of ticks that had fed on each of the 4 main hosts determined to be utilized by ticks in this study: mouse, shrew, squirrel/chipmunk and deer (Fig. 4). Nymphs that had fed on shrews and mice, but not squirrels/chipmunks, were associated with the prevalence of infected nymphs using linear regression. Feeding on deer appeared to be negatively associated, but this was not statistically significant. The prevalence of infected nymphs also had a positive association with adult ticks that had fed on mice as nymphs, but this was not statistically significant. Interestingly, there was no association between the identification of either shrews or squirrels as bloodmeal hosts in adults and infection in nymphal ticks. Once again, deer appeared to have a negative association, but this lacked statistical significance.
Fig. 4. The association between the prevalence of B. burgdorferi s.s.-infected nymphs at our field sites with bloodmeal host identification from nymphs and adults.

The prevalence of infection in nymphs at each of our field sites is graphed against proportion of ticks whose bloodmeal host was identified as: mice (A), shrews (B), squirrels/chipmunks (C), and deer (D). Bloodmeal identification data from adults are represented by open circles and nymphs by closed circles. Best fit linear regression lines are presented for adults (dotted) and nymphs (solid); those with significant associations are drawn in red. Note that squirrels= gray squirrels, chipmunks and other Sciuridae, and that the y-axis for panel C is different than the rest.
4. Discussion
We document significant differences in host utilization between larval and nymphal deer ticks by bloodmeal remnant analysis of questing nymphs and adults. Because the hosts upon which deer ticks feed depends largely upon the species that are available in their immediate vicinity, we attempted to control for any differences in host availability by collecting matched cohorts of ticks that had fed at the same site during the same summer season. This should account for population variation, such as the multiannual fluctuations that often occur in rodent populations (e.g., (Kesner and Linzey, 1997; Tryon and Snyder, 1973). However, because this is a field study, there are inherent differences that cannot be controlled for. For example, the larvae and nymphs at our field sites differ in the timing of greatest activity; nymphs are most active during May-June, and larvae during August and September. Therefore, it is likely that there will be monthly variation of available host populations due to recruitment over the summer months, particularly for mice. However, these differences occur every year and are part of the natural cycle that we seek to understand. Although nymphs have only fed once previously, adult ticks have fed twice and, theoretically, would contains remnants from both bloodmeals. Our previous work has shown that our ability to detect bloodmeal remnants wanes over time, making it unlikely that we are able to detect remnants from the larval host in adult ticks (Goethert et al., 2021). We detected similar rates of multiple bloodmeal hosts from both nymphs and adults (<5% at most sites) and conclude that we are only detecting the host upon which the tick had fed in the most recent stage. We demonstrate that whereas both subadult deer tick stages commonly feed on mice, the larvae were significantly more likely than nymphs to utilize shrews as bloodmeal hosts, and nymphs were significantly more likely to utilize birds as well as squirrels/chipmunks (Fig. 2). Previous field studies looking at infestation rates of trapped hosts have documented these differences (Hanincova et al., 2006; Mather et al., 1989; Schmidt et al., 1999). However, our study measures more than host infestation; we measure realized host contribution because the ticks have fed to repletion and successfully molted to the next stage. Infestation does not necessarily imply successful feeding: ticks may be removed by grooming or immunological rejection, or perish with the host if it is preyed upon.
Nymphs ingest a larger volume of blood than larvae while attached to a host. This should imply that we should more successfully detect bloodmeal remnants in adult ticks, and this may account for the apparent difference in sensitivity of our assay between adult and nymphal ticks (76% of ticks with successful host identification in adults vs 68% in nymphs). We do not adjust our data to attempt to correct for potential sensitivity differences because there are other factors that can affect our ability to detect bloodmeals, and we are unable to know the extent to which each would contribute to the negative results in our bloodmeal assay. First, our assay only tests for a limited number of species. There are other possible hosts, such as weasels, foxes, rats, cats and moles, among others, for which we do not assay due to the reduced likelihood (as demonstrated by the literature or by their known representation in a site) of their contribution relative to what we consider to be likely main hosts. Any tick that had fed on any of these hosts would be included in the unknown category. Another factor that contributes to negative results from our assay is the age of the tick. Our previous work has demonstrated that newly molted colony ticks yield a stronger signal than the field-collected ticks and that amplification success decreases as ticks are allowed to age in the laboratory, presumably, due to the continued digestion of bloodmeal remnants (Goethert et al., 2021). During our preliminary work for this study, we collected adult ticks in the fall as well as the following spring. The spring adults, which were older and had overwintered, consistently yielded extremely low success rates (<50%, data not shown) and, therefore, were excluded from the study. Larvae that feed early in the season can molt and overwinter as unfed nymphs, contributing to our lower success rate in nymphal ticks. Furthermore, ticks in our sites may comprise multiple cohorts, with an unknown number of ticks having survived from a previous year. There is no consensus on the longevity of the various stages. This is, in part, due to the complexities of the life cycle, such as whether a tick had found a bloodmeal host early or late in the season or at all, but also due to inconsistent results from survival experiments, which hold ticks outside in artificial enclosures for monitoring (Gray, 1982; Randolph et al., 2002; Walker, 2001; Yuval and Spielman, 1990). Environmentally stressful conditions may also contribute to rapid physiological aging. Although multiple methods have been developed for assessing physiological age, none are easily done on large numbers of ticks and also allow for DNA assays to be done on the same specimen (Balashov et al., 2010; Uspensky, 1995; Walker, 2001). Accordingly, there may be a sensitivity differential of our assay for analyzing adult and nymphal ticks, but nonetheless the general pattern of differences in host utilization between larvae and nymphs is readily apparent.
Consistent with the prevailing dogma that deer are incompetent hosts for B. burgdorferi s.s. (Telford III et al., 1988), we detected a zoo-prophylactic effect for ticks having fed on a deer (Fig. 3C and D, odds ratio 0.08 [95% CI, 0.02, 0.3] for nymphs, 0.4 [0.2, 0.7] for adults). Furthermore, sites with higher proportions of ticks that fed on deer tended to be associated with lower rates of infection in nymphs, though this association was not statistically significant (Fig. 4D). The lack of statistical significance may be due to the small numbers of infected ticks found to have fed on deer. We have identified deer bloodmeal remnants in small numbers of infected ticks in our other studies as well (Goethert et al., 2021; Goethert and Telford III, 2022b). Although it is well known that deer complement lyses B. burgdorferi s.s., it may be that transmission occurs without a systemic infection by cofeeding near an infected tick, similar to what has been shown to occur with sheep (Ogden et al., 1997). The numbers of ticks infected this way are small compared to those infected by rodent hosts and are not likely to significantly impact the force of transmission. Cofeeding could contribute to the enzootic cycle when rodent hosts are scarce.
Bloodmeal identification was conducted on infected adult ticks. Such ticks had fed twice (once as a larvae and again as a nymph) and could have acquired infection during their first bloodmeal as larvae. We cannot ascertain which feeding was infectious. However, the bloodmeal host identifications obtained from infected ticks (Fig. 3B) closely resemble the distribution of that for uninfected adult ticks (Fig. 2). It is thus puzzling that despite having generally served as the bloodmeal source for 40% of adult ticks (Fig. 3B), squirrels/chipmunks were not significantly associated with infection (Fig. 3D). The majority of the squirrel/chipmunk population was surely exposed to infection, yet they were not associated with infecting nymphs. Unfortunately, we did not trap animals and directly assess infection status and therefore cannot know the proportion of animals at our site that were infected. Previous analyses suggest that gray squirrels have poor reservoir capacity for B. burgdorferi s.s., whereas that for chipmunks appears much greater (Brunner et al., 2008; Hanincova et al., 2006; Keesing et al., 2009). It may be that our analysis is confounded by gray squirrels obscuring the contribution of chipmunks because our assay cannot distinguish between the two species. We continue to work to identify specific primer targets to differentiate the contributions of the sciurid hosts.
Because nymphal deer ticks have only fed once, and B. burgdorferi s.s. is not known to be transmitted transovarially, host bloodmeal identification of infected nymphs can determine which host served as the source of infection. In this study, larvae that had fed on mice and shrews were responsible for over 70% of the infected nymphs and feeding on these hosts was significantly associated with becoming infected (Figs. 3A and C). This is consistent with the paradigm of mice being the most important reservoir in the B. burgdorferi s.s. enzootic cycle (Levine et al., 1985; Mather et al., 1989). The role of shrews, however, has been underappreciated in the B. burgdorferi s.s. literature. An early study dismissed their potential role as reservoir hosts because they appeared to feed few ticks (Telford et al., 1990) even though they were infectious to ticks. Few studies have subsequently suggested otherwise (Bown et al., 2011; Brisson et al., 2008). We demonstrate that not only are shrews feeding a large proportion of larval deer ticks (Fig. 2), they also are responsible for infecting as many, or more, ticks than mice (Fig. 3A). Our prior studies using bloodmeal identification have documented only a few instances where mice were identified as the dominant host from nymphal deer ticks. We have, however, consistently detected the contribution of shrews as hosts for larval deer ticks as well as demonstrating their realized reservoir capacity for Babesia microti as well as deer tick virus (Goethert et al., 2021; Goethert and Telford III, 2022a). Shrews are difficult to study but their contribution to the enzootic cycles of the deer tick-transmitted agents requires more analysis across diverse sites.
Theoretical considerations have suggested that the force of B. burgdorferi s.s. transmission is greatest when larvae and nymphs feed on the same kind of host (Spielman et al., 1984); larval feeding is not diverted to hosts that did not receive infectious bites from nymphs. Our findings are consistent with this suggestion for mice. Similar proportions of larvae and nymphs fed on mice (Fig. 2), and the proportion of larvae is significantly associated with the prevalence of infection (Fig. 4A). The proportion of nymphs feeding on mice (detected as bloodmeal remnants in adults) also appears to have a positive, though nonsignificant, association with the prevalence of infection with more larvae acquiring infection from mice (detected as infection and bloodmeal remnants in nymphs). This pattern was not observed for shrews. As for mice, we found that larval deer ticks often fed on shrews, and the proportion of them doing so was positively associated with the prevalence of infection in the resulting nymphs (Fig. 4B). Bloodmeal remnants in adult ticks, however, demonstrated that nymphs did not commonly feed on shrews (Fig. 2); there was no association between the identification of shrew bloodmeal remnants in adults (evidence for what nymphs had fed upon) with the infection prevalence in nymphs (Fig. 4B). In other words, there was no association between nymphal ticks feeding on shrews and the ability of the shrews to infect larvae. We conclude that shrews may act as reservoir hosts without many infectious bites from nymphal ticks, which raises the question: How do enough shrews become infected such that they contribute so much to infection in host seeking nymphs? It may be that shrews are efficiently infected and few bites are required to render many individuals infectious within a site; on Nantucket, most individuals were found to be infected (Telford et al., 1990). Shrews may comprise “superspreaders”, a few individuals that not only feed the majority of ticks but also serve as the source of infection (Brunner and Ostfeld, 2008). We do not have good estimates for shrew abundance in our sites because our past trapping studies were focused on mice. Our bloodmeal assay cannot discriminate between individuals of the same species and therefore cannot determine how many different individuals the ticks have fed on. It may also be that shrews retain reservoir competence for extended durations. Shrews are as long lived, if not more so, than mice with 7% of those trapped in one summer being found the following summer; in the same study, only 4% of mice lived a year (Pearson, 1945). Only a small proportion of the mouse population maintains B. burgdorferi s.s. infection through the winter and can serve to infect ticks in the spring (Anderson et al., 1987; Lindsay et al., 1997); similar data are not available for shrews, but if they retain infectivity over winter and into the summer they could contribute infected ticks without exposure to new infectious nymphal bites. Previous models of relative reservoir competence based on infestation rates of captured animals and xenodiagnostic larvae consistently estimated that of shrews to be about half that of mice but it is thought that this is an underestimate of their reservoir capacity (Brunner et al., 2008; LoGiudice et al., 2003). We find in this and our prior studies that shrews are contributing as many, if not more, infected nymphs than mice.
Squirrels demonstrate the reverse pattern of infestation to that of shrews. These hosts fed many nymphs and few larvae. In fact, sciurids account for almost half of the bloodmeal hosts identified from adults (what the nymphs had fed on), but only 9% of the bloodmeal hosts identified from nymphs (what the larvae had fed on) (Fig. 2). Interestingly, squirrels/chipmunks do not appear to contribute significantly to the enzootic cycle of B. burgdorferi s.s. in our study sites. They contributed only 10% of the infected nymphs (Fig. 3A), and neither larvae or nymphs that had fed on squirrels/chipmunks were likely become infected (Fig. 3C and D). Furthermore, there was no association between either stage of deer tick having fed on squirrels/chipmunks and the prevalence of infection in nymphs (Fig. 4C). Our conclusions are limited given that our bloodmeal identification primers cannot discriminate among the diverse sciurids, and therefore gray squirrels, chipmunks, red squirrels and woodchucks are all possible hosts in our Rhode Island sites (only gray squirrels are present on Nantucket). Larval ticks likely have different preferences for these animals and any significant association with a specific host (e.g., chipmunk) is being obscured by the group specific identification. Previous studies find both chipmunks and squirrels to be infested with large numbers of subadult deer ticks (Levin et al., 2002; Mannelli et al., 1993; Schulze et al., 2005), and the reservoir capacity for chipmunks has been estimated to be similar to that of shrews (Brunner et al., 2008; Hanincova et al., 2006; LoGiudice et al., 2003). Although squirrels or chipmunks are key hosts for the enzootic B. burgdorferi s.s. cycle in some sites (Mannelli et al., 1993; Sidge et al., 2021), in our study sites, sciurids do not significantly contribute to the force of enzootic transmission.
We conclude that there is differential realized host utilization for subadult deer ticks. Although mice fed equal proportions of larvae and nymphs in our sites, this was not the case for shrews and squirrels/chipmunks. Larvae were more likely to have fed on shrews, and nymphs were more likely to have fed on squirrels/chipmunks. Whether our observations characterize enzootic transmission of B. burgdorferi s.s. in other sites remains to be determined; host communities differ across the large range of the deer tick.
Supplementary Material
Acknowledgments
We thank the Nantucket Conservation Foundation for access to field sites on Nantucket Island, and the Harvard Museum of Comparative Zoology for contributing the positive control material for developing the bird assay. We would like to acknowledge the technical contributions of Tucker Taylor, Laura Zimmerman and Jennifer Rotti.
Funding
We are supported by grants from the National Institutes of Health (R01 AI 130105, R01 AI 137424), the Global Lyme Alliance and the Rainwater Foundation; as well as by a generous gift from Catherine C. Lastavica.
Footnotes
CRediT authorship contribution statement
Heidi K. Goethert: Conceptualization, Methodology, Investigation, Writing – original draft, Writing – review & editing. Thomas N. Mather: Investigation. Alanna O’Callahan: Investigation. Sam R. Telford III: Investigation, Funding acquisition, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ttbdis.2023.102230.
Data availability
The data has been placed in an open repository and the link shared in the paper.
References
- Anderson JF, Johnson RC, Magnarelli LA, 1987. Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. J. Clin. Microbiol. 25, 1564–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashov Yu.S., Grigoryeva LA, Leonovich SA, 2010. Estimation of the biological age in females of the taiga tick Ixodes persulcatus by changes in the body shape and the surface of the integument. Entmol. Rev. 90, 251–254. 10.1134/S0013873810020107. [DOI] [PubMed] [Google Scholar]
- Bown KJ, Lambin X, Telford G, Heyder-Bruckner D, Ogden NH, Birtles RJ, 2011. The common shrew (Sorex araneus): a neglected host of tick-borne infections? Vector Borne Zoonotic Dis. 11, 947–953. doi: 10.1089/vbz.2010.0185. [DOI] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, Ostfeld RS, 2008. Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc. R. Soc. B 275, 227–235. 10.1098/rspb.2007.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner JL, LoGiudice K, Ostfeld RS, 2008. Estimating reservoir competence of Borrelia burgdorferi; hosts: prevalence and infectivity, sensitivity, and specificity. J. Med. Entomol. 45, 139–147. 10.1603/0022-2585(2008)45.139:ercobb.2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brunner JL, Ostfeld RS, 2008. Multiple causes of variable tick burdens on small-mammal hosts. Ecology 89, 2259–2272. 10.1890/07-0665.1. [DOI] [PubMed] [Google Scholar]
- Davidar P, Wilson M, Ribeiro JM, 1989. Differential distribution of immature Ixodes dammini (Acari: Ixodidae) on rodent hosts. J. Parasitol. 75, 898–904. [PubMed] [Google Scholar]
- Egyed L, 2017. Difference in susceptibility of small rodent host species to infestation by Ixodes ricinus larvae. Exp. Appl. Acarol. 72, 183–189. 10.1007/s10493-017-0121-2. [DOI] [PubMed] [Google Scholar]
- Goethert HK, 2021. Protocol for bloodmeal identification in ticks using retrotransposon-targeted Real Time PCR [WWW Document]. Protoc. Exch 10.21203/rs.3.pex-1736/v1. [DOI] [Google Scholar]
- Goethert HK, Mather TN, Buchthal J, Telford SR, 2021. Retrotransposon-based blood meal analysis of nymphal deer ticks demonstrates spatiotemporal diversity of Borrelia burgdorferi and Babesia microti reservoirs. Appl. Environ. Microbiol. 87 10.1128/AEM.02370-20 e02370-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethert HK, Telford III SR, 2022a. Limited capacity of deer to serve as zooprophylactic hosts for Borrelia burgdorferi in the northeastern United States. Appl. Environ. Microbiol. 88 10.1128/aem.00042-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goethert HK, Telford III SR, 2022b. Host contributions to the force of Borrelia burgdorferi and Babesia microti transmission differ at edges of and within a small habitat patch. Appl. Environ. Microbiol. 10.1128/aem.02391-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JS, 1982. The development and questing activity of Ixodes ricinus (L.) (Acari: Ixodidae) under field conditions in Ireland. Bull. Entomol. Res. 72, 263–270. 10.1017/S0007485300010567. [DOI] [Google Scholar]
- Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D, 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 12, 604–611. 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker W, Bishop F, Wood H, 1912. The life history of some north american ticks. in Bull. Bur. Entomol. USDA 1–239. [Google Scholar]
- James AM, Oliver JH, 1990. Feeding and host preference of immature Ixodes dammini, I. scapularis, and I. pacificus (Acari: Ixodidae). J. Med. Entomol. 27, 324–330. 10.1093/jmedent/27.3.324. [DOI] [PubMed] [Google Scholar]
- Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Vuong H, Ostfeld RS, 2009. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B-Biol. Sci rspb20091159. 10.1098/rspb.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner MH, Linzey AV, 1997. Modeling population variation in Peromyscus leucopus: an exploratory analysis. J. Mammal. 78, 643–654. 10.2307/1382915. [DOI] [Google Scholar]
- Levin ML, Nicholson WL, Massung RF, Sumner JW, Fish D, 2002. Comparison of the reservoir competence of medium-sized mammals and Peromyscus leucopus for Anaplasma phagocytophilum in Connecticut. Vector Borne Zoonotic Dis. 2, 125–136. 10.1089/15303660260613693. [DOI] [PubMed] [Google Scholar]
- Levine JF, Wilson ML, Spielman A, 1985. Mice as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 34, 355–360. 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, McEwen SA, Campbell GD, 1997. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. J. Wildl. Dis. 33, 766–775. 10.7589/0090-3558-33.4.766. [DOI] [PubMed] [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F, 2003. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 100, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main AJ, Carey AB, Carey MG, Goodwin RH, 1982. Immature Ixodes dammini (Acari: Ixodidae) on small animals in Connecticut, USA. J. Med. Entomol. 19, 655–664. 10.1093/jmedent/19.6.655. [DOI] [PubMed] [Google Scholar]
- Mannelli A, Kitron U, Jones CJ, Slajchert TL, 1993. Role of the eastern chipmunk as a host for immature Ixodes dammini (Acari: Ixodidae) in northwestern Illinois. J. Med. Entomol. 30, 87–93. 10.1093/jmedent/30.1.87. [DOI] [PubMed] [Google Scholar]
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT, 1996. Entomologic index for human risk of Lyme disease. Am. J. Epidemiol. 144, 1066–1069. [DOI] [PubMed] [Google Scholar]
- Mather TN, Wilson ML, Moore SI, Ribeiro JMC, Spielman A, 1989. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am. J. Epidemiol. 130, 143–150. 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Matuschka FR, Fischer P, Musgrave K, Richter D, Spielman A, 1991. Hosts on which nymphal Ixodes ricinus most abundantly feed. Am. J. Trop. Med. Hyg. 44, 100–107. [DOI] [PubMed] [Google Scholar]
- Occhibove F, Kenobi K, Swain M, Risley C, 2021. An eco-epidemiological modeling approach to investigate dilution effect in two different tick-borne pathosystems. Ecol. Appl. 10.1002/eap.2550 e2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden NH, Nuttall PA, Randolph SE, 1997. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitol 115, 591–599. 10.1017/S0031182097001868. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Levi T, Keesing F, Oggenfuss K, Canham CD, 2018. Tick-borne disease risk in a forest food. web. Ecol. 99, 1562–1573. 10.1002/ecy.2386. [DOI] [PubMed] [Google Scholar]
- Pearson OP, 1945. Longevity of the short-tailed shrew. Am. Midl. Nat. 34, 531–546. 10.2307/2421143. [DOI] [Google Scholar]
- Randolph SE, Green RM, Hoodless AN, Peacey MF, 2002. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasit. 32, 979–989. [DOI] [PubMed] [Google Scholar]
- Ratti V, Winter JM, Wallace D, 2021. Dilution and amplification effects in Lyme disease: modeling the effects of reservoir-incompetent hosts on Borrelia burgdorferi sensu stricto transmission. Ticks Tick-Borne Dis. 12, 101724 10.1016/j.ttbdis.2021.101724. [DOI] [PubMed] [Google Scholar]
- Schmidt KA, Ostfeld RS, Schauber EM, 1999. Infestation of Peromyscus leucopus and Tamias striatus by Ixodes scapularis (Acari: Ixodidae) in relation to the abundance of hosts and parasites. J. Med. Entomol. 36, 749–757. 10.1093/jmedent/36.6.749. [DOI] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, 2005. Host associations of Ixodes scapularis (Acari: Ixodidae) in residential and natural settings in a Lyme disease-endemic area in New Jersey. J. Med. Entomol. 42, 966–973. 10.1093/jmedent/42.6.966. [DOI] [PubMed] [Google Scholar]
- Shaw MT, Keesing F, McGrail R, Ostfeld RS, 2003. Factors influencing the distribution of larval blacklegged ticks on rodent hosts. Am. J. Trop. Med. Hyg. 68, 447–452. [PubMed] [Google Scholar]
- Sidge JL, Foster ES, Buttke DE, Hojgaard A, Graham CB, Tsao JI, 2021. Lake Michigan insights from island studies: the roles of chipmunks and coyotes in maintaining Ixodes scapularis and Borrelia burgdorferi in the absence of white-tailed deer. Ticks Tick-Borne Dis. 12, 101761 10.1016/j.ttbdis.2021.101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowik TJ, Lane RS, 2009. Feeding preferences of the immature stages of three western North American Ixodid ticks (Acari) for avian, reptilian, or rodent hosts. J. Med. Entomol. 46, 115–122. 10.1603/033.046.0115. [DOI] [PubMed] [Google Scholar]
- Spielman A, 1999. The role of surveillance in interventions directed against vector-borne disease. Ecosyst. Health 5, 141–145. 10.1046/j.1526-0992.1999.09923.x. [DOI] [Google Scholar]
- Spielman A, Clifford CM, Piesman J, Corwin MD, 1979. Human babesiosis on Nantucket Island, USA: description of the vector, Ixodes (Ixodes) dammini, n. sp. (Acarina: Ixodidae). J. Med. Entomol. 15, 218–234. [DOI] [PubMed] [Google Scholar]
- Spielman A, Etkind P, Piesman J, Ruebush TK, Juranek DD, Jacobs MS, 1981. Reservoir hosts of human babesiosis on Nantucket Island. Am. J. Trop. Med. Hyg. 30, 560–565. 10.4269/ajtmh.1981.30.560. [DOI] [PubMed] [Google Scholar]
- Spielman A, Levine JF, Wilson ML, 1984. Vectorial capacity of North American Ixodes ticks. Yale J. Biol. Med. 57, 507. [PMC free article] [PubMed] [Google Scholar]
- Telford SR III, Mather TN, Moore SI, Wilson ML, Spielman A, 1988. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 39, 105–109. [DOI] [PubMed] [Google Scholar]
- Telford SR 3rd, Mather TN, Adler GH, Spielman A, 1990. Short-tailed shrews as reservoirs of the agents of Lyme disease and human babesiosis. J. Parasitol. 76, 681–683. [PubMed] [Google Scholar]
- Tokarz R, Tagliafierro T, Cucura DM, Rochlin I, Sameroff S, Lipkin WI, 2017. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan virus in ticks by a multiplex real-time reverse transcription-PCR assay. mSphere 2. 10.1128/mSphere.00151-17 e00151–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryon CA, Snyder DP, 1973. Biology of the eastern chipmunk, Tamias striatus: life tables, age distributions, and trends in population numbers. J. Mammal 54, 145–168. 10.2307/1378877. [DOI] [Google Scholar]
- Uspensky I, 1995. Physiological age of ixodid ticks: aspects of its determination and application. J. Med. Entomol. 32, 751–764. [DOI] [PubMed] [Google Scholar]
- Van Oosten AR, Heylen DJA, Elst J, Philtjens S, Matthysen E, 2016. An experimental test to compare potential and realised specificity in ticks with different ecologies. Evol. Ecol. 30, 487–501. 10.1007/s10682-015-9816-1. [DOI] [Google Scholar]
- Walker AR, 2001. Age structure of a population of Ixodes ricinus (Acari: Ixodidae) in relation to its seasonal questing. Bull. Entomol. Res. 91, 69–78. [PubMed] [Google Scholar]
- Yuval B, Spielman A, 1990. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae). J. Med. Entomol. 27, 196–201. 10.1093/jmedent/27.2.196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data has been placed in an open repository and the link shared in the paper.


