Abstract
The nucleotide sequence of a plasmid-borne trimethoprim resistance gene from a commensal fecal Escherichia coli isolate revealed a new dihydrofolate reductase gene, dfrXV, which occurred as a gene cassette integrated in a site-specific manner in a class 1 integron. The new gene shows 84% nucleotide identity and the predicted protein shows 90% amino acid identity with dfrI and DHFR type I, respectively. Genes for spectinomycin resistance, aadA1 [ant (3′′)-Ia], and sulfonamide resistance, sulI, were located downstream of dfrXV in a manner identical to that in pLMO229.
Trimethoprim is an antimicrobial agent used on its own or in combination with sulfamethoxazole in the treatment of infections caused by gram-negative organisms. Trimethoprim selectively inhibits the bacterial dihydrofolate reductase (DHFR), thus preventing the reduction of dihydrofolate to tetrahydrofolate (8). The most common mechanism of resistance to trimethoprim in enterobacteria is the production of an additional plasmid-mediated DHFR which, unlike the chromosomal enzyme, is less sensitive to inhibition by trimethoprim (5). Sixteen trimethoprim resistance enzymes have been identified in enterobacteria and have been characterized and grouped on the basis of their nucleotide sequences and kinetic properties. The largest of these groups and by far the most prevalent are the type I-like enzymes, which include dfrI, dfrIb, dfrV, dfrVI, and dfrVII (14). This enzyme group is characterized by an open reading frame (ORF) of 157 amino acid residues, and the members of this group share between 64 and 88% amino acid sequence identity in this ORF (14). The majority of these enzymes have been found as gene cassettes inserted into the recombinationally active sites of integrons (22). In a survey of trimethoprim resistance in South Africa, 357 isolates of gram-negative, aerobic, commensal fecal flora were probed with oligonucleotide probes to determine the prevalence of DHFR resistance genes within the population (2, 3). Hybridization experiments revealed that contrary to all previous data, the most prevalent DHFR was type Ib (21.8%), followed by types VII (18.8%), I (14.6%), VIII (12.9%), XIII (12.3%), V (7.8%), and XII (0.3%) (1, 3). Forty-six of 357 isolates did not hybridize to any of the DHFR probes. One of these isolates, Escherichia coli UI14, which is highly resistant to trimethoprim (MIC, >2,048 μg/ml), was shown to transfer a 101-kb plasmid (pUK2317) which confers resistance to trimethoprim, spectinomycin, tetracycline, and sulfonamides to a recipient strain, E. coli J62-2, by conjugation (4).
MATERIALS AND METHODS
Purified pUK2317 DNA was restricted with PstI, and the fragments were ligated into PstI-restricted pGEM-3Zf(+) (Promega, Madison, Wis.) and electrotransformed (10) into E. coli JM109. A trimethoprim-resistant transformant, pUK2411, which contained a 9.4-kb PstI fragment was further restricted with BamHI, and the 6.74-kb fragment which contained the plasmid vector and a 3.54-kb cloned fragment were religated to produce trimethoprim-resistant clone pUK2412. A 1,280-bp PvuII fragment was further subcloned in both directions into the SmaI site of pGEM-3Zf(+) to produce trimethoprim-resistant clones pUK2413 and pUK2414. These two plasmids were then restricted with HindIII and were religated to produce trimethoprim-sensitive clones pUK2415 and pUK2416. Cloning was performed as described previously (23), and sequencing reactions were performed with both dGTP and dITP labels with the SEQUENASE, version 2.0, DNA sequencing kit (United States Biochemicals, Cleveland, Ohio). Sequence comparisons were made with the BLAST computer program (National Center for Biotechnology Information).
Nucleotide sequence accession number.
The nucleotide sequence of the type XV DHFR has been given EMBL accession no. Z8331.
RESULTS AND DISCUSSION
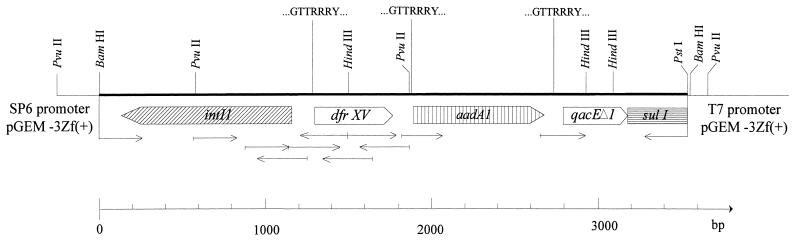
Figure 1 shows the restriction map, gene map, and direction of sequencing of pUK2412. From the partial sequence and restriction map, the trimethoprim resistance gene was shown to be preceded by the intI1 gene associated with a class 1 integron, as reported previously (12, 16, 19, 24). A primer (5′-AACGATGTTACGCAGCAG-3′) based on the sequence which occurs between the integrase ORF and the start of the first gene cassette (12, 16, 19, 24) was used to sequence the complementary strand upstream of the integrase gene. Upstream of the integrase ORF and its flanking structures, the nucleotide sequence revealed the DHFR ORF of 471 bp on the complementary strand. The DHFR ORF encoded 157 amino acids and was identified by its close nucleotide sequence homology with dfrI (84.4%) and the close amino acid homology (90 to 63%) that it shared with the trimethoprim-resistant type I-like DHFRs encoded by dfrI, dfrIb, dfrV, dfrV, and dfrVII (11, 24, 26, 29, 31). The ORF begin with the atypical E. coli start codon GTG at positions 357 to 359 (Fig. 2). Despite the unusual start codon which normally codes for valine, it is the only codon which is preceded by a plausible Shine-Dalgarno sequence (TAAGGAAGT). Since the ATG codon that is located five amino acids downstream of the GTG codon is not preceded by such a sequence, it is unlikely that this is the start codon. Furthermore, the use of alternative E. coli start codons GTG and TTG in other type I-like DHFRs such as dfrI, dfrV, and dfrVII has previously been demonstrated by experiments involving N-terminal amino acid sequencing and site-directed mutagenesis (11, 17, 26). The ORF ended with stop codon TAA at positions 828 to 830. The translated polypeptide for the DHFR is shown in Fig. 2. The new DHFR gene has been named dfrXV, and the encoded polypeptide has been designated the type XV DHFR (EMBL accession no. Z83311).
FIG. 1.
Restriction map, gene map, cassette boundaries, and direction of sequencing (→) of pUK2412.
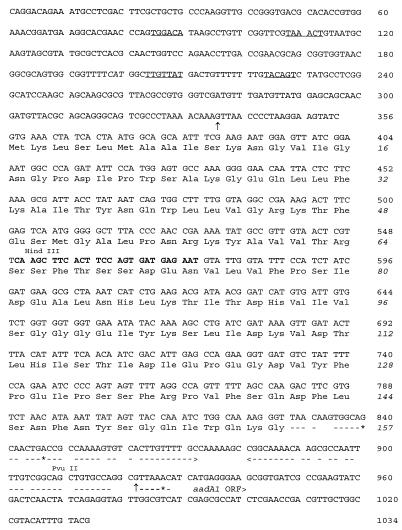
FIG. 2.
Nucleotide sequence and translated polypeptide of dfrXV. The sequence used as a gene probe is printed in boldface type. The gene cassette boundaries are marked (↑), and the imperfect inverted repeat (59-base element) is underlined (interrupted lines); gaps indicate mismatched bases, asterisks denote extra bases in the left half of the element which interrupt the inverted repeat, and arrows (---›) denote the direction of symmetry. The putative promoter sequences of the conserved element are underlined, and the start codon of intI1 is in italics.
dfrXV as a mobile cassette.
Gene cassettes inserted into the core site of class 1 integrons are usually flanked by a consensus sequence (GTTRRRY) on the 5′ end, which marks the point of insertion of the gene cassette into the integron, and an imperfect inverted repeat known as the 59-base element, situated 3′ to the resistance gene ORF (22). Similarly, dfrXV was flanked by both a core element, which was located 16 nucleotides 5′ to the DHFR ORF and which is presumed to mark the cassette boundary, and an inverted repeat (underlined in Fig. 2) from nucleotides 825 to 928 at the 3′ end of the DHFR ORF which marks the 3′ end of the gene cassette. The core element (GTTAACC) differed from the consensus sequence by a single nucleotide. Like most gene cassettes, no recognizable E. coli promoter was present between the core element and the start of the ORF. Sequence analysis of the upstream conserved element (intI1) revealed a promoter region previously identified to drive the expression of inserted gene cassettes (underlined in Fig. 2) (12, 15, 22). This particular promoter polymorphism was identified as a hybrid promoter which is a promoter with weak to moderate strength, as described previously (15). The first promoter is followed by a second promoter which has been identified in all class I integrons and which is thought to be nonfunctional due to the short spacing between the −35 and −10 hexamers (15). The nucleotide sequence downstream of the DHFR ORF was determined from pUK2412 with a primer that was constructed from nucleotides 849 to 866, which are located within the 59-base element. The core element at the end of this 59-base element marks the start of the next gene cassette (Fig. 2). From the nucleotide sequence, this cassette was identified as the gene for streptomycin and spectinomycin resistance aadA1 [ant(3′′)-Ia] (13, 32). The sequence flanking the PstI site of pUK2412 was identical to part of the ORF of sulI (nucleotides 986 to 1236 [24]). The junction between the putative aadA1 and sulI genes was sequenced and was found to be identical to the qacEΔ1 cassette which encodes a membrane efflux protein (21, 22) (nucleotides 211 to 480; EMBL accession no. X17479). With the exception of the DHFR gene cassette, in which dfrI is substituted with dfrXV, the order of the gene cassettes in pUK2317 is identical to that of pLMO229 (25).
Inhibition profiles of DHFR.
DHFR assays were performed by the method of Osborn and Huennekens (18) as described previously (6). From crude cell lysates (28), the specific activity of the DHFR, expressed in nanomoles of dihydrofolate (FH2) reduced per minute per milligram of protein, of E. coli UI14 was 14.3, a value 14-fold higher than the specific activity of the E. coli K-12 chromosomal enzyme. The specific activity of the enzyme from the J62-2:pUK2317 transconjugant (10.8 nmol of FH2 reduced/min/mg of protein) was lower, probably as a result of host-specific differences. As a result of the high copy number of pGEM3Zf(+) and the strong T7 promoter, the pUK2413 clone of dfrXV produced an approximately 1,000-fold increase in the specific activity of DHFR (1,049.1 nmol of FH2 reduced/min/mg of protein) in comparison to that of the E. coli JM109 host chromosomal DHFR (1.0 nmol of FH2 reduced/min/mg of protein). Partially purified DHFR was prepared by ultrasonic disruption, followed by ammonium sulfate precipitation and, finally, Sephadex G-75 gel exclusion chromatography as described previously (28). The approximate inhibitor profiles and kinetic properties of the type XV DHFR were similar to those obtained with partially purified extracts for other DHFR enzymes of this group (Table 1). DHFR activity was assayed in the presence of increasing concentrations of trimethoprim and methotrexate to determine the concentration required to inhibit the activity of the type XV DHFR by 50% (ID50). In comparison to the chromosomal DHFR of E. coli JM109 (ID50 = 0.007 μM), the type XV DHFR (ID50 = 22.4 μM) was more than 3,000 times more resistant to trimethoprim. The type XV DHFR was 1,500 times more resistant to inhibition by methotrexate (ID50 = 4.4 μM) than the chromosomal DHFR of E. coli JM109 (ID50 = 0.003 μM). The Lineweaver-Burke plots used to determine the Michaelis constant (Km) and the inhibitor constant for trimethoprim (Ki) showed that the Km for the type XV DHFR was calculated to be 16.7 μM FH2. The Ki values for trimethoprim at concentrations of 10 and 25 μM were 16.2 and 15.6 μM, respectively (mean Ki = 15.9 μM FH2). Unlike the other enzymes of this group, the type XV DHFR was found to be extremely heat stable, even at low protein concentrations, and could survive exposure to 45°C for more than 20 min without any significant loss of activity.
TABLE 1.
Biochemical properties of DHFR types I, Ib, V, VI, VII, and XVa
| DHFR | Tmp ID50 (μM) | Mtx ID50 (μM) | FH2Km (μM) | Tmp Ki (μM) | TD50 min |
|---|---|---|---|---|---|
| I | 57.0 | 4.4 | 5.6 | 7.4 | 0.5 |
| Ib | 32.0 | 2.8 | 11.0 | 41 | 1.2 |
| V | 23.0 | 3.5 | 15.5 | 3.2 | —b |
| VI | 200.0 | 7.3 | 31.2 | 75.0 | 0.4 |
| VII | 30.0 | 3.0 | 20.0 | 7.0 | 1.5 |
| XV | 22.4 | 4.4 | 16.7 | 15.9 | >20 |
Molecular epidemiology of dfrXV.
Dot blotting was performed as described previously (3). The nucleotide sequences of dfrI and dfrXV were aligned to determine regions of maximum heterogeneity, and a 30-mer oligonucleotide probe (5′-ATACATTCTCATCACTGGAAGTGAAGCTTG-3′) which contained nine nucleotide mismatches compared with the sequence of dfrI was selected for the detection of dfrXV (boldface type in Fig. 2). This region of nucleotide sequence heterogeneity encodes a predicted highly variable external loop that is located between two conserved regions of the secondary structure and that has been described previously for the discrimination and detection of closely related DHFR genes (1, 2, 31). Forty-six of 357 isolates of gram-negative commensal fecal flora did not hybridize to probes for other resistant DHFR types, and of these 26.1% (12 of 46) hybridized to the probe for dfrXV. The type XV DHFR was detected in isolates from all three regions that were sampled in South Africa: nine isolates were from urbanized communities in the province of Gauteng, two isolates were from rural populations in the Northern Province, and one isolate was from rural Mpumalanga. Of the 12 hybridization-positive isolates, an E. coli isolate from Gauteng did not transfer trimethoprim resistance to an E. coli J62-2 recipient strain. Five different EcoRI restriction profiles were obtained for the 11 plasmids from the transconjugants which harbored dfrXV. Six isolates harbored plasmids which shared identical restriction profiles (pUK2317) and resistance markers (trimethoprim, sulfamethoxazole, tetracycline, spectinomycin), and all were isolated from the same urban community in Gauteng. Two plasmids, pUK2370 and pUK2369, had similar restriction profiles, and both conferred resistance to ampicillin, trimethoprim, sulfamethoxazole, tetracycline, and spectinomycin. Plasmid pUK2370 was detected in two isolates, one from Gauteng and the other from the Northern Province, and pUK2369 was isolated from Gauteng. Plasmid pUK2403, isolated from the Northern Province, harbored another unique restriction profile and conferred resistance to ampicillin, trimethoprim, sulfamethoxazole, tetracycline, and spectinomycin. Eleven of 12 of the isolates which hybridized to the probe for dfrXV were identified as E. coli. The remaining isolate from Mpumalanga was a Klebsiella sp. which harbored a novel transferable plasmid pUK2322 which conferred resistance to trimethoprim and sulfamethoxazole. The absence of streptomycin and spectinomycin resistance determinants on this plasmid suggests that this may be a second integron context for the dfrXV cassette. The MICs of trimethoprim conferred by these plasmids were all greater than 2,048 mg/liter. To determine the association and position of dfrXV among the class 1 integrons, PCR was used to amplify the region between the intI1 and dfrXV ORFs. PCR products of 750 bp were obtained for all 11 isolates which harbored transferable plasmids. The size of the PCR product suggests that the dfrXV cassette was the most recent cassette to be inserted and was inserted immediately upstream of the integrase gene (9, 22). No PCR product was detected in the isolate which was unable to transfer resistance to the E. coli recipient strain. The use of sulfonamides in combination with trimethoprim appears to play a significant role in the selection of sulI-associated integrons and has presumably applied strong selection pressure for the uptake of new trimethoprim resistance cassettes by these elements.
REFERENCES
- 1.Adrian P V, Thomson C J, Klugman K P, Amyes S G B. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. A novel dihydrofolate reductase cassette inserted in an integron borne on a Tn21-like element, abstr. C87; p. 55. [Google Scholar]
- 2.Adrian P V, Thomson C J, Klugman K P, Amyes S G B. Prevalence of trimethoprim resistant dihydrofolate reductase genes identified with oligonucleotide probes in plasmids from South African isolates of commensal faecal flora. J Antimicrob Chemother. 1995;35:497–508. doi: 10.1093/jac/35.4.497. [DOI] [PubMed] [Google Scholar]
- 3.Adrian P V, Thomson C J, Klugman K P, Amyes S G B. Prevalence and genetic location of non-transferable trimethoprim resistant dihydrofolate reductase genes in South African commensal faecal flora. Epidemiol Infect. 1995;115:255–267. doi: 10.1017/s0950268800058386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amyes S G B, Gould I M. Trimethoprim resistance plasmids in faecal bacteria. Ann Microbiol (Inst Pasteur) 1984;135B:177–186. [PubMed] [Google Scholar]
- 5.Amyes S G B, Smith J T. R-factor trimethoprim resistance mechanism: an insusceptible target site. Biochem Biophys Res Commun. 1974;58:412–418. doi: 10.1016/0006-291x(74)90380-5. [DOI] [PubMed] [Google Scholar]
- 6.Amyes S G B, Smith J T. The purification properties of the trimethoprim-resistant dihydrofolate reductase mediated by the R-factor, R388. Eur J Biochem. 1976;61:597–603. doi: 10.1111/j.1432-1033.1976.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 7.Amyes S G B, Towner K J, Carter G I, Thomson C J, Young H-K. The type VII dihydrofolate reductase: a novel plasmid-encoded trimethoprim-resistant enzyme from gram-negative bacteria isolated in Britain. J Antimicrob Chemother. 1989;24:111–119. doi: 10.1093/jac/24.2.111. [DOI] [PubMed] [Google Scholar]
- 8.Burchall J J, Hitchings G H. Inhibitor binding analysis of dihydrofolate reductases from various species. Mol Pharmacol. 1965;1:126–136. [PubMed] [Google Scholar]
- 9.Collis C M, Grammaticopoulos G, Briton J, Stokes H W, Hall R M. Site-specific insertion of gene cassettes into integrons. Mol Microbiol. 1993;9:41–52. doi: 10.1111/j.1365-2958.1993.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 10.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fling M E, Richards C. Nucleotide sequence of the trimethoprim resistant dihydrofolate reductase gene harboured by Tn7. Nucleic Acids Res. 1983;11:5147–5158. doi: 10.1093/nar/11.15.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall R M, Vockler C. The region of the IncN plasmid R46 coding for resistance to β-lactam antibiotics, streptomycin/spectinomycin and sulphonamides is closely related to antibiotic resistance segments found in IncW plasmids and in Tn21-like transposons. Nucleic Acids Res. 1987;15:7491–7501. doi: 10.1093/nar/15.18.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollingshead S, Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenyltransferase. Plasmid. 1985;13:17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- 14.Huovinen P, Sundström L, Swedburg G, Sköld O. Trimethoprim and sulfonamide resistance. Antimicrob Agents Chemother. 1995;39:279–289. doi: 10.1128/aac.39.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lévesque C, Brassard S, Lapointe J, Roy P H. Diversity and relative strength of tandem promoters for the antibiotic-resistance genes of several integrons. Gene. 1994;142:49–54. doi: 10.1016/0378-1119(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 16.Martinez E, de la Cruz F. Transposon Tn21 encodes a RecA-independent site-specific integration system. Mol Gen Genet. 1988;211:320–325. doi: 10.1007/BF00330610. [DOI] [PubMed] [Google Scholar]
- 17.Novak P, Stone D, Burchall J J. R-plasmid dihydrofolate reductase with a dimeric subunit structure. J Biol Chem. 1983;258:10956–10959. [PubMed] [Google Scholar]
- 18.Osborn M J, Huennekens F M. Enzymatic reduction of dihydrofolic acid. J Biol Chem. 1958;233:969–974. [PubMed] [Google Scholar]
- 19.Ouellette M, Roy P H. Homology of ORFs from Tn2603 and from R46 to site-specific recombinases. Nucleic Acids Res. 1987;15:10055. doi: 10.1093/nar/15.23.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pattishall K H, Acar J, Burchall J, Goldstein F W, Harvey R J. Two distinct types of trimethoprim-resistant dihydrofolate reductase specified by R-plasmids of different compatibility groups. J Biol Chem. 1977;252:2319–2323. [PubMed] [Google Scholar]
- 21.Rådström P, Sköld O, Swedberg G, Flensburg J, Roy P H, Sundström L. Transposon Tn5090 of plasmid R751, which carries an integron, is related to Tn7, Mu, and the retroelements. J Bacteriol. 1994;176:3257–3268. doi: 10.1128/jb.176.11.3257-3268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Recchia G D, Hall R M. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–3027. doi: 10.1099/13500872-141-12-3015. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Sundström L, Rådström P, Swedberg G, Sköld O. Site-specific recombination promotes linkage between trimethoprim- and sulfonamide resistance genes. Sequence characterization of dhfrV and sulI and a recombination active locus of Tn21. Mol Gen Genet. 1988;213:191–201. doi: 10.1007/BF00339581. [DOI] [PubMed] [Google Scholar]
- 25.Sundström L, Sköld O. The dhfrI trimethoprim resistance gene of Tn7 can be found at specific sites in other genetic surroundings. Antimicrob Agents Chemother. 1990;34:642–650. doi: 10.1128/aac.34.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundström L, Swedberg G, Sköld O. Characterization of transposon Tn5086, carrying the site-specifically inserted gene dhfrVII mediating trimethoprim resistance. J Bacteriol. 1993;175:1796–1805. doi: 10.1128/jb.175.6.1796-1805.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomson C J, Amyes S G B. Biochemical properties of the type V plasmid-encoded trimethoprim resistant dihydrofolate reductase. J Pharm Pharmacol. 1988;40:21. [Google Scholar]
- 28.Wylie B A, Amyes S G B, Young H-K, Koornhof H J. Identification of a novel plasmid-encoded dihydrofolate reductase mediating high-level resistance to trimethoprim. J Antimicrob Chemother. 1988;22:429–435. doi: 10.1093/jac/22.4.429. [DOI] [PubMed] [Google Scholar]
- 29.Wylie B A, Koornhof H J. Nucleotide sequence of dihydrofolate reductase type VI. J Med Microbiol. 1991;35:214–218. doi: 10.1099/00222615-35-4-214. [DOI] [PubMed] [Google Scholar]
- 30.Young H-K, Amyes S G B. Characterisation of a new transposon-mediated trimethoprim-resistant dihydrofolate reductase. Biochem Pharmacol. 1985;34:4334–4337. doi: 10.1016/0006-2952(85)90296-5. [DOI] [PubMed] [Google Scholar]
- 31.Young H-K, Qumsieh M J, McIntosh M L. Nucleotide sequence and genetic analysis of the type Ib trimethoprim-resistant, Tn4132-encoded dihydrofolate reductase. J Antimicrob Chemother. 1994;34:715–725. doi: 10.1093/jac/34.5.715. [DOI] [PubMed] [Google Scholar]
- 32.Zühlsdorf M T, Wiedemann B. Functional and physiological characterisation of the Tn21 cassette for resistance genes in Tn2426. J Gen Microbiol. 1993;139:995–1002. doi: 10.1099/00221287-139-5-995. [DOI] [PubMed] [Google Scholar]