Abstract
Blood loss and transfusion of blood products are key concerns during liver transplantation. Whole-blood viscoelastic testing devices have been used to monitor hemostatic function and guide the transfusion of blood products in this patient population. The Quantra System with the QStat Cartridge is a new point-of-care, closed-system viscoelastic testing device that measures changes in clot stiffness during coagulation and fibrinolysis using ultrasound detection of resonance. The aim of this multicenter prospective observational study was to evaluate the Quantra System against the ROTEM delta device in monitoring coagulation and fibrinolysis in patients undergoing liver transplantation. One hundred twenty-five (125) adult subjects (above 18 y old) were enrolled across 5 medical centers in the US. Blood samples were collected at a minimum of 3-time points: preincision (baseline), during the anhepatic phase, and after the start of reperfusion. Performance was assessed as the correlation of equivalent measurements from the QStat Cartridge and ROTEM delta INTEM, EXTEM, and FIBTEM assays. In addition, a clinical concordance analysis was performed to assess the agreement between the 2 devices related to the detection of fibrinolysis. The correlation between the 2 viscoelastic testing devices was strong, with r-values ranging between 0.88 and 0.95, and the overall agreement with respect to detecting fibrinolysis was 90.3% (CI, 86.9%–93.2%). The results indicate that the Quantra with the QStat Cartridge provides comparable information as the ROTEM delta in the assessment of hemostatic function during a liver transplant. Quantra’s simplicity of use and availability of rapid results may provide clinicians with a faster, more convenient means to assess coagulation and fibrinolysis status in the operating room and critical care setting.
INTRODUCTION
The liver synthesizes and regulates the production of many of the procoagulant and anticoagulant proteins involved in maintaining balanced hemostasis. The hepatic dysfunction arising from the development of acute or chronic liver disease leads to a variety of physiologic changes resulting in a global decrease in coagulation proteins that regulate hemostasis, which predisposes patients with liver disease to experience several systemic complications.1–5 Over time, the changes in the coagulation system, coupled with thrombocytopenia and impaired platelet function, create a tenuous “rebalanced” system with a less robust functional capacity for regulating coagulation and fibrinolysis. As patients progress toward end-stage liver disease, this balance becomes fragile, and the capacity for maintaining hemostasis becomes increasingly inadequate, putting patients at higher risk of complications from bleeding and/or thrombosis.3,4 Liver transplantation for these patients poses a great challenge in coagulation and blood management. Although advancements in surgical and anesthetic techniques have been associated with a reduction in the amount of blood loss and the transfusion of allogenic blood products over time, the ability to monitor derangements in hemostasis throughout liver transplant surgery remains critical for providing a more targeted transfusion strategy both intraoperatively and postoperatively.5,6
Whole-blood viscoelastic testing (VET) devices, such as the ROTEM delta (Werfen, Bedford, MA) and TEG 5000 (Haemonetics, Braintree, MA), have been frequently used to assess the functional coagulation changes in patients with cirrhosis undergoing liver transplantation. These whole-blood assays demonstrate the multifactorial derangement in hemostasis that can lead to excessive bleeding and/or thrombotic complications perioperatively more closely than plasma-based standard laboratory tests. In a recent survey, the Quality & Standard Committee from the Society for the Advancement of Transplant Anesthesia (SATA) reported 95% of responding liver transplantation centers use VET for transfusion guidance during liver transplant surgery.7 The use of VET devices in liver transplantation has been associated with a reduction in intraoperative bleeding and blood product utilization when adopted in conjunction with a goal-directed treatment algorithm.8–13 Specifically, based on an international literature review of clinical evidence, VET use has received a “Strong” recommendation by the joint Enhanced Recovery after Surgery subgroup for Orthotopic Liver Transplant (ERAS4OLT) and International Liver Transplant Society (ILTS) consensus group.14 The utilization of these devices for the management of perioperative bleeding has also been recommended in the most recent guidelines on patient blood management issued by the STS/SCA/AmSECT/SABM.15
The QStat Cartridge is the second single-use disposable cartridge recently developed for use on the Quantra Hemostasis Analyzer (HemoSonics, LLC, Durham, NC) to assess blood coagulation and fibrinolysis at the point of care. The QStat Cartridge includes a test to assess fibrinolysis in addition to tests to monitor platelet function, coagulation factor consumption, and fibrinogen contribution to clot strength.16 The QStat Cartridge is intended to aid in the management of hypocoagulable and hypercoagulable conditions in the settings of trauma and liver transplantation. We hypothesized that results obtained from the Quantra with the QStat Cartridge would correlate with results obtained from the ROTEM delta across the various phases of liver transplantation in adult patients.
METHODS
Study design
This was a multicenter prospective, observational study in patients undergoing liver transplantation. The study was conducted at 5 academic medical centers in the US: the University of Virginia Health System (Charlottesville, VA), the Ohio State University Wexner Medical Center (Columbus, OH), the University of Michigan Medical Center (Ann Arbor, MI), the University of Florida Health Shands Hospital (Gainesville, FL), and the University of Texas Southwestern Medical Center (Dallas, TX). The study was reviewed and approved by a central Institutional Review Board (IRB) (Advarra, Pro 00041164) and by the local IRB at each of the participating clinical sites and registered on ClinicalTrials.gov (NCT04312958). All research was conducted in accordance with both the Declarations of Helsinki and Instabul. Written consent was given in writing by all participating subjects.
The objective of this study was to evaluate the Quantra with the QStat Cartridge in the clinical setting of liver transplantation as well as demonstrate correlation (method comparison) to the ROTEM delta platform.
Study population
The study population consisted of adult (above 18 y) male and female liver transplant recipients undergoing deceased donor (after brain or circulatory death), living donor, or simultaneous liver-kidney transplantation. Patients were excluded from the study if concurrently enrolled in a distinct study that could have confounded the results, if affected by a condition that, in the opinion of the surgical team, would have posed additional risks, if incarcerated at the time of enrollment, if pregnant, or if written consent could not be obtained.
In addition, a small number (n=5, accounting for roughly 3% of the total enrollment) of normal subjects was also enrolled to generate contrived samples with varied fibrinolytic activity. Contrived samples were run on both devices to assess correlation over a broader spectrum of conditions that cannot typically be achieved with clinical samples from the patient population.
Study protocol
For each enrolled subject, specific information was documented from the patient medical chart, including patient demographics (age, sex, and race), the indication for surgery, surgery duration; the time when blood samples were collected and the time that diagnostic tests were performed, results of diagnostic tests performed including QStat Cartridge, ROTEM delta, and standard laboratory coagulation testing (when available), and blood loss recorded within 24 hours of QStat Cartridge testing, blood products, and relevant medications administered within 24 hours of QStat Cartridge testing.
Blood samples were obtained at a minimum of 3 distinct time points throughout the course of surgery: (1) before the start of surgery, after the induction of anesthesia (baseline); (2) during the anhepatic phase; and (3) after the start of reperfusion. Additional samples may have been taken during the dissection phase or after surgery, in the intensive care unit, as directed by the clinical team. At each of these time points, a whole-blood sample was collected for analysis with the QStat Cartridge in parallel with a sample collected for the ROTEM delta. For these analyses, samples were collected in separate 2.7 mL evacuated tubes containing 3.2% sodium citrate (light blue top) using standard phlebotomy practices by venipuncture or from an existing central venous catheter. Additional samples may have been collected for routine coagulation testing in the laboratory per the site’s standard of care. Samples for QStat Cartridge analysis were kept at room temperature before testing.
At 4 clinical sites, ROTEM delta testing was performed within each site’s College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) certified central laboratory, and results were interpreted based on the locally established reference range intervals. At 1 clinical site, the ROTEM delta was performed by trained research staff in a research laboratory. The INTEM, EXTEM, and FIBTEM assays were run on the ROTEM for up to 60 minutes to complete the measurement of the parameters required for data analysis.
An electronic case report form was completed for each subject using the Medrio eClinical electronic data capture software (Medrio LLC, San Francisco, CA). The Principal Investigator at each site reviewed and approved each completed subject Casebook. After data were locked in for subjects across all study sites, study data were exported through SAS export file for statistical analysis.
Quantra and QStat cartridge
The Quantra and the QStat Cartridge were described previously.16–19 Briefly, Quantra uses an ultrasound-based technology called Sonic Estimation of Elasticity via Resonance (SEER) Sonorheometry to measure the evolution of the shear modulus (ie, stiffness) of a whole-blood sample over time. The QStat Cartridge is a closed-system disposable consumable that performs 4 measurements in parallel using citrated whole-blood samples and outputs 5 parameters representative of the hemostatic system of the patient. These parameters are summarized in Table 1.
TABLE 1.
QStat Cartridge output parameters
| Output parameter | Units | Description | Reportable ranges | Reference ranges |
|---|---|---|---|---|
| Clot Time (CT) | s | Clot time in citrated whole blood | 60–480 | 121–175 |
| Clot Stability to Lysis (CSL) | % | Reduction of clot stiffness that is likely due to the influence of fibrinolysis | 10–100 | 92–100a |
| Clot Stiffness (CS) | hPa | Stiffness of blood clot | 2–65 | 14.0–35.4 |
| Platelet Contribution to clot Stiffness (PCS) | hPa | Contribution of platelet activity to overall clot stiffness | 2–50 | 12.8–32.3 |
| Fibrinogen Contribution to clot Stiffness (FCS) | hPa | Contribution of functional fibrinogen to overall clot stiffness | 0.2–30 | 0.9–4.2 |
Validated threshold of <90% indicates the presence of fibrinolysis.
Abbreviation: hPa, hectoPascals.
Although the parameters CT, CS, FCS, and PCS have been previously evaluated in the context of the QPlus Cartridge,18 the Clot Stability to Lysis (CSL), which provides a quantitative measure of fibrinolysis, is unique to the QStat Cartridge. As previously reported, the computation of CSL mitigates the interfering effects of clot relaxation, often observed in viscoelastic testing systems as a reduction in clot stiffness not attributable to fibrinolysis but to the interactions between platelets and fibrinogen.16 On the basis of manufacturer data (not shown here), a CSL value below the threshold value of 90% was determined to indicate the presence of fibrinolysis.
The Quantra analyzers and the QStat Cartridges used in the study were labeled for Investigational Use Only (IUO), and the results generated by the system were blinded to the clinical team and not used to alter or influence existing standards of care.
Statistical analysis plan
Descriptive analyses included a summary of subject demographics, baseline characteristics, and summary statistics for clinical end points. These were generated using the eligible subject set consisting of all enrolled subjects whose data are included in the study database. Samples were eligible for inclusion in the analysis data set if data from the Quantra and at least one of the ROTEM assays are available to allow at least 1 Quantra to ROTEM comparison.
Pearson correlation coefficients were calculated and used to demonstrate the correlation between the Quantra and equivalent ROTEM test parameters. For correlation analysis, ROTEM clot stiffness amplitudes A20 were converted to clot elasticity (Pascals, Pa) using a formula as described.20,21 A simple linear regression model was used to evaluate the linear relationship between device measurements. Point estimates of Pearson correlation coefficient were reported and presented with simple linear regression fits for the above models. The interpretation of the strength of the correlation was based on the definitions presented by Schober et al.22
In addition, a clinical agreement between the QStat CSL parameter and ROTEM lysis parameters was determined for each blood sample using a 2×2 matrix in which the QStat CSL and ROTEM lysis parameters were assigned to “Yes” or “No” based on the following definitions:
For QStat, lysis was defined as “Yes” if CSL is below the threshold value of 90%. Conversely, lysis was defined as “No” if CSL is greater than or equal to the threshold.
For ROTEM, the clot lysis-positive sample was defined as EXTEM ML>15% when EXTEM ML was determined at 60 minutes. This definition has been previously reported in the trauma and liver transplant literature.8,13,23–25
Overall, “Yes” and “No” agreements were calculated, and 95% CIs were generated using a nonparametric bootstrap approach. CIs were defined as the 2.5th and 97.5th percentile of the empirical distribution for each limit.
Finally, logistic regression models were used to assess the ability of QStat parameters to discriminate a series of ROTEM-based values. AUC of the receiver operating characteristic (ROC) plots were created for each model, and optimal QStat cutoff values were obtained by using the Youden J value. CIs were estimated for cutoffs, sensitivity, specificity, and negative and positive predictive values.
All analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC) or R Version 4.1.2, https://www.r-project.org/).
Sample size calculation
A simulation study was performed to inform the required sample size to be sufficiently powered to achieve the primary acceptance criteria, which were >80% agreement between the fibrinolysis parameters measured by the Quantra and ROTEM devices. Data from a pilot study were used to inform the simulation study. A bootstrap method was applied to simulate 10,000 clinical trials using the observed data cell probabilities for a range of sample sizes 70–613 powered to meet the acceptance criteria. Power was determined as the percentage of trials that meet the acceptance criteria out of total simulated trials. The sample size estimated for this study was 115, with 80% power. Assuming a dropout rate of 10%, the total number of subjects required to be enrolled in this study is 127.
RESULTS
A total of 134 liver transplant patients were enrolled in the study. Nine patients were excluded due to the use of nonvalid cartridges (5), samples collected >2 hours apart for comparative analysis (1), no data was available for either Quantra or ROTEM delta (2), and other reasons (1). Thus, the eligible study population consisted of 125 liver transplant patients, which generated a total of 392 paired samples containing matching Quantra and ROTEM delta results. The patient demographics and surgical details are summarized in Tables 2 and 3, respectively. The primary indications for liver transplant were chronic viral hepatitis, alcohol-associated liver disease, and NASH, which accounted for ~77% of all cases. In addition, samples from 5 normal subjects were used to generate 24 contrived specimens (5.8% of the total study database) that exhibited increased fibrinolytic activity. The total database consisted of 416 paired measurements.
TABLE 2.
Study demographics of surgical population
| Variable | Value |
|---|---|
| Total eligible surgical patients, n (%) | 125 (100) |
| Female sex, n (%) | 52 (41.6) |
| Age (y) | 58 (48, 64) |
| Weight (kg) | 84.1 (72.1, 97.5) |
| Race, n (%) | |
| Caucasian | 111 (88.8) |
| Black/African American | 10 (8.0) |
| Asian | 1 (0.8) |
| Other | 3 (2.4) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 3 (2.4) |
| Not Hispanic or Latino | 122 (97.6) |
Note: Data are expressed as number (%) or median (interquartile range).
TABLE 3.
Surgical procedures summary and outcomes
| Variable | Value |
|---|---|
| MELD scores | 24 (17, 31) |
| Primary reason for liver transplant, n (%) | |
| Chronic viral hepatitis | 36 (28.8) |
| Alcohol-associated liver disease | 34 (27.2) |
| NASH | 26 (20.8) |
| HCC | 8 (6.4) |
| Cirrhosis | 8 (6.4) |
| Cholestatic liver disease | 4 (3.2) |
| Other | 9 (7.2) |
| Donor organ source, n (%) | |
| Deceased donor: brain death | 89 (71.2) |
| Deceased donor: cardiac death | 27 (21.6) |
| Living donor | 9 (7.2) |
| Donor age (y) | 37 (28, 53) |
| Combined liver-kidney transplant, n (%) | 14 (11.2) |
| Surgery duration (min) | |
| Total time | 369 (312, 432) |
| Anhepatic time | 58 (45, 73) |
| Cold ischemia time | 341 (212, 419) |
| Warm ischemia time | 32 (26, 41) |
| Outcome | |
| Estimated blood loss during surgery (cc) | 3529 (1500, 4000) |
| Cell saver (cc) | 961 (248, 1250) |
| Estimated blood loss 24 h postsurgery (cc) | 441 (0, 663)a |
| Discharged from hospital, n (%) | 98 (78.4) |
| Still admitted >15 d after surgery, n (%) | 26 (20.8) |
| Death, n (%) | 1 (0.8) |
| Hospital stay (d) | 7 (5, 9) |
| ICU stay (d) | 2 (2, 4) |
Note: Data are expressed as number (%) or median (interquartile range).
Data reported for 86 patients.
Abbreviations: ICU, intensive care unit; MELD, Model for End-stage Liver Disease.
Correlation analysis
Linear regression analysis demonstrated a very strong positive correlation between the 2 devices for the comparable output parameters: CT versus INTEM CT, CS versus EXTEM A20 (amplitude at 20 min after clot initiation), FCS versus FIBTEM A20, and PCS versus a parameter derived offline after nonlinear transformation of EXTEM A20 and FIBTEM A20 as described by Solomon et al.21 Scatter plots are shown in A–D of Figure 1. The correlation and bias observed in these comparisons are in general agreement with the results reported in similar studies in cardiac surgery and trauma patients.16,18
FIGURE 1.
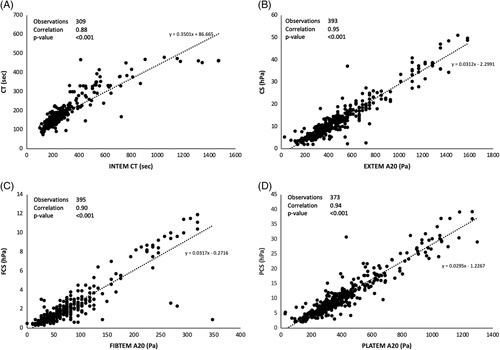
Scatter plots of QStat parameters versus corresponding ROTEM delta parameters. The value of EXTEM A20 and FIBTEM A20 were converted from clot amplitude in units of millimeter to elasticity in units of Pascals (Pa) using validated conversion formulas.20,21 PLATEM A20 is not a parameter output by the ROTEM delta, but instead it was calculated offline by subtracting EXTEM A20 and FIBTEM A20 after conversions to Pascals. Abbreviations: CS, clot stiffness; CT, clot time; PCS, platelet contribution to clot stiffness; FCS, fibrinogen contribution to clot stiffness.
The Supplemental Material, http://links.lww.com/LVT/A401, includes a subanalysis of QStat Cartridge parameters and correlation with ROTEM as a function of graft type.
Concordance analysis
Hyperfibrinolysis can occur during liver transplantation surgery and has been associated with perioperative bleeding.5 The results from the fibrinolysis concordance analysis are summarized in Table 4. The overall agreement between the 2 devices for the quantification of fibrinolysis was 90.3%, with similar agreements in each of the fibrinolysis-positive and negative subgroups. As shown in this table, 363 paired samples were used for the analysis of agreement; of these, 88 samples (24%) were classified as fibrinolysis positive using the Quantra, whereas only 69 samples (19%) met the criteria for the ROTEM delta.
TABLE 4.
Clinical agreement analysis of fibrinolysis
| ROTEM delta | |||
|---|---|---|---|
| EXTEM ML>15%a (fibrinolysis+) | EXTEM ML≤15%a (fibrinolysis−) | Total | |
| QStat Cartridge | |||
| CSL<90% (fibrinolysis+) | 61 | 27 | 88 |
| CSL≥90% (fibrinolysis−) | 8 | 267 | 275 |
| Total | 69 | 294 | 363 |
| Positive agreement | |||
| 88.4% (78.4%, 94.9%) | — | — | — |
| Negative agreement | |||
| 90.8% (86.9%, 93.9%) | — | — | — |
| Overall agreement | |||
| 90.3% (86.9%, 93.2%) | — | — | — |
Note: Agreement is expressed as percentage (95% CI).
Measured at 60 minutes after clot initiation.
Abbreviation: CSL, clot stability to lysis.
Twenty-seven samples were classified as positive for fibrinolysis by the Quantra CSL but as negative by the ROTEM delta. Of these, 11 samples had borderline CSL values between 86% and 89% (7 more samples had values between 80% and 84%), whereas 5 samples had borderline EXTEM ML values between 10% and 15%.
Similarly, of the 8 discordant samples for which fibrinolysis was classified as positive by the ROTEM delta but negative by the Quantra CSL, 4 had borderline positive EXTEM ML values between 16% and 19%. These 8 discordant results were further analyzed using additional test results from the ROTEM FIBTEM and APTEM assays when available (data not shown). This additional information suggested that 7 of the 8 discordant ROTEM results were likely to reflect clot relaxation or ROTEM analysis errors leading to a miscalculation by the EXTEM ML assay.
Of the 35 discordant samples, 18 (51.4%) were collected during the anhepatic phase, 9 (25.7%) during the reperfusion phase, and 7 (20%) at baseline. One (2.9%) sample was contrived.
ROC analysis
ROC analyses were conducted to determine the ability of selected QStat Cartridge parameters to discriminate specific values of corresponding ROTEM assays (Table 5). The ROTEM values represent threshold values typically used in goal-directed treatment algorithms for managing coagulopathic bleeding in liver transplant surgery. For every condition tested, the AUC was very high, with values ranging between 0.93 and 0.95. The calculated sensitivity and specificity values are similar to those previously reported in cardiac surgery patients.18 The optimal QStat CS and FCS cutoff values corresponding to each EXTEM and FIBTEM threshold are provided in Table 5.
TABLE 5.
ROC analysis of QStat parameters for various ROTEM thresholds
| Model | QStat cutoff value (hPa) | N (yes/no) | AUC | Sensitivity | Specificity | NPV |
|---|---|---|---|---|---|---|
| EXTEM A20<25 mm vs. CS | CS<3.8 (2.5, 5.1) | 10/383 | 0.94 (0.88, 1.00) | 0.90 (0.71, 1.00) | 0.86 (0.83, 0.90) | 0.99 (0.99, 1.00) |
| EXTEM A20<30 mm vs. CS | CS<5.3 (4.6, 6.0) | 26/367 | 0.95 (0.91, 0.95) | 0.88 (0.76, 1.00) | 0.9 (0.87, 0.93) | 0.99 (0.98, 1.00) |
| EXTEM A20<35 mm vs. CS | CS<6.9 (6.5, 7.3) | 58/335 | 0.95 (0.93, 0.97) | 0.95 (0.90, 1.00) | 0.86 (0.78, 0.95) | 0.99 (0.94, 1.00) |
| EXTEM A20<40 mm vs. CS | CS<7.9 (7.6, 8.2) | 106/287 | 0.95 (0.94, 0.95) | 0.91 (0.85, 0.96) | 0.90 (0.86, 0.93) | 0.96 (0.94, 0.99) |
| EXTEM A20<45 mm vs. CS | CS<11.1 (10.5, 11.7) | 178/215 | 0.95 (0.93, 0.97) | 0.97 (0.94, 0.99) | 0.84 (0.79, 0.89) | 0.97 (0.94, 0.99) |
| FIBTEM A20<4 mm vs. FCS | FCS<0.5 (0.4, 0.6) | 9/386 | 0.94 (0.89, 1.00) | 0.89 (0.68, 1.00) | 0.92 (0.90, 0.95) | 0.99 (0.99, 1.00) |
| FIBTEM A20<6 mm vs. FCS | FCS<0.8 (0.7, 0.9) | 57/338 | 0.94 (0.90, 0.97) | 0.91 (0.84, 0.99) | 0.85 (0.81, 0.89) | 0.98 (0.97, 1.00) |
| FIBTEM A20<8 mm vs. FCS | FCS<1.0 (0.9, 1.1) | 121/274 | 0.93 (0.91, 0.96) | 0.88 (0.82, 0.93) | 0.89 (0.86, 0.93) | 0.94 (0.91, 0.97) |
| FIBTEM A20<10 mm vs. FCS | FCS<1.4 (1.3, 1.5) | 181/214 | 0.94 (0.92, 0.96) | 0.94 (0.90, 0.97) | 0.79 (0.74, 0.85) | 0.94 (0.90, 0.97) |
| FIBTEM A20<12 mm vs. FCS | FCS<1.6 (1.5, 1.7) | 246/149 | 0.94 (0.91, 0.96) | 0.89 (0.85, 0.93) | 0.85 (0.79, 0.90) | 0.82 (0.76, 0.88) |
Note: Numbers in (,) represent the 95% CIs.
Abbreviations: CS, clot stiffness; FCS, fibrinogen contribution to clot stiffness. NPV, negative predictive value
DISCUSSION
This multicenter, prospective study aimed at characterizing the performance of this new cartridge and performing a correlation analysis with the ROTEM delta. The ROTEM delta is a well-established VET device for the management of perioperative bleeding in liver transplant patients, with several reports highlighting the clinical benefits of such a device, such as reduced intraoperative and postoperative use of allogeneic blood products, improved clinical outcomes, and reduced costs.8–13 Perioperative coagulation monitoring with the use of viscoelastic testing plays an important role in managing the complexity of the coagulopathy of end-stage liver disease in the setting of liver transplantation, which can result in significant blood loss. Data that can help guide clinicians in providing targeted transfusion therapy in this setting are extremely valuable.
The results presented here demonstrate that the Quantra QStat System and the ROTEM delta are strongly positively correlated, as indicated by the linear correlation coefficients reported in Figure 1. These findings are consistent with those previously reported on the multicenter evaluation of the Quantra with the QPlus Cartridge in patients undergoing cardiac and spine-reconstruction surgeries.18 Similarly, there was a strong concordance between the 2 devices for the detection of fibrinolysis, as demonstrated by an overall agreement between the QStat CSL parameter and EXTEM ML parameter of >90%. A similar level of agreement has been reported in the trauma population, albeit with a smaller number of fibrinolysis-positive samples.16 An EXTEM ML value >15% was used as a comparator to CSL, as this assay/parameter combination is often used for the diagnosis of fibrinolysis in trauma and liver transplant. Furthermore, analysis of the discordant samples, for which EXTEM ML predicted fibrinolysis but QStat CSL did not, highlighted the importance of measuring clot lysis with and without fibrinolysis inhibition to rule out the reduction in clot stiffness not attributable to clot lysis but rather to clot retraction/relaxation.26
The ROC analysis yielded QStat cutoff values that correspond, with high sensitivity and specificity, to ROTEM delta values that are typically used in goal-directed transfusion algorithms validated for liver transplant patients. Similar results were reported by Groves et al18 in the cardiac patient population. These data provide practical information to clinical teams considering adopting the Quantra System with the QStat Cartridge. The simplicity of interpretation of the information displayed in the Quantra dials allows for simple and streamlined treatment algorithms that could rapidly aid in the management of these patients. The proposed QStat cutoff values shown in Table 5 should be validated in future interventional studies to refine specific trigger and target values for clinical interventions in the liver transplant population (Figure 2).
FIGURE 2.
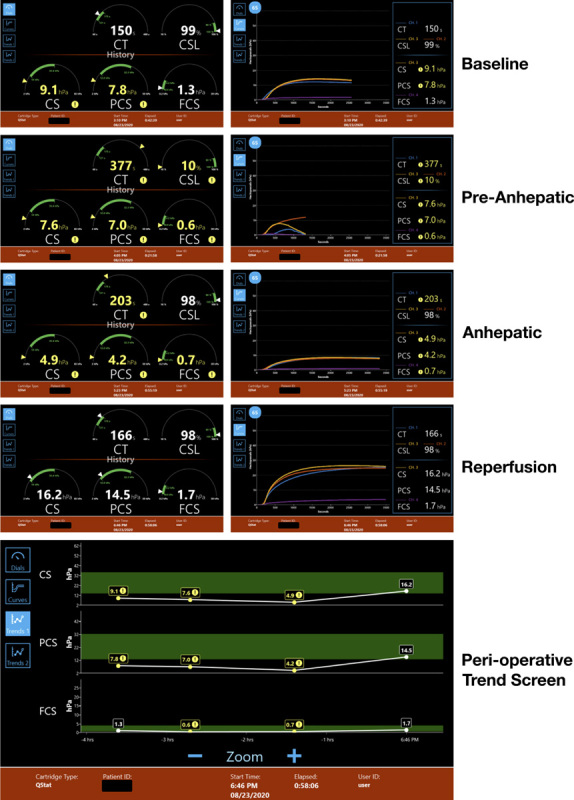
QStat Cartridge results (left column: Dial View, right column: corresponding Curve Screens) across 4-time points for one of the subjects enrolled in the study. The bottom panel shows the perioperative Trend View for the individual measurements of the clot stiffness-based parameters CS, FCS, and PCS. Abbreviations: CS, clot stiffness; CSL, clot stability to lysis; PCS, platelet contribution to clot stiffness; FCS, fibrinogen contribution to clot stiffness.
Our study had several limitations. As it was observational and the ROTEM delta was used for the clinical management of the enrolled subjects, the direct clinical impact of the QStat Cartridge could not be determined. With respect to the comparison between devices, not all the QStat and ROTEM delta parameters were compared, as each system parameters were not available across both platforms. Furthermore, even though the ROTEM EXTEM ML parameter was used as the comparator in this study, there is no widely accepted gold standard test/assay for the diagnosis of fibrinolysis. Finally, the number of enrolled subjects was not equal across the clinical sites, with 2 centers providing the majority of subjects.
In this multicenter prospective comparison of results obtained from the Quantra with the QStat Cartridge to the ROTEM delta to measure coagulation and fibrinolysis status of adult patients undergoing liver transplantation, we demonstrated a strong positive correlation between the results of the 2 VET devices. The ability to perform testing at the point of care with minimal sample handling requirements and rapidly obtain actionable results may provide additional clinical advantages and safety considerations over existing devices. However, additional prospective interventional studies are needed to fully characterize the impact of the Quantra system with QStat cartridge on patient outcomes following liver transplantation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Yohannes Tesfay for the support with data analysis.
FUNDING INFORMATION
The study was funded by HemoSonics, LLC.
CONFLICTS OF INTEREST
Antolin S. Flores received grants from Hemosonics. He consults for Werfen. Katherine T. Forkin received grants from Hemosonics. Sathish S. Kumar received grants from Edwards Life Sciences, Hemosonics, and PCORI. Deborah A. Winegar is employed by Hemosonics. Francesco Viola is employed by Hemosonics. Meghan M. Brennan has no conflicts to report.
Footnotes
Abbreviations: CLIA, Clinical Laboratory Improvement Amendments; CSL, clot stability to lysis; CS, clot stiffness; CT, clot time; CAP, College of American Pathologists; ERAS4OLT, Enhanced Recovery after Surgery Subgroup for Orthotopic Liver Transplant; FCS, fibrinogen contribution to clot stiffness; ICU, intensive care unit; IRB, Institutional Review Board; ILTS, International Liver Transplant Society; IUO, Investigational Use Only; MELD, Model for End-stage Liver Disease; ML, maximum lysis; Pa, Pascals; PCS, platelet contribution to clot stiffness; ROC, receiver operating characteristic; SABM, Society for the Advancement of Patient Blood Management; SATA, Society for the Advancement of Transplant Anesthesia; SCA, Society of Cardiovascular Anesthesiologists; STS, Society of Thoracic Surgeons; SEER, Sonic Estimation of Elasticity via Resonancer; VET, viscoelastic testing.
This study data was presented as a poster and oral presentation at the American Society of Anesthesiology 2022 annual meeting in New Orleans, LA.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.ltxjournal.com.
Contributor Information
Antolin S. Flores, Email: antolin.flores@osumc.edu.
Katherine T. Forkin, Email: KET2A@uvahealth.org.
Meghan M. Brennan, Email: mbrennan@anest.ufl.edu.
Sathish S. Kumar, Email: ssathish@med.umich.edu.
Deborah A. Winegar, Email: dwinegar@hemosonics.com.
Francesco Viola, Email: fviola@hemosonics.com.
REFERENCES
- 1.Becker RC. Cell-based models of coagulation: a paradigm in evolution. J Thromb Thrombolysis. 2005;20:65–8. [DOI] [PubMed] [Google Scholar]
- 2.Gaertner F, Massberg S. Blood coagulation in immunothrombosis—at the frontline of intravascular immunity. Semin Immunol. 2016;28:561–9. [DOI] [PubMed] [Google Scholar]
- 3.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–56. [DOI] [PubMed] [Google Scholar]
- 4.Caldwell SH, Hoffman M, Lisman T, Macik BG, Northup PG, Reddy KR, et al. Coagulation disorders and hemostasis in liver disease: pathophysiology and critical assessment of current management. Hepatology. 2006;44:1039–46. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann M, Szalai C, Saner FH. Hemostasis in liver transplantation: pathophysiology, monitoring, and treatment. World J Gastroenterol. 2016;22:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forkin KT, Colquhoun DA, Nemergut EC, Huffmyer JL. The coagulation profile of end-stage liver disease and considerations for intraoperative management. Anesth Analg. 2018;126:46–61. [DOI] [PubMed] [Google Scholar]
- 7.Crouch C, Sakai T, Aniskevich S, Damian D, De Marchi L, Kaufman M, et al. Adult liver transplant anesthesiology practice patterns and resource utilization in the United States: survey results from the society for the advancement of transplant anesthesia. Clin Transplant. 2022;36:e14504. [DOI] [PubMed] [Google Scholar]
- 8.Smart L, Mumtaz K, Scharpf D, Gray NO, Traetow D, Black S, et al. Rotational thromboelastometry or conventional coagulation tests in liver transplantation: comparing blood loss, transfusions, and cost. Ann Hepatol. 2017;16:916–23. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher C, Eismann H, Sieg L, Friedrich L, Scheinichen D, Vondran FW, et al. Use of rotational thromboelastometry in liver transplantation is associated with reduced transfusion requirements. Exp Clin Transplant. 2018;17:222–30. [DOI] [PubMed] [Google Scholar]
- 10.De Pietri L, Ragusa F, Deleuterio A, Begliomini B, Serra V. Reduced transfusion during OLT by POC coagulation management and TEG functional fibrinogen: a retrospective observational study. Transplantation direct. 2016;2:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leon-Justel A, Noval-Padillo JA, Alvarez-Rios AI, Mellado P, Gomez-Bravo MA, Álamo JM, et al. Point-of-care haemostasis monitoring during liver transplantation reduces transfusion requirements and improves patient outcome. Clinica Chimica Acta. 2015;446:277–83. [DOI] [PubMed] [Google Scholar]
- 12.Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590–93. [DOI] [PubMed] [Google Scholar]
- 13.Trzebicki J, Flakiewicz E, Kosieradzki M, Blaszczyk B, Kołacz M, Jureczko L, et al. The use of thromboelastometry in the assessment of hemostatsis during orthotopic liver transplantation reduces the demand for blood products. Ann Transplant. 2010;15:19–24. [PubMed] [Google Scholar]
- 14.Yoon U, Bartoszko J, Bezinover D, Biancofiore G, Forkin KT, Rahman S, et al. ERAS4OLT. org Working Group . Intraoperative transfusion management, antifibrinolytic therapy, coagulation monitoring and the impact on short‐term outcomes after liver transplantation—a systematic review of the literature and expert panel recommendations. Clinical Transplantation. 2022;36:e14637. [DOI] [PubMed] [Google Scholar]
- 15.Tibi P, McClure RS, Huang J, et al. STS/SCA/AmSECT/SABM update to the clinical practice guidelines on patient blood management. Ann Thorac Surg. 2021;112:981–1004. [DOI] [PubMed] [Google Scholar]
- 16.Michelson EA, Cripps MW, Ray B, Winegar DA, Viola F. Initial clinical experience with the Quantra QStat System in adult trauma patients. Trauma Surg Acute Care Open. 2020;5:e000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrante EA, Blasier KR, Givens TB, Lloyd CA, Fischer TJ, Viola F. A novel device for the evaluation of hemostatic function in critical care settings. Anesth Analg. 2016;123:1372–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groves DS, Welsby IJ, Naik BI, Tanaka K, Hauck JN, Greenberg CS, et al. Multicenter evaluation of the Quantra QPlus system in adult patients undergoing major surgical procedures. Anesth Analg. 2020;130:899–909. [DOI] [PubMed] [Google Scholar]
- 19.Leadbetter NH, Givens TB, Viola F. Unique approach to quality assurance in viscoelastic testing. J Appl Lab Med. 2020;5:1228–41. [DOI] [PubMed] [Google Scholar]
- 20.Lang T, Johanning K, Metzler H, Piepenbrock S, Solomon C, Rahe-Meyer N, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108:751–8. [DOI] [PubMed] [Google Scholar]
- 21.Solomon C, Ranucci M, Hochleitner G, Schochl H, Schlimp CJ. Assessing the methodology for calculating platelet contribution to clot strength (platelet component) in thromboelastometry and thrombelastography. Anesth Analg. 2015;121:868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–8. [DOI] [PubMed] [Google Scholar]
- 23.Schöchl H, Frietsch T, Pavelka M, Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67:125–31. [DOI] [PubMed] [Google Scholar]
- 24.Gall LS, Vulliamy P, Gillespie S, Jones TF, Pierre RS, Breukers SE, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg. 2019;269:1184–91. [DOI] [PubMed] [Google Scholar]
- 25.Guth C, Vassal O, Friggeri A, Wey PF, Inaba K, Decullier E, et al. Effects of modification of trauma bleeding management: a before and after study. Anaesth Crit Care Pain Med. 2019;38:469–76. [DOI] [PubMed] [Google Scholar]
- 26.Katori N, Tanaka KA, Szlam F, Levy JH. The effects of platelet count on clot retraction and tissue plasminogen activator-induced fibrinolysis on thrombelastography. Anesth Analg. 2005;100:1781–5. [DOI] [PubMed] [Google Scholar]


