Abstract
Objective
To evaluate the effects of light therapy on the alleviation of sleep disturbances, agitation and depression in people with dementia.
Methods
A search was performed in PubMed, Medline, SCOPUS, Web of Science, EMBASE, CINAHL, Cochrane Library, for studies published between 2000 and 2021.
Results
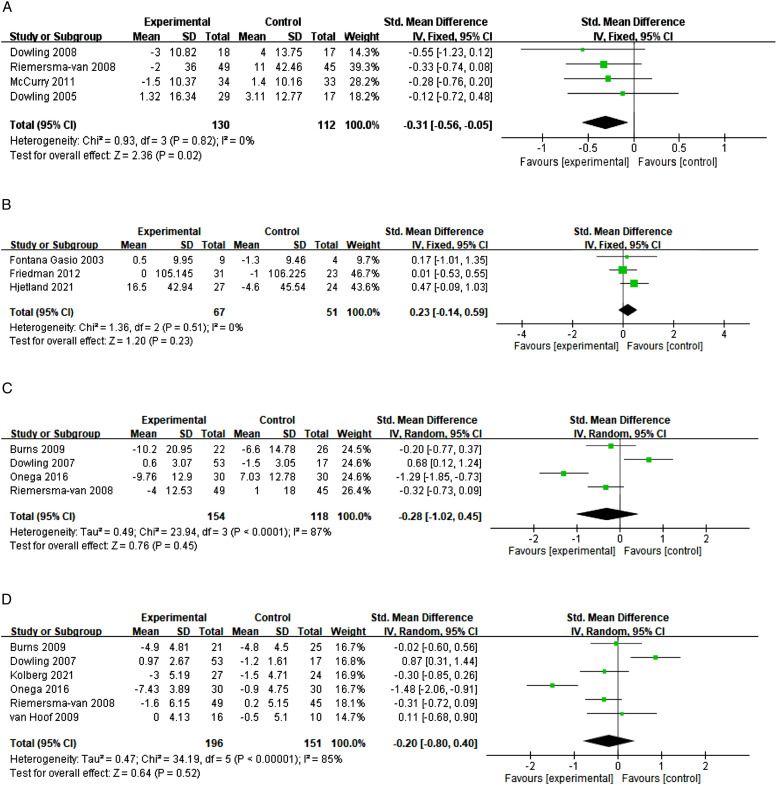
A total of 4315 articles were screened. Sixteen articles were eligible for this review and 11 randomized controlled studies were included in the meta-analysis. Light therapy had a significant effect on reducing the number of awakenings in sleep (n = 4; 95% CI = −.56, −.05; I2 = 0%; SMD = −.31) but was not significant in reducing the wake after sleep onset (n = 3; 95% CI = −.14, .59; I2 = 0%; SMD = .23), agitation (n = 4; 95% CI = −1.02, .45; I2 = 87%; SMD = −.28) and depression (n = 6; 95% CI = −.80, .40, I2 = 85%; SMD = −.20).
Conclusion
Light therapy appeared to be more effective in terms of alleviating sleep disturbances, rather than reducing agitation and depression, but its long-term effects remain unclear.
Keywords: light therapy, dementia, behavioral and psychological symptoms, sleep
Introduction
Human beings are synchronized to the circadian rhythms of our biological clocks; we sleep during the night and are typically awake and active during the day, following a 24-h solar day. 1 Up to 90% of people with dementia (PwD) may suffer from behavioral and psychological symptoms of dementia (BPSD), such as disturbed sleep-wake circadian patterns, nocturnal wandering, mood disorder such as agitation, depression, disinhibition, apathy, and physical or verbal abuse, delusions, etc.2,3 Among them, the most prevalent symptoms are apathy, agitation, irritability, anxiety, and depression.4,5
Sleep disturbance is a common symptom of BPSD in all types of dementia. Studies show that nearly half of PwD experience sleep disturbance, 6 such as difficulty falling asleep or frequent awakenings during the night.7,8 A recent meta-analysis reported that 20% of care home residents with dementia have clinically significant sleep disturbance but sleep disturbance was much higher (70%) when actigraphy is used for measurement. 9 Previous studies have shown that people with Alzheimer’s Disease (AD) will spend approximately 40% of the night awake and large portion of the day-time asleep; they wander around their homes at night, with or without aggressive or agitated behavior during the day because of poor sleep at night.10-12
The reasons suggested for sleep disturbance in PwD are not unitary. Behaviorally, PwD and older people tend to spend less time outside, meaning their exposure to high levels of daylight can become increasingly limited. When the indoor environment is poorly lit, this problem becomes even greater. The more severe the sleep disturbance, the higher the strain experienced by caregivers, which will eventually affect the quality of life of PwD. 13 Biologically, both older people and PwD may experience the degeneration of the suprachiasmatic nuclei (SCN), which could explain their disturbed sleep-wake circadian system. 14 The human visual system response to visible light occurs through four kinds of photoreceptors on the retina, each with different levels of spectral sensitivity. The four types of photoreceptors are conventionally grouped into two classes: rods and cones. In general, the visual photoreceptors are most sensitive to the middle-wavelength portion of the visible spectrum, peaking at around 555 nm (“green” light). 15 Light stimulates the photoreceptors in the eye, which sends signals to the SCN in the hypothalamus of the brain. The SCN then synchronizes the biological clock to the 24-h day. Apart from rods and cones, a newly discovered non-visual photoreceptor on the retina participates in circadian system, by converting light signals into neural signals for the biological clock, which have a response function peaking near 460 nm (“blue” light). 16 Age-related problems in the eye, such as cataracts and muscular degeneration, may also cause less light to reach the retina and further intensify the sleep problems experienced by PwD. Moreover, the circadian disruption is more pronounced in PwD than their healthy counterparts. 17 As a result, the light levels required by PwD to stimulate the SCN, which in turn helps to synchronize the circadian system, are significantly higher than the light levels required by other people in the community. 18
Evidence showed that bright light exposure (>1000 lux) during the morning improves night-time sleep, increases day-time wakefulness, reduces agitated behavior in the evenings, and restructures the day/sleep pattern of people with AD. 19 Light therapy (also known as phototherapy) consists of exposure to daylight or to specific wavelengths and intensity of light, administered for a prescribed amount of time and, in some cases, at a specific time of day. There are different forms of light therapy, using outdoor sunlight, use of bright light from artificial indoor light source, light visors worn on the head, etc., 20 and dawn-dusk simulation that mimics natural outdoor light conditions, 21 and the use of blue wavelength light therapy. 22 Light therapy may make use of, for example, a light box, a desk lamp, a wall-mount or ceiling fixture, which emits a high level of light at a specified distance, much brighter than a customary lamp. The light itself could be full-spectrum light that is similar in composition to sunlight or specific wavelengths of light from the blue (460 nm) to the green (525 nm) areas of the visible spectrum. For the treatment, the patient’s eyes should be at a prescribed distance from the light source, with the light striking the retina, but not necessarily looking directly into the light.
The effects of light therapy have been demonstrated in previous studies; bright light exposure during the morning (typically >1000 lux at the cornea) could improve the night-time sleep and increase the day-time wakefulness of PwD.23-30 However, this level of lighting is commonly not available in most indoor areas which the light intensities is 40–200 times lower than being outdoors during the day. 14 When the light source was tuned to the spectral sensitivity of the circadian system, such as short-wavelength “blue” light, lower light levels (30 lux at the cornea) administered for 2 hours in the evening were also shown to be effective in increasing sleep efficiency in PwD.31-33 Apart from using a light box, ambient lighting interventions can also be employed to increase the sleep efficiency of PwD, they do not limit the activity of the patients or require them to look in a particular direction. 34 In a previous study, custom luminaires using a bluish light source (CCT 9325 K) were built to light up the ceiling so that a minimum lighting level of 350–400 lux at the eye was achieved. 35 This was also found to significantly increase circadian entrainment and sleep efficiency, and significantly reduce symptoms of depression in PwD.
Recently, two systematic reviews and two meta-analyses have been published in the literature respectively, but conflicting results have been reported. Cibeira et al. 20 reviewed 36 studies with different study designs, including randomized controlled trials (RCTs) and quasi-experimental studies, and concluded that potential positive effects of light therapy on managing sleep, behavioral and mood disturbances, but limited effects had been shown on cognition and functions, in older adults with cognitive impairment. However, another systematic review of 32 articles concluded mixed results on sleep, cognition, mood, and behavior in patients with AD but with a general trend toward a positive effect. 36 The Cochrane review by Forbes et al. 37 examined all relevant RCTs and concluded that there was no effect of using bright light on cognitive function, sleep, challenging behavior, and psychiatric symptoms associated with dementia. However, Chiu et al. 38 concluded from a meta-analysis of nine RCTs that light therapy has a moderate effect on behavioural disturbances and depression, and a small effect on total sleep time at night. Moreover, different studies have recommended different light levels and exposure periods for light box therapy and ambient light intervention. It should be noted that the exact amount of light needed to effectively stimulate the circadian systems of PwD is still not known. 19 Therefore, the aim of this study is to evaluate the effects of light therapy on sleep, agitation and depression in PwD, reported in randomized controlled trials that were obtained by searching computer databases for relevant studies conducted between Jan 2000 and Dec 2021, so as to provide updated evidence for clinical practice.
Methods
Literature search
This study was carried out following the framework of the Preferred Reported Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 39 The literature search was performed using seven electronic databases: PubMed, Medline, SCOPUS, Web of Science, EMBASE, CINAHL, and the Cochrane Library. Search terms included dementia, Alzheimer’s, light stimulation, light therapy, and bright light therapy. “AND” and “OR” were also used in keyword combinations, to search for all potential studies. The literature search was carried out by two authors independently, to avoid missing potential studies.
Inclusion criteria
The inclusion criteria were set according to the PICOS search tool. (1) Participants: Patients diagnosed with dementia; (2) Intervention using any form of light stimulation; (3) Comparison: Participants were assigned to either a light stimulation group or a control group that adopted placebo light stimulation, standard care, or other conventional treatments, and did not receive light stimulation; (4) Outcomes: Outcome measures included the overall BPSD, or BPSD symptoms, such as depression, agitation, and sleep disturbance; (5) Study design: Randomized controlled trials (RCTs) only. Moreover, the included studies must be in English and available in full text. The publication period was limited from Jan 2000 to Dec 2021.
Data extraction
Data were extracted by two authors independently, including the authors, study design, diagnosis, sample size, age, gender, interventions, dosage, outcome, results, and follow-up information from the included studies. In cases where studies missed necessary data for the meta-analysis, we also tried to contact some of the authors directly.
Appraisal of methodological quality
The Physiotherapy Evidence Database (PEDro) scale was used to assess the methodological quality of the included studies – again, by two authors independently. 40 The PEDro scale covers a total of 11 items. The first item is a screening question with no score, and the second to 11th items are scoring items. For each item, one point is given when it meets the standard; when the condition is not met, zero points are given. The highest possible score is 10. The quality of studies with a PEDro score of nine or 10 was regarded as excellent, six-to-eight as good, four-to-five as fair, and below four as poor. 40
Data analysis
RevMan 5.3 was used to analyze the extracted data. 41 Since all of the outcomes were measured using different tools, the standard mean difference (SMD) for continuous outcomes was selected to estimate the pooled effect size. All statistical analyses were performed with under 95% confidence intervals (95%CI), using an I2 test and a chi-square test to analyze the statistical heterogeneity of the studies. 42 The I2 value was computed for each meta-analysis, and it indicated a high level of heterogeneity across studies if the value approached the value of 1 or 100%. If high heterogeneity with a significant result on the test of heterogeneity existed, the random-effect model was selected, and if low heterogeneity with an insignificant result on the test of heterogeneity existed, the fixed-effect model was selected. 43 The magnitude of the effect size corresponding to each SMD was interpreted according to the guideline: small, SMD = .2; medium, SMD = .5; and large, SMD = .8. 44 A funnel plot was used to assess publication bias.
Results
Study identification
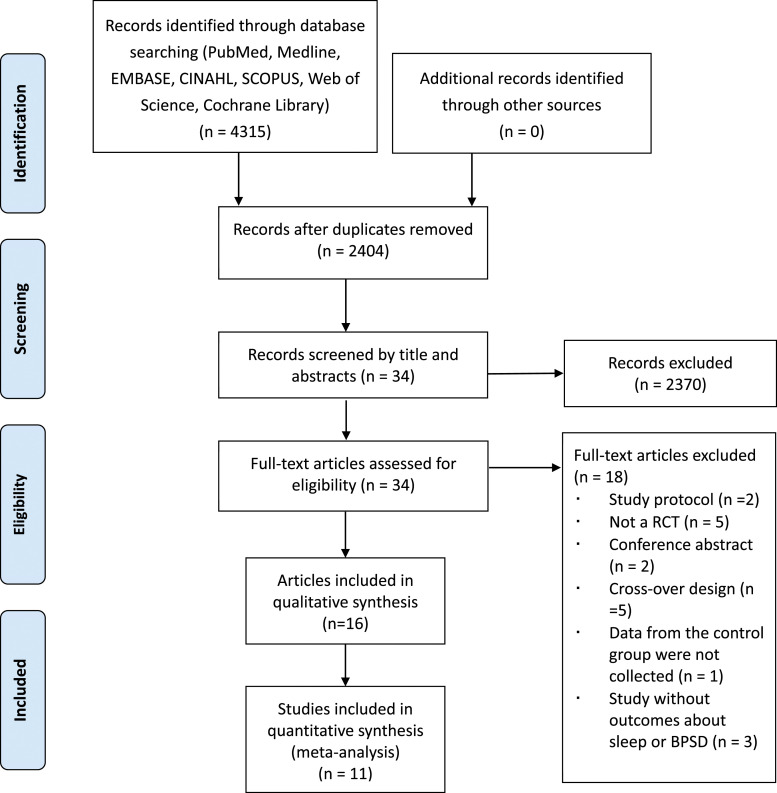
Figure 1 depicts the search process. A total of 4315 articles were identified from seven electronic databases, 2404 duplicates were removed, 2370 articles were excluded after screening by reading the titles and abstracts, and then the remaining 34 articles were selected by referring to the inclusion criteria. Of these, 18 articles were excluded because they were study protocols (n = 2), because they were conference abstract (n = 2), because they were not RCTs (n = 5), because they were cross-over studies (n = 5), because data from the control group were not collected (n = 1), and because the studies did not include outcomes about BPSD and sleep (n = 3). The general characteristics of the included studies are shown in Table 1. Finally, 16 articles were eligible for the current review, among them, one study was rated as excellent, nine studies as good, five as fair and one as poor. The results of the methodological quality assessment are shown in Table 2.
Figure 1.
PRISMA flow diagram of the study selection process.
Table 1.
The characteristics of the included studies.
| Study | Design | Diagnosis | Sample Size | Age (years) | Gender (Male, Female) | Intervention (E, C) | Dosage | Outcome Measures | Follow-Up (Period) |
|---|---|---|---|---|---|---|---|---|---|
| Ancoli-Israel et al., 2003a | RCT | Alzheimer disease | E1 = 30 | 82.3±7.6 | (29, 63) | E1: Morning bright light | 2500 lux, 2 h per day, 10 days | Actillume (activity level); Actigraphy (sleep) | Yes (5 days) |
| E2 = 31 | E2: Evening bright light | ||||||||
| C = 31 | C: morning dim red light | ||||||||
| Ancoli-Israel et al., 2003b | RCT | Alzheimer disease | E1 = 30 | 82.3±7.6 | (29, 63) | E1: Morning bright light | 2500 lux, 2 h per day, 10 days | CMAI; ABRS | Yes (5 days) |
| E2 = 31 | E2: Evening bright light | ||||||||
| C = 31 | C: morning dim red light | ||||||||
| Fontana Gasio et al., 2003 | RCT | Dementia | E = 9; C = 4 | E = 86.8±4.5; C = 83.0 ± 5.2 | (1, 12) | E: Dawn-dusk light therapy; | 280 lux for 1 h/day, 3 weeks | Actiwatch: Number of awakenings at night | Yes (3 weeks) |
| C: placebo normal light | |||||||||
| Dowling et al., 2005a | RCT | Alzheimer’s disease | E = 29; C = 17 | 84.0±10.0 | (10, 36) | E: bright light therapy; C: Placebo normal light | 2500 lux for 1 h/day, 5 days per week, 10 weeks | Actigraphy: Number of awakenings at night | No |
| Dowling et al., 2005b | RCT | Alzheimer’s disease | E1 = 29 | 84.0±10.0 | (13, 57) | E1: Bright light therapy in the morning; E2: Bright light therapy in the afternoon; C: Placebo normal light | 2500 lux for 1 h/day, 5 days per week, 10 weeks | Actigraphy: Number of awakenings at night | No |
| E2 = 24 | |||||||||
| C = 17 | |||||||||
| Dowling et al., 2007 | RCT | Alzheimer’s disease | E1 = 29 | 84.0±10.0 | (13, 57) | E1: Bright light therapy in the morning; E2: Bright light therapy in the afternoon; C: Placebo normal light | 2500 lux for 1 h/day, 5 days per week, 10 weeks | NPI-NH | No |
| E2 = 24 | |||||||||
| C = 17 | |||||||||
| Dowling et al., 2008 | RCT | Alzheimer’s disease | E = 18; C = 17 | E = 89.0±7.0; C = 82.0±10.0 | NA | E: bright light therapy; C: Placebo normal light | 2500 lux for 1 h/day, 5 days per week, 10 weeks | Actigraphy: Number of awakenings at night | No |
| Riemersma-van der Lek et al., 2008 | RCT | Dementia | E = 49; C = 45 | E = 85.0±6.0; C = 85.0±5.0 | (9, 85) | E: bright light therapy; C: Placebo normal light | 1000 lux for 8 h/day, 6 weeks | Actigraphy: Number of awakenings at night | No |
| CSDD; CMAI | |||||||||
| Burns et al., 2009 | RCT | Dementia | E = 22; C = 26 | E = 82.5; C = 84.5 | (16, 32) | E: bright light therapy; C: Placebo normal light | 10,000 lux for 2 h/day, 2 weeks | CMAI | Yes (4 weeks) |
| CSDD | |||||||||
| van Hoof et al., 2009 | RCT | Dementia | E = 16 | E = 86.3±7.6 | (7, 19) | E: Ambient bright light | 1750–1800 lx for 10 h/day, 3 weeks bluish light and 3 weeks yellowish light | Dutch behaviour observation scale for intramural psychogeriatrics | No |
| C = 10 | C = 84.4±5.7 | C: dim light | |||||||
| McCurry et al., 2011 | RCT | Dementia | E = 34; C = 33 | E = 80.6±7.3; C = 81.2±8.0 | NA | E: bright light therapy; C: Placebo normal light | 2500 lux for 1 h/day, 8 weeks | Actigraphy: Number of awakenings at night | Yes (4 months) |
| Friedman et al., 2012 | RCT | MCI; Alzheimer’s dementia; other dementia or diagnosable memory impairment | E = 31 | 68.8±12.7 | (31, 23) | E: bright light therapy | 4200 lux for 30-min per day, 2 weeks | Actigraphy (sleep) | No |
| C = 23 | C: dim red light | ||||||||
| Onega et al., 2016 | RCT | Dementia | E = 30 | 82.6±9.60 | (17, 43) | E: bright light therapy | 10,000 lux for 30-min twice a day, five times a week, 8 weeks | CSDD | No |
| C = 30 | C: low-intensity light | CMAI | |||||||
| Onega et al., 2018 | RCT | Dementia | E = 30 | 82.6±9.60 | (17, 43) | E: bright light therapy | 10,000 lux for 30-min twice a day, five times a week, 8 weeks | CSDD | No |
| C = 30 | C: low-intensity light | ||||||||
| Hjetland et al., 2021 | RCT | Dementia | E = 33 | E = 84.3±6.2 | (22, 47) | E: Ambient light | 1000 lux, 3 h per day, 24 weeks | Actigraphs (sleep) | No |
| C = 36 | C = 82.8±7.9 | C: standard light | |||||||
| Kolberg et al., 2021 | RCT | Dementia | E = 33 | E = 84.3±6.2 | (22, 47) | E: Ambient light | 1000 lux, 3 h per day, 24 weeks | NPI-NH | No |
| C = 36 | C = 82.8±7.9 | C: standard light | CSDD |
RCT: randomized controlled trial; NA: not available; E: experimental group; C: control group; CMAI: Cohen-Mansfield Agitation Inventory; CSDD: Cornell Scale for Depression in Dementia; NPI-NH: Neuropsychiatric Inventory-Nursing Home; MCI: Mild cognitive impairment. ABRS: Agitated Behavior Rating Scale.
Table 2.
The Physiotherapy Evidence Database (PEDro) Scale scores of the included studies.
| Studies | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total Score | Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ancoli-Israel et al., 2003a | YES | √ | √ | √ | √ | 4 | Fair (same study) | ||||||
| Ancoli-Israel et al., 2003b | YES | √ | √ | √ | √ | 4 | |||||||
| Fontana Gasio et al., 2003 | YES | √ | √ | √ | √ | √ | √ | 6 | Good | ||||
| Dowling et al., 2005a | YES | √ | √ | √ | √ | √ | √ | 6 | Good | ||||
| Dowling et al., 2005b | YES | √ | √ | √ | √ | √ | 5 | Fair (same study) | |||||
| Dowling et al., 2007 | YES | √ | √ | √ | √ | √ | 5 | ||||||
| Dowling et al., 2008 | YES | √ | √ | √ | √ | √ | √ | √ | √ | 8 | Good | ||
| Riemersma-van der Lek et al., 2008 | YES | √ | √ | √ | √ | √ | √ | √ | √ | √ | 9 | Excellent | |
| Burns et al., 2009 | YES | √ | √ | √ | √ | √ | √ | √ | 7 | Good | |||
| van Hoof et al., 2009 | YES | √ | √ | √ | 3 | Poor | |||||||
| McCurry et al., 2011 | YES | √ | √ | √ | √ | √ | √ | √ | √ | 8 | Good | ||
| Friedman et al., 2012 | YES | √ | √ | √ | √ | √ | 5 | Fair | |||||
| Onega et al., 2016 | YES | √ | √ | √ | √ | √ | √ | 6 | Good (same study) | ||||
| Onega et al., 2018 | YES | √ | √ | √ | √ | √ | √ | 6 | |||||
| Hjetland et al., 2021 | YES | √ | √ | √ | √ | √ | √ | 6 | Good (same study) | ||||
| Kolberg et al., 2021 | YES | √ | √ | √ | √ | √ | √ | 6 |
1) Eligibility criteria specified (item does not score); 2) random allocation of subjects to groups; 3) concealed allocation; 4) groups’ baseline comparability regarding the most important prognostic indicators; 5) blinding of all subjects; 6) blinding of all therapists; 7) blinding of all appraisers; 8) at least 1 outcome finding from >85% of the subjects; 9) intention-to-treat analysis; 10) between-group statistical comparisons; 11) provided point measures and measures of variability.
However, 11 studies were included in the meta-analysis only because the work of three studies were published in two articles separately (one for sleep and one for depression/agitation)24,25; 27,45; 46,47 and one study was published as two articles (one is the primary analysis and another is a subgroup analysis of depression based on severity) (Onega et al., 2016; Onega et al 2018).48,49 Both the studies by Ancoli-Israel et al.24,25 were excluded from our meta-analysis. Ancoli-Israel et al. 24 used wake after sleep onset (hours), however, there were errors in the published data and that the agitation score of Ancoli-Israel et al. 25 were also not reported because the data were not available from the authors.
Participants
The 11 studies in the meta-analysis included a total of 648 participants. Of them, five studies reported information about the participants’ gender (n = 546): 27% were male (n = 148) and 73% were female (n = 398). The sample size in each study ranged from 13 to 94.
Interventions
Among the 11 included studies in the meta-analysis, light therapy box with an exposure of at least 2500 lux was used to provide an intervention in four studies.12,26,27,45,50 Intervention sessions for these 4 studies were set to an average of 2 h a day, between 5 and 7 days a week, for between 2 and 10 weeks. Two studies used 10,000 lux for the light intensity,24,25,51 one of these used 2 h exposure per day for 2 weeks 51 while the other used 30 mins exposure twice a day, five times per week for 8 weeks.24,25 Two out of three studies provide light exposure with 1000 lux, 3 h daily for 24 weeks,46,47 but 1 study used it for 8 hours daily for 6 weeks. 52 One study used about 1800 lux 31 but another study used 4200 lux. 53
Outcomes
Actigraphy was used in most of the studies that assessed sleep disturbances in PwD (n = 9). The number of awakenings at night recorded by the wrist actigraphy and the time spent awake after sleep onset were selected to reflect the quality of sleep. Overall BPSD was measured using the Neuropsychiatric Inventory (NPI) (n = 2). BPSD symptoms, such as agitation and depression, were measured using the Cohen-Mansfield Agitation Inventory (CMAI) (n = 3) and the Cornell Scale for Depression in Dementia (CSDD) (n = 4).
The effects of light therapy on sleep
A total of 242 participants were recruited in the four selected studies, to enable an evaluation of the effects of light therapy on the reduction of the number of awakenings at night.26,27,50,52 The results of the pooled SMD showed that light therapy has a significant effect on reducing the number of awakenings at night in PwD compared with the control group (SMD = −.31; 95%CI = −.56, −.05; I2 = 0%; P = .02; fixed-effect model; Figure 2(a)). However, there was only one study which carried out a follow-up analysis of the long-term effects at 4 months (McCurry et al, 2011). 54 Publication bias might exist, according to the non-symmetrical results in the funnel plot.
Figure 2.
(A) The effects of light therapy on the reduction of the number of awakenings; (B) The effect of light therapy on the sleep quality measured by the wake after sleep onset; (C) The effects of light therapy on the reduction of agitation; (D) The effects of light therapy on the reduction of depression.
There were three studies which measured the sleep quality in terms of the time spent awake after sleep onset, involving a total of 118 participants,21,46,53 however, our results show that there was no significant effect in reducing the time spent awake after sleep onset (SMD = .23; 95%CI = −.14, .59; I2 = 0%; P = .23; fixed-effect model) (Figure 2(b)).
The effects of light therapy on agitation
A total of 272 participants were recruited for 4 studies evaluating the effects of light therapy on the reduction of agitation (Dowling et al, 2007; Burns et al, 2009; Onega et al, 2016; Riemersma-van der Lek et al, 2008).27,48,51,52 The results of a pooled SMD showed that the effects of light therapy in this respect was not significant (SMD = −.28; 95%CI = −1.02, .45; I2 = 87%; p = .45; random-effect model) (Figure 2(c)). There was no study evaluating its long-term effects on the reduction of agitation. The funnel plot analysis shows that publication bias might exist, according to the non-symmetrical results.
The effects of light therapy on depression
A total of 374 participants were recruited across 6 studies evaluating the effects of light therapy on the reduction of depression.27,34,47,48,51,52 Since Kolberg et al. 47 reported medians rather than means in the paper, and that the dataset could not be obtained from the authors, we hypothesized that the median was equal to the mean because of the large sample size among similar studies. The results of a pooled SMD showed that the effects of light therapy were not significant in reducing depression (SMD = −.20; 95% CI = −.80, .40, I2 = 85%; p = .52; random-effect model) (Figure 2(d)). Again, there was no study evaluating its long-term effects on the reduction of agitation. Publication bias may exist according to the non-symmetrical results in the funnel plot analysis.
Discussion
Sleep disturbance and BPSD are two of the greatest challenges faced in the course of providing dementia care. A total of 11 RCT studies in 16 papers were analyzed in the meta-analysis. The results show that light therapy significantly improved sleep disturbance in terms of reducing the number of awakenings, but did not significantly improve the sleep quality as measured by the wake after sleep onset, nor reduce BPSD symptoms such as agitation and depression. All RCT studies used bright light except the study by Fontana Gasio et al. 21 which used 24-h dynamic light exposure with an automatic lighting system – a dawn-dusk stimulation; the light levels changed according to the time of day. However, only 5 out of the 11 studies in this review included a follow-up study, ranged from 5 days, 3–4 weeks, and 4 months, with no evidence supporting the long-term effects of light therapy on the improvement of sleep.
Our findings were partially consistent with that of the Cochrane review by Forbes et al. 37 that the use of light therapy had no effect on challenging behaviour or psychiatric symptoms in PwD but significant small-to-moderate effect on sleep was found in our study. However, the findings are still not conclusive. Chiu et al. 38 in their review concluded that light therapy has a moderate effect on behavioural disturbances and depression, and a small effect on total sleep time at night. The reason for the inconsistency might be that a crossover study 7 was included in their review and that another RCT 52 was missed in their calculation; and that both BPSD and agitation was also pooled together in their outcome analysis. 38
Based on our findings, the effects of light therapy on the reduction of BPSD remain uncertain. In a single group study by Figueiro et al., 55 a tailored lighting intervention using low-level “bluish-white” lighting during the daytime for 4 weeks in nursing homes could increase sleep quality (sleep time and sleep efficiency) and reduce depression and agitation in PwD. 55 However, Dowling et al. 27 study participants’ agitation scores increased after bright light exposure, suggesting that we need to be more cautious in future clinical practice. The dysregulation of melatonin rhythms, core body temperature, and circadian rhythms is common in PwD. 12 All of these changes can lead to various forms of sleep problems, including discontinuous sleep and multiple awakenings at night.12,55 Moreover, different light intensities and durations of exposure were used in these studies, including 1 h per day for between 8 and 10 weeks, 5 days per week, at 2500 lux12,26,27,50; 3 h daily for 24 weeks46,47 and 8 h daily for 6 weeks at 1000 lux. 52 Two studies used 10,000 lux.24,25,51 or 30 min at 10,000 lux twice a day, five times per week for 8 weeks.24,25 Two studies adopted a different protocol of using 4200 lux or about 1800 lux respectively.34,53 On the other hand, the effects of light therapy for behavioral symptoms varied in patients with various medical conditions, for example, moderate-quality evidence suggests that blue-wavelength light therapy may be useful for post traumatic brain injury depression but not sleepiness and sleep disturbance. 22
Regarding the measurement, eight studies used actigraphy – a wearable wristwatch accelerometer for logging the motor activity during sleep, for the measurement of sleep quantitatively but not qualitatively. Because sleep disturbances are too common among the BPSD symptoms and that reducing sleep disturbances and improving sleep efficiency are the main reasons why light therapy has to be used in this population due to disrupted circadian rhythms. In future, the use of sleep-specific questionnaires for caregivers should be considered in addition to the use of actigraphy for a better understanding of the sleep disturbances of clients living at home or institutions. One of the suggested tools is the Pittsburgh Sleep Quality Index (PSQI), 56 a self-report questionnaire for staff or caregivers to report the subjective nature of the client’s sleep quality over a 1-month time interval, which requires only 5–10 min to complete, and provide a clear picture, including the subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction, etc. For clinicians and caregivers to use for research and clinical activities. 57 Nevertheless, two studies used the NPI for the measurement of overall BPSD symptoms, three studies used the CMAI for agitation, and four studies used the CSDD for depression. Although they are all standardized measures for BPSD, we suggest that these measures should be used together in the form of a uniform dataset for more accurate measurement of the behavioral symptoms with heterogeneous manifestation among older people with various types of dementia.
The presently available evidence suggests that stimulating the suprachiasmatic nucleus (SCN) of the hypothalamus with light can improve circadian rhythm disorders. 37 Those suffering from dementia may experience degeneration of the SCN, which could explain their disturbed sleep-wake cycles. Since the circadian system is dependent on the timing of light exposure, and the duration of light exposure needed to affect the system may take minutes. 16 In healthy older people, ambient light was shown to have a significant impact on night-time sleep, with a critical exposure threshold of 3000 lux. 58 On the other hand, older people and PwD tend to spend less time outside, meaning their exposure to high levels of daylight can become increasingly limited. This situation has worsened further during the 2020/21 COVID-19 pandemic, because older people are being encouraged to stay at home to reduce their risk of infection from social interactions with others. Light therapy can regulate circadian rhythms and environmental light-dark cycles through special neurons in the SCN, thus improving the sleep disturbances experienced by PwD.37,52
This review has several limitations: (1) the standard mean difference was selected to calculate the combined effect size, due to the variety of evaluation tools used; and (2) only RCT studies in English from 2000 to 2021 were included. This review provides implications for future studies, which should explore (1) whether or not the interventions are more effective for different levels of severity of dementia on sleep and BPSD, whether the effects are the same or different; (2) various forms of lighting in terms of light intensity, wavelengths, and duration of exposure, so as to provide optimal effects through interventions in regard to the alleviation of sleep disturbance and BPSD; and (3) the long-term effects of light therapy on PwD. The above suggestions will eventually provide clues to the underlying mechanisms of using light therapy, which may lead to successful care for PwD.
Conclusion
The current systematic review and meta-analysis show that light therapy has positive effects on sleep disturbances in PwD in terms of reducing the number of awakenings but not improving the wake after sleep onset nor reducing agitation and depression. The long-terms effects of light therapy are still unknown, due to insufficient evidence. In the future, more multicenter, well-designed RCT studies with large sample sizes are warranted.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by the Innovation and Technology Fund – Mid-stream Research Programme for Universities (Grant No.: MRP/011/17X), Innovation and Technology Commission, Hong Kong SAR.
ORCID iD
Kenneth NK Fong https://orcid.org/0000-0001-5909-4847
References
- 1.Moore RY. Circadian rhythms: basic neurobiology and clinical applications. Annu Rev Med. 1997;48:253-266. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendriks SA, Smalbrugge M, Galindo-Garre F, Hertogh CMPM, van der Steen JT. From admission to death: Prevalence and course of pain, agitation, and shortness of breath, and treatment of these symptoms in nursing home residents with dementia. J Am Med Dir Assoc. 2015;16(6):475-481. [DOI] [PubMed] [Google Scholar]
- 4.Cerejeira J, Lagarto L, Mukaetova-Ladinska EB. Behavioral and psychological symptoms of dementia. Front Neurol. 2012;3:73. DOI: 10.3389/fneur.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neville CC, Byrne GJA. Prevalence of disruptive behaviour displayed by older people in community and residential respite care settings. Int J Ment Health Nurs. 2007;16:81-85. [DOI] [PubMed] [Google Scholar]
- 6.Urrestarazu E, Iriarte J. Clinical management of sleep disturbances in Alzheimer’s disease: Current and emerging strategies. Nat Sci Sleep. 2016;8:21-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloane PD, Figueiro M, Garg S, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Light Res Technol. 2015;47(2):161-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bliwise DL. Sleep disorders in Alzheimer’s disease and other dementias. Clin Cornerstone. 2004;6(suppl 1A):S16-S28. [DOI] [PubMed] [Google Scholar]
- 9.Webster L, Costafreda Gonzalez S, Stringer A, et al. Measuring the prevalence of sleep disturbances in people with dementia living in care homes: A systematic review and meta-analysis. Sleep. 2020;43(4):zsz251. DOI: 10.1093/sleep/zsz251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Parker L, Sinaee R, Fell RL, Kripke DF. Sleep fragmentation in patients from a nursing home. J Gerontol. 1989;44(1):M18-M21. [DOI] [PubMed] [Google Scholar]
- 11.Vitiello MV, Poceta JS, Prinz PN. Sleep in Alzheimer’s disease and other dementing disorders. Can J Psychol. 1991;45(2):221-239. [DOI] [PubMed] [Google Scholar]
- 12.McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev. 2000;4(6):603-628. [DOI] [PubMed] [Google Scholar]
- 13.Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics. 2015;15(1):65-74. DOI: 10.1111/psyg.12069 [DOI] [PubMed] [Google Scholar]
- 14.Bonmati-Carrion MA, Arguelles-Prieto R, Martinez-Madrid MJ, et al. Protecting the melatonin rhythm through circadian healthy light exposure. Int J Mol Sci. 2014;15:23448-23500. DOI: 10.3390/ijms151223448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Lighting Handbook. New York: IESNA; 2011. [Google Scholar]
- 16.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070-1073. [DOI] [PubMed] [Google Scholar]
- 17.Figueiro MG, Hamner R, Higgins P, Hornick T, Rea MS. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31:711-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stopa EG, Volicer L, Kuo-Leblanc V, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J Neuropathol Exp Neurol. 1999;58(1):29-39. [DOI] [PubMed] [Google Scholar]
- 19.Hanford N, Figueiro M. Light therapy and Alzheimer’s disease and related dementia: Past, present, and future. J Alzheimers Dis. 2013;33(4):913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cibeira N, Maseda A, Lorenzo-López L, Rodriguez-Villamil JL, Lopez-Lopez R, Millan-Calenti JC. Application of light therapy in older adults with cognitive impairment: A systematic review. Geriatr Nurs. 2020;41(6):970-983. DOI: 10.1016/j.gerinurse.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 21.Fontana Gasio P, Kräuchi K, Cajochen C, et al. Dawn-dusk simulation light therapy of disturbed circadian rest-activity cycles in demented elderly. Exp Gerontol. 2003;38(1-2):207-216. [DOI] [PubMed] [Google Scholar]
- 22.Srisurapanont K, Samakarn Y, Kamklong B, et al. Blue-wavelength light therapy for post-traumatic brain injury sleepiness, sleep disturbance, depression, and fatigue: A systematic review and network meta-analysis. PLoS One. 2021;16(2):e0246172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. J Am Geriatr Soc. 2005;53(5):803-810. [DOI] [PubMed] [Google Scholar]
- 24.Ancoli-Israel S, Gehrman P, Martin JL, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22-36. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Martin JL, Gehrman P, et al. Effect of light on agitation in institutionalized patients with severe Alzheimer Disease. Am J Geriatr Psychiatr. 2003;11(2):194-203. [PubMed] [Google Scholar]
- 26.Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJW. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17(2):221-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowling GA, Graf CL, Hubbard EM, Luxenberg JS. Light treatment for neuropsychiatric behaviors in Alzheimer’s disease. West J Nurs Res. 2007;29(8):961-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fetveit A, Skjerve A, Bjorvatn B. Bright light treatment improves sleep in institutionalised elderly: An open trial. Int J Geriatr Psychiatr. 2003;18(6):520-526. [DOI] [PubMed] [Google Scholar]
- 29.Fetveit A, Bjorvatn B. Bright-light treatment reduces actigraphic-measured daytime sleep in nursing home patients with dementia: A pilot study. Am J Geriatr Psychiatr. 2005;13(5):420-423. [DOI] [PubMed] [Google Scholar]
- 30.Sloane PD, Williams CS, Mitchell CM, et al. High-intensity environmental light in dementia: Effect on sleep and activity. J Am Geriatr Soc. 2007;55(10):1524-1533. [DOI] [PubMed] [Google Scholar]
- 31.Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatr Clin Neurosci. 2000;54(3):352-353. [DOI] [PubMed] [Google Scholar]
- 32.Figueiro MG, Rea MS. LEDs: Improving the sleep quality of older adults. Leon, Spain. Proceedings of the CIE Midterm Meeting and International Lighting Congress. 2005. [Google Scholar]
- 33.Figueiro MG, Rea MS, Eggleston G. Light therapy and Alzheimer’s disease. Sleep Rev. 2003;4(1):24. [Google Scholar]
- 34.van Hoof J, Aarts MPJ, Rense CG, Schoutens AMC. Ambient bright Light in dementia: Effects on behavior and circadian rhythmicity. Build Environ. 2008;44(1):146-155. DOI: 10.1016/j.buildenv.2008.02.005 [DOI] [Google Scholar]
- 35.Figueiro MG, Hunter CM, Higgins P, et al. Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health. 2015;1(4):322-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitolo M, Tonon C, La Morgia C, Testa C, Carelli V, Lodi R. Effects of light treatment on sleep, cognition, mood, and behavior in Alzheimer’s Disease: A systematic review. Dement Geriatr Cognit Disord. 2018;46:371-384. DOI: 10.1159/000494921 [DOI] [PubMed] [Google Scholar]
- 37.Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014(2):Cd003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiu HL, Chan PT, Chu H, et al. Effectiveness of light therapy in cognitively impaired persons: A metaanalysis of randomized controlled trials. J Am Geriatr Soc. 2017;65(10):2227-2234. [DOI] [PubMed] [Google Scholar]
- 39.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J Physiother. 2009;55(2):129-133. [DOI] [PubMed] [Google Scholar]
- 41.The Cochrane Collaboration . Review Manager (RevMan). Version 5.3 [software]. The Nordic Cochrane Centre; 2014. [Google Scholar]
- 42.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo SH, Fong KNK, Chen Z, Chung RCK. Cognitive and psychological interventions for the reduction of post-concussion symptoms in patients with mild traumatic brain injury: A systematic review. Brain Inj. 2020;34:1305-1321. DOI: 10.1080/02699052.2020.1802668 [DOI] [PubMed] [Google Scholar]
- 44.Cohen J. A power primer. Psychol Bull. 1992;112:155-159. [DOI] [PubMed] [Google Scholar]
- 45.Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL. Effect of timed bright light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. Int J Geriatr Psychiatr. 2005;20(8):738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hjetland GJ, Kolberg E, Pallesen S, et al. Ambient bright light treatment improved proxy-rated sleep but not sleep measured by actigraphy in nursing home patients with dementia: a placebo-controlled randomised trial. BMC Geriatr. 2021;21:312. DOI: 10.1186/s12877-021-02236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolberg E, Hjetland GJ, Thun E, et al. The effects of bright light treatment on affective symptoms in people with dementia: a 24-week cluster randomized controlled trial. BMC Psychiatry. 2021;21:377. DOI: 10.1186/s12888-021-03376-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onega LL, Pierce TW, Epperly L. Effect of bright light exposure on depression and agitation in older adults with dementia. Issues Ment Health Nurs. 2016;37:660-667. DOI: 10.1080/01612840.2016.1183736 [DOI] [PubMed] [Google Scholar]
- 49.Onega LL, Pierce TW, Epperly L. Bright light therapy to treat depression in individuals with mild/moderate or severe dementia. Issues Ment Health Nurs. 2018;39(5):370-373. DOI: 10.1080/01612840.2018.1437648 [DOI] [PubMed] [Google Scholar]
- 50.Dowling GA, Burr RL, Van Someren EJW, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56(2):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: A randomized controlled trial. Int Psychogeriatr. 2009;21(4):711-721. [DOI] [PubMed] [Google Scholar]
- 52.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, Van Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299(22):2642-2655. [DOI] [PubMed] [Google Scholar]
- 53.Friedman L, Spira AP, Hernandez B, et al. Brief morning light treatment for sleep/wake disturbances in older memory-impaired individuals and their caregivers. Sleep Med. 2012;13:546-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCurry SM, Pike KC, Vitiello MV, Logsdon RG, Larson EB, Teri L. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer’s disease: Results of a randomized, controlled trial. J Am Geriatr Soc. 2011;59(8):1393-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Figueiro MG, Plitnick BA, Lok A, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193-213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 57.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52-73. doi: 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 58.Hood B, Bruck D, Kennedy G. Determinants of sleep quality in the healthy aged: The role of physical, psychological, circadian and naturalistic light variables. Age Ageing. 2004;33(2):159-165. [DOI] [PubMed] [Google Scholar]