Introduction
Crystalline nephropathies are an underdiagnosed cause of kidney disease characterized by the histologic finding of intrarenal crystal deposition primarily involving the tubulointerstitium.1 These disorders are particularly challenging. They require the pathologist to deduce the nature of the crystals from a variety of clues (polarization, color, fluorescence, etc.) before a direct chemical identification.
Clinically, patients may present crystalluria, proteinuria, and cylinduria, while tubulopathies, AKI, and CKD may also develop. Identification of crystals within the kidneys on biopsy is definitive for a diagnosis of crystalline nephropathy and necessitates evaluation of the underlying cause.2
Although excellent reviews summarizing crystal nephropathies have been published,2–4 we propose a practical and easy approach to guide the diagnosis on the basis of three steps: examination under polarized light, color of the crystals on hematoxylin and eosin and periodic acid–Schiff stains, and fluorescence.
Case
A 76-year-old man with dyslipidemia and ischemic heart disease presented with pancreatic cancer. Treatment with chemotherapy FOLFIRINOX (folinic acid, fluorouracil, irinotecan, and oxaliplatin) followed by pancreaticoduodenectomy and consolidation chemotherapy by LV5FU2 (fluorouacil and leucovorin) was proposed. His other medications included atenolol, acetylsalicylic acid, levothyroxine, paroxetine, pancreatic enzyme supplements 75,000 UI/d (since the surgery), and ascorbic acid 500 mg/d, up to 3 days a week (as needed). The postoperative course was marked by chronic diarrhea without weight loss and a progressive decline in kidney function (eGFR/Modification of Diet in Renal Disease 90 ml/min per operative and then 67, 45, and 33 ml/min at 4, 14, and 18 months, respectively, when a kidney biopsy was performed). Urinalysis revealed no proteinuria, hematuria, or leukocyturia. Kidney biopsy revealed tubulointerstitial fibrosis. Examination under polarized light revealed numerous birefringent and translucent crystals within the interstitium referring to calcium oxalate, sodium urate, or cystinosis crystals and drug-induced (acyclovir, indinavir, atazanavir, amoxicillin, and sulfadiazine). Patient's medical history did not reveal the use of such drugs nor pathologies, such as HIV or cystinosis. The diagnosis of oxalate crystal nephropathy is based on a history of duodenopancreatectomy and intake of ascorbic acid and hyperoxaluria (115 mg/24 hours and an oxalate/creatinine ratio of 78.7 mg/g). Treatment including low oxalate diet, calcium and potassium citrate supplementation, and avoidance of ascorbic acid intake resulted in stabilization of kidney function at 33 ml/min 6 months after the kidney biopsy.
How to Diagnose Crystalline Nephropathy
Crystalline nephropathies are a unique form of kidney disease. They are categorized by the cause, namely medication induced (sulfa-based medications, methotrexate, acyclovir, protease inhibitors, triamterene, ciprofloxacin, levofloxacin, amoxicillin, ascorbic acid, foscarnet, and sodium phosphate purgative), paraproteinemia induced (light chain proximal tubulopathy, crystalline cast nephropathy, crystalglobulinemia, and crystal storing histocytosis), and those associated with inherited disorders (Dent disease, primary hyperoxaluria, Lowe syndrome, and cystinosis).
The clinical presentations of the above syndromes can be variable and cannot provide too much insight into the cause of the nephropathy. AKI is present in most of the causes of this syndrome, but in some, there will also be nephrolithiasis and in some crystalluria. Fanconi syndrome is more associated with the light chain–associated crystalline nephropathies and many hereditary nephropathies (cystinosis, Dent disease, and Lowe syndrome). High-grade monoclonal proteinuria is a subtle clue in light chain crystalline cast nephropathy. Diagnosis of medication-induced crystalline nephropathies hinges on the knowledge of culprit medications, their clinical syndromes, and urine microscopic findings.
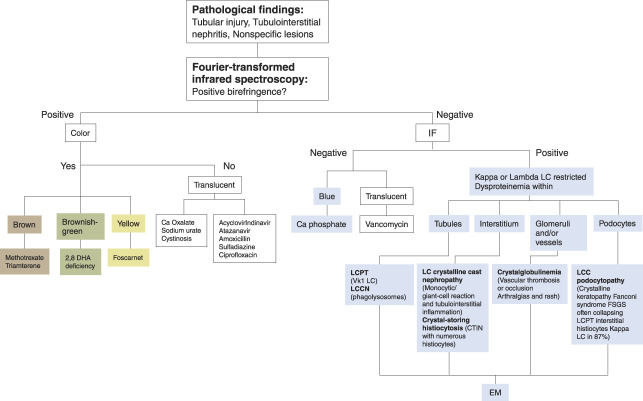
Urinary examination and microscopy is clinically useful in diagnosis of crystal nephropathies. The color, shape, and content of the crystals can be useful in the clinical diagnosis and clues the clinicians in to the cause of the syndrome. Figure 1 summarizes the various urine microscopic findings of drug-induced, light chain–induced, and genetic causes of this syndrome.
Figure 1.
Crystalline nephropathy: an illustrated algorithm approach. In the presence of specific lesions, the first step is an examination under polarized light. If there is a refringence under polarized light, the second step is to determine the color of the crystals. The color guide toward different categories of etiology: crystals with a positive birefringent polarization and brown, brownish-green, or yellow can lead to methotrexate or triamterene, 2,8 dihydroxyadenine deficit, and foscarnet crystals, respectively, whereas crystals birefringent and translucent evoke calcium oxalate, sodium urate, or cystinosis crystals and drug-induced (acyclovir, indinavir, atazanavir, amoxicillin, and sulfadiazine). Crystals without polarization and blue colored on PAS are phosphocalcic crystals. If crystals are colorless, vancomycin crystals could be the search. If none of the above methods are successful, the pathologist should perform immunofluorescence as the third step: a positive IF for kappa or lambda chains should lead to a search for LC-restricted dysproteinemia (LC cast nephropathy, LC proximal tubulopathy, crystal-storing histiocytosis, and crystalglobulinemia). Crystal topography aids diagnosis according to the nephron structure affected (tubules, LCPT, LCCN; interstitium, LC crystalline cast nephropathy, crystal-storing histiocytosis; podocytes/FSGS, LCC podocytopathy; glomeruli and/or vessels, crystalglobulinemia). Electron microscopic analysis is necessary in these cases. A negative IF indicates phosphocalcic (blue) or vancomycin (translucent) crystals. CTIN, chronic tubulointerstitial nephritis; EM, electron microscopy; IF, immunofluorescence; LC, light chain; LCCN, light chain crystalline cast nephropathy; LCPT, light chain proximal tubulopathy; PAS, periodic acid–Schiff.
Kidney-related imaging, such as computed tomography scan or ultrasound, can help only if there is nephrolithiasis associated with the syndrome. Pure crystal-related AKI may not be clinically diagnosed with imaging techniques.
It is not uncommon that most patients are asymptomatic and only show isolated elevated serum creatinine. In some cases, if the urine findings are not useful or are completely benign, kidney biopsy is the gold standard. If histologic findings are not obvious at the beginning and in the presence of specific lesions (acute tubular injury in nonatrophic tubules, interstitial fibrosis and tubular atrophy, and mild-to-moderate interstitial inflammation without eosinophilic infiltrate), the first step that the pathologist should systematically perform is an examination under polarized light (Figure 1). If there is a refringence under polarized light, the second step is to determine the color of the crystals. The color will guide the pathologist toward different categories of etiology: drug induced, metabolic, genetic, or others.
Crystals with a positive birefringent polarization and brown, brownish-green, or yellow can lead to methotrexate or triamterene, 2,8-dihydroxyadenine deficit, and foscarnet crystals, respectively, whereas crystals birefringent and translucent can evoke calcium oxalate, sodium urate, or cystinosis crystals and drug-induced (acyclovir, indinavir, atazanavir, amoxicillin, and sulfadiazine).
Crystals without polarization and blue colored on periodic acid–Schiff suggest phosphocalcic crystals. If crystals are colorless, vancomycin crystals could be the search (B).
If none of the above methods are successful, the pathologist should perform immunofluorescence as the third step.
A positive immunofluorescence for kappa or lambda chains should lead to a search for light chain–restricted dysproteinemia (light chain cast nephropathy, light chain proximal tubulopathy, crystal-storing histiocytosis, and crystalglobulinemia). Electron microscopic analysis is necessary in these cases. Nevertheless, the topography of the crystals helps in the diagnosis. Tubular involvement suggests light chain proximal tubulopathy (particularly in the case of Vk1 light chains) and/or light chain crystalline cast nephropathy (in the presence of phagolysosomes). Interstitial involvement points to light chain crystalline cast nephropathy, particularly in cases of associated monocytic/giant cell reaction and tubulointerstitial inflammation or crystal-storing histiocytosis in the presence of numerous histiocytes. Glomerular localization of crystals suggests crystalglobulinemia, particularly in cases of vascular thrombosis or occlusion and arthralgias and rash or light chain crystalline podocytopathy in cases of FSGS often collapsing in association with Fanconi syndrome, crystalline keratopathy, and kappa light chains.5
A negative immunofluorescence indicates phosphocalcic (blue) or vancomycin (translucent) crystals
Careful clinicopathologic correlation is important in the interpretation of crystalline nephropathies. Therefore, biopsy specimens could be analyzed by Fourier-transformed infrared spectroscopy to confirm the composition of crystals (Figure 1).
Disclosures
K.D. Jhaveri reports consultancy agreements with Calliditas, George Clinicals, GSK, PMV Pharmaceuticals, and Secretome; reports honoraria from the American Society of Nephrology and UpToDate.com; reports serving on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Journal of Onconephrology, Kidney International, and Nephrology Dialysis Transplantation; and reports serving as Editor-in-Chief of ASN Kidney News and section editor for onconephrology for Nephrology Dialysis Transplantation. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Isabelle Brocheriou, Hassan Izzedine.
Formal analysis: Isabelle Brocheriou, Hassan Izzedine.
Investigation: Isabelle Brocheriou.
Methodology: Isabelle Brocheriou, Hassan Izzedine, Kenar D. Jhaveri.
Resources: Hassan Izzedine.
Supervision: Isabelle Brocheriou, Hassan Izzedine.
Validation: Isabelle Brocheriou, Hassan Izzedine, Kenar D. Jhaveri.
Visualization: Hassan Izzedine.
Writing – original draft: Isabelle Brocheriou, Hassan Izzedine, Kenar D. Jhaveri.
Writing – review & editing: Isabelle Brocheriou, Hassan Izzedine, Kenar D. Jhaveri.
References
- 1.Yarlagadda SG, Perazella MA. Drug-induced crystal nephropathy: an update. Expert Opin Drug Saf. 2008;7(2):147–158. doi: 10.1517/14740338.7.2.147 [DOI] [PubMed] [Google Scholar]
- 2.Perazella MA, Herlitz LC. The crystalline nephropathies. Kidney Int Rep. 2021;6(12):2942–2957. doi: 10.1016/j.ekir.2021.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herlitz LC, D'Agati VD, Markowitz GS. Crystalline nephropathies. Arch Pathol Lab Med. 2012;136(7):713–720. doi: 10.5858/arpa.2011-0565-RA [DOI] [PubMed] [Google Scholar]
- 4.Nicholas Cossey L, Dvanajscak Z, Larsen CP. A diagnostician's field guide to crystalline nephropathies. Semin Diagn Pathol. 2020;37(3):135–142. doi: 10.1053/j.semdp.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Nasr SH Kudose S Javaugue V, et al. Pathological characteristics of light chain crystalline podocytopathy. Kidney Int. 2023;103(3):616–626. doi: 10.1016/j.kint.2022.11.026 [DOI] [PubMed] [Google Scholar]