Abstract
In contrast to significant advances in the management of patients with chronic heart failure over the past few years, there has been little change in how patients with acute heart failure are treated. Symptoms and signs of fluid overload are the primary reason for hospitalization of patients who experience acute decompensation of heart failure. Intravenous loop diuretics remain the mainstay of therapy in this patient population, with a significant subset of them showing suboptimal response to these agents leading to incomplete decongestion at the time of discharge. Combination diuretic therapy, that is, using loop diuretics along with an add-on agent, is a widely applied strategy to counter renal sodium avidity through sequential blockade of sodium absorption within renal tubules. The choice of the second diuretic is affected by several factors, including the site of action, the anticipated secondary effects, and the available evidence on their efficacy and safety. While the current guidelines recommend combination diuretic therapy as a viable option to overcome suboptimal response to loop diuretics, it is also acknowledged that this strategy is not supported by strong evidence and remains an area of uncertainty. The recent publication of landmark studies has regenerated the interest in sequential nephron blockade. In this article, we provide an overview of the results of the key studies on combination diuretic therapy in the setting of acute heart failure and discuss their findings primarily with regard to the effect on renal sodium avidity and cardiorenal outcomes.
Keywords: diuretics, heart failure, randomized controlled trials
Background
Fluid overload is the primary reason for admission of patients with acute heart failure.1 Irrespective of their ejection fraction, these patients have a similar profile of congestion and the goals for their management remain the same.2 While alterations in venous capacitance have been proposed as a potential factor that can provoke or modify the clinical presentation, most patients with acute heart failure are admitted due to excessive sodium and fluid retention with resulting fluid overload.3–5 Therefore, renal sodium avidity has long been the focus of investigations exploring potential approaches to improve decongestion process in acute heart failure.6
Diuretics and Natriuresis
When using diuretics in the setting of acute heart failure, a few clinical points about urinary sodium excretion merit attention: (1) the response to diuretics is highly variable, and the overall urinary sodium excretion tends to decrease rapidly with decongestion7–9; (2) spot urine sodium concentration portends prognostic value and can predict the likelihood of successful decongestion10; and (3) while loop diuretics are the mainstay of therapy, a significant subset of patients show suboptimal response.11 A widely accepted quantitative definition of diuretic resistance with utility in both clinical and research scenarios remains elusive.12 Qualitatively, diuretic resistance can be described as an inadequate rate/quantity of natriuresis despite an adequate diuretic regimen.13 Combination diuretic therapy, that is, using loop diuretics along with an add-on agent, has been used as a potential means to more efficiently counter renal sodium avidity through sequential blockade of sodium absorption within renal tubules. The choice of the second diuretic agent may be determined by several factors such as its site of action, the potential side effects, and the evidence from contemporary trials supporting their benefit.14,15
Sodium-Glucose Cotransporter-2 Inhibitors
The sodium-glucose cotransporter-2 inhibitors (SGLT-2i) gained much attention over the past decade because they were shown to offer important cardiorenal benefits in patients with kidney or cardiovascular disease with or without diabetes.16–19 SGLT-2 is a luminal transporter in the S1 and S2 segments of the proximal tubule that normally reabsorbs about 97% of filtered glucose, whereas SGLT-1 in the S3 segment reabsorbs the remainder.20 Most sodium is reabsorbed in the proximal tubule in exchange for hydrogen by sodium:hydrogen exchanger 3 (NHE3). While SGLT-2 increases activity of NHE3,21 the NHE3 can also increase the expression of SGLT-2.22 Thus, beyond osmotic diuresis, SGLT-2 is a component of an interactive proximal tubular absorptive platform whose blockade impairs sodium reabsorption profoundly even in the absence of excessive luminal glucose. Moreover, increased tubular fluid glucose and osmolality impairs water reabsorption and can dilute the tubular fluid sodium leading to paracellular secretion of sodium in the proximal tubules. It has been shown that angiotensin II can also increase SGLT-2 mRNA and protein expression in the kidney tissue, which may have an effect on proximal tubular absorption of sodium.23 While 1 week of SGLT-2i could increase urinary sodium excretion of loop diuretics by 36% in animal studies,24 they may induce significant natriuresis in patients with heart failure without neurohormonal activation.25,26
The Effects of Empagliflozin on Clinical Outcomes in Patients With Acute Decompensated Heart Failure (EMPA-RESPONSE-AHF) trial randomized 80 patients with acute heart failure to either receive empagliflozin or placebo.27 Patients who received empagliflozin had a tendency for better decongestion as evidenced by weight loss and reduction in N-terminal pro b-type natriuretic peptide (NT-proBNP), and their urine output and net fluid loss were noted to be significantly higher. However, none of the primary end points reached statistical significance, including dyspnea score (Table 1). The patients' eGFR was 55 ml/min at baseline, and the number of patients with rise in serum creatinine (RSC) was comparable in both arms. EMPA-RESPONSE was the first trial of SGLT-2i in acute heart failure and confirmed safety of these drugs in this setting while also suggesting some improvement in fluid balance. Interestingly, a post hoc analysis found that empagliflozin stimulates osmotic diuresis through increased glycosuria rather than natriuresis, leading to lower urinary sodium concentration despite increased sodium excretion (Figure 1).29 The Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF) trial randomized 60 patients with acute heart failure to empagliflozin 25-mg daily or placebo in addition to standard treatment.30 The investigators reported a 25% increase in cumulative urine output. Addition of empagliflozin also increased loop diuretic efficiency with a more pronounced decrease in NT-proBNP. The dosage of 25-mg empagliflozin, which is higher than the doses used for long-term cardiorenal benefit, was chosen to maximize potential diuretic effects and was shown to be safe.
Table 1.
Randomized controlled trials of dual nephron blockade in acute heart failure
| Secondary Nephron Site Blockade | Trial Name | Year of Publication | No. of Patients | Add-On Agent | Baseline Kidney Function | Primary End Points | Diuresis | Natriuresis | Weight Loss | Key Finding | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Proximal tubule (SGLT-2) | EMPA-RESPONSE-AHF | 2020 | 80 | Empagliflozin 10 mg |
eGFR: 55 ml/min | Change in dyspnea, diuretic response, LOS, NT-proBNP | 3442 versus 2400 ml at day 1,a 3449 ml difference in cumulative urine output at day 4a | NA | 2.83 versus 2.3 kg at day 4b | No difference in any of the four primary end points | Significantly improved heart failure readmission or WHF or death at 60 d |
| Proximal tubule (CA) | ADVOR | 2022 | 519 | Acetazolamide 500 mg |
eGFR: 39 ml/min | Successful decongestion (no edema, pleural effusion, and ascites) | 4600 versus 4100 ml at day 2a | 468 versus 369 mmol at day 2a | NA | Successful decongestion in 42.2% versus 30.5%a | Shorter LOS in intervention arm by 1.1 d |
| DCT (NCC) | CLOROTIC | 2022 | 230 | HCTZ 25, 50, and 100 mg |
eGFR: 43 ml/min | Change in body weight and dyspnea | 1775 versus 1440 ml in 24 hb | 64 versus 47 mmol/L at 96 ha | 2.3 versus 1.5 kg at 72 ha | Successful decongestion (weight, diuresis, natriuresis) without change in dyspnea | The trial was halted prematurely because of slow recruitment, higher weight loss per 40 mg of furosemide in the intervention arm |
| DCT (MR) | ATHENA-HF | 2017 | 360 | Spironolactone 100 mg |
eGFR: 56 ml/min | Change in NT-pro-BNP | 6086 versus 5584 ml at day 4b | NA | 3.3 versus 2.8 kg at 96 hb | No difference in the primary or secondary end points between the two groups | No difference in serum potassium or kidney function |
| Collecting duct (V2R) | EVEREST | 2007 | 4133 | Tolvaptan 30 mg |
Serum creatinine: 1.4 mg/dl | Change in global clinical status and body weight | NA | NA | 3.35 versus 2.73 kg at day 7a in trial A, 3.77 versus 2.79a kg in trial B | Improvement in weight, but not global clinical status | Improvement in dyspnea and edema |
Adapted from ref. 28. SGLT-2, sodium-glucose cotransporter-2; EMPA-RESPONSE-AHF, Effects of Empagliflozin on Clinical Outcomes in Patients With Acute Decompensated Heart Failure; LOS, length of hospital stay; NT-proBNP, N-terminal pro b-type natriuretic peptide; NA, not available; WHF, worsening heart failure; CA, carbonic anhydrase; ADVOR, Acetazolamide in Decompensated Heart Failure with Volume Overload; DCT, distal convoluted tubule; NCC, sodium-chloride cotransporter; CLOROTIC, Combination of Loop with Thiazide Diuretics for Decompensated Heart Failure; HCTZ, hydrochlorothiazide; MR, mineralocorticoid receptor; ATHENA-HF, Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure; V2R, vasopressin-2 receptor; EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan.
The intervention arm versus the control arm, any statistically significant P value of <0.05 reported by the authors.
The intervention arm versus the control arm, any statistically insignificant P value of 0.05 or more reported by the authors.
Figure 1.

Comparison of the diuresis on day 1 between loop diuretics and the intervention. The difference observed in urine sodium excretion among patients who received loop diuretics reflects the differences in the protocols that were used in each trial. For the EVEREST trial, it is the weight reduction on day 1 from trial A. ADVOR, Acetazolamide in Decompensated Heart Failure with Volume Overload; ATHENA-HF, Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure; CLOROTIC, Combination of Loop with Thiazide Diuretics for Decompensated Heart Failure; EMPA-RESPONSE-AHF, Effects of Empagliflozin on Clinical Outcomes in Patients With Acute Decompensated Heart Failure; EVEREST, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan.
Recently, the Empagliflozin in Patients Hospitalized for Acute Heart Failure (EMPULSE) trial randomized 530 patients with acute heart failure to either empagliflozin or placebo.31 The patients showed improvement in decongestion at all time points, which was assessed through several markers, including a clinical congestion score. For example, on day 15, the mean weight of patients who received empagliflozin was 1.97 kg less than those who received placebo (P < 0.0001), which remained the same after adjustment for loop diuretic dose. The improved decongestion was sustained for the next 3 months, and the investigators were able to show that it translated into clinical benefits, including a lower number of heart failure events.31–33
Because SGLT-2i have rapidly become a key component of quadruple therapy for chronic heart failure, initiating them at the time of admission for acute heart failure portends the added benefit that the patients would be more likely to continue them after discharge.34 Moreover, the benefits of SGLT-2i are evident as early as 30 days after initiation.35 Therefore, this class of medications should be strongly considered when patients with acute heart failure are admitted for congestive symptoms.
Carbonic Anhydrase Inhibitors
Over the past few years, there has been a growing body of evidence on a strong link between low serum chloride levels and adverse outcomes in heart failure, which may even be stronger than that of sodium.36–38 Maladaptive neurohormonal activation and diuretic resistance have been proposed as potential mechanisms to explain this phenomenon.15,39 These observations have generated interest in an old class of diuretics, the carbonic anhydrase (CA) inhibitors, that can increase serum chloride levels.40
In the proximal tubules, the hydrogen secreted into the lumen combines with bicarbonate to form carbonic acid, which is rapidly dehydrated to CO2 and H2O by CA. Carbon dioxide produced by dehydration of carbonic acid enters the proximal tubule cell, where it is then rehydrated back to carbonic acid, facilitated by intracellular CA. After dissociation of carbonic acid, hydrogen is available for transport by the apical NHE3, and the bicarbonate is transported out of the cell by a basolateral membrane transporter. CA inhibitors are a group of diuretics that cause significant bicarbonate loss in the urine, counterbalanced by an equivalent increase in plasma chloride concentration. These agents can also lead to enhanced natriuresis through downregulation of pendrin (a chloride/bicarbonate exchanger), which works in tandem with the epithelial sodium channel (ENaC) in distal nephron.41 From a clinical standpoint, progressive bicarbonate depletion leads to decreased serum bicarbonate levels, which further undermines the substrate availability for CA-mediated sodium reabsorption in the proximal tubules. Therefore, the diuretic efficacy of CA inhibitors may decrease with repeated use over several days.42
Acetazolamide was used in the Diamox to Increase the Urinary Excretion of Sodium: an Investigational Study in Congestive Heart Failure (DIURESIS-CHF) trial that randomized 34 patients with acute heart failure to receive either loop diuretic monotherapy or in combination with acetazolamide.43 Despite lower doses of loop diuretic in the combination diuretic therapy arm, the two groups experienced the same degree of decongestion. Acetazolamide was associated with a 62% improvement in loop diuretic efficiency. This pilot study set the stage for a large multicenter trial, Acetazolamide in Decompensated Heart Failure with Volume Overload (ADVOR).44 It randomized 519 patients with acute heart failure to receive intravenous acetazolamide (500 mg bolus daily) or placebo in addition to an intravenous furosemide regimen (twice the oral home dose). The median GFR of the patients was 39 ml/min, and more than 80% had an eGFR of <60 ml/min. Successful decongestion within 3 days was achieved more often in the acetazolamide arm than placebo (42.2 versus 30%, P < 0.001). At 48 hours, the cumulative diuresis and natriuresis were higher in the acetazolamide arm as well (by 0.5 L of urine and 98 mmol of sodium; Figure 1). Urine sodium concentration was 92 mmol/L in the overall population of ADVOR (unpublished data presented at the American Heart Association annual meeting 2022). The patients in the acetazolamide arm experienced a modest increment (27%) in their urine sodium. In fact, post hoc analysis revealed that urine sodium concentration was only 12 mmol/L higher in patients who received combination diuretic therapy, which is in line with the studies confirming that proximal tubular absorption of sodium has a negligible role in suboptimal response to loop diuretics in patients with heart failure (Figure 2).46 Moreover, acetazolamide was administered in this study for only 2 days after randomization or until the occurrence of complete decongestion. The patients who received acetazolamide had a shortened length of stay, but there was no difference in kidney end points, all-cause mortality, or heart failure readmission. Finally, it is noteworthy that ADVOR investigators defined successful decongestion (i.e., the primary end point of the study) by the absence of edema, ascites, and pleural effusion rather than the more common manifestations of volume overload such as dyspnea or jugular vein distension. So, a hypothetical patient who had trace pedal edema without pleural effusion or ascites would have been considered successfully decongested although he continued to have jugular vein distension. These limited criteria are likely to have affected the reported rate of decongestion in ADVOR, which is higher than previous studies.11
Figure 2.

Comparison of the urine sodium concentration generated by loop diuretics versus the intervention, which includes either an add-on diuretic or the ultrafiltrate. The difference observed in urine sodium excretion among patients who received loop diuretics reflects the differences in the protocols that were used in each trial. The data for ADVOR and CLOROTIC are obtained from the reported diuresis and natriuresis at 48 and 96 hours, respectively. The data for EMPA-RESPONSE are obtained from the mean daily spot urine sodium (day 1–4) from Boorsma et al.29 The data for ultrafiltration are adopted from Chung et al.45 (modified from ref. 28).
Thiazide and Thiazide-Like Diuretics
Only approximately 10% of the filtered sodium is reabsorbed in the distal convoluted tubule (DCT).47 Similar to the thick ascending loop of Henle, this segment is relatively impermeable to water; sodium reabsorption dilutes the tubular fluid. In the DCT, sodium absorption takes place through sodium-chloride cotransporter (NCC) located distal to macula densa. In the early segment, sodium transport is driven exclusively by NCC, whereas in the late segment, the ENaC also contributes.48
Because distal tubular compensatory sodium reabsorption due to prolonged exposure to loop diuretics is considered the primary driver of diuretic resistance in acute heart failure, thiazides are the most frequently used class of add-on diuretics.46,49 Metolazone is a quinazoline diuretic, with properties similar to the thiazide diuretics. It has a long duration of action and is effective in patients with low levels of eGFR. Beyond combination diuretic therapy to counter diuretic resistance, thiazide diuretics have also been proposed for upfront use in those patients with low eGFR to enhance natriuresis.50
Most clinical evidence to support the use of thiazides for sequential nephron blockade comes from small observational studies.50 Channer et al.51 compared the impact of two add-on thiazide diuretics (metolazone and bendrofluazide) in 33 consecutive patients with acute heart failure who were unresponsive to intravenous loop diuretics. They reported a median of 5.1–5.6 kg weight loss after adding thiazides. In a more recent randomized controlled trial, addition of oral hydrochlorothiazide (HCTZ) significantly increased loop diuretic efficiency.52
The largest study of add-on thiazides, Combination of Loop with Thiazide Diuretics for Decompensated Heart Failure (CLOROTIC), was a trial that randomized 230 patients with acute heart failure (median eGFR of 43 ml/min) to receive either oral HCTZ or placebo for 5 days in addition to a protocol-driven low-dose intravenous furosemide regimen.53 Patients assigned to HCTZ experienced a significantly greater weight loss (Table 1). Moreover, the HCTZ group had a 36% higher median urinary sodium excretion at 96 hours (Figure 2). The dosing scale and oral route of administration of HCTZ are likely to have played a role in the small differences seen between the two groups, whereas the low-dose loop diuretic is possibly the primary reason for overall modest natriuresis in both arms. There was no difference in dyspnea score, but more patients in the HCTZ arm experienced RSC. While it has been argued that the RSC in the HCTZ group can reflect more efficient decongestion, a recent study suggested that hemoconcentration has minimal contribution to changes in serum creatinine levels during the treatment of acute heart failure.54 A variety of metrics for rehospitalization and mortality were found to be similar in both groups. CLOROTIC was halted due to slow enrollment before the planned patient population of more than 300 was achieved; the post hoc analysis estimated the study power to be 81%.53
Similar to other add-on diuretics, thiazides have not been associated with lower risk of death. In fact, in a study on 13,898 admissions for acute heart failure, 1048 patients received adjuvant metolazone, which was found to be strongly associated with increased mortality after multivariable and propensity adjustment, while high‐dose loop diuretics were not associated with higher risk of death.55 The diuretic optimization strategies evaluation (DOSE) trial lends support to the general safety of high-dose intravenous loop diuretics.11
Mineralocorticoid Receptor Antagonists
Aldosterone interacts with the mineralocorticoid receptor, which is broadly expressed in several tissues such as the heart, the kidney, and the vasculature.56 In the kidney, it acts mainly in the distal nephron, which includes the late DCT, the connecting tubule, and the collecting duct system, accounting for 5%–10% of total sodium reabsorption.57,58 Within the principal cells, aldosterone increases the expression of sodium channels and sodium-potassium ATPase. The sodium channels on the luminal side allow sodium to passively diffuse into the principal cells.59 Meanwhile, potassium channels allow passive diffusion out of the cell into the tubular lumen whenever a sodium ion enters the cell. The net effect is sodium absorption from the lumen, which allows for water absorption, provided that arginine vasopressin (AVP) is present to make the cells permeable to water.60
Mineralocorticoid receptor antagonists (MRAs) are not potent natriuretic agents when administered as monotherapy.61 Their efficacy is typically greatest when aldosterone levels are excessively elevated (e.g., in heart failure). In a single-center prospective trial, 100 patients with acute heart failure were randomized to either receive standard medical therapy or combination diuretic therapy with spironolactone (50–100 mg/d).62 A greater proportion of patients who received spironolactone were found to be decongested. RSC was more prevalent in the control group, and there was no difference in serum potassium levels. Similarly, in a small prospective study of patients with acute heart failure and diuretic resistance, addition of high-dose spironolactone (100–200 mg/d) was associated with weight loss and reduced dyspnea, without worsening hyperkalemia or RSC.63
In 2017, the Aldosterone Targeted Neurohormonal Combined with Natriuresis Therapy in Heart Failure (ATHENA-HF) trial randomized 360 patients with acute heart failure to high-dose spironolactone (100 mg) versus placebo or 25-mg spironolactone (usual care).64 Spironolactone was discontinued after 96 hours, and further MRA use was left to the treating physician's discretion. The investigators did not observe a significant reduction in clinical congestion score or log NT-proBNP among those who received high-dose spironolactone compared with placebo. Similarly, urine output and weight change were found to be similar, as were clinical outcomes such as heart failure rehospitalization rate (Table 1). Short duration of therapy for a medication that is a prodrug and needs to be converted to its active metabolite (canrenone, half-life of 17 hours) has been proposed as a reason for the neutral findings.64 Future trials using intravenous formulation of canrenone are needed to more precisely explore the impact of diuretic doses of MRA.
Vasopressin Receptor Antagonists
There are three subtypes of AVP receptors that differ in localization and signal transduction (V1a, V1b, and V2).65 Vasopressin V2 receptors, which are found in the principal cells of the renal collecting and connecting tubules, mediate the hydro-osmotic effect of vasopressin in the kidney. Binding of AVP to V2 receptors activates aquaporin-2 water channels and promotes their trafficking to the apical membrane for exocytic insertion into the cell membrane.66 Nonosmotic release of AVP is a key component of maladaptive neurohormonal activation in heart failure.67 Therefore, vasopressin receptor antagonists (VRAs) can promote decongestion through urinary electrolyte-free water excretion (aquaresis).
The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) clinical status trial compared tolvaptan with placebo in 4133 patients admitted for acute heart failure within 48 hours of admission.68 The patients who received tolvaptan had lower weight on days 1 and 7 and reported improvement in their dyspnea as well. Interestingly, there was also a larger reduction in the median dose of loop diuretics at discharge, implying an accelerated transition to euvolemia. Despite promising results in the short term, the EVEREST outcome trial showed no effect for tolvaptan on long-term mortality or heart failure–related morbidity.69 Two other studies found that add-on tolvaptan leads to greater weight and fluid loss, albeit without an effect on dyspnea.70,71 It has been postulated that weight loss due to enhanced water excretion, if not accompanied by natriuresis, may not portend the anticipated salutary benefits. Moreover, because tolvaptan can also bind to V1a receptors, the lack of long-term clinical benefit may be the result of a compensatory increase in plasma AVP levels with subsequent enhanced V1a agonism, leading to increase in peripheral resistance and decrease in cardiac output, ultimately countering the benefits of aquaresis.72 The efficacy and safety of a novel dual-acting V1a/V2 VRA, pecavaptan, is currently under investigation (NCT03901729).73
Connecting the Dots
Management of diuretic resistance remains among the knowledge gaps in need of further research.74 There is no widely accepted quantitative definition for diuretic efficiency in the setting of acute heart failure. Not only are fluid balance and weight loss challenging metrics to accurately obtain in clinical practice but the correlation between them may also be limited.75 Furthermore, the inconsistency of the studies in terms of using weight, urine output, fluid balance, diuretic dosing regimen, etc., makes it an added challenge for interpretation and comparison of the available data. Whether more recent attempts to predict natriuretic response using timed urine collection would provide a reliable tool to guide diuretic therapy needs to be explored in large prospective trials.12
As seen in various studies above, regardless of the add-on agent, combination diuretic therapy is in general associated with a modest increase in urinary sodium excretion, weight reduction, and decongestion (Table 1). Enhanced distal sodium transport, more than proximal absorption, attenuates the maximal efficacy of loop diuretics.46,76,77 This nephron-specific element of diuretic resistance is more consequential than delivery of the loop diuretic to the site of action and forms the rationale for use of thiazide-type diuretics to augment furosemide-induced natriuresis.78 Review of the current guidelines of professional societies (Table 2) reveals that (1) the level of evidence behind combination diuretic therapy is not considered strong, (2) sequential nephron blockade is recommended only after intensification of loop diuretics has failed to improve congestion, and (3) the preferred agents are metolazone and thiazides.
Table 2.
Current guidelines for combination diuretic therapy in acute heart failure
| Professional Society | Level of Recommendation/Evidence | Recommendation |
|---|---|---|
| American Heart Association/American College of Cardiology/Heart Failure Society of America74 (2022) | Moderate recommendation Moderate-quality evidence |
In patients hospitalized with heart failure when diuresis is inadequate to relieve symptoms and signs of congestion, it is reasonable to intensify the diuretic regimen using either higher doses of intravenous loop diuretics or addition of a second diuretic |
| European Society of Cardiology79 (2021) | Moderate recommendation Moderate-quality evidence |
If the diuretic response remains inadequate despite doubling loop diuretic dose, concomitant administration of other diuretics acting at different sites, namely thiazides or metolazone or acetazolamide, may be considered |
| Canadian Cardiovascular Society80 (2017) | Weak recommendation Moderate-quality evidence |
For patients with persistent volume overload despite optimal medical therapy and increase in loop diuretics, cautious additional use of a second diuretic (a thiazide/low-dose metolazone) may be considered |
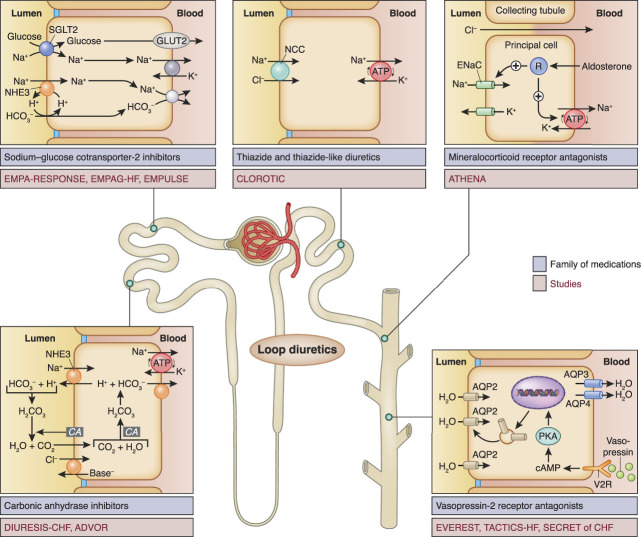
Distal to thiazides are the MRAs; more data are needed to determine whether the intravenous active form of spironolactone may change the landscape. Distal to MRAs, we have vaptans with the inherent disadvantage of being aquaretic rather than natriuretic. It remains unclear whether they will have a role in this setting.70,71 Moving proximally, CA inhibitors have generated much interest recently due to the encouraging results of ADVOR. Assuming that their mechanism of action is primarily through the increase of chloride in serum and macula densa, sodium-free chloride supplementation (NCT03446651) and hypertonic saline have been proposed as potentially viable options.81–83 Whether the inherent issue of decreased efficacy on multiple doses of CA inhibitors (tachyphylaxis) will translate into a clinical challenge needs to be explored. Finally, the indications for the use of SGLT-2i have been continuously expanding since they were first used in diabetes. With the encouraging results of the available data, there is much hope that they may have a role in the setting of acute heart failure in addition to their other side benefits. Figure 3 depicts the sites of action, medications, and studies related to sequential nephron blockade.
Figure 3.
The sites of action, family of medications, and clinical trials exploring combination diuretic therapy for acute heart failure. TACTICS-HF and SECRET of CHF are refs. 70 and 71, respectively. AQP, aquaporin; CA, carbonic anhydrase; DIURESIS-CHF, Diamox to Increase the Urinary Excretion of Sodium: an Investigational Study in Congestive Heart Failure; EMPAG-HF, Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure; EMPULSE, Empagliflozin in Patients Hospitalized for Acute Heart Failure; ENaC, epithelial sodium channel; NCC, sodium-chloride cotransporter; NHE3, sodium:hydrogen exchanger 3; V2R, vasopressin-2 receptor.
As of now, there is no large-scale head-to head comparison of diuretics across various classes to determine the best add-on agent for combination diuretic therapy. In a randomized trial on 60 patients with acute heart failure and diuretic resistance, metolazone, intravenous chlorothiazide, or tolvaptan was added to high-dose loop diuretics.84 Compared with metolazone, neither chlorothiazide nor tolvaptan resulted in more weight loss or higher urine output at 48 hours. As expected, tolvaptan resulted in a lower spot urine sodium (58 mmol/L) than both oral and intravenous thiazide diuretics (104 mmol/L for metolazone, P = 0.002, and 117 mmol/L for chlorothiazide, P < 0.001).
Finally, while widely used, combination diuretic therapy remains an area of uncertainty in the acute heart failure realm. It is within this context that mechanical removal of excess fluid (extracorporeal ultrafiltration) may prove helpful because it removes sodium at concentrations that are well beyond the capacity of any form of combination diuretic therapy (135–140 mmol/L; Figure 2). It also allows for precise adjustment of the amount of fluid removal28,85 and results in efficient decongestion and decreased rate of readmission.86–88 Marketing of portable or miniaturized devices may further potentiate the interest in this technology.89,90 Similar to combination diuretic therapy, however, there is no evidence so far for a positive effect on survival.
Disclosures
A. Kazory reports consultancy for CHF Solutions (NuWellis), Inc.; Scientific Advisory Board: Baxter, Elsevier, Horizon Inc., Relypsa Inc., and W. L. Gore; and honoraria from CHF Solutions (NuWellis), Inc., Horizon Inc., Relypsa Inc., and W. L. Gore.
Funding
None.
Author Contributions
Conceptualization: Amir Kazory.
Resources: Amir Kazory.
Writing – original draft: Amir Kazory.
Writing – review & editing: Amir Kazory.
References
- 1.Chioncel O, Mebazaa A, Harjola V-P, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017;19(10):1242–1254. doi: 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 2.Van Aelst LNL, Arrigo M, Placido R, et al. Acutely decompensated heart failure with preserved and reduced ejection fraction present with comparable haemodynamic congestion. Eur J Heart Fail. 2018;20(4):738–747. doi: 10.1002/ejhf.1050 [DOI] [PubMed] [Google Scholar]
- 3.Colombo PC, Doran AC, Onat D, et al. Venous congestion, endothelial and neurohormonal activation in acute decompensated heart failure: cause or effect? Curr Heart Fail Rep. 2015;12(3):215–222. doi: 10.1007/s11897-015-0254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganda A, Onat D, Demmer RT, et al. Venous congestion and endothelial cell activation in acute decompensated heart failure. Curr Heart Fail Rep. 2010;7(2):66–74. doi: 10.1007/s11897-010-0009-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbrugge FH, Dupont M, Steels P, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–495. doi: 10.1016/j.jacc.2013.04.070 [DOI] [PubMed] [Google Scholar]
- 6.Martens P, Tang WHW, Mullens W, et al. Renal sodium avidity, the prevailing renal target in heart failure. Eur Heart J. 2021;42(43):4478–4481. doi: 10.1093/eurheartj/ehab650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verbrugge FH, Nijst P, Dupont M, Penders J, Tang WW, Mullens W, et al. Urinary composition during decongestive treatment in heart failure with reduced ejection fraction. Circ Heart Fail. 2014;7(5):766–772. doi: 10.1161/CIRCHEARTFAILURE.114.001377 [DOI] [PubMed] [Google Scholar]
- 8.Verbrugge FH. Editor’s Choice-Diuretic resistance in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2018;7(4):379–389. doi: 10.1177/2048872618768488 [DOI] [PubMed] [Google Scholar]
- 9.Hodson DZ, Griffin M, Mahoney D, et al. Natriuretic response is highly variable and associated with 6-month survival: insights from the ROSE-AHF trial. JACC Heart Fail. 2019;7(5):383–391. doi: 10.1016/j.jchf.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens P, Chen HH, Verbrugge FH, Testani JT, Mullens W, Tang WW, et al. Assessing intrinsic renal sodium avidity in acute heart failure: implications in predicting and guiding decongestion. Eur J Heart Fail. 2022;24(10):1978–1987. doi: 10.1002/ejhf.2662 [DOI] [PubMed] [Google Scholar]
- 11.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805. doi: 10.1056/NEJMoa1005419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao VS, Ivey-Miranda JB, Cox ZL, et al. Natriuretic equation to predict loop diuretic response in patients with heart failure. J Am Coll Cardiol. 2021;77(6):695–708. doi: 10.1016/j.jacc.2020.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison DH, Felker GM, et al. Diuretic treatment in heart failure. N Engl J Med. 2017;377(20):1964–1975. doi: 10.1056/NEJMra1703100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox ZL, Rao VS, Testani JM, et al. Classic and novel mechanisms of diuretic resistance in cardiorenal syndrome. Kidney360. 2022;3(5):954–967. doi: 10.34067/KID.0006372021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazory A, Costanzo MR, et al. The dynamic relationship between serum chloride and cardiorenal syndrome. Rev Cardiovasc Med. 2020;21(1):25–29. doi: 10.31083/j.rcm.2020.01.6 [DOI] [PubMed] [Google Scholar]
- 16.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with Ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 17.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 18.Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 19.Herrington WG, Staplin N, Wanner C, et al.; The EMPA-KIDNEY Collaborative Group, Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vrhovac I, Balen Eror D, Klessen D, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467(9):1881–1898. doi: 10.1007/s00424-014-1619-7 [DOI] [PubMed] [Google Scholar]
- 21.Coady MJ, El Tarazi A, Santer R, et al. MAP17 is a necessary activator of renal na+/glucose cotransporter SGLT2. J Am Soc Nephrol. 2017;28(1):85–93. doi: 10.1681/ASN.2015111282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onishi A, Fu Y, Darshi M, et al. Effect of renal tubule-specific knockdown of the Na+/H+ exchanger NHE3 in Akita diabetic mice. Am J Physiol Renal Physiol. 2019;317(2):F419–F434. doi: 10.1152/ajprenal.00497.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes-Pardo H, Bautista R, Vargas-Robles H, Rios A, Sánchez D, Escalante B, et al. Role of sodium/glucose cotransporter inhibition on a rat model of angiotensin II-dependent kidney damage. BMC Nephrol. 2019;20(1):292. doi: 10.1186/s12882-019-1490-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75–R83. doi: 10.1152/ajpregu.00357.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC, et al. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc. 2018;7(4):e007046. doi: 10.1161/JAHA.117.007046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin M, Rao VS, Ivey-Miranda J, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142(11):1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22(4):713–722. doi: 10.1002/ejhf.1713 [DOI] [PubMed] [Google Scholar]
- 28.Kazory A, Ronco C. Tackling congestion in acute heart failure; is it the primetime for “combo diuretic therapy”? Cardiorenal Med. 2023;14. doi: 10.1159/000529646 [DOI] [PubMed] [Google Scholar]
- 29.Boorsma EM, Beusekamp JC, Maaten JM, et al. Effects of empagliflozin on renal sodium and glucose handling in patients with acute heart failure. Eur J Heart Fail. 2021;23(1):68–78. doi: 10.1002/ejhf.2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze PC, Bogoviku J, Westphal J, et al. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG-HF). Circulation. 2022;146(4):289–298. doi: 10.1161/CIRCULATIONAHA.122.059038 [DOI] [PubMed] [Google Scholar]
- 31.Biegus J, Voors AA, Collins SP, et al. Impact of empagliflozin on decongestion in acute heart failure: the EMPULSE trial. Eur Heart J. 2023;44(1):41–50. doi: 10.1093/eurheartj/ehac530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voors AA, Damman K, Teerlink JR, et al. Renal effects of empagliflozin in patients hospitalized for acute heart failure: from the EMPULSE trial. Eur J Heart Fail. 2022;24(10):1844–1852. doi: 10.1002/ejhf.2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28(3):568–574. doi: 10.1038/s41591-021-01659-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao VN, Murray E, Butler J, et al. In-hospital initiation of sodium-glucose cotransporter-2 inhibitors for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;78(20):2004–2012. doi: 10.1016/j.jacc.2021.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixit NM, Ziaeian B, Fonarow GC. SGLT2 inhibitors in heart failure: early initiation to achieve rapid clinical benefits. Heart Fail Clin. 2022;18(4):587–596. doi: 10.1016/j.hfc.2022.03.003 [DOI] [PubMed] [Google Scholar]
- 36.Grodin JL, Simon J, Hachamovitch R, et al. Prognostic role of serum chloride levels in acute decompensated heart failure. J Am Coll Cardiol. 2015;66(6):659–666. doi: 10.1016/j.jacc.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 37.Grodin JL, Verbrugge FH, Ellis SG, Mullens W, Testani JM, Tang WHW. Importance of abnormal chloride homeostasis in stable chronic heart failure. Circ Heart Fail. 2016;9(1):e002453. doi: 10.1161/CIRCHEARTFAILURE.115.002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kazory A, Ronco C. Emergence of chloride as an overlooked cardiorenal connector in heart failure. Blood Purif. 2020;49(1-2):219–221. doi: 10.1159/000503774 [DOI] [PubMed] [Google Scholar]
- 39.Rivera FB, Alfonso P, Golbin JM, et al. The role of serum chloride in acute and chronic heart failure: a narrative review. Cardiorenal Med. 2021;11(2):87–98. doi: 10.1159/000515604 [DOI] [PubMed] [Google Scholar]
- 40.Kataoka H. Acetazolamide as a potent chloride-regaining diuretic: short- and long-term effects, and its pharmacologic role under the “chloride theory” for heart failure pathophysiology. Heart Vessels. 2019;34(12):1952–1960. doi: 10.1007/s00380-019-01433-x [DOI] [PubMed] [Google Scholar]
- 41.Zahedi K, Barone S, Xu J, Soleimani M. Potentiation of the effect of thiazide derivatives by carbonic anhydrase inhibitors: molecular mechanisms and potential clinical implications. PLoS One. 2013;8(11):e79327. doi: 10.1371/journal.pone.0079327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarafidis PA, Georgianos PI, Lasaridis AN. Diuretics in clinical practice. Part I: mechanisms of action, pharmacological effects and clinical indications of diuretic compounds. Expert Opin Drug Saf. 2010;9(2):243–257. doi: 10.1517/14740330903499240 [DOI] [PubMed] [Google Scholar]
- 43.Verbrugge FH, Martens P, Ameloot K, et al. Acetazolamide to increase natriuresis in congestive heart failure at high risk for diuretic resistance. Eur J Heart Fail. 2019;21(11):1415–1422. doi: 10.1002/ejhf.1478 [DOI] [PubMed] [Google Scholar]
- 44.Mullens W, Dauw J, Martens P, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;387(13):1185–1195. doi: 10.1056/NEJMoa2203094 [DOI] [PubMed] [Google Scholar]
- 45.Chung ES, O'Brien TM, Menon S, Bartone C, Mazur W, Kereiakes DJ. A pilot study of target weight guided treatment in acute heart failure using ultrafiltration or usual care: effect on sodium removal. Korean Circ J. 2014;44(3):156–161. doi: 10.4070/kcj.2014.44.3.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao VS, Planavsky N, Hanberg JS, et al. Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol. 2017;28(11):3414–3424. doi: 10.1681/ASN.2016111178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandberg MB, Maunsbach AB, McDonough AA, et al. Redistribution of distal tubule Na+-Cl- cotransporter (NCC) in response to a high-salt diet. Am J Physiol Renal Physiol. 2006;291(2):F503–F508. doi: 10.1152/ajprenal.00482.2005 [DOI] [PubMed] [Google Scholar]
- 48.Obermüller N, Bernstein P, Velázquez H, et al. Expression of the thiazide-sensitive Na-Cl cotransporter in rat and human kidney. Am J Physiol Renal Physiol. 1995;269(6):F900–F910. doi: 10.1152/ajprenal.1995.269.6.F900 [DOI] [PubMed] [Google Scholar]
- 49.Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96(3-4):132–143. doi: 10.1159/000047397 [DOI] [PubMed] [Google Scholar]
- 50.Jentzer JC, DeWald TA, Hernandez AF, et al. Combination of loop diuretics with thiazide-type diuretics in heart failure. J Am Coll Cardiol. 2010;56(19):1527–1534. doi: 10.1016/j.jacc.2010.06.034 [DOI] [PubMed] [Google Scholar]
- 51.Channer KS, McLean KA, Lawson-Matthew P, Richardson M, et al. Combination diuretic treatment in severe heart failure: a randomised controlled trial. Heart. 1994;71(2):146–150. doi: 10.1136/hrt.71.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piardi DS, Butzke M, Mazzuca ACM, et al. Effect of adding hydrochlorothiazide to usual treatment of patients with acute decompensated heart failure: a randomized clinical trial. Sci Rep. 2021;11(1):16474. doi: 10.1038/s41598-021-96002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trullàs JC, Morales-Rull JL, Casado J, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. 2023;44(5):411–421. doi: 10.1093/eurheartj/ehac689 [DOI] [PubMed] [Google Scholar]
- 54.Maulion C, Chen S, Rao VS, et al. Hemoconcentration of creatinine minimally contributes to changes in creatinine during the treatment of decompensated heart failure. Kidney360. 2022;3(6):1003–1010. doi: 10.34067/KID.0007582021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brisco-Bacik MA, Ter Maaten JM, Houser SR, et al. Outcomes associated with a strategy of adjuvant metolazone or high-dose loop diuretics in acute decompensated heart failure: a propensity analysis. J Am Heart Assoc. 2018;7(18):e009149. doi: 10.1161/JAHA.118.009149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42(2):152–161. doi: 10.1093/eurheartj/ehaa736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pearce D, Manis AD, Nesterov V, Korbmacher C, et al. Regulation of distal tubule sodium transport: mechanisms and roles in homeostasis and pathophysiology. Pflugers Arch. 2022;474(8):869–884. doi: 10.1007/s00424-022-02732-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10(4):676–687. doi: 10.2215/CJN.12391213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valinsky WC, Touyz RM, Shrier A, et al. Aldosterone and ion channels. Vitamins Horm. 2019;109:105–131. doi: 10.1016/bs.vh.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 60.Arroyo JP, Ronzaud C, Lagnaz D, Staub O, Gamba G, et al. Aldosterone paradox: differential regulation of ion transport in distal nephron. Physiology (Bethesda). 2011;26(2):115–123. doi: 10.1152/physiol.00049.2010 [DOI] [PubMed] [Google Scholar]
- 61.Sica DA. Mineralocorticoid receptor antagonists for treatment of hypertension and heart failure. Methodist DeBakey Cardiovasc J. 2015;11(4):235–239. doi: 10.14797/mdcj-11-4-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ferreira JP, Santos M, Almeida S, Marques I, Bettencourt P, Carvalho H, et al. Mineralocorticoid receptor antagonism in acutely decompensated chronic heart failure. Eur J Intern Med. 2014;25(1):67–72. doi: 10.1016/j.ejim.2013.08.711 [DOI] [PubMed] [Google Scholar]
- 63.Bansal S, Munoz K, Brune S, Bailey S, Prasad A, Velagapudi C, et al. High-dose spironolactone when patients with acute decompensated heart failure are resistant to loop diuretics: a pilot study. Ann Intern Med. 2019;171(6):443–447. doi: 10.7326/M18-3285 [DOI] [PubMed] [Google Scholar]
- 64.Butler J, Anstrom KJ, Felker GM, et al. Efficacy and safety of spironolactone in acute heart failure: the ATHENA-HF randomized clinical trial. JAMA Cardiol. 2017;2(9):950–958. doi: 10.1001/jamacardio.2017.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Natochin YV, Golosova DV. Vasopressin receptor subtypes and renal sodium transport. Vitamins Horm. 2020;113:239–258. doi: 10.1016/bs.vh.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez AA, Salinas-Parra N, Cifuentes-Araneda F, Reyes-Martinez C, et al. Vasopressin actions in the kidney renin angiotensin system and its role in hypertension and renal disease. Vitamins Horm. 2020;113:217–238. doi: 10.1016/bs.vh.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Vinod P, Krishnappa V, Chauvin AM, Khare A, Raina R. Cardiorenal syndrome: role of arginine vasopressin and vaptans in heart failure. Cardiol Res. 2017;8(3):87–95. doi: 10.14740/cr553w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gheorghiade M, Konstam MA, Burnett JC, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297(12):1332–1343. doi: 10.1001/jama.297.12.1332 [DOI] [PubMed] [Google Scholar]
- 69.Konstam MA, Gheorghiade M, Burnett JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–1331. doi: 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 70.Felker GM, Mentz RJ, Cole RT, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69(11):1399–1406. doi: 10.1016/j.jacc.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 71.Konstam MA, Kiernan M, Chandler A, et al. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol. 2017;69(11):1409–1419. doi: 10.1016/j.jacc.2016.12.035 [DOI] [PubMed] [Google Scholar]
- 72.Izumi Y, Miura K, Iwao H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J Pharmacol Sci. 2014;124(1):1–6. doi: 10.1254/jphs.13r13cp [DOI] [PubMed] [Google Scholar]
- 73.Goldsmith SR, Burkhoff D, Gustafsson F, et al. Dual vasopressin receptor antagonism to improve congestion in patients with acute heart failure: design of the AVANTI trial. J Card Fail. 2021;27(2):233–241. doi: 10.1016/j.cardfail.2020.10.007 [DOI] [PubMed] [Google Scholar]
- 74.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American heart association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895-e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 75.Testani JM, Brisco MA, Kociol RD, et al. Substantial discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am J Med. 2015;128(7):776–783.e4. doi: 10.1016/j.amjmed.2014.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ellison DH. Clinical pharmacology in diuretic use. Clin J Am Soc Nephrol. 2019;14(8):1248–1257. doi: 10.2215/CJN.09630818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol. 2015;5(1):45–98. doi: 10.1002/cphy.c140002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ter Maaten JM, Rao VS, Hanberg JS, et al. Renal tubular resistance is the primary driver for loop diuretic resistance in acute heart failure. Eur J Heart Fail. 2017;19(8):1014–1022. doi: 10.1002/ejhf.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 80.Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 comprehensive update of the canadian cardiovascular society guidelines for the management of heart failure. Can J Cardiol. 2017;33(11):1342–1433. doi: 10.1016/j.cjca.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 81.Hanberg JS, Rao V, Ter Maaten JM, et al. Hypochloremia and diuretic resistance in heart failure: mechanistic insights. Circ Heart Fail. 2016;9(8):e003180. doi: 10.1161/CIRCHEARTFAILURE.116.003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griffin M, Soufer A, Goljo E, et al. Real world use of hypertonic saline in refractory acute decompensated heart failure: a U.S. center’s experience. JACC Heart Fail. 2020;8(3):199–208. doi: 10.1016/j.jchf.2019.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kazory A. Chloride and cardiorenal interactions in heart failure. Nephron. 2023;147(1):6–8. doi: 10.1159/000524987 [DOI] [PubMed] [Google Scholar]
- 84.Cox ZL, Hung R, Lenihan DJ, Testani JM, et al. Diuretic strategies for loop diuretic resistance in acute heart failure: the 3T trial. JACC Heart Fail. 2020;8(3):157–168. doi: 10.1016/j.jchf.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kazory A. Ultrafiltration therapy for heart failure: balancing likely benefits against possible risks. Clin J Am Soc Nephrol. 2016;11(8):1463–1471. doi: 10.2215/CJN.13461215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6):675–683. doi: 10.1016/j.jacc.2006.07.073 [DOI] [PubMed] [Google Scholar]
- 87.Costanzo MR, Negoianu D, Jaski BE, et al. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail. 2016;4(2):95–105. doi: 10.1016/j.jchf.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 88.Jain A, Agrawal N, Kazory A, et al. Defining the role of ultrafiltration therapy in acute heart failure: a systematic review and meta-analysis. Heart Fail Rev. 2016;21(5):611–619. doi: 10.1007/s10741-016-9559-2 [DOI] [PubMed] [Google Scholar]
- 89.Sgarabotto L, Kazory A, Brendolan A, Di Lullo L, Zanella M, Ronco C, et al. The science of extracorporeal ultrafiltration: introducing a novel miniaturized device. Cardiorenal Med. 2023;13:46–55. doi: 10.1159/000529613 [DOI] [PubMed] [Google Scholar]
- 90.Murugan R, Kazory A, Sgarabotto L, Ronco C, et al. Fluid overload and precision net ultrafiltration in critically ill patients. Cardiorenal Med. 2023;13:9–18. doi: 10.1159/000527390 [DOI] [PMC free article] [PubMed] [Google Scholar]