Visual Abstract
Keywords: geriatric nephrology, hemodialysis, patient-centered care
Abstract
Background
Potentially inappropriate medications, or medications that generally carry more risk of harm than benefit in older adults, are commonly prescribed to older adults receiving dialysis. Deprescribing, a systematic approach to reducing or stopping a medication, is a potential solution to limit potentially inappropriate medications use. Our objective was to identify clinicians and patient perspectives on factors related to deprescribing to inform design of a deprescribing program for dialysis clinics.
Methods
We conducted rapid qualitative analysis of semistructured interviews and focus groups with clinicians (dialysis clinicians, primary care providers, and pharmacists) and patients (adults receiving hemodialysis aged 65 years or older and those aged 55–64 years who were prefrail or frail) from March 2019 to December 2020.
Results
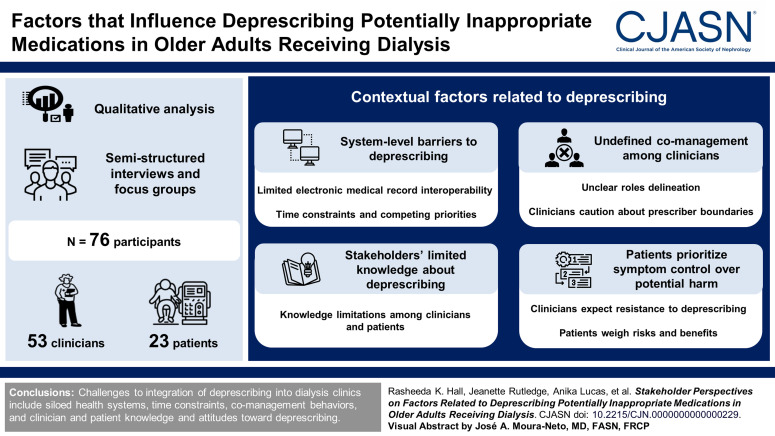
We interviewed 76 participants (53 clinicians [eight focus groups and 11 interviews] and 23 patients). Among clinicians, 24 worked in dialysis clinics, 18 worked in primary care, and 11 were pharmacists. Among patients, 13 (56%) were aged 65 years or older, 14 (61%) were Black race, and 16 (70%) reported taking at least one potentially inappropriate medication. We identified four themes (and corresponding subthemes) of contextual factors related to deprescribing potentially inappropriate medications: (1) system-level barriers to deprescribing (limited electronic medical record interoperability, time constraints and competing priorities), (2) undefined comanagement among clinicians (unclear role delineation, clinician caution about prescriber boundaries), (3) limited knowledge about potentially inappropriate medications (knowledge limitations among clinicians and patients), and (4) patients prioritize symptom control over potential harm (clinicians expect resistance to deprescribing, patient weigh risks and benefits).
Conclusions
Challenges to integration of deprescribing into dialysis clinics included siloed health systems, time constraints, comanagement behaviors, and clinician and patient knowledge and attitudes toward deprescribing.
Introduction
Potentially inappropriate medications, or medications that carry more risk of harm than benefit in older adults, are prescribed to approximately 40%–60% of older adults receiving dialysis.1–3 Psychoactive potentially inappropriate medications, such as sedatives, muscle relaxants, and opioids, higher risk for geriatric syndromes contributing to functional decline, limited quality of life, and mortality in older adults.4–6 Adults receiving dialysis who are approaching age 65 and are frail also are at risk of potentially inappropriate medication adverse effects.7,8 One approach to reduce potentially inappropriate medication use is deprescribing, a systematic process to reducing or stopping a medication.9 To design a deprescribing program intervention to reduce potentially inappropriate medication use, we need to understand how potentially inappropriate medication deprescribing is currently operationalized.
Recent studies show deprescribing is feasible in dialysis settings through use of deprescribing tools tailored for hemodialysis clinics.10,11 However, deprescribing is not considered standard of care. Deprescribing involves a cyclical process: (1) medication reconciliation, (2) medication review, (3) shared decision making, (4) deprescribing initiation, and (5) monitoring (Figure 1). Ideally, the deprescribing process is optimized for shared decision making through adequate time, appropriate information available for all parties (i.e., educational materials), stakeholder input, and consideration of patient values and preferences.9,12 Deprescribing models used in other clinical settings may not fit the dialysis population because care involves multiple prescribers across separate health settings who must be attentive to appropriate dosing for kidney failure. In addition, the disproportionate burden of polypharmacy, high pill burden, and functional impairment in patients receiving dialysis affect quality of life and may yield different medication preferences from the general older adult population.13–15 As a result, it is critical to uncover unique aspects of patients and the environment influencing the deprescribing process.
Figure 1.
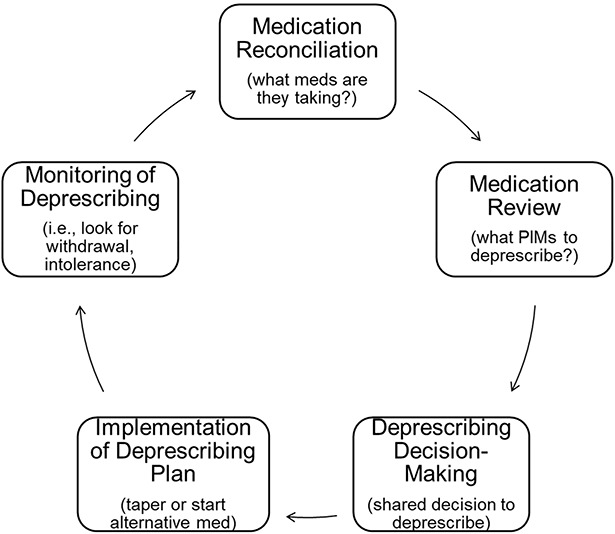
The deprescribing process. A schematic of the deprescribing process provided during clinician focus groups and interviews. Adapted from ref. 9, with permission. PIMs, potentially inappropriate medications.
To address this research gap, we conducted a qualitative study with key stakeholders (clinicians working in dialysis or primary care settings, pharmacists, and patients) to identify contextual factors (e.g., environment, behaviors, preferences) related to integration of deprescribing into dialysis clinics.
Methods
Study Design and Participant Population
We conducted separate qualitative inquiries with clinicians and patients. Clinicians included a convenience sample of primary care physicians (PCPs), pharmacists, and dialysis clinicians. For patient participants, our purposive sampling plan included adults aged 55 years or older with at least two individuals in each of the following categories: (1) potentially inappropriate medication use (taking one versus none), (2) dialysis vintage (≥6 or <6 months) because dialysis-related symptoms commonly treated with potentially inappropriate medications may be more common in patients with ≥6 months of dialysis,16 and (3) independence in medication management (independent or dependent). Patients aged 55–64 were required to be frail or prefrail (based on the Study of Osteoporotic Fractures criteria) for study inclusion.17 Exclusion criteria included those with advanced dementia, those receiving hospice, and non–English-speaking patients. Adhering to the Declaration of Helsinki, all participants provided informed consent. The study protocol was approved by the Duke Institutional Review Board (Pro00100184). We report this study according to the consolidated criteria for reporting qualitative research.18
Recruitment and Data Collection Procedures
Clinician focus groups and interviews occurred in 2019. Dialysis clinicians and pharmacists were identified through emails to registrants for the 2019 National Kidney Foundation Annual Clinical Meeting in Boston, Massachusetts. Those interested in participating attended one of four focus groups held during the National Kidney Foundation meeting. PCPs were identified from clinics affiliated with Duke University School of Medicine. Patient interviews occurred from January 2019 to December 2020. Patients were recruited from seven local hemodialysis clinics proximate to Duke University in Durham, North Carolina. Participants could choose to have a caregiver present for the interview. Caregivers who did participate signed an information sheet acknowledging their agreement to participate in an audio-recorded interview.
For all stakeholders, focus group sessions (clinicians only) and semistructured interviews were held on a day/time convenient for study participants. Some patient interviews were completed by phone during the coronavirus disease 2019 pandemic. Sessions lasted up to 1 hour and were led by trained interviewers. Sessions were audio-recorded and later transcribed by an experienced transcriptionist. Focus groups and/or interviews continued until saturation was achieved.
We obtained the following information from clinicians: (1) demographics (age, sex, race, ethnicity as mandated by the National Institutes of Health), (2) number of dialysis patients in their practice, (3) practice setting (rural versus urban), (4) proportion of time spent in patient care, and (5) employment length. We obtained the following information from patient participants: (1) demographics (as in clinicians); (2) time receiving dialysis; (3) current potentially inappropriate medications use (potentially inappropriate medication categories: sedatives, anticholinergics, muscle relaxants, opioids, alpha blockers, and central alpha agonists) based on medical records; (4) cognitive function using the Trails Making Test A and B, and Saint Louis University Mental Status instrument, or modified Montreal Cognitive Assessment test (used over the telephone during coronavirus disease 2019 pandemic)19–21; (5) the revised Patients' Attitudes Towards Deprescribing questionnaire22; and (6) among those aged 55–64 years, frailty status based on Study of Osteoporotic Fractures criteria.17 Payment for participation for patients and clinicians were valued at $30 and $75, respectively.
Interview Guides
We developed interview guides based on the Interprofessional Shared Decision-Making (IP-SDM) model, which describes the influence of both environment and multiple clinicians in shared decision making.12 For clinicians, additional questions explored how to incorporate the deprescribing process into their current environment. Clinicians received a description of the deprescribing process (Figure 1),9 a list of potentially inappropriate medications, and potential barriers to deprescribing. For patient participants, additional questions explored their experience with medication-related side effects, potentially inappropriate medication use, and deprescribing program preferences.
Data Analysis
We used rapid analysis methodology to identify themes.23,24 Rapid analysis involves a deductive and explanatory approach to theme identification through completion of a template to summarize each interview. The summary templates included neutral domains (based on the IP-SDM framework) each corresponding to at least one interview question. After development of each template, two individuals summarized each transcript. We met to address discrepancies. We transferred summaries into tables (participant in each column, domain in each row) to identify trends within and across stakeholder groups. On review of the tables, we determined meaning saturation was achieved because participant responses provided a full picture for each domain.25 We used summary statements from these matrices to identify themes. We provided a summary of findings to clinician participants through email to request feedback; respondents reported the summary was consistent with their impressions. We did not present a summary of findings to patients because of concern for excessive burden for recontacting them.
See Supplemental Material for additional methods.
Results
Cohort Characteristics
There were 76 participants in this study, 53 clinicians and 23 patients. Among clinicians, 24 worked in dialysis clinics (12 physicians, three nurses, and nine advanced practice providers), 18 worked in primary care (14 physicians and four advanced practice providers), and 11 were pharmacists (Table 1). Among patient participants, most (n=13) were aged 65 and older, nine of the ten aged 55–64 years were considered frail or prefrail, 18 (78%) had cognitive impairment, and 14 (61%) were Black race (Table 2). Eleven participants had a caregiver present for their interview. Sixteen participants (70%) were taking at least one potentially inappropriate medication, and the most common potentially inappropriate medications were sedatives (39%) and opioids (30%). Figure 2 shows patient participant responses to items from the revised Patients' Attitudes Towards Deprescribing questionnaire.
Table 1.
Characteristics of clinicians who participated in focus groups or interviews
| Characteristic | Clinicians (N=53) | ||
|---|---|---|---|
| Dialysis N=24 (45%) |
Primary Care N=18 (34%) |
Pharmacist N=11 (21%) |
|
| Sex, N (%) | |||
| Male | 7 (29) | 8 (44) | 4 (36) |
| Female | 17 (71) | 10 (56) | 7 (64) |
| Race, N (%) | |||
| Black | 2 (8) | 3 (17) | 0 (0) |
| White | 12 (50) | 10 (55) | 9 (82) |
| Othera | 10 (42) | 5 (28) | 2 (18) |
| Ethnicity, N (%) | |||
| Hispanic or Latino | 1 (4) | 0 (0) | 0 (0) |
| Role with respect to this study, N (%) | |||
| Nephrologist | 12 (50) | 0 (0.0) | 0 (0) |
| Nurse | 3 (12) | 0 (0.0) | 0 (0) |
| Advanced practice provider | 9 (38) | 4 (22) | 0 (0) |
| Primary care physician | 0 (0) | 14 (78) | 0 (0) |
| Years in current job | |||
| Median (IQR) | 9.0 (3.0–12.5) | 5.0 (3.5–11.0) | 5.0 (3.0–20.0) |
| Practice area, N (%) | |||
| Rural | 3 (12) | 4 (22) | 1 (9) |
| Urban | 21 (88) | 14 (78) | 10 (91) |
| Time spent in patient care, N (%) | |||
| 1%–25% | 2 (8) | 1 (6) | 2 (18) |
| 26%–50% | 1 (4) | 0 (0) | 4 (36) |
| 51%–75% | 6 (25) | 2 (11) | 2 (18) |
| 76%–100% | 15 (63) | 15 (83) | 3 (27) |
| No. of dialysis patients you have prescribed medications to, N (%) b | |||
| None | 4 (17) | 0 (0) | 6 (55 |
| 1–5 | 0 (0) | 11 (65) | 0 (0) |
| 6–15 | 2 (8) | 5 (29) | 0 (0) |
| >15 | 18 (75) | 1 (6) | 5 (45) |
| Dialysis patients seen in past year, N (%) | |||
| None | 0 (0) | 0 (0) | 1 (9) |
| 1–5 | 0 (0) | 10 (56) | 1 (9) |
| 6–15 | 1 (4) | 7 (39) | 0 (0) |
| >15 | 23 (96) | 1 (5) | 9 (82) |
IQR, interquartile range.
Other race includes Asian, Native American, Native Hawaiian, or Other or more than one race.
One primary care provider did not report a response to “number of dialysis patients you have prescribed medications to,” so proportions are based on n=17.
Table 2.
Characteristics of patients who participated in interviews
| Characteristic | Patients (N=23) |
|---|---|
| Age, yr, mean (SD) | 69 (9) |
| Age group, N (%) | |
| Age 65 and older | 13 (57) |
| Age 55–64 | 10 (43) |
| Sex, N (%) | |
| Male | 10 (43) |
| Female | 13 (57) |
| Race, N (%) | |
| Black | 14 (61) |
| White | 9 (39) |
| Ethnicity, N (%) | |
| Hispanic or Latino | 0 (0) |
| Length of time on dialysis, yr, median (IQR) | 2.4 (0.8–6) |
| <6 mo | 3 (13) |
| ≥6 mo | 20 (87) |
| Current potentially inappropriate medication use, N (%) | 16 (70) |
| Independent with medications, N (%) | 18 (78) |
| Presence of cognitive impairmenta, N (%) | 18 (78) |
IQR, interquartile range.
Cognitive impairment determined from SLUMS <27 or Montreal Cognitive Assessment <26; N=23 except where noted.
Figure 2.
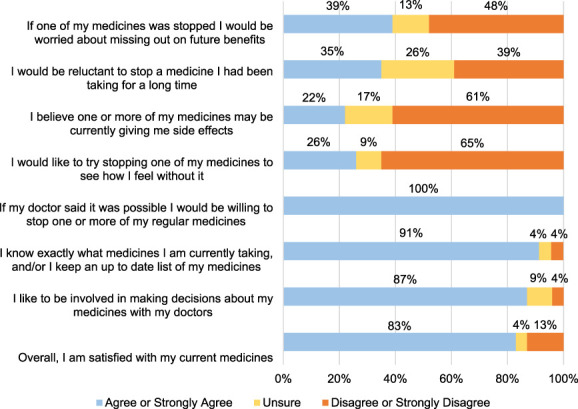
Patient responses from Patients' Attitudes towards Deprescribing questionnaire. This is a bar graph of participant responses to items included in the revised Patients' Attitudes towards Deprescribing questionnaire. Participants could choose one or none of “Strongly disagree” (1), “Disagree” (2), “Unsure” (3), “Agree” (4), “Strongly agree” (5). Shown here are combined responses for agree/strongly agree and disagree/strongly disagree. N=23 for all items.
Summary of Themes
Our qualitative analyses revealed four major themes that reflect contextual factors affecting deprescribing implementation in dialysis clinics. Table 3 shows exemplary quotes corresponding to these themes.
Table 3.
Exemplary quotes from clinicians and patients by themes
| Theme 1: System-level barriers to deprescribing | |
|
Limited electronic medical record interoperability Clinician quotes only | |
| 1a: “I think some of the external barriers is the electronic medical record. For us, it would be amazing if we had access, but even our clinic to dialysis to hospital, none of them… talk to each other.” (Dialysis clinician focus group) 1b: “…it's very challenging comanaging dialysis patients because we don't share any of the records even when you have like nephrologists [with access to the same EMR], who are, you know, their dialysis physician you know, so it's a lot of barriers to like direct communication.” (PCP focus group) 1c: “I mean I think that the most important thing from my perspective on [deprescribing] is just the importance of an accurate medication list and I think one of the biggest challenges in a dialysis unit is that a lot of our EMRs…don't cross, so…unfortunately they rely on faxed records...” (Pharmacist) | |
|
Time constraints and competing priorities Clinician quotes only | |
| 1d: “I don't think [nephrologists] have the – even if they do have the willingness, they just don't have the time to do it… their days so packed in, they have to drive from hospital to hospital or practice to practice that they just don't have time to talk and explain to the patient.” (Dialysis clinician focus group) 1e: “The visits are 20 minutes, it can take 10 minutes, 15 minutes to even room the patient and then when you go in the room, you have 5 minutes left, 10 minutes left and they have all these other things that are going on and it's not just possible.” (PCP focus group) 1f: “Bringing up [deprescribing decision making] can be a very involved process and you know, sometimes … you ideally would want to get a patient back…you may be really limited in that …adherence to follow up and close follow up and there's transportation issues that the patients then face.” (PCP focus group) 1g: “… there's so many other competing responsibilities or competing problems that probably, [deprescribing is] probably the last one that, you know, the providers are [concerned about].” (Dialysis clinician) 1h: “Deprescribing is best done when you don't have an acute issue or don't have an active issue and you want to take a step back and step through all the problems and all the medications and that takes, that itself takes a lot of time so if you're competing with another issue that you're trying to address, that becomes very challenging.” (PCP focus group) 1i: “The med rec process in our outpatient unit is kind of the nurses going through you know, presumably every thirty days, going through that checklist, but…there's not a formalized process of [calling pharmacies] to make sure that we have everything accurate…the nurses are great and everything, but they've got a million other things on their plate.” (Pharmacist focus group) | |
| Theme 2: Undefined comanagement among clinicians | |
| Unclear roles delineation | |
| Clinician quotes | Patient quotes |
| 2c: “…accountability…is unclear. Is the nephrologist worried about diabetes? Some of them do and some of them don't …sometimes we end up repeating each other's work. I don't think the patients know either….if they come and their blood pressure is really high and I say well is your nephrologist adjusting your medicine and they don't know.” (PCP focus group) 2d: “I have some patients that will come to me and say my primary care wants to do this, are you okay with it? And then I have the flip side where I ask, talk to a patient and say, I think we should do this, but oh, I need to talk to my primary care about it.” (Dialysis clinician focus group) 2e: “So, I really think, as with everything in medicine, it truly is like person, patient, dependent on the conversation, for anything including deprescribing and who that particular patient feels is their main you know, trust in what they should do and I think for many patients, that is their dialysis physician, but sometimes it is still that primary care. So, especially in the older patients, if they're in a community or setting where they've been seeing them for you know, 40 plus years, they still just want that approval.” (Dialysis clinician focus group) |
2f: “I would always get a second opinion [about deprescribing from either primary care or dialysis doctor], regardless.” (61-year-old woman) 2g: “I be asking [my PCP] what is [the medication] for and do I really need to take it.” (57-year-old woman) 2h: “I don't discuss any medications [with my dialysis doctor], not unless there's a change or I feel like something is going wrong.” (57-year-old woman) |
|
Clinician caution about prescriber boundaries Clinician quotes only | |
| 2i: “If I didn't start it, I would think long and hard before I stop it because my presumption is somebody that did start it had a good reason to do so.” (Dialysis clinician) 2j: “You know, what I think a lot of times what we encounter is that people are hesitant to discontinue in situations where they feel that they're stepping on somebody else's toes and so… ideally we would have everybody onboard before we made that decision to deprescribe.” (Pharmacist) 2k: “A lot of patients are okay with deprescribing as long as their doctor agrees with it, so they also want to have their you know, doctor buy in to the whole plan.” (Pharmacist) | |
| Theme 3: Stakeholders' limited knowledge about deprescribing potentially inappropriate medications | |
|
Knowledge limitations among clinicians Clinician quotes |
Knowledge limitations among patients Patient quotes |
| 3a: “The other barrier is educational as well. So, let's say someone is on oxycodone and I want to give them NSAIDS. Now I have a question in my mind, how much can I give somebody safely on hemodialysis? I don't have that answer clear in my mind.” (PCP focus group) 3b: “Once we define a few things, we speak with the nephrologist attending or the physician on board and we give them the plan because most of the time…they are not aware of the alternatives.” (Pharmacist) |
3c: In response to “tell me why you don't ask any questions [about your medications]”: “Because he should know what he's doing …I don't have anybody else to trust about this stuff.” (61-year-old woman) 3d: “How will it [affect] me, positively or negatively. Will it have side effects to it? Is it gonna make a difference with … my quality of life?” (82-year-old man) 3e: “I want to know why they want to cut back or do away with this medication. Now, that's the first thing I want to know and then if somebody tell me, I can do some research or something like that to find out why all this going on.” (67-year-old man) |
| Theme 4: Patients prioritize symptom control over potential harm | |
|
Clinicians expect resistance to deprescribing Clinician quotes | |
| 4a: “Most dialysis patients will tell you, they've had all the other alternatives and whatever they're on now is what they're comfortable with and what works and they don't want any more change.” (Dialysis clinician focus group) 4b: “They'd rather take the consequences of … the side effects of the medications than get rid of them.” (PCP focus group) 4c: “Yes, they are probably psychologically dependent upon it and they're convinced that they cannot go without them…that's the hardest, probably the greatest barrier to deprescribing some of these agents.” (Pharmacist) 4d: “If you tell them you're gonna [deprescribe and] counsel on good sleep hygiene… but …patients just want the pill to take at night to help them sleep.” (Pharmacist focus group) 4e: “Sometimes the patients just do not want to be involved. They sort of disassociate with their care and you have no choice but to talk to the [caregiver].” (Dialysis clinician) 4f: “[Family is] comfortable with mom being comfortable sitting in a recliner and sleeping after dialysis, rather than having to take her to rehab….We have seen some of those barriers with family member being busy also and having family member caregiver fatigue.” (Dialysis clinician focus group) | |
|
Patients weigh risks and benefits Patient quotes | |
| 4g: “Well, if you advised me not to take it, I have pain, what are you gonna give me in place of it?” (76-year-old woman) 4h: “Yeah, that would be okay, as long as it was just as affective [for pain].” (Response to replacing pain pill with one with fewer side effects) (66-year-old woman) 4i: “That's a hard question cause it's a yes and no. If I need it, well am I benefitting from it or am I not benefitting from it? ....That's something I really have to ponder on. I can't give you no definite answers to that.” (Response to question: What would you think if you knew that a medication that you are taking has side effects that can make you rely more on others for your daily activities?) (76-year-old woman) | |
EMR, electronic medical record; PCP, primary care provider; NSAIDs, nonsteroidal anti-inflammatory drugs.
System-Level Barriers to Deprescribing
Limited Electronic Medical Record Interoperability
All clinician groups acknowledged the deprescribing process can be challenging because it involves engaging multiple prescribers across clinical settings with different electronic medical records (EMR) (Table 3, quote 1a). Clinicians caring for an individual patient may not have access to the same EMR as other clinicians, limiting their ability to identify patients with potentially inappropriate medications. In addition, some PCPs believed communication about deprescribing decisions with dialysis clinicians was challenging because of separate clinical settings (Table 3, 1b). Pharmacists who work in dialysis reported strategies to work around separate EMRs (e.g., letter or a phone call to another prescriber on behalf of the nephrologist).
Time Constraints and Competing Priorities
After reviewing the deprescribing process, most clinicians reported “it was a valuable thing to do,” but described inadequate time and personnel to undertake the process. Dialysis clinicians were concerned about not having enough time to have shared decision-making discussions with their current workflow. Similarly, PCPs lacked time because of short encounters (approximately 5–10 minutes) and difficulty getting a patient back to clinic more often (Table 3, 1e, 1f), which hindered their motivation to engage in deprescribing discussions. Because of time constraints, both PCPs and dialysis clinicians acknowledged deprescribing discussions often would have to be delayed because the patient presented with a competing priority (e.g., uncontrolled diabetes, complications of missed dialysis) (Table 3, 1g, 1h). This was particularly challenging for PCPs who had less frequent encounters with dialysis patients and needed to address acute concerns, chronic conditions, and health care maintenance. To address time constraints, dialysis clinicians and pharmacists expressed a need for additional personnel (e.g., pharmacist). Dialysis nurses were considered as a potential solution to this need; however, nurses have limited time and resources to achieve an accurate medication list (Table 3, 1i).
Undefined Comanagement among Clinicians
Unclear Role Delineation
Both PCPs and dialysis clinicians noted that they do not explicitly delineate their roles in areas where there is overlapping expertise. As a result, clinicians justified delaying deprescribing discussions further because “accountability… is unclear.” PCPs described lack of clarity around who is responsible for management of specific issues: “sometimes we end up repeating each other's work” (Table 3, 2c). Because of these unclear roles, patients varied in their preferred clinician for decision making (e.g., dialysis clinician or PCP) (Table 3, 2d, 2e). This preference may arise from previous clinical encounters such that some patients report they seldom discuss their medications with their dialysis clinician, while others want buy-in from their dialysis clinician (Table 3, 2f–2h).
Clinician Caution about Prescriber Boundaries
Dialysis clinicians stated often a patient is taking a potentially inappropriate medication prescribed by another clinician (e.g., PCP, psychiatrists, pain specialists). When it comes to deprescribing in such circumstances, some were uncomfortable deprescribing a medication prescribed by another clinician (Table 3, 2i, 2j). By contrast, PCPs did not express the same concerns about stopping another provider's prescription. Regardless, pharmacists and PCPs emphasized the value of getting buy-in from the prescribing clinician (Table 3, 2k).
Limited Knowledge about Potentially Inappropriate Medications
Knowledge Limitations among Clinicians
Both nephrology and PCP clinicians acknowledged they do not know all the potential harms of potentially inappropriate medications or how to deprescribe them (i.e., tapering algorithms). Dialysis clinicians expressed concern that other clinicians (e.g., PCP, emergency, subspecialists) often inappropriately dose or prescribe medications, including potentially inappropriate medications, in patients receiving dialysis. PCPs endorsed a need for additional education on medication dose adjustments for patients receiving dialysis and training on deprescribing, including alternative medications (Table 3, 3a). Pharmacists acknowledged their role in filling these knowledge gaps (Table 3, 3b).
Knowledge Limitations among Patients
Clinicians from all three disciplines reported better patients' understanding of medications would facilitate medication reconciliation and deprescribing discussions. Specifically, one dialysis clinician noted patients may not understand deprescribing could be helpful: “What do you mean, less dosing might work better? I'm scared, I don't want to let go.”
Patient interviews revealed various preferences on understanding medications. Some patients noted that they do not ask questions when approached about medication initiation or deprescribing. When asked why, some stated they “trust the doctor” or expressed agreement: “I am doing what they want me to do.” By contrast, some patients wanted detailed information (e.g., side effects, indication) (Table 3, 3d, 3e) for decision making.
Patients Prioritize Symptom Control over Potential Harm
Clinicians Expect Resistance to Deprescribing
Clinicians generally expressed concern that it is challenging to sell it (i.e., discuss deprescribing) because patients were often hesitant to stop a familiar medication that relieves symptoms (Table 3, 4a–4d). Clinicians believed patients valued benefits of the potentially inappropriate medication or have a habit of taking it. Because of this expected challenge, one dialysis clinician noted low drive to deprescribe because “I have to be ready to engage them sometimes… mentally ready… to have that conversation, actually listen to what they are saying.” Caregivers were considered valuable when patients did not want to discuss medications with their clinician on their own (Table 3, 4e). However, dialysis and PCP clinicians both pointed out caregivers may also be hesitant to deprescribing potentially inappropriate medications because potentially inappropriate medications could alleviate symptoms (e.g., sleeplessness), which could lead to caregiver relief (Table 3, 4f).
Table 4.
Implications of qualitative study findings
| Theme | Subtheme | How Theme Should Inform Strategies to Optimize Deprescribing for Dialysis Patients |
|---|---|---|
| System-level barriers to deprescribing | Limited interoperability | • Integrated electronic health records (ideal) • Optimize access to EMRs at both settings • Establish routines for asynchronous communication between clinicians |
| Time constraints and competing priorities | • Allot resources (time, training, personnel) for sufficient medication reconciliation • Assign specific personnel (e.g., pharmacist, care coordinator) and time for deprescribing process |
|
| Undefined comanagement among clinicians | Unclear role delineation | • Establish and communicate clear roles and responsibilities for each clinician |
| Clinician caution about prescriber boundaries | • Engage all relevant prescribers in the deprescribing discussion | |
| Stakeholders' limited knowledge about deprescribing potentially inappropriate medications | Limited knowledge among clinicians | • Educate clinicians on risk of potentially inappropriate medications • Provide decision support to clinicians (education materials, EMR tool, or detailed recommendations) |
| Limited understanding among patients | • Patient-centered education materials and/or decision aids | |
| Patients prioritize symptom control over potential harm | Clinicians expect resistance to deprescribing | • Acknowledge other issues may need to be addressed and/or multiple conversations before a patient agrees to deprescribe |
| Patients weigh risks and benefits | • Engage in shared decision making that is sensitive to patient values/preferences and provides sufficient information exchange |
EMR, electronic medical record.
Patients Weigh Risks and Benefits
Many patients endorsed potential hesitancy to stopping a medication for concern that symptoms, especially pain, will not be managed (Table 3, 4g–4i) (Figure 2). However, patient interviews suggested openness to deprescribing: Most patients did not favor experiencing medication side effects (e.g., sleepiness, dizziness). Some patients would consider deprescribing a medication that could threaten their independence. Most patients preferred taking fewer medications: One patient aged 65 years or older stated: “I would welcome it.” All 23 patients endorsed being “willing to stop one or more of my regular medicines” (Figure 2).
To address patient preference for symptom control, some PCPs and pharmacists emphasized that deprescribing discussions should inform patients of alternative approaches for symptom management (e.g., “having an option that the patient understands could be effective” [PCP focus group]). However, clinicians noted that few alternatives exist (e.g., limited alternatives to opioids for pain), and access to complementary symptom management strategies (e.g., cognitive behavioral therapy) was limited.
Discussion
This qualitative study on deprescribing potentially inappropriate medications revealed contextual factors related to the deprescribing process. The factors included health system barriers, patient and clinician knowledge and attitudes toward deprescribing potentially inappropriate medications, and current approaches to medication discussions. This study's findings highlight areas to address for optimal deprescribing in dialysis clinics.
The contextual factors identified in this study are similar to previous studies. As in a Canadian qualitative study on deprescribing in hemodialysis clinics,26 we identified support of deprescribing from clinicians and patients, but hesitations due to limited time, resource constraints, and competing priorities. Studies conducted in general older adult populations have highlighted similar issues, as well as fragmented care, patient perceptions, and clinician self-efficacy or belief in their ability to conduct the deprescribing process.27–30 Our study builds on this literature by adding data from key stakeholders (patients, caregivers, clinicians from multiple care settings [dialysis, primary care, and pharmacist]) and using the IP-SDM model to identify system- and individual-level factors relevant to deprescribing potentially inappropriate medications.
Fragmented care has long been a problem for health care delivery for the dialysis population,31 so our findings reiterate a need to minimize fragmented care to optimize deprescribing across care settings. Interoperable EMRs would optimize medication review for deprescribing and would promote delivery of primary care in dialysis settings.32,33 Because pharmacists endorsed both routine communication with PCPs and noted their expertise in medication management could be applied in a deprescribing program, engaging pharmacists in deprescribing programs may help bridge the communication gap among clinicians and relieve the pressure of time constraints. Another potential approach to minimize fragmented care is initiation of deprescribing discussions during the interdisciplinary, patient care plan meetings that occur annually, at a minimum, in dialysis clinics.34 If combined with pharmacist engagement, these initial discussions can be followed by pharmacist-led shared decision making and deprescribing.35 However, research evaluating the effect of these system-level changes on deprescribing is warranted.
Our findings demonstrate clinician and patient knowledge, attitudes, and behavior are contextual factors that are associated with deprescribing. Based on theoretical models of behavior change, efforts to enhance knowledge and change attitudes can potentially yield intentional behavior change for optimal deprescribing (Table 4).36 Efforts to enhance clinician knowledge about deprescribing include clinical tools, such as potentially inappropriate medication Check, an online potentially inappropriate medication screening tool, or EMR nudges.37,38 Consistent with prior evidence, PCPs in this study care for a limited number of patients receiving dialysis39; therefore, novel decision support tools to support PCPs for prescribing in kidney failure may be beneficial.40 Clinical champions embedded in the dialysis care setting may also help clinicians' deprescribing skill set.41 Decision-making guides, such as decision aids, algorithms, and deprescribing manuals, serve to promote clinician self-efficacy and patient understanding.42–44 The Advancing Kidney Disease through Optimal Medication Management initiative is developing clinician resources relevant for deprescribing.45 With respect to clinician concern about prescriber boundaries, this concern could be mitigated through improving communication across multiple disciplines and care coordination agreements.46,47
This study has clinical implications. Consistent with other survey results of older adults with polypharmacy, all our patient participants were willing to have a medication deprescribed.48 However deprescribing shared decision making should be individualized by (1) allowing flexibility in which clinician communicates with the patient about deprescribing because some patients may prefer PCP over dialysis clinician and (2) engaging caregivers.49 Factors that play a role in decision making in some patients (e.g., cognition, psychological, social, financial)50 may also lead other patients to defer decision making to their clinicians.51 Therefore, clinicians should encourage patients to express their opinions to ensure a shared decision-making experience. To address concerns about how symptoms will be controlled when a medication is deprescribed, clinicians should (1) express reassurance that the plan can be re-evaluated if deprescribing is not tolerated and (2) provide alternative therapies for symptom management. Because this study confirms prior evidence that cognitive impairment is common in older adults receiving dialysis, clinicians should acknowledge that cognitive impairment may affect a patient's understanding of deprescribing and can facilitate the participation of these individuals in deprescribing with inclusive approaches (e.g., use short sentences, present one idea at a time).52
This study's strength lies in broad representation of perspectives from multiple stakeholder groups (e.g., dialysis clinicians, PCPs, pharmacists, patients, caregivers) who are integral for the deprescribing process. However, this qualitative study has limitations. We did not have robust representation from other dialysis staff (e.g., nurses, technicians). Still, we identified important concerns, and ongoing qualitative studies concurrent with a pilot deprescribing study will fill this knowledge gap. Second, all patient participants received care from one geographic area, clinicians involved in kidney care were attendees at an academic meeting, and clinicians beyond dialysis were an academic-affiliated, geographically limited PCP group. This selection bias limits generalizability to the broader patient population, all dialysis clinicians, or other specialists who prescribe for the dialysis population and who reside in other geographic areas. While the themes uncovered are informative for deprescribing initiatives in dialysis clinics across the United States, additional environmental or cultural factors may need to be considered for optimal adaptation.
We identified contextual factors that inform how to optimize deprescribing for the dialysis setting. These findings can be used to promote system-level changes and behavioral changes to enhance deprescribing shared decision making. Additional research is needed for integration of deprescribing into routine care.
Supplementary Material
Acknowledgments
Thank you to Donna Crabtree, Holly Hough, and Megan Reaves for technical assistance. Thank you to Margaret Falkovic for interview assistance.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The findings and interpretation do not necessarily represent the official views of the American Society of Nephrology.
Footnotes
See related editorial, “The Life-Changing Magic of Tidying Up the Medication List,” on pages 1254–1256.
Disclosures
C. Colón-Emeric reports consultancy for Amgen and Novartis, research funding from UCB, co-inventor 2 use patents for bisphonate indication in cardiovascular diseases, and advisory or leadership roles for Amgen and Novartis. L.J. Fish reports advisory or leadership role as Board President of Be the Village. R.K. Hall reports consultancy for Bayer, Chinook, Goldfinch, Inside Edge, Otsuka, Reata Pharmaceuticals, Third Bridge, Travere Pharmaceuticals, and United Health Group; ownership interest in Pfizer and Vertez; advisory or leadership role for the CJASN Editorial Board, Journal of American Geriatrics Society Editorial Board, Kidney360, and Pediatric Nephrology; speakers bureau for Inside Edge and Otsuka; and other interests or relationships as owner of Internal Shifts Coaching LLC. A. Lucas reports an advisory or leadership role for American Kidney Fund Medical Advisory Committee. W. St. Peter reports the consultancy for CSL-Vifor Pharma, GSK, and Total Renal Care, Inc.; honoraria from American Nephrology Nursing Association, Integritas Group, Letters and Sciences, and OptumLabs; advisory or leadership role as Scientific Advisory Board Member for National Kidney Foundation; and other interests or relationships with Centers for Medicare and Medicaid Services Technical Expert Panel on Development of a Quality Measure Assessing Delay in Progression of Chronic Kidney Disease (CKD), NKF and ASN Task Force on eGFR and Race, and Technical Expert Panel for Quality Insights Kidney Care Pilot project. J. St. Clair Russell reports consultancy for AdheaRx and Renalytix. J. Rutledge reports research funding from NephroNet, Inc. and Vifor Pharma, Inc. The remaining author has nothing to disclose.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases from R01DK133509 (C.K. Liu), American Society of Nephrology (R.K. Hall), American Kidney Fund Clinical Scientist in Nephrology Program (A. Lucas), and National Institute on Aging from K76AG059930, R24AG064025, P30AG028716, K23AG057813 (R.K. Hall, C. Colón-Emeric, and C.K. Liu).
Author Contributions
Conceptualization: Cathleen Colón-Emeric, Rasheeda K. Hall.
Data curation: Rasheeda K. Hall, Christine K. Liu, Jeanette Rutledge, Jennifer St. Clair Russell.
Formal analysis: Cathleen Colón-Emeric, Laura J. Fish, Rasheeda K. Hall, Christine K. Liu, Anika Lucas, Jeanette Rutledge, Jennifer St. Clair Russell.
Funding acquisition: Rasheeda K. Hall.
Investigation: Rasheeda K. Hall.
Methodology: Cathleen Colón-Emeric, Laura J. Fish, Rasheeda K. Hall, Wendy St. Peter.
Project administration: Rasheeda K. Hall, Jeanette Rutledge.
Resources: Rasheeda K. Hall.
Supervision: Cathleen Colón-Emeric, Laura J. Fish, Wendy St. Peter.
Writing – original draft: Rasheeda K. Hall, Christine K. Liu, Jeanette Rutledge.
Writing – review & editing: Cathleen Colón-Emeric, Laura J. Fish, Rasheeda K. Hall, Christine K. Liu, Anika Lucas, Jeanette Rutledge, Jennifer St. Clair Russell, Wendy St. Peter.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/CJN/B792.
References
- 1.Kondo N Nakamura F Yamazaki S, et al. Prescription of potentially inappropriate medications to elderly hemodialysis patients: prevalence and predictors. Nephrol Dial Transplant. 2015;30(3):498–505. doi: 10.1093/ndt/gfu070 [DOI] [PubMed] [Google Scholar]
- 2.Parker K, Aasebo W, Stavem K. Potentially inappropriate medications in elderly haemodialysis patients using the STOPP criteria. Drugs Real World Outcomes. 2016;3(3):359–363. doi: 10.1007/s40801-016-0088-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daubresse M, Alexander GC, Crews DC, Segev DL, McAdams-DeMarco MA. Trends in opioid prescribing among hemodialysis patients, 2007-2014. Am J Nephrol. 2019;49(1):20–31. doi: 10.1159/000495353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol. 2018;29(7):1970–1978. doi: 10.1681/ASN.2018010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Psychoactive medications and adverse outcomes among older adults receiving hemodialysis. J Am Geriatr Soc. 2019;67(3):449–454. doi: 10.1111/jgs.15740 [DOI] [PubMed] [Google Scholar]
- 6.Mina D, Johansen KL, McCulloch CE, Steinman MA, Grimes BA, Ishida JH. Muscle relaxant use among hemodialysis patients: prevalence, clinical indications, and adverse outcomes. Am J Kidney Dis. 2019;73(4):525–532. doi: 10.1053/j.ajkd.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 7.Hall RK Blumenthal JB Doerfler RM, et al. Risk of potentially inappropriate medications in adults with CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2021;78(6):837–845.e1. doi: 10.1053/j.ajkd.2021.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randles MA, O'Mahony D, Gallagher PF. Frailty and potentially inappropriate prescribing in older people with polypharmacy: a bi-directional relationship? Drugs Aging. 2022;39(8):597–606. doi: 10.1007/s40266-022-00952-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process. Br J Clin Pharmacol. 2014;78(4):738–747. doi: 10.1111/bcp.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George JS, Joseph R, Thomas ETA, John GP, Siby A, Nair MM. Active deprescribing program in chronic kidney disease patients undergoing haemodialysis. Nephrology (Carlton). 2021;26(11):890–897. doi: 10.1111/nep.13936 [DOI] [PubMed] [Google Scholar]
- 11.McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611–618. doi: 10.1053/j.ajkd.2017.02.374 [DOI] [PubMed] [Google Scholar]
- 12.Légaré F Stacey D Pouliot S, et al. Interprofessionalism and shared decision-making in primary care: a stepwise approach towards a new model. J Interprof Care. 2011;25(1):18–25. doi: 10.3109/13561820.2010.490502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colombijn JMT Bonenkamp AA van Eck van der Sluijs A, et al. Impact of polypharmacy on health-related quality of life in dialysis patients. Am J Nephrol. 2021;52(9):735–744. doi: 10.1159/000518454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall RK, Cary MP, Jr., Washington TR, Colón-Emeric CS. Quality of life in older adults receiving hemodialysis: a qualitative study. Qual Life Res. 2020;29(3):655–663. doi: 10.1007/s11136-019-02349-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi: 10.2215/CJN.00290109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1057–1064. doi: 10.2215/CJN.00430109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ensrud KE Ewing SK Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168(4):382–389. doi: 10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 18.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 19.Nasreddine ZS Phillips NA Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 20.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Reitan Neuropsychology Laboratory; 1986. [Google Scholar]
- 21.Tariq SH, Tumosa N, Chibnall JT, Perry MH, III, Morley JE. Comparison of the Saint Louis University Mental Status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder—a pilot study. Am J Geriatr Psychiatry. 2006;14(11):900–910. doi: 10.1097/01.JGP.0000221510.33817.86 [DOI] [PubMed] [Google Scholar]
- 22.Reeve E, Low LF, Shakib S, Hilmer SN. Development and validation of the revised patients' attitudes towards deprescribing (rPATD) questionnaire: versions for older adults and caregivers. Drugs Aging. 2016;33(12):913–928. doi: 10.1007/s40266-016-0410-1 [DOI] [PubMed] [Google Scholar]
- 23.Hamilton AB, Finley EP. Qualitative methods in implementation research: an introduction. Psychiatry Res. 2019;280:112516. doi: 10.1016/j.psychres.2019.112516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skillman M, Cross-Barnet C, Friedman Singer R, Rotondo C, Ruiz S, Moiduddin A. A framework for rigorous qualitative research as a component of mixed method rapid-cycle evaluation. Qual Health Res. 2019;29(2):279–289. doi: 10.1177/1049732318795675 [DOI] [PubMed] [Google Scholar]
- 25.Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: how many interviews are enough? Qual Health Res. 2017;27(4):591–608. doi: 10.1177/1049732316665344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bondurant-David K Dang S Levy S, et al. Issues with deprescribing in haemodialysis: a qualitative study of patient and provider experiences. Int J Pharm Pract. 2020;28(6):635–642. doi: 10.1111/ijpp.12674 [DOI] [PubMed] [Google Scholar]
- 27.Gillespie R, Mullan J, Harrison L. Factors which influence the deprescribing decisions of community-living older adults and GPs in Australia. Health Soc Care Community; 2022;30(6):e6206–e6216. doi: 10.1111/hsc.14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeve E, Low LF, Hilmer SN. Beliefs and attitudes of older adults and carers about deprescribing of medications: a qualitative focus group study. Br J Gen Pract. 2016;66(649):e552–e560. doi: 10.3399/bjgp16X685669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linsky A, Zimmerman KM. Provider and system-level barriers to deprescribing: interconnected problems and solutions. Public Policy Aging Rep. 2018;28(4):129–133. doi: 10.1093/ppar/pry030 [DOI] [Google Scholar]
- 30.Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544. doi: 10.1136/bmjopen-2014-006544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan CE Zhong J Mohottige D, et al. Fragmentation of care as a barrier to optimal ESKD management. Semin Dial. 2020;33(6):440–448. doi: 10.1111/sdi.12929 [DOI] [PubMed] [Google Scholar]
- 32.Sutton PR, Payne TH. Interoperability of electronic health information and care of dialysis patients in the United States. Clin J Am Soc Nephrol. 2019;14(10):1536–1538. doi: 10.2215/CJN.05300419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beers KH Sperati CJ Weisman DS, et al. Improving primary care delivery for patients receiving maintenance hemodialysis. Am J Kidney Dis. 2021;78(6):886–891. doi: 10.1053/j.ajkd.2021.02.340 [DOI] [PubMed] [Google Scholar]
- 34.Mandel EI, Bernacki RE, Block SD. Serious illness conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854–863. doi: 10.2215/CJN.05760516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzancic I, Kummer I, Drzaic M, Ortner Hadziabdic M. Community-based pharmacists' role in deprescribing: a systematic review. Br J Clin Pharmacol. 2022;88(2):452–463. doi: 10.1111/bcp.14947 [DOI] [PubMed] [Google Scholar]
- 36.Fan Y Zhang S Li Y, et al. Development and psychometric testing of the knowledge, attitudes and practices (KAP) questionnaire among student tuberculosis (TB) patients (STBP-KAPQ) in China. BMC Infect Dis. 2018;18(1):213. doi: 10.1186/s12879-018-3122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persell SD Brown T Doctor JN, et al. Development of high-risk geriatric polypharmacy electronic clinical quality measures and a pilot test of EHR nudges based on these measures. J Gen Intern Med. 2022;37(11):2777–2785. doi: 10.1007/s11606-021-07296-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desnoyer A Blanc AL Pourcher V, et al. PIM-Check: development of an international prescription-screening checklist designed by a Delphi method for internal medicine patients. BMJ Open. 2017;7(7):e016070. doi: 10.1136/bmjopen-2017-016070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silver SA Bota SE McArthur E, et al. Association of primary care involvement with death or hospitalizations for patients starting dialysis. Clin J Am Soc Nephrol. 2020;15(4):521–529. doi: 10.2215/CJN.10890919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tawadrous D, Shariff SZ, Haynes RB, Iansavichus AV, Jain AK, Garg AX. Use of clinical decision support systems for kidney-related drug prescribing: a systematic review. Am J Kidney Dis. 2011;58(6):903–914. doi: 10.1053/j.ajkd.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 41.Parchman ML, Perloff J, Ritter G. Can clinician champions reduce potentially inappropriate medications in people living with dementia? Study protocol for a cluster randomized trial. Implement Sci. 2022;17(1):63. doi: 10.1186/s13012-022-01237-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bayliss EA Shetterly SM Drace ML, et al. Deprescribing education vs usual care for patients with cognitive impairment and primary care clinicians: the OPTIMIZE pragmatic cluster randomized trial. JAMA Intern Med. 2022;182(5):534–542. doi: 10.1001/jamainternmed.2022.0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann NK Schmiedl S Mortsiefer A, et al. Development of a deprescribing manual for frail older people for use in the COFRAIL study and in primary care. Ther Adv Drug Saf. 2022;13:204209862211226. doi: 10.1177/20420986221122684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefebvre MJ Ng PCK Desjarlais A, et al. Development and validation of nine deprescribing algorithms for patients on hemodialysis to decrease polypharmacy. Can J Kidney Health Dis. 2020;7:205435812096867. doi: 10.1177/2054358120968674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxson R, St Peter WL. Optimizing patient medication management within value-based kidney care models. Renal Physicians Association News. Rockville, MD;2022:1,6.
- 46.Carrier E, Dowling MK, Pham HH. Care coordination agreements: barriers, facilitators, and lessons learned. Am J Manag Care. 2012;18(11):e398–e404. PMID: 23198751. [PubMed] [Google Scholar]
- 47.Duong MH McLachlan AJ Bennett AA, et al. Iterative development of clinician guides to support deprescribing decisions and communication for older patients in hospital: a novel methodology. Drugs Aging. 2021;38(1):75–87. doi: 10.1007/s40266-020-00820-8 [DOI] [PubMed] [Google Scholar]
- 48.Reeve E, Wolff JL, Skehan M, Bayliss EA, Hilmer SN, Boyd CM. Assessment of attitudes toward deprescribing in older medicare beneficiaries in the United States. JAMA Intern Med. 2018;178(12):1673–1680. doi: 10.1001/jamainternmed.2018.4720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Linsky A, Meterko M, Bokhour BG, Stolzmann K, Simon SR. Deprescribing in the context of multiple providers: understanding patient preferences. Am J Manag Care. 2019;25(4):192–198. PMID: 30986016. [PMC free article] [PubMed] [Google Scholar]
- 50.Todd A, Jansen J, Colvin J, McLachlan AJ. The deprescribing rainbow: a conceptual framework highlighting the importance of patient context when stopping medication in older people. BMC Geriatr. 2018;18(1):295. doi: 10.1186/s12877-018-0978-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russ AJ, Kaufman SR. Discernment rather than decision-making among elderly dialysis patients. Semin Dial. 2012;25(1):31–32. doi: 10.1111/j.1525-139X.2011.01047.x [DOI] [PubMed] [Google Scholar]
- 52.Alsawy S, Mansell W, McEvoy P, Tai S. What is good communication for people living with dementia? A mixed-methods systematic review. Int Psychogeriatr. 2017;29(11):1785–1800. doi: 10.1017/S1041610217001429 [DOI] [PubMed] [Google Scholar]