Visual Abstract
Keywords: immunology, immunosuppression, membranous nephropathy, proteinuria, risk factors
Abstract
Background
The 2021 Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend following anti-phospholipase A2 receptor (PLA2R) antibody levels as a marker of treatment response in membranous nephropathy; however, the optimal timing to evaluate antibody levels and how to combine them with other clinical variables are currently unknown.
Methods
We used a cohort of 85 patients from the Membranous Nephropathy Trial Of Rituximab (MENTOR) with anti-PLA2R antibodies ≥14 RU/ml to identify risk factors for not experiencing proteinuria remission after 12 months of treatment with cyclosporine or rituximab. Three landmark times were considered: at baseline and after 3 and 6 months of treatment. Logistic regression model performance was evaluated using C-statistics and model fit (Akaike information criterion [AIC], R2).
Results
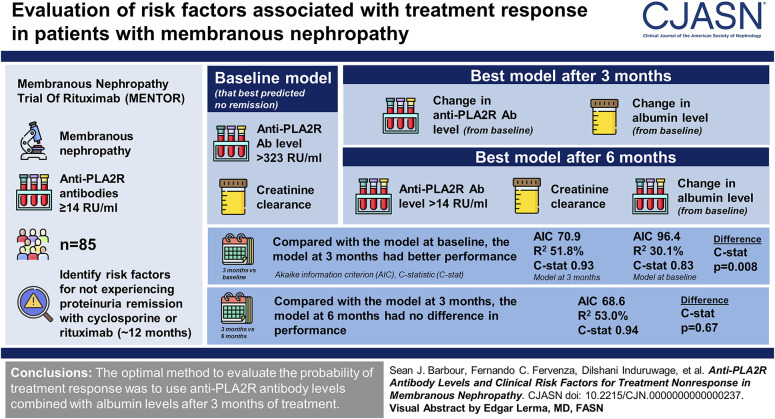
The model at baseline that best predicted no remission included anti-PLA2R antibodies >323 RU/ml and creatinine clearance; the best model after 3 months included the change from baseline in both antibody and albumin levels; and the best model after 6 months included antibody levels >14 RU/ml, creatinine clearance, and the change from baseline in albumin. Compared with the model at baseline, the model at 3 months had better model fit (AIC 70.9 versus 96.4, R2 51.8% versus 30.1%) and higher C-statistic (0.93 versus 0.83, P = 0.008). The model at 6 months had no difference in performance compared with the model at 3 months (AIC 68.6, R2 53.0%, C-statistic 0.94, P = 0.67).
Conclusions
In patients with membranous nephropathy treated with cyclosporine or rituximab in the MENTOR trial, we found that the optimal method to evaluate risk factors for the probability of treatment response was to use anti-PLA2R antibody levels combined with albumin levels after 3 months of treatment, which was significantly better than using antibody levels alone or risk factor evaluation at baseline, with no added benefit of waiting until 6 months of treatment.
Podcast
This article contains a podcast at https://dts.podtrac.com/redirect.mp3/www.asn-online.org/media/podcast/CJASN/2023_10_09_CJN0000000000000237.mp3
Introduction
Patients with primary membranous nephropathy and high-risk features, including persistent proteinuria >3.5 g/d and/or declining kidney function, have a 20%–50% risk of progression to end-stage kidney disease over 10 years.1–6 As such, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend treating such patients with immunosuppression, with a goal of achieving complete or partial proteinuria remission because these surrogate outcomes are highly correlated with a long-term benefit on kidney survival.6–8 However, proteinuria in membranous nephropathy is slow to improve after treatment, taking 12–24 months or longer to observe maximum reduction after treatment with rituximab, calcineurin inhibitors, or alkylating agents.9–14 This exposes patients to prolonged and potentially unnecessary immunosuppression before treatment response can be determined. Shorter-term markers are needed to better identify both patients responding to treatment who are likely to achieve proteinuria remission and those in whom treatment is not effective and alternative therapy may be considered.
Since the discovery that 75%–80% of idiopathic membranous nephropathy is associated with anti-phospholipase A2 receptor (PLA2R) antibodies, it has been hypothesized that recurrent measurement of antibody levels over time may be an effective earlier biomarker of treatment response.15–17 Multiple studies have demonstrated that with immunosuppression, anti-PLA2R antibodies decrease before proteinuria and immunologic remission precedes proteinuria remission by 6–12 months.18–21 However, the current literature is limited by the use of observational studies without standardized assessments; the use of antibody assays that are not readily available in clinical practice or antibody thresholds on the basis of assay characteristics instead of correlation with clinical outcomes, such as proteinuria remission; the use of measurements at 6 months but not earlier time points after staring immunosuppression; or also not considering changes in readily available clinical variables, such as proteinuria, albumin, and kidney function. As such, the optimal use of repeated measures of anti-PLA2R antibodies over time and how they should be combined with clinical variables to monitor treatment response remain uncertain, with the KDIGO guidelines providing expert opinion but no evidence-based recommendations.7
We therefore used the Membranous Nephropathy Trial Of Rituximab (MENTOR) cohort to evaluate the longitudinal measurement of clinical variables and anti-PLA2R antibody levels at baseline, 3 months, and 6 months using the standard EUROIMMUN assay and their association with subsequent complete or partial proteinuria remission 1 year after treatment with rituximab or cyclosporine.
Methods
Study Population
This was a secondary analysis of the MENTOR trial, which randomized patients with primary membranous nephropathy to cyclosporine for 12 months or rituximab at baseline and 6 months and has been previously described.11 We included patients from the trial with baseline anti-PLA2R antibody levels ≥14 RU/ml using the EUROIMMUN ELISA assay. This cutoff was chosen on the basis of the manufacturer threshold used to define a positive test and to be consistent with the 2021 KDIGO guidelines.7
Variable Definitions
Data were available at baseline (time of randomization), 3, 6, and 12 months, including a centralized measurement of serum creatinine and albumin and proteinuria and creatinine clearance from 24-hour urine collections. The primary outcome for this analysis was remission status at 12 months, which included both complete (proteinuria ≤0.3 g/d and albumin ≥35 g/L) and partial (proteinuria reduction by ≥50% from baseline and ≤3.5 g/d) remission. Anti-PLA2R IgG antibody levels were measured every 3 months using the EUROIMMUN ELISA assay.22 By comparison, an in-house ELISA assay was previously used in the original publication of the MENTOR trial, for which values >40 RU/ml are considered borderline and values >80 RU/ml are considered positive.23 There was strong correlation between the EUROIMMUN and in-house ELISA assay results, with correlation coefficient R-values between 0.49 and 0.89 at the three landmark times (baseline, 3 months, and 6 months, see Supplemental Figure 1).
Statistical Analyses
Logistic regression was used to model the absence of complete or partial remission at 12 months (given it was less frequent than the presence of remission). Three landmark times were considered: baseline, 3 months, and 6 months. Predictor variables were considered at each landmark on the basis of the available literature as being associated with disease severity or remission outcomes, including anti-PLA2R antibody levels, creatinine clearance, proteinuria, albumin, use of immunosuppression before trial enrollment, and treatment allocation in the trial (rituximab or cyclosporine).2,6,11,18,20,24–27 Model fit was assessed using R2 and the Akaike information criterion (AIC) and model discrimination using C-statistic. Continuous variables were transformed as necessary or categorized using a method that identifies the optimal cut point that maximally differentiates the outcome status.28 The best parameterization for each continuous variable was based on model fit. At baseline, for proteinuria, this was a cut point of >10 g/d; for albumin, it was a cut point of ≤22 g/L; and for creatinine clearance, it was as a continuous variable. At 3 and 6 months, the best parameterization for proteinuria and albumin was the change from baseline as continuous variables. For creatinine clearance, at 3 months, it was the absolute value, and at 6 months, it was the change from baseline using a cut point of >8 ml/min per 1.73 m2. Multivariable models were constructed for each landmark time using a forward selection strategy. Because of high collinearity between changes in proteinuria and changes in albumin at 3 and 6 months, only changes in albumin were included in multivariable models.
Additional details regarding the study population, variables, and statistical analyses are available in the Supplemental Methods.
Results
The cohort included 85 patients (Supplemental Figure 2), with characteristics summarized in Table 1. After 12 months of treatment with rituximab or cyclosporine, 47 patients were in remission (nine complete remission, 38 partial remission). The primary outcome for this analysis was not experiencing a complete or partial remission at 12 months, with the aim of comparing risk factors for the primary outcome at three different landmark times: at baseline before treatment and after 3 and 6 months of treatment according to the trial protocol.
Table 1.
Characteristics of the analytic cohort at baseline
| Baseline Characteristics | Overall Cohort | No Remission at 12 mo | Remission at 12 mo |
|---|---|---|---|
| Number of patients | 85 | 38 | 47 |
| Age, yr | 50 (43–61) | 54 (44–64) | 47 (42–61) |
| Male sex, n (%) | 65 (77) | 33 (87) | 32 (68) |
| Prior immunosuppression, n (%) | 22 (26) | 11 (29) | 11 (23) |
| Treatment allocation, n (%) | |||
| Rituximab | 46 (54) | 19 (50) | 27 (57) |
| Cyclosporine | 39 (46) | 19 (50) | 20 (43) |
| Albumin, g/dl | 2.4 (2.1–2.9) | 2.4 (2.0–2.9) | 2.5 (2.2–3.0) |
| Proteinuria, g/d | 9.8 (7.1–13.9) | 11.4 (8.2–14.3) | 8.9 (7–13.7) |
| Creatinine clearance, ml/min per 1.73 m2 | 80 (65–103) | 73 (51–87) | 87 (75–118) |
| PLA2R antibody level, RU/ml | 165 (59–380) | 346 (134–590) | 116 (52–186) |
| PLA2R antibody levels (categories), n (%) | |||
| 14–20 RU/ml | 2 (2) | 1 (3) | 1 (2) |
| ≥20 RU/ml | 83 (98) | 37 (97) | 46 (98) |
| Time from biopsy to baseline, mo | 4.8 (3–8.9) | 4.7 (2.9–7.9) | 4.9 (3–9.7) |
| Interstitial fibrosis/tubular atrophy, n (%) | |||
| <10% | 47 (55) | 18 (47) | 29 (62) |
| 10%–20% | 32 (38) | 17 (45) | 15 (32) |
| >20% | 6 (7) | 3 (8) | 3 (6) |
| Global glomerulosclerosis (% of glomeruli) | 0 (0–11) | 4 (0–10) | 0 (0–14) |
| Segmental glomerulosclerosis (% of glomeruli) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
Baseline values were obtained at the time of randomization before the allocation of treatment according to the MENTOR trial protocol. Data are presented as count (frequency) or median (interquartile range). MENTOR, Membranous Nephropathy Trial Of Rituximab.
Anti-PLA2R antibody and albumin levels over time are shown in Figure 1 on the basis of remission status at 12 months, with considerable overlap in baseline levels but more distinct separation starting at 3 months. Antibody levels and clinical variables after 3 months of treatment are presented in Table 2. There was a larger reduction in antibody levels over 3 months in patients who did not achieve remission, likely because they started with higher levels at baseline (Table 1). In those who did not remit at 12 months, proteinuria and albumin at 3 months were worse and showed less absolute improvement from baseline. Antibody levels and clinical variables after 6 months of treatment followed a similar pattern (Supplemental Table 1).
Figure 1.
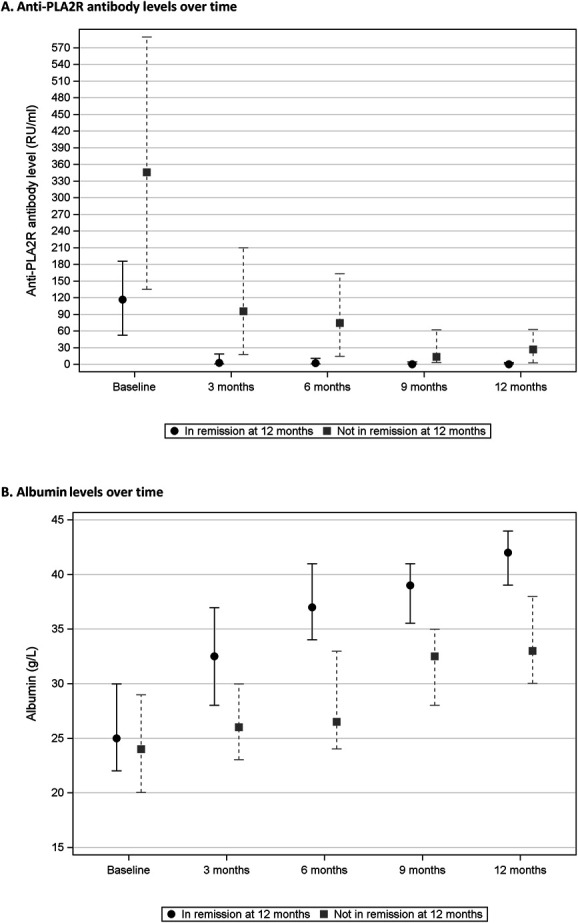
Anti-PLA2R antibody and albumin levels over time based on remission status at 12 months. Data in the figure are presented as median and interquartile range. (A) Anti-PLA2R antibody levels over time. (B) Albumin levels over time.
Table 2.
Characteristics of the analytic cohort after 3 months of treatment with cyclosporine or rituximab as part of the MENTOR trial
| Characteristics at 3 mo | Overall Cohort | No Remission at 12 mo | Remission at 12 mo |
|---|---|---|---|
| Albumin at 3 mo, g/dl | 3.0 (2.4–3.4) | 2.6 (2.3–3.0) | 3.2 (2.8–3.7) |
| Albumin change from baseline to 3 mo, g/dl | 0.4 (0.1–0.7) | 0.2 (0–0.4) | 0.6 (0.2–0.9) |
| Proteinuria at 3 mo, g/d | 6.5 (3.3–9.5) | 9.3 (6.5–12.1) | 3.6 (2.3–7.1) |
| Proteinuria change from baseline to 3 mo, g/d | −4.2 (−6.8 to −2) | −2.5 (−4.4 to 0.2) | −5.2 (−7.3 to −3.3) |
| Creatinine clearance at 3 mo, ml/min per 1.73 m2 | 80 (65–98) | 70 (47–94) | 86 (76–105) |
| Creatinine clearance change from baseline to 3 mo, ml/min per 1.73 m2 | −1 (−21 to 11) | −2 (−21 to 9) | 0 (−21 to 11) |
| PLA2R antibody level at 3 mo, RU/ml | 12 (2–84) | 95 (17–210) | 3 (0–19) |
| PLA2R antibody levels at 3 mo, n (%) | |||
| <14 RU/ml | 43 (51) | 8 (21) | 35 (75) |
| 14–20 RU/ml | 3 (4) | 2 (5) | 1 (2) |
| ≥20 RU/ml | 39 (46) | 28 (74) | 11 (23) |
| PLA2R antibody change from baseline to 3 mo, RU/ml | −136 (−321 to −57) | −234 (−419 to −80) | −82 (−170 to −51) |
Data are presented as count (frequency) or median (interquartile range). MENTOR, Membranous Nephropathy Trial Of Rituximab.
Identifying the Optimal Way to Model Anti-PLA2R Antibody Levels
At each landmark time, different ways of modeling antibody levels were compared to identify which is the best risk factor for not achieving remission at 12 months. The optimal cut points for the absolute antibody values at each landmark time that were maximally associated with the risk of no remission at 12 months were >323 RU/ml at baseline, >58 RU/ml at 3 months, and >14 RU/ml at 6 months. These were compared using the absolute antibody value as a continuous variable or using the change from baseline to 3 or 6 months as either continuous variables or on the basis of cutoff values. The parameterization of anti-PLA2R antibody levels at each landmark time that optimally predicts no remission at 12 months was chosen on the basis of the lowest AIC and highest R2 (Supplemental Table 2). At baseline and 6 months, the best parameterization of antibody levels was using the values at the landmark times and a cutoff of >323 RU/ml at baseline and a cutoff of >14 RU/ml at 6 months (adjusted for baseline values). At the 3-month landmark time, the best parameterization of antibody levels was the absolute change from baseline as a continuous variable (adjusted for baseline values). This implies that after 3 months of treatment, there is not a single threshold reduction in anti-PLA2R antibody level that optimally predicts remission status at 12 months, instead it is necessary to consider the absolute reduction on a continuous scale.
Combining Clinical Variables with Anti-PLA2R Antibody Levels as Risk Factors for Subsequent Remission
We next sought to determine whether remission status at 12 months is best predicted using antibody levels alone or in combination with clinical risk factors. The following variables were considered: sex; immunosuppression use before entering the trial; treatment allocation to rituximab or cyclosporine in the trial; and creatinine clearance, albumin, proteinuria, and the optimal parameterization of antibody levels identified above at each landmark time. Univariable, bivariable, and multivariable models describing the association between these variables and the risk of no remission at 12 months are shown at the baseline, 3-month, and 6-month landmark times (Table 3, Supplemental Table 3, and Supplemental Table 4, respectively).
Table 3.
Univariable, bivariable, and multivariable model results for the risk of no remission at 12 months using risk factors at a 3-month landmark time
| Risk Factors | Univariable or Bivariable Modelsa | Multivariable Model | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| PLA2R antibody change from baseline to 3 mo (per RU/ml decrease)a | 0.97 (0.95 to 0.99) | 0.007 | 0.96 (0.93 to 0.98) | 0.002 |
| PLA2R antibody level at baseline (per RU/ml)a | 1.03 (1.01 to 1.05) | 0.008 | 1.04 (1.02 to 1.07) | 0.001 |
| Proteinuria change from baseline to 3 mo (per g/d decrease)a | 0.60 (0.47 to 0.75) | <0.001 | — | — |
| Proteinuria at baseline (per g/d)a | 1.42 (1.20 to 1.69) | <0.001 | — | — |
| Creatinine clearance at 3 mo >52 ml/min per 1.73 m2a | 0.12 (0.02 to 0.66) | 0.02 | 0.24 (0.02 to 2.81) | 0.26 |
| Creatinine clearance at baseline (per 10 ml/min per 1.73 m2 decrease)a | 1.08 (0.90 to 1.30) | 0.42 | 1.25 (0.92 to 1.69) | 0.16 |
| Albumin change from baseline to 3 mo (per 0.1 g/dl increase)a | 0.75 (0.65 to 0.87) | <0.001 | 0.71 (0.55 to 0.92) | 0.008 |
| Albumin at baseline (per 0.1 g/dl)a | 1.08 (0.99 to 1.19) | 0.09 | 1.02 (0.89 to 1.18) | 0.75 |
| Male sex | 3.1 (1.0 to 9.5) | 0.05 | 6.22 (0.81 to 47.6) | 0.08 |
| Prior immunosuppression use | 1.3 (0.5 to 3.5) | 0.56 | — | — |
| Treatment allocation to rituximab (versus cyclosporine) | 0.7 (0.3 to 1.8) | 0.49 | — | — |
OR, odds ratio; CI, confidence interval.
Variables with metrics at 3 months and at baseline were included together in bivariable models to adjust for baseline values.
In the multivariable model at baseline, antibody levels >323 RU/ml and lower creatinine clearance at baseline were both associated with a higher probability of not being in remission at 12 months (odds ratio [OR], 11.1 and 1.22, respectively, both P < 0.05, Supplemental Table 3). Changes in proteinuria and albumin over time were highly collinear, as such, only albumin was included in the multivariable models at the 3- and 6-month landmark times. The benefit of using albumin instead of proteinuria was confirmed using a clustering analysis and LASSO regression (Supplemental Methods and Supplemental Table 5). In the multivariable model at the 3-month landmark time, a larger decrease in antibody levels over 3 months and a larger increase in albumin over 3 months were both associated with a lower probability of not being in remission at 12 months (OR, 0.96 and 0.71, respectively, both P < 0.05), whereas higher antibody levels at baseline were associated with a higher probability of not being in remission at 12 months (OR, 1.04, P = 0.001, Table 3). In the multivariable model at the 6-month landmark time, antibody levels >14 RU/ml at 6 months and lower baseline creatinine clearance were associated with a higher probability of not being in remission at 12 months (OR, 7.2 and 1.41, respectively, both P < 0.05), whereas a larger increase in albumin over 6 months was associated with a lower probability of not being in remission (OR, 0.74, P = 0.002, Supplemental Table 4).
At each landmark time, measures of model fit (AIC, R2) and C-statistics were used to compare the multivariable models described above with univariable or bivariable models that included only the optimal parameterizations for anti-PLA2R antibody levels. At each landmark time, the multivariable models that included clinical variables and antibody levels had better model fit with lower AIC and higher R2 values compared with the models that included only antibody levels (Table 4). The C-statistics at each landmark time were significantly higher in the models that included clinical variables with antibody levels compared with those including antibody levels alone: 0.83 versus 0.72 (P = 0.005) at baseline, 0.93 versus 0.83 (P = 0.01) at 3 months, and 0.94 versus 0.81 (P = 0.008) at 6 months (Figure 2). These results demonstrate that compared with using anti-PLA2R antibody levels alone, the probability of not being in remission at 12 months can be better assessed at each landmark time by also considering the clinical risk factors that were included in the multivariable models described above.
Table 4.
Model fit for univariable, bivariable, and multivariable models for the risk of no remission at 12 months at different landmark times
| Model | AIC | R2, % |
|---|---|---|
| Baseline landmark time | ||
| Univariable: anti-PLA2R Ab only | 100.3 | 21.5 |
| Multivariable: anti-PLA2R Ab+creatinine clearance | 96.4 | 30.1 |
| 3-mo landmark time | ||
| Bivariable: anti-PLA2R Ab onlya | 88.7 | 33.1 |
| Multivariable: anti-PLA2R Aba + albumina | 70.9 | 51.8 |
| 6-mo landmark time | ||
| Bivariable: anti-PLA2R antibody onlya | 88.6 | 33.2 |
| Multivariable: anti-PLA2R Aba + Creatinine clearancea + albumina | 68.6 | 53.0 |
Results are presented for the risk of not experiencing remission at 12 months using the univariable, bivariable, and multivariable models from Supplemental Table 3 at a baseline landmark time, Table 3 at a 3-month landmark time, and Supplemental Table 4 at a 6-month landmark time. Only predictor variables with P < 0.05 in the multivariable models are listed in the table. A lower AIC and higher R2 values indicate better model fit. AIC, Akaike information criterion; PLA2R, phospholipase A2 receptor; Ab, antibody levels.
Includes adjustment for baseline values.
Figure 2.
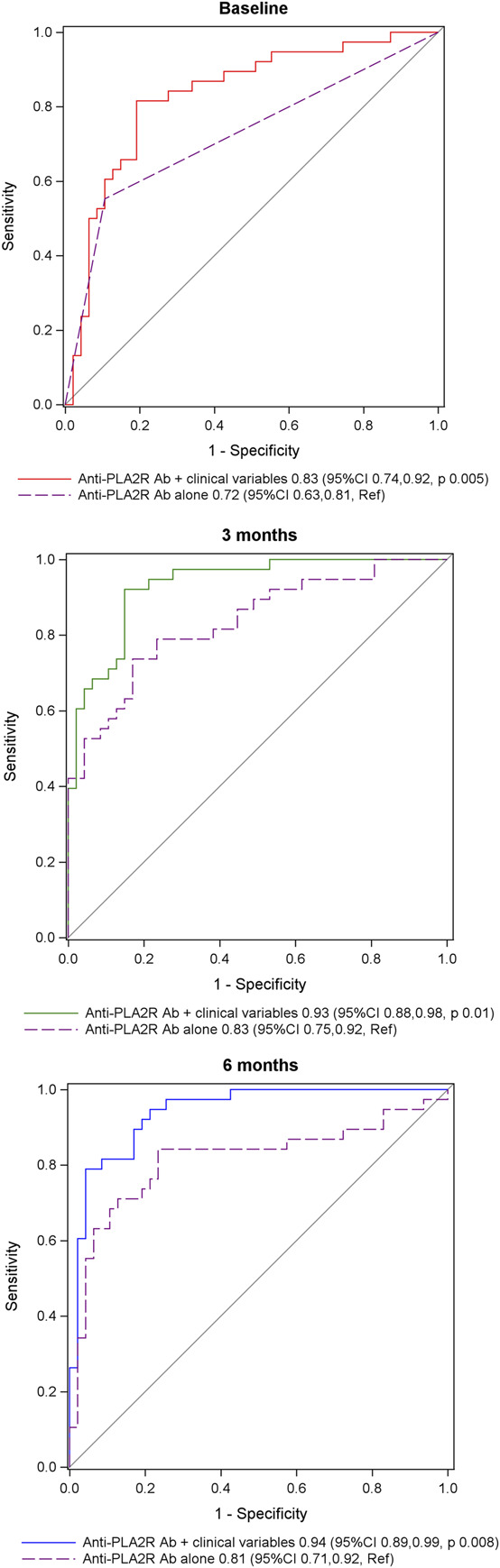
Receiver-operating curves comparing models that include only anti-PLA2R antibody levels to multivariable models that also include clinical variables at each landmark time. The univariable, bivariable, and multivariable models are those from Table 3 and Supplemental Tables 3 and 4. This figure allows comparison, at each landmark time, of the receiver-operating curves for models with anti-PLA2R antibody levels alone with models that include antibody levels and clinical variables. The significant clinical predictor variables (P < 0.05) in the multivariable model at baseline include creatinine clearance, in the multivariable model at 3 months include albumin, and in the multivariable model at 6 months include creatinine clearance and albumin. The C-statistic values for each model are provided in the figure legends. Ab, antibodies; CI, confidence interval; PLA2R, phospholipase A2 receptor; Ref, reference model. Figure 2 can be viewed in color online at www.cjasn.org.
Determining the Earliest Landmark Time That Allows Prediction of Remission Status at 12 months
There was better model fit for the multivariable model at 3 months with clinical variables and antibody levels compared with the multivariable model at baseline, as indicated by lower AIC and higher R2 values (Table 4). The difference in AIC and R2 values between the multivariable models at 3 months and 6 months were negligible. The C-statistic for the multivariable model at 3 months was higher than the model at baseline (0.93 versus 0.83, P = 0.008) but similar to the model at 6 months (0.93 versus 0.94, P = 0.67) (Figure 3). Collectively, these results demonstrate that by combining anti-PLA2R antibody levels with the clinical risk factors that were included in the multivariable models, the probability of not being in remission at 12 months can be better assessed at the 3-month landmark time compared with baseline, and there is no additional benefit to waiting until the 6-month landmark time.
Figure 3.
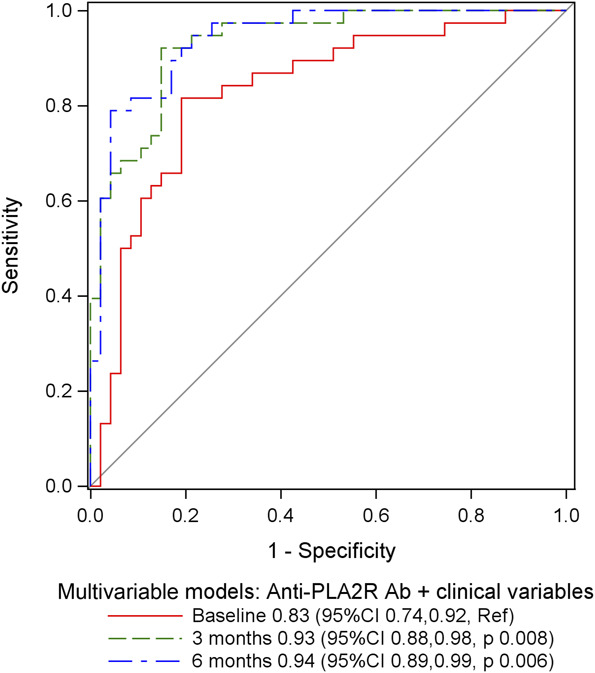
Receiver-operating curves comparing multivariable models at each landmark time that include clinical variables and anti-PLA2R antibody levels. The multivariable models are those from Table 3 and Supplemental Tables 3 and 4. This figure allows comparison of the receiver-operating curves for models with anti-PLA2R antibody levels and clinical variables across different landmark times. The significant clinical predictor variables (P < 0.05) in the multivariable model at baseline include creatinine clearance, in the multivariable model at 3 months include albumin, and in the multivariable model at 6 months include creatinine clearance and albumin. The C-statistic values for each model are provided in the figure legend. Figure 3 can be viewed in color online at www.cjasn.org.
Additional Analyses
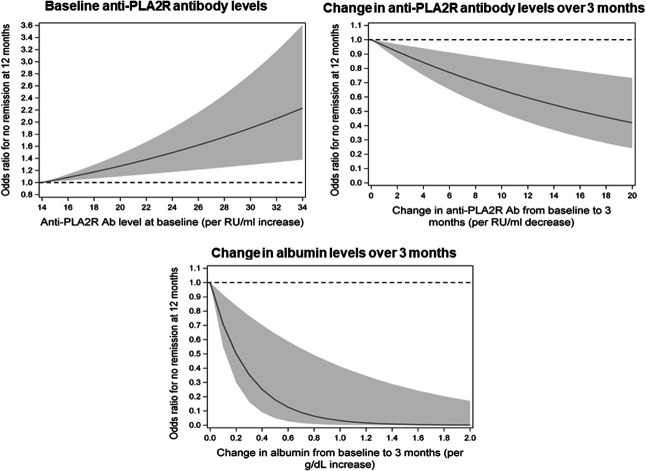
The relative risks of not being in remission at 12 months associated with baseline anti-PLA2R antibody levels and with changes in antibody and albumin levels from baseline to 3 months are shown in Figure 4. Each unit of higher antibody levels at baseline and each unit of improvement in antibody and albumin levels over 3 months of treatment were associated with significant changes in the relative risk of subsequent remission, which demonstrates the importance of considering these risk factors independently and on a continuous scale. Treatment allocation was forced into the multivariable model as a main effect (Supplemental Table 6), with similar results as the primary analysis. When treatment was included in interaction terms with each risk factor in the multivariable model at 3 months, none of the interaction terms were significant (Supplemental Table 7). These results suggest that the risk factors identified in the primary analysis were independent of and not modified by treatment allocation to rituximab or cyclosporine. The results were similar when the models were repeated in the full trial cohort, irrespective of anti-PLA2R antibody status (Supplemental Table 8).
Figure 4.
The relative risk of not being in remission at 12 months associated with anti-PLA2R antibody levels at baseline and changes in anti-PLA2R antibody and albumin levels over 3 months. The y-axis is the odds ratio for the risk not being in remission at 12 months on the basis of the multivariable model in Table 3 at the 3-month landmark time. The shaded region is the 95% confidence interval.
Discussion
Using a cohort with primary membranous nephropathy and anti-PLA2R antibody levels ≥14 RU/ml that were treated with cyclosporine or rituximab as part of the MENTOR trial, we have shown that changes in antibody and albumin levels over the first 3 months of treatment are the best risk factors for subsequent remission status after 12 months of therapy. Importantly, it was possible to assess the risk of subsequent remission after the first 3 months, with no added benefit of waiting until treatment had been given for 6 months.
Our results have potential implications for the clinical management of patients with membranous nephropathy. After 3 months of immunosuppression, changes in anti-PLA2R antibody and albumin levels can be used as risk factors for proteinuria remission after 12 months of treatment with either cyclosporine or rituximab. Each unit of improvement in antibody and albumin levels after 3 months are independently associated with a lower risk of not experiencing subsequent remission (Figure 4). This demonstrates the importance of considering changes in antibody and albumin levels on a continuous scale and as separate independent risk factors for subsequent remission. It is also important to account for the baseline value when considering the magnitude of change in antibody or albumin levels over 3 months because those who start with worse values require larger improvements over time to have a higher probability of subsequent remission. Our results show that it is not necessary to wait for 6 months of treatment to assess the probability of remission. This is consistent with the trajectory of antibody and albumin levels over time (Figure 1), in which there was a distinct separation between remission groups at 3 months but with no further separation after 6 months. Early changes in antibody and albumin levels after 3 months of treatment with cyclosporine or rituximab could be used to identify patients most likely to benefit from continuing therapy and to identify those at low risk of subsequent remission who may benefit from modifying their treatment. When following response to immunosuppression, proteinuria and albumin tend to improve in parallel. Our results indicate that increasing albumin is a better predictor of treatment response than decreasing proteinuria, which is supported by both clustering analysis and LASSO regression (Supplemental Methods and Supplemental Table 5). Small changes in albumin over 3 months of treatment (2–6 g/L) had a more significant effect on the probability of subsequent remission compared with larger changes in proteinuria (2.5–5.2 g/d, Table 2). There was a trend toward male sex increasing the risk of not experiencing remission (OR, 6.22 at 3 months, 95% confidence interval, 0.81 to 47.6, P = 0.08). This requires further evaluation in larger cohorts to generate a more precise risk estimate. Collectively, we used a well-defined clinical trial cohort treated with cyclosporine or rituximab and a data-driven approach to identify short-term changes in anti-PLA2R antibody and albumin levels over 3 months as the best predictors of treatment-induced remission at 12 months.
Although the specific antibody cutoff values identified in our analysis require external validation, they can be used to provide general insights into the immunologic trajectory of PLA2R-mediated membranous nephropathy after treatment with cyclosporine or rituximab. Several studies have demonstrated that after immunosuppression, antibody levels decline earlier than proteinuria and immunologic remission precedes proteinuria remission.18–21 We identified data-driven cutoffs for antibody levels at different landmark times that were maximally associated with subsequent proteinuria remission, instead of using thresholds that define EUROIMMUN assay results. After 3 months of treatment, the best cutoff for antibody levels was 58 RU/ml, which was a better predictor of remission status than either the threshold used to define a positive assay result (14 RU/ml) or the lower limit of assay detectability (3 RU/ml), but not as good as considering the relative change from baseline on a continuous scale. After 6 months of treatment, the best measure of antibody levels maximally associated with subsequent remission was a cutoff of 14 RU/ml. These results suggest that a favorable anti-PLA2R antibody trajectory during immunosuppression treatment should be an early reduction in levels after 3 months to at least a value below 58 RU/ml and a negative assay result of <14 RU/ml after 6 months, which can be expected to result in proteinuria remission by up to 1 year or longer.
Our results should be considered in the context of existing studies and current guideline recommendations on the kinetics of anti-PLA2R antibodies after immunosuppression treatment. The 2021 KDIGO guidelines include a Practice Point to evaluate antibodies after 6 months of immunosuppression, suggesting that patients with values <2 RU/ml should stop treatment; those with declining values to <50 RU/ml should complete a longer course of their current treatment; and those with values ≥50 RU/ml should change to an alternative therapy. This recommendation is based on expert opinion; is limited by a lack of data to support these threshold values; and does not consider changes in clinical variables, such as albumin, earlier landmark times before 6 months, or how close the baseline antibody level is to the proposed threshold of 50 RU/ml. Prior studies instead used antibody levels <14 RU/ml or relative percent change from baseline, and no study has evaluated a threshold value of 50 RU/ml.21,29,30 To improve applicability in clinical practice, our results need to be converted into a formal prediction model and evaluated using appropriate analytics, which will require larger and more diverse cohorts exposed to different treatments. However, previous studies used to inform KDIGO guidelines have similar limitations, and our data-driven analysis has advantages compared with the existing literature because it is based on treatment response instead of assay characteristics. In our analysis, the threshold value for antibody levels after 6 months of treatment associated with the lowest probability of remission was >14 RU/ml; however, earlier assessment of treatment response can be achieved by considering baseline and absolute changes in both albumin and anti-PLA2R antibody levels over the first 3 months of therapy.
There are several limitations to consider. Our results apply to patients who meet the inclusion criteria of the MENTOR trial with anti-PLA2R antibody levels ≥14 RU/ml treated with cyclosporine or rituximab according to the trial. We used 12-month remission as the outcome for this analysis because our primary purpose was to identify risk factors for an initial response to treatment. There are two implications compared with using 24-month remission, which was the primary outcome in the trial. First, late response to rituximab between 12 and 24 months may have been missed, although overall remission did not change in this time frame (60% at both 12 and 24 months). Second, we could not assess durability of treatment response after stopping therapy because this would require a separate analysis strategy to evaluate relapse risk in those who first achieved remission. None of our results can inform the optimal duration of treatment with cyclosporine or rituximab because all patients completed a full treatment course as per the trial protocol. Although better creatinine clearance at baseline was associated with a higher probability of subsequent remission, changes in creatinine clearance over 3 or 6 months were not. This may be because there was only a modest 8 ml/min per 1.73 m2 decrease over 6 months and additional study is required in cohorts with more rapid decline in kidney function. The severity of disease in the MENTOR trial, given by baseline proteinuria, albumin, and anti-PLA2R antibody levels, was worse than in other clinical trials.31,32 Nonetheless, the inclusion criteria were consistent with current guideline recommendations regarding moderate-to-high–risk patients being considered for immunosuppression.7 Our cohort was not large enough to support separate multivariable models in subgroups based on treatment with cyclosporine or rituximab. However, the results in Supplemental Tables 6 and 7 suggest that the risk factors identified in our analysis were independent of and not modified by treatment allocation. These limitations are offset by several strengths, including the use of a prospective trial cohort, random allocation of treatment, standardized follow-up, and regular measurements of anti-PLA2R antibodies and clinical risk factors using assays commonly available in clinical practice.
In summary, using the MENTOR trial cohort with membranous nephropathy and anti-PLA2R antibody levels ≥14 RU/ml, changes in antibody and albumin levels over the first 3 months of treatment with cyclosporine or rituximab can be used to predict proteinuria remission at 12 months. Using both albumin and antibody levels together to assess the probability of subsequent remission is superior to using antibody levels alone, and using 3-month values provides similar benefit compared with waiting for 6 months of treatment.
Supplementary Material
Acknowledgments
Genentech and the Fulk Family Foundation provided funding for the MENTOR trial. Genentech and EUROIMMUN provided funding for assay measurements. Genentech also provided rituximab, and EUROIMMUN provided in-kind measurement of anti-PLA2R antibody levels. None of the funding sources had a role in the conduct of the MENTOR trial or in the current analysis.
Footnotes
MENTOR Trial investigators Executive Committee: F.C. Fervenza (Principal Investigator), D.C. Cattran (Co-Principal Investigator), J. Appel, D. Gipson, M. Kretzler, B. Rovin. Study Investigators and Collaborators: United States – Mayo Clinic, Rochester, MN: F.C. Fervenza, J.C. Lieske, N. Leung, S.B. Erickson; Columbia University, New York, NY: J. Radhakrishnan, A. Bomback, J. Hogan, P. Canetta, W. Ahn; Stanford University, Stanford, CA: R. Lafayette, N. Arora, P. Nargund; Ohio State University, Columbus, OH: B. Rovin, A. Alvarado, S. Parikh, L.A. Hebert; Mayo Clinic, Jacksonville, FL: N. Aslam, I. Porter; University of Michigan Medical Center, Ann Arbor, MI: P. Gipson, M. Kretzler, B. Plattner, D. Gipson, L. Mariani, P. Garg, P. Rao; Case Western Reserve University, Cleveland, OH: J. Sedor, J. O'Toole; University of Washington Medical Center, Seattle, WA: J.A. Jefferson, P.J. Nelson; Kansas University Medical Center, Kansas City, KS: E. McCarthy, S. Yarlagadda, N. Jain; University of Alabama at Birmingham, Birmingham, AL: D. Rizk; Cleveland Clinic, Cleveland, OH: J. Simon, S. Gebreselassie; Medical College of Wisconsin, Froedtert Hospital, Milwaukee, WI: S. Blumenthal; New York University Medical Center, New York, NY: L. Beara-Lasic, O. Zhdanova; Mayo Clinic, Scottsdale, AZ: L. Thomas, I. Cohen, M. Keddis; University of Arizona, Tucson, AZ: A. Sussman, B. Thajudeen; University of Mississippi Medical Center, Jackson, MS: T. Fulop, I. Craici, S. Wagner, A. Dreisbach, D. Monga; University of Miami, Miami, FL: D. Green, A. Mattiazzi, A. Nayer, D. Thomas, L. Barisoni; Washington University School of Medicine, St. Louis, MO: T. Li, A. Vijayan; Central Arkansas Veterans Healthcare System, Little Rock, Arkansas: L. Juncos Canada – University Health Network, Toronto General Hospital, Toronto, ON: D.C. Cattran, H. Reich, M.A. Hladunewich; Providence Health Care, St. Paul's Hospital, Vancouver, BC: S. Barbour, A. Levin; Centre hospitalier universitaire de Québec, Québec City, QC: D. Philibert, F. Mac-Way, S. Desmeules; Saudi Arabia – King Abdulaziz University, Jeddah: G. Ankawi. Pathology Adjudication: S. Sethi, C. Avila-Casado. Anti-PLA2R Laboratory: P. Brenchley. Quality of Life Measures: H. Beanlands.
Contributor Information
Collaborators: F.C. Fervenza, D.C. Cattran, J. Appel, D. Gipson, M. Kretzler, B. Rovin, F.C. Fervenza, J.C. Lieske, N. Leung, S.B. Erickson, J. Radhakrishnan, A. Bomback, J. Hogan, P. Canetta, W. Ahn, R. Lafayette, N. Arora, P. Nargund, B. Rovin, A. Alvarado, S. Parikh, L.A. Hebert, N. Aslam, P. Gipson, M. Kretzler, B. Plattner, D. Gipson, L. Mariani, P. Garg, P. Rao, J. Sedor, J. O’Toole, J.A. Jefferson, P.J. Nelson, E. McCarthy, S. Yarlagadda, N. Jain, D. Rizk, J. Simon, S. Gebreselassie, S. Blumenthal, L. Beara-Lasic, O. Zhdanova, L. Thomas, I. Cohen, M. Keddis, A. Sussman, B. Thajudeen, T. Fulop, I. Craici, S. Wagner, A. Dreisbach, D. Monga, D. Green, A. Mattiazzi, A. Nayer, D. Thomas, L. Barisoni, T. Li, A. Vijayan, L. Juncos, D.C. Cattran, H. Reich, M. Hladunewich, S. Barbour, A. Levin, D. Philibert, F. Mac-Way, S. Desmeules, G. Ankawi, S. Sethi, C. Avila-Casado, and P. Brenchley
Disclosures
G.B. Appel reports consultancy for Achillion, Alexion, Apellis, Arrowhead, Aurinia, Bristol Myers Squibb, Chemocentryx, Chinook, EMD Serono, Genentech, Genzyme-Sanofi, GlaxoSmithKline, E. Lilly, Mallinkrodt, Merck, Novartis, Omeros, Pfizer, Reata, Travere Therapeutics, and Vertex Therapeutics; research funding from Achillion-Alexion, Apellis, Calliditas, Chemocentryx, Equillium, Genentech-Roche, Goldfinch, Mallinkrodt, Novartis, Reata, Sanofi-Genzyme, and Vertex—all through Columbia University; honoraria from Aurinia, Calliditas, and GlaxoSmithKline; royalties from UpToDate; advisory or leadership roles for UpToDate Editorial Board and Med Advisory board for Alexion, Alexion-Achillion, Apellis, Arrowhead, Aurinia, BM Squib, Chinook, Genentech, GlaxoSmithKline, Lilly, Reata, Roche, and Sanofi—no role as officer or board member of any pharmaceutical or other; and speakers bureau for Aurinia lectures on lupus nephritis, for GSK for lectures on lupus nephritis, and Calliditas for lecture on the gut and IgAN. N. Aslam reports ownership interest in Doximity; research funding from AstraZeneca, Baxter, Idorsia, Novartis, and Otsuka; and advisory or leadership roles for Chinook Advisory Board, Florida Society of Nephrology Board of Directors, and Travere Therapeutics Advisory Board. S.J. Barbour reports consultancy for Achillion, Alexion, Eledon, HIBio, Inception Sciences, Novartis, Pfizer, Vera, and Visterra; research funding from Alexion, Novartis, and Roche; and honoraria from Alexion and Roche. D.C. Cattran reports consultancy for Alexi, Alnylam, Aurinia, Calliditis, Chemocentrx, Chinook Therapeutics, Forsee, Horizon, Reistone, Vera Therapeutics, and Zyversa Therapeutics; research funding from Alnylam; honoraria from Alexion, Calliditis, and Kyowa Hakko Kirin Co; advisory or leadership roles for Alnylam, Calliditis, NephCure, SONG-GD, UpToDate, and Vera; and other interests or relationships with Aurinea, Dimerix, Novartis, and Vera Therapeutics. F.C. Fervenza reports employment with Mayo Clinic; consultancy for Alexion Pharmaceuticals, ByoCrystal, Galapagos, GSK, Novartis, Otsuka, and Takeda; research funding from Chemocentryx, Genentech, Hoffman La Roche, Janssen Pharmaceutical, Morphosys, and Retrophin; honoraria from UpToDate; and advisory or leadership roles for JASN, Kidney International, Nephrology, Nephrology Dialysis and Transplantation, and UpToDate. M.A. Hladunewich reports research funding from Calliditas Therapeutics, Chemocentryx, Chinook, Ionis, Pfizer, and Roche; honoraria from UpToDate; and other interests or relationships as medical lead for the Glomerular Disease Ontario Renal Network. D. Induruwage reports employment with the BC Provincial Renal Agency. K. Kiryluk reports consultancy for Calvariate and HiBio and research funding from Aevi Genomics, AstraZeneca, Bioporto, Vanda, and Visterra. R. Lafayette reports consultancy for Alexion, Inc., Aurinia, Calliditas, Inc., Chinook, Inc., Novartis, Omeros, Inc., Otsuka, Inc., Travere, Inc., Vera, Inc., and Visterra, Inc. and research funding from Apellis, Calliditas, Chemocentryx, Chinook, NIH, Omeros, Otsuka, Pfizer, Roche, Travere, and Vera. H.N. Reich reports consultancy for Calliditas, Chinook, Novartis, Omeros, Pfizer, and Retrophin (Travere); research funding from clinical trial recruitment: Alnylam, Calliditas, Chemocentryx, Omeros, and Pfizer and national coordinating investigator: Calliditas and Chinook; advisory or leadership roles for Academic advisory—Omeros, Academic leadership committee—Calliditas, Calliditas, Chinook, and Kidney International Editorial Board, and Steering Committee—Eledon; peer-reviewed funding from the Kidney Foundation of Canada; other interests or relationships with Canadian Institutes for Health Research and Pearson Foundation; fellowship support from the Fast Foundation; and consultation for the Canadian Agency for Drugs and Technologies in Health. B. Rovin reports consultancy for Alexion, AstraZeneca, Aurinia, Biocryst, Biogen, BMS, Calliditas, Chemocentryx, Corrona, EMD-Serono/Merck, Exagen, Galapagos, Genentech, Horizon, Human Genome Sciences (GSK), Idorsia, Janssen, Morphosys, MedImmune, Novartis, Omeros, Otsuka, Resonance, Retrophin, RILITE Foundation, Roche, and Vistera; research funding from Biogen; honoraria from Alexion, AstraZeneca, Aurinia, Biocryst, Biogen, BMS, Calliditas, Chemocentryx, Corrona, EMD-Serono/Merck, Exagen, Galapagos, Genentech, Horizon, Human Genome Sciences (GSK), Idorsia, Janssen, MedImmune, Morphosys, Novartis, Omeros, Otsuka, Resonance, Retrophin, RILITE Foundation, Roche, and Vistera; advisory or leadership roles for ASN Kidney Week, CureGN, KDIGO, Kidney International, Kidney International Reports, Lupus Foundation of America, Nephrology Dialysis and Transplantation, and UpToDate; a lot of work with the ASN, mostly educational courses; work with the NKF and the ISN; and work with the LFA. L. Zand reports employment with Mayo Clinic, Rochester, MN, and research funding from Genentech, Janssen Pharmaceuticals, and Mallinckrodt. All remaining authors have nothing to disclose.
Funding
This work was supported by Genentech and a generous gift from Mr. Scott Brittingham to the Mayo Nephrology Collaborative Group.
Author Contributions
Conceptualization: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza.
Data curation: Paul E. Brenchley, Daniel C. Cattran, Fernando C. Fervenza, Dilshani Induruwage, Lili Liu.
Formal analysis: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza, Dilshani Induruwage.
Funding acquisition: Daniel C. Cattran, Fernando C. Fervenza.
Investigation: Gerald B. Appel, Nabeel Aslam, Sean J. Barbour, Paul E. Brenchley, Daniel C. Cattran, Fernando C. Fervenza, Michelle A. Hladunewich, Dilshani Induruwage, Krzysztof Kiryluk, Richard Lafayette, Lili Liu, Heather N. Reich, Brad Rovin, Ladan Zand.
Methodology: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza, Dilshani Induruwage.
Project administration: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza.
Resources: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza.
Software: Dilshani Induruwage, Lili Liu.
Supervision: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza.
Visualization: Gerald B. Appel, Nabeel Aslam, Sean J. Barbour, Paul E. Brenchley, Daniel C. Cattran, Fernando C. Fervenza, Michelle A. Hladunewich, Dilshani Induruwage, Krzysztof Kiryluk, Richard Lafayette, Lili Liu, Heather N. Reich, Brad Rovin, Ladan Zand.
Writing – original draft: Sean J. Barbour, Daniel C. Cattran, Fernando C. Fervenza.
Writing – review & editing: Gerald B. Appel, Nabeel Aslam, Sean J. Barbour, Paul E. Brenchley, Daniel C. Cattran, Fernando C. Fervenza, Michelle A. Hladunewich, Dilshani Induruwage, Krzysztof Kiryluk, Richard Lafayette, Lili Liu, Heather N. Reich, Brad Rovin, Ladan Zand.
Supplemental Material
Supplemental Table 1. Characteristics of the analytic cohort after 6 months of treatment with cyclosporine or rituximab as part of the MENTOR trial.
Supplemental Table 2. Model fit for univariable and bivariable models for the risk of no remission at 12 months using different parameterizations for anti-PLA2R antibody measurements at each landmark time.
Supplemental Table 3. Univariable and multivariable model results for the risk of no remission at 12 months using risk factors at a baseline landmark time.
Supplemental Table 4. Univariable, bivariable, and multivariable model results for the risk of no remission at 12 months using risk factors at a 6-month landmark time.
Supplemental Table 5. Multivariable model results using LASSO regression.
Supplemental Table 6. Multivariable model results that include treatment allocation to rituximab versus cyclosporine as part of the MENTOR trial.
Supplemental Table 7. Interaction terms between treatment allocation to rituximab versus cyclosporine as part of the MENTOR trial and each risk factor in the multivariable model at the 3-month landmark time.
Supplemental Table 8. Multivariable model results in the overall MENTOR trial cohort.
Supplemental Figure 1. Correlation between EUROIMMUN ELISA anti-PLA2R antibody levels used in this analysis and in-house ELISA antibody levels that were used in the MENTOR trial publication.
Supplemental Figure 2. Derivation of the analytic cohort.
References
- 1.Cattran DC, Reich HN, Kim SJ, Troyanov S. Have we changed the outcome in membranous nephropathy? A propensity study on the role of immunosuppressive therapy. Clin J Am Soc Nephrol. 2011;6(7):1591–1598. doi: 10.2215/CJN.11001210 [DOI] [PubMed] [Google Scholar]
- 2.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 1997;51(3):901–907. doi: 10.1038/ki.1997.127 [DOI] [PubMed] [Google Scholar]
- 3.Schieppati A Mosconi L Perna A, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329(2):85–89. doi: 10.1056/nejm199307083290203 [DOI] [PubMed] [Google Scholar]
- 4.Polanco N Gutierrez E Covarsi A, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21(4):697–704. doi: 10.1681/ASN.2009080861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McQuarrie EP, Stirling CM, Geddes CC. Idiopathic membranous nephropathy and nephrotic syndrome: outcome in the era of evidence-based therapy. Nephrol Dial Transplant. 2012;27(1):235–242. doi: 10.1093/ndt/gfr220 [DOI] [PubMed] [Google Scholar]
- 6.Troyanov S, Wall CA, Scholey JW, Miller JA, Cattran DC. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66(3):1199–1205. doi: 10.1111/j.1523-1755.2004.00873.x [DOI] [PubMed] [Google Scholar]
- 7.Rovin BH Adler SG Barratt J, et al. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4):S1–S276. doi: 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 8.Thompson A, Cattran DC, Blank M, Nachman PH. Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol. 2015;26(12):2930–2937. doi: 10.1681/ASN.2015010091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponticelli C Zucchelli P Passerini P, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48(5):1600–1604. doi: 10.1038/ki.1995.453 [DOI] [PubMed] [Google Scholar]
- 10.Ruggenenti P Cravedi P Chianca A, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(8):1416–1425. doi: 10.1681/ASN.2012020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fervenza FC Appel GB Barbour SJ, et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381(1):36–46. doi: 10.1056/nejmoa1814427 [DOI] [PubMed] [Google Scholar]
- 12.Fervenza FC Abraham RS Erickson SB, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5(12):2188–2198. doi: 10.2215/CJN.05080610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fervenza FC Cosio FG Erickson SB, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73(1):117–125. doi: 10.1038/sj.ki.5002628 [DOI] [PubMed] [Google Scholar]
- 14.Howman A Chapman TL Langdon MM, et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet. 2013;381(9868):744–751. doi: 10.1016/s0140-6736(12)61566-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck LH Jr. Bonegio RG Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21. doi: 10.1056/nejmoa0810457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoxha E Harendza S Zahner G, et al. An immunofluorescence test for phospholipase-A(2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26(8):2526–2532. doi: 10.1093/ndt/gfr247 [DOI] [PubMed] [Google Scholar]
- 17.Qin W Beck LH Jr. Zeng C, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22(6):1137–1143. doi: 10.1681/ASN.2010090967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck LH Jr. Fervenza FC Beck DM, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22(8):1543–1550. doi: 10.1681/ASN.2010111125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bech AP, Hofstra JM, Brenchley PE, Wetzels JF. Association of anti-PLA(2)R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2014;9(8):1386–1392. doi: 10.2215/CJN.10471013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25(6):1357–1366. doi: 10.1681/ASN.2013040430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggenenti P Debiec H Ruggiero B, et al. Anti-Phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26(10):2545–2558. doi: 10.1681/ASN.2014070640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahnrich C Komorowski L Probst C, et al. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421:213–218. doi: 10.1016/j.cca.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 23.Kanigicherla D Gummadova J McKenzie EA, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83(5):940–948. [DOI] [PubMed] [Google Scholar]
- 24.Hofstra JM Debiec H Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23(10):1735–1743. doi: 10.1681/ASN.2012030242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz-Polski B Debiec H Rousseau A, et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol. 2018;29(2):401–408. doi: 10.1681/ASN.2017070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprangers B Bomback AS Cohen SD, et al. Idiopathic membranous nephropathy: clinical and histologic prognostic features and treatment patterns over time at a tertiary referral center. Am J Nephrol. 2012;36(1):78–89. doi: 10.1159/000339628 [DOI] [PubMed] [Google Scholar]
- 27.Xie J Liu L Mladkova N, et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat Commun. 2020;11(1):1600. doi: 10.1038/s41467-020-15383-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams B, Mandrekar J, Mandrekar S, Cha S, Furth A. Finding optimal cutpoints for continuous covariates with binary and time-to-event outcomes, Vol 79. Department of Health Sciences Research Mayo Clinic Tecnical Report Series; 2006:1–26. [Google Scholar]
- 29.Pourcine F Dahan K Mihout F, et al. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: a single-centre study over 14 years. PLoS One. 2017;12(3):e0173201. doi: 10.1371/journal.pone.0173201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segarra-Medrano A Jatem-Escalante E Carnicer-Caceres C, et al. Evolution of antibody titre against the M-type phospholipase A2 receptor and clinical response in idiopathic membranous nephropathy patients treated with tacrolimus. Nefrologia. 2014;34(4):491–497. doi: 10.3265/Nefrologia.pre2014.Jun.12536 [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Juarez G Rojas-Rivera J Logt AV, et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 2021;99(4):986–998. doi: 10.1016/j.kint.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 32.Scolari F Delbarba E Santoro D, et al. Rituximab or cyclophosphamide in the treatment of membranous nephropathy: the RI-CYCLO randomized trial. J Am Soc Nephrol. 2021;32(4):972–982. doi: 10.1681/ASN.2020071091 [DOI] [PMC free article] [PubMed] [Google Scholar]