Summary:
Infections are problematic in postmastectomy implant-based reconstruction with infection rates as high as 30%. Strategies to reduce the risk of infection have demonstrated various efficacies. A prolonged course of systemic, oral antibiotics has not shown evidence-based benefit. Although absorbable antibiotic beads have been described for orthopedic procedures and pressure wounds, their use has not been well studied during breast reconstruction, particularly for prepectoral implant placement. The purpose of this study was to evaluate the selective use of prophylactic absorbable calcium sulfate antibiotic beads during high-risk implant-based, prepectoral breast reconstruction after mastectomy. Patients who underwent implant-based, prepectoral breast reconstruction between 2019 and 2022 were reviewed. Groups were divided into those who received antibiotic beads and those who did not. Outcome variables included postoperative infection at 90 days. A total of 148 patients (256 implants) were included: 15 patients (31 implants) who received biodegradable antibiotic beads and 133 patients (225 implants) in the control group. Patients who received antibiotic beads were more likely to have a history of infection (66.7%) compared with the control group (0%) (P < 0.01). Surgical site infection occurred in 3.2% of implants in the antibiotic bead group compared with 7.6%, but this did not reach statistical significance. The incidence of infection in high-risk patients who have absorbable antibiotic beads placed during the time of reconstruction seems to be normalized to the control group in this pilot study. We present a novel use of prophylactic absorbable antibiotic beads in prepectoral breast implant reconstruction.
Takeaways
Question: Does selective use of locally delivered prophylactic absorbable calcium sulfate antibiotic beads benefit patients during high-risk implant-based, prepectoral breast reconstruction after mastectomy?
Findings: There was no significant difference seen in surgical site infection or implant loss in high-risk patients receiving prophylactic absorbable antibiotic beads during implant-based, prepectoral breast reconstruction compared with the control group.
Meaning: We present a novel use of prophylactic absorbable antibiotic beads in prepectoral breast implant reconstruction and show that the incidence of infection in high-risk patients who have absorbable antibiotic beads placed during reconstruction seems to be normalized to the control group.
LOCAL PROPHYLACTIC ANTIBIOTICS FOR IMPLANT RECONSTRUCTION
Postoperative infection is problematic in implant-based breast reconstruction after mastectomy for breast cancer, with an incidence as high as 30%.1 Implant infection significantly impacts patient quality of life and increases overall healthcare costs.2 Risk factors of patients associated with higher rates of infected breast implants include age, higher body mass index (BMI), presence of diabetes mellitus, and active smoking.3,4 Strategies to reduce the risk of infection include antibiotic irrigation and “no-touch” technique during implant delivery.5–9 A prolonged course of systemic, oral antibiotics has not shown evidence-based benefit.10,11
Selective, prophylactic, local delivery of antibiotics may provide the therapeutic effect of antibiotics while avoiding systemic side effects and other issues of antibiotic overuse.12,13 Absorbable antibiotic beads have been well described for orthopedic procedures for osteomyelitis, as well as pressure sore reconstruction for ulcer recurrence, and vascular graft infection.14–16 The use of absorbable antibiotic beads during prepectoral breast reconstruction has not been well studied.17,18 The purpose of our study was to evaluate the selective use of prophylactic absorbable antibiotic beads in high-risk patients undergoing implant-based prepectoral breast reconstruction.
A single institutional retrospective review of patients who underwent implant-based breast reconstruction by a single surgeon was performed between 2019 and 2022. Patients were divided into two groups: high-risk and control group. “High risk” patients included patients with a history of breast implant infection, higher BMI, and history of diabetes or active smoking and had prophylactic absorbable antibiotic beads placed during reconstruction. The control group were patients who did not receive antibiotic beads and underwent implant-based breast reconstruction during the study interval. Baseline characteristics included demographics, diabetes, BMI, and smoking status. History of infection, chemoradiation, and implant plane were also obtained. Outcome that varies included postoperative infection or implant loss at 90 days. Statistical analyses were performed within IBM SPSS Version 28 (IBM Corp., Armonk, N.Y.). Two-tailed values of P less than 0.05 were considered significant. Categorical variables were analyzed using Fisher exact test. Continuous variables were compared with t tests.
The study included 148 patients (256 implants) who underwent implant-based breast reconstruction. There were 15 patients (31 implants) in the antibiotic bead group and 133 patients (225 implants) in the control group. The two groups had no significant differences in demographic data, mastectomy type, or treatment (Table 1).
Table 1.
Baseline Characteristics and Treatment Outcomes
| Antibiotic Bead (n = 15 Patients, 31 Implants) | Control Group (n = 133 Patients, 225 Implants) | P | |
|---|---|---|---|
| Average age (y) | 45.53 (SD 12.1) | 47.13 (10.8) | P = 0.57 |
| Body mass index (kg/m2) | 30.79 (SD 7.8) | 27.98 (SD 5.8) | P = 0.09 |
| Diabetes mellitus | 3 (20%) | 7 (5.3%) | P = 0.0658 |
| Smoker | 1 (6.7%) | 5 (3.8%) | P = 0.479 |
| Nipple sparing mastectomy | 8 (53.3%) | 85 (63.9%) | P = 0.416 |
| Skin sparing mastectomy | 7 (46.7%) | 48 (36.1%) | P = 0.416 |
| Prepectoral implant | 15 (100%) | 133 (100%) | P = 1 |
| Neoadjuvant chemotherapy | 1 (6.7%) | 48 (36.1%) | P = 0.02 |
| Prior radiation | 1 (6.7%) | 5 (3.6%) | P = 0.479 |
| Adjuvant chemotherapy | 3 (20%) | 38 (28.6%) | P = 0.761 |
| Adjuvant radiation | 4 (26.7%) | 40 (30.1%) | P = 1 |
| Prior infection | 10 (66.7%) | 0 (0%) | P = 0.00001 |
| Implant loss (total expanders) | 1 (6.7%) | 22 (16.5%) | P = 0.468 |
| Infection at 90 days | 1 (6.7%) | 17 (12.8%) | P = 0.695 |
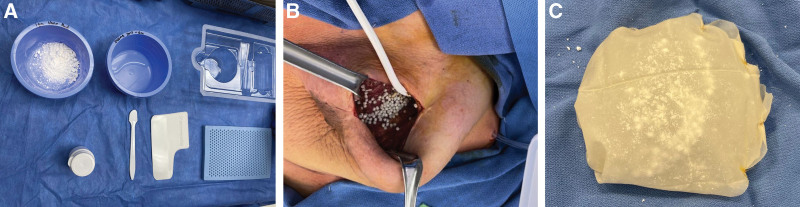
Absorbable calcium sulfate antibiotic beads (Stimulan; Biocomposites Ltd., United Kingdom) were used for prophylactic placement during implant-based reconstruction. A resting base is combined with 1 g of vancomycin and 240 mg of liquid gentamicin and mixed thoroughly into a fine paste. This paste is then distributed across a mold that contains pockets to create 3–6 mm beads, which solidify for 2–5 minutes before application (Fig. 1). For prepectoral reconstruction, antibiotic beads are placed behind the implant over the pectoralis major muscle and anterior to the implant sparsely between the subcutaneous fat and the implant. (See Video [online], which displays the placement of prophylactic absorbable calcium sulfate antibiotic beads during prepectoral implant-based reconstruction). To place the beads uniformly around the implant, Vicryl mesh can be used to circumferentially wrap the implant with beads between the Vicryl mesh and implant. Drains were placed in all patients who received antibiotic beads because of seroma risk.
Fig. 1.
Absorbable antibiotic beads for prepectoral breast implant reconstruction. A, The calcium sulfate beads are reconstituted with vancomycin and gentamycin. B, For prepectoral reconstruction, beads are placed over the pectoralis major muscle under the implant. C, Alternatively, beads can be uniformly placed around the implant using a Vicryl mesh wrap.
Video 1. displays the placement of prophylactic absorbable calcium sulfate antibiotic beads during prepectoral implant-based reconstruction.
All patients in the antibiotic bead group and control group underwent prepectoral breast reconstruction. In the antibiotic bead group, 66.7% of patients had a history of infection compared with 0% in the control group (P < 0.05). There was no significant difference in mean BMI in the antibiotic bead group (30.8 kg/m2 compared with 28.0 kg/m2 in the control group; P = 0.09). Diabetes was present in 20.0% of antibiotic bead patients compared with 5.3% of control patients (P = 0.07). There was no significant difference in predictive variables of nipple or skin sparing mastectomy, chemotherapy, or radiation between the two groups. Implants were used in 33.3% and tissue expanders in 67.7% in the high-risk group at the time of antibiotic bead placement. Surgical site infection occurred in 3.2% (one implant) in the antibiotic bead group compared with 7.6% (22 implants) in the control group, but this did not reach statistical significance.
DISCUSSION
Infection after breast implant reconstruction is problematic.19,20 Prophylactic antibiotic beads have been described in other disciplines, including vascular graft infection, treatment of osteomyelitis, orthopedic, and urologic implant procedures.21–23 Absorbable antibiotic beads have not been well studied for implant-based breast reconstruction.
A study by Johnstone et al used a nonabsorbable poly methyl methacrylate plate impregnated with vancomycin and tobramycin placed prophylactically over the pectoralis major muscle nonselectively in prepectoral tissue expander reconstruction.24 They found a decrease of surgical site infection in tissue expanders, from 14% to 4%.24 Absorbable calcium sulfate beads exhibit eluted antibiotics at a higher concentration than the minimum inhibitory concentration for up to 40 days with a zone of inhibition that began to taper between day 26-40.25,26 Therefore, these absorbable antibiotic beads are effective for up to 40 days and resorb between 30 and 60 days. Nonabsorbable (poly methyl methacrylate) antibiotic beads show loss of zone of inhibition by day 12.26 Another advantage of absorbable beads is the potential for placing a breast implant rather than tissue expander, because a stage to remove the antibiotic beads/cement is not required. One-third of patients in our study who received antibiotic beads had implants placed. A study performed by Kenna et al assessed prophylactic antibiotic beads during subpectoral breast implant reconstruction and demonstrated a significant reduction in implant loss.18
Patients who were deemed high risk, primarily because of previous infection, received antibiotic beads in our study. This suggests that use of absorbable antibiotic beads may normalize the risk of infection in high-risk breast cancer patients undergoing immediate implant-based reconstruction in this pilot study. A limitation of our pilot study is the small sample size homogeneity of patients receiving antibiotic beads.
Similar to other surgical disciplines such as orthopedic surgery, we used antibiotic beads selectively to limit cost. In addition, absorbable calcium sulfate has been shown to be effective in vitro for biofilm prevention and elimination.26 There is potential for other novel applications of antibiotic beads, such as capsular contracture and breast implant illness. Future horizons for innovations in prepectoral breast implant reconstruction may define indications for absorbable antibiotic beads.
DISCLOSURES
The authors have no financial interest to declare in relation to the content of this article. This work was supported by the U.S. National Institutes of Health (NIH) grants R01AI165958 and R21AI171932 to Dr. Sinha, and U.S. Department of Defense grants W81XWH2110135 and NIH K08HL167164 to Dr. Hassanein.
Footnotes
Published online 16 October 2023.
Presented at the Plastic Surgery Research Council 68th Annual Meeting, April 16, 2023, Cleveland, Ohio.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Lester and Hassanein contributed equally to this work.
REFERENCES
- 1.Francis SH, Ruberg RL, Stevenson KB, et al. Independent risk factors for infection in tissue expander breast reconstruction. Plast Reconstr Surg. 2009;124:1790–1796. [DOI] [PubMed] [Google Scholar]
- 2.Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143:53–60; discussion 61. [DOI] [PubMed] [Google Scholar]
- 3.Pinsolle V, Grinfeder C, Mathoulin-Pelissier S, et al. Complications analysis of 266 immediate breast reconstructions. J Plast Reconstr Aesthet Surg. 2006;59:1017–1024. [DOI] [PubMed] [Google Scholar]
- 4.Disa JJ, Ad-El DD, Cohen SM, et al. The premature removal of tissue expanders in breast reconstruction. Plast Reconstr Surg. 1999;104:1662–1665. [DOI] [PubMed] [Google Scholar]
- 5.Bamba R, Tran PC, Mailey BA, et al. Comparison of breast reconstruction outcomes using oxychlorosene versus triple antibiotic solution for pocket irrigation. Plast Reconstr Surg Glob Open. 2022;10:e3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mladick RA. “No-touch” submuscular saline breast augmentation technique. Aesthetic Plast Surg. 1993;17:183–192. [DOI] [PubMed] [Google Scholar]
- 7.Morkuzu S, Ozdemir M, Leach GA, et al. Keller funnel efficacy in “No Touch” breast augmentation and reconstruction: a systematic review. Plast Reconstr Surg Glob Open. 2022;10:e4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen JB, Carroll C, Tenenbaum MM, et al. Breast implant-associated infections: the role of the national surgical quality improvement program and the local microbiome. Plast Reconstr Surg. 2015;136:921–929. [DOI] [PubMed] [Google Scholar]
- 9.Dawson SE, Bamba R, Tran PC, et al. Implant-based breast reconstruction outcomes using oxychlorosene for pocket irrigation. Plast Reconstr Surg. 2021;148:518e–520e. [DOI] [PubMed] [Google Scholar]
- 10.Rothe K, Munster N, Hapfelmeier A, et al. Does the duration of perioperative antibiotic prophylaxis influence the incidence of postoperative surgical-site infections in implant-based breast reconstruction in women with breast cancer? A retrospective study. Plast Reconstr Surg. 2022;149:617e–628e. [DOI] [PubMed] [Google Scholar]
- 11.Sisco M, Kuchta K, Alva D, et al. Oral antibiotics do not prevent infection or implant loss after immediate prosthetic breast reconstruction. Plast Reconstr Surg. 2023;151:730e–738e. [DOI] [PubMed] [Google Scholar]
- 12.Baker JE, Seitz AP, Boudreau RM, et al. Doxycycline-coated silicone breast implants reduce acute surgical-site infection and inflammation. Plast Reconstr Surg. 2020;146:1029–1041. [DOI] [PubMed] [Google Scholar]
- 13.Makarewicz N, Lipman K, Johnstone T, et al. Use of local antibiotic delivery systems in tissue expander and implant-based breast reconstruction: a systematic review of the literature. Eplasty. 2023;23:e24. [PMC free article] [PubMed] [Google Scholar]
- 14.Calhoun JH, Mader JT. Treatment of osteomyelitis with a biodegradable antibiotic implant. Clin Orthop Relat Res. 1997:206–214. [PubMed]
- 15.Khansa I, Barker JC, Ghatak PD, et al. Use of antibiotic impregnated resorbable beads reduces pressure ulcer recurrence: a retrospective analysis. Wound Repair Regen. 2018;26:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGuinness B, Ali KP, Phillips S, et al. A scoping review on the use of antibiotic-impregnated beads and applications to vascular surgery. Vasc Endovascular Surg. 2020;54:147–161. [DOI] [PubMed] [Google Scholar]
- 17.Sherif RD, Ingargiola M, Sanati-Mehrizy P, et al. Use of antibiotic beads to salvage infected breast implants. J Plast Reconstr Aesthet Surg. 2017;70:1386–1390. [DOI] [PubMed] [Google Scholar]
- 18.Kenna DM, Irojah BB, Mudge K, et al. Absorbable antibiotic beads prophylaxis in immediate breast reconstruction. Plast Reconstr Surg. 2018;141:486e–492e. [DOI] [PubMed] [Google Scholar]
- 19.Pittet B, Montandon D, Pittet D. Infection in breast implants. Lancet Infect Dis. 2005;5:94–106. [DOI] [PubMed] [Google Scholar]
- 20.Ooi A, Song DH. Reducing infection risk in implant-based breast-reconstruction surgery: challenges and solutions. Breast Cancer (Dove Med Press). 2016;8:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barth RE, Vogely HC, Hoepelman AI, et al. “To bead or not to bead?” Treatment of osteomyelitis and prosthetic joint-associated infections with gentamicin bead chains. Int J Antimicrob Agents. 2011;38:371–375. [DOI] [PubMed] [Google Scholar]
- 22.Chen AF, Parvizi J. Antibiotic-loaded bone cement and periprosthetic joint infection. J Long Term Eff Med Implants. 2014;24:89–97. [DOI] [PubMed] [Google Scholar]
- 23.Droggin D, Shabsigh R, Anastasiadis AG. Antibiotic coating reduces penile prosthesis infection. J Sex Med. 2005;2:565–568. [DOI] [PubMed] [Google Scholar]
- 24.Johnstone T, Lipman K, Makarewicz N, et al. Use of antibiotic-impregnated polymethylmethacrylate (PMMA) plates for prevention of periprosthetic infection in breast reconstruction. Plast Reconstr Surg Glob Open. 2023;11:e4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts R, McConoughey SJ, Calhoun JH. Size and composition of synthetic calcium sulfate beads influence dissolution and elution rates in vitro. J Biomed Mater Res B Appl Biomater. 2014;102:667–673. [DOI] [PubMed] [Google Scholar]
- 26.Howlin RP, Brayford MJ, Webb JS, et al. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 2015;59:111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]