Abstract
Acute coronary syndrome (ACS) is an urgent clinical condition of cardiovascular diseases. The present study evaluated the predictive efficacy of the hemoglobin to serum creatinine ratio (Hgb/Cr) on long-term mortality in patients with ACS. The ratio, representing the proportion of the 2 values, is cheap, practical, and very easy to calculate at the bedside. Our study included 475 patients who were admitted to the coronary intensive care unit with a diagnosis of ACS and who underwent coronary angiography. The Hgb/Cr ratio was calculated by dividing the admission hemoglobin by the admission serum creatinine. All patient data were collected from the electronic hospital information system, patient files, and the hospital’s archive. A comparison of the patients laboratory findings revealed that the Hgb/Cr ratios differed significantly between the survivor and non-survivor group [16.6 (7.7–49) vs 13.8 (4.91–32.8), respectively; P < .001]. A univariate Cox regression analysis showed that the Hgb/Cr ratio was statistically significant in predicting long-term mortality (0.836; 95% confidence interval [CI]: 0.781–0.895; P < .001). After adjusting the model by adding clinically and statistically significant variables, the Hgb/Cr ratio was still an independent predictor of long-term mortality (0.886; 95% CI: 0.815–0.963; P = .004). The Hgb/Cr ratio’s discriminant ability was tested with an receiver operating characteristic curve analysis. The Hgb/Cr ratio’s area under the curve value was 0.679 (95% CI: 0.609–0.750; P < .001). A survival analysis using the Kaplan–Meier curve of the 2 Hgb/Cr ratio groups (according to cutoff value) revealed that the low-Hgb/Cr group had a significantly higher mortality rate than high-Hgb/Cr group. The Hgb/Cr ratio was found to be an independent predictor of long-term mortality in ACS patients.
Keywords: anemia, creatinine, hemoglobin, hemoglobin to creatinine ratio, mortality
1. Introduction
Acute coronary syndrome (ACS) is an urgent clinical condition of cardiovascular diseases, embracing ST-elevation myocardial infarction (STEMI), non-ST-elevation myocardial infarction (NONSTEMI), and unstable angina. According to 2021 data from the American Heart Association, 805,000 people experience ACS in the United States annually, including 605,000 new and 200,000 recurrent attacks.[1,2]
Many scorings and risk assessment algorithms have been developed to predict and reduce the mortality of coronary artery disease (CAD). The evaluated values of scores such as Killip and Pursuit in ACS patients were identified entirely by clinical findings, whereas scores such as GRACE and CRUSADE were further supported by laboratory findings.[3–6] Both hemoglobin and serum creatinine are parameters used in CAD scoring and algorithms.
Anemia, defined as a reduction of the red blood cells that are responsible for tissue oxygenation, is a disease that increases the heart’s workload due to cardiovascular compensation. In a review by Guedeney et al[7] that analyzed 15 studies, anemia was found to be 10.5% to 46.4% more common in patients with ACS.[8]
The other parameter, serum creatinine, is a laboratory finding used in the diagnosis and follow-up of chronic kidney disease (CKD) in clinical practice. The frequency of chronic kidney injury which is known to increase cardiovascular events for many reasons, such as chronic inflammation, oxidative stress, endothelial dysfunction, and calcification increases up to 20%-25% in ACS patients.[9,10]
As suggested by these findings, CAD is often accompanied by anemia and impaired renal function. This association, which is frequently seen in patients with ACS, has been evaluated in studies, and a relationship has been shown of anemia and impaired renal function with increased early and long-term mortality and adverse outcomes in patients with ACS.[7,11–15]
Hemoglobin and serum creatinine are 2 simple parameters that are routinely evaluated in patients presenting with ACS. The hemoglobin to serum creatinine ratio (Hgb/Cr) ratio, representing the proportion of the 2 values, is cheap, practical, and easy calculate at the bedside. Our study evaluated the predictive effect of Hgb/Cr ratio on long-term mortality in patients with ACS.
2. Materials and methods
2.1. Patients
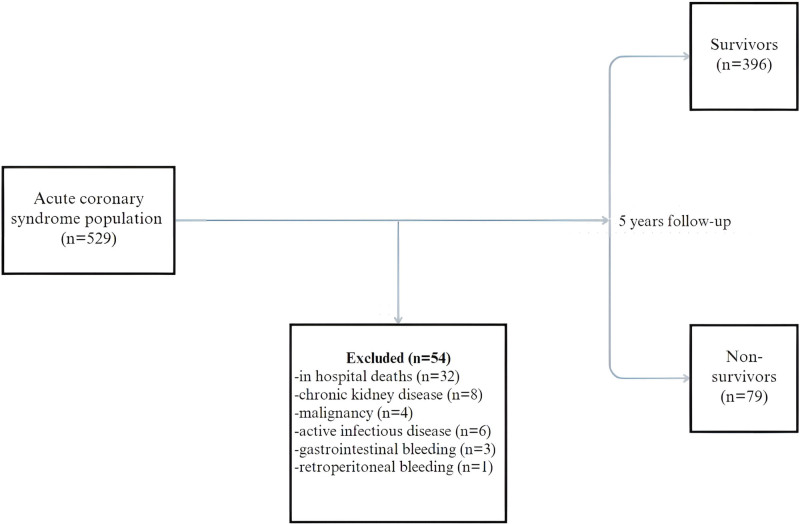
Using a hospital electronic data information system and patient files, this cross-sectional study retrospectively analyzed 529 patients who were diagnosed with ACS by a cardiologist, were hospitalized in coronary intensive care, and underwent coronary angiography from January 2015 through January 2018. STEMI and NONSTEMI patients aged 18 to 90 years were included in the study. Fifty-four patients were excluded who had active malignancy, severe hepatic and renal failure (typically a serum creatinine > 2.0 mg/dL), active or recent internal bleeding, known bleeding diathesis, or other significant comorbidities as well as patients who died in hospital or received erythrocyte transfusion upon hospitalization (Fig. 1).
Figure 1.
Diagram of the patient flowchart.
2.2. Data collection
We analyzed the patients’ peripheral venous blood samples in the hematology laboratory, and complete blood count parameters were calculated by an automated blood counter (Beckman Coulter, Brea, CA). Urea, creatinine, glomerular filtration rate (GFR), albumin, alanine aminotransferase, total cholesterol, high-density lipoprotein, low-density lipoprotein (LDL), total protein, troponin I, and blood glucose levels were analyzed in the biochemistry laboratory for all patients upon admission.
The patients demographic characteristics and clinical and laboratory data were recorded. Medical treatments, echocardiographic and angiographic findings, and in hospital and long-term endpoints were collected from the electronic hospital information system, patient files, and the hospital’s archive. The patients date-of-death information was obtained via the hospital data system.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (Non-interventional Clinical Research Ethics Committee, decision no. 2023/07-27).
2.3. Statistical analysis
We analyzed the data using SPSS version 23.0 for Windows (IBM Corp., Armonk, NY). The parametric data are presented as mean 6 standard deviations and as medians (minimum to maximum) as appropriate. The categorical variables are shown as numbers and percentages. The differences among the categorical variables of the groups were analyzed using the chi-squared test. The normality of distribution was investigated with the Shapiro–Wilk test for parametric data, and the homogeneity of the data was analyzed using Levene test. The differences in the numerical variables for the independent groups were analyzed with either the t test or the Mann–Whitney U test. The discriminant abilities and cutoff points of the Hgb/Cr ratio in predicting long-term mortality were compared using the area under the receiver operating characteristic.
The survival analysis was done using the Kaplan–Meier product limit estimate method according to cutoff value. Univariate and multivariate Cox regression models were used to evaluate the independent association of the Hgb/Cr ratio with long-term mortality; the unadjusted and adjusted hazard ratios are reported with their respective 95% confidence intervals (CIs). Clinically and statistically significant variables were also included in the variants in the Cox regression analysis. The level of significance was set at P < .05.
3. Results
Our study included 475 patients, 128 (27%) women and 347 (73%) men, who were hospitalized with a diagnosis of ACS and underwent coronary angiography. The mean age was 59.4 ± 13 years, and the mean follow-up period was 53.8 ± 16.3 months. It was observed that 79 (16.6%) of the patients died during follow-up. The patients were divided into 2 groups as survivors and non-survivors.
Age was higher in non-survivors than in survivors (70.4 ± 12.8 years vs 57.2 ± 11.9 years, respectively; P < .001), also ejection fraction was lower [44 (15–68) vs 50.4 (20–70), respectively; P < .001]. It was observed that female gender (24.2% vs 41.8%; P = .001), history of hypertension (HT) (29.8% vs 43%; P = .021), and history of cerebrovascular event (2% vs 7.6%; P = .017) were significantly associated with mortality. When the medications of the patients were compared, it was found that statin (44.3% vs 58.1%; P = .024) and antiaggregant therapy (89.9% vs 98%; P = .002) usage rates were significantly lower in non-survivors. A comparison of the patients laboratory findings revealed that hemoglobin [14.2 (8.1–18.3) vs 13.1 (8.5–17.2); P < .001], serum creatinine [0.91 (0.25–1.56) vs 1.02 (0.5–1.94); P = .001], blood glucose [154 (57–453) vs 185.4 (83–452); P = .011] values and Hgb/Cr ratios [16.6 (7.7–49) vs 13.8 (4.91–32.8); P < .001] differed significantly between the survivor and non-survivor group, respectively. Table 1 shows the baseline clinical, demographic, and laboratory characteristics of the study population according to survival status. There were no significant differences in myocardial infarction (MI) type, lipid profile, white blood cell, platelet, or troponin parameters between the survivor and non-survivor upon admission.
Table 1.
Baseline characteristics of the acute coronary syndrome population according to survival.
| Characteristics | Survivors (n = 396) | Non-survivors (n = 79) | P value |
|---|---|---|---|
| Age (yr), mean ± SD | 57.2 ± 11.9 | 70.4 ± 12.8 | <0001 |
| Sex, female % | 24 | 41.8 | .001 |
| Diabetes mellitus, % | 27.3 | 34.2 | .214 |
| Hypertension, % | 29.8 | 43 | .021 |
| Prior cerebrovascular event, % | 2 | 7.6 | .017 |
| Peripheral artery disease, % | 0.8 | 3.8 | .061 |
| MI type (STEMI), % | 58.1 | 50.6 | .870 |
| Antiaggregants, % | 98 | 89.9 | .002 |
| Beta-blockers, % | 48.7 | 45.6 | .607 |
| ACE inhibitors- ARB, % | 72.7 | 70.9 | .738 |
| Statins, % | 58.1 | 44.3 | .024 |
| Ejection fraction (%), median (min–max) | 50.4 (20–70) | 44 (15–68) | <.001 |
| Blood glucose (mg/dL), median (min–max) | 154 (57–453) | 185.4 (83–452) | .011 |
| Serum creatinine (mg/dL), median (min–max) | 0.91 (0.25–1.56) | 1.02 (0.5–1.94) | .001 |
| Total cholesterol (mg/dL), median (min–max) | 184 (87–402) | 180.4 (82–299) | .558 |
| LDL-C(mg/dL), median (min–max) | 114.2 (21.20–330) | 115.7 (37–221) | .780 |
| HDL-C (mg/dL), median (min–max) | 38.6 (5–85) | 38.9 (18–68) | .790 |
| Triglyceride (mg/dL), median (min–max) | 156.6 (11–896) | 127.7 (36–381) | .004 |
| WBC (×103 µL), median (min–max) | 11 (4.1–26.9) | 11.5 (4.7–22.4) | .311 |
| Hemoglobin (g/dL), median (min–max) | 14.2 (8.1–18.3) | 13.1 (8.5–17.2) | <.001 |
| Platelet (×103 µL), median (min–max) | 235.4 (56–566) | 247.2 (64–542) | .227 |
| Neutrophyl (×103 µL), median (min–max) | 7.9 (2.2–23.4) | 8.7 (0.4–20.4) | .099 |
| ALT (U/L), median (min–max) | 29.9 (9–369) | 26.7 (7–116) | .358 |
| Albumin (g/L), median (min–max) | 40.6 (20–52.6) | 39.2 (28–47.7) | .088 |
| Troponin (ng/mL), median (min–max) | 9837 (0.17–99,000) | 8508 (0.20–94,000) | .625 |
| Hgb/Cr ratio, median (min–max) | 16.6 (7.7–49) | 13.8 (4.91–32.8) | <.001 |
ACE = angiotensin-converting enzyme, ALT = alanine aminotransferase, ARB = angiotensin receptor blocker, HDL-C = high-density lipoprotein-cholesterol, Hgb/Cr = hemoglobin to-serum creatitine ratio, LDL-C = low density lipoprotein-cholesterol, MI = myocardial infarction, STEMI = ST elevated myocardial infarction, WBC = white blood cell.
According to the univariate Cox regression analysis, the Hgb/Cr ratio was statistically significant in predicting long-term mortality (0.836; 95% CI: 0.781–0.895; P < .001). After adjusting the model by adding clinically and statistically significant variables (age, gender, ejection fraction, HT, history of diabetes mellitus [DM], cerebrovascular event, LDL, statin, and antiaggregant use), the Hgb/Cr ratio remained an independent predictor of long-term mortality (0.886; 95% CI: 0.815–0.963; P = .004) (Table 2).
Table 2.
Unadjusted and adjusted Cox proportional hazards model for post-acute coronary syndrome 5 year all-cause mortality.
| Variables | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% Cl) | P value | HR (95% Cl) | P value | |
| Age | 1.082 (1.061–1.102) | <.001 | 1.062 (1.037–1.088) | <.001 |
| Gender | 0.481 (0.308–0.753) | .001 | NS | |
| HT | 1.707 (1.093–2.665) | .019 | NS | |
| DM | 1.329 (0.835–2.116) | .230 | NS | |
| Prior CVE | 3.556 (1.545–8.182) | .003 | NS | |
| LDL- cholesterol | 1.001 (0.995–1.006) | .749 | NS | |
| Ejection fraction | 0.955 (0.935–0.975) | <.001 | 0.960 (0.938–0.983) | .001 |
| Antiaggregants | 0.229 (0.110–0.477) | <.001 | NS | |
| Statins | 0.592 (0.380–0.923) | .021 | NS | |
| Hgb/Cr ratio | 0.836 (0.781–0.895) | <.001 | 0.886 (0.815–0.963) | .004 |
CVE = cerebrovascular event, DM = diabetes mellitus, Hgb/Cr = Hemoglobin to-serum creatinine ratio, HT = hypertension, LDL = low density lipoprotein.
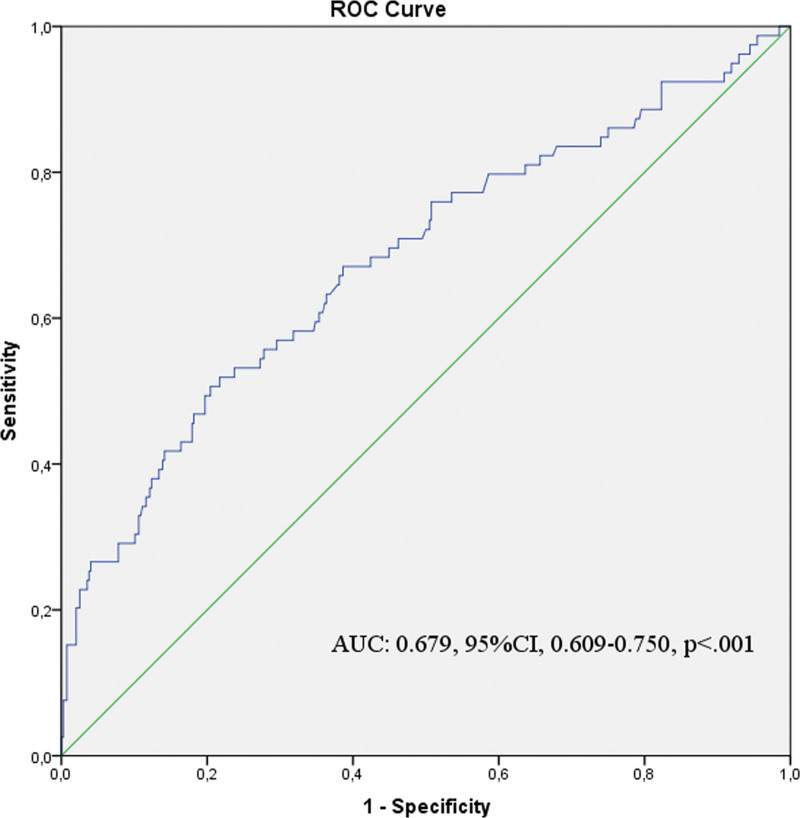
The Hgb/Cr ratio’s discriminant ability was tested with receiver operating characteristic curve analysis. The Hgb/Cr ratio’s area under the curve value was 0.679 (95% CI: 0.609–0.750; P < .001). The cutoff value of the Hgb/Cr ratio was 14.86, with a sensitivity of 63.7% and a specificity of 64.1% (Fig. 2).
Figure 2.
Receiver–operating characteristic (ROC) curve analysis plot to determine the cutoff value of Hgb/Cr ratio in the prediction of mortality.
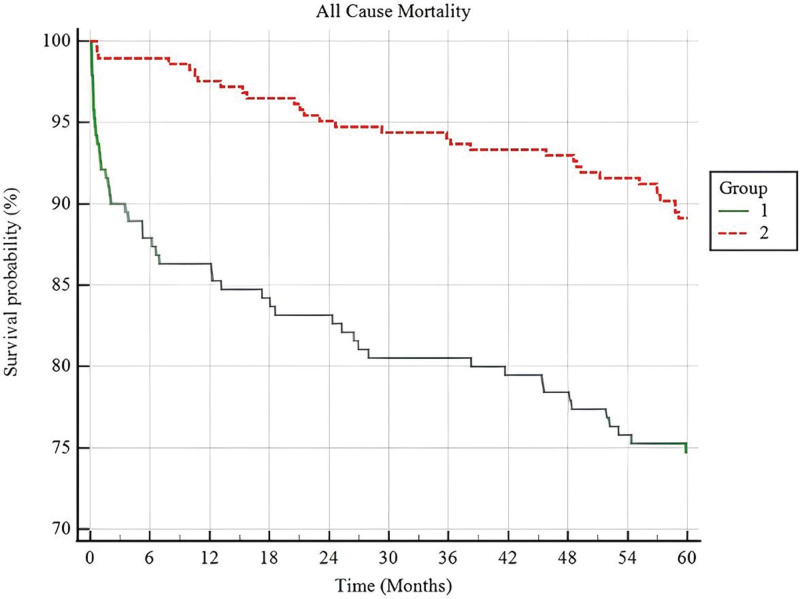
A survival analysis of the 2 Hgb/Cr ratio groups (according to cutoff value) using the Kaplan–Meier curve revealed that the low-Hgb/Cr group had a significantly higher mortality rate than the high-Hgb/Cr group (Fig. 3).
Figure 3.
Kaplan–Meier survival curves of 5-year mortality post-ACS according to Hgb/Cr ratio groups. ACS = acute coronary syndrome.
4. Discussion
Our study demonstrated the effect of Hgb/Cr ratio on long-term mortality in patients with ACS, defined as a sudden decrease in blood flow to the heart that is often caused by rupture of the lipid-burden coronary artery plaque followed by platelet aggregation and coronary artery obstruction.[1,16] The most important risk factors for ACS have been identified as age, smoking, DM, HT, hyperlipidemia, and body mass index. The incidence of CAD patients is expected to increase in the future due to obesity, unhealthy lifestyles, and especially the aging population.[1,17] In the past century, the average survival rate in patients with ACS has improved with the increase in medical treatment agents that have demonstrated an effect on mortality in CAD and with the advance of percutaneous intervention methods. However, CAD still accounts for about 1-3rd of all deaths worldwide.[17] Many blood parameters have shown an association with mortality in ACS patients, including high blood sugar at admission, increased troponin levels, increased inflammatory markers, low hemoglobin level, and increased serum creatinine level.[18–20]
Anemia is defined as a decrease in red blood cells and is evaluated by a decrease in hemoglobin in clinical practice. It is common both in the general population and in CAD patients and is a disease with a multifactorial etiology. Anemia, which various randomized studies have found to be present in 10% to 43% of ACS patients, is associated with poor prognosis in both the early and late periods of ACS.[21,22]
Anemia in ACS patients may predate hospitalization or develop during or after hospitalization. Anemia in CAD patients is typically normocytic, normochromic, and hypo-proliferative,[22] and it can have many causes. Although a slight increase in erythropoietin (EPO) levels has been observed in CAD patients, it remains quite low compared to anemia levels.[22–24] In addition, about 10% to 20% of these patients have erythropoietin stimulating agent resistance, and decreased EPO levels may also lead to a decrease in the cardioprotective effects of EPO.[21–24]
Other important causes of anemia include the hemodilution observed in heart failure patients and the use of antiaggregants and anticoagulants in ACS patients. Especially in the elderly ACS population, nutritional deficiencies, such as iron, b12, and folic acid, often play a more prominent role in the etiology of anemia.[21,25] Because anemic patients are often older and often use insufficient antiaggregant or anticoagulants, patient profile also has a negative impact on prognosis.[21,26]
Furthermore, inflammatory cytokines that occur in ACS patients, such as IL-1, IL 6, and TNFα, affect anemia through the inflammatory response by decreasing the red blood cells half-life, lowering the EPO level and reducing iron bioavailability. The anemia of chronic disease can also be seen in ACS through the same pathways.[21,27] Anemia leads to a decrease in myocardial oxygen supply, exacerbation of ischemia, and formation of myocardial necrosis and fibrosis, ultimately adversely affecting prognosis in patients with CAD.[12,21]
The Myocardial Ischemia National Audit Project registry published by Mamas et al[28] evaluated the relationship between anemia and mortality in patients with ACS. Anemia was found to be independently associated with 30-day and 1-year mortality in ACS patients, and a significant correlation was observed between hemoglobin levels and death outcomes. A review by Sabatine et al[12] analyzed about 40,000 patients included in 16 clinical ACS studies to compare the levels of anemia and cardiovascular outcomes. It found that hemoglobin under 14 mg/dL in STEMI patients significantly and progressively increased cardiovascular mortality and congestive heart failure, while hemoglobin under 11 mg/dL in NONSTEMI patients also showed an independent relationship with death, MI, and recurrent ischemia. In a study involving 936 patients, female patients with suspected ischemia were compared to evaluate anemia and adverse cardiovascular outcomes. Anemia was identified as an independent predictor of adverse cardiovascular outcomes, with the risk increasing by 20% for each 1 g/dL reduction in hemoglobin.[29]
Serum creatinine concentration, a common measure of glomerular filtration rate, is a clinical indicator of kidney function.[10] Age, DM, HT, and smoking, which are risk factors for CAD, are also risk factors for CKD. Additionally, CKD has been identified as a major risk factor for CAD.[2,3,30]
When prospective studies of chronic kidney patients are evaluated, cardiac events and increased incidence of CAD can be explained by changes in fibrinogen and homocysteine metabolism, increased lipoprotein (a) levels, hypoalbuminemia, increased inflammatory mediators, increased oxidized LDL, high-density lipoprotein dysfunction, the development of micro- and macrocalcification in coroner arteries, and decreased plaque stabilization.[30–32] Intimal calcification, which leads to a high frequency of cardiac events, is more common in patients with advanced CKD, but medial calcification due to a longer hemodialysis time and serum calcium phosphate abnormalities is a more common finding in patients of younger age.[33,34] The extent of coronary arterial calcification in CKD patients has been associated with a greater coronary plaque burden compared to the general population. In addition, the chronic inflammatory process in CKD patients, the inhibition of nitrite oxide synthesis, and uremic myocardial damage without coronary stenosis are other leading causes of adverse cardiovascular events in these patients.[34]
Factors that may affect the prognosis of CAD patients with CKD include a delay in diagnosis due to atypical findings, the defensive approach that is occasionally taken due to concerns about contrast nephropathy in invasive procedures, and the insufficient use of drugs such as antiplatelets, angiotensin-converting enzyme inhibitors, etc.[35]
There are many studies on the negative prognostic effect of impaired renal function in patients with ACS.[36] An article by Reddan et al[37] analyzed 13,707 ACS patients from 2 clinical studies and found that every 10 mL increase in GFR reduced mortality in these patients. Another study, the Valsartan in Acute Myocardial Infarction Trial (VALIANT), included 14,527 ACS patients and found that every 10-unit reduction in estimated GFR was associated with an increased risk ratio (1:10) for death and nonfatal cardiovascular outcomes.[38] A study by Qi et al[35] included 1840 patients who were categorized and compared in 3 groups as GFR > 90, GFR 60 to 90, and GFR < 60. A low GFR was associated with an unfavorable prognosis and high mortality.
In Numasawa et al[39] comprehensive a Japanese Nationwide Registry study, the relationship between hemoglobin creatinine ratio, a new parameter, and in hospital mortality was evaluated in 157,978 patients who did not undergo dialysis but underwent percutaneous coronary intervention. It found that the Hgb/Cr ratio was independently associated with both in hospital mortality and bleeding complications. A study by Çamci et al[40] compared contrast nephropathy and Hgb/Cr in 500 patients who underwent percutaneous coronary intervention and found that the Hgb/Cr ratio is also an independent predictor of contrast nephropathy.
ACS is a clinical condition with high early and late mortality. The aim of treatment in these patients is to reduce mortality in the late as well as the early period, so it is crucial to conduct risk assessment in these patients starting from diagnosis. In this respect, the ratio of hemoglobin and serum creatinine, which are routinely evaluated in each patient, can provide additional information for risk scoring as a simple, easily calculated bedside evaluable parameter. In patients with CAD, who are often more anemic, older, and have higher rates of inadequate treatment due to impaired renal function, such an assessment can inform the planning of closer follow-up and the selection of more intensive treatment strategies for those with a higher risk of mortality.
5. Limitations
This study has several limitations. First, the study was designed as a retrospective study. Second, our number of patients is inadequate, so there is a need for studies with a larger number of patients. Third, we did not have accurate data on the individual etiology of anemia and CKD in patients with low hemoglobin and high creatinine levels. Fourth limitation of the study is that the discharge hemoglobin and serum creatinine parameters of the patients are not routinely checked and their data are incomplete, and they are not included in the study due to the lack of follow-up data. The last, there were insufficient data on whether individual CAD and heart failure treatment were optimal.
6. Conclusion
The Hgb/Cr ratio was found to be an independent predictor of long-term mortality in ACS patients. The Hgb/Cr ratio is a simple, inexpensive bedside index and appears to be successful in mortality scoring or risk assessment. It can be widely used in daily practice in the near future with the support of prospective studies with larger patient numbers.
Author contributions
Conceptualization: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Data curation: Mevlut Demir, Taner Sen, Mehmet Ali Astarcioglu.
Formal analysis: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Funding acquisition: Mevlut Demir, Fatih Kahraman, Taner Sen.
Investigation: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Methodology: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Project administration: Mevlut Demir, Taner Sen, Mehmet Ali Astarcioglu.
Resources: Mevlut Demir, Fatih Kahraman.
Software: Mevlut Demir, Fatih Kahraman, Mehmet Ali Astarcioglu.
Supervision: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Validation: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Visualization: Mevlut Demir, Fatih Kahraman, Taner Sen, Mehmet Ali Astarcioglu.
Writing – original draft: Mevlut Demir, Taner Sen, Mehmet Ali Astarcioglu.
Writing – review & editing: Mevlut Demir, Taner Sen, Mehmet Ali Astarcioglu.
Abbreviations:
- ACS
- acute coronary syndrome
- CAD
- coronary artery disease
- CI
- confidence intervals
- CKD
- chronic kidney disease
- DM
- diabetes mellitus
- EPO
- erythropoietin
- GFR
- glomerular filtration rate
- Hgb/Cr
- hemoglobin to-serum creatinine ratio
- HT
- hypertension
- LDL
- low density lipoprotein
- NONSTEMI
- non-ST-elevation myocardial infarction
- STEMI
- ST-elevation myocardial infarction
The authors had no funding and have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
How to cite this article: Demir M, Kahraman F, Sen T, Astarcioglu MA. The relationship of the hemoglobin to serum creatinine ratio with long-term mortality in patients with acute coronary syndrome: A retrospective study. Medicine 2023;102:41(e35636).
Contributor Information
Fatih Kahraman, Email: drfkahraman@gmail.com.
Taner Sen, Email: taner.sen@ksbu.edu.tr.
Mehmet Ali Astarcioglu, Email: mehmetali.astarcioglu@ksbu.edu.tr.
References
- [1].Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327:662–75. [DOI] [PubMed] [Google Scholar]
- [2].Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics–2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–743. [DOI] [PubMed] [Google Scholar]
- [3].Boersma E, Pieper KS, Steyerberg EW, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: results from an international trial of 9461 patients. Circulation. 2000;101:2557–67. [DOI] [PubMed] [Google Scholar]
- [4].Subherwal S, Bach RG, Chen AY, et al. Baseline risk of major bleeding in non–ST-segment–elevation myocardial infarction: the CRUSADE (can rapid risk stratification of unstable angina patients suppress AD verse outcomes with early implementation of the ACC/AHA guidelines) bleeding score. Circulation. 2009;119:1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit: a two-year experience with 250 patients. J Am Coll Cardiol. 1999;20:457–64. [DOI] [PubMed] [Google Scholar]
- [6].Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ. 2006;333:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guedeney P, Sorrentino S, Claessen B, et al. The link between anemia and adverse outcomes in patients with acute coronary syndrome. Expert Rev Cardiovasc Ther. 2019;17:151–9. [DOI] [PubMed] [Google Scholar]
- [8].Rymer JA, Rao SV. Anemia and coronary artery disease: pathophysiology, prognosis, and treatment. Coron Artery Dis. 2018;29:161–7. [DOI] [PubMed] [Google Scholar]
- [9].Di Mauro M, Fiorentini V, Mistrulli R, et al. Acute coronary syndrome and renal impairment: a systematic review. Rev Cardiovasc Med. 2022;23:49. [DOI] [PubMed] [Google Scholar]
- [10].Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–53. [PubMed] [Google Scholar]
- [11].Lawler PR, Filion KB, Dourian T, et al. Anemia and mortality in acute coronary syndromes: a systematic review and meta-analysis. Am Heart J. 2013;165:143–153.e5. [DOI] [PubMed] [Google Scholar]
- [12].Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–9. [DOI] [PubMed] [Google Scholar]
- [13].Santopinto JJ, Fox KA, Goldberg RJ, et al. Creatinine clearance and adverse hospital outcomes in patients with acute coronary syndromes: findings from the global registry of acute coronary events (GRACE). Heart. 2003;89:1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pitsavos C, Kurlaba G, Panagiotakos DB, et al. Association of creatinine clearance and in-hospital mortality in patients with acute coronary syndromes the GREECS study. Circ J. 2007;71:9–14. [DOI] [PubMed] [Google Scholar]
- [15].Masoudi FA, Plomondon ME, Magid DJ, et al. Renal insufficiency and mortality from acute coronary syndromes. Am Heart J. 2004;147:623–9. [DOI] [PubMed] [Google Scholar]
- [16].Higuma T, Soeda T, Abe N, et al. A combined optical coherence tomography and intravascular ultrasound study on plaque rupture, plaque erosion, and calcified nodule in patients with ST-segment elevation myocardial infarction: incidence, morphologic characteristics, and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:1166–76. [DOI] [PubMed] [Google Scholar]
- [17].Liu KT, Lee SWH, Selvaraj SG, et al. An update on acute coronary syndrome and myocardial infarction registries among member countries of the Asian Pacific Society of Cardiology. J Asian Pac Soc Cardiol. 2022;1:e27. [Google Scholar]
- [18].Antman EM, Tanasijevic MJ, Thompson B, et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–9. [DOI] [PubMed] [Google Scholar]
- [19].Heidenreich PA, Alloggiamento T, McDonald KM, et al. The prognostic value of troponin in patients with non-ST elevation acute coronary syndromes: a meta-analysis. J Am Coll Cardiol. 2001;38:478–85. [DOI] [PubMed] [Google Scholar]
- [20].Zhang S, Diao J, Qi C, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: a meta-analysis. BMC Cardiovasc Disord. 2018;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Satpathy C, Mohanty NK. Anemia in acute coronary syndrome: an overview. Indian J Cardiovasc Dis Women-Wincars. 2021;06:194–8. [Google Scholar]
- [22].Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012;23:1631–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vlagopoulos PT, Tighiouart H, Weiner DE, et al. Anemia as a risk factor for cardiovascular disease and all-cause mortality in diabetes: the impact of chronic kidney disease. J Am Soc Nephrol. 2005;16:3403–10. [DOI] [PubMed] [Google Scholar]
- [24].Sanchis-Gomar F, Garcia-Gimenez JL, Pareja-Galeano H, et al. Erythropoietin and the heart: physiological effects and the therapeutic perspective. Int J Cardiol. 2014;171:116–25. [DOI] [PubMed] [Google Scholar]
- [25].Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications, and management. Blood. 2018;131:505–14. [DOI] [PubMed] [Google Scholar]
- [26].Meneveau N, Schiele F, Seronde MF, et al. Anemia for risk assessment of patients with acute coronary syndromes. Am J Cardiol. 2009;103:442–7. [DOI] [PubMed] [Google Scholar]
- [27].Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. [DOI] [PubMed] [Google Scholar]
- [28].Mamas MA, Kwok CS, Kontopantelis E, et al. Relationship between anemia and mortality outcomes in a national acute coronary syndrome cohort: insights from the UK myocardial ischemia national audit project registry. J Am Heart Assoc. 2016;5:e003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Arant CB, Wessel TR, Olson MB. Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women’s Ischemia Syndrome Evaluation Study. J Am Coll Cardiol. 2004;13:12–2014. [DOI] [PubMed] [Google Scholar]
- [30].Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1823–38. [DOI] [PubMed] [Google Scholar]
- [31].Al Suwaidi J, Reddan DN, Williams K, et al. Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–80. [DOI] [PubMed] [Google Scholar]
- [32].Jankowski J, Floege J, Fliser D, et al. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143:1157–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. [DOI] [PubMed] [Google Scholar]
- [34].Widimsky P, Rychlik I. Renal disease and acute coronary syndrome. Heart. 2010;96:86–92. [DOI] [PubMed] [Google Scholar]
- [35].Qi L, Liu H, Cheng L, et al. Impact of renal insufficiency on prognosis of patients with acute coronary syndrome. Int J Gen Med. 2021;14:8919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rodrigues FB, Bruetto RG, Torres US, et al. Effect of kidney disease on acute coronary syndrome. Clin J Am Soc Nephrol. 2010;5:1530–6. [DOI] [PubMed] [Google Scholar]
- [37].Reddan DN, Szczech L, Bhapkar MV, et al. Renal function, concomitant medication use and outcomes following acute coronary syndromes. Nephrol Dial Transplant. 2005;20:2105–12. [DOI] [PubMed] [Google Scholar]
- [38].Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95. [DOI] [PubMed] [Google Scholar]
- [39].Numasawa Y, Inohara T, Ishii H, et al. Association of the hemoglobin to Serum creatinine ratio with In-hospital adverse outcomes after percutaneous coronary intervention among Non-dialysis patients: insights from a Japanese nationwide registry (J-PCI Registry). J Clin Med. 2020;9:3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Çamci S, Kinik M, Ari S, et al. The predictive value of hemoglobin to creatinine ratio for contrast-induced nephropathy in percutaneous coronary interventions. Clin Chem Lab Med (CCLM). 2022;60:1455–62. [DOI] [PubMed] [Google Scholar]