Abstract
Introduction:
A high salt diet is a significant risk factor for hypertension, and scholarly investigations into this relationship have garnered considerable attention worldwide. However, bibliometric analyses in this field remain underdeveloped. This study aimed to conduct a bibliometric and visual analysis of research progress on the link between high salt and hypertension from 2011 to 2022 with the goal of identifying future research trends and providing valuable insights for this field.
Methods:
High salt and hypertension data were obtained from the Web of Science Core Collection database. Microsoft Excel, Scimago Graphica, CiteSpace, and VOSviewer software were employed to analyze publication output trends, the most productive countries or regions, journals, authors, co-cited references, and keywords.
Results:
After screening, 1470 papers met the inclusion criteria. Relevant publications increased annually by 3.66% from 2011 to 2022. The United States led in research productivity, with The Journal of Hypertension publishing the most papers, and David L. Mattson as the most prolific author. Oxidative stress has emerged as a prominent research topic, and extensive investigations have been conducted on related mechanisms. “Oxidative stress,” “gut microbiota,” and “kidney injury” are recent hotspots that are expected to remain so, and this study carefully characterizes the mechanism of high salt-induced hypertension based on these hotspots.
Conclusion:
This study utilized bibliometric and visualization analysis to identify the development trends and hotspots of publications related to high salt and hypertension. The findings of this study offer valuable insights into the forefront of emerging trends and future directions in this field.
Keywords: bibliometric, gut microbiota, high salt, hypertension, kidney injury
1. Introduction
Hypertension, also known as high blood pressure, is a common medical condition, and it has emerged as a major global health challenge, affecting millions of individuals across the world.[1,2] It is difficult to detect and manage, leading to severe complications such as other cardiovascular diseases (CVDs) and kidney injury.[3,4] Over the past several decades, the number of people with high blood pressure in the world has increased by 90%.[5] In 2010, hypertension had already become the leading cause of global mortality,[6] and it is projected that by 2025, approximately 1.56 billion adults will be affected by hypertension.[7] In order to address this issue, the governments around the world have implemented various policies and measures over the past decades to prevent and control hypertension. For instance, one of the most significant policies implemented by the Chinese government in the past 2 decades is the establishment of the National Center for CVDs in 2000. Schemes of the center aim to enhance the prevention and control of CVDs such as hypertension, improve their diagnosis and treatment, and promote scientific research and education in the field.[8] Notwithstanding these endeavors, there are still challenges that need to be overcome in order to effectively tackle the issue of hypertension on a global scale. The increasing prevalence of hypertension in developing countries is closely related to people awareness and acceptance of treatment and their choice of treatment.[9] The situation demands sustained and coordinated efforts to promote hypertension prevention and management.
Numerous studies have linked high salt intake with hypertension, especially those affecting the cardiovascular system.[10,11] A series of studies that have attracted the attention of scholars in this field indicate that high sodium or salt intake is associated with the pathogenesis of hypertension.[12–14] Meanwhile, epidemiological and intervention studies on human subjects have also indicated that high salt intake is associated with increased blood pressure.[15] Reducing salt intake can lower blood pressure and decrease the risk of CVDs.[16] In 2003, the World Health Organization recommended a daily sodium intake of 2.0 grams for adults (equivalent to 5 grams of salt per day) based on the evaluation of the best available evidence.[17] Since 2011, an increasing number of scholars have started researching the relationship between high salt intake and hypertension. With the rapid development of new technologies and the impact of global health challenges, researchers of this field have made significant progress in researching the link between high salt intake and hypertension. In the domain, numerous articles have summarized in this field, particularly the mechanisms involved.[18–25] As a result, there is a growing necessity for a systematic analysis of past research in this area to gain a better understanding of current research trends and hotspots. Up to now, a systematic summary of these abundant papers has yet to be compiled.
Bibliometric analysis is an emerging, important, and fundamental research analysis method used in the scientific research field. In the early 21st century, bibliometric analysis significantly developed with the promotion of the internet.[26] Combined with visual analysis, it highlights the impact of a large number of relevant studies on the field, and presents meaningful insights into research prospects. In the medical field, papers based on bibliometric analysis to investigate the current state of a particular topic have garnered the interest of academics.[27,28] It uses quantitative statistics to summarize the current status, trends, and hotspots of research topics, as well as the contributions of authors, journals, institutions, or countries.[29] Recently, scholars have used bibliometric analysis to examine the effects of certain dietary habits on physiology or pathology, such as ketogenic diet therapies for neurological diseases and cancer,[30,31] the Mediterranean diet on cancer,[32] and time-restricted eating.[33] Additionally, some scholars have conducted bibliometric analyses in certain areas related to CVDs, such as exosomes in CVD,[34] the gut microbiome and coronary heart disease and the use of acupuncture in heart disease.[35,36] These articles have played a positive role in advancing research in these fields. While scholars have conducted bibliometric analyses on global research trends, there have been few studies in the field of high salt and hypertension.[37] In this study, we conducted a comprehensive bibliometric analysis of research literature on high salt and hypertension from 2011 to 2022, visualizing trends in annual publications, countries, international collaborations, journals, and authors. Additionally, we performed keyword analysis to identify hotspots in research on hypertension related to high salt. This study has the potential to provide insights into the emerging trends and cutting-edge future directions in this field.
2. Materials and Methods
2.1. Data retrieval and collection
All literatures were extracted from Clarivate analysis Web of Science Core collection (WoSCC) Database including editions of SCI-EXPANDED (2003-present) and SSCI (2003-present) via the Chengdu University of Traditional Chinese Medicine Library website. The WoSCC, covering more than 12,000 of high-quality scientific journals, is known to harbor relatively reliable database and also considered as the optimal database in previous bibliometric studies.[38,39] Only articles and reviews written in English were included from 2011 to 2022 (retrieved on February 24, 2023). The retrieved papers were exported and saved as plain text files that stored in download_txt format. The detail of search strategy and literature filtering are presented in Supplementary Table 1, http://links.lww.com/MD/K174 and Figure 1, respectively.
Figure 1.
Flow chart of literature retrieval research.
2.2. Data analysis
All valid documents retrieved from Web of Science Core Collection were converted to Microsoft Excel 2019, VOSviewer, and CiteSpace to perform visual analysis. VOSviewer 1.6.18 was employed to scrutinize nations, regions, and authors. Utilizing network visualization, VOSviewer categorizes countries, regions, or authors into distinct clusters and colors them based on the temporal progression of their appearance, superimposing time onto the co-occurrence networks of countries, organizations, or authors. Data concerning national cooperation were transformed into GML format by VOSviewer and imported into Scimago Graphica Beta software 1.0.28 to display geographic distribution and national clusters. CiteSpace software 6.1.R6 was used to analyze co-cited references and keywords. In the co-occurrence graph, the size of the circle represents the number of co-citations, and centrality and burstiness were prominently denoted by the outer purple and red rings of the nodes, respectively. Key points in CiteSpace are nodes with centrality >0.1. The co-occurring keyword cluster analysis utilized the long-likelihood ratio algorithm. In the clustering diagram, a square enclosed the members of the same cluster, and the cluster name was displayed based on the cluster proportion. We used Microsoft Office Excel 2019 to analyze the trend of the number of articles published in the year.
3. Result
3.1. The trend of publication outputs
The annual number of published papers reflects the pace of subject knowledge and is a significant indicator for studying the trends in the field. It shows the worldwide trend in publications and cumulative number of publications about the relationship between hypertension and high salt diet from 2011 to 2022 in Figure 2. We can draw directly that the number of related literatures has increased year by year on the whole in these years. Especially, there was a noticeable increase in the annual volume of pertinent publications, and more than 100 publications were released annually every year. The result indicates that the research interest in this field has been steadily increasing.
Figure 2.
The annual publication and cumulative number of publications.
3.2. Distribution of countries/regions
Analyzing the distribution of published articles can help identify high-output countries or regions that have contributed to the field. The publications relating to this topic that have been published are mainly distributed in 82 different nations/regions. There were 26 nations included in our analysis of global cooperation using the VOSviewer when the lowest number of papers was restricted to more than 5. According to the global productivity map geographical distribution, as shown in Figure 3A, these papers were primarily published in North America, Asia, and European nations. Table 1 lists the top ten nations/ regions relating to this topic, which also shows that USA produced the most publications (36.547%, 561), followed by China (24.756%, 380), and Japan (15.309%, 235). The publications from United States have the most citations of 14,925 times. Each node represents a country/region, and the node size is proportional to the number of the publications. Line thickness between nodes indicates link strength of a collaboration. Total cooperation intensity (TCI) refers to the thickness of the lines connecting nodes, representing the level of international co-authorship.[40] Regarding international cooperation between countries/regions, USA enjoys cooperation from many countries, among which the 2 most important countries are China and Japan, respectively (Fig. 3A). It shows that the co-authorship visualization map showed that the top 5 TCI were the USA (TCI = 293), China (TCI = 148), Germany (TCI = 87), Japan (TCI = 73) and Australia (TCI = 61) in Figure 3B. This discovery suggests that these countries may have played a crucial role in the research of this field.
Figure 3.
Distribution of countries/regions. (A) The cluster of all contributing countries by Scimago Graphica. (B) Country/region collaboration map generated by Scimago Graphica software. Each node represents a country/region, and the node size is proportional to the number of the publications. Line thickness between nodes indicates link strength of a collaboration. Total cooperation intensity (TCI) refers to the thickness of the lines connecting nodes, representing the level of international co-authorship.
Table 1.
The top 10 countries or regions contributing to publications.
| Rank | Country | Counts | Percentage | Citations | Average citation per paper | Total link strength |
|---|---|---|---|---|---|---|
| 1 | USA | 561 | 0.365472313 | 14925 | 26.60427807 | 561 |
| 2 | China | 380 | 0.247557003 | 4908 | 12.91578947 | 380 |
| 3 | Japan | 235 | 0.153094463 | 4256 | 18.1106383 | 235 |
| 4 | Germany | 74 | 0.048208469 | 3400 | 45.94594595 | 74 |
| 5 | Australia | 61 | 0.039739414 | 1458 | 23.90163934 | 61 |
| 6 | Brazil | 49 | 0.031921824 | 713 | 14.55102041 | 49 |
| 7 | Canada | 49 | 0.031921824 | 1069 | 21.81632653 | 49 |
| 8 | England | 49 | 0.031921824 | 2059 | 42.02040816 | 49 |
| 9 | Netherlands | 39 | 0.025407166 | 716 | 18.35897436 | 39 |
| 10 | France | 38 | 0.0247557 | 1348 | 35.47368421 | 38 |
3.3. Journals and co-cited academic journals
The analysis of the distribution of the source of published articles is helpful to identify highly influential academic journals. A total of 1470 articles related to this field were published in 417 academic journals. The journal of HYPERTENSION (96, 6.53%) had the highest number of outputs, followed by AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY (60, 4.08%), JOURNAL OF HYPERTENSION (53, 3.60%), HYPERTENSION RESEARCH (52, 3.53%), and Scientific Reports (48, 3.27%). Among the top 10 journals, HYPERTENSION has the highest impact factor (IF: 9.897), followed by NUTRIENTS with an IF of 6.706 (Table 2). Co-citation analysis is designed to measure the degree of relationship between articles. The impact of a journal depends on its co-citation frequency, which reflects the influence of a journal in a specific research field. Among 5591 co-cited journals, 11 journals were cited over 1000 times. As is shown in Table 2, HYPERTENSION (7227) was the most frequently cited journal, followed by AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY (2527) and JOURNAL OF HYPERTENSION (1812). Among the top 10 journals, CIRCULATION has the highest IF (39,918), followed by CIRCULATION RESEARCH with an IF of 23.213. The dual-map overlay of journals shows the distribution of relationships between journals, citing journals on the left and cited journals on the right. The colored paths between them indicate the cited relationships. As is shown in Figure 4, there are 4 main citation paths, including 2 orange paths and 2 green path. The orange path indicates that studies published in Molecular/Biology/Genetics journals and Health/Nursing/Medicine journals are cited for studies in Molecular, Biology Immunology journals. The green path means that studies published in Molecular/Biology/Genetics journals and Health/Nursing/Medicine journals are also cited for studies in Medicine/Medical/Clinical journals. There were 4 colored primary citation pathways in this map. These findings suggest that the research in this field has a high impact and its focus may be on studying related mechanisms.
Table 2.
Top 10 journals and co-cited journals related to hypertension concerned with high salt.
| RANK | Journal | Documents | Count | IF (2022) | JCR | Co-cited journal | Citation | IF (2022) | JCR |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Hypertension | 96 | 6.50% | 9.897 | Q1 | Hypertension | 7227 | 9.897 | Q1 |
| 2 | American Journal of Physiology-Renal Physiology | 60 | 4.10% | 4.097 | Q2 | American Journal of Physiology-Renal Physiology | 2527 | 4.097 | Q2 |
| 3 | Journal of Hypertension | 53 | 3.60% | 4.776 | Q2 | Journal of Hypertension | 1812 | 4.776 | Q2 |
| 4 | Hypertension Research | 52 | 3.50% | 5.528 | Q2 | Circulation | 1593 | 39.918 | Q1 |
| 5 | Plos One | 48 | 3.30% | 3.752 | Q2 | Kidney Int | 1427 | 18.998 | Q1 |
| 6 | American Journal of Hypertension | 35 | 2.40% | 3.08 | Q3 | American Journal of Physiology-Heart and Circulatory Physiology | 1381 | 5.125 | Q1 |
| 7 | American Journal of Physiology-Regulatory Integrative and Comparative Physiology | 32 | 2.20% | 3.21 | Q2 | Journal of the American Society of Nephrology | 1367 | 14.978 | Q1 |
| 8 | Nutrients | 30 | 2.00% | 6.706 | Q1 | American Journal of Physiology-Regulatory Integrative and Comparative Physiology | 1307 | 3.21 | Q2 |
| 9 | Scientific Reports | 29 | 2.00% | 4.996 | Q2 | Circulation Research | 1152 | 23.213 | Q1 |
| 10 | American Journal of Physiology-Heart and Circulatory Physiology | 26 | 1.80% | 5.125 | Q1 | Journal of Clinical Investigation | 1135 | 19.456 | Q1 |
IF = impact factor.
Figure 4.
The dual-map overlay of journals on hypertension related to high salt.
3.4. Authors and co-cited authors
Analyzing the most prolific/cited authors in a certain field can help us understand the current research hotspots and trends in that field. The top 10 most productive authors in the field of hypertension concerned with high salt are presented in Table 3. Among them, Mattson, David L. in USA ranked first (n = 25) is the most prolific author, closely followed by Kang, Yu-Ming in China (n = 24). Furthermore, Fujita, Toshiro, Cowley, Allen W., Jr, and Mattson, David L were the top 3 authors with the highest average number of citations (36 vs 35 vs 33 times, respectively). A co-authorship overlay visualization map was generated using VOSviewer software, and the threshold for the minimum number of documents by an author was set to 5. The color of each circle corresponds to the average publication year of the author, the size of a circle is proportional to the number of literatures published by the author, and the thickness of the connecting line indicates the cooperation frequency. Totally, 222 authors who met the threshold were identified, and the largest set of connected items consists of 154 items that shows in Figure 5A. Kang, Yu-Ming and Yu, Xiao-Jing were shown to have cooperated closely. The size of a circle is proportional to the total number of citations of the author, and the thickness of the connecting line indicates the strength of the co-citation link.[41] A citation visualization map (Fig. 5B) was also generated using VOSviewer software, and the threshold for the minimum number of citations of an author was set to 50. There were 89 authors who met the threshold identified, and it could be seen that He, Feng J., Weinberger, Myron H., and Mattson, D.L. had made significant contributions to the field. In fact, the contributions of these authors have had a considerable impact on the field.
Table 3.
The top 10 most productive authors in the field.
| Rank | Author | Country | Article counts | Total number of citations | Average number of citations |
|---|---|---|---|---|---|
| 1 | Mattson, David L. | USA | 25 | 837 | 33.48 |
| 2 | Kang, Yu-Ming | China | 24 | 395 | 16.45833333 |
| 3 | Mu, Jian-Jun | China | 22 | 281 | 12.77272727 |
| 4 | Fujita, Toshiro | Japan | 21 | 756 | 36 |
| 5 | Cowley, Allen W., Jr. | USA | 21 | 743 | 35.38095238 |
| 6 | Geurts, Aron M. | USA | 19 | 569 | 29.94736842 |
| 7 | Nishiyama, Akira | Japan | 19 | 349 | 18.36842105 |
| 8 | Wang, Yang | China | 19 | 216 | 11.36842105 |
| 9 | Liang, Mingyu | USA | 18 | 541 | 30.05555556 |
| 10 | Pollock, David M. | USA | 18 | 283 | 15.72222222 |
Figure 5.
Authors and co-cited authors visualization maps. (A) Author co-authorship overlay visualization map. The color of each circle corresponds to the average publication yr of the author, the size of a circle is proportional to the number of literatures published by the author, and the thickness of the connecting line indicates the cooperation frequency. (B) Author co-citation visualization map. The size of a circle is proportional to the total number of citations of the author, and the thickness of the connecting line indicates the strength of the co-citation link.
3.5. Co-cited references and references burst
Co-citation analysis indicated that 2 references appeared in the reference list of a third citation article, and then the 2 references formed a co-citation relationship. In Supplementary Table 2, http://links.lww.com/MD/K175, we listed the 20 most frequently cited references related to research on this field. Among the 46,300 cited references, 20 references were cited more than 50 times (Supplementary Table 2, http://links.lww.com/MD/K175). Figure 6 shows the top 25 references with the most robust citation bursts. It can be seen that the first reference with citation bursts was in 2011. In recent years, the majority of hypertension references related to high salt intake have primarily emphasized the reliability of their citations, indicating the potential for further expansion of this research in the future.
Figure 6.
Top 25 references with the strongest citation bursts involved in hypertension related in high salt on CiteSpace.
3.6. The analysis of hotspots and the frontiers
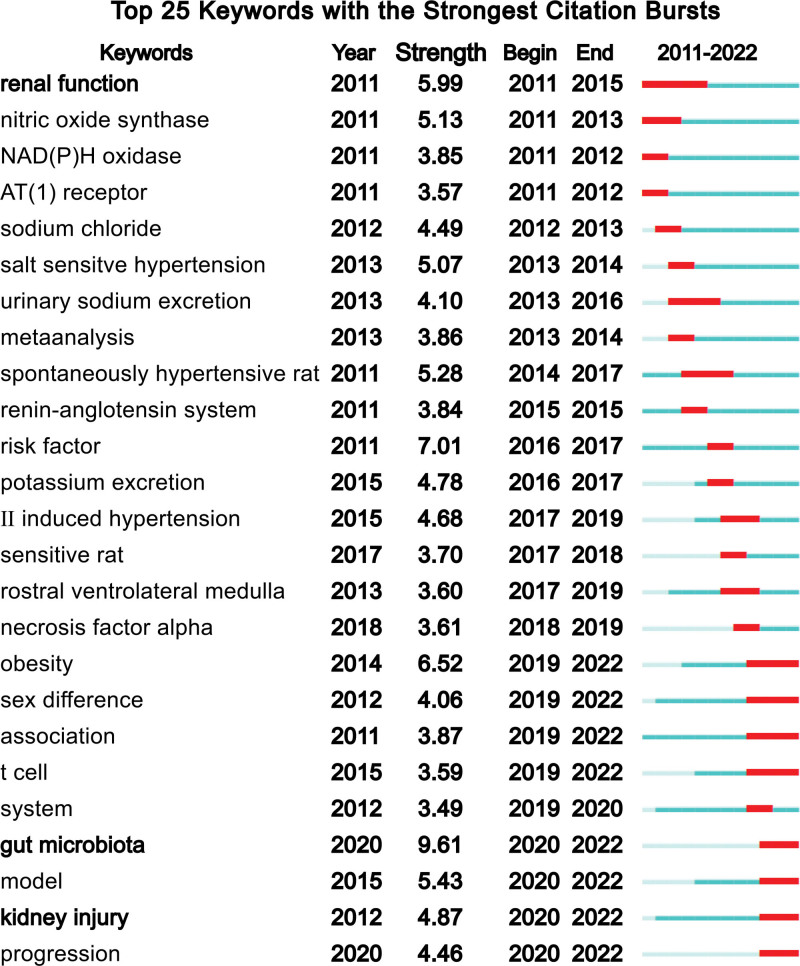
Through the keyword co-occurrence, we may learn about the areas of interest and future research directions in this discipline. Key terms encompass publications’ major subject matters, 117 (20) out of 5890 retrieved keywords from abstracts and titles satisfied the threshold (Fig. 7). The graph reveals that research on mechanisms related to oxidative stress (OS) is a major area of focus in this field. It is useful to define the important concepts like OS and to create a framework for studies on the relationship of hypertension and high salt by analyzing the associated keywords. Another crucial sign of the study frontiers, hotspots, and rising trends throughout time was the strength of the keywords’ bursts (Fig. 8). Most significantly, the citation burst time of terms such as “gut microbiota” (2020–2021), and “kidney injury” (2020–2021) has persisted into 2022 and the signals are still occurring, demonstrating that these fields have recently received a lot of attention.
Figure 7.
VOSviewer visualization map of keywords clustering analysis on hypertension related to high salt.
Figure 8.
The top 25 keywords with the strongest citation bursts.
4. Discussion
4.1. General information
With the evolution of human lifestyles, dietary habits have emerged as a significant determinant of overall health.[42] Particularly, a high-sodium diet has garnered attention as a prominent contributor to health issues.[43] To present the current research on the worldwide scientific output on the hypertension articles related to high salt, utilizing data from 2010 to 2022, we employed bibliometric analysis as a novel technique to manage and elucidate the knowledge structure in this field. Our findings indicate that this field has entered a golden era of research, as the volume of publications has witnessed a rapid increase since 2014. The United States and China have much more publications than other nations/regions and are the most productive countries in this subject, according to an examination of countries/regions. However, Germany stands out as the country with the highest research quality in this field, as it exhibits the highest average citation count for the papers produced in the region. According to the citation network map, the United States has the highest TCI, indicating that its articles may have a higher connection. Mattson, David L produced the most papers and held major leadership roles in the field, and HYPERTENSION, AMERICAN JOURNAL OF PHYSIOLOGY-RENAL PHYSIOLOGY and JOURNAL OF HYPERTENSION might be the main journals for this topic. Typically, this information can provide valuable insights for gaining an overall understanding of the research field concerning the link between high salt intake and hypertension.
From a clinical standpoint, the research can contribute to our understanding of the relationship between salt intake and hypertension. It can shed light on the mechanisms by which excessive salt consumption contributes to the development and progression of hypertension, offering valuable information for healthcare professionals and researchers working in the field. This knowledge can potentially lead to improved diagnostic and therapeutic strategies for managing hypertension caused by high salt. On the fundamental level, bibliometrics research provides a quantitative assessment of the impact of these articles, such as the number of citations they receive, the journals they are published in, and their overall visibility within the scientific community. This analysis helps researchers evaluate the influence and significance of the findings, identify research gaps, and guide future investigations. Overall, the research has clinical value by enhancing our understanding of the condition and informing medical practices. It also holds fundamental value by quantitatively assessing the impact and recognition of these articles within the scientific community, thus influencing future research and progress in the field.
4.2. Hotpots
Through bibliometric analysis of keywords with being mentioned and the strongest citation bursts, we have identified 3 research hotspots: gut microbiota, OS and renal function or kidney injury. In conjunction with some of the highly cited and relevant articles mentioned above, we can provide a brief summary of the important mechanisms underlying high blood pressure caused by high salt intake.
4.2.1. The gut microbiota has been regarded as an important pathway underlying high blood pressure caused by high salt intake.
The gut microbiota plays a crucial role in the occurrence and development of hypertension. Throughout history, salt has been used as a vital component for preservation and sterilization.[44] Consequently, the impact of a high-salt diet on the ecological balance of the human gastrointestinal microbiota is likely to be one of the significant pathways through which high salt intake leads to hypertension. The modulation of blood pressure by gut microbiota represents a significant aspect of the current research interest in this field (Fig. 9). In 2015, an experimental study demonstrated a correlation between the composition and abundance of gut microbiota and blood pressure in Dahl rats, and Lactobacillus has been identified as a key participant among the microbial species, influencing blood pressure through immune-dependent mechanisms.[45] Study suggested that moderate high-salt diet reduced the survival rate of Lactobacillus spp. and the administration of Lactobacillus murinus prevented the exacerbation of actively induced experimental salt-sensitive hypertension induced by salt intake, through the modulation of TH17 cells.[46] The gut microbiota can also influence blood pressure by modulating human metabolites. It has been discovered that a high-salt diet can decrease the abundance of Bacteroides fragilis and arachidonic acid in the gut. This reduction led to increased corticosterone production in the intestine, ultimately resulting in elevated corticosterone levels in both the intestine and bloodstream. This process ultimately led to an increase in blood pressure.[47] Furthermore, the nervous and endocrine systems also play crucial roles as intermediaries in the gut microbiota-mediated high-salt-induced hypertension. Research indicates that after high-salt consumption, the gut microbiota interacts with the sympathetic nervous system in the brain, as well as the neuroendocrine and immune systems, influencing hypertension.[48]
Figure 9.
Based on the bibliometric analysis, a schematic diagram of the mechanism involving high salt, gut microbiota, and hypertension has been proposed. The gut microbiota can affect hypertension by activating the sympathetic nervous system within the brain and interacting with the neuroendocrine and immune systems. High salt intake can reduce the survival rate of Lactobacillus spp. and Bacteroides fragilis in the gut and increase Th17 cells, thereby raising blood pressure. Lactulose and (poly)phenols can alleviate gut microbiota dysbiosis caused by high salt intake, and (poly)phenols can also alleviate hypertension. After entering the gastrointestinal tract, bile acid (BAs) undergo chemical modification from primary to secondary BAs through the gut microbiota. Chenodeoxycholic acid (CDCA) can alleviate hypertension in rats. Chlorogenic acid can alleviate high salt-induced hypertension in mice by regulating gut microbiota and BA metabolism. In hypertensive rat models, the gut microbiota that produce short-chain fatty acids (SCFAs) are reduced.
The metabolites produced by the gut microbiota serve as important vehicles in mediating the link between high-salt diet and hypertension. Short-chain fatty acids (SCFAs) are the most extensively studied compounds in this field. They are the main metabolites produced by gut microbiota through the fermentation of dietary fiber in the gastrointestinal tract. In hypertensive rat models, the reduction of gut microbiota producing butyrate and propionate indicates the significant role of SCFAs in the development of hypertension.[49] Research indicates that a high-salt diet leads to a reduction in gut microbiota producing SCFA, resulting in decreased SCFA levels in the intestines and bloodstream.[49,50] The G protein-coupled receptor and endothelial G protein-coupled receptor 41 are 2 crucial receptors. The former can improve salt-induced hypertension by influencing renin generation, while the latter can ameliorate hypertension caused by a high-salt diet by altering vascular endothelial function.[51,52] Additionally, SCFAs play a critical role in the immune pathway and may also be one of the essential mechanisms in salt-induced hypertension.[53,54] In recent years, there has been growing interest in the role of bile acid (BA) in salt-sensitive hypertension models. Upon entering the gastrointestinal tract, primary BA undergo chemical modifications to become secondary BA by the gut microbiota.[55] The natural ligand of Farnesoid X receptor, chenodeoxycholic acid, has been shown to alleviate hypertension in rats.[56] Chlorogenic acid can alleviate high-salt induced hypertension in mice by regulating gut microbiota and BA metabolism.[57] Concomitantly, a research has also indicated that lactulose can mitigate salt-sensitive hypertension.[58] And (poly)phenols in berries and Sacha inchi (Plukenetia volubilis L.) shell extract can also alleviate gut microbiota dysbiosis caused by high salt intake.[59] The gut microbiota has been a recent focus of research, and its association with hypertension, which is widely recognized as a major consequence of excessive salt intake, has gained significant attention. The mechanisms underlying the impact of the gut microbiota on high salt-induced hypertension are worth exploring.
4.2.2. The mechanisms related to OS are a major topic of research in this field.
Alterations in OS function may represent another research focus in understanding the relationship between high salt intake and hypertension. Through bibliometric analysis, the keyword “OS” appeared in 266 research papers. Alterations in OS function, represented by excessive production of reactive oxygen species (ROS), may be one of the crucial mechanisms underlying hypertension induced by high salt consumption.[60] Some experiments have already shown that salt acutely affects human OS on salt-sensitive hypertension.[61] Consumption of high-salt diet may increase the production of ROS in the rostral ventrolateral medulla, thereby activating central sympathetic outflow and increasing the risk of hypertension.[62–64] Experiments have shown that long-term consumption of capsaicin may prevent high-salt-induced OS, increase the production of nitric oxide, and lower blood pressure.[65] This effect may be caused by the activation of transient receptor potential vanilloid type 1.[65] Experimental evidence suggests that supplementing with alpha-lipoic acid and tert-butylhydroquinone can alleviate OS in the paraventricular nucleus and reduce high-salt-induced hypertension.[66,67] Furthermore, lowering OS and/or increasing the bioavailability of nitric oxide can ameliorate hypertension and cardiorenal damage induced by high salt intake.[68] These studies collectively demonstrate that excessive ROS production resulting from high salt intake can disrupt the body OS function, consequently leading to hypertension. These findings hold significant value in providing a theoretical foundation, experimental methodologies, and research directions for further investigations on the relationship between high salt consumption and hypertension.
4.2.3. Renal function and renal injury are the main concerns of researchers regarding hypertension induced by high salt intake.
Renal function emerged as a keyword with the strongest citation bursts in 2011 to 2015, and in recent years, scholars in this field have been interested in exploring the mechanisms underlying kidney injury associated with high blood pressure induced by high salt intake (Fig. 10). The kidney serves as the primary excretion site for sodium ions, and kidney function impairment is closely associated with hypertension caused by high salt intake. There is already evidence indicating that high salt intake can promote hypertension and kidney injury.[69,70] Patients with salt-sensitive hypertension are more susceptible to severe target organ damage caused by hypertension, which may increase their risk of kidney and CVD.[70] In 2012, a pioneering study was conducted to explore the physiological characteristics of a model of high nephron number for the first time.[71] It was discovered that a high nephron endowment confers a protective effect against salt-induced hypertension.[71] Evidence suggested that epigenetic regulation of WNK lysine deficient protein kinase 4 transcription is associated with the development of salt-sensitive hypertension. In rat models of salt-sensitive hypertension and sympathetic nervous system overactivity, salt loading suppresses renal WNK lysine deficient protein kinase 4 expression, activates the Na+-Cl− cotransporter, and induces salt-dependent hypertension.[72] In salt-sensitive hypertension models, ras-related C3 botulinum toxin substrate 1 GTPase induces elevated blood pressure and renal injury through a pathway dependent on mineralocorticoid receptor.[73] The activation of the nicotinamide adenine dinucleotide phosphate oxidase 4 Gene(NOX4)/H2O2/mammalian target of rapamycin 1 pathway also leads to salt-induced hypertension and renal injury.[74] In models of salt-sensitive hypertension, there is an increase in expression of the nicotinamide adenine dinucleotide phosphate oxidase subunit p67phox in the renal medulla.[75] A high-salt diet triggers lymphocyte activation in the postischemic kidney, exacerbating renal inflammation and fibrosis.[76] The activation of transient receptor potential vanilloid type 1 exerts anti-inflammatory and anti-OS effects in preventing renal tissue damage after ischemia/reperfusion injury and in salt-induced hypertension.[77] Lowered expression of external transcribed spacer 1 in the kidneys prevented renal injury associated with hypertension in Dahl salt-sensitive rats.[78] Orally administered analogues of active epoxyeicosatrienoic acid can alleviate renal injury in hypertensive Dahl salt-sensitive rat.[79] Pathways associated with renal function or injury continue to be a research hotspot, with increasing numbers of mechanisms being studied.
Figure 10.
Based on a bibliometric analysis, a schematic diagram of the mechanisms involved in high salt intake, kidney function damage, and hypertension has been proposed. High salt intake is known to promote hypertension and kidney damage, and this may be associated with the inhibition of WNK lysine deficient protein kinase 4 (WNK4) expression, activation of the NOX4/H2O2/mTORC1 pathway, and activation of renal lymphocytes. Additionally, activation of transient receptor potential vanilloid type 1 (TRPV1), downregulation of external transcribed spacer 1 (ETS-1) expression, and oral administration of active epoxyeicosatrienoic acid analogs can alleviate kidney injury. mTORC = mammalian target of rapamycin, NOX4 = NADPH oxidase 4 Gene.
5. Conclusion
Researches on the relationship between hypertension and high salt diet possess essential research value and application prospects in cardiovascular field. In this study, we utilized software such as CiteSpace and VOSviewer to collect literature on high salt intake and hypertension, and conducted bibliometric and visualization analysis to investigate the development trends and research hotspots in this field. The research interest in this field has generally increased year by year, with the investigation of its related mechanisms being a hot topic among scholars, and is likely to continue to be so. Overall, we believe that our research provides insights into the future trends and hotpots of high salt-induced hypertension to some extent.
Author contributions
Data curation: Jie Wang, Xile Peng.
Formal analysis: Kaidi Nie, Li Chen.
Writing – original draft: Zhixuan Chen.
Writing – review & editing: Luming Qi, Lina Xia.
Supplementary Material
Abbreviations:
- BA
- bile acid
- CVD
- cardiovascular diseases
- IF
- impact factor
- OS
- oxidative stress
- ROS
- reactive oxygen species
- SCFAs
- short-chain fatty acids
- TCI
- total cooperation intensity
ZC and LQ contributed equally to this work.
This work was financially supported by the National Natural Science Foundation of China (82274384, 81774387 and 82205240).
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Ethical approval is not required, because our research does not involve human and other animal experiments.
How to cite this article: Chen Z, Qi L, Wang J, Nie K, Peng X, Chen L, Xia L. Research trends and hotpots on the relationship between high salt and hypertension: A bibliometric and visualized analysis. Medicine 2023;102:41(e35492).
References
- [1].Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Forouzanfar MH, Afshin A, Alexander LT, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. [DOI] [PubMed] [Google Scholar]
- [4].Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet (London, England). 2016;387:435–43. [DOI] [PubMed] [Google Scholar]
- [5].Zhou B, Bentham J, Di Cesare M, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet. 2017;389:37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- [8].Report on Cardiovascular Health and Diseases in China 2021: an updated summary. Biomed Environ Sci. [DOI] [PubMed] [Google Scholar]
- [9].Ibrahim MM, Damasceno A. Hypertension in developing countries. Lancet (London, England). 2012;380:611–9. [DOI] [PubMed] [Google Scholar]
- [10].Roberts WC. High salt intake, its origins, its economic impact, and its effect on blood pressure. Am J Cardiol. 2001;88:1338–46. [DOI] [PubMed] [Google Scholar]
- [11].He FJ, MacGregor GA. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. 2010;52:363–82. [DOI] [PubMed] [Google Scholar]
- [12].Swaye PS, Gifford RW, Berrettoni JN. Dietary salt and essential hypertension. Am J Cardiol. 29:33–8. [DOI] [PubMed] [Google Scholar]
- [13].Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52:146–51. [DOI] [PubMed] [Google Scholar]
- [14].Meneely GR, Battarbee HD. High sodium-low potassium environment and hypertension. Am J Cardiol. 1976;38:768–85. [DOI] [PubMed] [Google Scholar]
- [15].Kawano Y, Ando K, Matsuura H, et al. Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (1) Rationale for Salt Restriction and Salt-Restriction Target Level for the Management of Hypertension. Hypertens Res. 2007;30:879–86. [DOI] [PubMed] [Google Scholar]
- [16].Appel LJ, Frohlich ED, Hall JE, et al. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–43. [DOI] [PubMed] [Google Scholar]
- [17].Weltgesundheitsorganisation, FAO, Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a WHO-FAO Expert Consultation; [Joint WHO-FAO Expert Consultation on Diet, Nutrition, and the Prevention of Chronic Diseases, 2002, Geneva, Switzerland]. World Health Organization; 2003. [Google Scholar]
- [18].Kurtz TW, DiCarlo SE, Pravenec M, et al. An alternative hypothesis to the widely held view that renal excretion of sodium accounts for resistance to salt-induced hypertension. Kidney Int. 2016;90:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tang WHW, Bäckhed F, Landmesser U, et al. Intestinal microbiota in cardiovascular health and disease. J Am Coll Cardiol. 2019;73:2089–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. JASN. 2017;28:1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He FJ, Burnier M, MacGregor GA. Nutrition in cardiovascular disease: salt in hypertension and heart failure. Eur Heart J. 2011;32:3073–80. [DOI] [PubMed] [Google Scholar]
- [22].Aaron KJ, Sanders PW. Role of dietary salt and potassium intake in cardiovascular health and disease: a review of the evidence. Mayo Clin Proc. 2013;88:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].He FJ, Tan M, Ma Y, et al. Salt reduction to prevent hypertension and cardiovascular disease. J Am Coll Cardiol. 2020;75:632–47. [DOI] [PubMed] [Google Scholar]
- [24].Hunter RW, Dhaun N, Bailey MA. The impact of excessive salt intake on human health. Nat Rev Nephrol. 2022;18:321–35. [DOI] [PubMed] [Google Scholar]
- [25].Morris RC, Schmidlin O, Sebastian A, et al. Vasodysfunction that involves renal Vasodysfunction, not abnormally increased renal retention of sodium, accounts for the initiation of salt-induced hypertension. Circulation. 2016;133:881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bar-Ilan J. Informetrics at the beginning of the 21st century—a review. Journal of Informetrics. 2008;2:1–52. [Google Scholar]
- [27].Sugimoto CR, Ahn YY, Smith E, et al. Factors affecting sex-related reporting in medical research: a cross-disciplinary bibliometric analysis. Lancet (London, England). 2019;393:550–9. [DOI] [PubMed] [Google Scholar]
- [28].Brandt JS, Hadaya O, Schuster M, et al. A bibliometric analysis of top-cited Journal articles in obstetrics and gynecology. JAMA Netw Open. 2019;2:e1918007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hassan W, Yekta BG, Nabavi SM, et al. The progress and research trends of statin medications: advanced epidemiological and bibliometrical assessment. Curr Probl Cardiol. 2023;48:101638. [DOI] [PubMed] [Google Scholar]
- [30].Wang Y, Zhang J, Zhang Y, et al. Bibliometric analysis of global research profile on ketogenic diet therapies in neurological diseases: beneficial diet therapies deserve more attention. Front Endocrinol. 2023;13:1066785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li R, Huang Q, Ye C, et al. Bibliometric and visual analysis in the field of ketogenic diet on cancer from 2012 to 2021. Front Nutr. 2022;9:1060436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Liu Y, Lu J. A bibliometric analysis of Mediterranean diet on cancer from 2012 to 2021. Front Nutr. 2023;10:1128432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang S, Lin X, Guan Y, et al. Bibliometric and visual analysis of time-restricted eating. Front Nutr. 2022;9:979702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ma D, Guan B, Song L, et al. A bibliometric analysis of exosomes in cardiovascular diseases from 2001 to 2021. Front Cardiovasc Med. 2021;8:734514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Long D, Mao C, Zhang X, et al. Coronary heart disease and gut microbiota: a bibliometric and visual analysis from 2002 to 2022. Front Cardiovasc Med. 2022;9:949859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li X, Yin Z, Ling F, et al. The application of acupuncture in cardiopathy: a bibliometric analysis based on Web of Science across ten recent years. Front Cardiovasc Med. 2022;9:920491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Devos P, Ménard J. Trends in worldwide research in hypertension over the period 1999–2018: a bibliometric study. Hypertension. 2020;76:1649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cheng K, Guo Q, Shen Z, et al. Bibliometric analysis of global research on cancer photodynamic therapy: focus on nano-related research. Front Pharmacol. 2022;13:927219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cheng K, Zhang H, Guo Q, et al. Emerging trends and research foci of oncolytic virotherapy for central nervous system tumors: a bibliometric study. Front Immunol. 2022;13:975695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wu Z, Cheng K, Shen Z, et al. Mapping knowledge landscapes and emerging trends of sonodynamic therapy: a bibliometric and visualized study. Front Pharmacol. 2023;13:1048211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wu H, Cheng K, Tong L, et al. Knowledge structure and emerging trends on osteonecrosis of the femoral head: a bibliometric and visualized study. J Orthop Surg Res. 2022;17:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fukumoto Y. Nutrition and cardiovascular diseases. Nutrients. 2021;14:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Malta D, Petersen KS, Johnson C, et al. High sodium intake increases blood pressure and risk of kidney disease. From the Science of Salt: a regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J Clin Hypertens (Greenwich). 2018;20:1654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sofos JN. Antimicrobial effects of sodium and other ions in foods: a review. J Food Saf. 1984;6:45–78. [Google Scholar]
- [45].Yang T, Richards EM, Pepine CJ, et al. The gut microbiota and the brain–gut–kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14:442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wilck N, Matus MG, Kearney SM, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yan X, Jin J, Su X, et al. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res. 2020;126:839–53. [DOI] [PubMed] [Google Scholar]
- [48].Mell B, Jala VR, Mathew AV, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bier A, Braun T, Khasbab R, et al. A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 2018;10:1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pluznick JL, Protzko RJ, Gevorgyan H, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Natarajan N, Hori D, Flavahan S, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics. 2016;48:826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li M, van Esch BCAM, Wagenaar GTM, et al. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52–9. [DOI] [PubMed] [Google Scholar]
- [54].Parada Venegas D, De la Fuente MK, Landskron G, et al. Short Chain Fatty Acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Li C, Li J, Weng X, et al. Farnesoid X receptor agonist CDCA reduces blood pressure and regulates vascular tone in spontaneously hypertensive rats. J Am Soc Hypertens. 2015;9:507–516.e7. [DOI] [PubMed] [Google Scholar]
- [57].Zhu Q, Zhu Y, Liu Y, et al. Moderation of gut microbiota and bile acid metabolism by chlorogenic acid improves high-fructose-induced salt-sensitive hypertension in mice. Food Funct. 2022;13:6987–99. [DOI] [PubMed] [Google Scholar]
- [58].Zhang Z, Zhao J, Tian C, et al. Targeting the gut microbiota to investigate the mechanism of Lactulose in negating the effects of a high-salt diet on hypertension. Mol Nutr Food Res. 2019;63:1800941. [DOI] [PubMed] [Google Scholar]
- [59].Gomes A, Oudot C, Macià A, et al. Berry-enriched diet in salt-sensitive hypertensive rats: metabolic fate of (Poly)Phenols and the role of gut microbiota. Nutrients. 2019;11:2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res. 2011;34:431–40. [DOI] [PubMed] [Google Scholar]
- [61].Laffer CL, Bolterman RJ, Romero JC, et al. Effect of salt on isoprostanes in salt-sensitive essential hypertension. Hypertension. 2006;47:434–40. [DOI] [PubMed] [Google Scholar]
- [62].Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens Res. 2011;34:407–12. [DOI] [PubMed] [Google Scholar]
- [63].Koga Y, Hirooka Y, Araki S, et al. High salt intake enhances blood pressure increase during development of hypertension via oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertens Res. 2008;31:2075–83. [DOI] [PubMed] [Google Scholar]
- [64].Kishi T, Hirooka Y. Oxidative stress in the brain causes hypertension via sympathoexcitation. Front Physio. 2012;3:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hao X, Chen J, Luo Z, et al. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflugers Archiv 2011;461:345–53. [DOI] [PubMed] [Google Scholar]
- [66].Su Q, Liu JJ, Cui W, et al. Alpha lipoic acid supplementation attenuates reactive oxygen species in hypothalamic paraventricular nucleus and sympathoexcitation in high salt-induced hypertension. Toxicol Lett. 2016;241:152–8. [DOI] [PubMed] [Google Scholar]
- [67].Bai J, Yu XJ, Liu KL, et al. Tert-butylhydroquinone attenuates oxidative stress and inflammation in hypothalamic paraventricular nucleus in high salt-induced hypertension. Toxicol Lett. 2017;281:1–9. [DOI] [PubMed] [Google Scholar]
- [68].Carlström M, Brown RD, Yang T, et al. L-arginine or tempol supplementation improves renal and cardiovascular function in rats with reduced renal mass and chronic high salt intake. Acta Physiol (Oxf). 2013;207:732–41. [DOI] [PubMed] [Google Scholar]
- [69].Morimoto A, Uzu T, Fujii T, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet (London, England). 1997;350:1734–7. [DOI] [PubMed] [Google Scholar]
- [70].Bihorac A, Tezcan H, Ozener C, et al. Association between salt sensitivity and target organ damage in essential hypertension. Am J Hypertens. 2000;13:864–72. [DOI] [PubMed] [Google Scholar]
- [71].Walker KA, Cai X, Caruana G, et al. High nephron endowment protects against salt-induced hypertension. Am J Physiol Renal Physiol. 2012;303:F253–8. [DOI] [PubMed] [Google Scholar]
- [72].Mu S, Shimosawa T, Ogura S, et al. Epigenetic modulation of the renal β-adrenergic–WNK4 pathway in salt-sensitive hypertension. Nat Med. 2011;17:573–80. [DOI] [PubMed] [Google Scholar]
- [73].Shibata S, Mu S, Kawarazaki H, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor–dependent pathway. J Clin Invest. 2011;121:3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kumar V, Kurth T, Zheleznova NN, et al. NOX4/H2O2/mTORC1 pathway in salt-induced hypertension and kidney injury. Hypertension. 2020;76:133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Feng D, Yang C, Geurts AM, et al. Increased expression of NAD(P)H oxidase subunit p67phox in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab. 2012;15:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mehrotra P, Patel JB, Ivancic CM, et al. Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT-1R antagonism. Kidney Int. 2015;88:776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yu SQ, Ma S, Wang DH. Activation of TRPV1 prevents salt-induced kidney damage and hypertension after renal ischemia-reperfusion injury in rats. Kidney Blood Press Res. 2018;43:1285–96. [DOI] [PubMed] [Google Scholar]
- [78].Feng W, Chen B, Xing D, et al. Haploinsufficiency of the Transcription Factor Ets-1 Is Renoprotective in Dahl Salt-Sensitive Rats. J Am Soc Nephrol. 2017;28:3239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Hye Khan MA, Neckář J, Manthati V, et al. Orally active epoxyeicosatrienoic acid analog attenuates kidney injury in hypertensive Dahl salt–sensitive rat. Hypertension. 2013;62:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]