Abstract
TonB couples the energized state of the cytoplasmic membrane to the operation of outer membrane receptors responsible for Fe(III) siderophore uptake across the outer membrane of gram-negative bacteria. A tonB mutant of Pseudomonas aeruginosa deficient in iron siderophore uptake was shown in the present study to be hypersusceptible to a wide variety of antibiotics, reminiscent of the phenotype of mutants defective in the mexAB-oprM antibiotic efflux operon. This was not related to influences of a tonB mutation on the iron status of the cell, and indeed, intrinsic antibiotic susceptibility and mexAB-oprM expression were unaffected by iron levels in the growth medium. The presence of tonB on a multicopy plasmid increased the level of resistance of a MexAB-OprM+ strain but not that of a MexAB-OprM− strain to a variety of antimicrobial agents. mexAB-oprM expression was not, however, altered in a tonB deletion mutant, indicating that any influence of TonB on MexAB-OprM-mediated multidrug resistance was at the level of pump activity. Consistent with this, drug accumulation assays revealed that the tonB deletion mutant exhibited decreased levels of drug efflux. Still, the multidrug resistance of a nalB strain was not wholly abrogated by a tonB mutation, indicating that it is likely not an essential component of the efflux apparatus. Similarly, elimination of tonB from an nfxB strain only partially compromised MexCD-OprJ-mediated multidrug resistance. Intriguingly, the drug susceptibility of a mexAB-oprM deletion strain was increased following deletion of tonB, suggesting that TonB may also influence antibiotic resistance mediated by determinants other than MexAB-OprM (and MexCD-OprJ). Thus, TonB plays an important role in both intrinsic and acquired antibiotic resistance in P. aeruginosa.
Energy-dependent receptor-mediated uptake of nutrients across the outer membrane of gram-negative bacteria requires the product of a gene, tonB (40). Anchored via its N terminus to the cytoplasmic membrane, TonB extends into the periplasm, where it apparently interacts with receptor proteins present in the outer membrane (see references 4 and 40 and references therein). This organization of the protein reflects its presumed role in coupling of the energized state of the cytoplasmic membrane to these outer membrane receptors (4, 40). These receptors are described as gated channels whose activities (i.e., channel opening) are modulated by TonB (18, 20, 41) and a ligand (18, 28). Characterized by shared regions of homology, these so-called TonB-dependent receptors include receptors for ferric siderophores (4), vitamin B12 (4), heme (14), and transferrin (5).
The recently described Pseudomonas aeruginosa TonB protein (TonBP.a.) is highly homologous to a variety of previously described TonB polypeptides in other gram-negative bacteria (39), exhibiting a conserved proline-rich motif and C-terminal region characteristic of these proteins. The P. aeruginosa tonB gene complemented tonB mutations in both Escherichia coli and Pseudomonas putida WCS358 (39), highlighting the interchangeability of TonB proteins and, therefore, a conserved mechanism of action in different organisms. P. aeruginosa tonB mutants are deficient in siderophore-mediated iron acquisition (39), consistent with the expected TonB dependence of this process. TonBP.a. is, however, unique in that it possesses an unusual N-terminal extension not shared by other examples of TonB proteins (39), making it markedly larger than other TonB proteins. The significance of this extension has yet to be elucidated.
Although TonB proteins are generally implicated in uptake or import processes across the outer membrane, a TonB-like molecule identified in Aeromonas hydrophila was shown to play a role in the export of the exotoxin aerolysin (17). Although it was not clear whether this molecule was truly a TonB protein, it did suggest that energy-dependent export might occur at the level of the outer membrane and that it might be mediated by TonB. In this regard, intrinsic antibiotic resistance in P. aeruginosa is afforded, in part, by the operation of a multidrug efflux pump encoded by the mexA-mexB-oprM operon (9, 24, 34, 35), in which oprM encodes an outer membrane (9, 26, 35) porin-like protein which is predicted to export antibiotics across the outer membrane. Channel-forming activity for OprM has not been tested, although a homologous outer membrane protein, OprJ, part of a second multidrug efflux system in P. aeruginosa encoded by the mexCD-oprJ genes (33), failed to form channels in planar lipid bilayer membranes (32), suggesting that it (and OprM) may form gated channels. It is possible, therefore, that drug efflux via these pumps may require a TonB or a TonB-like protein for the operation of the outer membrane components. In this report we show that the intrinsic multidrug resistance of P. aeruginosa is compromised in a tonB deletion strain and that the operation of MexAB-OprM is, indeed, facilitated by the TonB protein. Still, it is clear that TonB is not essential for expression of MexAB-OprM-mediated resistance and, as such, may influence antibiotic resistance in P. aeruginosa via unknown effects on MexAB-OprM and/or other resistance mechanisms.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are described in Table 1. K880 was constructed by disruption of the mexB gene of PAO6609 with an ΩHg cartridge. Briefly, mexB was recovered on a 4.5-kb SalI fragment from pPV20 and was cloned into the unique SalI site in pSUP202ΔTc. The ΩHg cartridge, obtained on a BamHI fragment from pHP45ΩHg, was subsequently inserted into the unique BamHI site present within the mexB-coding region. Following transformation of this construct, dubbed pNIN5, into E. coli S17-1, the vector was mobilized into P. aeruginosa PAO6609 via conjugation as described previously (34), and colonies resistant to streptomycin and HgCl2 were selected on Luria (L) agar after 24 to 48 h of growth at 37°C. Streptomycin- and HgCl2-resistant PAO6609 colonies were then screened on carbenicillin plates, and those which were susceptible to carbenicillin carried a chromosomal mexB::ΩHg mutation, as determined by immunoblotting with a MexB-specific antiserum (45). nalB multidrug-resistant derivatives of PAO6609 were isolated by plating 200 μl of an overnight L-broth culture onto L agar containing both ciprofloxacin (0.4 μg/ml) and cefoperazone (8 μg/ml). The colonies that arose were screened for the resistance phenotype characteristic of nalB strains, and MexA-MexB-OprM overexpression was confirmed via Western immunoblotting with an anti-MexB antiserum. Similarly, nfxB mutants were selected by plating overnight cultures in L broth (100 μl) onto L agar containing 1 μg of levofloxacin (Daiichi Pharmaceutical) per ml and screening for the resistance phenotype characteristic of nfxB mutants (26). Deletion of the mexAB-oprM-coding region present on plasmid pPVR20 was carried out previously to yield pRS14 (46). In order to introduce this deletion into strain PAO6609, P. aeruginosa DNA carrying this deletion was recovered from pRS14 on a ca. 4.6-kb DraI-SmaI fragment and was cloned into SmaI-restricted pEX100T. The resultant recombinant, pQZ03, was transformed into E. coli S17-1 and was mobilized into P. aeruginosa PAO6609 as described previously (34). Following isolation of streptomycin-resistant, carbenicillin-resistant transconjugants, strains carrying a chromosomal deletion of mexAB-oprM were selected on 10% (wt/vol) sucrose and were screened for the absence of MexAB-OprM with specific antisera. The tonB gene was recovered from plasmid pTON-4 on a 2.5-kb EcoRI-HindIII fragment and was cloned into pSL1180. Digestion of the resultant vector with MscI removed ca. 500 bp from within the tonB-coding sequence (corresponding to residues G117 to S297). The vector and associated tonB sequences was purified free of the 500-bp MscI fragment and were ligated with a ca. 5-kb SmaI fragment of pHP45ΩHg carrying the ΩHg cartridge. The partially deleted tonB gene now carrying the ΩHg insertion was recovered on a PstI fragment and was cloned into PstI-restricted pEX100T-Pst (to yield pQZ01). Following transformation of E. coli S17-1, the vector was mobilized into P. aeruginosa PAO6609 via conjugation (34). Conjugation mixtures were plated onto L agar containing streptomycin and HgCl2 and were incubated overnight at 37°C. A few of the colonies obtained were then streaked onto L agar supplemented with sucrose (10% [wt/vol]) and HgCl2. Sucrose-resistant, HgCl2-resistant colonies were then screened for the loss of plasmid-mediated carbenicillin resistance and were assessed for the presence of the tonB phenotype, consistent with the presence of a ΩHg-tagged tonB deletion in the chromosome. Briefly, 1-ml cultures of iron-deficient minimal medium with or without iron or ethylenediamine di(o-hydroxyphenylacetic acid) (EDDHA)-supplemented iron-deficient minimal medium with or without pyoverdine were inoculated with prospective mutants, and the mutants were grown overnight at 37°C. tonB mutants grew poorly in iron-deficient medium and not at all in the pyoverdine-supplemented medium, while the parent strain grew well in both media.
TABLE 1.
Bacterial strains and plasmids
| Strains and plasmids | Genotype or descriptiona | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Prototroph | |
| A2 | PAO1 fur | 13 |
| PAO6609 | met-9011 amiE200 rpsL pvd-9 | 15 |
| K883 | PAO6609 tonB::ΩHg | 39 |
| K880 | PAO6609 mexB::ΩHg | This study |
| K1040 | PAO6609 ΔtonB | This study |
| K1034 | nalB derivative of PAO6609 | This study |
| K1035 | K1034 ΔtonB | This study |
| K1032 | PAO6609 ΔmexA-mexB-oprM | This study |
| K1033 | K1032 ΔtonB | This study |
| PAM1350 | nfxB derivative of PAO6609 | This study |
| PAM1387 | PAM1350 mexB::ΩHg, obtained via transduction of mexB::ΩHg from K879 | This study |
| PAM1375 | PAM1350 tonB::ΩHg obtained via transduction of tonB::ΩHg from K883 | This study |
| E. coli | ||
| DH5α | endA hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR [φ80 dlacΔ(lacZ)M15] | 1 |
| S17-1 | thi pro hsdR recA Tra+ | 44 |
| MM294 | supE44 λ− rfbD1 spoT thi-1 endA1 hsdR17 pro | 30 |
| Plasmids | ||
| pSL1180 | Superlinker phagemid cloning vector | Pharmacia |
| pPV20 | Derivative of the E. coli-P. aeruginosa shuttle cloning vector pAK1900 carrying the mexA-mexB-oprM efflux operon on a 8.5-kb HindIII insert | 35 |
| pEX100T | pUC19-based gene replacement vector; Apr | 43 |
| pEX100T-Pst | pEX100T derivative in which the unique SmaI cloning site has been replaced with a unique PstI site | 39 |
| pRK2013 | Broad-host-range helper vector; Kmr | 8 |
| pSUP202ΔTc | Derivative of the gene replacement vector pSUP202 carrying a deletion within the tet gene removing the unique BamHI and HindIII sites in this vector; Apr or Cbr Cmr | 5a |
| pHP45ΩHg | Derivative of pHP45:Ω in which the Smr and Spcr of the Ω interposon is replaced by the HgCl2 resistance operon of Tn501 | 7 |
| pRK415 | Broad-host-range cloning vector; Tcr | 19 |
| pTON-4 | pRK415 derivative carrying tonB on a 2.5-kb PstI insert | 39 |
| pQZ07 | pRK415 derivative carrying tonB on a 1.4-kb PstI-EcoRI insert | This study |
| pRSP14 | pAK1900::ΔmexAB-oprM; Apr | 46 |
| pQZ01 | pEX100T-Pst::ΔtonB | This study |
| pQZ03 | pEX100T::ΔmexAB-oprM | This study |
| pMMB206 | Broad-host-range, low-copy-number cloning vector; Cmr | 29 |
| pQZ04 | pMMB206 derivative carrying tonB on a 1.4-kb PstI-HindIII fragment | This study |
| pMXR5 | pMP190 derivative carrying a mexA-lacZ transcriptional fusion; low-copy-number Cmr | 38 |
ΩHg, HgCl2-resistance derivative of interposon Ω; Apr, ampicillin resistance; Cbr, carbenicillin resistance; Tcr, tetracycline resistance, Cmr, chloramphenicol resistance; Kmr, kanamycin resistance; Smr, streptomycin resistance; Spcr, spectinomycin resistance.
Plasmids.
The plasmids used in this study are listed in Table 1. pQZ04 was constructed by cloning a 1.4-kb PCR amplification product of the tonB plasmid pTON-4 into pMMB206. By using primer tonB-PstI (5′-TATACTGCAGAGATCGCCATCGGCTGGTCG-3′), which anneals upstream of the tonB gene, and primer tonB-HindIII (5′-AATTAAGCTTCGTCTGCGAGACCTACC-3′), which anneals downstream of tonB, the tonB gene was amplified with Vent polyamerase (New England Biolabs, Mississauga, Ontario, Canada). Reaction mixtures (100 μl) contained 0.5 μM each primer, 0.4 mM each deoxynucleoside triphosphate, 4 mM MgCl2, 10% (vol/vol) dimethyl sulfoxide, 10 ng of pTON-4 DNA as template, 1 U of Vent polymerase, and 1× Vent polymerase buffer. The mixture was incubated at 94°C for 2 min, followed by 30 cycles of 1 min at 94°C, 1 min at 58°C, and 2 min at 72°C, before finishing the reaction with 10 min at 72°C. The 1.4-kb PCR product was purified with the Qiaquick-spin PCR purification kit (Qiagen), digested with PstI and HindIII, and cloned into PstI-HindIII-restricted pMMB206. The same tonB-carrying PCR product was amplified from pTON-4 as described above and was cloned into pRK415 to yield pQZ07, with the exception that an EcoRI cleavage site replaced the HindIII site in the second primer, and, thus, the PstI-EcoRI-restricted PCR product was cloned into PstI-EcoRI-restricted pRK415.
Growth conditions.
L broth (Difco) or tryptic soy broth (TSB; Difco) was used as the rich medium throughout the study except for the cultivation of strains for use in β-galactosidase assays, in which brain heart infusion broth (Difco) was used. Where indicated, FeCl3 was included in rich media at 100 μM. Iron-deficient succinate minimal (36) and King B (21) media have been described previously. The former was supplemented with methionine (1 mM) as necessary and with FeCl3 (100 μM), EDDHA (150 μg/ml), or pyoverdine (100 μg/ml), as indicated. The following antibiotics, except where indicated otherwise, were included in the growth media: carbenicillin (200 μg/ml), streptomycin (500 μg/ml), HgCl2 (15 μg/ml), tetracycline (E. coli, 10 μg/ml; P. aeruginosa, 100 μg/ml), and chloramphenicol (E. coli, 30 μg/ml; P. aeruginosa, 200 μg/ml).
DNA methods.
Protocols for the preparation of plasmid DNA, restriction digests, ligations, transformations, and isolation of restriction fragments from agarose gels have been described previously (39, 42). Plasmids were constructed in E. coli DH5α prior to their introduction into P. aeruginosa.
Transductions.
tonB::ΩHg and mexB::ΩHg mutations were transduced from K883 and K879, respectively, by using phage F116L by a previously described protocol (23).
Assays.
MIC determinations were carried out by the broth dilution technique as described previously (24) or by agar diffusion. In the latter method, bacteria (100 μl of an overnight culture) were added to 3 ml of molten top agar (0.7% [wt/vol] Bacto Agar; Difco) and were spread over L agar (L broth solidified with 1.5% [wt/vol] Bacto Agar). After solidification of the top agar, sterile concentration disks (0.25 in.; Difco) were impregnated with 2 to 10 μl of antibiotic solution and were placed on the surfaces of the agar plates. Following overnight incubation of the plates, the diameters of the zones of bacterial growth inhibition surrounding the filter disks were measured. The relative susceptibilities of different strains to the various antibiotics tested were correlated with the sizes of the zones of inhibition, with increased zone size reflecting increased susceptibility. β-Galactosidase assays were carried out on logarithmic-phase cells cultivated in brain heart infusion broth in the presence of the appropriate antibiotics as described previously (27). The level of accumulation of [3H]tetracycline (NEN/Dupont) was assayed exactly as described previously (24).
Cell envelopes, SDS-polyacrylamide gel electrophoresis, and Western immunoblotting.
Cell envelopes were prepared from overnight cultures of P. aeruginosa grown in L broth following disruption via sonication and harvesting of the cell membrane fraction by centrifugation as described previously (45). Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (with 9% [wt/vol] acrylamide in the running gel) and immunoblotting with a rabbit anti-MexB antiserum (45) or rabbit anti-OprM antiserum (48) have also been described previously (45).
Triparental matings.
Introduction of plasmids pRK415, pMMB206, pMP190, and their derivatives into P. aeruginosa required a triparental mating procedure with the helper vector pRK2013 (8). One hundred microliters of overnight cultures of plasmid-containing E. coli DH5α, pRK2013-containing E. coli MM294, and PAO6609 derivatives of P. aeruginosa were pelleted together in a microcentrifuge tube, resuspended in 25 μl of L broth, and spotted onto the center of an L-agar plate. Following incubation overnight at 37°C, bacterial growth was resuspended in 1 ml of L broth, and appropriate dilutions were plated onto L agar containing streptomycin (to counterselect the E. coli strains) and 20 μg (for K1032) to 100 μg (for PAO6609) of chloramphenicol (for pMMB206 and its derivatives) per ml, 20 μg (for K1032) to 100 μg (for PAO6609) of tetracycline (for pRK415 and its derivatives) per ml or 20 μg (for K1040) to 100 μg (for PAO6609) of chloramphenicol (for pMP190 and its derivatives) per ml. Plasmid DNA was prepared from P. aeruginosa recipients by the miniprep procedure to confirm successful plasmid transfer.
RESULTS
tonB mutants are hypersusceptible to multiple antibiotics.
The tonB gene of P. aeruginosa has recently been cloned and characterized, and a mutant, K883, in which the tonB gene was inactivated was constructed (39). Early attempts at introducing plasmids into this strain by selection with antibiotics at concentrations conventionally used for this organism invariably failed, suggesting that the tonB mutation may have lowered the intrinsic antibiotic resistance of P. aeruginosa. Indeed, the use of lower concentrations of antibiotic permitted ready selection of plasmids in K883. To test directly whether a tonB mutation compromises intrinsic resistance and because the tonB mutation in K883 was somewhat unstable, a tonB deletion strain, strain K1040, was constructed. Compared to the PAO6609 parent strain, K1040 was markedly more susceptible to tetracycline, chloramphenicol, quinolones, macrolides, novobiocin, and β-lactams (except for imipenem) (Table 2).
TABLE 2.
Influence of a tonB mutation on the antibiotic susceptibility of P. aeruginosa
| Strain | Relevant property | MIC (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CAR | CEF | NOR | CIP | NOV | ERY | TET | CAM | IMP | ||
| PAO6609 | Parent | 64 | 2 | 0.5 | 0.1 | 256 | 512 | 8 | 32 | 1 |
| K1040 | ΔtonB | 2 | 0.06 | 0.125 | 0.025 | 4 | 16 | 2 | 8 | 1 |
| K1034 | nalB | 512 | 8 | 2 | 0.4 | >512 | 1,024 | 64 | 512 | 1 |
| K1035 | nalB ΔtonB | 4 | 0.06 | 0.5 | 0.1 | 128 | 32 | 8 | 32 | 1 |
| K880 | mexB::ΩHg | 1 | 0.25 | 0.25 | 0.05 | 16 | 64 | 2 | 2 | 1 |
| PAM1350 | nfxB | 32 | 8 | 16 | 3.2 | 256 | >512 | 128 | 128 | ND |
| PAM1375 | nfxB ΔtonB | 2 | 0.5 | 4 | 0.8 | 32 | 64 | 4 | 256 | ND |
| PAM1387 | nfxB mexB::ΩHg | 2 | 2 | 8 | 1.6 | 64 | 512 | 8 | 256 | ND |
| K1032 | ΔmexAB-oprM | 0.5 | 0.5 | 0.25 | 0.025 | 16 | 32 | 1 | 2 | 1 |
| K1033 | ΔmexAB-oprM ΔtonB | 0.5 | 0.06 | 0.125 | ≤0.013 | 4 | 16 | 0.5 | 0.5 | 1 |
The MICs reported here were determined in both L broth and TSB containing 100 μM FeCl3 for strains PAO6609, K1040, K1034, and K1035 and in TSB containing 100 μM FeCl3 for all other strains. CAR, carbenicillin; CEF, cefepime; NOR, norfloxacin; CIP, ciprofloxacin; NOV, novobiocin; ERY, erythromycin; TET, tetracycline; CAM, chloramphenicol; IMP, imipenem; ND, not determined.
Involvement of TonB in MexA-MexA-OprM-dependent multidrug resistance.
To assess whether TonB contributes to the antibiotic resistance afforded by the mexAB-oprM-encoded efflux system, the influence of multiple copies of the cloned tonB gene on the resistance afforded by this operon was examined. Introduction of the multicopy tonB plasmids pQZ04 or pQZ07 into the MexAB-OprM+ strain PAO6609 but not the MexAB-OprM− strain K1032 provided a slight (twofold) but reproducible increase in the MICs of several antibiotics including cefepime, norfloxacin, ciprofloxacin, and tetracycline (data not shown). By the more sensitive agar diffusion method, PAO6609 carrying pQZ04 was shown to be consistently more resistant to all agents tested than PAO6609 without the tonB vector (Table 3). In contrast, the susceptibility of K1032 remained unchanged upon introduction of the tonB-carrying plasmid (Table 3), indicating that TonB specifically enhanced MexAB-OprM-mediated antibiotic resistance.
TABLE 3.
Influence of the cloned tonB gene on antibiotic resistance in P. aeruginosaa
| Strain | Plasmid | Diam (mm) of zone of inhibitionb
|
||||||
|---|---|---|---|---|---|---|---|---|
| CAR | CEF | NOR | CIP | ERY | NOV | TET | ||
| PAO6609 | pMMB206 | 23 | 20 | 28 | 26 | 26 | 18 | 17 |
| PAO6609 | pQZ04 | 19 | 16 | 25 | 23 | 20 | 15 | 14 |
| K1032 | pMMB206 | 20 | 20 | 30 | 18 | 20 | 15 | 11 |
| K1032 | pQZ04 | 20 | 20 | 29 | 18 | 20 | 15 | 10 |
Filter discs impregnated with the indicated antibiotics were overlaid on agar plates inoculated with the MexAB-OprM+ strain PAO6609 and the MexAB-OprM− strain K1032 carrying plasmid pMMB206 or pMMB206::tonB (pQZ04). Following incubation overnight, the diameter of the circle of growth inhibition was measured. The reported values are representative of the values from two experiments.
Antibiotic abbreviations were as described in the legend to Table 2. The amounts of antibiotic spotted onto the filter discs in 2 to 10 μl were as follows: for PAO6609, carbenicillin, 160 μg; cefepime, 5 μg; norfloxacin, 5 μg; ciprofloxacin, 2.5 μg; erythromycin, 2,560 μg; novobiocin, 1,280 μg; and tetracycline, 80 μg; for K1032, carbenicillin, 2.5 μg; cefepime, 1.25 μg; norfloxacin, 1.25 μg; ciprofloxacin, 0.3 μg; erythromycin, 160 μg; novobiocin, 80 μg; tetracycline, 2.5 μg.
In an attempt to further assess a contribution of TonB to MexAB-OprM-mediated antibiotic resistance, a nalB strain of PAO6609 hyperexpressing mexAB-oprM (K1034) was isolated, and the influence of the elimination of tonB on drug susceptibility was assessed. Although the tonB deletion did render the nalB strain more susceptible to all drugs tested, in several instances resistance levels still exceeded those seen in the ΔtonB strain (compare the levels of resistance of K1035 and K1040) (Table 2), indicating that the enhanced multidrug resistance afforded by the nalB mutation was not wholly abrogated by deletion of tonB. Thus, although compromised by its absence, it appeared that MexAB-OprM-mediated multidrug resistance was not absolutely dependent on TonB.
Iron influences on multidrug resistance.
TonB mutants are severely compromised as regards their ability to transport iron (via siderophores) and, as such, are iron limited even under iron-rich conditions (31, 40). Indeed, K1040 expressed the normally iron-repressible FpvA ferric pyoverdine receptor protein (37) during growth in L broth or TSB, media in which the TonB+ strain PAO6609 failed to elicit FpvA production (data not shown). As such, the tonB mutation might be influencing antibiotic susceptibility as a result of its influence on the iron status of the cell. Inclusion of 100 μM FeCl3 in TSB repressed expression of FpvA in K1040 (data not shown), overcoming the iron effects of the tonB mutation, although the antibiotic susceptibilities of PAO6609 and K1040 were unaltered in this medium (Table 2). Thus, the primary effect of a tonB deletion on antibiotic susceptibility in P. aeruginosa is independent of its influence on iron acquisition.
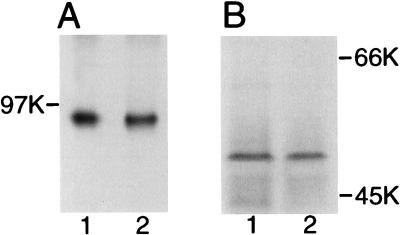
The observation that iron did not influence the drug susceptibility of the TonB+ strain was intriguing, given previous suggestions that MexAB-OprM was iron regulated (34). Still, Western immunoblotting revealed that MexB and OprM levels in cell envelopes of strain PAO6609 were not influenced by the iron richness of the growth medium (Fig. 1). Moreover, a fur mutant, which like the tonB mutant elicited expression of iron-repressible gene products under iron-rich conditions (i.e., in L broth) (2) (data not shown), exhibited no change in antibiotic susceptibility relative to that of its Fur+ parent strain (data not shown). Thus, the iron status of the cell is not a significant determinant as regards the intrinsic antibiotic resistance of P. aeruginosa or expression of MexAB-OprM.
FIG. 1.
Influence of iron on expression of components of the MexAB-OprM multidrug efflux system. Cell envelopes of P. aeruginosa PAO6609 cultured in iron-limited (lanes 1) and iron-supplemented (100 μM FeCl3) (lanes 2) succinate minimal medium were electrophoresed on SDS-polyacrylamide gels, immunoblotted, and developed with antisera raised against purified MexB (A) or OprM (B). Molecular mass markers are indicated (in kilodaltons).
tonB mutants are not altered in mexAB-oprM gene expression.
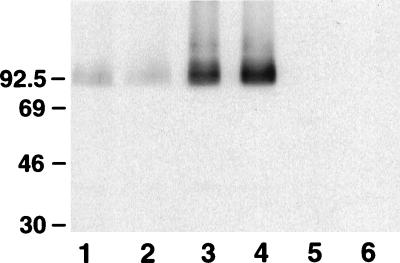
The influence of TonB on MexAB-OprM-mediated multidrug resistance could be manifested by an effect on the level of expression and/or activity of the efflux pump. To rule out any influence of a tonB mutation on expression of the MexAB-OprM components, then, mexAB-oprM gene expression was assessed in PAO6609 and its tonB mutant K1030 with a plasmid-borne mexA-lacZ fusion (pMXR5). Both strains showed substantial levels of expression of mexA-lacZ, consistent with the known constitutive expression of mexAB-oprM, although no differences were seen between these strains (PAO6609, 2,726 Miller units; K1040, 2,544 Miller units). Similarly, when expression of pump components was examined directly, no differences in the level of MexB (Fig. 2) or OprM (data not shown) were observed between PAO6609 or its nalB derivative K1034 and their tonB derivatives.
FIG. 2.
Influence of a tonB deletion on production of the MexB component of the MexA-MexB-OprM efflux pump. Cell envelopes of P. aeruginosa PAO6609 (lane 1), K1034 (nalB) (lane 3), K1032 (ΔmexAB-oprM) (lane 5), and their ΔtonB derivatives K1040 (lane 2), K1035 (lane 4), and K1033 (lane 6), respectively, were electrophoresed on SDS-polyacrylamide gels, immunoblotted, and developed with an antiserum raised against purified MexB (28). Molecular mass markers are indicated at the left (in kilodaltons).
Role of TonB in antibiotic efflux.
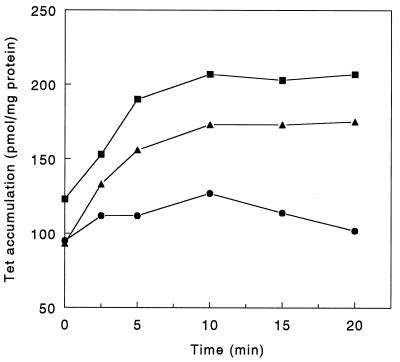
In light of the apparent influence of TonB on MexAB-OprM-mediated multidrug resistance, the increased antibiotic susceptibility of ΔtonB strain K1040 likely results from a decrease in drug efflux. To assess this, drug (tetracycline) accumulation assays were performed with K1040 and its TonB+ parent strain PAO6609. As seen in Fig. 3, the tonB mutant accumulated more tetracycline than did PAO6609, consistent with a defect in drug efflux in K1040. The addition of the uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) increased the levels of tetracycline accumulation in both strains (to 320 pmol/mg of protein in K1040 and to 400 pmol/mg of protein in PAO6609 after 30 min) indicating, however, that some drug efflux was still occurring in the ΔtonB strain. The levels of tetracycline accumulated by K1040 were comparable to that seen for the mexB::ΩHg mutant K880 deficient in the MexAB-OprM efflux system (Fig. 3), indicating that both mutants were similarly compromised as regards tetracycline efflux. Indeed, drug susceptibility testing also indicated that these two strains were indistinguishable as regards drug resistance (Table 2), indicating that the same resistance mechanism was likely being affected in K880 and K1040. These data were consistent with previous suggestions that the TonB status of the cell somehow influences MexAB-OprM-mediated drug efflux.
FIG. 3.
Accumulation of [3H]tetracycline (Tet) by P. aeruginosa PAO6609 (•), K1040 (ΔtonB) (▴), and K880 (mexB::ΩHg) (■). Radiolabelled tetracycline (5 μM) was added to logarithmic-phase cells, and cellular drug accumulation was determined as a function of time. The data presented here are representative of three separate experiments. The addition of CCCP (final concentration, 0.2 mM) at 10 min in control experiments resulted in the accumulation of tetracycline to ≥400 pmol/mg of protein after 30 min for all strains.
Influence of a tonB deletion on MexC-MexD-OprJ-mediated multidrug resistance.
To assess the influence of the TonB status of the cell on the antibiotic resistance afforded by other multidrug efflux systems in P. aeruginosa, a tonB deletion was introduced into the MexCD-OprJ-overproducing nfxB strain PAM1350 (to yield PAM1375). As seen in Table 2, the enhanced multidrug resistance of PAM1350 relative to that of its PAO6609 parent strain was substantially compromised by the tonB deletion. Still, PAM1375 remained more resistant (to certain agents) than the ΔtonB derivative of PAO6609 (K1040) (Table 2). Elimination of the MexAB-OprM efflux system in this nfxB strain (by insertional inactivation of mexB; see data for PAM1387 in Table 2) had a much more modest impact on antibiotic susceptibility, indicating that the increased susceptibility of PAM1375 compared with the susceptibility of PAM1350 was not due primarily to an impact of the tonB deletion on MexAB-OprM-mediated resistance. As such, the antibiotic resistance afforded by the MexCD-OprJ multidrug efflux system would appear to be similarly compromised, but not wholly abrogated, by the elimination of TonB.
TonB contributes to antibiotic resistance independent of MexAB-OprM.
In an attempt to gain further insight into the role of TonB in the antibiotic resistance of P. aeruginosa, the influence of a tonB mutation on the resistance of a strain already lacking the mexAB-oprM operon was assessed. Elimination of tonB in the ΔmexAB-oprM strain K1032 (to yield K1033) caused an increase in the level of susceptibility to most antibiotics tested (Table 2). Since MexCD-OprJ is not expressed in K1032 (31), these data suggested that antibiotic resistance mechanisms other than efflux mediated by MexAB-OprM or MexCD-OprJ were compromised by the elimination of TonB.
DISCUSSION
The intrinsic antibiotic resistance of P. aeruginosa results from a synergy between a low level of outer membrane permeability and drug efflux (10, 25). The former limits the rate of antibiotic uptake into the cell, permitting the latter to accommodate the levels of drug which accumulate intracellularly. It is not hard to imagine, however, that this low outer membrane permeability might also restrict the efficient release of material from the cell into the extracellular milieu. Thus, the efflux systems responsible for intrinsic (MexAB-OprM) (9, 24, 34, 35) and acquired (MexAB-OprM in nalB mutants [24, 26, 38], MexCD-OprJ in nfxB mutants [26, 33], and MexEF-OprN in nfxC mutants [22, 26]) multidrug resistance in P. aeruginosa include an outer membrane constituent apparently responsible for breaching the outer membrane barrier to permit efficient extrusion of antibiotics (and, presumably, the natural substrates of these pumps) across the outer membrane. Indeed, the negative influence of the outer membrane barrier on drug efflux was aptly demonstrated by studies of the TetA tetracycline efflux pump in which resistance was compromised by the decreased level of outer membrane permeability of porin-deficient E. coli (47).
Although the outer membrane components of the P. aeruginosa efflux pumps have not been characterized in detail, OprJ has been purified (16) and reconstituted in planar lipid bilayer membranes where channel-forming activity was not observed (32). A possible explanation for this is that OprJ (and OprM) form gated channels. Such gating would make sense since maintenance of low outer membrane permeability in P. aeruginosa seems to be of some importance (the activity of the major porin F channels in this organism seems to be severely curtailed [3] and substrate-specific porins are low-conductance channels of limited permeability [11, 12]), and it is likely that “open” channels of a size that would be necessary to accommodate those antibiotics known to be substrates for the MexAB-OprJ and MexCD-OprM pumps would be incompatible with this. The functioning of a gated channel in drug efflux would, however, require a mechanism for gate opening to permit antibiotic exit across the outer membrane. The only known examples of gated channels in bacteria are the TonB-dependent receptors for Fe siderophores, vitamin B12, heme, and transferrin (4, 5, 14), in which energy-dependent channel opening is mediated by the TonB-ExbB-ExbD complex (4). The demonstration here that MexAB-OprM-mediated multidrug resistance is enhanced by TonB and that mexB and tonB mutants were similarly compromised as regards drug resistance and efflux is strongly consistent with an involvement of TonB in the operation of the MexAB-OprM efflux system, perhaps as an energy coupler to a gated OprM channel. Certainly, the tonB influence on MexAB-OprM-mediated antibiotic resistance is not an indirect effect of the tonB mutation on iron availability and/or energization, since compensation for the iron defect failed to alleviate the observed drug hypersensitivity of the mutant. Moreover, susceptibility to aminoglycosides such as kanamycin was not altered by the tonB mutation (31), even though this class of antibiotics requires membrane energization for uptake. Still, the observation that the multidrug resistance of a nalB mutant hyperexpressing MexAB-OprM was not wholly abrogated by a tonB deletion indicates that TonB is likely not essential to the operation of this efflux system and, therefore, that OprM is unlikely to be a TonB-dependent gated channel. As such, the absence of tonB may be indirectly affecting the activity of the MexAB-OprM efflux system via some as yet unknown mechanism that is unrelated to the known effects of a tonB mutation on the iron status of P. aeruginosa. In light of the recent identification of a second tonB gene in P. aeruginosa (Pseudomonas Genome Project), however, a TonB molecule may play a direct role in the operation of the MexAB-OprM (and MexCD-OprJ) systems, and the failure of the tonB deletion described here to wholly abrogate the multidrug resistance of nalB and nfxB strains might be explained by the fact that the product of this most recent tonB gene partially compensates for the absence of the first one.
It is likely that the increased level of drug (tetracycline) accumulation observed in a tonB deletion strain resulted from a defect in efflux and was not due to an increase in uptake, resultant, for example, from some alteration in the barrier property of the P. aeruginosa outer membrane. Given that a tonB deletion negatively affected the level of resistance afforded by a known multidrug efflux transporter (MexAB-OprM), one would, in fact, expect an effect on drug efflux. Moreover, the increased level of antibiotic resistance of the tonB deletion strain K1040 was shown to be drug specific (e.g., imipenem susceptibility was not affected), arguing that a general alteration in permeability cannot explain the resistance phenotype of a tonB mutant. Finally, the levels of tetracycline accumulated by the ΔtonB strain in the presence of an uncoupler were not higher than those observed for the TonB+ strain PAO6609. If the difference in the levels of drug accumulation between PAO6609 and K1040 was due to an increase in the level of drug uptake in the latter (as a result of a decrease in the outer membrane permeability barrier), the absence of efflux mechanisms in the presence of CCCP should have provided a higher net level of accumulation of tetracycline in K1040 relative to that in PAO6609.
The observation here that the levels of the components of the MexAB-OprM multidrug efflux system were not increased during the growth of P. aeruginosa in iron-limited medium was surprising, given the previous report of iron-regulated expression of mexAB-oprM (47). Still, the antibiotic susceptibility of P. aeruginosa in this study was not influenced by iron levels in the growth medium, as would be expected if iron did not affect MexAB-OprM levels. The previous study assessed mexAB-oprM expression with a plasmid-borne mexA (then called ORFA)-phoA fusion in strains cultured in iron-rich and iron-poor media. In light of recent evidence demonstrating that mexAB-oprM expression is growth regulated (increases in the logarithmic to late-logarithmic phase [6]), it is possible that the iron-rich and iron-poor cells of the previous study were, in fact, at different phases of growth at the time that mexAB-oprM expression was assayed. Thus, differences in the levels of expression observed for iron-rich versus iron-poor cells may have reflected growth phase differences and not the differences in medium iron levels.
Regardless of the nature of the involvement of TonB in antibiotic resistance in P. aeruginosa, it is clear that resistance mediated by a variety of determinants is compromised in a tonB mutant strain. As such, this protein plays an important role in the antibiotic resistance of this organism and is thus an attractive target for therapeutic intervention. It will be of interest, too, to see what role, if any, the recently identified second tonB gene plays in antibiotic resistance in P. aeruginosa.
ACKNOWLEDGMENTS
We thank N. Bianco for construction of strain K880 and D. Biek, V. Lee, and M. Schmid for reading the manuscript and providing helpful comments.
This work was supported by an operating grant from the Medical Research Council of Canada. X.-Z.L. is the holder of a Canadian Cystic Fibrosis Foundation Studentship. R.S. is a Natural Sciences and Engineering Research Council (NSERC) Postdoctoral Fellow. K.P. is an NSERC University Research Fellow.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 2.Barton H, Johnson Z, Cox C D, Vasil A I, Vasil M L. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol. 1996;21:1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x. [DOI] [PubMed] [Google Scholar]
- 3.Bellido F, Martin N L, Siehnel R J, Hancock R E W. Reevaluation, using intact cells, of the exclusion limit and role of porin OprF in Pseudomonas aeruginosa outer membrane permeability. J Bacteriol. 1992;174:5196–5203. doi: 10.1128/jb.174.16.5196-5203.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 5.Cornelisson C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5a.Dean C R, Poole K. Expression of the ferric enterobactin receptor (PfeA) of Pseudomonas aeruginosa: involvement of a two-component regulatory system. Mol Microbiol. 1993;8:1095–1103. doi: 10.1111/j.1365-2958.1993.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 6.Evans, K., and K. Poole. Unpublished data.
- 7.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 8.Figurski D H, Helinski E R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh N, Tsujimoto H, Poole K, Yamagishi J-I, Nishino T. The outer membrane protein OprM of Pseudomonas aeruginosa is encoded by oprK of the mexA-mexB-oprK multidrug resistance operon. Antimicrob Agents Chemother. 1995;39:2567–2569. doi: 10.1128/aac.39.11.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock R E W. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1998;5:37–42. doi: 10.1016/S0966-842X(97)81773-8. [DOI] [PubMed] [Google Scholar]
- 11.Hancock R E W, Poole K, Benz R. Outer membrane protein P of Pseudomonas aeruginosa: regulation by phosphate deficiency and formation of small anion-specific channels in lipid bilayer membranes. J Bacteriol. 1982;150:730–738. doi: 10.1128/jb.150.2.730-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hancock R E W, Egli C, Benz R, Siehnel R J. Overexpression in Escherichia coli and functional analysis of a novel PPi-selective porin, OprO, from Pseudomonas aeruginosa. J Bacteriol. 1992;174:471–476. doi: 10.1128/jb.174.2.471-476.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassett D J, Sokol P A, Howell M L, Ma J-F, Schweizer H T, Ochsner U, Vasil M L. Ferric uptake regulator (Fur) mutants of Pseudomonas aeruginosa demonstrate defective siderophore-mediated iron uptake, altered aerobic growth, and decreased superoxide dismutase and catalase activities. J Bacteriol. 1996;178:3996–4003. doi: 10.1128/jb.178.14.3996-4003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson D P, Payne S M. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J Bacteriol. 1994;176:3269–3277. doi: 10.1128/jb.176.11.3269-3277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohnadel D, Haas D, Meyer J-M. Mapping of mutations affecting pyoverdine production in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1986;36:195–199. [Google Scholar]
- 16.Hosaka M, Gotoh N, Nishino T. Purification of a 54-kilodalton protein (OprJ) produced in NfxB mutants of Pseudomonas aeruginosa and production of a monoclonal antibody specific to OprJ. Antimicrob Agents Chemother. 1995;39:1731–1735. doi: 10.1128/aac.39.8.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howard S P, Meiklejohn H G, Shivak D, Jahagirdar R. A TonB-like protein and a novel membrane protein containing an ATP-binding cassette function together in exotoxin secretion. Mol Microbiol. 1996;22:595–604. doi: 10.1046/j.1365-2958.1996.d01-1713.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Payne M A, Cao Z, Foster S B, Feix J B, Newton S M C, Klebba P E. Ligand-specific opening of a gated-porin channel in the outer membrane of living bacteria. Science. 1997;276:1261–1264. doi: 10.1126/science.276.5316.1261. [DOI] [PubMed] [Google Scholar]
- 19.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 20.Killmann H, Benz R, Braun V. Conversion of the FhuA transport protein into a diffusion channel through the outer membrane of Escherichia coli. EMBO J. 1993;12:3007–3016. doi: 10.1002/j.1460-2075.1993.tb05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King E O, Ward M K, Raney D F. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 22.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J-C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 23.Krishnapillai V. A novel transducing phage: its role in recognition of a possible new host-controlled modification system in Pseudomonas aeruginosa. Mol Gen Genet. 1971;114:134–143. doi: 10.1007/BF00332784. [DOI] [PubMed] [Google Scholar]
- 24.Li X-Z, Nikaido H, Poole K. Role of MexA-MexB-OprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma D, Cook D N, Hearst J E, Nikaido H. Efflux pumps and drug resistance in gram-negative bacteria. Trends Microbiol. 1994;2:489–493. doi: 10.1016/0966-842x(94)90654-8. [DOI] [PubMed] [Google Scholar]
- 26.Masuda N, Sakagawa E, Ohya S. Outer membrane proteins responsible for multiple drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:645–649. doi: 10.1128/AAC.39.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller J H. A laboratory manual and handbook for Escherichia coli and related bacteria. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. A short course in bacterial genetics; pp. 72–74. [Google Scholar]
- 28.Moeck G S, Tawa P, Xiang H, Ismail A A, Turnbull J L, Coulton J W. Ligand-induced conformational change in the ferrichrome-iron receptor of Escherichia coli K-12. Mol Microbiol. 1996;22:459–471. doi: 10.1046/j.1365-2958.1996.00112.x. [DOI] [PubMed] [Google Scholar]
- 29.Morales V M, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 30.Neidhardt F C. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1987. [Google Scholar]
- 31.Poole, K. Unpublished data.
- 32.Poole, K., N. Gotoh, and R. E. W. Hancock. Unpublished data.
- 33.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J-I, Li X-Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB multidrug resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 34.Poole K, Heinrichs D E, Neshat S. Cloning and sequence analysis of an EnvCD homologue in Pseudomonas aeruginosa: regulation by iron and possible involvement in the secretion of the siderophore pyoverdine. Mol Microbiol. 1993;10:529–544. doi: 10.1111/j.1365-2958.1993.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 35.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole K, Neshat S, Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991;78:1–5. [PubMed] [Google Scholar]
- 37.Poole K, Neshat S, Krebes K, Heinrichs D E. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol. 1993;175:4597–4604. doi: 10.1128/jb.175.15.4597-4604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs D, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole K, Zhao Q, Neshat S, Heinrichs D E, Dean C R. The tonB gene of Pseudomonas aeruginosa encodes a novel TonB protein. Microbiology. 1996;142:1449–1458. doi: 10.1099/13500872-142-6-1449. [DOI] [PubMed] [Google Scholar]
- 40.Postle K. TonB protein and energy transduction between membranes. J Bioenerget Biomembr. 1993;25:591–602. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 41.Rutz J M, Liu J, Lyons J A, Goranson J, Armstrong S K, McIntosh M, Feix J B, Klebba P E. Formation of a gated channel by a ligand-specific transport protein in the bacterial outer membrane. Science. 1992;258:471–475. doi: 10.1126/science.1411544. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 43.Schweizer H P, Hoang T T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 44.Simon R, Priefer U, Puehler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 45.Srikumar R, Kon T, Gotoh N, Poole K. Expression of Pseudomonas aeruginosa multidrug efflux pumps MexA-MexB-OprM and MexC-MexD-OprJ in Escherichia coli. Antimicrob Agents Chemother. 1998;42:65–71. doi: 10.1128/aac.42.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srikumar R, Li X-Z, Gotoh N, Poole K. The inner membrane efflux components are responsible for the β-lactam specificity of multidrug efflux pumps in Pseudomonas aeruginosa. J Bacteriol. 1997;179:7875–7881. doi: 10.1128/jb.179.24.7875-7881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thanassi D G, Suh G S, Nikaido H. Role of the outer membrane barrier in efflux-mediated tetracycline resistance of Escherichia coli. J Bacteriol. 1995;177:998–1007. doi: 10.1128/jb.177.4.998-1007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Q, Li X-Z, Srikumar R, Poole K. Contribution of outer membrane efflux protein OprM to antibiotic resistance in Pseudomonas aeruginosa independent of MexAB. Antimicrob Agents Chemother. 1998;42:1682–1688. doi: 10.1128/aac.42.7.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]