ABSTRACT
Beta‐synuclein is a promising cerebrospinal fluid and blood biomarker of synaptic damage. Here we analysed its accuracy in the discrimination between sporadic Creutzfeldt–Jakob disease (n = 150) and non‐prion rapidly progressive dementias (n = 106). In cerebrospinal fluid, beta‐synuclein performed better than protein 14‐3‐3 (AUC 0.95 vs. 0.89) and, to a lesser extent, than total tau (AUC 0.92). Further, the diagnostic value of plasma beta‐synuclein (AUC 0.91) outperformed that of plasma tau (AUC 0.79) and neurofilament light chain protein (AUC 0.65) and was comparable to that of cerebrospinal fluid biomarkers. Beta‐synuclein might represent the first highly accurate blood biomarker for the diagnosis of sporadic Creutzfeldt–Jakob disease.
Introduction
The use of cerebrospinal fluid (CSF) biomarkers of neurodegeneration and prion pathology as well as brain magnetic resonance imaging (MRI) have significantly improved the diagnostic assessment of sporadic Creutzfeldt–Jakob disease (sCJD). 1 , 2 , 3 , 4 Nevertheless, sCJD diagnosis remains challenging due to the wide disease heterogeneity, including six major disease subtypes with distinct clinicopathological features and its clinical overlap with other forms of rapidly progressive dementia (RPD). 5 , 6 Moreover, CSF markers are not ideal for screening purposes considering the invasiveness of the lumbar puncture (LP), and reaching a high accuracy in the diagnosis using brain MRI requires a level of operator experience that may not be present at all hospitals. 4 , 7 , 8
In this context, developing a highly accurate blood biomarker might present considerable advantages in terms of minimal invasiveness, wide applicability for early diagnosis, and tracking of disease progression. However, available markers, such as blood neurofilament light chain protein (NfL) and tau protein, although showing a good prognostic value, 8 , 9 , 10 are of limited utility in discriminating prion disease from other RPDs. 8 , 10 , 11
Recently, we have reported elevated levels of the presynaptic protein beta‐synuclein (beta‐syn) in CSF and blood of patients with prion disease as a surrogate marker of synaptic damage. 12 , 13 , 14 Here, we compared CSF and plasma beta‐synuclein performance to that of classic surrogate biomarkers [CSF total tau (t‐tau), CSF 14‐3‐3 protein, plasma tau, and plasma NfL] in the discrimination between sCJD and non‐prion RPD forms in the clinical setting and evaluated beta‐syn prognostic value in sCJD.
Methods
Case classification
We analysed retrospectively CSF and plasma samples from patients with RPD submitted to the neuropathology laboratory at the Institute of Neurological Sciences of Bologna between 2004 and 2022. Detailed inclusion criteria are reported in supplementary methods. We included 150 patients with sCJD and 106 participants representative of the most common etiologies of non‐prion RPD. 6 The sCJD group encompassed 82 definite and 68 probable cases with a significant number of patients from the three most prevalent sCJD subtypes [MM(V)1, VV2, and MV2K]. 1 , 5 For the analysis according to the molecular subtype, we merged the subjects with definite sCJD with those with a probable diagnosis and a high level of certainty for a given subtype (supplementary methods). 4 , 15 Diagnoses of non‐prion RPD cases are provided in Table S1.
Survival was defined as the time (in months) from LP or blood collection to (i) death or (ii) akinetic mutism if life‐extending treatments (e.g., enteral/parenteral nutrition, tracheostomy) were performed. 10
The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines and approved by the ethics committee “Area Vasta Emilia Centro” (approval number AVEC:18025, 113/2018/OSS/AUSLBO). Written informed consent was given by study participants or the next of kin.
Biomarker analyses
CSF and EDTA plasma samples were collected, aliquoted, and stored at −80°C according to standard procedures. We measured CSF t‐tau, CSF 14‐3‐3 gamma isoform, plasma tau, and plasma NfL as reported. 7 , 10 We analysed CSF and plasma beta‐syn using in‐house established classic and digital ELISAs, respectively (supplementary methods). 13 , 14 All CSF samples were tested by the second‐generation CSF prion Real‐Time Quaking‐Induced Conversion assay (RT‐QuIC). 16 sCJD participants were positive, whereas non‐prion RPD cases were negative by RT‐QuIC. All biomarkers were analyzed by operators blinded to patients' diagnoses.
Statistical analysis
We used the Mann–Whitney U or Kruskal–Wallis test (followed by Dunn‐Bonferroni post hoc test) to compare continuous variables. Spearman's correlations and linear regression analyses were used to test the possible associations between variables. Each marker's diagnostic accuracy was calculated using receiver operating characteristic (ROC) analyses. The DeLong test was used to compare the different areas under the curve (AUCs). Univariate and multivariate Cox regression analyses tested the associations between survival and CSF or plasma beta‐syn, and/or known prognostic factors in sCJD. 10 We performed survival analyses in the whole cohort of sCJD cases and in the most prevalent clinicopathological subtypes. Statistical tests were two‐tailed, and p‐values were considered statistically significant at <0.05. Further details are reported in supplementary methods.
Results
CSF and plasma beta‐syn levels in the diagnostic groups
Demographic features and biomarker results in the diagnostic groups are shown in Table 1. Associations between demographic variables and biomarkers and among biomarkers are reported in supplementary results.
Table 1.
Demographic variables and distribution of CSF and plasma biomarkers across sporadic Creutzfeldt–Jakob disease and non‐prion RPDs.
| Sporadic Creutzfeldt–Jakob disease (n = 150) | Non‐prion RPD n = 106) | p value | |
|---|---|---|---|
| Age (years) mean ± SD | 67.56 ± 9.77 | 72.15 ± 10.37 | <0.001 |
| Female % | 48.7 | 53.8 | 0.421 |
| Time from onset to sample collection (months) mean ± SD | 3.74 ± 3.93 | – | |
| CSF beta‐syn (pg/mL) | 4307 (1724–7301) | 297 (195–655) | <0.001 |
| Median (IQR) | |||
| Plasma beta‐syn (pg/mL) | 104.6 (44.7–186.6) | 10.3 (2.8–27.9) | <0.001 |
| Median (IQR) | |||
| CSF t‐tau (pg/mL) | 6392 (2343–10828) | 619 (384–1268) | <0.001 |
| Median (IQR) | |||
| CSF 14–3‐3 (AU/mL) | 69200 (33225–139250) | 9695 (6215–18425) | <0.001 |
| Median (IQR) | |||
| Plasma tau (pg/mL) | 7.5 (4.1–22.3) | 2.7 (1.8–4.7) | <0.001 |
| Median (IQR) | |||
| Plasma NfL (pg/mL) | 112.6 (60.4–208.8) | 59.2 (27.9–156.3) | <0.001 |
| Median (IQR) |
Beta‐syn, beta‐synuclein; CSF, cerebrospinal fluid; IQR, interquartile range; NfL, neurofilament light chain; RPD, rapidly progressive dementia; SD, standard deviation; t‐tau, total tau protein.
sCJD patients showed higher CSF and plasma beta‐syn levels compared to non‐prion RPD subjects (p < 0.001 for both) (Fig. 1A,B). Moreover, different CSF and blood beta‐syn distributions were observed for the most prevalent sCJD subtypes (Fig. 1C,D, and Table S2). Both sCJD MM(V)1 and VV2 subgroups demonstrated significantly higher CSF beta‐syn levels compared to the MV2K group (p < 0.001 for both comparisons). In plasma, instead, the marker reached the highest values in sCJD MM(V)1 (p < 0.001 vs. both VV2 and MV2K) with no significant differences between the VV2 and MV2K groups. All findings mentioned above remained significant after the exclusion of probable cases (supplementary results). The profiles of classic CSF and blood surrogate biomarkers in sCJD subtypes and non‐prion RPD groups are reported in Table 1, supplementary results, Table S3. The distributions of CSF, plasma beta‐syn, and other biomarker values across non‐prion RPD etiologies are shown in Table 2, supplementary results, and Table S4.
Figure 1.
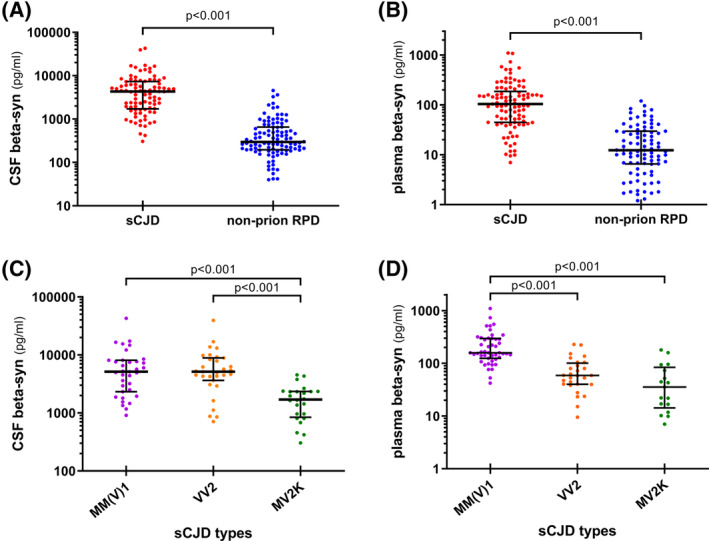
CSF and plasma beta‐syn levels in the diagnostic groups. (A) CSF and (B) plasma beta‐syn values in sporadic Creutzfeldt–Jakob disease and non‐prion RPD; (C) CSF and (D) plasma beta‐syn levels in sporadic Creutzfeldt–Jakob disease most prevalent molecular subtypes [MM(V)1, VV2, and MV2K]. β‐syn values are presented as log(pg/mL); median, and interquartile range are shown for the biomarker levels. p values of Mann–Whitney or Dunn's post hoc test after Kruskal–Wallis test are reported. CSF, cerebrospinal fluid; RPD, rapidly progressive dementia; sCJD, sporadic Creutzfeldt–Jakob disease.
Table 2.
CSF and blood beta‐syn distribution in non‐prion RPD etiologies.
| Diagnostic categories of non‐prion RPD patients | n | CSF beta‐syn (pg/mL) Median (IQR) | n | Plasma beta‐syn (pg/mL) Median (IQR) |
|---|---|---|---|---|
| All neurodegenerative diseases | 52 | 268 (184–454) | 50 | 10.8 (3.3–25.9) |
| Alzheimer's disease | 42 | 281 (200–467) | 40 | 11.6 (3.0–26.8) |
| Dementia with Lewy bodies | 8 | 131.5 (71.5–252.5) | 8 | 8.7 (3.1–18.7) |
| Frontotemporal dementia | 2 | 243, 82 | 2 | 9.4, 36.2 |
| Vascular/mixed dementia and stroke | 10 | 201 (76.5–629) | 10 | 19.6 (1.5–65.4) |
| Immuno‐mediated, infectious encephalitis and other inflammatory/infective diseases | 27 | 493 (221–1284) | 25 | 7.3 (1.4–34.2) |
| Toxic/metabolic encephalopathies | 10 | 521 (193–952) | 10 | 13.2 (5.3–30.0) |
| Central nervous system malignancy | 5 | 659 (387–2772) | 3 | 2.8, 4.0, 0.8 |
Beta‐syn, beta‐synuclein; CSF, cerebrospinal fluid; IQR, interquartile range; RPD, rapidly progressive dementia.
Diagnostic and prognostic value of CSF and blood beta‐syn
The ROC curve data of all CSF and blood biomarkers in the differential diagnosis between sCJD and non‐prion RPD are reported in Table 3. Specifically, the discriminative performances of CSF (AUC 0.949 ± 0.014) and plasma beta‐syn (AUC 0.914 ± 0.019) were the highest observed for their respective compartments. At Youden index calculated cutoffs (Table 3), the positive predictive values of CSF and plasma beta‐syn were 77.8% and 83.8%, respectively, whereas the negative predictive values were 96.3% and 84.8%, respectively. Furthermore, CSF beta‐syn performed better than CSF 14‐3‐3 (AUC 0.892 ± 0.022) and, to a lesser extent, than CSF t‐tau (AUC 0.922 ± 0.017). Moreover, the diagnostic value of plasma beta‐syn was higher than that of plasma tau (AUC 0.791 ± 0.032) and plasma NfL (AUC 0.654 ± 0.040), and comparable to that of classic CSF surrogate markers.
Table 3.
Diagnostic accuracy of CSF and plasma beta‐syn in comparison with that of other CSF and blood biomarkers in the discrimination between sporadic Creutzfeldt‐Jakob disease and non‐prion RPDs.
| AUC | Cutoff | Sens (%) | Spec (%) | DeLong p vs. CSF beta‐syn | DeLong p vs. plasma beta‐syn | ||
|---|---|---|---|---|---|---|---|
| CSF beta‐syn | 0.949 ± 0.014 | > | 1313 pg/mL | 84.4 | 92.2 | – | 0.135 |
| Plasma beta‐syn | 0.914 ± 0.019 | > | 37.5 pg/mL | 84.7 | 84.0 | 0.135 | – |
| CSF t‐tau | 0.922 ± 0.017 | > | 1770 pg/mL | 86.0 | 87.7 | 0.212 | 0.765 |
| CSF 14‐3‐3 | 0.892 ± 0.013 | > | 16550 AU/mL | 94.0 | 73.6 | 0.028 | 0.447 |
| Plasma tau | 0.791 ± 0.032 | > | 4.1 pg/mL | 77.3 | 69.7 | <0.001 | 0.001 |
| Plasma NfL | 0.654 ± 0.040 | > | 50.2 pg/mL | 88.0 | 47.5 | <0.001 | <0.001 |
AUC, area under the curve; beta‐syn, beta‐synuclein; CSF, cerebrospinal fluid; NfL, neurofilament light chain; sens, sensitivity, spec, specificity; t‐tau, total tau protein.
At cut‐off values favouring sensitivity (99%) over specificity, as in the context of a screening test (Table S5), CSF and plasma beta‐syn showed the highest specificities (60.8% and 46.0%, respectively) compared to the other biomarkers.
The ROC curves for the distinction between sCJD and major non‐prion RPD etiologies are shown in Table S6 and confirm the same results.
Overall, CSF and plasma beta‐syn concentrations were significantly associated with survival in sCJD subjects at univariate and multivariate analyses after accounting for age, disease duration (time from onset to sampling), and PRNP codon 129 genotype (Table S7). However, the association did not remain significant after adjustment for the clinicopathological subtype nor in the sub‐analysis considering a single molecular subgroup (Table S7).
Discussion
Beta‐syn is emerging as a promising CSF and blood biomarker for synaptic damage in several neurodegenerative diseases. 12 , 13 , 14 In the present study, we reported higher CSF and plasma beta‐syn levels in sCJD compared to non‐prion RPDs. In prion diseases, the massive synaptic degeneration might lead to beta‐syn release from the presynaptic terminals into the extracellular space and then in biofluids. The finding of the decreased beta‐syn levels in the brain tissue of sCJD patients 14 and the marked increase in CSF and blood supports this hypothesis. Compared to other synaptic markers, which show extracerebral synthesis (alpha‐synuclein) or lack a sensitive blood assay [neurogranin and synaptosomal‐associated protein 25 (SNAP‐25)], 14 , 17 , 18 beta‐syn is CNS‐specific 19 and can be reliably measured in blood using a digital ELISA. 14
As reported for tau protein, 10 CSF beta‐syn showed similar levels in sCJD MM(V)1 and VV2, whereas plasma concentrations were higher in the former group than in the latter. To explain this behaviour, we previously hypothesized 10 that, due to the early cortical neuronal and synaptic damage, the brains of subjects with MM(V)1 manifest a higher spillover of neuronal markers in the blood. Indeed, the VV2 subtype shows a predominant subcortical pathology with only late cerebral cortex involvement. 5 , 10 , 20
Most interestingly, CSF beta‐syn performed better than CSF 14‐3‐3 and, to a lesser extent, than CSF t‐tau, and, if adopted as a screening test, correctly excluded a higher number of non‐prion RPDs than all other biomarkers. Thus, while the CSF RT‐QuIC still represents the most sensitive and specific biofluid marker for sCJD, CSF beta‐syn might represent a highly performant first‐level test in patients with suspected sCJD for those laboratories performing the prion seeding assay as a confirmative test when other tests are suggestive of prion disease. 4 , 7 , 10
Nevertheless, the significant added value of beta‐syn mainly relies on the maintenance of a high diagnostic performance in the blood. Blood NfL and tau showed poor discriminative performance in the clinical setting of RPDs. 8 , 10 , 11 Conversely, blood beta‐syn might accurately estimate noninvasively the degree of synaptic/neuronal damage with relatively low cost and a potential application for screening and monitoring to a broad population.
In the context of prognostication, CSF and plasma beta‐syn concentrations, like other CSF and blood markers, 10 might also represent complementary predictive factors for survival together with other variables (i.e., age, PRNP codon 129 genotype, etc.). Here, the lack of associations between the biomarker values and survival in the molecular subtypes might partially rely on the small sample size of each subgroup. Moreover, another potential limitation of the study is the lack of clinical measurements of functional status and progression rate, which previously correlated with biomarker values. 9 , 11
In conclusion, by providing evidence that the diagnostic value of plasma beta‐syn is comparable to that of classic CSF surrogate markers (i.e., t‐tau and 14‐3‐3), the present results suggest that beta‐syn might represent the first accurate blood marker for the diagnostic screening of patients with suspected sporadic Creutzfeldt–Jakob disease in vivo. Further, the marker might contribute to patient stratification and prognostication.
Author Contributions
Conception and design of the study: Samir Abu‐Rumeileh, Markus Otto, and Piero Parchi; acquisition and analysis of data: Samir Abu‐Rumeileh, Steffen Halbgebauer, Giuseppe Mario Bentivenga, Lorenzo Barba, Simone Baiardi, Andrea Mastrangelo, Patrick Oeckl, Petra Steinacker, Angela Mammana, Sabina Capellari, Markus Otto, and Piero Parchi; drafting of the manuscript: Samir Abu‐Rumeileh, Markus Otto, and Piero Parchi.
Conflict of Interest Statement
M.O. gave scientific advice to Axon, Biogen Idec, Fujirebio and Roche, all unrelated to the work presented in this paper. The foundation of the state Baden–Wuerttemberg handed in a patent for the measurement of β‐synuclein in neurological diseases. Relevant authors are M.O., S.H., and P.O. The other authors report no conflicts of interest.
Supporting information
Data S1 Supporting Information.
Acknowledgments
The authors wish to thank Barbara Polischi and Katrin Schulz for their valuable technical assistance.
This research was supported by the Ministero della Salute (Ricerca Corrente), the #NextGenerationEU (NGEU) funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006), the German Federal Ministry of Education and Research (projects: FTLDc 01GI1007A), the EU Joint Program‐Neurodegenerative Diseases (JPND) network, the EU Moodmarker program (01EW2008), Roux program of the Martin Luther University Halle‐Wittenberg, the Foundation of the State Baden‐Württemberg (D.3830), Genfi‐Prox (01ED2008A), the German Research Foundation/DFG (SFB1279), the Boehringer Ingelheim Ulm University BioCenter (D.5009) and the Thierry Latran foundation (D.2468). S.A.R. was supported by the Medical Faculty of Martin‐Luther‐University of Halle‐Wittenberg (Clinician Scientist‐Programme No. CS22/06).
Funding Statement
This work was funded by EU Joint Programme ‐ Neurodegenerative Disease Research grant 01EW2008; Genfi‐Prox grant 01ED2008A; German Federal Ministry of Education and Research grant FTLDc 01GI1007A; Ministero della Salute grant Ricerca Corrente; Ministero dell'Università e della Ricerca (MUR), National Recovery and Resilience Plan (NRRP) project MNESYS grant PE0000006; EU Moodmarker program grant 01EW2008; Roux program of the Martin Luther University Halle‐Wittenberg; Foundation of the State Baden‐Württemberg grant D.3830; German Research Foundation/DFG grant SFB1279; Boehringer Ingelheim Ulm University BioCenter grant D.5009; Thierry Latran foundation grant D.2468; Medical Faculty of Martin‐Luther‐University of Halle‐Wittenberg grant ClinicianScientist‐ProgrammCS22/06.
References
- 1. Hermann P, Appleby B, Brandel JP, et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt‐Jakob disease. Lancet Neurol. 2021;20(3):235‐246. doi: 10.1016/S1474-4422(20)30477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudge P, Hyare H, Green A, Collinge J, Mead S. Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry. 2018;89(5):461‐466. doi: 10.1136/jnnp-2017-316853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watson N, Hermann P, Ladogana A, et al. Validation of revised international Creutzfeldt‐Jakob disease surveillance network diagnostic criteria for sporadic Creutzfeldt‐Jakob disease. JAMA Netw Open. 2022;5(1):e2146319. doi: 10.1001/jamanetworkopen.2021.46319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mastrangelo A, Mammana A, Baiardi S, et al. Evaluation of the impact of CSF prion RT‐QuIC and amended criteria on the clinical diagnosis of Creutzfeldt‐Jakob disease: a 10‐year study in Italy. J Neurol Neurosurg Psychiatry. 2023;94(2):121‐129. doi: 10.1136/jnnp-2022-330153 [DOI] [PubMed] [Google Scholar]
- 5. Parchi P, Giese A, Capellari S, et al. Classification of sporadic Creutzfeldt‐Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol. 1999;46(2):224‐233. [PubMed] [Google Scholar]
- 6. Hermann P, Zerr I. Rapidly progressive dementias ‐ aetiologies, diagnosis and management. Nat Rev Neurol. 2022;18(6):363‐376. doi: 10.1038/s41582-022-00659-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abu‐Rumeileh S, Baiardi S, Polischi B, et al. Diagnostic value of surrogate CSF biomarkers for Creutzfeldt‐Jakob disease in the era of RT‐QuIC. J Neurol. 2019. Dec;266(12):3136‐3143. doi: 10.1007/s00415-019-09537-0 [DOI] [PubMed] [Google Scholar]
- 8. Zerr I, Villar‐Piqué A, Hermann P, et al. Diagnostic and prognostic value of plasma neurofilament light and total‐tau in sporadic Creutzfeldt‐Jakob disease. Alzheimers Res Ther. 2021;13(1):86. doi: 10.1186/s13195-021-00815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Staffaroni AM, Kramer AO, Casey M, et al. Association of blood and cerebrospinal fluid tau level and other biomarkers with survival time in sporadic Creutzfeldt‐Jakob disease. JAMA Neurol. 2019;76(8):969‐977. doi: 10.1001/jamaneurol.2019.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abu‐Rumeileh S, Baiardi S, Ladogana A, et al. Comparison between plasma and cerebrospinal fluid biomarkers for the early diagnosis and association with survival in prion disease. J Neurol Neurosurg Psychiatry. 2020;91(11):1181‐1188. doi: 10.1136/jnnp-2020-323826 [DOI] [PubMed] [Google Scholar]
- 11. Thompson AGB, Anastasiadis P, Druyeh R, et al. Evaluation of plasma tau and neurofilament light chain biomarkers in a 12‐year clinical cohort of human prion diseases. Mol Psychiatry. 2021;26(10):5955‐5966. doi: 10.1038/s41380-021-01045-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oeckl P, Halbgebauer S, Anderl‐Straub S, et al. Targeted mass spectrometry suggests Beta‐synuclein as synaptic blood marker in Alzheimer's disease. J Proteome Res. 2020;19:1310‐1318. doi: 10.1021/acs.jproteome.9b00824 [DOI] [PubMed] [Google Scholar]
- 13. Halbgebauer S, Oeckl P, Steinacker P, et al. Beta‐synuclein in cerebrospinal fluid as an early diagnostic marker of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2021;92(4):349‐356. doi: 10.1136/jnnp-2020-324306 [DOI] [PubMed] [Google Scholar]
- 14. Halbgebauer S, Abu‐Rumeileh S, Oeckl P, et al. Blood β‐synuclein and neurofilament light chain during the course of prion disease. Neurology. 2022;98(14):e1434‐e1445. doi: 10.1212/WNL.0000000000200002 [DOI] [PubMed] [Google Scholar]
- 15. Abu‐Rumeileh S, Barschke P, Oeckl P, et al. Prodynorphin and proenkephalin in cerebrospinal fluid of sporadic Creutzfeldt‐Jakob disease. Int J Mol Sci. 2022;23(4):2051. doi: 10.3390/ijms23042051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franceschini A, Baiardi S, Hughson AG, et al. High diagnostic value of second generation CSF RT‐QuIC across the wide spectrum of CJD prions. Sci Rep. 2017;7(1):10655. doi: 10.1038/s41598-017-10922-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Vos A, Jacobs D, Struyfs H, et al. C‐terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease. Alzheimers Dement. 2015;11(12):1461‐1469. doi: 10.1016/j.jalz.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 18. Halbgebauer S, Steinacker P, Hengge S, et al. CSF levels of SNAP‐25 are increased early in Creutzfeldt‐Jakob and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2022;93:1059‐1065. doi: 10.1136/jnnp-2021-328646 [DOI] [PubMed] [Google Scholar]
- 19. Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue‐based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 20. Baiardi S, Magherini A, Capellari S, et al. Towards an early clinical diagnosis of sporadic CJD VV2 (ataxic type). J Neurol Neurosurg Psychiatry. 2017;88(9):764‐772. doi: 10.1136/jnnp-2017-315942 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information.


