Abstract
Objective
Circadian and multidien cycles of seizure occurrence are increasingly discussed as to their biological underpinnings and in the context of seizure forecasting. This study analyzes if patient reported seizures provide valid data on such cyclical occurrence.
Methods
We retrospectively studied if circadian cycles derived from patient‐based reporting reflect the objective seizure documentation in 2003 patients undergoing in‐patient video‐EEG monitoring.
Results
Only 24.1% of more than 29000 seizures documented were accompanied by patient notifications. There was cyclical underreporting of seizures with a maximum during nighttime, leading to significant deviations in the circadian distribution of seizures. Significant cyclical deviations were found for focal epilepsies originating from both, frontal and temporal lobes, and for different seizure types (in particular, focal unaware and focal to bilateral tonic–clonic seizures).
Interpretation
Patient seizure diaries may reflect a cyclical reporting bias rather than the true circadian seizure distributions. Cyclical underreporting of seizures derived from patient‐based reports alone may lead to suboptimal treatment schemes, to an underestimation of seizure‐associated risks, and may pose problems for valid seizure forecasting. This finding strongly supports the use of objective measures to monitor cyclical distributions of seizures and for studies and treatment decisions based thereon.
Introduction
Epileptic seizures appear to occur unpredictably, yet not at random times. Individual patients may have predominant occurrence times during the night, at awakening, or during certain periods of the day, week, or month. 1 This may have major implications for both, timely adapted treatment schemes and for risk assessment and decisions on patient monitoring. Moreover, the cyclical structure of the manifestation of epileptic seizures is relevant in several regards. The distribution of seizures over time may provide data to model endogenous 2 , 3 , 4 or exogenous 5 , 6 factors modulating brain excitability. Cyclical seizure distributions may be relevant for SUDEP risk assessment. 7 , 8 They may also be used to timely adapt treatment schemes (“chronotherapy,” e.g., Ref. 9 ). Furthermore, cyclical distributions of seizures, when assessed over sufficient periods of time, may be used to model seizure propensity and forecast seizure occurrence. In recent years, a number of publications have reported statistically significant prediction of seizure occurrences, based on seizure diaries, 10 based on non‐EEG data derived from wearables in combination with seizure occurrence times reported by patients, 11 and based on ultralong‐term EEG recordings. 12 , 13 , 14 The validity of patient‐based seizure documentation alone has, however, been questioned as patients may not be aware of seizures, may have complete or partial retrograde amnesia of seizure manifestations 15 or may document at false time points. 14 , 16 Two studies analyzing seizure reporting during in‐hospital monitoring found an underreporting of seizures across seizure types. 17 , 18 Under ambulatory conditions, a lack of correlation between patient‐based seizure documentation and seizure patterns obtained from intracranial and subcutaneous long‐term recordings has been reported. 19 , 20
The question thus arises if underreporting of seizures by patients affects the apparent cyclical distribution of seizures in time, and if so, if the impact needs to be taken into consideration when seizure diaries are used as outcome measures, to assess seizure‐associated risks, or as a basis for seizure forecasting.
To address this issue, we analyzed in‐hospital recordings from a large patient cohort undergoing video‐EEG monitoring for seizure documentation. We retrospectively assessed patient‐based seizure reporting as available from time stamps from patients pushing an alarm button within a time window of 10 min around documented seizures, and we compared the timing of patient‐reported seizures with the circadian distribution of objectively documented seizures based on the expert analysis of simultaneous EEG and video‐recordings. We hypothesized that incomplete patient‐based seizure reporting may affect the apparent circadian distribution of seizure occurrence.
Methods
Data from n = 2003 patients undergoing long‐term video‐EEG monitoring for a mean duration of 5 days at the Freiburg Epilepsy Center, a European Reference Center (EpiCare), 32 were retrospectively reviewed for the period January 2010–February 2022. About 1214 patients (mean age: 32.8, range: 10–83, 49.6% female) presented seizures during monitoring.
Not all epilepsies of these patients were classified. Among the 1057 patients who had seizures and classification information, 89.2% had focal epilepsy, of which 45.7% had a temporal and 14.8% had a frontal lobe origin; 11.5% of patients had idiopathic generalized epilepsy (3 patients had both types of epilepsy). Similarly, seizure types were classified as to the state of awareness only when sufficient information from patient testing was available.
An electronic EEG database was retrospectively analyzed for seizure markers identified by members of the clinical team; these markers document the consensus found in seizure conferences with board‐certified clinical neurophysiologists, based on the thorough visual EEG and video‐analysis performed by a team of an epileptologist and a technician. Markers include seizure onset times as well as automatically stored times of push‐button events triggered by patients. Only clinically manifest seizures but no subclinical electrographic events were included in the analysis (for differentiating features see Ref. 22 ). All patients had been instructed to push a button placed in their monitoring bed, to report each of their seizures whenever they had a feeling suggesting a seizure. Patients aged less than 10 years were excluded from the analysis as misunderstanding or inability to comply with performing seizure reporting might have biased the results.
For patients with more than five seizures recorded during monitoring, all except five randomly selected seizures were excluded from all subsequent analyses, in order to avoid overrepresentation of patients with a high seizure frequency.
In an additional analysis, onset times of all seizures and seizure‐associated button presses were first averaged using the circular mean on a per‐patient basis, then the circular means and medians of these averages were calculated.
Patient alarms and expert documentation of seizure onsets were analyzed, and the circadian cyclical timing of patient alarms was compared to objective timings of seizure onsets. Patient‐triggered button presses were considered associated with objective seizure timing when markers of button presses were within a time window of 180 s before to 420 s after video‐EEG‐based markers of seizure onset. A time window of 3 min prior to the onset of EEG changes and objective seizure manifestations was allowed to correctly classify ictal manifestations based on subjective experiences (auras) as seizure related.
For seizures classified as focal aware, focal unaware, and bilateral tonic–clonic, percentages of correctly notified seizures were calculated separately.
The circadian occurrence of patient‐reported and of objectively documented seizures was compared using Watson's two‐sample test of homogeneity. Comparisons with circular uniform distributions were done using Watson's goodness of fit test. Tests were run on the original time points, without binning. Due to the limited number of seizures per patient, cyclical reporting was studied only across patients rather than intra‐individually.
Results
Out of a total of 29142 seizures recorded in 1214 patients, 7037 (24.1%) were reported by button presses. The median number of seizures recorded per patient was 9 (range: 1–619, mean: 24.0). The fraction of seizures reported across individuals was, minimal = 0%, maximal = 100%, mean = 31.7%, median = 16.7%. The total number of tonic–clonic seizures in the complete sample of 29142 seizures was 801, representing 2.7% of all seizures.
After limiting the maximum number of seizures included per patient to five, 5033 seizures, of which 1781 (35.4%) were reported by button presses, were used in subsequent analyses. From this subset, classification information was available for 2251 of these seizures, 533 of which (23.7%) were classified as focal aware, 600 (26.7%) as focal unaware, 419 (18.6%) as generalized tonic–clonic, and 700 (31.2%) were unclassified.
Patients reported by button press 71.1% of focal aware seizures, 26.8% of focal unaware seizures, and 26.3% of bilateral tonic–clonic seizures. About 15.8% of patients with seizures correctly reported 80% or more of their seizures, 8.8% correctly reported all seizures.
The analysis of circadian circular distributions of hourly seizure occurrences showed a significant deviation from a uniform distribution for both patient‐reported and objective seizure documentation (p < 0.01 in either case, Watson's goodness of fit test for the circular uniform distribution), demonstrating nonuniform circadian distributions for both, seizures reported by patients and for objectively documented seizures. Rates of video‐EEG‐documented seizures were highest during nighttime and the early morning hours, with a circular mean between at 5.36 AM (Fig. 1A), whereas rates of patient‐reported seizure occurrences were highest during daytime, with a circular mean at 2.46 PM (Fig. 1B). A large temporal difference was also present when averaging seizure onset times and button press times, respectively, across all the seizures of individual patients and using the circular mean or median of these per patient averages: mean seizure onset time: 06:00 AM, median: 05:05 AM; mean and median button press time: 03:15 PM.
Figure 1.
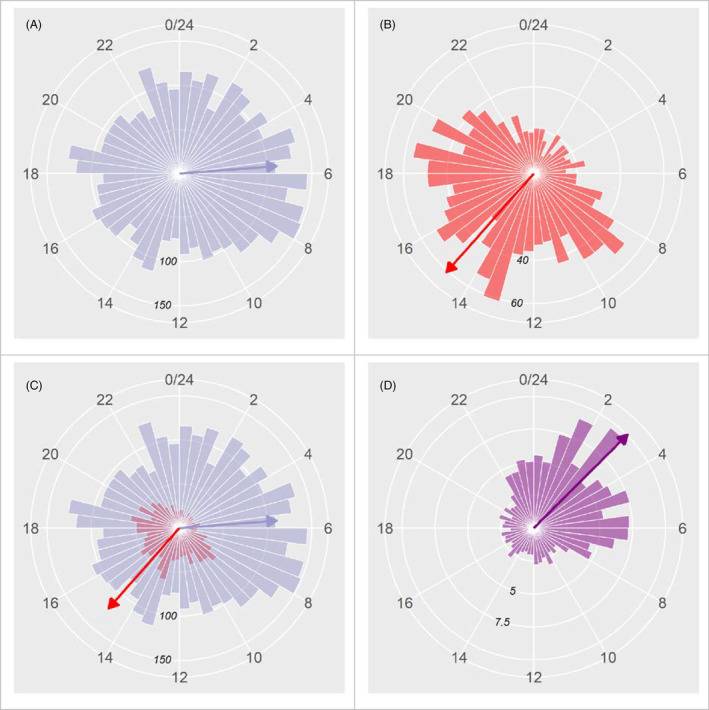
Rose plots showing the circadian distribution of objectively documented seizures (blue) and patient alarms (red). (A) Circadian distribution of objectively documented seizures as occurring during time bins of 30 min. (B) Circadian distribution of patient‐alarms. (C) Superposition of patient reporting and video‐EEG‐based seizure documentation. (D) Ratio of the number of documented seizures and patient‐reported seizures for every time bin. The time of day (0–24 h) is indicated by the numbers on the outermost circle. The numbers on the concentric inner circles indicate the event counts associated with bars of the same length as the radius of the respective circle. The arrows are resultant vectors scaled down to 1/3 (A and C) or 1/6 (B and D) of their real length to make them fit into the plots. Note that not only is the number of seizures objectively documented considerably higher at all times of the day and night, but also that the circadian distributions differ, with a maximum of patient‐reported events during the afternoon, and maximum objectively documented seizures during the night and the early morning hours. There is a cyclical underreporting of seizures by patients with maximum underreporting during nighttime.
There was a significant difference between the circadian distributions of seizure‐associated button presses and objectively documented seizures, respectively (p < 0.001, Watson's two‐sample test of homogeneity; Fig. 1C); the fraction of seizures missed for patient‐based reporting reached a maximum during nighttime (Fig. 1D).
Cyclical underreporting in the pattern of seizure occurrence times was identified for several seizure types in focal epilepsy (Figs 2 and 3). The temporal distributions of reported and documented seizures were significantly different in focal to bilateral tonic–clonic seizures (Watson's test for homogeneity on two samples of circular data, p < 0.01). A considerable part or focal seizures was unclassified as to whether awareness was preserved for the complete seizure period. The temporal distributions of reported and documented seizures were significantly different for the whole group of focal seizures, and for the subgroups of unclassified focal seizures and focal unaware seizures, respectively (Watson's test for homogeneity on two samples of circular data p < 0.01 for the whole group, p < 0.001 for unclassified seizures, and p < 0.05 for focal unaware seizures), but not for focal aware seizures. Patients who reported at least 80% of their seizures had their seizures far more frequently during daytime (Fig. 4A) and showed no significant difference in the distributions of documented and reported seizures.
Figure 2.

Circadian distributions of video‐EEG and patient‐based seizure documentation for bilateral tonic–clonic seizures (A and B), all focal seizures (C and D), focal seizures unclassified to the state of awareness (E and F). The left column of plots (A, C, and E) represents seizure times (blue) and button press times (red) separately whereas the right column (B, D, and F) shows the quotient of the number of seizures and the number of button presses for every time bin, using the same data as the plot to its left. The time of day (24 h) is indicated by the numbers on the outermost circle. The bin size is 30 min in A, C, and E, and 60 min in B, D, and F. The numbers on the concentric inner circles indicate the event counts associated with bars of the same length as the radius of the respective circle. The arrows are resultant vectors scaled down to 1/3 (B–F), 1/5 (A) of their real length to make them fit into the plots.
Figure 3.

Circadian distributions of video‐EEG and patient‐based seizure documentation for focal aware seizures (A and B), and focal unaware seizures (C and D). The left column of plots (A and C) represents seizure times (blue) and button press times (red) separately whereas the right column (B and D) shows the quotient of the number of seizures and the number of button presses for every time bin, using the same data as the plot to its left. The time of day (24 h) is indicated by the numbers on the outermost circle. The bin size is 30 min in A and C and 60 min in B and D. The numbers on the concentric inner circles indicate the event counts associated with bars of the same length as the radius of the respective circle. The arrows are resultant vectors scaled down to 1/3 (B–D) or 1/6 (A) of their real length to make them fit into the plots.
Figure 4.

Circadian distributions of video‐EEG and patient‐based seizure documentation for patients with ≥80% success rate (A and B), temporal lobe epilepsy (C and D), and frontal lobe epilepsy (E and F). The left column of plots (A, C, and E) represents seizure times (blue) and button press times (red) separately, whereas each plot in the right column (B, D, and F) shows the quotient of the number of seizures and the number of button presses for every time bin, using the same data as the plot to its left. The time of day (24 h) is indicated by the numbers on the outermost circle. The bin size is 30 min in A, C, and E and 60 min in B, D, and F. The numbers on the concentric inner circles indicate the event counts associated with bars of the same length as the radius of the respective circle. The arrows are resultant vectors scaled down (except for b) to 1/3 (C, D, and F) or 1/6 (A and E) of their real length to make them fit into the plots.
Major differences between the timing of patient‐reported versus documented seizures were present also in subgroups of patients with identified temporal lobe epilepsy, frontal lobe epilepsy, and idiopathic generalized epilepsy. About 33.3% of seizures of temporal origin were reported by patients, in contrast to only 19.9% of frontal lobe seizures and 13.2% of primarily generalized seizures. Differences in circular means of reported and documented seizures were largest in temporal lobe epilepsy (3.12 PM in reported vs. 8.34 AM in documented seizures; Fig. 4C), in the range of several hours in frontal lobe epilepsy (0.56 AM for reported vs. 3:45 AM for documented seizures; Fig. 4E), but with an almost symmetrical circular distribution for reported seizure times. The temporal distributions of reported seizures were significantly different from those of documented seizures in all three subgroups (Watson's test for homogeneity on two samples of circular data, temporal lobe epilepsy: p < 0.001; frontal lobe epilepsy: p < 0.01; idiopathic generalized epilepsy: p < 0.05).
Discussion
The analysis of more than 29000 seizures from more than 1200 patients with ascertained epilepsy provides evidence of a cyclical circadian pattern of underreporting of seizures. Not only was the majority of seizures unreported by patients; this underreporting resulted in a major deviation of the objective circadian distribution of seizures from what patients reported. Diurnal variation in patient reporting of seizure events was almost the inverse of the diurnal variation of objectively documented seizures based on video‐EEG. Of note, the study did not include false‐positive patient‐based seizure reporting, which may additionally widen the gap between objective and subjective timing of seizures. 19
A correct reporting of 24% of the complete set of seizures (or 35.4% of a subgroup of seizures randomly selected for statistical analysis) by patients as found here is in the lower range of earlier reports 17 , 18 , 23 , 25 which revealed an overall correct documentation of, respectively, 28–49% of seizures in smaller patient cohorts undergoing in‐patient video‐EEG monitoring. This may depend on the epilepsy syndromes assessed, and might reflect a lower percentage of focal epilepsies of temporal origin in our study. An even lower percentage (18.3%) of seizures correctly reported was, however, found under ambulatory conditions when using objective documentation with the NeuroVista implant based on combined intracranial EEG and acoustic recordings 19 and using ultralong subscalp EEG recordings. 12 Thus, underreporting of seizures was confirmed in our analysis in a larger cohort comprising patients across ages from 10 years and above. Problems resulting for treatment decisions, in medicolegal issues like permission to drive, and for the validity of clinical trials have been highlighted recently. 27 , 28
A preferred reporting of seizures by patients during daytime, as found in our cohort, corresponds to the results of an analysis using the electronic seizure diary “Seizure Tracker” in a very large patient cohort, 27 similar to in‐hospital studies. 18 Entries in seizure diaries thus appear to support the notion of cyclical dependencies of seizure occurrence, as reported by Karoly et al. 10 and discussed in several recent studies of seizure forecasting. 11 , 30 , 33 , 35 The circadian cycles with daytime preponderance of seizure occurrence, reported on the basis of unvalidated patient‐provided timing information, may, however, at least in part, reflect a cyclical reporting bias rather than the true circadian seizure distributions. Future studies addressing sleep stages and the brain areas involved in ictal spread may contribute to a better understanding of mechanisms involved in unawareness and amnesia for seizures.
A limitation of this study is that it was performed under in‐hospital conditions which differ from an outpatient setting, and that push‐button events during video‐EEG monitoring may not necessarily be identical to entries into seizure diaries; in particular, patients might retrospectively enter seizure occurrence data in a diary outside the 10‐min time window chosen here to classify button‐presses as seizure related. This limitation is inherent in using video‐EEG‐monitoring considered the gold standard to identify and document seizures; seizures neither documented during the early ictal nor during the immediate postictal phase are, however, certainly candidates for underreporting also in a seizure diary. The identification of clinical events is limited even in an in‐hospital setting; thus very mild seizure forms may have occurred which are underrepresented in this study (see also Ref. 22 ). Depending on the presence of other persons, seizure diaries which additionally include caregiver or family member entries may better reflect the circadian distribution than found in the setting of in‐patient video‐EEG monitoring (see, however, underreporting also by parents (Akman et al. 2009)). 31 In addition, patients may retrospectively report seizure types with overlasting postictal impairments, in particular following bilateral tonic–clonic seizures, at the time of awakening. Thus, quantitatively the peri‐ictal time period of 10 min may overestimate the degree of underreporting. On the other hand, bilateral tonic–clonic seizures were a limited subgroup of seizure, and retrospective reporting of seizures with long delays cannot be expected to solve the problem of an incorrect circadian attribution of seizure time points.
This study addressed circadian cycles rather than multidien cycles of weeks to months, due to the limited duration of in‐patient recordings. 32 Thus, forecasting on a daily basis as reported by Goldenholz et al. 33 using seizure diaries and as used in the study by Proix et al. (2021) using data from intracranial EEG recordings may be less affected by cyclical seizure underreporting. 34
The finding of a cyclical underreporting of seizures is of considerable relevance for studies analyzing biological mechanisms underlying seizure cycles, for studies assessing treatment effects on seizure cycles, 35 and for studies aiming at forecasting seizures. Obviously in these settings, the additional consideration of objective data for establishing the time distribution of seizures appears appropriate in order to identify valid time points of seizure occurrence (e.g., Ref. 36 ). In particular, hourly forecasting approaches based only on seizures which are reported by patients may be suboptimal in the vast majority of cases, as up to 75% of seizures would then remain neglected. Importantly, we found a circadian bias in patient reporting. In‐hospital optimization of seizure forecasting algorithms is insufficient in many patients, 21 the use of outpatient data in an ambulatory setting appears imperative, and the use of seizure cycles, circadian or multidien, have become of high interest in such long‐term recordings. Using diaries only, however, may lead to inaccurate estimation of forecasting performance, if correctly predicted seizure occurrence periods are falsely considered seizure‐free periods due to inadequate patient documentation. Circadian underreporting, on the other hand, may lead to suboptimal training of forecasting algorithms, if distributions over time are based on patient alarms only. New options to perform continuous ultralong objective seizure assessment in outpatient settings 12 , 14 , 31 , 41 will allow to study intra‐individual cycles, as do studies with intracranially implanted devices assessing EEG and clinical seizure features. 38
Information as to how accurately patients are able to document seizures is also critical for employing seizure diaries for clinical decisions, including the assessment of SUDEP risk, 7 , 8 , 24 particularly given the circadian underreporting of more severe seizure types. It may also be crucial to define subpopulations which are appropriate to conduct research. For the majority of patients, the circadian underreporting of seizures as revealed here strongly calls for basing the analyses of seizure occurrence patterns on datasets integrating complementary information on seizure occurrence times, for example, using data obtained by wearables or ultralong EEG recording systems. 14 Time stamps integrating objective information may considerably better serve as ground truths for the assessment of the performance of detection and prediction algorithms, 33 by reducing the effects of cyclical underreporting of seizures by patients.
Whereas the unawareness of epilepsy patients for the majority of their seizures may be considered a disease‐specific feature, differences between the subjective and objective severity of disease manifestations are relevant also to other neurological diseases, calling for studies on the relative role of patient‐based reporting and objective assessment also in other contexts (e.g., Refs. 32 , 34 ).
Funding Information
This study did not receive external funding.
Author Contributions
ASB and MR contributed to the conception and design of the study. ASB, MR, AB, and MD contributed to the acquisition and analysis of data. ASB, MR, AB, NZ, MD, and VSAA contributed to drafting of the manuscript. All authors approved the final draft of the manuscript.
Conflict of Interest
ASB has received research funding from EU and American Epilepsy Foundation for the assessment of seizure occurrence using wearables and EEG recordings, and receives research funding from the company UNEEG for an ongoing clinical multicenter trial on subcutaneous ultralong‐term EEG recordings. MR has received research funding from EU and American Epilepsy Foundation for the assessment of seizure occurrence using wearables and EEG recordings. The other authors have nothing to report.
Acknowledgments
The authors would like to express their gratitude to the team of technicians and medical doctors involved in seizure documentation, ictal testing, and classification of seizures during in‐patient video‐EEG monitoring. Open Access funding enabled and organized by Projekt DEAL.
[Correction added on 12 September 2023, after first online publication: The reference citation numbers were corrected throughout the article.]
Contributor Information
Andreas Schulze‐Bonhage, Email: andreas.schulze-bonhage@uniklinik-freiburg.de.
Mark P. Richardson, Email: mark.richardson@kcl.ac.uk.
Armin Brandt, Email: armin.brandt@uniklinik-freiburg.de.
Nicolas Zabler, Email: nicolas.zabler@uniklinik-freiburg.de.
Matthias Dümpelmann, Email: matthias.duempelmann@uniklinik-freiburg.de.
Victoria San Antonio‐Arce, Email: victoria.san.antonio@uniklinik-freiburg.de.
References
- 1. Griffiths G, Fox JT. Rhythm in epilepsy. Lancet. 1938;232(5999):409‐416. [Google Scholar]
- 2. Khan S, Khurana M, Vyas P, Vohora D. The role of melatonin and its analogues in epilepsy. Rev Neurosci. 2020;32:49‐67. doi: 10.1515/revneuro-2019-0088 [DOI] [PubMed] [Google Scholar]
- 3. Kreitlow BL, Li W, Buchanan GF. Chronobiology of epilepsy and sudden unexpected death in epilepsy. Front Neurosci. 2022;16:936104. doi: 10.3389/fnins.2022.936104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Campen JS, Valentijn FA, Jansen FE, Joëls M, Braun KP. Seizure occurrence and the circadian rhythm of cortisol: a systematic review. Epilepsy Behav. 2015;47:132‐137. doi: 10.1016/j.yebeh.2015.04.071 [DOI] [PubMed] [Google Scholar]
- 5. Haut SR, Hall CB, Masur J, Lipton RB. Seizure occurrence: precipitants and prediction. Neurology. 2007;69(20):1905‐1910. doi: 10.1212/01.wnl.0000278112.48285.84 [DOI] [PubMed] [Google Scholar]
- 6. Tényi D, Janszky J, Jeges S, Schulze‐Bonhage A. Food intake precipitates seizures in temporal lobe epilepsy. Sci Rep. 2021;11:16515. doi: 10.1038/s41598-021-96106-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryvlin P, Rheims S, Lhatoo SD. Risks and predictive biomarkers of sudden unexpected death in epilepsy patient. Curr Opin Neurol. 2019;32(2):205‐212. doi: 10.1097/WCO.0000000000000668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serrand C, Rheims S, Faucanié M, et al. Stratifying sudden death risk in adults with drug‐resistant focal epilepsy: the SUDEP‐CARE score. Eur J Neurol. 2023;30:22‐31. doi: 10.1111/ene.15566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun S, Wang H. Clocking epilepsies: a chronomodulated strategy‐based therapy for rhythmic seizures. Int J Mol Sci. 2023;24(4):4223. doi: 10.3390/ijms24044223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Karoly PJ, Cook MJ, Maturana M, et al. Forecasting cycles of seizure likelihood. Epilepsia. 2020;61:776‐786. doi: 10.1101/2019.12.19.19015453 [DOI] [PubMed] [Google Scholar]
- 11. Karoly PJ, Rao VR, Gregg NM, et al. Cycles in epilepsy. Nat Rev Neurol. 2021;17:267‐284. [DOI] [PubMed] [Google Scholar]
- 12. Hirsch M, Novitskaya Y, Schulze‐Bonhage A. Value of ultra‐longterm subcutaneous EEG monitoring for treatment decisions in temporal lobe epilepsy. Epilepsia Open. 2023. 8 (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stirling RE, Grayden DB, D'Souza W, et al. Forecasting seizure likelihood with wearable technology. Front Neurol. 2021;15(12):704060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viana PF, Duun‐Henriksen J, Glasstetter M, et al. 230 days of ultra long‐term subcutaneous EEG: seizure cycle analysis and comparison to patient diary. Ann Clin Transl Neurol. 2021;8:288‐293. doi: 10.1002/acn3.51261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mielke H, Meissner S, Wagner K, Joos A, Schulze‐Bonhage A. Which seizure elements do patients memorize? A comparison of history and seizure documentation. Epilepsia. 2020;61:1365‐1375. doi: 10.1111/epi.16550 [DOI] [PubMed] [Google Scholar]
- 16. Maiwald T, Blumberg J, Timmer J, Schulze‐Bonhage A. Are prodromes preictal events? A prospective PDA‐based study. Epilepsy Behav. 2011;21:184‐188. doi: 10.1016/j.yebeh.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 17. Blum DE, Eskola J, Bortz JJ, Fisher RS. Patient awareness of seizures. Neurology. 1996;47:260‐264. doi: 10.1212/wnl.47.1.260 [DOI] [PubMed] [Google Scholar]
- 18. Hoppe C, Poepel A, Elger CE. Epilepsy: accuracy of patient seizure counts. Arch Neurol. 2007;64(11):1595‐1599. doi: 10.1001/archneur.64.11.1595 [DOI] [PubMed] [Google Scholar]
- 19. Cook MJ, O'Brien TJ, Berkovic SF, et al. Prediction of seizure likelihood with a long‐term, implanted seizure advisory system in patients with drug‐resistant epilepsy: a first‐in‐man study. Lancet Neurol. 2013;12:563‐571. doi: 10.1016/S1474-4422(13)70075-9 [DOI] [PubMed] [Google Scholar]
- 20. Weisdorf S, Duun‐Henriksen J, Kjeldsen MJ, Poulsen FR, Gangstad SW, Kjær TW. Ultra‐long‐term subcutaneous home monitoring of epilepsy‐490 days of EEG from nine patients. Epilepsia. 2019;60:2204‐2214. doi: 10.1111/epi.16360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schulze‐Bonhage A. Seizure prediction: time for new, multimodal and ultra‐long‐term approaches. Clin Neurophysiol. 2022;133:152‐153. doi: 10.1016/j.clinph.2021.10.006 [DOI] [PubMed] [Google Scholar]
- 22. Castillo Rodriguez MLA, Brandt A, Schulze‐Bonhage A. Differentiation of subclinical and clinical electrographic events in long‐term electroencephalographic recordings. Epilepsia. 2022. doi: 10.1111/epi.17401 [DOI] [PubMed] [Google Scholar]
- 23. Kerling F, Mueller S, Pauli E, Stefan H. When do patients forget their seizures? An electroclinical study. Epilepsy Behav. 2006;9:281‐285. doi: 10.1016/j.yebeh.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 24. Poochikian‐Sarkissian S, Tai P, del Campo M, et al. Patient awareness of seizures as documented in the epilepsy monitoring unit. Can J Neurosci Nurs. 2009;31:22 23. [PubMed] [Google Scholar]
- 25. Elger CE, Hoppe C. Diagnostic challenges in epilepsy: seizure under‐reporting and seizure detection. Lancet Neurol. 2018;17:279‐288. doi: 10.1016/S1474-4422(18)30038-3 [DOI] [PubMed] [Google Scholar]
- 26. Fisher RS, Blum DE, DiVentura B, et al. Seizure diaries for clinical research and practice: limitations and future prospects. Epilepsy Behav. 2012;24:304‐310. doi: 10.1016/j.yebeh.2012.04.128 [DOI] [PubMed] [Google Scholar]
- 27. Ferastraoaru V, Goldenholz DM, Chiang S, Moss R, Theodore WH, Haut SR. Characteristics of large patient‐reported outcomes: where can one million seizures get us? Epilepsia Open. 2018;3:364‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baud MO, Kleen JK, Mirro EA, et al. Multi‐day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9:88. doi: 10.1038/s41467-017-02577-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karoly PJ, Goldenholz DM, Freestone DR, et al. Circadian and circaseptan rhythms in human epilepsy: a retrospective cohort study. Lancet Neurol. 2018;17(11):977‐985. [DOI] [PubMed] [Google Scholar]
- 30. Rao VR, Leguia MG, Tcheng TK, Baud MO. Cues for seizure timing. Epilepsia. 2021;62(Suppl 1):S15‐S31. doi: 10.1111/epi.16611 [DOI] [PubMed] [Google Scholar]
- 31. Akman CI, Montenegro MA, Jacob S, Eck K, Chiriboga C, Gilliam F. Seizure frequency in children with epilepsy: factors influencing accuracy and parental awareness. Seizure. 2009;18(7):524‐529. doi: 10.1016/j.seizure.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 32. Schulze‐Bonhage A, Bruno E, Brandt A, et al. Diagnostic yield and limitations of in‐hospital documentation in patients with epilepsy. Epilepsia. 2022. doi: 10.1111/epi.17307 [DOI] [PubMed] [Google Scholar]
- 33. Goldenholz DM, Goldenholz SR, Romero J, Moss R, Sun H, Westover B. Development and validation of forecasting next reported seizure using e‐diaries. Ann Neurol. 2020;88:588‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Proix T, Truccolo W, Leguia MG, et al. Forecasting seizure risk in adults with focal epilepsy: a development and validation study. Lancet Neurol. 2021;20:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gregg NM, Sladky V, Nejedly P, et al. Thalamic deep brain stimulation modulates cycles of seizure risk in epilepsy. Sci Rep. 2021;11:24250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gregg NM, Pal Attia T, Nasseri M, et al. Seizure occurrence is linked to multiday cycles in diverse physiological signals. Epilepsia. 2023;64:1627‐1639. doi: 10.1111/epi.17607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Böttcher S, Vieluf S, Bruno E, et al. Data quality evaluation in wearable monitoring. Sci Rep. 2022;12(1):21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karoly PPJ, Ung H, Grayden DB, et al. The circadian profile of epilepsy improves seizure forecasting. Brain. 2017;140:2169‐2182. [DOI] [PubMed] [Google Scholar]
- 39. Purnell BS, Thijs RD, Buchanan GF. Dead in the night: sleep‐wake and time‐of‐day influences on sudden unexpected death in epilepsy. Front Neurol. 2018;9:1079. doi: 10.3389/fneur.2018.01079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khodakarami H, Shokouhi N, Horne M. A method for measuring time spent in bradykinesia and dyskinesia in people with Parkinson's disease using an ambulatory monitor. J Neuroeng Rehabil. 2021;18:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lipsmeier F, Taylor KI, Kilchenmann T, et al. Evaluation of smartphone‐based testing to generate exploratory outcome measures in a phase 1 Parkinson's disease clinical trial. Mov Disord. 2018;33:1287‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]


