Abstract
We have determined the nucleotide sequence of the blaS gene encoding the carbapenem-hydrolyzing L-1 β-lactamase from Stenotrophomonas maltophilia GN12873. Analysis of the DNA and deduced amino acid sequences identified a product of 290 amino acids. Comparisons of the L-1 amino acid sequence with those of other zinc β-lactamases showed 88.6% identity with the L-1 enzyme from S. maltophilia IID1275 and less than 20% identity with other class B metalloenzymes.
Two β-lactamases, L-1 and L2, cause β-lactam resistance in Stenotrophomonas maltophilia. The native L-1 metalloenzyme has an isoelectric point of 6.9 and a molecular mass of 118 kDa. The L-1 enzyme from S. maltophilia IID1275 has been sequenced and has low identity with all other known metallo-β-lactamase enzymes (18).
We have reported the cloning in Escherichia coli of the blaS gene producing the chromosomal L-1 β-lactamase from S. maltophilia GN12873 having biochemical properties and an isoelectric point similar to those of the enzyme expressed in the parental strain (5). We present here the nucleotide sequence of the blaS gene and comparisons between the L-1 enzymes from strains IID1275 and GN12873, showing the heterogeneity of S. maltophilia enzymes. A multiple alignment defined the conserved amino acid boxes found in all class B metalloenzymes.
The bacterial strains and plasmids used in this study are described in Table 1. E. coli DH5α was the recipient strain used for construction, maintenance, and propagation of recombinant plasmids. Bacterial cells were routinely grown on tryptic soy agar (Difco Laboratories, Detroit, Mich.) containing appropriate antibiotics (ampicillin, 50 μg/ml; imipenem, 10 μg/ml; kanamycin, 50 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| E. coli DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 15 |
| S. maltophilia GN12873 | bla (L-1 and L2) | 14 |
| Plasmids | ||
| pBGS18+ | KmrlacZ | 15 |
| pMON01 | 2.6-kb Sau3A fragment cloned into pACYC184Cmr | 5 |
| pMON13 | Kmr Apr; 1.6-kb HindIII-SalI fragment of pMON01 in pBGS18+ | This work |
Abbreviations: Apr, ampicillin resistance; Cmr, chloramphenicol resistance; Kmr, kanamycin resistance.
Large plasmid DNA preparations were done by using the Qiagen Maxi Kit (Qiagen, Chatsworth, Calif.). Restriction enzyme, T4 ligase, and DNA-modifying enzyme reactions were done as recommended by the manufacturer (New England Biolabs, Inc., Beverly, Mass.). Construction of recombinant molecules and transformation and selection of bacterial clones were done by standard procedures (15).
Nucleotide sequence determinations were done on an Applied Biosystems 373 DNA sequencer using ABI Prism dye terminator cycle sequencing ready reaction kits with the AmpliTaq DNA polymerase protocol as recommended by the manufacturer (Perkin-Elmer, Mississauga, Ontario, Canada). Sequencing primers were usually 21-mers selected from the last 50 nucleotides read from chromatograms and synthesized on a Beckman Oligo1000 DNA synthesizer.
Electrophoretograms were visualized by using Factura, Gene Navigator, and AutoAssembler software (ABI). DNA sequence analysis was done with the Genetics Computer Group software package (Wisconsin Package Version 9.0; Genetics Computer Group, Madison, Wis.). Molecular masses were predicted from amino acid sequences as previously described (2). Multiple alignments were done with PileUp and CLUSTAL W (16).
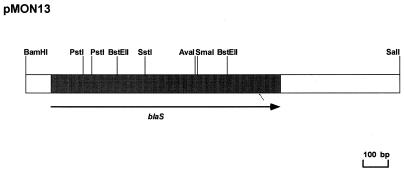
The physical map of encoding L-1-pMON13 is shown in Fig. 1. Restriction endonuclease sites deduced from the DNA sequences matched the physical map previously reported (5), except for one PstI site not identified as two restriction sites separated by only 12 nucleotides.
FIG. 1.
Physical map of recombinant plasmid pMON13. The open boxes represent the 1.4-kb BamHI-SalI DNA fragment from pMON01 cloned into pBGS18+. The shaded box represents the position of the blaS gene in the cloned moiety, and the arrow indicates the direction of transcription.
The complete nucleotide sequence obtained is shown in Fig. 2 and is 1,425 bp long. Analysis of the nucleotide sequence revealed an open reading frame (ORF), selected by a BLAST search with the PIR database, long enough to encode the putative L-1 polypeptide. We determined that the G+C content was 56.6% for the 5′ upstream region of the ORF, 68.7% within the ORF, and 68.4% for the 3′ downstream region. A putative ribosome binding site was found 10 bp upstream of an ATG start codon (positions 100 to 102 in Fig. 2), but no putative promoter was identified. One possible terminator was localized at the end of the ORF (nucleotide positions 947 to 963 in Fig. 2), and a second terminator forming a loop structure was localized 260 bp downstream of the termination codon (positions 1229 to 1250). The deduced polypeptide was 290 amino acids long with a calculated putative signal peptide of 33 amino acids which agreed with the previously published N-terminal peptide sequence, except for 8 amino acids (1). The molecular weight of the L-1 metallo-β-lactamase from strain GN12873 was similar to the value obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (1, 14).
FIG. 2.
Nucleotide and deduced amino acid sequences of blaS encoding the L-1 metallo-β-lactamase from S. maltophilia GN12873. The proposed Shine-Dalgarno ribosome binding site (RBS) is identified. The 33-amino-acid leader peptide underlined has been identified previously (1). The termination codon is indicated by an asterisk, and the putative terminators, ter1 and ter2, for secondary loop structures are underlined. Vertical arrows identify the cutting sites mapped for restriction endonucleases.
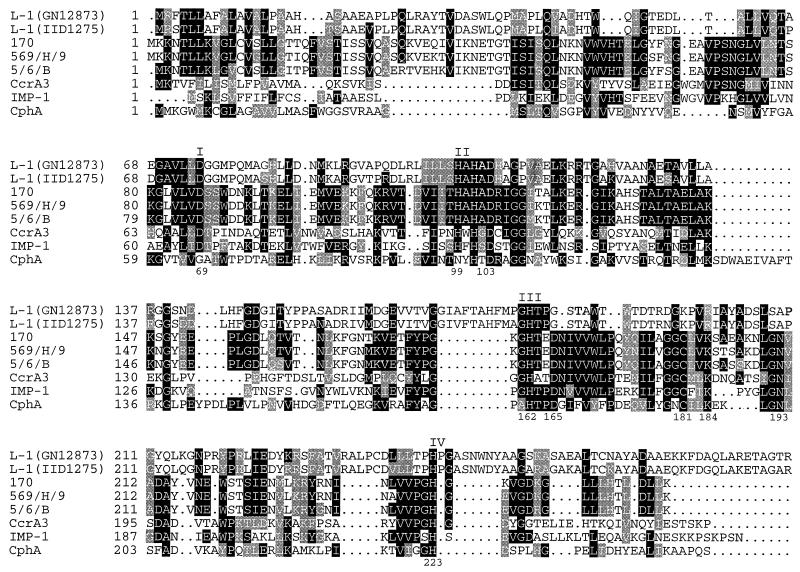
Curiously, 12 to 14% heterogeneity was observed between the nucleotide and amino acid sequences of the L-1 enzymes from strains GN12873 and IID1275. We found 88.7% DNA identity between both of the blaS structural genes (data not shown). Comparisons between the L-1 amino acid sequences showed 33 amino acid changes in a total of 290 amino acids, mostly in regions outside conserved amino acid boxes that we identified (Fig. 3). Such heterogeneity is unique to the family of L-1 chromosomal enzymes; other β-lactamase types, such as class C chromosomal enzymes, have not been shown to have such heterogeneity within the same species of bacteria.
FIG. 3.
Alignment of the L-1 amino acid sequences with the sequences of eight class B metallo-β-lactamases. The black boxes identify amino acids conserved in four of the eight polypeptides, and the gray boxes indicate amino acids that are similar in four of the eight polypeptides. Amino acids that are conserved in seven of the eight metallo-β-lactamases and known to be important in the crystal structure available are represented as boxes I to IV. Numbering of amino acids follows that of the B. fragilis CcrA3 enzyme (4). Sequences: L-1 (GN12873), from S. maltophilia GN12873 (5; this work) (GenBank accession no. AF010282); L-1 (IID1275), from S. maltophilia IID1275 (18) (GenBank accession no. S45349); 170, from alkalophilic Bacillus sp. strain 170 (7) (GenBank accession no. M15350); 569/H/9, from B. cereus 569/H/9 (6) (GenBank accession no. M11189); 5/6/B, from B. cereus 5/6/B (8) (GenBank accession no. M19530); CcrA3, from B. fragilis QMCN3 (12); IMP-1, from S. marcescens TN9106 (10) (GenBank accession no. D50438) (identical to the amino acid sequence of the K. pneumoniae metallo-β-lactamase from plasmid pDK4-4 [GenBank accession no. D29636]); CphA, from A. hydrophila AE036 (23) (GenBank accession no. X57102).
Additional comparisons were done between the GN12873 L-1 enzyme and all other known class B metalloenzymes, and the results are summarized in Table 2. Amino acid identity between the family of L-1 β-lactamases and all other class B enzymes was low. CcrA (11; GenBank accession no. M63556), CfiA (17), CcrA3 (12), and CcrA4 (12) differed by zero to four amino acids (13). CphA2 (GenBank accession no. U60294) and CphA (9; GenBank accession no. X57102) shared greater than 95% amino acid identity (13). Thus, only CphA and CcrA3 were included in Table 2.
TABLE 2.
Percent pairwise identities between class B metallo-β-lactamase sequences
| β-Lactamase | % Identitya between amino acid sequences
|
|||||||
|---|---|---|---|---|---|---|---|---|
| L-1 of GN12873 | L-1 of IID1275 | 569/H/9 | 170 | 5/6/B | IMP-1 | CcrA3 | CphA | |
| L-1 of GN12873 | ||||||||
| L-1 of IID1275 | 88.6 | |||||||
| 569/H/9 | 19.0 | 19.7 | ||||||
| 170 | 19.0 | 19.3 | 93.0 | |||||
| 5/6/B | 18.1 | 19.1 | 90.3 | 89.5 | ||||
| IMP-1 | 14.5 | 13.5 | 30.1 | 30.1 | 30.9 | |||
| CcrA3 | 12.5 | 11.2 | 25.5 | 24.0 | 25.2 | 32.2 | ||
| CphA | 12.5 | 10.9 | 23.8 | 24.6 | 24.6 | 18.6 | 22.9 | |
Identity values are expressed as percentages calculated with the complete amino acid sequences, including the signal peptides. The values were derived by dividing the number of perfect matches by the length of the shorter sequence, excluding gaps, in a multiple-alignment method.
The alignment obtained for class B β-lactamases is shown in Fig. 3. Amino acids conserved in four of eight polypeptides are shaded black, and amino acids similar in four of eight polypeptides are shaded gray; these identified four regions conserved among seven of the eight metalloenzymes used in the multiple alignment. The multiple alignment was constructed by taking into consideration the three-dimensional structures of the Bacillus cereus 569/H/9 and Bacteroides fragilis (CcrA3) β-lactamases (3, 4) and using the numbering scheme of CcrA3. The alignment obtained was similar to what others have proposed for class B β-lactamases when considering active-site elements (3, 4, 13). The difference in our alignment appeared as a gap in the C-terminal region and was introduced by the family of L-1 enzymes used in the alignment.
The alignment shown in Fig. 3 identified conserved features among class B metalloenzymes that can be correlated to structure and function. The blocks of amino acids conserved in box I contained the triad Leu, Val, and Ile-Asp that is found in all class B enzymes including Asp69, which is known to be buried in the structure and has been implicated in zinc ligand positioning (4). Box II contained the His Xaa His Xaa Asp consensus sequence which has been proposed as the ligand of Zn1 and Zn2 in the CcrA3 enzyme structure (4). Box III contained the consensus sequence Gly His162 Thr, which, along with His99 and His101, forms the ligand of Zn1. Box IV contained His223, which is the ligand of Zn2 (4).
Nucleotide sequence accession number.
The sequence reported here has been assigned GenBank accession no. AF010282.
Acknowledgments
We thank Y. Saino, Gunma University, Maebashi, Japan, for S. maltophilia GN12873; J. Renaud, Université Laval, for technical assistance in nucleotide sequencing; O. Herzberg, CARB, University of Maryland, College Park, for comments regarding the L-1 structure; and J.-M. Frère, Université de Liège, Liège, Belgium, for confirmation of the L-1 sequence reported here.
This study was supported by grants to R.C.L. from the Canadian Center of Excellence as a member of the Canadian Bacterial Diseases Network. R.C.L. is a Research Scholar of Exceptional Merit of and J.D. obtained a studentship from Le Fonds de la Recherche en Santé du Québec.
REFERENCES
- 1.Bicknell R, Emanuel E L, Gagnon J, Waley S G. The production and molecular properties of the zinc β-lactamase of Pseudomonas maltophilia IID1275. Biochem J. 1985;229:791–797. doi: 10.1042/bj2290791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjellqvist B G, Hughes J, Pasquali C, Paquet N, Ravier F, Sanchez J-C, Frutiger S, Hochstrasser D F. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis. 1993;14:1023–1031. doi: 10.1002/elps.11501401163. [DOI] [PubMed] [Google Scholar]
- 3.Carfi A, Pares S, Dunee E, Galleni M, Duez C, Frere J M, Dideberg O. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 1995;14:4914–4921. doi: 10.1002/j.1460-2075.1995.tb00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Concha N O, Rasmussen B A, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc β-lactamase from Bacteroides fragilis. Structure. 1996;4:823–836. doi: 10.1016/s0969-2126(96)00089-5. [DOI] [PubMed] [Google Scholar]
- 5.Dufresne J, Vézina G, Levesque R C. Cloning and expression of the imipenem-hydrolyzing β-lactamase operon from Pseudomonas maltophilia in Escherichia coli. Antimicrob Agents Chemother. 1988;32:819–826. doi: 10.1128/aac.32.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain M, Carlino A, Madonna M J, Lampen J O. Cloning and sequencing of the metallothioprotein β-lactamase II gene of Bacillus cereus 569/H in Escherichia coli. J Bacteriol. 1985;164:223–229. doi: 10.1128/jb.164.1.223-229.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato C, Kudo T, Watanabe K, Horikoshi K. Nucleotide sequence of the β-lactamase gene of alkalophilic Bacillus sp. strain 170. J Gen Microbiol. 1985;131:3317–3324. doi: 10.1099/00221287-131-12-3317. [DOI] [PubMed] [Google Scholar]
- 8.Lim H M, Pene J J, Shaw R. Cloning, nucleotide sequence, and expression of the Bacillus cereus 5/B/6 β-lactamase II structural gene. J Bacteriol. 1988;170:2873–2878. doi: 10.1128/jb.170.6.2873-2878.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massida O, Rossolini G M, Satta G. The Aeromonas hydrophilia cphA gene: molecular heterogeneity among class B metallo-β-lactamases. J Bacteriol. 1991;173:4611–4671. doi: 10.1128/jb.173.15.4611-4617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osano E, Arakawa Y, Wacharotayankun R, Ohta M, Horii T, Ito H, Yoshimura F, Kato N. Molecular characterization of an enterobacterial metallo-β-lactamase found in a clinical isolate of Serratia marcescens that shows imipenem resistance. Antimicrob Agents Chemother. 1994;38:71–78. doi: 10.1128/aac.38.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen B A, Gluzman Y, Tally F P. Cloning and sequencing of the class B β-lactamase gene (ccrA) from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1990;34:1590–1592. doi: 10.1128/aac.34.8.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen B A, Gluzman Y, Tally F P. Escherichia coli chromosomal mutations that permit direct cloning of the Bacteroides fragilis metallo-β-lactamase gene, ccrA. Mol Microbiol. 1991;5:1211–1219. doi: 10.1111/j.1365-2958.1991.tb01895.x. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen B A, Bush K. Carbapenem-hydrolyzing β-lactamases. Antimicrob Agents Chemother. 1997;41:223–232. doi: 10.1128/aac.41.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saino Y, Kobayashi F, Inoue M, Mitsuhashi S. Purification and properties of inducible penicillin β-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982;22:564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 16.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson J S, Malamy M H. Sequencing the gene for an imipenem-cefoxitin-hydrolyzing enzyme (CfiA) from Bacteroides fragilis TAL2480 reveals strong similarity between CfiA and Bacillus cereus β-lactamase II. J Bacteriol. 1990;172:2584–2593. doi: 10.1128/jb.172.5.2584-2593.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh T R, Hall L, Assinder S J, Nichols W W, Cartwright S J, MacGowan A P, Bennett P M. Sequence analysis of the L1 metallo-β-lactamase from Xanthomonas maltophila. Biochim Biophys Acta. 1994;1218:199–201. doi: 10.1016/0167-4781(94)90011-6. [DOI] [PubMed] [Google Scholar]