ABSTRACT
Objective:
COVID-19 has been associated with a significant burden to those who survive the acute phase. We aimed to describe the quality of life and symptoms of anxiety, depression, and posttraumatic stress disorder (PTSD) at 90 days after hospital discharge of COVID-19 patients.
Methods:
Patients with COVID-19 admitted to a private hospital in the city of São Paulo, Brazil, between April of 2020 and April of 2021 were interviewed by telephone at 30 and 90 days after discharge to assess the quality of life and symptoms of depression, anxiety, and PTSD.
Results:
A total of 2,138 patients were included. The mean age was 58.6 ± 15.8 years, and the median length of hospital stay was 9.0 (5.0-15.8) days. Between the two time points, depression increased from 3.1% to 7.2% (p < 0.001), anxiety increased from 3.2% to 6.2% (p < 0.001), and PTSD increased from 2.3% to 5.0% (p < 0.001). At least one physical symptom related to COVID-19 diagnosis persisted in 32% of patients at day 90.
Conclusions:
Persistence of physical symptoms was high even at 90 days after discharge. Although the prevalence of symptoms of anxiety, depression, and PTSD was low, these symptoms persisted for three months, with a significant increase between the time points. This finding indicates the need to identify at-risk patients so that they can be given an appropriate referral at discharge.
Keywords: COVID-19; Anxiety; Depression; Stress disorders, post-traumatic; Quality of life; Critical care outcomes
RESUMO
Objetivo:
A COVID-19 tem sido associada a um fardo significativo para aqueles que sobrevivem à fase aguda. Nosso objetivo foi descrever a qualidade de vida e sintomas de ansiedade, depressão e transtorno de estresse pós-traumático (TEPT) 90 dias após a alta hospitalar em pacientes com COVID-19.
Métodos:
Pacientes com COVID-19 internados em um hospital privado na cidade de São Paulo (SP) entre abril de 2020 e abril de 2021 foram entrevistados por telefone 30 e 90 dias após a alta para avaliar a qualidade de vida e sintomas de depressão, ansiedade e TEPT.
Resultados:
Foram incluídos 2.138 pacientes. A média de idade foi de 58,6 ± 15,8 anos, e a mediana do tempo de internação hospitalar foi de 9,0 (5,0-15,8) dias. Entre os dois momentos, a depressão aumentou de 3,1% para 7,2% (p < 0,001), a ansiedade, de 3,2% para 6,2% (p < 0,001), e o TEPT, de 2,3% para 5,0% (p < 0,001). Pelo menos um sintoma físico relacionado ao diagnóstico de COVID-19 persistia em 32% dos pacientes no 90º dia.
Conclusões:
A persistência dos sintomas físicos foi elevada mesmo 90 dias após a alta. Embora a prevalência de sintomas de ansiedade, depressão e TEPT tenha sido baixa, esses sintomas persistiram por três meses, com aumento significativo entre os momentos. Esse achado indica a necessidade de identificar os pacientes de risco para que possam receber o encaminhamento adequado no momento da alta.
Descritores: COVID-19, Ansiedade, Depressão, Transtornos de estresse pós-traumático, Qualidade de vida, Resultados de cuidados críticos
INTRODUCTION
During the first two waves of the COVID-19 pandemic in Brazil, approximately half of patients hospitalized with the disease were admitted to an ICU. During these two waves, 44% to 55% of these critically ill patients experienced ARDS and required invasive mechanical ventilation, and mortality rates were higher than 75%. 1
Overall, survivors of COVID-19 now amount to more than 400 million worldwide. 2 There is a high probability that at least some of these patients experience new or worsened physical, cognitive, and mental health symptoms that persist after hospital discharge, especially those who were admitted to the ICU (post-intensive care syndrome). 3 , 4 Severely ill patients with COVID-19 are often discharged home with some degree of functional disability. 5 These patients will most likely benefit from meticulous follow-up with rehabilitation programs aiming at physical and neuropsychological recovery. 6
Pre-COVID-19 epidemiological studies have shown that up to 75% of patients undergoing mechanical ventilation in the ICU suffer from delirium during their stay. 7 COVID-19 patients have been proven to be especially vulnerable to delirium, with more than 54% of those developing it during the first 21 days of ICU in a multicenter cohort. 8 As a result, these patients may be at an increased risk of long-term cognitive impairment. 9
There is now evidence of the psychiatric implications of COVID-19 both during the acute phase and after hospital discharge. 4 , 10 A single-center study has shown that sequelae of COVID-19 were common (in 49% of patients), and symptoms, such as anxiety or depression, were present in 26% 1 year after the diagnosis of COVID-19. Moreover, even patients who have physically recovered from symptoms are at risk of suffering from long-term mental health problems such as posttraumatic stress disorder (PTSD), anxiety, and depression, which can negatively impact their quality of life (QoL). 11 , 12 However, most studies have evaluated either the persistence of symptoms or the QoL after COVID-19. Therefore, we aimed to describe the QoL and symptoms of anxiety, depression, and PTSD at 90 days after hospital discharge in COVID-19 patients using validated instruments.
METHODS
Study design and participants
This is a single-center retrospective cohort study performed at the Hospital Sírio-Libanês, a private tertiary health care center in São Paulo, Brazil. We included all adult patients (≥ 18 years of age) with laboratory-confirmed COVID-19 by RT-PCR who were hospitalized and discharged alive between April of 2020 and April of 2021. All eligible patients received a telephone call at 30 and 90 days after hospital discharge and were invited to answer specific questions on their health status. This telephone follow-up interview was part of an institutional initiative to evaluate outcomes for COVID-19 patients and generate benchmarking and research data. The study protocol was approved by the local research ethics committee (Hospital Sirio-Libanês, approval number CAAE 51460021.6.0000.5461), which waived the need for informed consent.
Data collection during hospital-stay
The following demographic and clinical data were collected during hospital stay: age, sex, symptoms at admission, level of education, comorbidities, need for ICU admission, hospital/ICU length of stay (LOS), respiratory support (oxygen therapy, high flow nasal cannula, noninvasive mechanical ventilation, invasive mechanical ventilation, and extracorporeal membrane oxygenation), hemodynamic support (vasopressors, intra-aortic balloon pump, and extracorporeal membrane oxygenation), nosocomial infections, treatments (antibiotics, corticosteroids, and others), need for tracheostomy, use of psychological support, need for physical rehabilitation, and vital status at hospital discharge.
30- and 90-day follow-up of COVID-19 survivors
A structured telephone interview questionnaire was administered twice (at 30 and 90 days after hospital discharge). The questionnaire contained questions on demographics, persistence of symptoms, need for oxygen therapy, need for dialysis, need for specific medical or rehabilitation care, return to work, among others.
Three specific validated questionnaires were also administered. To assess the quality of life, including usual activities of daily living, we used the EuroQol Group 5-Dimension 3-Level questionnaire (EQ-5D-3L), 13 , 14 which has five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) that are scored on a categorical scale ranging from 1 to 3, where 1 indicates no problem, 2 indicates some problems, and 3 indicates a severe problem. To screen for depression, we administered the Patient Health Questionnaire-2 (PHQ-2), 15 , 16 a two-item questionnaire about the frequency of depressed mood and anhedonia in the past two weeks, ranging from 0 to 6 (scores ≥ 3 indicate a high likelihood of major depression). To screen for anxiety, we administered the Generalized Anxiety Disorder-2 (GAD-2), 17 a two-item questionnaire about the frequency of anxiety and worrying symptoms in the past two weeks, ranging from 0 to 6 (scores ≥ 3 indicate a high likelihood of generalized anxiety disorder). Finally, PTSD was defined according to the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) criteria. 18
Statistical analysis
Absolute and relative frequencies were used for qualitative variables and mean ± standard deviation or median (IQR), as appropriate, was used to describe quantitative characteristics. The association with the outcomes of qualitative variables was assessed using the chi-square test or exact tests (Fisher’s exact test or likelihood ratio), and that of quantitative variables was assessed with the Student’s t-test. The analyses were performed with R (R version 4.0.2) and R Studio version 1.3.959 (The R Foundation for Statistical Computing, Vienna, Austria). Significance was set at p < 0.05.
RESULTS
A total of 3,086 patients with PCR-confirmed COVID-19 were admitted to the Hospital Sírio-Libanês during the study period. Of these, 2,952 (95.7%) were discharged alive. The mean age of survivors was lower than that of nonsurvivors (58.6 ± 15.8 years vs. 80.8 ± 10.5 years; p < 0.001). Additionally, survivors had shorter hospital lengths of stay as compared with nonsurvivors-median: 9.0 (5.0-15.8) days vs. 25.0 (13.0-48.0) days; p < 0.001). A total of 735 (23.8%) of patients were admitted to the ICU with a median ICU length of stay of 10 (5-17) days, 396 (53.9%) of whom required invasive mechanical ventilation for a median of 10 (6-21) days. The in-hospital mortality of patients admitted to the ICU was 14.6%.
Of the 2,952 patients discharged alive, 1,122 (38.0%) completed the interview on both occasions (at 30 and 90 days after discharge), 670 (22.6%) completed only the first interview (day 30), and 346 (11.7%) completed only the second interview (day 90). A total of 2,138 (72.3%) of patients completed the interview at least once after hospital discharge (Figure 1). The characteristics of the patients included at each time point are described in Table 1. There was no statistically significant difference between the groups of patients who completed the interview at 30 days (30-day group), at 90 days (90-day group), and on both occasions (30+90-day group), except for the use of corticosteroids during hospital stay, which was lower in the 90-day group.
Figure 1. Flow chart of patient inclusion at 30- and 90-day follow-up interviews.
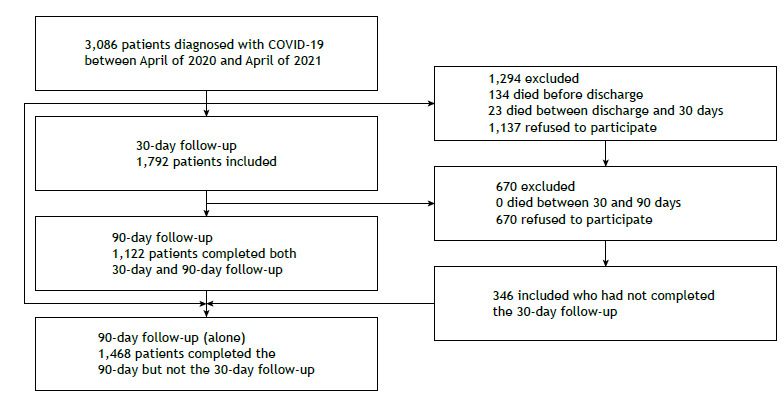
Table 1. Characteristics of the patients.a .
| Characteristic | Group | p | ||
|---|---|---|---|---|
| 30-day | 90-day | 30+90 day | ||
| (n = 1,792) | (n = 1,468) | (n = 1,122) | ||
| Age, years | 59.3 ± 15.8 | 59.6 ± 15.5 | 59.9 ± 15.6 | 0.59 |
| Sex at birth, female | 626 (34.9) | 491 (33.4) | 374 (33.3) | 0.57 |
| Ethnicity, White | 1,571 (95.6) | 1,306 (96.0) | 990 (96.2) | 0.52 |
| Marital status, married | 1,438 (81.1) | 1,184 (81.5) | 923 (83.2) | 0.4 |
| Higher education | 1,509 (86.2) | 1,257 (87.2) | 963 (87.5) | 0.38 |
| Comorbidities | 1,399 (78.1) | 1,148 (78.2) | 879 (78.3) | 0.98 |
| Arterial hypertension | 702 (39.2) | 587 (40.0) | 456 (40.6) | 0.72 |
| Diabetes | 381 (21.3) | 324(22.1) | 251 (22.4) | 0.74 |
| Cardiovascular disease | 325 (18.1) | 285 (19.4) | 222 (19.8) | 0.47 |
| Cerebrovascular disease | 48 (2.7) | 39 (2.7) | 30 (2.7) | 0.99 |
| Corticosteroids | 1,385 (77.3) | 1,015 (69.1)*,† | 839 (74.8) | < 0.001 |
| High-flow nasal cannula | 323 (18.0) | 227 (15.5) | 188 (26.8) | 0.15 |
| NIV | 446 (24.9) | 327 (22.3) | 278 (24.8) | 0.17 |
| Invasive MV | 195 (10.0) | 137 (9.3) | 111 (9.9) | 0.28 |
| Vasopressor use | 162 (9) | 130 (8.9) | 107 (9.5) | 0.83 |
| Psychological support during hospitalization | 213 (11.9) | 157 (10.7) | 125 (11.2) | 0.55 |
| Rehabilitation support during hospitalization | 1,414 (79.0) | 1,127 (76.8) | 875 (78.1) | 0.34 |
| Need of ICU admission | 391 (21.8) | 326 (22.2) | 252 (22.4) | 0.91 |
| ICU LOS, days | 15 [10-25] | 15 [9-24] | 16 [10-26] | 0.15 |
| Hospital LOS, days, | 8 [5-14] | 8 [5-13] | 8 [5-14] | 0.62 |
NIV: noninvasive ventilation; MV: mechanical ventilation, and LOS: length of stay. aValues expressed as n (%), mean ± SD, or median [IQR]. bOnly for patients admitted to the ICU. To correct for multiple comparison between two groups of study participants, we used a Bonferroni corrected p-value of 0.0167 for statistical significance. *p < 0.001 (90-day vs. 30-day). †p = 0.002 (90-day vs. 30+90-day.
On day 30, 41.2% of non-ICU patients reported persistence of at least one physical symptom related to COVID-19 diagnosis. Although a significant decrease was shown on day 90, 30.3% of the patients reported persistence of symptoms (p < 0.001). This decrease was also evident in the patients who had been admitted to the ICU-56.9% and 37.8% reported persistence of symptoms on day 30 and on day 90, respectively (p < 0.001; Table 2).
Table 2. Outcomes at 30 days and at 90 days after discharge.
| Outcome | Total sample | Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 30-day | 90-day | ||||||||
| (n = 2,138) | (n = 1,792) | (n = 1,468) | |||||||
| 30-day | 90-day | p | Non-ICU | ICU | p | Non-ICU | ICU | p | |
| EQ-5D-3L | |||||||||
| Mobility | 0.1 | < 0.001 | < 0.001 | ||||||
| I have no problems in walking about | 1,458/1,783 (81.8%) | 1,237/1,462 (84.6%) | 1,192/1,394 (85.5%) | 266/389 (68.4%) | 981/1,138 (86.2%) | 256/324 (79%) | |||
| I have some problems in walking about | 292/1,783 (16.4%) | 202/1,462 (13.8%) | 184/1,394 (13.2%) | 108/389 (27.8%) | 146/1,138 (12.8%) | 56/324 (17.3%) | |||
| I am confined to bed | 33/1,783 (1.8%) | 23/1,462 (1.6%) | 18/1,394 (1.3%) | 15/389 (3.9%) | 11/1,138 (1%) | 12/324 (3.7%) | |||
| Self-care | 0.87 | < 0.001 | < 0.001 | ||||||
| I have no problems with self-care | 1,640/1,783 (92%) | 1,347/1,463 (92.1%) | 1,306/1,394 (93.7%) | 334/389 (85.9%) | 1,062/1,138 (93.3%) | 285/325 (87.7%) | |||
| I have some problems washing or dressing myself | 104/1,783 (5.8%) | 81/1,463 (5.5%) | 66/1,394 (4.7%) | 38/389 (9.8%) | 58/1,138 (5.1%) | 23/325 (7.1%) | |||
| I am unable to wash or dress myself | 39/1,783 (2.2%) | 35/1,463 (2.4%) | 22/1,394 (1.6%) | 17/389 (4.4%) | 18/1,138 (1.6%) | 17/325 (5.2%) | |||
| Usual activities | 0.95 | < 0.001 | 0.04 | ||||||
| I have no problems with performing my usual activities | 1,565/1,781 (87.9%) | 1,289/1,461 (88.2%) | 1,258/1,391 (90.4%) | 307/389 (78.7%) | 1,012/1,137 (89%) | 277/324 (85.5%) | |||
| I have some problems with performing my usual activities | 178/1,781 (10%) | 142/1,461 (9.7%) | 111/1,391 (8%) | 67/389 (17.2%) | 107/1,137 (9.4%) | 35/324 (10.8%) | |||
| I am unable to perform my usual activities | 38/1,781 (2.1%) | 30/1,461 (2.1%) | 22/1,391 (1.6%) | 16/389 (4.1%) | 18/1,137 (1.6%) | 12/324 (3.7%) | |||
| Pain/Discomfort | <0.001 | 0.03 | 0.95 | ||||||
| I have no pain or discomfort | 1,623/1,772 (91.69%) | 1,223/1,455 (84.1%) | 1,279/1,386 (92.3%) | 344/386 (88.4%) | 953/1,133 (84.1%) | 270/322 (83.8%) | |||
| I have moderate pain or discomfort | 134/1,772 (7.6%) | 198/1,455 (13.6%) | 99/1,386 (7.1%) | 35/386 (9%) | 153/1,133 (13.5%) | 45/322 (14%) | |||
| I have extreme pain or discomfort | 15/1,772 (0.8%) | 34/1,455 (2.3%) | 8/1,386 (0.6%) | 7/386 (1.8%) | 27/1,133 (2.4%) | 7/322 (2.2%) | |||
| Anxiety/Depression | < 0.001 | 0.82 | 0.4 | ||||||
| I am not anxious or depressed | 1,529/1,765 (85.7%) | 1,151/1,451 (79.4%) | 1,198/1,379 (86.9%) | 331/386 (85.1%) | 902/1,130 (79.8%) | 249/321 (77.5%) | |||
| I am moderately anxious or depressed | 195/1,765 (11%) | 256/1,451 (17.6%) | 149/1,379 (10.8%) | 46/386 (11.8%) | 192/1,130 (17%) | 64/321 (20%) | |||
| I am extremely anxious or depressed | 41/1,765 (2.3%) | 44/1,451 (3%) | 32/1,379 (2.3%) | 9/386 (2.3%) | 36/1,130 (3.2%) | 8/321 (2.5%) | |||
| GAD-2 ≥ 3 | 48/1,478 (3.2%) | 77/1,245 (6.2%) | < 0.001 | 36/1,190 (3%) | 10/287 (3.5%) | 0.68 | 63/987 (6.4%) | 14/258 (5.4%) | 0.57 |
| PHQ-2 ≥ 3 | 46/1,477 (3.1%) | 90/1,247 (7.2%) | < 0.001 | 42/1,191 (3.5%) | 6/287 (2.1%) | 0.21 | 69/989 (7%) | 21/258 (8.1%) | 0.52 |
| Persistence of any physical symptoms related to the COVID-19 diagnosis | 795/1,779 (44.6%) | 466/1,457 (32%) | < 0.001 | 573/1,389 (41.2%) | 222/390 (56.9%) | < 0.001 | 343/1132 (30.3%) | 123/325 (37.8%) | 0.01 |
| Post-traumatic stress disorder | |||||||||
| Repetitive and disturbing thoughts, memories or images | 34/1,476 (2.3%) | 63/1,251 (5%) | < 0.001 | 22/1,188 (1.9%) | 12/288 (4.2%) | 0.02 | 38/990 (3.8%) | 25/260 (9.6%) | < 0.001 |
| Physical symptoms related to the hospitalization | 45/1,476 (3%) | 37/1,243 (3%) | 0.89 | 34/1189 (2.9%) | 11/287 (3.8%) | 0.38 | 22/985 (2.2%) | 15/258 (5.8%) | 0.002 |
EQ-5D-3L: EuroQol Group 5-Dimension 3-Level questionnaire; GAD-2: Generalized Anxiety Disorder-2; and PHQ-2: Patient Health Questionnaire-2.
The temporal trends in the EQ-5D-3L domains, GAD-2 score, PHQ-2 score, and other symptoms in non-ICU and ICU patients are shown in Table 2. Between 30 and 90 days after hospital discharge, the proportion of ICU patients with mobility problems decreased (from 31.6% to 21.0%, p = 0.003), as did the proportion of ICU patients with problems in performing usual activities (from 21.3% to 14.5%; p = 0.047), whereas there was no difference in the self-care and pain/discomfort domains of the EQ-5D-3L. Non-ICU patients had similar mobility, self-care, and usual activities domain results, but there was a decrease in the proportion of non-ICU patients without pain or discomfort (from 92.3% to 84.1%; p < 0.001) between 30 and 90 days after hospital discharge. In both the ICU and non-ICU groups, there was a decrease in the number of patients without anxiety or depression feelings (anxiety/depression domain of the EQ-5D-3L), from 85.1% to 77.5% (p = 0.012) and from 86.9% to 79.8% (p < 0.001), respectively.
In a sensitivity analysis, we obtained similar results when we restricted all comparisons to the 1,122 patients in the 30+90-day group (Table 3).
Table 3. Patients who completed the questionnaires at 30 and 90 days after discharge (n = 1,122).a .
| Total | Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30-day | 90-day | ||||||||
| (n = 1,122) | (n = 1,122) | (n = 1,122) | |||||||
| 30 days | 90 days | p | Non-ICU | ICU | p | Non-ICU | ICU | p | |
| EQ-5D-3L | |||||||||
| Mobility | 0.112 | < 0.001 | < 0.001 | ||||||
| I have no problems in walking about | 918 (82.2) | 944 (84.5) | 746 (85.9) | 174 (69.3) | 746 (85.9) | 201 (79.8) | |||
| I have some problems in walking about | 175 (15.7) | 152 (13.6) | 111 (12.8) | 64 (25.5) | 113 (13.0) | 39 (15.5) | |||
| I am confined to bed | 24 (2.1) | 21 (1.9) | 11 (1.3) | 13 (5.2) | 9 (1.0) | 12 (4.8) | |||
| Self-Care | 0.584 | < 0.001 | < 0.001 | ||||||
| I have no problems with self-care | 1,030 (92.2) | 1,025 (91.8) | 817 (94.2) | 215 (85.3) | 807 (93.0) | 221 (87.7) | |||
| I have some problems washing or dressing myself | 61 (5.5) | 60 (5.4) | 37 (4.3) | 24 (9.5) | 46 (5.3) | 14 (5.6) | |||
| I am unable to wash or dress myself | 26 (2.3) | 32 (2.9) | 13 (1.5) | 13 (5.2) | 15 (1.7) | 17 (6.7) | |||
| Usual activities | 0.803 | < 0.001 | 0.011 | ||||||
| I have no problems with performing my usual activities | 975 (87.4) | 985 (88.3) | 784 (90.4) | 194 (77.0) | 774 (89.3) | 214 (84.9) | |||
| I have some problems with performing my usual activities | 117 (10.5) | 105 (9.4) | 70 (8.1) | 47 (18.7) | 79 (9.1) | 26 (10.3) | |||
| I am unable to perform my usual activities | 24 (2.2) | 26 (2.3) | 13 (1.5) | 11 (4.4) | 14 (1.6) | 12 (4.8) | |||
| Pain/Discomfort | < 0.001 | 0.057 | 0.800 | ||||||
| I have no pain or discomfort | 1,020 (92.3) | 927 (83.9) | 804 (93.2) | 222 (89.2) | 722 (83.4) | 212 (85.1) | |||
| I have moderate pain or discomfort | 75 (6.8) | 150 (13.6) | 54 (6.3) | 22 (8.8) | 121 (14.0) | 31 (12.4) | |||
| I have extreme pain or discomfort | 10 (0.9) | 28 (2.5) | 5 (0.6) | 5 (2.0) | 23 (2.7) | 6 (2.4) | |||
| Anxiety/Depression | < 0.001 | 0.700 | 0.452 | ||||||
| I am not anxious or depressed | 971 (88.2) | 869 (78.9) | 760 (88.4) | 218 (87.2) | 688 (79.8) | 190 (76.3) | |||
| I am moderately anxious or depressed | 114 (10.4) | 201 (18.3) | 89 (10.3) | 27 (10.8) | 150 (17.4) | 52 (20.9) | |||
| I am extremely anxious or depressed | 16 (1.5) | 31 (2.8) | 11 (1.3) | 5 (2.0) | 24 (2.8) | 7 (2.8) | |||
| GAD-2 ≥ 3 | 17 (1.9) | 50 (5.6) | < 0.001 | 15 (2.0) | 4 (2.2) | 0.777 | 42 (5.6) | 9 (4.7) | 0.623 |
| PHQ-2 ≥ 3 | 25 (2.8) | 66 (7.4) | < 0.001 | 19 (2.5) | 6 (3.2) | 0.610 | 48 (6.4) | 18 (9.4) | 0.144 |
| Persistence of any physical symptoms related to the COVID-19 diagnosis | 509 (45.9) | 364 (32.9) | < 0.001 | 367 (42.5) | 143 (56.7) | < 0.001 | 264 (30.6) | 102 (40.5) | 0.003 |
| Post-traumatic stress disorder | |||||||||
| Repetitive and disturbing thoughts, memories or images | 19 (2.1) | 52 (5.8) | < 0.001 | 13 (1.7) | 8 (4.3) | 0.049 | 33 (4.4) | 22 (11.4) | < 0.001 |
| Physical symptoms related to the hospitalization | 26 (2.9) | 22 (2.5) | 0.608 | 20 (2.7) | 6 (3.2) | 0.663 | 13 (1.7) | 12 (6.3) | 0.001 |
EQ-5D-3L: EuroQol Group 5-Dimension 3-Level questionnaire; GAD-2: Generalized Anxiety Disorder-2; and PHQ-2: Patient Health Questionnaire-2. aValues expressed as n (%).
There was an increase in the number of patients with a PHQ-2 score ≥ 3 from day 30 to day 90 in both the non-ICU (from 3.5% to 7.0%; p < 0.001) and ICU groups (from 2.1% to 8.1%; p = 0.001; Table 2). Only in the non-ICU group was there a significant increase in the number of patients with a GAD-2 score ≥ 3 from day 30 to day 90 (from 3.0% to 6.4%; p < 0.001). The number of patients with symptoms of PTSD, that is, with disturbing thoughts referring to the recent illness, increased from day 30 to day 90 in both non-ICU (from 1.9% to 3.8%; p = 0.004) and ICU groups (from 4.2% to 9.6%; p = 0.01). Conversely, there was no significant difference in physical symptoms related to the hospitalization from day 30 to day 90 in either group.
The worsening of PTSD (p = 0.931), GAD-2 score, and PHQ-2 score showed no significant correlations with the inability of patients to return to work. However, a noticeable deterioration in mobility (p < 0.001) and an increase in pain or discomfort (p = 0.013) were significantly correlated with the inability to return to work.
DISCUSSION
In this large Brazilian cohort treated at a private hospital, we showed that the persistence of physical symptoms related to COVID-19 diagnosis decreased over time but still affected 3 in 10 patients 90 days after hospital discharge. Also, patients who had been admitted to the ICU showed improved mobility and usual activities within this period. The number of patients experiencing subjective symptoms, such as pain, discomfort, anxiety, and depression, increased over time, mostly among non-ICU patients. This increase had no association with whether patients could return to work or not.
We described the prevalence of anxiety and depression at 90 days after discharge. Some studies have evaluated psychiatric disorders after COVID-19. 4 , 10 , 19 From the clinical perspective, the essence of COVID-19 should be seen as a sepsis-induced viral infection and has the essential characteristics of sepsis induced by other pathogens. 20 We showed that COVID-19, like sepsis and septic shock, 21 leads to long-lasting neuropsychiatric consequences, with almost 1 in 10 patients still experiencing anxiety, depression, and PTSD three months after discharge. We, however, found the prevalence of these conditions to be lower than that found by others. Huang et al. 4 found that 23% of patients admitted to the hospital due to COVID-19 had anxiety or depression six months after discharge, whereas a meta-analysis showed a pooled prevalence of depression and anxiety of 45-47% after COVID-19 infection. 22 Several reasons could underlie this difference. First, many studies used the EuroQol-5D to determine the prevalence of anxiety and depression, while we used the GAD-2 and PHQ-2 questionnaires. When measured by the EuroQol-5D (20.5%), the prevalence of anxiety and depression was similar to that in the study by Huang et al., 4 but still considerably lower than that described by Deng et al. 22 Second, we studied patients from a hospital that serves a population of high socioeconomic status, with full access to health care both before and after hospitalization. The differences in prevalence may result from differences in accessibility to health care.
Our study has several limitations. First, it was a single-center, retrospective study in a hospital which is not representative of most of Brazilian health care institutions. Thus, the generalizability of our findings is limited, at least to most people living in low- and middle-income countries. Second, we used an administrative database not originally designed for research purposes to assess 30- and 90-day outcomes. However, this database is systematically fed by dedicated personnel with previous training on administering different questionnaires over the phone. Third, we could only obtain 90-day follow-up interviews of two-thirds of the patients who participated in the telephone interview at 30 days. However, in a sensitivity analysis in which we restricted all comparisons to patients who completed the interviews on both occasions, the results were not appreciably altered. Finally, we used simplified versions of questionnaires for anxiety (GAD-2) and depression (PHQ-2), which are mainly used as screening, not diagnostic tools.
In conclusion, we found that hospital admission due to COVID-19 seems to be associated with a relatively low prevalence of anxiety and depression 90 days after hospital discharge. However, of concern is the fact that there was an increase over time in the prevalence of anxiety and depression 90 days after discharge, which points to the necessity to identify patients at risk of developing anxiety/depression so that they can be given an appropriate referral at discharge.
ACKNOWLEDGMENTS
We would like to thank the entire team of the Hospital Outcomes Department for their effort and engagement, which made this study possible.
Footnotes
Financial support: None.
Study carried out at the Hospital Sírio-Libanês, São Paulo (SP) Brasil.
REFERENCES
- 1.Zeiser FA, Donida B, da Costa CA, Ramos GO, Scherer JN, Barcellos NT. First and second COVID-19 waves in Brazil A cross-sectional study of patients' characteristics related to hospitalization and in-hospital mortality. Lancet Reg Health Am. 2022;6:100107–100107. doi: 10.1016/j.lana.2021.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization [homepage on the Internet] WHO Coronavirus (COVID 19) Dashboard. Geneva: WHO; c2021. https://covid19.who.int [Google Scholar]
- 3.Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H. Improving long-term outcomes after discharge from intensive care unit report from a stakeholders' conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y. 1-year outcomes in hospital survivors with COVID-19 a longitudinal cohort study [published correction appears in. Lancet. 2022;399(10337):1778–1778. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi LU, Avelino-Silva TJ, Dias MB, Jacob-Filho W, Aliberti MJR. Patient-Centered Outcomes Following COVID-19 Frailty and Disability Transitions in Critical Care Survivors. Crit Care Med. 2022;50(6):955–963. doi: 10.1097/CCM.0000000000005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60–60. doi: 10.1038/s41572-020-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotfis K, Marra A, Ely EW. ICU delirium - a diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol Intensive Ther. 2018;50(2):160–167. doi: 10.5603/AIT.a2018.0011. [DOI] [PubMed] [Google Scholar]
- 8.Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D) a multicentre cohort study [published correction appears in Lancet Respir Med. 2021 Jan 27;:]. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duggan MC, Van J, Ely EW. Delirium Assessment in Critically Ill Older Adults Considerations During the COVID-19 Pandemic. Crit Care Clin. 2021;37(1):175–190. doi: 10.1016/j.ccc.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X. 6-month consequences of COVID-19 in patients discharged from hospital a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome a systematic review. Psychosom Med. 2008;70(4):512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bienvenu OJ, Colantuoni E, Mendez-Tellez PA, Shanholtz C, Dennison-Himmelfarb CR, Pronovost PJ. Cooccurrence of and remission from general anxiety, depression, and posttraumatic stress disorder symptoms after acute lung injury a 2-year longitudinal study. Crit Care Med. 2015;43(3):642–653. doi: 10.1097/CCM.0000000000000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabin R, de Charro F. EQ-5D a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 14.Santos M, Cintra MA, Monteiro AL, Santos B, Gusmão-Filho F, Andrade MV. Brazilian Valuation of EQ-5D-3L Health States Results from a Saturation Study. Med Decis Making. 2016;36(2):253–263. doi: 10.1177/0272989X15613521. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2 validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 16.de Lima Osório F, Vilela Mendes A, Crippa JA, Loureiro SR. Study of the discriminative validity of the PHQ-9 and PHQ-2 in a sample of Brazilian women in the context of primary health care. Perspect Psychiatr Care. 2009;45(3):216–227. doi: 10.1111/j.1744-6163.2009.00224.x. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Löwe B. Anxiety disorders in primary care prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 18.APAPsycNet [homepage on the Internet] DSM-IV sourcebook, Vol. 3. Worcester (MA): American Psychological Association; c2022. https://psycnet.apa.org/record/1997-97543-000 [Google Scholar]
- 19.Writing Committee for the COMEBAC Study GroupMorin L.Savale L.Pham T.Colle R.Figueiredo S Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19 [published correction appears in. JAMA. 2021;326(18):1874–1874. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin HY. The severe COVID-19 A sepsis induced by viral infection? And its immunomodulatory therapy. Chin J Traumatol. 2020;23(4):190–195. doi: 10.1016/j.cjtee.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng J, Zhou F, Hou W, Silver Z, Wong CY, Chang O. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients a meta-analysis. Ann N Y Acad Sci. 2021;1486(1):90–111. doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]


