Abstract
The details of valvular leaflet healing following valvuloplasty and leaflet perforation from endocarditis are poorly understood. In this study, the synthesis and turnover of valvular extracellular matrix due to healing of a critical sized wound was investigated. 29 sheep were randomized to either CTRL (n=11) or HOLE (n=18), in which a 2.8-4.8 mm diameter hole was punched in the posterior mitral leaflet. After 12 weeks, posterior leaflets were harvested and histologically stained to localize extracellular matrix components. Immunohistochemistry was also performed to assess matrix components and markers of matrix turnover. A semi-quantitative grading scale was used to quantify differences between HOLE and CTRL. After 12 weeks the hole diameter was reduced by 71.3±1.4% (p<0.001). Areas of remodeling surrounding the hole contained more activated cells, greater expression of proteoglycans and markers of matrix turnover (prolyl 4-hydroxylase, metalloproteases, and lysyl oxidase, each p≤0.025), along with fibrin accumulation. Two distinct remodeling regions were evident surrounding the hole, one directly bordering the hole rich in versican and hyaluronan and a second adjacent region with abundant collagen and elastic fiber turnover. The remodeling also caused reduced delineation between valve layers (p=0.002), more diffuse staining of matrix components and markers of matrix turnover (p<0.001), and disruption of the collagenous fibrosa. In conclusion, acute valve injury elicited distinct, heterogeneous alterations in valvular matrix composition and structure, resulting in partial wound closure. Because these changes could also affect leaflet mechanics and valve function, it will be important to determine their impact on healing wounds.
Keywords: mitral valve, collagen, proteoglycans, wound healing, mitral regurgitation
Introduction
The restoration of valvular integrity after valve perforation secondary to endocarditis or surgical procedures depends on proper valvular wound healing. Abnormal tissue growth during healing can lead to stenosis [1] and inadequate mechanical integrity of the wound can lead to valve failure [2]. Proper valve healing is especially important given that valve repair is generally preferred over replacement, particularly given that mitral replacement can lead to persistent pulmonary hypertension [3]. While much is known regarding wound repair in other connective tissues, the relatively limited vasculature in adult valves [4] and large proportion of extracellular matrix (ECM) relative to cells makes valvular wound healing unique [5]. Furthermore, valvular wound healing occurs in the presence of repetitive mechanical stresses on the wound site, such as tension, compression, bending, and shear, that could disrupt the healing process. Previous investigations of valvular wound healing have been limited to the pathology of slicing injury and sutures in valves [5, 6], denudation injury in a valve organ culture system [7], and a “scratch” wound in cultured valve cells [8]. Although perforation injuries are routinely used to study wound healing in other tissues [9], no studies have been performed to examine valvular healing in this setting. The aforementioned previous studies in valvular wound healing have provided important insight into the formation and cellular makeup of different granulation tissues, but the role of specific ECM components in valve wound healing remains unknown.
The ECM is integrally involved in valve maintenance and function, both biologically and mechanically. Turnover of collagen, which provides the valve with tensile strength, occurs throughout life within normal valves [10] and is altered in valve disease [11]. Moreover, collagen synthesis within valves has been linked to mechanical stimulation [12] and therefore would likely respond to altered strain magnitudes around a wound site. Proteoglycans (PGs) and glycosaminoglycans (GAGs) not only contribute to the material behavior of the valve [13], but are also responsive to mechanical stimulation [14]. PGs and GAGs are also involved in a number of cellular processes required for wound healing including cell growth and migration, growth factor regulation, and collagen fibrillogenesis [15]. In an in vitro model of valve wound healing, the PG versican was rapidly expressed by valvular interstitial cells (VICs) upon application of a scratch wound, and had sustained expression during the formation of a provisional wound matrix [8]. Because ECM undergoes mechanobiological regulation, is involved in processes necessary to wound healing, and contributes to valve function, it was hypothesized that the ECM would be altered following acute valve injury and that this remodeling would promote the restoration of tissue integrity.
Given the clinical relevance of understanding valve wound repair, and the paucity of in vivo valve wound repair experiments, in this study a 2.8-4.8 mm diameter hole was punched in the center of the posterior leaflet of the mitral valve (PML) of sheep. Unlike a slicing injury or the small perforation caused by a suture needle, a hole-punch creates a critical size defect (one that is unlikely to heal completely by itself) and hence could be considered representative of an exacerbated acute valve injury complete with altered hemodynamics. After 12 weeks, the PML in the region of the hole-punch was sectioned and analyzed using histology and immunohistochemistry. The quantity and distribution of PGs, GAGs, collagen, enzymes regulating matrix turnover, regional cell density, and the layered microstructure within the PML was analyzed and compared with a sham control group in which animals underwent the same surgery without creation of a hole in the PML.
Materials and methods
Experimental Animal Protocol
All animals received humane care in accordance with the guidelines developed by the National Institutes of Health (US Department of Health and Human Services NIH Publ. 85-23, Revised 1985) and a protocol approved by the Stanford University Institutional Animal Care and Use Committee. These studies were therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Twenty-nine 1-2 year-old sheep were randomized to either control (CTRL, n=11) or experimental (HOLE, n=18) groups. A left thoracotomy and atriotomy were employed to access the mitral valve, and after establishment of cardiopulmonary bypass a 2.8-4.8 mm diameter hole was created in the central scallop of the PML of HOLE animals with an aortic hole-puncher [16]. CTRL animals underwent the exact same operation without the hole-punch. On a weekly basis, a blinded echocardiographer performed transthoracic echocardiography and graded the mitral regurgitation (MR) on the basis of color Doppler regurgitant jet extent and width [17]. Animals were monitored daily for signs of distress after the hole-punch procedure, but did not show any. After 12 weeks, the upper bound of range for time required for ovine valve wound healing [5], the mitral valves were harvested and fixed in formalin.
Histology and Immunohistochemistry
The PML was isolated and a 5 mm wide strip cut from annulus to free edge (Fig. 1A), embedded in paraffin, and sectioned to a thickness of five microns. Each sample was stained histologically to demonstrate the general collagen, elastic fiber, and PG/GAG content and to distinguish between the different layers of the mitral valve. Movat pentachrome stained sections were used to assess layer delineation and collagen distribution. Masson’s trichrome stain was performed to assess fibrin accumulation and to grossly demonstrate neovascularization. Picrosirius red staining was performed to examine the collagen content and alignment and to infer type of collagen (red=collagen I, yellow/green=reticular collagen III) [18]. Immunohistochemistry (IHC) was also performed on CTRL and HOLE with the larger hole-punch diameters of 4-4.8 mm (n=15 from the total HOLE group) to demonstrate the PGs decorin and biglycan (DCN, BGN, courtesy of Dr. Larry Fisher, NIH), versican (VC, clone 2B1, Seikagaku, Japan), and the glycosaminoglycan (GAG) hyaluronan (HA, Seikagaku); elastic fiber-related proteins elastin, fibrillin (both Abcam, Cambridge, MA), and lysyl oxidase (LOX, Imgenex, San Diego, CA, involved in crosslinking both collagen and elastin); collagen type III (Col III, courtesy of Larry Fisher, NIH) which tends to be dysregulated in remodeling tissues [19]; markers of collagen synthesis prolyl 4-hydroxylase (P4H, Chemicon, Temecula, CA) and heat shock protein-47 (HSP47, Abcam); markers of matrix degradation matrix metalloproteases (MMPs)-1, -2, -9, and -13 (all Assay Designs, Ann Arbor, MI, except MMP13, Chemicon); and markers of valve cell activation smooth muscle alpha-actin (SMaA, Dakocytomation, Denmark) and non-muscle myosin (NMM, Covance, Berkeley, CA). For the preceding stains, a semi-quantitative grading scale from 0 to 4 was used to assess staining intensity and delineation of stain between valve layers for all markers except SMaA and NMM, for which staining was very localized. A single blinded observer performed grading both for the leaflet as a whole and for the specific regions of the annulus, mid-leaflet/hole-punch region, and free edge (Fig. 1B, C). The hole-punch region was further subdivided into the region immediately proximal (closer to the valve annulus) and distal (closer to the free edge) to the hole.
Fig. 1.
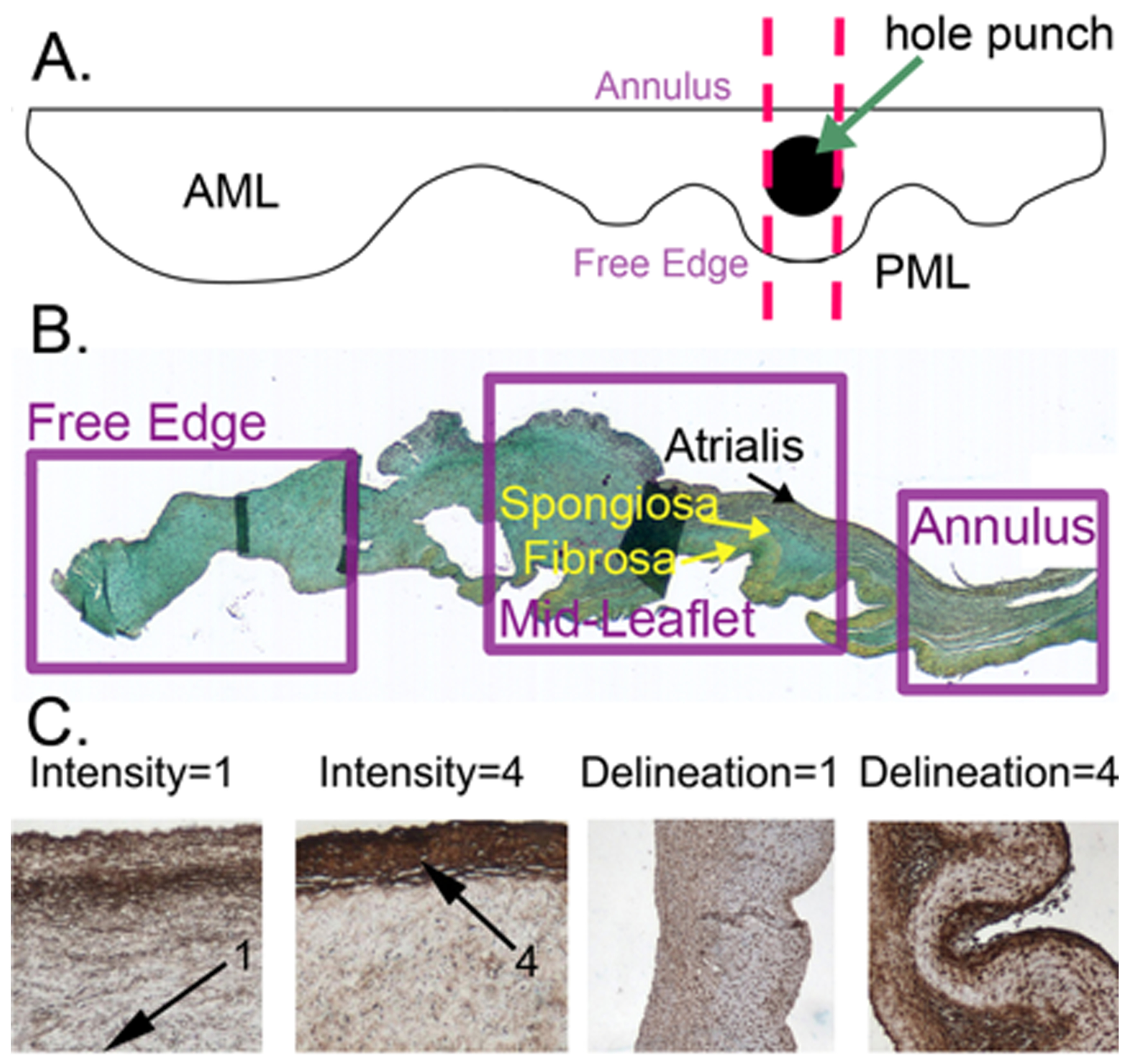
A) Diagram showing location from which PML cross-sections were taken. AML indicates anterior leaflet of MV. Sections were cut from annulus to free edge, as indicated by the dashed lines. B) Anatomy of the normal MV posterior leaflet illustrating valve regions and histological layers in a Movat-stained section. In Movat pentachrome stain black=elastic fibers, blue=PGs/GAGs, yellow=collagen, red=muscle or fibrin. C) Examples of grading rubrics for the characteristics staining intensity and delineation. For the characteristic delineation, “1” indicates little differentiation in intensity between layers. “4” indicates maximum for each characteristic. Separate rubrics were made for the delineation of different regions of the valve (annulus, mid-leaflet, and free edge).
To demonstrate additional characteristics of the cells, the CTRL and HOLE valve sections were immunohistochemically stained for CD45 (clone 1.11.32, Serotec, Kidlington, UK), as a leukocyte marker to demonstrate the infiltration of blood cells, and CD31 (Santa Cruz Biotechnology, Dallas, TX), as a marker for endothelial cells in the neovasculature. For CD45, staining was very light and the blue and brown channels had to be split in order to visualize positive staining. Channels were split and positive staining was quantified using ImageJ (NIH, Bethesda, MD) as previously published [20]. To assess CD31-stained microvasculature near the hole-punch (proximally or distally) or in the mid-leaflet of the CTRL valves, these regions were digitally imaged in a manner that avoided the hole boundary, and the blinded images were assessed semi-quantitatively for abundance of microvessels by 2 observers.
Assessment of Cell Density, Leaflet Thickness, and Hole Closure
Cell density was determined by counting valvular interstitial cell nuclei in images (captured using a Leica DM LS2 microscope (Wetzlar, Germany)) using Image Pro software (Media Cybernetics, Bethesda, MD). Regions assessed included the annulus, mid-leaflet, and the regions proximal and distal to the hole. Leaflet thickness was evaluated using Image Pro software for each of the above regions as well as the free edge. The increase in thickness adjacent to the hole-punch was determined as the greatest thickness just proximal or distal to the hole divided by the adjacent thinner “necked” region. Percent hole closure (% closure) was calculated as the difference between the original hole diameter (diameter of aortic hole-punch) and final hole diameter normalized to the original hole diameter. The final hole diameter was measured as the maximum distance between the leaflet tissues proximal and distal to the hole on the paraffin embedded tissue blocks (see Fig. 2A).
Fig. 2.
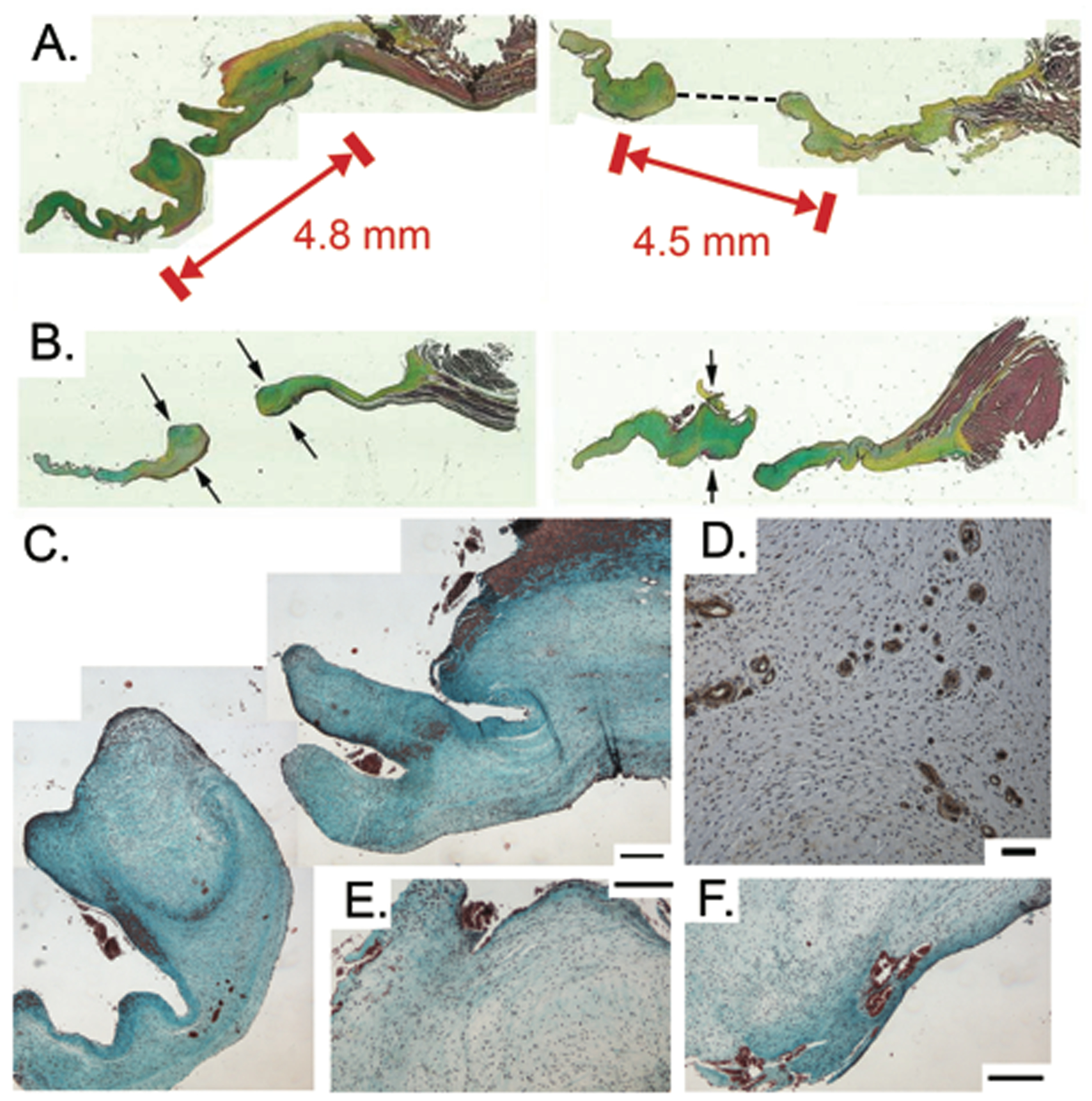
A) Substantial areas of remodeling significantly decreased hole diameter (Movat pentachrome stain). Red block markers spanned by arrows indicate initial diameter of hole punch; the black dashed line on the right tissue section indicates the measurement made to determine the final hole diameter. B) Areas surrounding hole punch show significant thickening as indicated by arrows (Movat pentachrome stain). Thickening was not always symmetric around the hole, as demonstrated by right image. C). Masson’s trichrome stain demonstrating accumulation of fibrin around hole. Scale bars indicate 200 μm. D) CD31-positive cells surrounding a rare demonstration of microvasculature within a HOLE leaflet. Scale bar indicates 50 μm. E-F) Masson’s trichrome stain illustrating examples of fibrin accumulation adjacent to hole. Scale bars indicate 200 μm.
Statistical Analysis
Data are presented as mean and standard error of the mean, unless otherwise noted. Multifactorial analysis of variance (ANOVA) with post-hoc Tukey testing was performed using SigmaStat (SPSS, Chicago, IL) with rank transformation for data sets that were not normally distributed. For all tests a significance level of 0.05 was used. Correlations were calculated using a Pearson product moment correlation for normally distributed data and a Spearman test for non-normally distributed data.
Results
Remodeling in Thickened, Cell-Dense Areas Reduced Hole Diameter
MR was greater in HOLE vs. CTRL throughout the study (MR at 12 weeks: CTRL 0.5±0.04 vs. HOLE 2.6±0.05, p<0.001). The hole-punch resulted in a regurgitant jet that was predominantly centrally oriented. As reported previously [16, 21], this MR in the HOLE valves was accompanied by a greater mitral annulus area, end-diastolic volume index, and end-systolic volume index after 12 weeks. There was also a significant correlation between the grade of MR and VIC activation in the mitral anterior leaflet. The full details of the cardiac and anterior mitral leaflet remodeling in these animals have been published [16, 21]. Substantial matrix production occurred around the hole-punch reducing the hole diameter by 71.3±1.4% from its original dimensions (p<0.001, Figs. 2A and 3A). The leaflet thickness in these areas of remodeling was 228±60% greater in the region proximal to the hole and 219±48% greater in the region distal to the hole compared to adjacent leaflet regions (Fig. 2B). Fibrin accumulation was commonly found adjacent to the hole (Figs. 2C,E,F). On rare occasion, microvessels were observed in the wound healing regions of HOLE valves (Figs. 2D,F), but these structures were only minimally present, and there was no difference between their abundance in CTRL and HOLE valves.
Fig. 3.
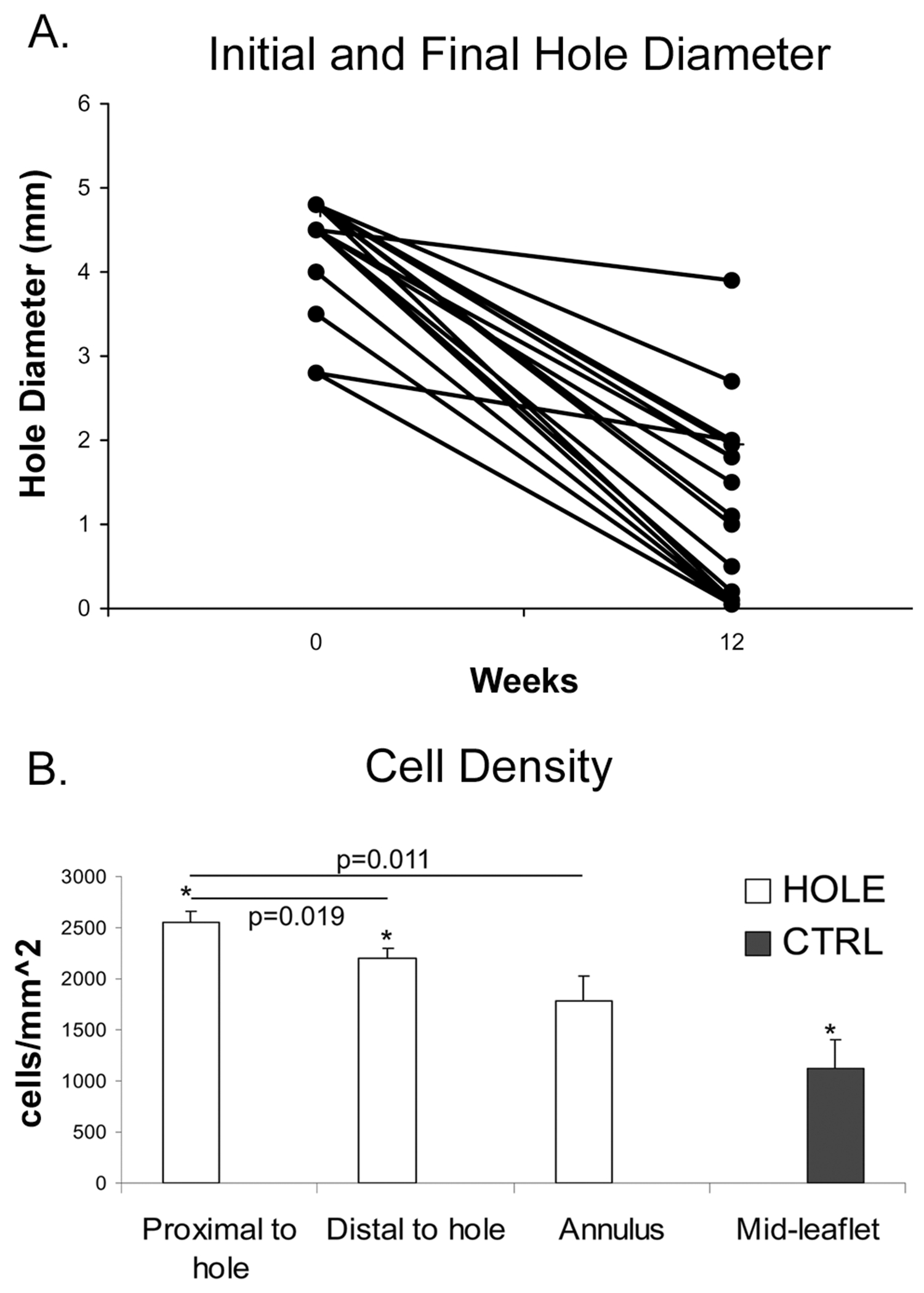
A) Initial and final hole diameters for HOLE samples. B) Cell density was increased in areas of remodeling compared to the annulus of the same HOLE leaflet as well as compared to CTRL. *=p<0.001. Error bars indicate the standard error of the mean.
At 12 weeks, the final hole dimensions ranged from approximately 0-4 mm in diameter (Fig. 3A). Average MR positively correlated with final hole diameter (r=0.55, p=0.024) and, for samples with hole diameters of 4-4.8 mm, inversely correlated with the percentage of hole closure (r=−0.66, p=0.011). Within PMLs with hole diameters of 4-4.8 mm, cell density in the region proximal to the hole was greater than in the region distal to the hole (p=0.019, Fig. 3B) and greater than in the annular region (p=0.011). The regions proximal and distal to the hole also had considerably higher cell densities than in the mid-leaflet of CTRL PMLs (p<0.001). To determine whether this higher cell density was due to an inflammatory infiltrate, the tissue sections were stained to detect the leukocyte common antigen (CD45), but the staining for this marker was very low (on 1-2% of all cells) in both HOLE and CTRL groups.
Matrix Turnover and Cell Activation Near the Hole-Punch
Compared to the equivalent region in CTRL PMLs, the hole-punch region showed significantly greater expression of VC, BGN, DCN and HA (each p≤0.006, Fig. 4A) and markers of matrix metabolism P4H, the MMPs (1, 2, 9, 13), and LOX (each p≤0.025, Fig. 4B). NMM and SMaA often co-localized with the MMPs, suggesting an activated myofibroblast phenotype.
Fig. 4.
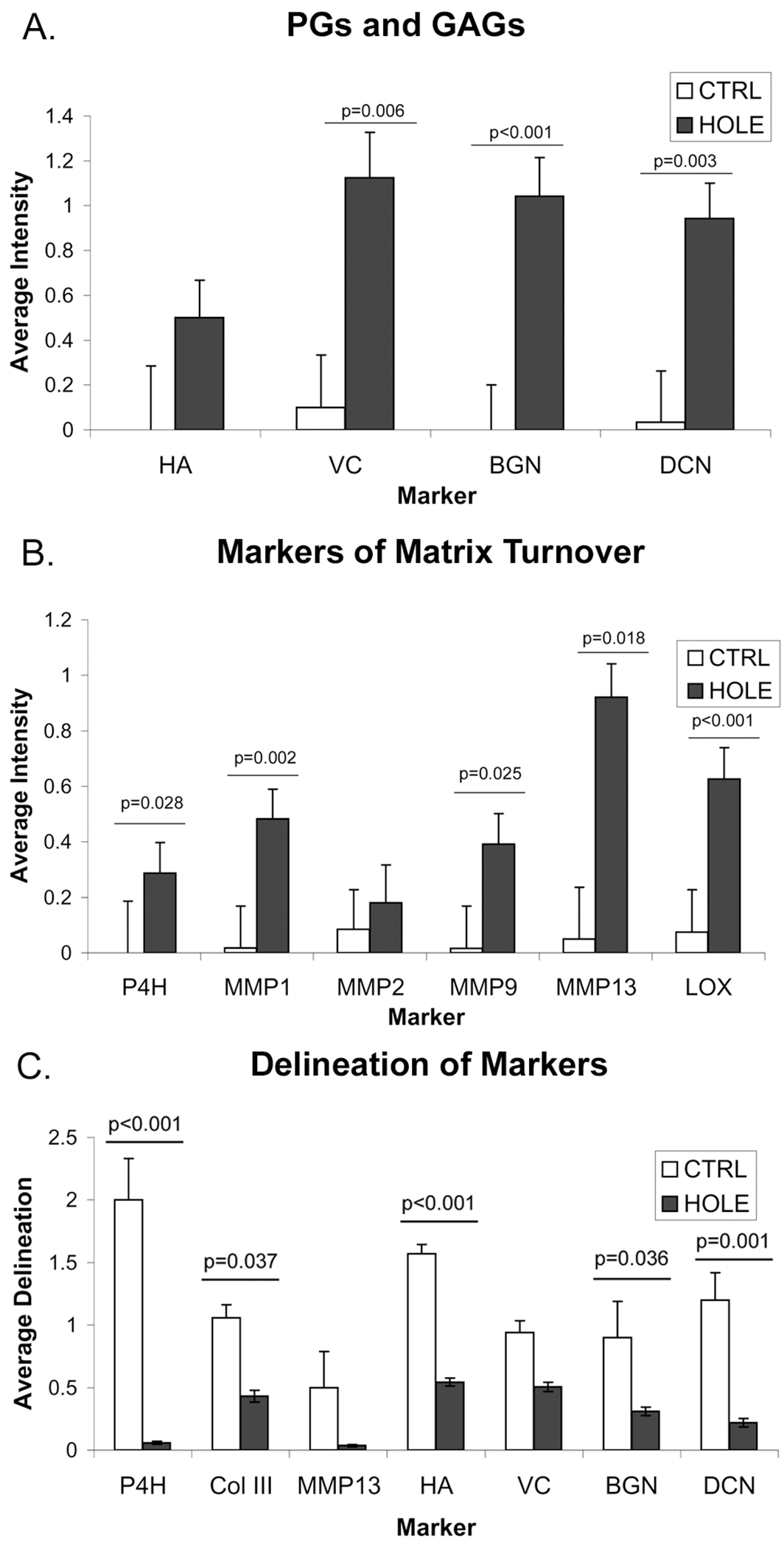
A) Intensity of PG and GAG staining in region around hole-punch in HOLE PML versus comparable regions in CTRL..B) Intensity of collagen-related marker staining in regions around hole-punch in HOLE PML versus comparable regions in CTRL. C) Average delineation of markers between layers in HOLE PML compared to CTRL. Error bars indicate the standard error of the mean. In each of A, B, and C, the ANOVA for the overall comparison of all related markers was p<0.001; the p-values above bars are from the post-hoc analyses.
Even outside the hole-punch regions, there was strong and localized staining of MMPs in the HOLE leaflets, such as in the atrialis layer of the annulus of many HOLE PMLs (data not shown). The free edge of HOLE leaflets also showed localized expression of several matrix turnover enzymes including MMPs, most commonly MMP1 and MMP9. Several samples also demonstrated a localized increase in elastin (co-localized with MMP9 and LOX) in the leaflet free edge.
Patterns of Remodeling
Two distinct regions, each containing different ECM, were evident within the remodeling tissue surrounding the hole-punch. Region 1 (R1) directly bordered the hole, whereas region 2 (R2) was adjacent to R1 but located slightly further from the hole–punch (Fig. 5A). R1 was apparent in Movat-stained sections as a pocket of alcian blue staining, which was predominantly composed of VC and HA (Fig. 5A). VC was primarily localized to R1 while HA was more diffusely present. In contrast, R2 showed strong staining for MMPs, DCN, and to a lesser extent BGN. Elastin staining in R2 was consistently co-localized with MMP9 and LOX, suggesting elastic fiber remodeling.
Fig. 5.
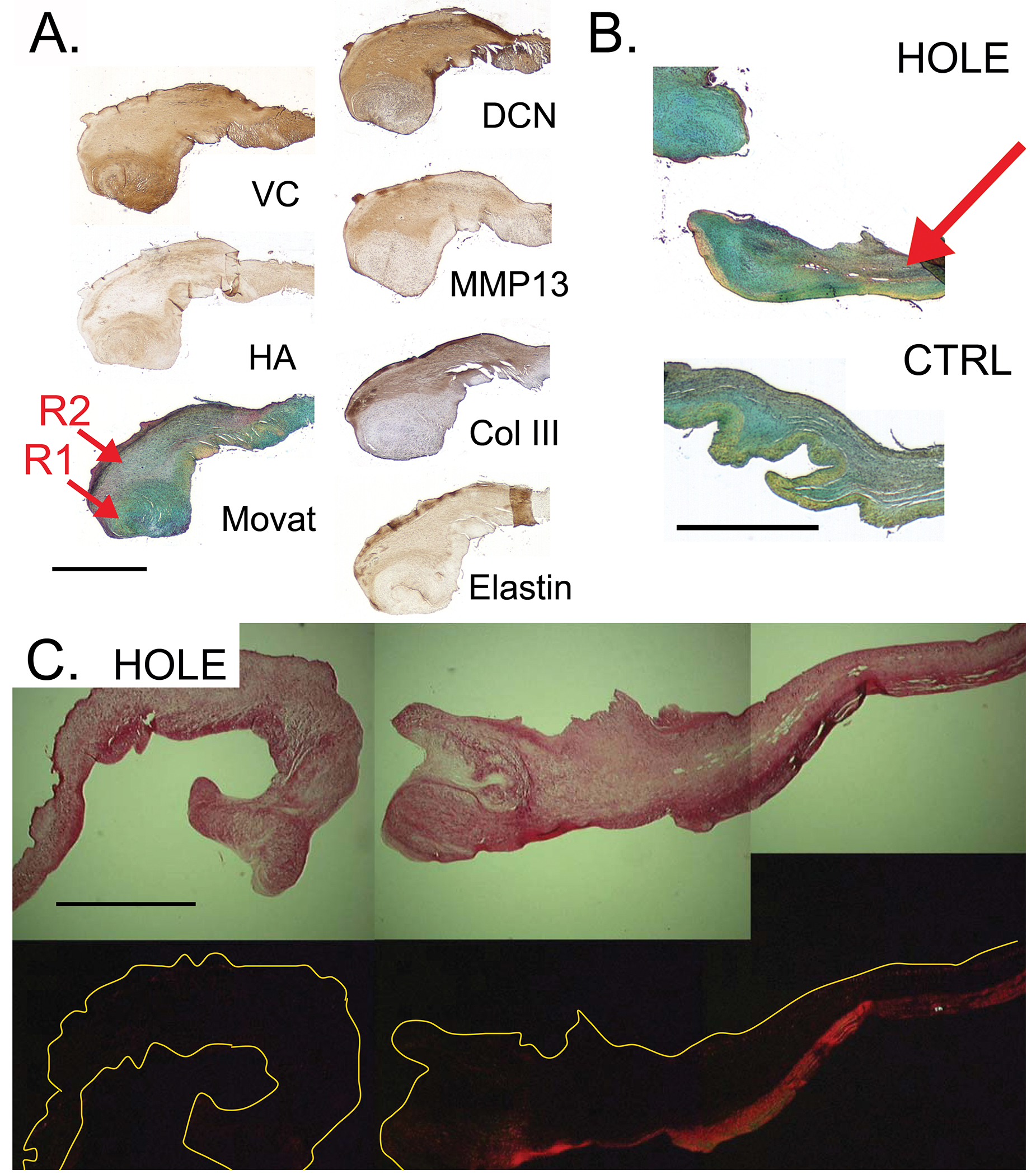
A) Two regions of remodeling (R1, R2) were evident as indicated by the red arrows: R1=immediately adjacent to the hole, rich in VC, HA, and demonstrating alcian-blue staining in Movat-stained sections; R2=located interior to R1 relative to the hole-punch, rich in DCN, MMPs, Col III, and elastin. B) Decreased delineation (indicated by red arrow) in HOLE PML compared to CTRL. C) Visualization of picrosirius red-stained tissue under polarized light demonstrates disruption of collagen backbone in HOLE PML. Scale bars for all images represent 1 mm.
There was also a difference in remodeling between the HOLE leaflets depending upon the degree of hole closure. Compared to samples with ≤60% closure (n=4), those with >90% closure (n=5) demonstrated more P4H, MMP13, and Col III (1.39±0.11 for more closure, 0.95±0.09 for less closure, p=0.009), less PG and HA (0.77±0.11 vs. 1.26±0.07, p=0.002), and less delineation of PGs and HA staining in the hole region (0.20±0.12 vs. 0.64±0.08, p=0.007).
Structural and Global Leaflet Changes
The hole-punch also affected the layered structure of the PML. In the hole region, Movat-stained sections showed decreased delineation between layers (HOLE 0.6±0.3, CTRL 1.9±0.3, p=0.002, Fig. 5B) and staining for PG, HA, P4H, Col III, and MMP13 was more diffuse in the HOLE samples (Fig. 4C). Visualization of picrosirius red-stained tissues under polarized light illustrated disruption of the collagenous fibrosa in HOLE PML (Fig. 5C). Consistent with the changes noted above for MMPs, changes in the ECM of the HOLE PML were not limited to the immediate vicinity of the hole-punch. PGs, Col III, and total collagen (from Movat staining) were more diffusely present throughout the leaflet in HOLE vs. CTRL (HOLE 0.7±0.1, CTRL 1.1±0.1, p=0.004), and there was a slight reduction in overall BGN, DCN, and elastin staining (HOLE 1.39±0.03, CTRL 1.55±0.05, p=0.037) in HOLE vs. CTRL.
Discussion
This research examined the ability for wound healing in mitral valve leaflets subjected to a critical size defect causing mitral regurgitation. After 12 weeks, the hole-punch-wounded PML demonstrated significant remodeling that substantially reduced the hole diameter and thickened the leaflet adjacent to the hole. Areas of remodeling contained elevated densities of activated cells, abundant PGs, greater ECM turnover, and fibrin accumulation. Samples with greater hole closure displayed greater collagen turnover and less expression of PGs and HA. Two distinct remodeling regions surrounded the hole-punch, one directly bordering the hole that was rich in highly hydrated GAGs and PGs (VC and HA), and a second adjacent region rich in enzymes regulating collagen and elastic fiber turnover. The remodeling also affected leaflet structure, causing reduced delineation between valve layers and disruption of the collagenous fibrosa.
Increased Collagen Turnover in Hole-Punch Region
The increase in markers of collagen synthesis (P4H, LOX), collagen degradation (MMPs), and the PGs BGN and DCN (given their role in collagen fibrillogenesis [15]) in the mid-leaflet/hole portion of HOLE compared to CTRL indicates increased collagen fiber turnover in the hole-punch region. Increased collagen turnover is further evidenced by co-localization of markers of collagen synthesis and collagen degradation as well as the disruption of the collagen-rich fibrosa evident in picrosirius red stained sections of HOLE leaflets. Such collagen turnover has been shown to be present to a limited extent in normal porcine valves throughout development and aging [10], is increased in other types of non-acute valve injury [21], and appears greatly increased in this case of injury. Both MMPs and DCN have been reported to be important to dermal wound healing, and to scarless wound healing in particular [22, 23]. The localized increase in MMPs in the annulus atrialis and free edge of HOLE found in this study likely reflect altered stresses in these regions resulting from hole-punch and the consequent MR.
Cells in Remodeling Tissue Show an Activated Phenotype
The colocalization of NMM- and SMaA-positive VICs, along with MMPs, in the areas of remodeling suggests that these cells are myofibroblasts. These results are consistent with reports of SMαA expression by cells at the cut edges of dissected portions of mitral valves cultured in an organ system [7] as well as SMαA expression in granulation tissue of a mitral valve slice wound [5]. Elevated SMαA expression has also been reported in chronic valve diseases as well as throughout development [11, 24]. Therefore, the expression of SMαA by valvular cells appears to be necessary for normal valve growth and acute repair, but is also involved in remodeling responses to chronic or acute injury.
The increased cell density found surrounding the wound in this study was also observed in the slice injury study [5] and increased cell proliferation was reported at the site of dissection in the organ culture study [7]. This evidence for cell proliferation, in conjunction with data regarding cell migration in valve injury [11, 25] suggests that the greater cell density local to the wound found in this study could be due to both cell migration and proliferation. It is also interesting to note that in the suture wound study, healing started on the annular side of the injury and was delayed in the free edge [6]. Correspondingly, we found significantly greater abundance of cells on the proximal side of the wound (closer to the annulus) compared to the distal side. Interestingly, the cells in this region were only minimally stained for the leukocyte common antigen marker CD45. This result suggests that by 12 weeks, the tissue remodeling was being mediated by the VICs, as opposed to leukocytes or blood-derived fibrocytes. Areas of remodeling also demonstrated some degree of fibrin accumulation, consistent with previous reports [5].
Distinct Areas of Remodeling Surrounding Hole-Punch
The existence of two regions surrounding the hole-punch (the hydrated GAG-rich R1 region bordering the hole and the adjacent R2 region of collagen and elastic fiber remodeling) suggests that remodeling occurs in two separate or at least temporally distinct processes. The distinct nature of these processes is supported by the strong segregation of matrix and enzyme expression. These regions are also consistent with previous studies of valve slice wounds in which two types of granulation tissue were reported: a “fibrous” variety and a “myxoid” variety that appeared to contain more PGs [5]. The two regions demonstrated here are also reminiscent of findings in artery-vein anastomoses in which PG staining was found closer to the lumen while collagen and elastin were found deeper within the intima [26]. HA and VC have been reported as principal components of the provisional matrix in dermal wounds [27, 28] and elastin has also been reported to be present in dermal wounds [29], although neither in those studies nor in the anastomoses study [26] were distinct regions reported. More recently, however, an analysis of the mitral leaflets following chronic implantation (>90 days) of the MitraClip device to reduce MR demonstrated heterogeneous remodeling, with myxomatous expansion of the middle spongiosa layer and fibroelastic thickening of the outer ventricular and atrial surfaces [30]. Given the myriad roles of VC and HA in cell migration [31], their presence in the R1 remodeling region may facilitate the migration of different cell types during the repair process [5], as noted in a demonstration of VC expression following scratch wounding of VICs in vitro [8]. Indeed, a generalized model of wound healing postulates that granulation tissue composed of HA and fibrin forms a temporary matrix into which fibroblasts and endothelial cells migrate [32, 33].
Characteristics of Greater Wound Closure
HOLE leaflets with greater percentage wound closure showed more characteristics of collagen fiber turnover, less expression of hydrated PGs and GAGs, and less delineation of PGs and HA staining in the hole-punch region. One interpretation for these results could be that collagen turnover promotes wound repair better than hydrated PGs and HA; there is debate in the dermal wound literature regarding whether various PGs inhibit or promote wound healing [27]. In light of the discussion of R1 and R2 above, another interpretation could be that the wounds showing greater closure are more mature, i.e., they have transitioned from a preliminary HA/VC matrix (R1) to collagen remodeling (R2). This interpretation would be consistent with previous reports of PGs increasing in abundance in valve slice wounds up to 12 weeks and collagen bundle formation increasing through 18 weeks [5]. Given that HOLE leaflets with the most hole closure tended to have a lower average grade of MR, there would also be a reduced degree of mechanical stimulation (from the regurgitant jet) in the hole region to drive the R1-type of remodeling.
Limitations
The creation of a critical size defect is a useful method to investigate remodeling in the mitral leaflet, so long as the results are considered within the appropriate context. This work compared the distribution of the different ECM components and unique remodeling regions in CTRL and HOLE specimens at the 12-week time point only; in the future, it will be important to examine these changes temporally. It should also be noted that the healing process in diseased valve tissue may be significantly different from that of normal tissues, both in terms of time course and the nature of ECM remodeling. In terms of the relevance to valve leaflet perforation in endocarditis, this model lacks the bacterial component and immunological sequelae of endocarditis. In the histological analysis, the main limitation was the subjective nature of grading, which was combated by the use of a grading rubric and by evaluating all leaflets stained for a given marker together. Variability in staining intensities between batches of IHC was avoided by staining all PML together. Although some variability remained, statistically significant differences between groups were found.
The use of a hole-punch to create the acute injury, standard in dermal wound studies [34], has multiple benefits, but also some limitations. One advantage of the hole-punch injury was it simulated an critical size defect complete with MR. The circular geometry of the hole-punch facilitated quantifying the amount of remodeling tissue produced, but does not directly mimic the types of incisions used in valvuloplasties and may result in different forces being applied to the tissue. Future studies could further investigate the interaction of the acute injury wound healing process with the altered hemodynamics such a defect creates, as well as examine additional aspects of remodeling such as the activation of growth factor-associated signaling pathways [35] and the Wnt-β-catenin pathway [25]. Furthermore, determining the percentage closure is a limited measure of the adequacy of healing (and may simply reflect differences in the speed of healing), as successful healing has a number of components including restoration of the mechanical integrity of the tissue. Regardless, these measurements did illustrate the changes in HOLE animals.
In conclusion, this study provides a detailed characterization of valvular ECM remodeling in response to acute injury in valves in terms of a wide range of individual matrix proteins, as well as markers of matrix synthesis and degradation. Distinct remodeling processes, including one rich in hydrated GAGs and another related to collagen and elastic fiber turnover, were noted and may prove necessary for the restoration of valve integrity after acute injury.
Acknowledgements
The authors sincerely thank the members of the Grande-Allen lab, especially Joshua Carroll and Luis Lazaro, as well as Dr. Scott Baggett for his statistical expertise and Dr. Marci Kang for assistance with figure layout. Funding comes in part by a Hertz Graduate Fellowship (EHS), Grants HL-29589 and HL-67025 from the National Heart Lung and Blood Institute (DCM), the McConnell Cardiovascular Surgical Research Fellowship program (TCN, AI), Thoracic Society Foundation Research Education Fellowship (TCN), and Uehara Memorial Foundation Research Fellowship, Japan (AI).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Dekker A, Black H, Von Lichtenberg F (1968) Mitral valve restenosis: a pathologic study. J Thorac Cardiovasc Surg 55:434–446 [PubMed] [Google Scholar]
- 2.Gillinov A, Cosgrove D, Lytle B, Taylor P, Stewart R, McCarthy P, Smedira N, Muehrcke D, Apperson-Hansen C, Loop F (1997) Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg 113:467–473 [DOI] [PubMed] [Google Scholar]
- 3.Briongos Figuero S, Moya Mur JL, Garcia-Lledo A, Centella T, Salido L, Acena Navarro A, Garcia Martin A, Garcia-Andrade I, Oliva E, Zamorano JL (2015) Predictors of persistent pulmonary hypertension after mitral valve replacement. Heart Vessels, in press. [DOI] [PubMed] [Google Scholar]
- 4.I-Ida T, Tamura K, Tanaka S, Asano G (2001) [Blood vessels in normal and abnormal mitral valve leaflets]. J Nippon Med Sch 68:171–180 [DOI] [PubMed] [Google Scholar]
- 5.Tamura K, Jones M, Yamada I, Ferrans VJ (2000) Wound healing in the mitral valve. J Heart Valve Dis 9:53–63 [PubMed] [Google Scholar]
- 6.Tamura K, Murakami M, Washizu M (2007) Healing of wound sutures on the mitral valve: an experimental study. Gen Thorac Cardiovasc Surg 55:98–104 [DOI] [PubMed] [Google Scholar]
- 7.Lester WM, Damji A, Gedeon I, Tanaka M (1993) Interstitial cells from the atrial and ventricular sides of the bovine mitral valve respond differently to denuding endocardial injury. In Vitro Cell Dev Biol - Animal 29A:41–50 [DOI] [PubMed] [Google Scholar]
- 8.Carthy JM, Boroomand S, McManus BM (2012) Versican and CD44 in in vitro valvular interstitial cell injury and repair. Cardiovasc Pathol 21:74–82 [DOI] [PubMed] [Google Scholar]
- 9.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E (2010) Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A 107:5845–5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens E, Grande-Allen K (2007) Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis 16:672–682 [PubMed] [Google Scholar]
- 11.Rabkin E, Aikawa M, Stone JR, Fukumoto Y, Libby P, Schoen FJ (2001) Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 104:2525–2532 [DOI] [PubMed] [Google Scholar]
- 12.Merryman WD, Youn I, Lukoff HD, Krueger PM, Guilak F, Hopkins RA, Sacks MS (2006) Correlation between heart valve interstitial cell stiffness and transvalvular pressure: implications for collagen biosynthesis. Am J Physiol Heart Circ Physiol 290:H224–231 [DOI] [PubMed] [Google Scholar]
- 13.Tseng H, Kim EJ, Connell PS, Ayoub S, Shah JV, Grande-Allen KJ (2013) The tensile and viscoelastic properties of aortic valve leaflets treated with a hyaluronidase gradient. Cardiovasc Eng Technol 4:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta V, Grande-Allen KJ (2006) Effects of static and cyclic loading in regulating extracellular matrix synthesis by cardiovascular cells. Cardiovasc Res 72:375–383 [DOI] [PubMed] [Google Scholar]
- 15.Kinsella MG, Bressler SL, Wight TN (2004) The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr 14:203–234 [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TC, Itoh A, Carlhall CJ, Bothe W, Timek TA, Ennis DB, Oakes RA, Liang D, Daughters GT, Ingels NB Jr., Miller DC (2008) The effect of pure mitral regurgitation on mitral annular geometry and three-dimensional saddle shape. J Thorac Cardiovasc Surg 136:557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helmcke F, Nanda N, Hsiung M, Soto B, Adey C, Goyal R, Gatewood R Jr (1987) Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation 75:175–183 [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Sun L, Wang W, Ma X (2006) Quantitative analysis of fibrosis formation on the microcapsule surface with the use of picro-sirius red staining, polarized light microscopy, and digital image analysis. J Biomed Mater Res 76A:120–125 [DOI] [PubMed] [Google Scholar]
- 19.Lis Y, Burleigh MC, Parker DJ, Child AH, Hogg J, Davies MJ (1987) Biochemical characterization of individual normal, floppy and rheumatic human mitral valves. Biochem J 244:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balaoing LR, Post AD, Liu H, Minn KT, Grande-Allen KJ (2014) Age-related changes in aortic valve hemostatic protein regulation. Arterioscler Thromb Vasc Biol 34:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens EH, Nguyen TC, Itoh A, Ingels NB Jr., Miller DC, Grande-Allen KJ (2008) The effects of mitral regurgitation alone are sufficient for leaflet remodeling. Circulation 118:S243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Järveläinen H, Puolakkainen P, Pakkanen S, Brown E, Höök M, Iozzo R, Sage E, Wight T (2006) A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen 14:443–452 [DOI] [PubMed] [Google Scholar]
- 23.Peled Z, Phelps E, Updike D, Chang J, Krummel T, Howard E, Longaker M (2002) Matrix metalloproteinases and the ontogeny of scarless repair: the other side of the wound healing balance. Plast Reconstr Surg 110:801–811 [DOI] [PubMed] [Google Scholar]
- 24.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ (2006) Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113:1344–1352 [DOI] [PubMed] [Google Scholar]
- 25.Li C, Xu S, Gotlieb AI (2011) The response to valve injury. A paradigm to understand the pathogenesis of heart valve disease. Cardiovasc Pathol 20:183–190 [DOI] [PubMed] [Google Scholar]
- 26.Swedberg S, Brown B, Sigley R, Wight T, Gordon D, Nicholls S (1989) Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation 80:1726–1736 [DOI] [PubMed] [Google Scholar]
- 27.Cattaruzza S, Perris R (2005) Proteoglycan control of cell movement during wound healing and cancer spreading. Matrix Biol 24:400–417 [DOI] [PubMed] [Google Scholar]
- 28.Singer A, Clark R (1999) Cutaneous wound healing. N Engl J Med 341:738–746 [DOI] [PubMed] [Google Scholar]
- 29.Ashcroft G, Kielty C, Horan M, Ferguson M (1997) Age-related changes in the temporal and spatial distributions of fibrillin and elastin mRNAs and proteins in acute cutaneous wounds of healthy humans. J Pathol 183:80–89 [DOI] [PubMed] [Google Scholar]
- 30.Ladich E, Michaels MB, Jones RM, McDermott E, Coleman L, Komtebedde J, Glower D, Argenziano M, Feldman T, Nakano M, Virmani R, Endovascular Valve Edge-to-Edge Repair Study I (2011) Pathological healing response of explanted MitraClip devices. Circulation 123:1418–1427 [DOI] [PubMed] [Google Scholar]
- 31.Ricciardelli C, Russell D, Ween M, Mayne K, Suwiwat S, Byers S, Marshall V, Tilley W, Horsfall D (2007) Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem 282:10814–10825 [DOI] [PubMed] [Google Scholar]
- 32.Weigel P, Fuller G, LeBoeuf R (1986) A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol 119:219–234 [DOI] [PubMed] [Google Scholar]
- 33.Weigel P, Frost S, McGary C, LeBoeuf R (1988) The role of hyaluronic acid in inflammation and wound healing. Int J Tissue React 10:355–365 [PubMed] [Google Scholar]
- 34.Davis T, Amare M, Naik S, Kovalchuk A, Tadaki D (2007) Differential cutaneous wound healing in thermally injured MRL/MPJ mice. Wound Repair Regen 15:577–588 [DOI] [PubMed] [Google Scholar]
- 35.Han L, Gotlieb AI (2012) Fibroblast growth factor-2 promotes in vitro heart valve interstitial cell repair through the Akt1 pathway. Cardiovasc Pathol 21:382–389 [DOI] [PubMed] [Google Scholar]


