Abstract
The Asteraceae family is well known for its toxic and repellent activity against mosquitoes. In this study, essential oils (EOs) extracted from the aerial parts of both wild and cultivated Artemisia campestris L. plants were tested for larvicidal activity against Culex pipiens (Diptera: Culicidae), a pest mosquito widely suspected to be the vector responsible for West Nile virus transmission. The research aims at comparing the chemical composition and insecticidal activity of cultivated and wild A. campestris EOs. The EOs were obtained by hydrodistillation from the plant's aerial parts and were analyzed using GC-MS. Furthermore, the larviciding experiment was carried out following the standard WHO protocol. The result showed that wild and cultivated plant EOs differed only quantitatively, while the qualitative profile revealed a nearly identical chemical composition. Camphor (18.98%), car-3-en-5-one (11.25%), thujone (6.36%), chrysanthenone (6.24%), filifolone (4.56%), and borneol (3.56%) dominate the wild plant EO. Camphor (21.01%), car-3-en-5-one (17%), chrysanthenone (10.15%), filifolone (7.90%), borneol (3.38%), and thujone (3.08%) are the major compounds of the cultivated plant. Cultivation did not affect the EO production since the yield of the cultivated plant was 0.5 ± 0.1% and 0.6 ± 0.2% for the wild plant. The cultivated A. campestris EO had the highest insecticidal activity (LC50 = 9.79 µg/ml), and no significant difference was noticed between wild and cultivated A. campestris EO in terms of LC90. These findings could pave the way for a new method of producing biocides to control major disease vectors and offer a potential alternative for pest control.
1. Introduction
Mosquitoes are a major threat to millions of people worldwide. They transmit various diseases, including malaria, yellow fever, dengue fever, West Nile fever, and chikungunya [1]. The West Nile virus (WNV) (family Flaviviridae and genus Flavivirus) can infect humans, birds, and horses, and it is primarily transmitted by the genus Culex [2].
The Culex genus is the most important vector of viruses causing Japanese encephalitis, St. Louis encephalitis, and West Nile fever [3]. The most common mosquito insect in rural and urban areas is Culex pipiens (Diptera: Culicidae) [4]. This insect, also known as the house mosquito, is one of the most widely distributed mosquitoes worldwide, and several studies have implicated C. pipiens in West Nile virus transmission [5, 6]. Moreover, it is an important pest for humans, causing allergic reactions such as local skin reactions and systemic reactions such as angioedema and urticaria [7]. Also, it has been linked to disease spread in several countries [8], including Morocco in 1996 [9] and more recently in 2010 [10].
Besides, the most efficient way to avoid mosquito bites [11, 12] is to use effective vector management strategies to control and prevent the propagation of mosquito-borne diseases [13].
Several methods (physical, genetic, and chemical) have been studied to control this mosquito in both its larval and adult forms. Over the last few decades, genetic control, a term that refers to a variety of methodologies such as sterile insect technique (SIT) or chemosterilization, the release of hybrids or insects with translocations, has been used as a mosquito control method [14, 15]. Furthermore, the most effective of these strategies is chemical control, but it poses serious risks to the environment and human health [16], one of which is pesticide resistance [17, 18].
As a result, replacing pesticides with biological alternatives based on natural products, especially aromatic plants, and EOs, is the ideal solution [19, 20]. Previous research on the plant's essential oils (EOs) against C. pipiens larvae has been conducted [21–23].
Artemisia is a large and important member of the Asteraceae family, with over 500 species found in Europe, North America, Asia, and South Africa [24]. These species are known for their diverse medicinal and therapeutic properties and include a variety of biologically active plants [25, 26], including Artemisia herba-alba, Artemisia annua L., Artemisia judaica L., and Artemisia arborescens [27, 28]. These plants are well known for their ability to treat a variety of ailments, including inflammation, hepatitis, cancer, malaria, infections, and diabetes [29–31]. A. herba-alba, commonly known as desert mugwort, found in arid regions, has traditionally been used to treat respiratory and parasitic infections (including helminths) and digestive issues, among other things [32–34]. A. annua L. is notable for its artemisinin content [35], whereas A. judaica L., commonly known as “Beithran” in Arabic, is used to treat stomach upset, heart disease, and diabetes [36]. Anti-inflammatory, antihistaminic, and antiviral properties are provided by A. arborescens [37]. Furthermore, Artemisia maritima and Artemisia nilagirica have powerful pharmacological effects and are effective against disease-carrying mosquitoes such as Aedes albopictus and C. pipiens [38–40].
Morocco is known for its diverse plant life, with a wide variety of species found throughout the country. Many of these plants have been traditionally used in Moroccan medicine, cooking, and other daily activities. The genus Artemisia contains 14 species, eight of which are endemic [40]. Artemisia campestris L is one of these endemic species, commonly known as “degoufet,” “tgouft,” or “alala.”. It is known for its strong, pungent aroma and has many medicinal properties, including biological properties such as antileishmania [41], antivenom, anticancer, antidiabetic, antihypertensive, anthelmintic, antimicrobial, antifungal, and insecticidal properties [42, 43], and it has been used to treat a variety of disorders, including digestive, respiratory, cutaneous, and genital diseases [44].
The valorization of EO from A. campestris refers to the process of utilizing the plant's EOs for practical applications, such as vector control. Indeed, EOs generally contain a high concentration of monoterpenes and sesquiterpenes, as well as flavonoids, phenolic acids, coumarins, and fatty acids [45]. A. campestris EO has been found to have numerous pharmacological activities, including antioxidant, antifungal, insecticidal, antibacterial, antimutagenic, antitumor, anthelmintic, and antihypertensive properties [46]. Over the years, EOs have been extracted from a wide range of plant species, and several types of research have concentrated on their biological properties [47], particularly their insecticidal activity [48, 49]. Furthermore, multiple studies in the pharmaceutical [50], agricultural [51, 52], and food [53] sectors have highlighted their potential use as alternatives to synthetic compounds. Also, several studies have found that EOs extracted from A. campestris have strong insecticidal activity [54, 55], specifically larvicidal activity against C. pipiens [56, 57].
Some of the compounds that have been identified in the EO of A. campestris include terpenes such as 1,8-cineole, borneol, and terpinen-4-ol and sesquiterpenes such as beta-caryophyllene, alpha-humulene, and germacrene D, as well as other compounds such as p-cymene, thujone, and caryophyllene oxide [46, 58–60].
The current study was carried out for the first time in Morocco in order to valorize the Moroccan endemic species A. campestris through the cultivation and to highlight the chemical components of EOs extracted from wild and cultivated A. campestris populations in Morocco, as well as to evaluate the larvicidal toxicity of these EOs against C. pipiens (Diptera: Culicidae). To the best of our knowledge, no study has been conducted to investigate the effect of the cultivation of A. campestris EOs on larvicidal activity against C. pipiens. Furthermore, our findings could serve as preliminary data for researchers interested in the valorization of other endemic plants and aid in the development of a biolarvicide, offering a natural and eco-friendly alternative to chemical insecticides. Additionally, it may play a crucial role in integrated vector management programs, paving the way for a natural product-based pest control method.
2. Materials and Methods
2.1. Plant Material
The aerial parts of two endemic Moroccan plants, wild and cultivated, were harvested at the flowering stage, during the period of May and July of 2022, and their entire details are listed in Table 1. Professor Badr Satrani, a botanist at the Forest Research Center in Rabat, made the identification. Both species' aerial parts were dried in a ventilated environment (in the shade in an airy space) for ten days before being extracted. We successfully cultivated this plant under the following conditions: we used the cutting propagation method, with the cuttings originating from a natural specimen at the Lmarija site (Guercif Province). These cuttings (stems), which were about 10 cm long and had two to three leaves, were planted directly in the field. To reduce transpiration, we covered the cuttings with plastic bottles. We provided regular watering at an ambient temperature and allowed the plant to grow in soil with a slightly alkaline limestone profile.
Table 1.
The main information on the two-plant species whose larvicidal activity was tested in this study.
| Species | Harvest date | Site | GPS coordinates | Altitude (m) |
|---|---|---|---|---|
| Wild A. campestris | May 2022 | Lmarija (Guercif Province) | 33°58′56.0″ N 3°17′31.9″ W | 816 |
| Cultivated A. campestris | July 2022 | Ouled Aid (Boulemane Province) | 33°29′55.0″N 3°39′50.4″W | 732 |
2.2. Extraction of Essential Oils and Chemical Characterization
The aerial part of each plant was divided into small plots and placed in a flask with 1 L of distilled water for a total of 100 g. Thereafter, EOs were extracted using a Clevenger apparatus [61] through a three-hour hydrodistillation process. After removing any remaining water with anhydrous sodium sulfate, they were kept at 4°C. Gas chromatography-mass spectrometry (GC-MS) was used to chemically analyze the EOs.
2.2.1. GC-MS Analysis
Gas chromatography-mass spectrometry (GC-MS) was used to determine the precise composition of EOs. This technique allows for compounds to be identified by their mass-to-charge ratio. The GC-MS analysis was performed using a Trace GC Ultra apparatus equipped with a triple quadrupole detector, a splitless injector, and an RTxi-5 Sil MS capillary apolar column (30 m × 0.25 mm ID × 0.25 m). The operational conditions were as follows: the column was held at 50°C for 2 minutes and then heated at a rate of 5°C/min to 160°C for 2 minutes and then to 280°C for 2 minutes. The connection to the Polaris QMS mass spectrometer was made at 280°C. The injection temperature was 250°C, the injection volume was 1 µl, the pressure was 37.1 kPa·mL/min, the carrier gas was helium, and the solvent was hexane. The identification of the different phytochemical components of the essential oil was obtained by determining their retention indices and comparing them with literature data [62, 63].
2.3. Mosquito Larvae Collection
C. pipiens larvae were collected from the Oued El-Mehraz region (altitude: 423 m; 34°02′13.74″N, 4°59′59.279″W). The larvae were gathered in a rectangular plastic dish and then kept in the entomology unit at the Regional Public Health Laboratory of Fez under consistent conditions, in the breading site water, with a water temperature of 24.6°C ± 2°C and a relative humidity of 50%–70%. The third and fourth-instar larvae were used in the experiments. We used two tools to identify mosquito larvae based on their morphological characteristics: the Moroccan identification key for Culicidae [64] and the African Mediterranean mosquito identification software [65].
2.4. Larvicidal Bioassays
The larviciding tests were carried out in accordance with the WHO recommendations, with minor modifications [66]. A sequence of exploratory experiments was carried out in order to establish an appropriate range of EO concentrations for both wild and cultivated A. campestris. Ethanol was used as a solvent, and the concentrations tested for wild A. campestris were 4, 8, 12.5, 20, 25, 30, and 50 µg/ml, while the concentrations tested for cultivated A. campestris were 2.5, 10, 20, 30, and 40 µg/ml. For each concentration, three replicates were prepared. In the experiment, one milliliter of each produced suspension was placed in a beaker containing 99 milliliters of distilled water and twenty third- and fourth-instar larvae of C. pipiens. A control test was also conducted by combining 1 ml of ethanol with 99 ml of distilled water and 20 C. pipiens larvae. Each larvicidal experiment, as well as the control test, received three replicates. Mortality of all concentrations was determined after 24 hours of treatment. If the control mortality test exceeds 5%, the mortality rate of larvae exposed to EOs must be corrected using Abbott's formula [67], and if the control mortality test exceeds 20%, the larvicide tests are invalid and must be repeated.
| (1) |
2.5. Statistical Data Processing
Statistical tests were performed using the log-probit program from CIRAD-CA/MABIS [68]. Finney's mathematical procedures were used to calculate lethal concentration levels, along with 95 percent confidence limits and the Chi2 test (LC50 and LC90), and a Tukey post hoc test was used with Origin Pro 2021 software to examine significant differences between average values (a probability of p ≤ 0.05 was considered statistically significant).
3. Results and Discussion
3.1. Yield of Essential Oils
The yield obtained from the samples of A. campestris was 0.6 ± 0.2% and 0.5 ± 0.1% for wild and cultivated plants (Figure 1), respectively. No significant differences (p > 0.05) were noticed between the wild and cultivated plants in terms of the average yield. The results indicated that cultivation did not affect EO production.
Figure 1.
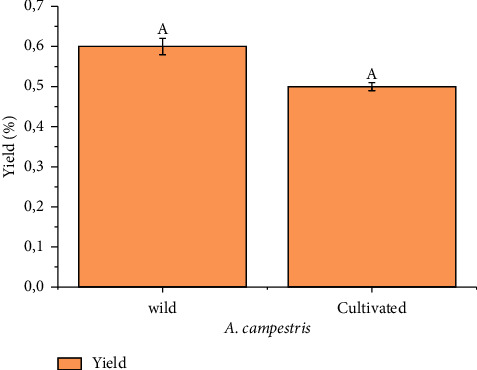
Average yield of A. campestris essential oil for the wild and cultivated plants. According to the Tukey test, each column represented by a different letter demonstrated a significant difference (p < 0.05).
Our findings are consistent with those obtained in Morocco by Aljaiyash et al. [24], who demonstrated that there is no significant difference in the yield of EO from cultivated and wild A. herba-alba and that cultivation does not affect oil production.
3.2. Chemical Composition of Essential Oils
Our findings showed that cultivation had little effect on the quantitative oil production of A. campestris. EO components of the plants investigated in this study are reported in Table 2. A total of thirty-five compounds were identified, representing 99.99% of wild A. campestris EO. The most prevalent components were camphor (18.98%), followed by car-3-en-5-one (11.25%), thujone (6.36%), chrysanthenone (6.24%), filifolone (4.56%), ledol (4.23%), chrysanthenyl acetate (3.84%), borneol (3.56%), and eucalyptol (3.45%). Moreover, thirty-three compounds were detected in the EO of cultivated A. campestris, accounting for 100% of the total composition. This oil was characterized by high levels of camphor (21.01%), followed by car-3-en-5-one (17%), chrysanthenone (10.15%), filifolone (7.90%), spathulenol (3.65%), borneol (3.38%), and thujone (3.08%).
Table 2.
Chemical compounds derived from both wild and cultivated A. campestris aerial parts (%).
| No | Compounds | aKI | bKI Lit | m/z ratio | Wild | Cultivated |
|---|---|---|---|---|---|---|
| 1 | 1,3-Cyclopentadiene, 1,2,5,5-tetramethyl- | 878 | 834 | 122 | Tr | 0.47 ± 0.03 |
| 2 | 1-Ethyl-5,5-dimethylcyclopenta-1,3-diene | 887 | 856 | 122 | Tr | 2.34 ± 0.05 |
| 3 | Camphene | 943 | 946 | 136 | 2.46 ± 0.04 | 1.70 ± 0.06 |
| 4 | Hemellitol | 1020 | 1018 | 120 | 1.68 ± 0.01 | 0.77 ± 0.05 |
| 5 | Pseudocumene | 1020 | 976 | 120 | Tr | 0.50 ± 0.05 |
| 6 | Cymenene | 1042 | 1066 | 132 | 1.74 ± 0.03 | 0.58 ± 0.04 |
| 7 | Eucalyptol | 1059 | 1057 | 154 | 3.45 ± 0.07 | 2.24 ± 0.05 |
| 8 | Thujone | 1062 | 1059 | 152 | 6.63 ± 0.08 | 3.08 ± 0.06 |
| 9 | Cis-p-menth-2-en-1-ol | 1109 | 1115 | 154 | 1.74 ± 0.03 | 1.06 ± 0.07 |
| 10 | Ocimenone | 1112 | 1225 | 150 | 1.57 ± 0.06 | 1.74 ± 0.08 |
| 11 | Pinocarvone | 1114 | 1121 | 150 | Tr | 1.91 ± 0.10 |
| 12 | Carvone | 1114 | 1187 | 150 | Tr | 1.31 ± 0.04 |
| 13 | Rosefuran | 1115 | 1081 | 150 | 0.45 ± 0.07 | 0.44 ± 0.10 |
| 14 | Neoalloocimene | 1118 | 1117 | 136 | 0.40 ± 0.08 | Tr |
| 15 | Verbenone | 1119 | 1190 | 150 | Tr | 1.74 ± 0.06 |
| 16 | Car-3-en-5-one | 1119 | 1114 | 150 | 11.25 ± 0.08 | 17 ± 0.25 |
| 17 | Filifolone | 1119 | 1107 | 150 | 4.56 ± 0.05 | 7.90 ± 0.08 |
| 18 | Chrysanthenone | 1119 | 1123 | 150 | 6.24 ± 0.08 | 10.15 ± 0.07 |
| 19 | Camphor | 1121 | 1117 | 153 | 18.98 ± 0.12 | 21.01 ± 0.19 |
| 20 | Chrysanthenol | 1136 | 1134 | 152 | 2.46 ± 0.11 | Tr |
| 21 | Terpinen-4-ol | 1137 | 1164 | 154 | 1.10 ± 0.05 | 1.43 ± 0.08 |
| 22 | Borneol | 1138 | 1136 | 139 | 3.56 ± 0.07 | 3.38 ± 0.11 |
| 23 | Piperitone | 1158 | 1221 | 152 | 2.63 ± 0.06 | Tr |
| 24 | Piperitol | 1175 | 1176 | 154 | 2.58 ± 0.11 | Tr |
| 25 | Carvotanacetone | 1190 | 1213 | 152 | 0.71 ± 0.11 | Tr |
| 26 | 2-hydroxyphenyl-ethanol | 1210 | 1410 | 138 | 1.54 ± 0.07 | 2.07 ± 0.11 |
| 27 | Butylphenyl | 1228 | 1500 | 204 | 0.38 ± 0.06 | Tr |
| 28 | Chrysanthemic acid | 1256 | 1283 | 168 | 0.40 ± 0.1 | Tr |
| 29 | Chrysanthenyl acetate | 1276 | 1267 | 194 | 3.84 ± 0.08 | 1.17 ± 0.06 |
| 30 | Fenchyl acetate | 1277 | 1253 | 196 | 1.16 ± 0.07 | Tr |
| 31 | Filifolide a | 1293 | 1318 | 166 | 2.14 ± 0.05 | Tr |
| 32 | Copaen-4-α-ol | 1367 | 1576 | 220 | 0.60 ± 0.10 | Tr |
| 33 | Isobornyl acrylate | 1390 | 1374 | 208 | 1.50 ± 0.10 | 1.83 ± 0.08 |
| 34 | 1-Acetoxy-p-menth-3-one | 1488 | 1471 | 212 | 1.44 ± 0.08 | 0.41 ± 0.06 |
| 35 | Bicyclogermacrene | 1499 | 1495 | 204 | Tr | 0.52 ± 0.08 |
| 36 | Germacrene D | 1515 | 1510 | 204 | 0.54 ± 0.03 | 1.91 ± 0.14 |
| 37 | Ledol | 1530 | 1565 | 222 | 4.23 ± 0.08 | 0.77 ± 0.06 |
| 38 | Palustrol | 1530 | 1548 | 222 | 0.65 ± 0.1 | Tr |
| 39 | Spathulenol | 1536 | 1549 | 220 | 2.19 ± 0.05 | 3.65 ± 0.1 |
| 40 | Carveol acetate | 1629 | 1629 | 152 | 3.07 ± 0.11 | Tr |
| 41 | Widdrol | 1651 | 1597 | 222 | 0.77 ± 0.07 | 1.01 ± 0.11 |
| 42 | Cariophylladienol I | 1677 | 2801 | 220 | Tr | 1.00 ± 0.21 |
| 43 | Globulol | 1681 | 1608 | 222 | 0.61 ± 0.01 | Tr |
| 43 | (1R,7S,E)-7-Isopropyl-4,10-dimethylenecyclodec-5-enol | 1699 | 1664 | 220 | Tr | 0.85 ± 0.08 |
| 44 | 7R,8R-8-Hydroxy-4-isopropylidene-7- | 1754 | 1754 | 220 | Tr | 0.43 ± 0.03 |
| 45 | Methyl 3-methyl-2-butenoate | 1980 | 1184 | 114 | 0.74 ± 0.06 | Tr |
| Total | 99.99 ± 0.01 | 100 ± 0.00 |
Tr: trace. Data are expressed as average ± standard deviation of triplicates. Data are expressed as the mean ± standard deviation of three replicates. aKI denotes Kovats retention indices experimentally calculated by retention indices for a homologous series of C8–C28 alkanes. bKI Lit denotes literature-based Kovats retention indices [69–71].
The EOs of the wild and cultivated plants showed a simple quantitative difference, particularly in the amounts of the major compounds (eucalyptol, thujone, car-3-en-5-one, filifolone, chrysanthenone, and camphor). The qualitative profile, on the other hand, revealed a nearly identical chemical composition. The oil obtained from the cultivated plant contained high levels of the four major compounds: camphor (21.01%), car-3-en-5-one (17%), chrysanthenone (10.15%), and filifolone (7.90%), whereas the oil obtained from the wild plant mainly contained camphor (18.98%), car-3-en-5-one (11.25%), chrysanthenone (6.24%), and thujone (6.63%).
Our discovery supports previous research that found camphor in the majority of A. campestris EOs [72, 73]. This bicyclic compound has numerous biological activities, including insecticidal, analgesic, antimicrobial, antiviral, anticancer, and antitussive properties [50].
Furthermore, several studies have been carried out and have revealed the presence of other chemical profiles in the EO of A. campestris. The EO of A. campestris from Algeria [58] revealed the presence of β-pinene (25.6%), sabinene (17%), and α-pinene (9.9%) as major compounds.
Similarly, Aicha et al. [74] discovered the presence of β-pinene (41.0%), p-cymene (9.9%), α-terpinene (7.9%), and limonene (6.5%) as the main compounds. In Morocco, Al Jahid et al. [59] discovered a high concentration of o-cymene (5.4%), limonene (7.0%), α-pinene (7.5%), spathulenol (10.8%), and β-pinene (12.0%). Also, in Tunisia, the main components of the EO of A. campestris are β-pinene (32.95%), limonene (15.13%), ∝-pinene (12.25%), g-terpinene (7.6%), and β-myrcene (5.51%) [41]. According to Rocha et al. [75], the main compounds of the EO of A. campestris aerial parts from Portugal are β-pinene (54.5%), cadin-4-en-7-ol (9.5%), Z-β-ocimene (6.0%), and y-terpinene (4.6%). A sample of Tunisian EO of A. campestris showed that the major constituents were β-pinene (36.40%), 2-undecanone (14.7%), limonene (10.57%), and benzene (6.3%) [76].
This disparity could be explained in part by qualitative and/or quantitative variations in the chemical composition of the EOs, which are primarily related to the cultivation conditions such as geographical factors, climate, and physical and chemical properties of the soil [77]. EO production is influenced by a variety of factors, including genetics and plant developmental stage. Environmental conditions also play an important role, causing biochemical and physiological changes that affect both the quantity and quality of EOs [78]. These changes can adversely affect aromatic plant production and subsequently reduce the overall quality of the obtained EOs. Therefore, it becomes imperative to explore and implement effective agronomical management techniques to enhance EO production and optimize compound concentration. Another critical factor influencing EO production is the presence of plant growth regulators or hormones. These substances, whether naturally occurring within plants or applied externally, can significantly influence both the production and chemical composition of EOs. By comprehending and controlling these variables, it becomes possible to achieve more consistent and improved EO products [79].
The synthesis of secondary metabolites is genetically controlled, but their production is greatly influenced by environmental conditions, harvesting, and postharvest factors. Precipitation, temperature, light, and humidity also affect the volatile oil yield and the content of principal components [80]. Plant development stage and specific organ development, as well as exogenous factors (biotic and abiotic), all play a role in shaping essential oil production [81]. Sangwan et al. [78] conducted research on EO production regulation and identified several influential factors affecting both the quantity and quality of these compounds. Such factors include ontogeny, photosynthetic rate, photoperiod, light quality, climatic and seasonal variations, nutritional availability, humidity, salinity, temperature, storage conditions, and growth regulators. Farooqi and Shukla's study [82] also confirmed the impact of growth regulators, or plant hormones, on the quality and quantity of EO.
3.3. Larvicidal Activity of Essential Oils against Culex pipiens
The two plant EOs were tested to evaluate their larvicidal activity against C. pipiens (Table 3), and the tested oils revealed various mortality percentages at different concentrations. Results are represented as mean ± standard error (SE) after 24 h of exposure. The data obtained from the EO of wild A. campestris show that the highest percentage mortality was recorded at 50 µg/ml and was evaluated at 100 ± 0.00%, and 40 µg/ml for the EO of the cultivated plant with a mortality rate of 100 ± 0.00%, after 24 h of treatment (Figures 2 and 3). Our findings were consistent with those found in the literature, which show that mortality increases with dose and contact time [83]. The most effective activity was obtained by the cultivated A. campestris EO (LC50 = 9.79 μg/ml).
Table 3.
Lethal concentrations of EOs (LC50 and LC90) from wild and cultivated A. campestris against C. pipiens.
| Essential oils | LC50 (µg/ml) (Ll-Ul)∗ | LC90 (µg/ml) (Ll-Ul)∗ | Equation of the regression line | Calculated Chi2 |
|---|---|---|---|---|
| Cultivated A. campestris | 9.79 (3.47 ± 16.52) | 38.57 (21.69 ± 59.77) | Y = −2.13334 + 2.15282∗ X | 32.800 |
| Wild A. campestris | 19.07 (13.57 ± 23.38) | 35.63 (28.37 ± 60.46) | Y = −6.04918 + 4.72392∗ X | 13.871 |
LC50 and LC90 = lethal concentrations that kill 50% and 90% of the exposed larvae; ∗Ll-Ul: lower limit-upper limit.
Figure 2.
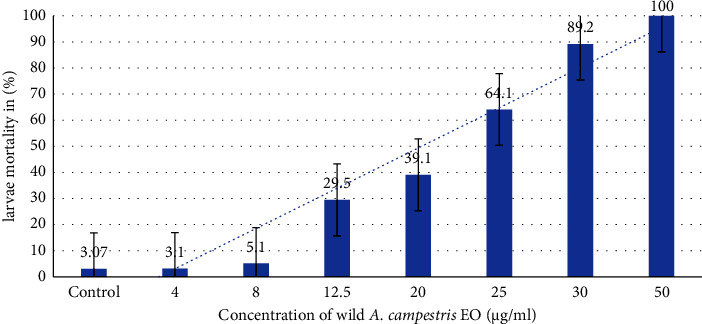
Mortality rates of C. pipiens larvae after 24 h of exposure to wild A. campestris EO at different concentrations.
Figure 3.
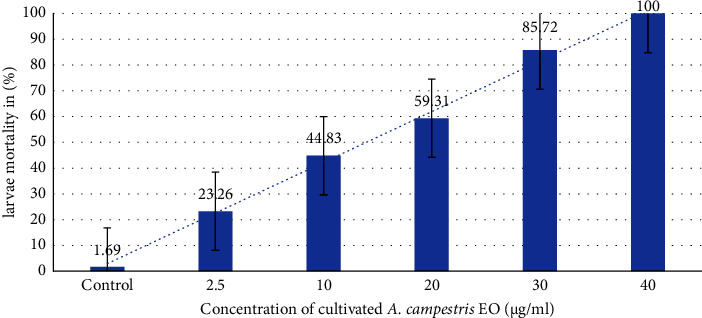
Mortality rates of C. pipiens larvae after 24 h of exposure to cultivated A. campestris EO at different concentrations.
The larvicidal activities against C. pipiens obtained from the wild samples of A. campestris were 19.07 ± 2.57 µg/ml and 35.63 ± 03.37 µg/ml for LC50 and LC90, respectively. Regarding lethal concentrations, the cultivated samples of A. campestris reported 9.79 ± 1.47 µg/ml and 38.57 ± 03.69 µg/ml for LC50 and LC90, respectively. Figure 4 illustrates the significant differences (p < 0.05) in LC50 of wild and cultivated A. campestris EOs. However, no significant difference (p > 0.05) was noticed among wild and cultivated A. campestris EOs in terms of LC90.
Figure 4.
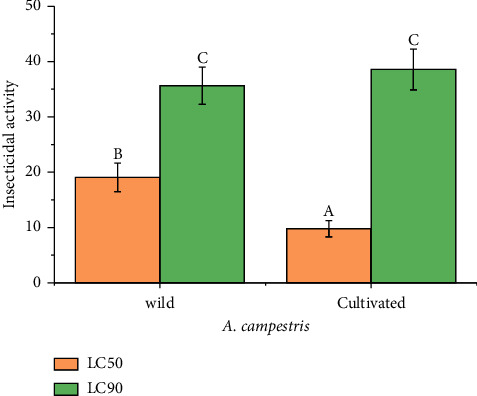
Average insecticidal activity of A. campestris EO for wild and cultivated plants. According to the Tukey test, each column represented by a different letter (A, B, and C) illustrated a significant difference (p < 0.05).
The results of the experiment revealed that both oils had interesting insecticidal properties against C. pipiens larvae. The insecticidal efficacy of both EOs is due to the high concentration of camphor, an oxygenated monoterpene well known for its strong insecticidal activity [84].
Almost 2,000 plant species with insecticidal activity have already been identified [85]. This activity could be explained by the development of chemical substances such as (phenols, polyphenols, terpenoids, and alkaloids) by plants [86]. In general, several authors state that products with LC50 values less than 100 μg/mL are considered active [87–89].
Sneha et al. [90] found that EOs derived from four Ocimum species (Ocimum basilicum, Ocimum gratissimum, Ocimum tenuiflorum, and Ocimum canum) were highly effective against three disease-carrying mosquitos: Aedes aegypti, Culex tritaeniorhynchus, and Armigeres subalbatus. Allspice EOs (Pimenta dioica) were also found to have strong insecticidal and larvicidal activities, with LC50 values of 18.5 ± 1.2 µg/mL against Aedes aegypti, 28.9 ± 1.6 µg/mL against Culex quinquefasciatus, and 55.1 ± 3.1 µg/mL against Armigeres subalbatus [91]. Narayanankutty et al. [92] also demonstrated that Cinnamomum verum EO has antibacterial, insecticidal, and larvicidal properties.
As far as we know, no previous studies have specifically investigated the larvicidal effects of EO from wild and cultivated A. campestris against the C. pipiens mosquito. As a result, we conducted a comparison of our findings with previous research on Artemisia's insecticidal properties against various Culicidae family species.
Several studies have investigated the insecticidal properties of EOs from wild and cultivated plants [93–95]. Also, several studies have been conducted to investigate the larvicidal activity of plants of the genus Artemisia [24, 96, 97].
Our results corroborate with those found in Morocco by Aljaiyash et al. [24], who proved that cultivated A. herba-alba EO was more toxic than the wild plants against adults of the insect pest T. castaneum. In the same context, EOs from both wild and cultivated Ruta chalepensis plants were found to have very high toxicity against Aedes albopictus larvae with LC50 values of 35.66 ppm and 33.18 ppm, respectively [95].
Also, both EOs present remarkable toxicity similar to that found by Ammar et al. [54] against C. quinquefasciatus with a value of LC50 of 45.8 mg/L.
From the presented results, we can conclude that the insecticidal activity could be expressed by the variety of bioactive molecules that define each EO.
Even if the two plants are of the same origin, the difference in chemical composition and insecticidal properties could be explained by the influence of several factors that act on cultivation conditions, including climatic factors, soil properties, and altitude [24].
4. Conclusion
This study focused on the larvicidal effect of essential oils from cultivated and wild populations of A. campestris, which are endemic to Morocco, against the mosquito Culex pipiens, which transmits the West Nile virus. The results revealed the efficacy of essential oils, as the chemical components within these oils displayed potent larvicidal activity. The cultivated A. campestris revealed the highest larvicidal activity. These active compounds are recommended as natural, plant-based alternatives with notable insecticidal properties. However, further studies should be conducted to explore the effect of essential oils from other species of the same genus, as well as their synergistic effects, to optimize their larvicidal potential. Additionally, the potential toxicity of these products on wildlife should also be studied.
Acknowledgments
The authors express special gratitude to Professor Badr Satrani, who identified the plant species, and to Ansong Dhaud Odei who corrected the manuscript's English.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
Authors' Contributions
A.A. conceived and designed the experiments, analyzed and interpreted the data, performed the experiments, and wrote the paper; S.A. and F.E. helped in the statistical treatment and analyzed the data; Y.E. helped in the experimental part and validation and contributed to writing, supervision, and editing; A.E.O.L. and A.F. contributed to conception, writing, supervision, and editing and performed the experiments, supervision, and data curation. All authors have read and approved the final manuscript.
Supplementary Materials
The supplementary material file submitted along with the manuscript that summarize the steps involved in the biological part of the study is a graphical abstract.
References
- 1.Mehlhorn H. Parasites and their world records in their fight for survival. In: Mehlhorn H., editor. Progress in Parasitology . Berlin, Heidelberg: Springer Berlin Heidelberg; 2011. [Google Scholar]
- 2.Hubálek Z. Mosquito-borne viruses in Europe. Parasitology Research . 2008;103:29–43. doi: 10.1007/s00436-008-1064-7. [DOI] [PubMed] [Google Scholar]
- 3.El-Sabrout A. M., Zoghroban A. A. M., Abdelgaleil S. A. M. Chemical composition and effects of four essential oils on mortality, development and physiology of the West Nile virus vector, Culex pipiens. International Journal of Tropical Insect Science . 2020;40(4):789–799. doi: 10.1007/s42690-020-00133-8. [DOI] [Google Scholar]
- 4.Zahran H. E.-D. M., Abou-Taleb H. K., Abdelgaleil S. A. M. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. Journal of Asia-Pacific Entomology . 2017;20(1):133–139. doi: 10.1016/j.aspen.2016.12.006. [DOI] [Google Scholar]
- 5.Al-Mekhlafi F. A., Abutaha N., Farooq M., Al-Wadaan M. Insecticidal effect of solenostemma argel extracts against Culex pipiens. Journal of the American Mosquito Control Association . 2018;34(3):217–223. doi: 10.2987/17-6725.1. [DOI] [PubMed] [Google Scholar]
- 6.El-Akhal F., Guemmouh R., Ez Zoubi Y., El Ouali Lalami A. Larvicidal activity of Nerium oleander against larvae West Nile vector mosquito Culex pipiens (Diptera: Culicidae) Journal of Parasitology Research . 2015;2015:5. doi: 10.1155/2015/943060.943060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng S. S., Huang C. G., Chen W. J., Kuo Y. H., Chang S.-T. Larvicidal activity of tectoquinone isolated from red heartwood-type Cryptomeria japonica against two mosquito species. Bioresource Technology . 2008;99(9):3617–3622. doi: 10.1016/j.biortech.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Medrouh B., Lafri I., Beck C., et al. First serological evidence of West Nile virus infection in wild birds in Northern Algeria. Comparative Immunology, Microbiology and Infectious Diseases . 2020;69 doi: 10.1016/j.cimid.2020.101415.101415 [DOI] [PubMed] [Google Scholar]
- 9.Murgue B., Murri S., Triki H., Deubel V., Zeller H. G. West nile in the mediterranean basin: 1950-2000. Annals of the New York Academy of Sciences . 2006;951(1):117–126. doi: 10.1111/j.1749-6632.2001.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 10.Schuffenecker I., Peyrefitte C. N., el Harrak M., Murri S., Leblond A., Zeller H. G. West nile virus in Morocco. Emerging Infectious Diseases . 2005;11(2):306–309. doi: 10.3201/eid1102.040817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benelli G., Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitology Research . 2016;115(5):1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization and Food and Agriculture Organization of the United Nations. Global Situation of Pesticide Management in Agriculture and Public Health: Report of a 2018 WHO-FAO Survey . Geneva, Switzerland: World Health Organization; 2019. https://apps.who.int/iris/handle/10665/329971 . [Google Scholar]
- 13.Huang Y.-J., Higgs S., Vanlandingham D. Biological control strategies for mosquito vectors of arboviruses. Insects . 2017;8(1):p. 21. doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knipling E. F., Laven H., Craig G. B., et al. Genetic control of insects of public health importance. Bulletin of the World Health Organization . 1968;38(3):421–438. [PMC free article] [PubMed] [Google Scholar]
- 15.Hasaballah A. I. Impact of gamma irradiation on the development and reproduction of Culex pipiens (Diptera; Culicidae) International Journal of Radiation Biology . 2018;94(9):844–849. doi: 10.1080/09553002.2018.1490040. [DOI] [PubMed] [Google Scholar]
- 16.Aouinty B., Oufara S., Mellouki F., Mahari S. Évaluation préliminaire de l’activité larvicide des extraits aqueux des feuilles du ricin (Ricinus communis L.) et du bois de thuya (Tetraclinis articulata (Vahl) Mast.) sur les larves de quatre moustiques culicidés: Culex pipiens (Linné), Aedes caspius (Pallas), Culiseta longiareolata (Aitken) et Anopheles maculipennis (Meigen) Biotechnology, Agronomy, Society and Environment . 2006;5 [Google Scholar]
- 17.Kioulos I., Kampouraki A., Morou E., Skavdis G., Vontas J. Insecticide resistance status in the major West Nile virus vector Culex pipiens from Greece: resistance status of Culex pipiens from Greece. Pest Management Science . 2014;70(4):623–627. doi: 10.1002/ps.3595. [DOI] [PubMed] [Google Scholar]
- 18.El Ouali Lalami A., El-Akhal F., El Amri N., Maniar S., Faraj C. État de la résistance du moustique Culex pipiens vis-à-vis du téméphos au centre du Maroc. Bulletin de la Société de Pathologie Exotique . 2014;107(3):194–198. doi: 10.1007/s13149-014-0361-x. [DOI] [PubMed] [Google Scholar]
- 19.El-Akhal F., Greche H., Chahdi F. O., Guemmouh R., Lalami A. E. O. Composition chimique et activité larvicide sur Culex pipiens d’huile essentielle de Thymus vulgaris cultivées au Maroc Chemical composition and larvicidal activity of Culex pipiens essential oil of Thymus vulgaris grown in Morocco. Environmental science . 2015;6 [Google Scholar]
- 20.Abdelhakim E. O. L., Fouad E. A., Wissal O., Fouad C., Rajae G., Hassane G. Composition chimique et activité antibactérienne des huiles essentielles de deux plantes aromatiques du centre nord marocain. Thymus vulagris et Thymus satureioïdis . 2013;8:p. 7. [Google Scholar]
- 21.Baz M. M., Selim A., Radwan I. T., Alkhaibari A. M., Khater H. F. Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. Scientific Reports . 2022;12(1):p. 4406. doi: 10.1038/s41598-022-08223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Kasem Bosly H. A. Larvicidal and adulticidal activity of essential oils from plants of the Lamiaceae family against the West Nile virus vector, Culex pipiens (Diptera: Culicidae) Saudi Journal of Biological Sciences . 2022;29(8) doi: 10.1016/j.sjbs.2022.103350.103350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khater H., E Soliman D., Slim A., Debboun M., M Baz M. Larvicidal efficacy of fifteen plant essential oils against Culex pipiens L. Mosquitoes in Egypt. Egyptian Journal of Veterinary Science . 2023;54(2):183–192. doi: 10.21608/ejvs.2022.161941.1395. [DOI] [Google Scholar]
- 24.Aljaiyash A., Kasrati A., Alaoui Jamali C., Chaouch A. Effect of cultivation on chemical composition and bioactivities of essential oils from Artemisia herba-alba Asso grown in Morocco. Biochemical Systematics and Ecology . 2018;81:74–79. doi: 10.1016/j.bse.2018.10.001. [DOI] [Google Scholar]
- 25.Ghasemi Pirbalouti A., Firoznezhad M., Craker L., Akbarzadeh M. Essential oil compositions, antibacterial and antioxidant activities of various populations of Artemisia chamaemelifolia at two phenological stages. Revista Brasileira de Farmacognosia . 2013;23(6):861–869. doi: 10.1590/S0102-695X2013000600002. [DOI] [Google Scholar]
- 26.Bora K. S., Sharma A. The genus Artemisia: a comprehensive review. Pharmaceutical Biology . 2011;49(1):101–109. doi: 10.3109/13880209.2010.497815. [DOI] [PubMed] [Google Scholar]
- 27.Salaroli R., Andreani G., Bernardini C., et al. Anticancer activity of an Artemisia annua L. hydroalcoholic extract on canine osteosarcoma cell lines. Research in Veterinary Science . 2022;152:476–484. doi: 10.1016/j.rvsc.2022.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Su S. H., Sundhar N., Kuo W. W., et al. Artemisia argyi extract induces apoptosis in human gemcitabine-resistant lung cancer cells via the PI3K/MAPK signaling pathway. Journal of Ethnopharmacology . 2022;299 doi: 10.1016/j.jep.2022.115658.115658 [DOI] [PubMed] [Google Scholar]
- 29.Ez Zoubi Y., Lairini S., Farah A., Taghzouti K., El Ouali Lalami A. Antioxidant and antibacterial activities of Artemisia herba-alba asso essential oil from middle atlas, Morocco. Phytothérapie . 2018;16:S48–S54. doi: 10.3166/phyto-2018-0057. [DOI] [Google Scholar]
- 30.Ez-Zoubi A., Ez Zoubi Y., Bentata F., et al. Preparation and characterization of a biopesticide based on Artemisia herba-alba essential oil encapsulated with succinic acid-modified beta-cyclodextrin. Journal of Chemistry . 2023;2023:8. doi: 10.1155/2023/3830819.3830819 [DOI] [Google Scholar]
- 31.Nezhadali A., Akbarpour M., Shirvan B. Z. Chemical composition of the essential oil from the aerial parts of Artemisia herba. E-Journal of Chemistry . 2008;5(3):557–561. doi: 10.1155/2008/730453. [DOI] [Google Scholar]
- 32.Belhattab R., Amor L., Barroso J. G., Pedro L. G., Cristina Figueiredo A. Essential oil from Artemisia herba-alba Asso grown wild in Algeria: variability assessment and comparison with an updated literature survey. Arabian Journal of Chemistry . 2014;7(2):243–251. doi: 10.1016/j.arabjc.2012.04.042. [DOI] [Google Scholar]
- 33.Seddiek S. A., Ali M. M., Khater H. F., El-Shorbagy M. M. Anthelmintic activity of the white wormwood, artemisia herba-alba against heterakis gallinarum infecting turkey poults. Journal of Medicinal Plants Research . 2020;5 [Google Scholar]
- 34.Aziz A. T., Alshehri M. A., Panneerselvam C., et al. The desert wormwood (Artemisia herba- alba)– from Arabian folk medicine to a source of green and effective nanoinsecticides against mosquito vectors. Journal of Photochemistry and Photobiology B: Biology . 2018;180:225–234. doi: 10.1016/j.jphotobiol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Agrawal P. K., Agrawal C., Blunden G. Artemisia extracts and artemisinin-based antimalarials for COVID-19 management: could these Be effective antivirals for COVID-19 treatment? Molecules . 2022;27(12):p. 3828. doi: 10.3390/molecules27123828. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Abu-Darwish M. S., Cabral C., Gonçalves M. J., et al. Chemical composition and biological activities of Artemisia judaica essential oil from southern desert of Jordan. Journal of Ethnopharmacology . 2016;191:161–168. doi: 10.1016/j.jep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Rose J., Earle S. The World of Aromatherapy: An Anthology of Aromatic History, Ideas, Concepts, and Case Histories . Berkeley, CA, USA: Frog, Ltd; 1996. [Google Scholar]
- 38.Stappen I., Wanner J., Tabanca N., et al. Chemical composition and biological effects of Artemisia maritima and Artemisia nilagirica essential oils from wild plants of western himalaya. Planta Medica . 2014;80(13):1079–1087. doi: 10.1055/s-0034-1382957. [DOI] [PubMed] [Google Scholar]
- 39.Ahameethunisa A. R., Hopper W. Antibacterial activity of Artemisia nilagirica leaf extracts against clinical and phytopathogenic bacteria. BMC Complementary and Alternative Medicine . 2010;10(1):p. 6. doi: 10.1186/1472-6882-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benabid A. Flore Et Écosystèmes Du Maroc: Évaluation Et Préservation De La Biodiversité . Paris, France: Ibis press Librairie et éd. Kalila wa Dimna; 2000. [Google Scholar]
- 41.Aloui Z., Messaoud C., Haoues M., et al. Asteraceae Artemisia campestris and Artemisia herba-alba essential oils trigger apoptosis and cell cycle arrest in Leishmania infantum promastigotes. Evidence-based Complementary and Alternative Medicine . 2016;2016:15. doi: 10.1155/2016/9147096.9147096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houicher A., Hechachna H., Özogul F. In Vitro determination of the antifungal activity of Artemisia campestris essential oil from Algeria. International Journal of Food Properties . 2016;19(8):1749–1756. doi: 10.1080/10942912.2015.1107734. [DOI] [Google Scholar]
- 43.Erel Ş. B., Reznicek G., Şenol S. G., Yavaşoğlu N. Ü. K., Konyalioğlu S., Zeybek A. U. Antimicrobial and antioxidant properties of Artemisia L. species from western Anatolia. Turkish Journal of Biology . 2012;36 doi: 10.3906/biy-0912-27. [DOI] [Google Scholar]
- 44.Miara M. D., Bendif H., Ouabed A., et al. Ethnoveterinary remedies used in the Algerian steppe: exploring the relationship with traditional human herbal medicine. Journal of Ethnopharmacology . 2019;244 doi: 10.1016/j.jep.2019.112164.112164 [DOI] [PubMed] [Google Scholar]
- 45.Masyita A., Mustika Sari R., Dwi Astuti A., et al. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chemistry X . 2022;13 doi: 10.1016/j.fochx.2022.100217.100217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dib I., Angenot L., Mihamou A., Ziyyat A., Tits M. Artemisia campestris L.: ethnomedicinal, phytochemical and pharmacological review. Journal of Herbal Medicine . 2017;7:1–10. doi: 10.1016/j.hermed.2016.10.005. [DOI] [Google Scholar]
- 47.Raut J. S., Karuppayil S. M. A status review on the medicinal properties of essential oils. Industrial Crops and Products . 2014;62:250–264. doi: 10.1016/j.indcrop.2014.05.055. [DOI] [Google Scholar]
- 48.Boulogne I., Petit P., Ozier-Lafontaine H., Desfontaines L., Loranger-Merciris G. Insecticidal and antifungal chemicals produced by plants: a review. Environmental Chemistry Letters . 2012;10(4):325–347. doi: 10.1007/s10311-012-0359-1. [DOI] [Google Scholar]
- 49.Fusani P., Ronga D., Carminati D., et al. Composition and biological activity of essential oils from Artemisia roxburghiana Besser and Elsholtzia fruticosa Rehder cultivated in Italy. Industrial Crops and Products . 2022;187 doi: 10.1016/j.indcrop.2022.115317.115317 [DOI] [Google Scholar]
- 50.Zielińska-Błajet M., Feder-Kubis J. Monoterpenes and their derivatives—recent development in biological and medical applications. Indian Journal of Management Science . 2020;21(19):p. 7078. doi: 10.3390/ijms21197078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raveau R., Fontaine J., Lounès-Hadj Sahraoui A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: a review. Foods . 2020;9(3):p. 365. doi: 10.3390/foods9030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godlewska K., Ronga D., Michalak I. Plant extracts- importance in sustainable agriculture. Italian Journal of Agronomy . 2021;16(2) doi: 10.4081/ija.2021.1851. [DOI] [Google Scholar]
- 53.Diniz do Nascimento L., Moraes A. A. B. D., Costa K. S. D., et al. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: new findings and potential applications. Biomolecules . 2020;10(7):p. 988. doi: 10.3390/biom10070988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ammar S., Noui H., Djamel S., et al. Essential oils from three Algerian medicinal plants (Artemisia campestris, Pulicaria arabica, and Saccocalyx satureioides) as new botanical insecticides? Environmental Science & Pollution Research . 2020;27(21):26594–26604. doi: 10.1007/s11356-020-09064-w. [DOI] [PubMed] [Google Scholar]
- 55.Murugesan S., Gunasekaran P., Tenzin G., et al. Laboratory Evaluation of Asteraceae Species Tagetes erecta Linnaeus and Tridax Procumbens Linnaeus for Their Toxicity against the Larvae of Culex quinquefasciatus Say 1823 (Diptera: Culicidae) Delhi, India: International Journal of Mosquito Research; 2016. [Google Scholar]
- 56.Masotti V., De Jong L., Moreau X., Rabier J., Laffont-Schwob I., Thiéry A. Larvicidal activity of extracts from Artemisia species against Culex pipiens L. mosquito: comparing endemic versus ubiquist species for effectiveness. Comptes Rendus Biologies . Jan. 2012;335(1):19–25. doi: 10.1016/j.crvi.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Saida D., Hadria B. Etude bibliographique de l’effet larvicide de l’huile essentielle d’Artemisia campestris à l’égard de Culex pipiens . Tébessa, Algeria: Universite laarbi tebessi tebessa; 2020. [Google Scholar]
- 58.Bakchiche B., Gherib A., Maatallah M., Miguel M. G. Chemical composition of essential oils of Artemisia campestris and juniperus phoenicea from Algeria. International Journal of Innovation and Applied Studies . 2014;9(4) [Google Scholar]
- 59.Al Jahid A., Elamrani A., Lahlou F. A., et al. Chemical composition and antibacterial activity of the essential oil isolated from the seeds of Moroccan Artemisia campestris L. Journal of Essential Oil-Bearing Plants . 2017;20(2):375–384. doi: 10.1080/0972060X.2016.1266969. [DOI] [Google Scholar]
- 60.Mathlouthi A., Belkessam M., Sdiri M., et al. Chemical composition and anti-leishmania major activity of essential oils from artemesia spp. grown in Central Tunisia. Journal of Essential Oil-Bearing Plants . Sep 2018;21(5):1186–1198. doi: 10.1080/0972060X.2018.1526128. [DOI] [Google Scholar]
- 61.Clevenger J. F. Apparatus for the determination of volatile oil. Journal of the American Pharmaceutical Association . 1928;17(4):345–349. doi: 10.1002/jps.3080170407. [DOI] [Google Scholar]
- 62.Adams R. P. Identification of Essential Oil Components by Gas Chromatography/quadrupole Mass Spectroscopy . 3rd. Carol Stream, IL, USA: Allured Pub. Corporation; 2001. [Google Scholar]
- 63.Joulain D., König W. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons . Hamburg, Germany: E.B.-Verlag; 1998. [Google Scholar]
- 64.Himmi O., Dakki M., Trari B., El Agbani M. A. Les Culicidae du Maroc: clés d’identification, avec données biologiques et écologiques, Tous les formats et éditions . Rabat, Morocco: Institut Scientifique; 1995. [Google Scholar]
- 65.Brunhes J., Rhaim A., Geoffroy B., Gueugnot J., Killick-Kendrick R., Camicas J. L. Didactiques. Tunis-Belvédère . Tunis, Tunisia: Institut Pasteur (Tunis); 2000. Les culicidae de l’Afrique méditerranéenne: un programme d’identification et d’enseignement. [Google Scholar]
- 66.World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. 2005. https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13 .
- 67.Abbott W. S. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology . 1925;18(2):265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 68.Giner M., Vassal M., Vassal C., Chiroleu F. Z., Kouaik Z. WinDL Software Version 2.0 . Montpellier, France: CIRAD-CA URBI/MABIS; 1999. [Google Scholar]
- 69.Babushok V. I., Linstrom P. J., Zenkevich I. G. Retention indices for frequently reported compounds of plant essential oils. Journal of Physical and Chemical Reference Data . 2011;40(4) doi: 10.1063/1.3653552.043101 [DOI] [Google Scholar]
- 70.Zhou X., Yu Q., Gong H., Tian S. GC-MS analysis of Ziziphora clinopodioides essential oil from North xinjiang, China. Natural Product Communications . 2012;7(1) doi: 10.1177/1934578X1200700128.1934578X1200700 [DOI] [PubMed] [Google Scholar]
- 71.Younessi-Hamzekhanlu M., Sanjari S., Dejahang A., et al. Evaluation of essential oil from different Artemisia fragrans willd. Populations: chemical composition, antioxidant, and antibacterial activity. Journal of Essential Oil-Bearing Plants . 2020;23(6):1218–1236. doi: 10.1080/0972060X.2020.1854129. [DOI] [Google Scholar]
- 72.Akrout A., Chemli R., Chreïf I., Hammami M. Analysis of the essential oil of Artemisia campestris L.: essential oil of artemisia campestris. Flavour and Fragrance Journal . 2001;16(5):337–339. doi: 10.1002/ffj.1006. [DOI] [Google Scholar]
- 73.Belhattab R., Boudjouref M., Barroso J G., Pedro L P., Figueirido A C. Essential oil composition from Artemisia campestris grown in Algeria. Advances in Environmental Biology . 2011;45:429–432. [Google Scholar]
- 74.Aicha N., Ines S., Mohamed B. S., et al. Chemical composition, mutagenic and antimutagenic activities of essential oils from (Tunisian) Artemisia campestris and Artemisia herba-alba. Journal of Essential Oil Research . 2008;20(5):471–477. doi: 10.1080/10412905.2008.9700061. [DOI] [Google Scholar]
- 75.Rocha M. I., Gonçalves M. J., Cavaleiro C., et al. Chemical characterization and bioactive potential of Artemisia campestris L. subsp. maritima (DC) Arcang. essential oil and hydrodistillation residual water. Journal of Ethnopharmacology . 2021;276 doi: 10.1016/j.jep.2021.114146.114146 [DOI] [PubMed] [Google Scholar]
- 76.Abidi A., Sebai E., Dhibi M., et al. Chemical analyses and anthelmintic effects of Artemisia campestris essential oil. Veterinary Parasitology . 2018;263:59–65. doi: 10.1016/j.vetpar.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 77.El Hamdaoui A., Msanda F., Boubaker H., et al. Essential oil composition, antioxidant and antibacterial activities of wild and cultivated Lavandula mairei Humbert. Biochemical Systematics and Ecology . 2018;76:1–7. doi: 10.1016/j.bse.2017.11.004. [DOI] [Google Scholar]
- 78.Sangwan N. S., Farooqi A. H. A., Shabih F., Sangwan R. S. Regulation of essential oil production in plants. Plant Growth Regulation . 2001;34(1):3–21. doi: 10.1023/A:1013386921596. [DOI] [Google Scholar]
- 79.Prins C. L., Vieira I. J. C., Freitas S. P. Growth regulators and essential oil production. Brazilian Journal of Plant Physiology . 2010;22(2):91–102. doi: 10.1590/S1677-04202010000200003. [DOI] [Google Scholar]
- 80.Blank A. F., Costa A. G., Arrigoni-Blank M. D. F., et al. Influence of season, harvest time and drying on Java citronella (Cymbopogon winterianus Jowitt) volatile oil. Revista Brasileira de Farmacognosia . 2007;17(4):557–564. doi: 10.1590/S0102-695X2007000400014. [DOI] [Google Scholar]
- 81.Gobbo-Neto L., Lopes N. P. Plantas medicinais: fatores de influência no conteúdo de metabólitos secundários. Química Nova . 2007;30(2):374–381. doi: 10.1590/S0100-40422007000200026. [DOI] [Google Scholar]
- 82.Shukla A., Abad Farooqi A. Utilization of plant growth regulators in aromatic plant production. Current Research on Medicinal and Aromatic Plants . 1990;50:152–157. [Google Scholar]
- 83.Ramzi A., Farah A., Ez Zoubi Y., Annemer S., El Ouali Lalami A. Aroma profile and fumigant toxicity of two Moroccan Lavandula species essential oils against Culex pipiens (Diptera: Culicidae) International Journal of Tropical Insect Science . 2022;42(3):2663–2672. doi: 10.1007/s42690-022-00795-6. [DOI] [Google Scholar]
- 84.Pavela R., Sajfrtová M., Sovová H., Bárnet M., Karban J. The insecticidal activity of Tanacetum parthenium (L.) Schultz Bip. extracts obtained by supercritical fluid extraction and hydrodistillation. Industrial Crops and Products . 2010;31(3):449–454. doi: 10.1016/j.indcrop.2010.01.003. [DOI] [Google Scholar]
- 85.Arnason J. T., Philogène B. J. R., Morand P. ACS Symposium Series, No. 387 . Washington, DC, USA: American Chemical Society; 1989. Insecticides of plant origin. [Google Scholar]
- 86.Dubey N. K. Natural Products in Plant Pest Management . Oxfordshire, UK: CABI; 2011. [Google Scholar]
- 87.Cheng S., Chang H. T., Chang S. T., Tsai K. H., Chen W. J. Bioactivity of selected plant essential oils against the yellow fever mosquito Aedes aegypti larvae. Bioresource Technology . 2003;89(1):99–102. doi: 10.1016/S0960-8524(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 88.Komalamisra N., Trongtokit Y., Rongsriyam Y., Apiwathnasorn C. Screening for larvicidal activity in some Thai plants against four mosquito vector species. Southeast Asian Journal of Tropical Medicine and Public Health . 2005;36(6):1412–1422. [PubMed] [Google Scholar]
- 89.Luz T. R. S. A., de Mesquita L. S. S., Amaral F. M. M. D., Coutinho D. F. Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Tropica . 2020;212 doi: 10.1016/j.actatropica.2020.105705.105705 [DOI] [PubMed] [Google Scholar]
- 90.Sneha K., Narayanankutty A., Job J. T., et al. Antimicrobial and larvicidal activities of different Ocimum essential oils extracted by ultrasound-assisted hydrodistillation. Molecules . 2022;27(5):p. 1456. doi: 10.3390/molecules27051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narayanankutty A., Kuttithodi A. M., Alfarhan A., Rajagopal R., Barcelo D. Chemical composition, insecticidal and mosquito larvicidal activities of allspice (pimenta dioica) essential oil. Molecules . 2021;26(21):p. 6698. doi: 10.3390/molecules26216698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Narayanankutty A., Kunnath K., Alfarhan A., Rajagopal R., Ramesh V. Chemical composition of Cinnamomum verum leaf and flower essential oils and analysis of their antibacterial, insecticidal, and larvicidal properties. Molecules . 2021;26(20):p. 6303. doi: 10.3390/molecules26206303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El Abdouni Khiyari M., Kasrati A., Jamali C. A., et al. Chemical composition, antioxidant and insecticidal properties of essential oils from wild and cultivated Salvia aucheri subsp. blancoana (Webb. & Helder)), an endemic, threatened medicinal plant in Morocco. Industrial Crops and Products . 2014;57:106–109. doi: 10.1016/j.indcrop.2014.03.029. [DOI] [Google Scholar]
- 94.Navarro-Rocha J., F Barrero A., Burillo J., Olmeda A. S., González-Coloma A. Valorization of essential oils from two populations (wild and commercial) of Geranium macrorrhizum L. Industrial Crops and Products . 2018;116:41–45. doi: 10.1016/j.indcrop.2018.02.046. [DOI] [Google Scholar]
- 95.Conti B., Leonardi M., Pistelli L., Profeti R., Ouerghemmi I., Benelli G. Larvicidal and repellent activity of essential oils from wild and cultivated Ruta chalepensis L. (Rutaceae) against Aedes albopictus Skuse (Diptera: Culicidae), an arbovirus vector. Parasitology Research . 2013;112(3):991–999. doi: 10.1007/s00436-012-3221-2. [DOI] [PubMed] [Google Scholar]
- 96.Julio L. F., Burillo J., Giménez C., et al. Chemical and biocidal characterization of two cultivated Artemisia absinthium populations with different domestication levels. Industrial Crops and Products . 2015;76:787–792. doi: 10.1016/j.indcrop.2015.07.041. [DOI] [Google Scholar]
- 97.Bailen M., Julio L. F., Diaz C. E., et al. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Industrial Crops and Products . 2013;49:102–107. doi: 10.1016/j.indcrop.2013.04.055. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The supplementary material file submitted along with the manuscript that summarize the steps involved in the biological part of the study is a graphical abstract.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


