Abstract
Background
The American Heart Association recently updated Life’s Essential 8 (LE8) score. This amalgamation of health factors, recognized for their individual associations with chronic kidney disease (CKD) risk, provides a robust tool to assess overall cardiovascular health (CVH), which could potentially be extrapolated to predict CKD risk.
Objectives
This study aimed to investigate the association between levels of CVH, as measured by the LE8 score, and risk of CKD in the UK Biobank.
Methods
A total of 147,988 participants free of CKD and cardiovascular disease from the UK Biobank were included in this prospective study. CVH levels were categorized as low (0–49), moderate (50–79), and high (80–100) using LE8 score. An adjusted Cox proportional hazard model was used to investigate the association between LE8 and CKD. The population attributable-risk (PAR) was also calculated.
Results
During a median follow-up of 10 y, 1936 CKD cases were documented. A higher LE8 score was associated with a significant lower risk of CKD (P < 0.001), and a linear dose–response relationship was observed. Similar patterns were also found in the associations of the LE8 behavior and biological subscale scores with CKD. Compared with participants with a low CVH category, participants with a moderate CVH were associated with a 39% lower risk of developing CKD (hazard ratio [HR]: 0.61; 95% confidence interval [CI]: 0.52, 0.72); and those with a high CVH had a 57% lower risk of CKD incidence (HR: 0.43; 95% CI: 0.35, 0.53) after adjustment for covariates. Among 8 distinct metrics of LE8 score, the BMI metric had the highest PAR (24.6%; 95% CI: 18.8, 30.2). Of the total CKD risk, 3.2% (95% CI: 1.4, 5.0) was attributable to inadequate or excessive sleep duration.
Conclusions
High CVH, defined by LE8, is significantly associated with a lower risk of CKD. These results suggest that promoting optimal cardiovascular health may lower the burden of CKD.
Keywords: Life’s Essential 8, cardiovascular health, CKD, lifestyle, PAR, UK Biobank
Introduction
The substantial burden of premature mortality and health loss due to chronic kidney disease (CKD) is well documented [1]. CKD is a major health issue that is linked to an increased risk of cardiovascular disease (CVD) and mortality around the globe [2,3]. CKD may also be promoted concurrently by CVD, creating a vicious cycle [4]. In a 1990–2016 Global Burden of Disease study, CKD incidence had increased by 89%, prevalence by 87%, mortality by 98%, and disability-adjusted life years by 62% [5]. Therefore, reducing the burden of CKD continues to be pivotal from a public health view.
The American Heart Association (AHA) recently updated an enhanced approach to assessing cardiovascular health (CVH), Life’s Essential 8 (LE8), and created a new tool conceptualizing CVH [6]. Not merely a measure of CVH, the LE8 score encompasses 8 important health metrics, making it unique. Besides diet, physical activity, smoking, BMI, blood lipids, blood glucose, and blood pressure (BP), sleep duration is newly introduced as a novel element, based on emerging evidence that emphasizes the significant tie between sleep length and cardiometabolic health [7,8]. Significantly, there are consistent findings linking sleep duration and quality with CKD risk [9,10]. Despite the well-established connection between LE8 score and CVD [11], no study has evaluated the relationship between LE8 score and risk of CKD. Given the intricate interplay between CVD and CKD, our study for first time explored the potential association between LE8 score and the long-term risk of CKD.
In current study, we aimed to investigate the association between the level of CVH, defined using LE8 score, and incident CKD among adult participants from the UK Biobank. We also calculated the population attributable-risk (PAR) for each component of LE8, providing new insights into the proportional contribution of each health metric to CKD risk.
Methods
Study design and population
The UK Biobank is a large, prospective cohort study that provides comprehensive genetic and health information [12,13]. Comprehensive information about the UK Biobank’s design and study population can be found in previously published literature [13]. In brief, over 500,000 participants, aged 37 to 73, were enrolled in the baseline study between 2006 and 2010 from 22 assessment centers located in England, Scotland, and Wales. Participants provided a range of health-related data through touchscreen questionnaires, physical examinations, and biological samples. From 2009 to 2012, a total of 210,962 participants completed at least one 24-h dietary recall of the previous day. This study received approval from both the National Health Service (NHS) National Research Service (ref: 11/NW/0382) and the Institutional Review Board of Tulane University (2018-1872). Each participant provided written informed consent.
In the current study, the baseline was defined as the time when the first 24-h diet recall was completed. Among those with at least one typical 24-h diet recall, we excluded 18,476 participants who had prevalent CVD or CKD at baseline and 44,498 participants with incomplete data on one or more components of LE8. This resulted in a total of 147,988 participants included in the analyses (Supplemental Figure 1).
CVH metrics assessment with LE8
A modified version of the original AHA LE8 score was developed in this study (Supplemental Table 1). Briefly, LE8 combines both behavioral (healthy diet, engagement in physical activity, avoidance of nicotine, healthy sleep, and healthy weight) and biological (healthy levels of blood lipids, blood glucose, and BP) targets [6]. Using the data collected from the 24-h dietary recall, the Dietary Approaches to Stop Hypertension (DASH) diet score was applied to determine what constitutes a healthy diet [14]. Participants who reported that their diet was out of the ordinary that day due to illness, fasting, or other circumstances were not included in the 24-h dietary assessments. In this study, the mean values of each dietary component were used. Baseline data on frequency and duration of moderate and vigorous physical activity, nicotine exposure, and sleep duration were collected through touchscreen questionnaires. During the assessment visit, height and weight were measured, and BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Total cholesterol was assessed by Cholesterol oxidase/Peroxidase (CHOD-POD) analysis; HDL cholesterol was assessed by enzyme immunoinhibition on a Beckman Coulter AU5800. Glycated hemoglobin (HbA1c) was assessed using a plasma sample collected at baseline through a high-performance liquid chromatography method on a Bio-Rad VARIANT II Turbo (Bio-Rad Laboratories, Inc). Trained nurses at the assessment center measured BP twice using an electronic BP monitor (Omron 705 IT, OMRON Healthcare Europe B.V.) or a mercury sphygmomanometer when automated instruments were unavailable. We calculated average systolic and diastolic BP levels as the mean of the 2 measurements. Each health metric was given a score between 0 and 100, with a lower number suggesting a lower level of health and a higher number indicating a higher level of health; the aggregate score is also scaled from 0 to 100 points. We also divided the CVH scores into 3 categories based on the AHA advisory: high CVH (80–100), moderate CVH (50–79), and low CVH (0–49) [6]. Additionally, in accordance with the AHA’s recommendations, we also created behavior subscales based on behavioral metrics (diet, physical activity, nicotine exposure, sleep health, and BMI) and biological metrics (blood lipids, blood glucose, and BP) [6].
Ascertainment of CKD
The International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes were used to define incident CKD cases (N18, N18.1, N18.2, N18.3, N18.4, N18.5, and N18.9) [15,16]. We defined CKD to include all 5 stages. Hospital inpatient records were acquired through connections to the Hospital Episode Statistics for England, Scottish Morbidity Records for Scotland, and Patient Episode Database for Wales. By connecting to the death registry, deaths from CKD were identified. Follow-up time in this study was computed from the date of baseline to diagnosis of CKD, the censoring date, or death (until May 2021), whichever occurred first. Detailed information on the ascertainment of outcomes is available online at =https://biobank.ctsu.ox.ac.uk/showcase/label.cgi?id=2000.
To identify prevalent CKD, we used the ICD-10 codes mentioned previously or an estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2 or albuminuria above 3 mg/mmol at baseline.
Covariates assessment
Potential confounders include age, sex, ethnicity, Townsend deprivation index (based on individual’s postcode, a composite measure of deprivation based on unemployment, non–car ownership, non–home ownership, and household overcrowding; a lower value represents higher socioeconomic status) [17], education levels, average household income, C-reactive protein (CRP) concentration, and cancer at baseline. Age, sex, and ethnicity were obtained from baseline using a touchscreen questionnaire. Participants’ ethnic backgrounds were classified into 6 categories: White, mixed, Asian, Black, Chinese, and other. The mixed category combined the responses of the UK Biobank ethnicity questions of “mixed,” “white and black Caribbean,” “white and black African,” “white and Asian,” along with “any other mixed backgrounds.” Townsend deprivation index was collected from local NHS Primary Care Trust registries and the name of the recruitment center. Education and average household income were obtained by touchscreen questionnaire during the initial visit at the assessment center. Blood samples were obtained at baseline, and levels of serum CRP were determined using a high sensitivity immunoturbidimetric assay conducted on a Beckman Coulter AU5800. The (CKD Epidemiology Collaboration) 2021 creatinine equation was used to calculate eGFR [18]. Baseline urine samples were used to measure urinary microalbumin levels via immunoturbidimetric methods (Randox Bioscience), with a detection limit of 6.7 mg/L. Urine creatinine levels were also measured by enzymatic methods (Beckman Coulter UK, Ltd). Albuminuria, represented by urinary albumin-to-creatinine ratio (ACR), was computed using paired microalbumin and creatinine measurements from the same sample. Assessment of history of CVD, diabetes, and cancer can be found in the Supplemental Methods. The definition of DASH diet score and its distribution can be found in Supplemental Table 2. Missing data for continuous and categorical covariates were addressed with mean values and a missing indicator category, respectively. For a comprehensive understanding of the count and proportion of study participants impacted by missing covariates, refer to Supplemental Table 3.
Statistical analysis
Cox proportional hazard models with the follow-up time (i.e., calendar time) as the time scale were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of the association between LE8 and risks of CKD. Specifically, we enumerated the number of CKD cases and calculated the total person-years of follow-up for each CVH category. The Schoenfeld residuals method and the Kaplan–Meier method were used to test the proportional hazards assumption; we did not observe violation of the assumption. We adjusted several potential confounders in these models. Model 1 was adjusted for age, sex, and ethnicity. Model 2 was further adjusted for Townsend deprivation index, education levels, and average household income. Model 3 was further adjusted for CRP and cancer at baseline. We repeated the analyses for behavior and biological subscale scores; behavior scale and biological scale were mutually adjusted in these models.
To conduct the dose–response analyses, we utilized restricted cubic spline functions with 3 knots. These analyses were conducted for the continuous LE8 score, the behavior subscale score, and the biological subscale score. For these analyses, we set the score of 0 as the referent (i.e., estimated HR: 1.00). We adjusted the analyses for age, sex, ethnicity, Townsend deprivation index, education, average household income, CRP, and cancer at baseline. The statistical significance for linearity was assessed using the Wald test, with P values < 0.001 for all the analyses indicating significant linear relationships.
We calculated the PAR to estimate the percentage of development of CKD in the UK Biobank population that theoretically would not have occurred if all participants were in the low-risk category, assuming causality. The PAR was computed for total LE8 score; behavior and biological subscale scores with mutual adjustment for each other in the model; and each individual component of the LE8, with all the 8 metrics simultaneously included in the model. For these analyses, we used categorical variables and defined the low-risk category as scores from 80 to 100.
We also conducted stratified analyses by covariables: age (<60 or ≥60 y), sex (female or male), ethnicity (White or non-White), Townsend deprivation index (Quintile 1, Quintiles 2–4, Quintile 5), education level (college/university degree or less than college/university degree), and average household income (< median or ≥ median), CRP (< median or ≥ median), and baseline cancer (no or yes). To assess interactions between the levels of CVH, defined by the LE8 score, and these factors, multiplicative interaction was evaluated by adding interaction terms to the original Cox models. Additionally, we tested whether other factors of sleep pattern except for sleep duration, which included morning/evening person (chronotype) (evening or morning), sleeplessness/insomnia (yes or no), snoring (yes or no), and daytime dozing/sleeping (yes or no), interacted with levels of CVH on risk of CKD.
Three sensitivity analyses were performed to test the robustness of the findings. First, missing data for all covariates were imputed using multivariate imputation by chained equations algorithm. Second, further sensitivity analysis was conducted to exclude individuals within 2 y of CKD onset to reduce the possibility of reverse causation. Finally, we conducted sensitivity analysis by excluding each individual component of the LE8 from the total LE8 score.
All statistical analyses were analyzed using SAS version 9.4 (SAS Institute Inc.), and a 2-sided P < 0.05 was considered as statistically significant.
Results
Baseline characteristics of participants according to low, moderate, and high CVH are presented in Table 1. Among the 147,988 participants with complete data on LE8 and no prevalent CKD and CVD at baseline, mean age was 56, and 55% were female. Compared with participants with lower CVH, participants with a high CVH were younger, more likely to be female and White, had lower levels of BMI, HbA1c, systolic BP, diastolic BP, CRP, less deprived Townsend deprivation index, and had higher DASH diet score, education levels and better eGFR, longer sleep duration, more moderate and vigorous physical activity, and higher percentage of high household income. A higher LE8 score represents a higher CVH level. Participants with a high CVH level seemed to have more protective factors. Of all the study participants, 20.3% (n = 30,088) were in the high CVH category, and 74.6% (n = 110,425) met the moderate CVH criteria.
TABLE 1.
Baseline characteristics according to the categories of Life’s Essential 8
| Life’s Essential 8 |
|||
|---|---|---|---|
| Low | Moderate | High | |
| Number of participants, % | 5.1 | 74.6 | 20.3 |
| Age, y | 55.1 (7.4) | 56.2 (7.8) | 53.8 (8.1) |
| Female, % | 40.8 | 51.0 | 73.6 |
| White, % | 95.2 | 96.0 | 96.7 |
| BMI, kg/m2 | 32.6 (5.5) | 27.2 (4.2) | 23.4 (2.5) |
| Townsend deprivation index | −0.8 (3.2) | −1.7 (2.8) | −1.8 (2.7) |
| HbA1c, % | 5.8 (0.9) | 5.4 (0.5) | 5.2 (0.3) |
| DASH diet score | 19.9 (3.8) | 23.9 (4.7) | 28.4 (4.2) |
| Sleep duration, h/d | 6.8 (1.5) | 7.1 (1.0) | 7.3 (0.8) |
| Moderate physical activity, min/wk | 97.7 (283.0) | 243.3 (393.4) | 295.1 (402.4) |
| Vigorous physical activity, min/wk | 28.0 (127.8) | 82.5 (161.4) | 111.7 (160.4) |
| SBP, mmHg | 157.2 (43.2) | 144.5 (33.3) | 126.2 (20.9) |
| DBP, mmHg | 96.0 (25.9) | 86.5 (19.6) | 76.2 (11.7) |
| eGFR, mL/min/1.73 m2 | 93.7 (12.5) | 97.4 (12.1) | 101.7 (11.2) |
| College/university degree, % | 36.5 | 46.0 | 56.9 |
| Household income, % | |||
| Less than £18,000 | 19.7 | 14.4 | 10.9 |
| £18,000 to £30,999 | 23.6 | 24.1 | 21.2 |
| £31,000 to £51,999 | 28.5 | 29.1 | 28.9 |
| £52,000 to £100,000 | 22.3 | 25.0 | 7.3 |
| Greater than £100,000 | 5.3 | 7.3 | 10.3 |
| CRP, mg/L | 4.0 (4.8) | 2.3 (3.8) | 1.4 (3.2) |
| Baseline cancer, % | 6.7 | 7.2 | 6.8 |
Data are n (%) unless otherwise indicated.
BMI: body mass index; CRP, C-reactive protein; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rat; HbA1c, glycated hemoglobin; SBP, sysolic blood pressure.
Over a median follow-up of 10 y, a total of 1936 incident events of CKD were documented. In Figure 1, the cumulative incidence displayed graded relationships with the 3 levels of CVH categories during follow-up (Log-rank test < 0.001). The associations between LE8 score and risk of CKD are shown in Table 2. In the analysis, we observed 168, 1542, and 226 CKD cases over 78,915.6, 1,175,936.4, and 322,509.6 person-y in the low, moderate, and high CVH categories, respectively. In the age, sex, and ethnicity adjusted model, a higher CVH, indicating by the LE8 score, was significantly associated with a lower risk of CKD, with HRs of 1 (reference), 0.54 (95% CI: 0.46, 0.63), and 0.35 (95% CI: 0.29, 0.43) for the CVH categories of low, moderate, and high, respectively. After further adjustment for Townsend deprivation index, education levels, and average household income, the associations were somewhat attenuated yet retained significance. It was observed that a higher CVH was notably linked with a diminished risk of CKD. The HRs were found to be 1 (reference), 0.58 (95% CI: 0.49, 0.68), and 0.40 (95% CI: 0.33, 0.49) for the CVH categories of low, moderate, and high, respectively. Following an additional adjustment accounting for CRP and baseline cancer, compared to participants with a low CVH category, those with a moderate CVH were associated with a 39% lower risk of developing CKD (HR: 0.61; 95% CI: 0.52, 0.72). Participants with a high CVH had a 57% lower risk of CKD incidence (HR: 0.43; 95% CI: 0.35, 0.53).
FIGURE 1.
Cumulative incidence of chronic kidney disease (CKD) according to cardiovascular health categories. Log-rank tests P < 0.001.
TABLE 2.
Life’s Essential 8 and incidence of chronic kidney disease
| Cases/person-y | Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) |
P |
HR (95% CI) |
P |
HR (95% CI) |
P |
||
| CKD | |||||||
| Cardiovascular health metrics | |||||||
| Low | 168/78,915.6 | Ref | Ref | Ref | |||
| Moderate | 1542/1,175,936.4 | 0.54 (0.46, 0.63) | <0.001 | 0.58 (0.49, 0.68) | <0.001 | 0.61 (0.52, 0.72) | <0.001 |
| High | 226/322,509.6 | 0.35 (0.29, 0.43) | <0.001 | 0.40 (0.33, 0.49) | <0.001 | 0.43 (0.35, 0.53) | <0.001 |
| Per SD increment in LE8 | 0.16 (0.12, 0.22) | <0.001 | 0.21 (0.15, 0.27) | <0.001 | 0.23 (0.18, 0.31) | <0.001 | |
| Subscale | |||||||
| Per SD increment in behavior scale | 0.26 (0.21, 0.33) | <0.001 | 0.31 (0.24, 0.39) | <0.001 | 0.34 (0.27, 0.43) | <0.001 | |
| Per SD increment in biological scale | 0.66 (0.54, 0.80) | <0.001 | 0.68 (0.56, 0.83) | <0.001 | 0.70 (0.57, 0.85) | <0.001 | |
CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio; LE8, Life’s Essential 8; Ref, reference; SD, standard deviation.
Model 1 was adjusted for age, ethnicity, and sex.
Model 2: Model 1 + Townsend deprivation index, education, and average household income.
Model 3: Model 2 + C-reactive protein and cancer at baseline.
Behavior scale and biological scale were mutually adjusted for subscale analyses.
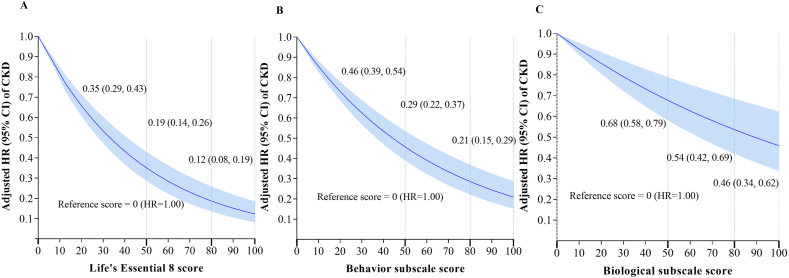
Associations of continuous LE8 score, behavior subscale score, and biological subscale score with risk of CKD are shown in Figure 2A–C. Overall, in the dose–response analyses, associations of the LE8 score, behavior subscale score, and biological subscale score with CKD all appeared to be linear (P for linearity < 0.001 for all). A higher LE8 score, behavior subscale score, and biological subscale score were all associated with a lower risk of CKD. For example, we used 0 as the referent score (estimated HR: 1.00), and with an increased LE8 score, participants with a score of 50 had a 65% (HR: 0.35; 95% CI: 0.29, 0.43) lower risk of incident CKD. Those who achieved LE8 scores of 80 and 100 experienced an 81% (HR: 0.19; 95% CI: 0.14, 0.26) and 88% (HR: 0.12; 95% CI: 0.08, 0.19) reduction in risk of incident CKD, respectively (Figure 2A). Both behavior and biological subscale scores demonstrated a similar protective trend with increased LE8 score (Figure 2B, C). Additionally, the HR for each SD increment in LE8 score was 0.23 (95% CI: 0.18, 0.31) for CKD after adjustment for age, sex, ethnicity, Townsend deprivation index, education level, average household income, CRP, and cancer at baseline. The behavior and biological subscale scores were both significantly associated with decreased risk of CKD, similar to the overall LE8 score, but with attenuated magnitudes (Table 2). In the sensitivity analysis, after employing multiple imputations to handle all missing covariates, the results of the analysis remained stable, reaffirming the robustness of our findings (Supplemental Table 4). Additionally, after excluding individuals within 2 y of CKD onset, we also found similar associations between LE8 score and risk of CKD (Supplemental Table 5).
FIGURE 2.
Dose–response relationships between continuous Life’s Essential 8 score (A), behavior subscale score (B), biological subscale score (C), and incidence risk of CKD. Results were adjusted for age, ethnicity, sex, Townsend deprivation index, education, average household income, C-reactive protein, and cancer at baseline. Vertical dotted lines indicate the cutoff points by the American Heart Association. 0 as the referent score (estimated HR = 1.00). P for linearity <0.001 for all. CI, confidence interval; CKD, chronic kidney disease; HR, hazard ratio.
When we calculated the PAR for the total LE8 score, behavior and biological subscale scores, and each LE8 component separately, all of them except smoking were significantly associated with risk of developing CKD in this population. All 8 factors contributing to overall LE8 score could have accounted for 70.4% (95% CI: 67.7, 98.8) of incident CKD in this cohort assuming causality. Participants who did not adhere to 5 low-risk behavioral metrics appeared to have higher PAR (25.0%; 95% CI: 17.8, 32.0) compared with those exhibiting 3 high-risk biological metrics (23.1%; 95% CI: 16.9, 29.2) (Supplemental Table 6). Among 8 distinct metrics of the LE8 score, BMI had the highest PAR (24.6%; 95% CI: 18.8, 30.2). Of the total CKD risk, 3.2% (95% CI: 1.4, 5.0) was attributable to inadequate or excessive sleep duration (Figure 3). Moreover, we also analyzed the associations with CKD by excluding each individual component of the LE8. Excluding each component of the LE8 score separately all showed slightly attenuated associations with risk of CKD after adjustment for all covariables, compared with the full LE8 score (Supplemental Table 7, compared to Table 2).
FIGURE 3.
The population attributable-risk of each individual component of the Life’s Essential 8.
Model was adjusted for age, ethnicity, sex, Townsend deprivation index, education, average household income, C-reactive protein, and cancer at baseline. CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; PAR, population attributable-risk.
We also conducted stratified analyses according to potential risk factors including age, sex, ethnicity, Townsend deprivation index, education level, average household income, CRP, and cancer at baseline (Table 3) to evaluate whether these variables modified the relation between the level of CVH, defined by the LE8 score, and risk of CKD. We did not find significant interactions between these factors and the level of CVH on risk of CKD. In addition, no significant interactions were observed between other sleep factors (i.e., chronotype, insomnia, snoring, and daytime dozing) and the level of CVH on risk of CKD (Supplemental Table 8).
TABLE 3.
Stratified analyses for association between Life’s Essential 8 and hazard of chronic kidney disease
| Subgroups | Cardiovascular health metrics |
||||
|---|---|---|---|---|---|
| Low | Moderate | High | P-trend | P-interaction | |
| Age (y) | 0.168 | ||||
| <60 | 1 (reference) | 0.58 (0.45, 0.76) | 0.42 (0.30, 0.58) | <0.001 | |
| ≥60 | 1 (reference) | 0.66 (0.53, 0.81) | 0.43 (0.33, 0.56) | <0.001 | |
| Sex | 0.468 | ||||
| Female | 1 (reference) | 0.60 (0.47, 0.72) | 0.45 (0.34, 0.60) | <0.001 | |
| Male | 1 (reference) | 0.61 (0.50, 0.76) | 0.40 (0.29, 0.54) | <0.001 | |
| Ethnicity | 0.401 | ||||
| Non-White | 1 (reference) | 0.68 (0.24, 1.96) | 0.49 (0.13, 1.83) | 0.290 | |
| White | 1 (reference) | 0.61 (0.51, 0.71) | 0.43 (0.35, 0.53) | <0.001 | |
| Townsend deprivation index | 0.669 | ||||
| Low (Q1) | 1 (reference) | 0.50 (0.34, 0.73) | 0.38 (0.24, 0.60) | <0.001 | |
| Intermediate (Q2–Q4) | 1 (reference) | 0.58 (0.47, 0.71) | 0.41 (0.32, 0.54) | <0.001 | |
| High (Q5) | 1 (reference) | 0.79 (0.56, 1.11) | 0.52 (0.32, 0.83) | 0.005 | |
| Education | 0.790 | ||||
| College/university degree | 1 (reference) | 0.66 (0.54, 0.79) | 0.45 (0.35, 0.58) | <0.001 | |
| Under college/university degree | 1 (reference) | 0.49 (0.36, 0.67) | 0.36 (0.25, 0.53) | <0.001 | |
| Household income | 0.052 | ||||
| < Median | 1 (reference) | 0.60 (0.50, 0.72) | 0.45 (0.37, 0.56) | <0.001 | |
| ≥ Median | 1 (reference) | 0.61 (0.44, 0.86) | 0.59 (0.40, 0.86) | <0.001 | |
| CRP | 0.060 | ||||
| < Median | 1 (reference) | 0.66 (0.43, 0.86) | 0.47 (0.30, 0.76) | <0.001 | |
| ≥ Median | 1 (reference) | 0.63 (0.53, 0.75) | 0.50 (0.39, 0.65) | <0.001 | |
| Baseline cancer | 0.383 | ||||
| No | 1 (reference) | 0.60 (0.51, 0.71) | 0.45 (0.37, 0.56) | 0.683 | |
| Yes | 1 (reference) | 0.74 (0.42, 11.33) | 0.28 (0.13, 0.62) | <0.001 | |
Data are expressed as hazard ratio and 95% confidence interval. Model was adjusted for age, ethnicity, sex, Townsend deprivation index, education, average household income, CRP, and cancer at baseline. CRP, C-reactive protein; Q, quartile.
Discussion
In this large prospective cohort, the relationship between the level of CVH, defined by the LE8 score, and risk of CKD was investigated in individuals free of CKD and CVD at baseline. We observed that higher CVH level was associated with significantly lower risk of CKD. Compared with participants with low CVH, those with moderate and high CVH had a 39% and 57% lower risk of developing CKD, respectively. Among 8 distinct metrics of the LE8 score, BMI had the highest PAR, and 3.2% (95% CI: 1.4, 5.0) of the total CKD risk was attributable to inadequate or excessive sleep duration.
To our knowledge, this is the first prospective study to investigate the association of the new CVH metrics, defined by LE8, and risk of CKD. Our results are consistent with the current knowledge that risk of CKD is inversely associated with CVH levels. Several previous studies applied the AHA Life’s Simple 7 (LS7) to assess CVH and risk of CKD [[19], [20], [21]]. In the Atherosclerosis Risk in Communities cohort study, a higher LS7 score was significantly associated with lower risk of CKD [21]. Such associations were also present across populations of different ethnic backgrounds. In the Multi-Ethnic Study of Atherosclerosis cohort (aged from 45–84 y), LS7 score could identify individuals who were prone to numerous chronic nonvascular diseases, including CKD [20]. However, as the predecessor of LE8 score, definitions for LS7 features might not be able to capture all current health-related behaviors and practices. There are restrictions to how the measurements are quantified, according to research [6]. Because of the simplified categories of ideal, intermediate, and poor classification of each individual component, the algorithm defined by LS7 definition lacks interindividual sensitivity and cannot be utilized to evaluate dose–response effects. In contrast, the LE8 score employs a continuous scale (ranging from 0–100 for each component), offering greater sensitivity to interindividual differences compared with LS7.
The PAR analyses in the current study provided additional evidence for the association between CVH and CKD. We first found that HRs in the association of the behavior subscale score with CKD appeared to decrease more sharply than that with the biological subscale score. In other words, addressing 5 modifiable behavioral metrics might offer greater protective effects against the burden of CKD in this population, emphasizing the particular importance of health behavioral interventions in prevention of CKD. In addition, sleep is newly added, which also significantly accounted for a substantial part of incident CKD in this cohort. The exact underlying mechanism of association between sleep and CKD remains unknown. Several lines of evidence suggested a potential adverse impact of short/prolonged sleep on the development of CKD. Systemic inflammation [22,23], sympathetic nervous system activity, and salt retention [24] may arise from either insufficient or excessive sleep and sleep disorders, potentially leading to glomerular endothelial dysfunction and subsequent renal dysfunction [25].
Although the precise interrelationship between the total LE8 components and CKD remains unclear, each individual component has been previously investigated. Research has established associations of CKD with healthy lifestyles and metabolic syndrome, both of which are integral behavioral and biological metrics within the LE8 framework [[26], [27], [28], [29]]. The association between modifiable behaviors and CKD has long been noted [28,30,31]. In line with our findings, prior research has demonstrated that adopting an optimal health behavior profile, healthy dietary pattern, being physically active, avoiding smoking, maintaining a healthy body weight, and adhering to a proper sleep pattern can be instrumental in preventing the onset and progression of CKD. Several important pathways with all these behavioral factors included increased inflammation (CRP and total white blood cell count) [32], renal blood flow [33], activity of the sympathetic nervous system, and alterations of glucose metabolism [34], which may act on incident CKD. As for the 3 biological metrics, first, because of hyperfiltration damage, increased glycosylation end products and reactive oxygen species and glucose impairment could be risk factors in kidney disease. Numerous cytokines, growth factors, and hormones, including transforming growth factor-β and angiotensin II, contribute to the pathologic alterations linked to diabetic nephropathy on a molecular level [35]. Second, hypertension is a well-established risk factor for CKD. Intraglomerular capillary pressure is influenced by systemic hypertension, which causes glomerulosclerosis and kidney function loss [35]. Third, our findings align with existing evidence indicating that non-HDL cholesterol management could yield renal advantages [36]. Plasma non-HDL cholesterol concentration exhibits a positive correlation with both plasma LDL cholesterol and triglyceride levels. Dyslipidemia is linked to a heightened probability of eGFR decline [37]. Taken together, this evidence supports the observed associations between the composite LE8 score and incident CKD, indicating that maintaining high CVH could benefit by reducing the burden of CKD. Future research is needed to develop effective interventions for improving overall CVH and lowering CKD.
The main strengths of this study include the use of the updated LE8 score to evaluate CVH, the large size of the study sample, and a prospective assessment of CKD outcome. In addition, we examined the CVH and CKD dose–response relationship and determined the minimal threshold for the beneficial association. Potential limitations of our study should be considered. First, the assessments of behavioral factors were based on self-report questionnaires, which are subject to information bias such as measurement errors or misclassification. Second, most study participants were Caucasian people, which could affect the generalizability of the study results; thus, future studies in other racial/ethnic groups are warranted. Third, only one time point was used to calculate LE8. During the follow-up to the current investigation, we were unable to take prospective lifestyle changes into consideration. Fourth, due to the healthy volunteer bias, disease prevalence and incidence rates might not be representative of the general population [38]. However, this would not compromise the analyses’ internal validity. Fifth, we recognize that data on the ACR was accessible only for a subset of the population. This factor could potentially influence its applicability as a confounding variable. Finally, although we considered several potential confounders, there might be residual confounding.
In conclusion, our findings from a large perspective cohort study lend support to a significant and graded inverse association between the LE8-defined CVH and risk of incident CKD. These findings feature the importance of improving CVH by adopting the AHA LE8 as a potential comprehensive prevention approach to reducing the burden of CKD.
Author contributions
The authors’ responsibilities were as follows—LQ, RT: concept and design; LQ, RT: acquisition, analysis, or interpretation of data; RT: statistical analysis; LQ, RT: wrote the manuscript; RT, XW, XL, HM, ZL, YH, LQ: critical revision of the manuscript for important intellectual content; LQ: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Conflicts of interest
The authors report no conflicts of interest.
Funding
The study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616). The funding sources and sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data availability
This study has been conducted using the UK Biobank Resource, approved project number 29256. The UK Biobank will make the source data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.08.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cockwell P., Fisher L.A. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662–664. doi: 10.1016/S0140-6736(19)32977-0. [DOI] [PubMed] [Google Scholar]
- 2.Foley R.N., Murray A.M., Li S., Herzog C.A., McBean A.M., Eggers P.W., et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. 2005;16(2):489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 3.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Weiner D.E., Tabatabai S., Tighiouart H., Elsayed E., Bansal N., Griffith J., et al. Cardiovascular outcomes and all-cause mortality: exploring the interaction between CKD and cardiovascular disease. Am. J. Kidney Dis. 2006;48(3):392–401. doi: 10.1053/j.ajkd.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Xie Y., Bowe B., Mokdad A.H., Xian H., Yan Y., Li T., et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D.M., Allen N.B., Anderson C.A.M., Black T., Brewer L.C., Foraker R.E., et al. Life’s Essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146(5):e18–e43. doi: 10.1161/CIR.0000000000001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St-Onge M.P., Grandner M.A., Brown D., Conroy M.B., Jean-Louis G., Coons M., et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanagasabai T., Ardern C.I. Contribution of inflammation, oxidative stress, and antioxidants to the relationship between sleep duration and cardiometabolic health. Sleep. 2015;38(12):1905–1912. doi: 10.5665/sleep.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao Q., Xie M., Zhu L., Dou Y., Dai M., Wu Y., et al. Association of sleep duration with chronic kidney disease and proteinuria in adults: a systematic review and dose–response meta-analysis. Int. Urol. Nephrol. 2020;52(7):1305–1320. doi: 10.1007/s11255-020-02488-w. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto R., Shinzawa M., Isaka Y., Yamakoshi E., Imai E., Ohashi Y., et al. Sleep quality and sleep duration with CKD are associated with progression to ESKD. Clin. J. Am. Soc. Nephrol. 2018;13(12):1825–1832. doi: 10.2215/CJN.01340118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Ma H., Wang X., Feng H., Qi L. Life’s Essential 8, genetic susceptibility, and incident cardiovascular disease: a prospective study. Arterioscler. Thromb. Vasc. Biol. 2023;43(7):1324–1333. doi: 10.1161/ATVBAHA.123.319290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 13.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLOS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung T.T., Chiuve S.E., McCullough M.L., Rexrode K.M., Logroscino G., Hu F.B. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch. Intern. Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 15.Roy L., Zappitelli M., White-Guay B., Lafrance J.P., Dorais M., Perreault S. Agreement between administrative database and medical chart review for the prediction of chronic kidney disease G category. Can. J.Kidney Health Dis. 2020;7(7) doi: 10.1177/2054358120959908. 2054358120959908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlasschaert M.E.O., Bejaimal S.A.D., Hackam D.G., Quinn R., Cuerden M.S., Oliver M.J., et al. Validity of administrative database coding for kidney disease: a systematic review. Am. J. Kidney Dis. 2011;57(1):29–43. doi: 10.1053/j.ajkd.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Yousaf S., Bonsall A. UK Data Service; Colchester, UK: 2017. UK Townsend Deprivation Scores from 2011 census data. [Google Scholar]
- 18.Inker L.A., Eneanya N.D., Coresh J., Tighiouart H., Wang D., Sang Y., et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N. Engl. J. Med. 2021;385(19):1737–1749. doi: 10.1056/NEJMoa2102953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez R., Lash J.P., Burrows B., Wilund K.R., Mattix-Kramer H.J., Peralta C., et al. The association of positive affect and cardiovascular health in Hispanics/Latinos with chronic kidney disease: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Prev. Med. Rep. 2019;15:100916. doi: 10.1016/j.pmedr.2019.100916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogunmoroti O., Allen N.B., Cushman M., Michos E.D., Rundek T., Rana J.S., et al. Association between Life’s Simple 7 and noncardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2016;5(10) doi: 10.1161/JAHA.116.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebholz C.M., Anderson C.A.M., Grams M.E., Bazzano L.A., Crews D.C., Chang A.R., et al. Relationship of the American Heart Association’s Impact Goals (Life’s Simple 7) with risk of chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) Cohort Study. J. Am. Heart Assoc. 2016;5(4) doi: 10.1161/JAHA.116.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier-Ewert H.K., Ridker P.M., Rifai N., Regan M.M., Price N.J., Dinges D.F., et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 23.Nadeem R., Molnar J., Madbouly E.M., Nida M., Aggarwal S., Sajid H., et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J. Clin. Sleep Med. 2013;9(10):1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusardi P., Mugellini A., Preti P., Zoppi A., Derosa G., Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am. J. Hypertens. 1996;9(5):503–505. doi: 10.1016/0895-7061(95)00389-4. [DOI] [PubMed] [Google Scholar]
- 25.Futrakul N., Sridama V., Futrakul P. Microalbuminuria—a biomarker of renal microvascular disease. Ren. Fail. 2009;31(2):140–143. doi: 10.1080/08860220802595948. [DOI] [PubMed] [Google Scholar]
- 26.Jang S.Y., Kim I.H., Ju E.Y., Ahn S.J., Kim D.K., Lee S.W. Chronic kidney disease and metabolic syndrome in a general Korean population: the Third Korea National Health and Nutrition Examination Survey (KNHANES III) Study. J. Public Health (Oxf.) 2010;32(4):538–546. doi: 10.1093/pubmed/fdp127. [DOI] [PubMed] [Google Scholar]
- 27.Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int. Suppl. 2011;3(4):368–371. doi: 10.1038/kisup.2013.79. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrauben S.J., Apple B.J., Chang A.R. Modifiable lifestyle behaviors and CKD progression: a narrative review. Kidney360. 2022;3(4):752–778. doi: 10.34067/KID.0003122021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsimihodimos V., Mitrogianni Z., Elisaf M. Dyslipidemia associated with chronic kidney disease. Open Cardiovasc. Med. J. 2011;5:41–48. doi: 10.2174/1874192401105010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng T., Li X., Ma H., Heianza Y., Qi L. Adherence to a healthy sleep pattern and risk of chronic kidney disease: the UK Biobank study. Mayo Clin. Proc. 2022;97(1):68–77. doi: 10.1016/j.mayocp.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly J.T., Su G., Carrero J.J. Lifestyle interventions for preventing and ameliorating CKD in primary and secondary care. Curr. Opin. Nephrol. Hypertens. 2021;30(6):538–546. doi: 10.1097/MNH.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 32.Wang C.P., Lu Y.C., Hung W.C., Tsai I.T., Chang Y.H., Hu D.W., et al. Inter-relationship of risk factors and pathways associated with chronic kidney disease in patients with type 2 diabetes mellitus: a structural equation modelling analysis. Public Health. 2021;190:135–144. doi: 10.1016/j.puhe.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Savino A., Pelliccia P., Chiarelli F., Mohn A. Obesity-related renal injury in childhood. Horm. Res. Paediatr. 2010;73(5):303–311. doi: 10.1159/000308161. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel K., Leproult R., L’Hermite-Balériaux M., Copinschi G., Penev P.D., Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 35.Lea J.P., Nicholas S.B. Diabetes mellitus and hypertension: key risk factors for kidney disease. J. Natl. Med. Assoc. 2002;94(8):7S–15S. suppl. [PMC free article] [PubMed] [Google Scholar]
- 36.Sugimoto K., Isobe K., Kawakami Y., Yamada N. The relationship between non-HDL cholesterol and other lipid parameters in Japanese subjects. J. Atheroscler. Thromb. 2005;12(2):107–110. doi: 10.5551/jat.12.107. [DOI] [PubMed] [Google Scholar]
- 37.Liang X., Ye M., Tao M., Zheng D., Cai R., Zhu Y., et al. The association between dyslipidemia and the incidence of chronic kidney disease in the general Zhejiang population: a retrospective study. BMC Nephrol. 2020;21(1):252. doi: 10.1186/s12882-020-01907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fry A., Littlejohns T.J., Sudlow C., Doherty N., Adamska L., Sprosen T., et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study has been conducted using the UK Biobank Resource, approved project number 29256. The UK Biobank will make the source data available to all bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply.