Abstract
Background
Body composition assessment aids evaluation of energy stores and the impact of diseases and interventions on child growth. Current United States pediatric reference ranges from the National Health and Nutrition Examination Survey (NHANES) include 20% of children with obesity, body mass index of ≥95th percentile.
Objectives
This study aimed to develop dual energy X-ray absorptiometry (DXA) based reference ranges in a diverse cohort with low-obesity prevalence from the Bone Mineral Density in Childhood Study (BMDCS).
Methods
This is a secondary analysis of a longitudinal, prospective, observational cohort. Healthy children (height and BMI within 3rd to 97th percentiles, ages 5–19 y at enrollment), from 5 United States centers were measured annually for ≤7 visits. Whole body scans were acquired using Hologic scanners. A subsample underwent repeat measurements to determine precision. We generated reference ranges for appendicular and total lean soft tissue mass index (LSTM Index), fat mass index (FMI), and other body composition measures. Resulting curves were compared to NHANES and across subgroups. Sex and age-specific equations were developed to adjust body composition Z-scores for height Z score.
Results
We obtained 9846 scans of 2011 participants (51% female, 22% Black, 17% Hispanic, 48% White, 7% Asian/Pacific Islander, and 6% with obesity). Precision (percent coefficient of variation) ranged from 0.7% to 1.96%. Median and–2 standard deviation curves for BMDCS and NHANES were similar, but NHANES +2 standard deviation LSTM Index and FMI curves were distinctly greater than the respective BMDCS curves. Subgroup differences were more extreme for appendicular LSTM Index-Z (mean ± SD: Asian −0.52 ± 0.93 compared with Black 0.77 ± 0.87) than for FMI-Z (Hispanic 0.29 ± 0.98 compared with Black −0.14 ± 1.1) and were smaller for Z-scores adjusted for height Z-score.
Conclusions
These reference ranges add to sparse normative data regarding body composition in children and adolescents and are based on a cohort with an obesity prevalence similar to current BMI charts. Awareness of subgroup differences aids in interpreting results.
Keywords: DXA, pediatric, reference, lean soft tissue mass index, fat mass index
Introduction
Measurement of body composition provides valuable information for nutritional assessment. In particular, body composition is useful for determining energy requirements because lean tissue is more metabolically active than adipose tissue [1], and quantifying fat mass (FM) aids in determining inadequate or excess energy stores [2]. In addition, measurement of lean and FM can be informative for determining medication doses [3,4], disease effects [5,6], and other types of biological research [7].
The broad use of body composition in nutritional assessment and clinical care has been limited for several reasons. First, the multiple methods for body composition assessment, such as hydrodensitometry, air displacement plethysmography, bioelectrical impedance analyses, and dual energy X-ray absorptiometry (DXA), are based on different properties and assumptions about tissue compartments, and their results are not interchangeable [8]. Second, even within the same method, such as bioelectrical impedance analysis or DXA, different manufacturers and software versions produce different results [9,10]. In children, use of body composition measures is challenged further by the need for age- and sex-specific reference ranges due to expected changes with growth and maturation [2]. Characteristics of the reference group and determination of reference ranges have important implications when interpreting body composition results.
Previously, we published lean BMI (LBMI, more precisely known as lean soft tissue mass index [LSTM Index]) and fat mass index (FMI) reference ranges for youth aged 8–20 y from DXA data collected by the NHANES [11]. These reference ranges were based on a nationally representative sample of the United States population with standardized data collection. Both LSTM Index and FMI are indices, similar to BMI, that account for stature and thereby provide a measure of a tissue mass relative to overall body size. However, there are several disadvantages to the NHANES reference ranges. First, the data were collected from 1999 to 2004, after the onset of the obesity epidemic among children in United States, and 20% of the sample had a BMI of ≥95th percentile for age. In contrast, obesity prevalence was 5% in the sample used to construct the Centers for Disease Control and Prevention (CDC) 2000 BMI charts, which are commonly used to screen for underweight, healthy weight, overweight, and obesity [12]. Thus, these 2 reference sources for nutritional assessment are not comparable, and excess adiposity could be underrecognized when using the NHANES body composition reference ranges. Second, DXA scans were not obtained in children aged <8 y. Third, LSTM Index excludes bone, but does not distinguish between skeletal muscle and organ tissue. The loss or gain of skeletal muscle mass in children is often the rationale for body composition assessment, such as in physical activity promotion programs or monitoring the effects of drug treatment or loss of ambulation [13,14]. For pediatric bone health assessment, measurement of skeletal muscle mass is particularly important because of the synchrony between bone strength and muscle mass. Indeed, the International Society of Clinical Densitometry’s pediatric position statement on reporting bone densitometry results recommends evaluation of muscle mass for interpretation of bone density measurements [15,16].
To address these shortcomings, we developed DXA based pediatric body composition reference ranges for ages 5–20 y from a large, longitudinal, geographically diverse cohort of healthy children with weight, height, and BMI within the 3rd to 97th percentiles of the CDC growth reference [17] at enrollment. This is a secondary analysis of data from the National Institute of Child Health and Human Development Bone Mineral Density in Childhood Study (BMDCS) that generated pediatric bone density reference ranges for US children. These body composition data are complimentary to bone density data that have been published from this cohort [[18], [19], [20], [21]]. We also aimed to evaluate precision in DXA body composition measures, differences in body composition according to self-identified race and ethnicity, and the association of variation in stature on body composition outcomes in children, adolescents, and emerging adults.
Sample and Methods
Study design and sample
The BMDCS was a multicenter mixed-longitudinal cohort study to establish pediatric reference ranges for bone density and has been described previously [21,22]. This is a secondary analysis of data collected for that study. The 5 clinical centers were Cincinnati Children’s Hospital Medical Center, Cincinnati, OH; Los Angeles Children’s Hospital, Los Angeles, CA; The Children's Hospital of Philadelphia, Philadelphia, PA; Creighton University, Omaha, NE; and Columbia University, New York, NY. The initial cohort consisted of healthy females aged 6–15 y and males aged 6–16 y at enrollment. Study visits occurred annually for ≤6 y (7 visits). An additional cohort was enrolled at the fourth visit consisting of 5-y-old and 19-y-old children who were followed annually for 3 y. The first visit occurred in July, 2002 and the last visit occurred December, 2009.
Eligibility criteria at enrollment were designed to identify children in good health, with normal growth and maturation, and free of health conditions and medication use that might contribute to suboptimal bone accrual or growth. The full list of inclusion and exclusion criteria is shown in Supplementary Table 1. At study completion, the health history for each participant was reviewed for changes in the baseline eligibility criteria. Data from study visits were excluded from the development of reference ranges if participants met the following criteria: steroid exposure (n = 268 visits), use of anticonvulsants or oral isotrentinoin (n = 74 visits), inflammatory bowel disease, cancer, or endocrine disorder (n = 8 visits), pregnancy (n = 35 visits), use of DepoProvera or Norplant (n = 46 visits), use of a psychiatric drug >180 d (n = 97 visits), use of stimulants >180 d (n = 106 visits).
The protocol was reviewed and approved by the institutional review board at each institution. Written informed consent was obtained from legal guardians for study participants aged <18 y and from study participants aged ≥18 y. Assent was obtained from study participants aged <18 y.
Data collection
Demographic characteristics and growth
Legal guardians or study participants provided demographic information including self-identified race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, and White) and ethnicity (Hispanic or Latino – yes, no, or unknown) by questionnaire based on the designated categories used by the NIH at the time of data collection. Trained personnel completed measurements of height, sitting height, and weight with the study participant wearing light clothing and shoes removed. Z-scores for height (HAZ), weight (WAZ), and body mass index (BMIZ) were calculated using the CDC 2000 growth charts [23].
Body composition by DXA
Whole body DXA scans were acquired with Hologic bone densitometers (QDR4500A, QDR4500W, and Delphi A models) by trained personnel following standard positioning and scan acquisition guidelines. Scans with movement were repeated if possible. All scans were analyzed centrally and inspected for validity at the University of California San Francisco (JAS) using Hologic Discovery 12.3 software at baseline and Apex 2.1 software at follow-up using the compare feature. There are no differences in these software versions in the analysis of whole body scans for study participants aged <20 y. Scans with movement or other artifacts were excluded from analyses. Scans were analyzed without the NHANES body composition analysis feature based on the publication by Schoeller et al. [24] because this analysis feature has not been validated in children. At the first visit, repeat scans with repositioning were acquired in 153 participants to calculate precision.
In the present study, appendicular LSTM Index and FMI were the primary outcomes. Appendicular LSTM was calculated as the sum of the lean mass (excluding bone mineral content) of the arms and legs. Appendicular LSTM Index was calculated as appendicular LSTM (kg) divided by height (meters) squared. FMI was calculated as total body FM (kg) divided by height (meters) squared. The secondary outcomes were total LSTM Index (kg/m2), subtotal (whole body excluding the head) LSTM Index (kg/m2), total LSTM (kg), subtotal LSTM (kg), appendicular LSTM (kg), leg LSTM (kg), and total FM (kg). These secondary outcomes were included because subregion DXA values may not be available or because they may be of interest to researchers or clinicians under special circumstances.
Statistical analysis
The distributions of continuous variables were inspected, and transformations applied where necessary. Reference ranges were determined using the Lambda Mu Sigma (LMS) method [25] and implemented in R (version 4.2.1, R Foundation for Statistical Computing) using the Generalized Additive Models for Location Scale and Shape function and the Box-Cox Cole Green distribution family. Models with different degrees of freedom were calculated and inspected visually. The number of degrees of freedom per parameter ranged from 0 to 5. The Aikike Information Criterion (AIC) was used to select the optimal, most parsimonious model (AIC value within 1% of the lowest AIC). Age-specific L (power for the Box-Cox transformation), M (median) and S (SD) values were calculated separately for males and females, and reference percentiles were determined using Equation 1
| Equation 1 |
where Z is the Z-score that corresponds to a given percentile. Z-scores were calculated for each primary and secondary outcome using Equation 2
| Equation 2 |
where X is the measured body composition outcome. All available ages were used to generate the curves. Due to the sparse number of values for individuals aged >20 y, the reference values presented were restricted to aged 5–20 y.
All further statistical analyses were conducted in Stata 16.1 (StataCorp). We calculated the precision (percent coefficient of variation, %CV) and root mean square error (RMSE) of each primary and secondary body composition outcome for the subset of 153 study participants who were scanned twice, with repositioning, on the same day. Precision and RMSE were calculated for the entire cohort and by predesignated age ranges (6–9.9 y, 10–13.9 y, and 14–16 y) to inspect for age-related differences in precision.
Subgroups based on self-identified race and ethnicity were compared for differences in body composition Z-scores using mixed effects models to account for the multiple observations per person. In addition, reference ranges were calculated separately for Black and non-Black youth to be consistent with previously published bone density reference ranges for this cohort.
Residual effects of short or tall stature (as determined by HAZ) on appendicular LSTM Index and FMI were examined by mixed effects regression analysis to account for the multiple observations per person. To further inspect this relationship, we repeated the analysis within subgroups based on age (5–9.9, 10–14.9, and ≥15 y). To test for association of height status with body composition outcomes, we used sex-specific regression models with age group-by-HAZ interactions to test for potential differences in the relationship between HAZ and body composition over the age range. If present, we developed age- and sex-specific HAZ adjustment equations as previously described [21,26] for total LSTM, subtotal LSTM, appendicular LSTM, and FM. The adjustment method involves calculating the age- and sex-specific predicted body composition Z-score based on the HAZ prediction equation. The HAZ-adjusted body composition outcome is calculated as:
| Equation 3 |
| Equation 4 |
where Z-score is the relevant body composition Z-score relative to age and sex (e.g., appendicular LSTM-Z score). HAZ adjustment equations were not calculated for body composition index measures (i.e., those divided by the square of height such as LSTM Index) because those indices largely account for height effects.
Results
Sample characteristics
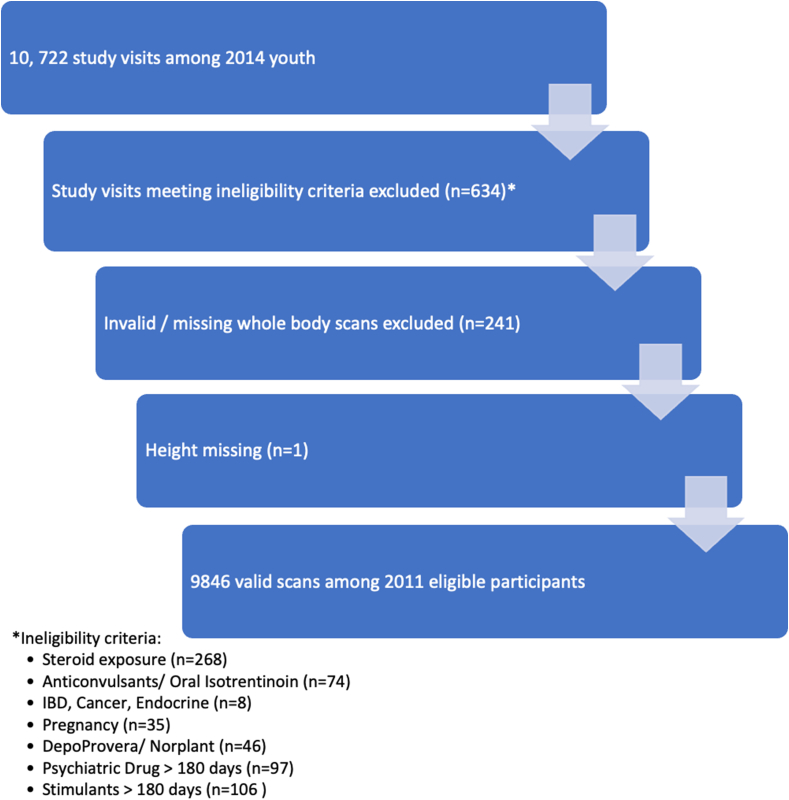
A total of 2014 children were enrolled in the BMDCS and completed ≤7 study visits resulting in a total of 10,722 visits, as described previously [21]. After exclusion of data due to study participants not meeting eligibility criteria at the time of the visit (n = 634), invalid whole or missing body scans (n = 241), and one individual missing a height measurement, 9846 complete whole body measurements from 2011 individuals were used in these analyses (Figure 1).
FIGURE 1.
Consort diagram showing enrollment of study participants and exclusions that resulted in the final data set used to create body composition reference ranges. IBD, inflammatory bowel disease.
The mean ± SD age at enrollment was 11.3 ± 4.4 y; the mean age for all observations was 13.6 ± 4.2 y, range 5–23.4 y (Table 1). The average number of observations per person was 5.8. The cohort was 51% female. The self-identified race and ethnicity distribution was 22% Black, 17% Hispanic, 48% White, 7% Asian/Pacific Islander, and 6% Mixed Race/Other/Unknown. The mean ± SD HAZ was 0.15 ± 0.85. Sixteen percent of the cohort had a BMI in the overweight category (BMI ≥85th and <95th percentile for sex and age), and 6% had a BMI in the obese category (BMI ≥95th percentile for sex and age).
TABLE 1.
Demographic, lifestyle, growth, and body composition characteristics of the Bone Mineral Density in Childhood Study cohort for all study visits
| Variable | |
|---|---|
| Study design | |
| Sample size | 2011 |
| Observations Visits per person | 9846 |
| 1 or 2 visits | 292 (15%) |
| 3 to 6 visits | 929 (26%) |
| 7 visits | 790 (39%) |
| Demographic and lifestyle | |
| Age, y | 13.6 ± 4.2 |
| Female sex observations | 5021 (51%) |
| Race and ethnicity groups | |
| American Indian/Alaskan Native | 13 (0.1%) |
| Asian/Pacific Islander | 694 (7%) |
| Black | 2175 (22%) |
| Hispanic | 1653 (17%) |
| White | 4737 (48%) |
| Other/Unknown | 11 (0.1%) |
| Mixed | 563 (6%) |
| Dietary calcium, mg1 | 849 ± 533 |
| Physical activity, h/wk2 | 14.2 ± 10.8 |
| Growth, maturation, and obesity status | |
| Height, cm | 154.5 ± 18.7 |
| Height Z-score | 0.15 ± 0.85 |
| Weight, kg | 50.67 ± 18.29 |
| Weight Z-score | 0.35 ± 0.83 |
| BMI, kg/m2 | 20.4 ± 3.9 |
| BMI Z-score | 0.31 ± 0.87 |
| BMI category observations | |
| Healthy weight (BMI <85th percentile) | 7719 (78%) |
| Overweight (BMI 85th to 94th percentile) | 1553 (16%) |
| Obese (BMI ≥95th percentile) | 574 (6%) |
| Tanner stage observations3 | |
| Stage 1 | 2408 (27%) |
| Stage 2 to 4 | 2532 (27%) |
| Stage 5 | 4133 (46%) |
| Body composition | |
| Appendicular lean soft tissue mass index, kg/m2 | 6.6 ± 1.5 |
| Fat mass index, kg/m2, mean ± SD | 4.9 ± 2.3 |
| Lean soft tissue mass index, kg/m2 | 14.9 ± 2.7 |
| Total lean body mass, kg | 37.2 ± 13.9 |
| Subtotal lean body mass, kg | 34.1 ± 13.5 |
| Total fat mass, kg | 12.0 ± 6.6 |
Values are mean ± SD or n (%).
n = 9837
n = 9843
Tanner stage available for n = 9073
Body composition precision and reference ranges
The precision (%CV) of body composition outcomes (Table 2) ranged from 0.70% to 1.96% for all ages combined. The precision as reflected by the RMSEs was similar across age groups. However, the %CV was slightly worse in the youngest age group (0.90%–2.50%) compared with older children (0.52%–1.85%), owing to the small mean values in the youngest children.
TABLE 2.
Precision of body composition outcomes in children
| All ages (n = 153) |
Ages 6–9.9 y (n = 49) |
Ages 10–13.9 y (n = 53) |
Ages 14–16.0 y (n = 51) |
|||||
|---|---|---|---|---|---|---|---|---|
| %CV | RMSE | %CV | RMSE | %CV | RMSE | %CV | RMSE | |
| Appendicular LSTM index, kg/m2 | 1.60 | 0.09 | 1.97 | 0.10 | 1.42 | 0.09 | 1.38 | 0.10 |
| FMI, kg/m2 | 1.95 | 0.08 | 2.50 | 0.08 | 1.40 | 0.07 | 1.84 | 0.07 |
| Total LSTM, kg | 0.70 | 0.21 | 0.90 | 0.18 | 0.65 | 0.22 | 0.52 | 0.23 |
| Total LSTM index, kg/m2 | 0.70 | 0.09 | 0.90 | 0.11 | 0.65 | 0.09 | 0.52 | 0.08 |
| Subtotal LSTM, kg | 0.80 | 0.21 | 1.09 | 0.19 | 0.66 | 0.20 | 0.59 | 0.24 |
| Subtotal LSTM index, kg/m2 | 0.80 | 0.10 | 1.09 | 0.11 | 0.66 | 0.09 | 0.59 | 0.09 |
| Appendicular LSTM, kg | 1.60 | 0.22 | 1.97 | 0.17 | 1.42 | 0.22 | 1.38 | 0.27 |
| Leg LSTM, kg | 1.96 | 0.22 | 2.22 | 0.14 | 1.79 | 0.21 | 1.85 | 0.28 |
| Total FM, kg | 1.95 | 0.17 | 2.50 | 0.14 | 1.40 | 0.15 | 1.84 | 0.21 |
%CV, percent coefficient of variation (SD/mean×100); FM, fat mass; FMI, fat mass index; LSTM, lean soft tissue mass; RMSE, root mean square error √(∑squared deviations/n).
LMS values and reference percentiles were created for the primary outcomes, appendicular LSTM Index and FMI (see Table 3 for ranges per year of age and Supplementary Tables 2 and 3 for ranges per decimal years), and secondary outcomes (total LSTM Index, subtotal LSTM Index, total LSTM, subtotal LSTM, appendicular LSTM, leg LSTM, and FM, see Supplementary Tables 4–10).
Table 3.
LMS values and reference ranges for appendicular lean soft tissue mass and fat mass index
| Age, y | Appendicular lean soft tissue mass index, kg/m2 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females |
Males |
|||||||||||||
| L | S | −2 SD | −1 SD | 0 SD (M) | 1 SD | 2 SD | L | S | −2 SD | −1 SD | 0 SD (M) | 1 SD | 2 SD | |
| 5.0–5.9 | 0.967 | 0.120 | 3.200 | 3.700 | 4.203 | 4.708 | 5.215 | −0.072 | 0.116 | 3.615 | 4.053 | 4.550 | 5.112 | 5.749 |
| 6.0–6.9 | 0.847 | 0.121 | 3.396 | 3.921 | 4.458 | 5.004 | 5.559 | −0.077 | 0.117 | 3.804 | 4.270 | 4.798 | 5.398 | 6.079 |
| 7.0–7.0 | 0.727 | 0.123 | 3.592 | 4.141 | 4.711 | 5.300 | 5.908 | −0.082 | 0.118 | 3.992 | 4.486 | 5.048 | 5.685 | 6.411 |
| 8.0–8.9 | 0.607 | 0.125 | 3.796 | 4.369 | 4.974 | 5.608 | 6.272 | −0.087 | 0.120 | 4.177 | 4.699 | 5.293 | 5.970 | 6.742 |
| 9.0–9.9 | 0.487 | 0.126 | 4.019 | 4.617 | 5.258 | 5.941 | 6.667 | −0.092 | 0.121 | 4.367 | 4.919 | 5.547 | 6.265 | 7.085 |
| 10.0–10.9 | 0.367 | 0.127 | 4.263 | 4.886 | 5.565 | 6.299 | 7.093 | −0.097 | 0.122 | 4.582 | 5.167 | 5.835 | 6.598 | 7.473 |
| 11.0–11.9 | 0.248 | 0.128 | 4.513 | 5.160 | 5.874 | 6.661 | 7.524 | −0.102 | 0.124 | 4.843 | 5.467 | 6.182 | 7.001 | 7.941 |
| 12.0–12.0 | 0.128 | 0.128 | 4.740 | 5.405 | 6.151 | 6.984 | 7.914 | −0.107 | 0.125 | 5.159 | 5.831 | 6.601 | 7.484 | 8.501 |
| 13.0–13.9 | 0.008 | 0.129 | 4.921 | 5.597 | 6.366 | 7.239 | 8.231 | −0.112 | 0.126 | 5.512 | 6.235 | 7.064 | 8.018 | 9.117 |
| 14.0–14.0 | −0.112 | 0.129 | 5.050 | 5.731 | 6.516 | 7.422 | 8.470 | −0.117 | 0.126 | 5.860 | 6.632 | 7.518 | 8.539 | 9.718 |
| 15.0–15.9 | −0.232 | 0.130 | 5.133 | 5.814 | 6.609 | 7.543 | 8.645 | −0.122 | 0.127 | 6.160 | 6.972 | 7.907 | 8.985 | 10.230 |
| 16.0–16.0 | −0.352 | 0.131 | 5.182 | 5.859 | 6.662 | 7.621 | 8.776 | −0.127 | 0.127 | 6.387 | 7.230 | 8.200 | 9.321 | 10.616 |
| 17.0–17.9 | −0.472 | 0.133 | 5.210 | 5.882 | 6.690 | 7.673 | 8.884 | −0.132 | 0.127 | 6.544 | 7.407 | 8.401 | 9.549 | 10.878 |
| 18.0–18.9 | −0.592 | 0.134 | 5.225 | 5.891 | 6.702 | 7.708 | 8.977 | −0.138 | 0.127 | 6.650 | 7.524 | 8.533 | 9.697 | 11.046 |
| 19.0–19.9 | −0.712 | 0.136 | 5.229 | 5.887 | 6.701 | 7.729 | 9.059 | −0.143 | 0.126 | 6.727 | 7.609 | 8.625 | 9.799 | 11.160 |
| Age, y | Fat mass index, kg/m2 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females |
Males |
|||||||||||||
| L | S | −2 SD | −1 SD | 0 SD (M) | 1 SD | 2 SD | L | S | −2 SD | −1 SD | 0 SD (M) | 1 SD | 2 SD | |
| 5.0–5.9 | −0.226 | 0.266 | 2.440 | 3.116 | 4.035 | 5.311 | 7.118 | −0.235 | 0.277 | 1.949 | 2.508 | 3.279 | 4.364 | 5.930 |
| 6.0–6.9 | −0.241 | 0.280 | 2.429 | 3.132 | 4.106 | 5.486 | 7.491 | −0.270 | 0.296 | 1.894 | 2.468 | 3.280 | 4.465 | 6.251 |
| 7.0–7.0 | −0.256 | 0.294 | 2.435 | 3.171 | 4.209 | 5.711 | 7.952 | −0.306 | 0.316 | 1.878 | 2.475 | 3.347 | 4.667 | 6.757 |
| 8.0–8.9 | −0.270 | 0.307 | 2.461 | 3.233 | 4.342 | 5.981 | 8.493 | −0.342 | 0.336 | 1.904 | 2.538 | 3.489 | 4.988 | 7.496 |
| 9.0–9.9 | −0.282 | 0.319 | 2.497 | 3.308 | 4.489 | 6.268 | 9.063 | −0.379 | 0.356 | 1.947 | 2.618 | 3.655 | 5.358 | 8.381 |
| 10.0–10.9 | −0.288 | 0.328 | 2.541 | 3.388 | 4.636 | 6.546 | 9.603 | −0.417 | 0.374 | 1.967 | 2.663 | 3.769 | 5.656 | 9.224 |
| 11.0–11.9 | −0.288 | 0.335 | 2.598 | 3.482 | 4.796 | 6.822 | 10.101 | −0.453 | 0.388 | 1.938 | 2.636 | 3.770 | 5.780 | 9.821 |
| 12.0–12.0 | −0.281 | 0.339 | 2.689 | 3.618 | 5.001 | 7.142 | 10.614 | −0.486 | 0.400 | 1.859 | 2.535 | 3.654 | 5.701 | 10.063 |
| 13.0–13.9 | −0.271 | 0.341 | 2.823 | 3.808 | 5.275 | 7.540 | 11.197 | −0.512 | 0.408 | 1.756 | 2.397 | 3.473 | 5.491 | 10.001 |
| 14.0–14.0 | −0.259 | 0.341 | 2.987 | 4.036 | 5.594 | 7.991 | 11.835 | −0.529 | 0.414 | 1.664 | 2.274 | 3.305 | 5.272 | 9.812 |
| 15.0–15.9 | −0.248 | 0.340 | 3.154 | 4.267 | 5.916 | 8.443 | 12.470 | −0.533 | 0.417 | 1.612 | 2.206 | 3.216 | 5.154 | 9.691 |
| 16.0–16.0 | −0.241 | 0.340 | 3.305 | 4.476 | 6.209 | 8.858 | 13.064 | −0.523 | 0.419 | 1.612 | 2.211 | 3.231 | 5.188 | 9.751 |
| 17.0–17.9 | −0.238 | 0.341 | 3.431 | 4.653 | 6.460 | 9.223 | 13.609 | −0.502 | 0.421 | 1.653 | 2.278 | 3.339 | 5.361 | 9.988 |
| 18.0–18.9 | −0.239 | 0.343 | 3.534 | 4.796 | 6.668 | 9.537 | 14.104 | −0.470 | 0.424 | 1.714 | 2.376 | 3.497 | 5.608 | 10.302 |
| 19.0–19.9 | −0.243 | 0.345 | 3.611 | 4.905 | 6.830 | 9.792 | 14.531 | −0.432 | 0.426 | 1.773 | 2.477 | 3.661 | 5.862 | 10.588 |
LMS analysis was implemented using the Generalized Additive Models for Location Scale and Shape (GAMLSS) function in R to generate values for L (lambda), S (sigma) and M(mu). L, M, and S are used to calculate smoothed distributions using the formula: M (1 + LSZ)1/L, where Z is a Z-score in the normal distribution. The smoothed distributions for −2, −1, 0 (median, i.e., mu), 1 and 2 SD are shown in the table. L, M, and S also are used to calculate exact Z-scores using the formula Z = [((X/M)L) – 1]/L•S, where X is the measured value.
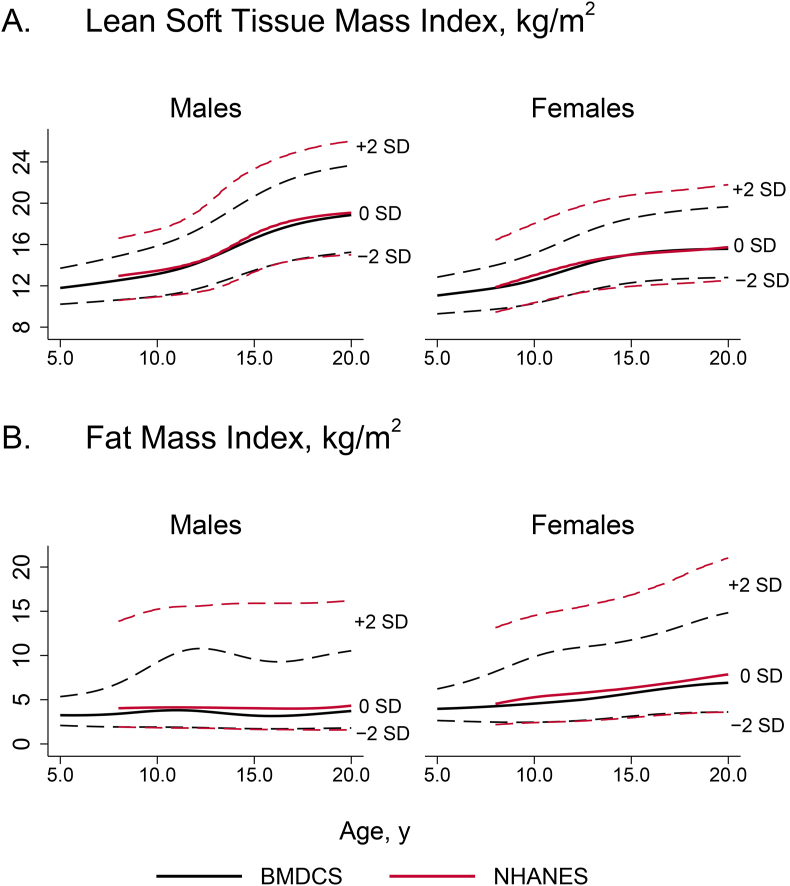
Comparison with NHANES reference ranges
We graphically compared our reference ranges to those previously published by Weber et al. [11] for total LSTM Index and FMI using NHANES data. As shown in Figure 2, for both indices, the age- and sex-specific values for the median (0 SD) and–2 SD curves for BMDCS and NHANES are similar. However, the NHANES +2 SD curves are distinctly greater than the BMDCS +2 SD curves, consistent with the difference in obesity prevalence between the 2 cohorts (20% for NHANES and 6% for BMDCS).
FIGURE 2.
Comparison of (A) lean soft tissue mass index and (B) fat mass index distributions for the Bone Mineral Density in Childhood Study (black lines) and the NHANES (1999–2004) published by Weber et al. [11]. Shown are the reference curves for −2 SD, 0 SD, and +2 SD for each source. Reference ranges are not based on the NHANES body composition adjustment.
Associations of stature with body composition outcomes
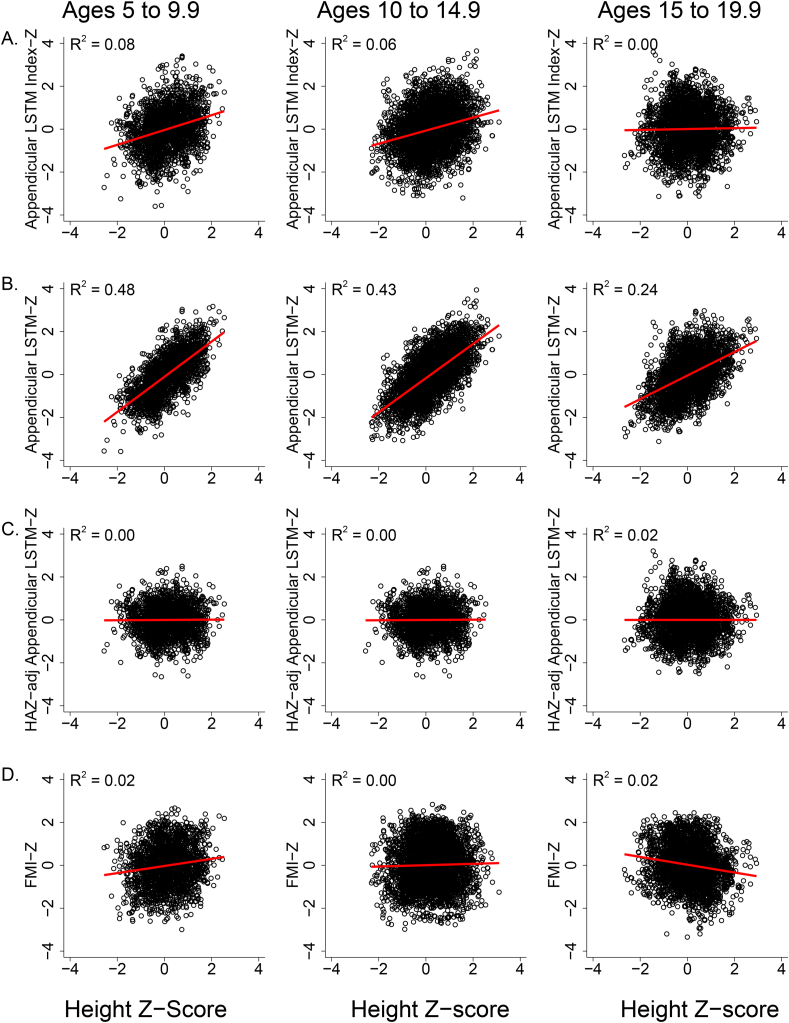
Similar to BMI, appendicular LSTM Index and FMI are body composition indices that minimize the association of body compartment size with stature by dividing the tissue mass by height2. We tested for a residual effect of stature on the Z-scores for these tissue compartments by examining the associations of appendicular LSTM Index-Z and FMI-Z with HAZ. As shown in Figure 3A, a small, but statistically significant positive association (R2 = 0.06 to 0.07, P < 0.001) between appendicular LSTM Index-Z and HAZ was found at younger ages (suggesting a minor residual effect of stature) but not among 15- to 20-y-olds. FMI-Z was positively associated with HAZ in younger children and negatively associated with HAZ in older children, but the associations were modest (Figure 3D).
FIGURE 3.
Scatterplots and regression lines illustrating the relationships of height-for-age Z-score (HAZ) with appendicular lean soft tissue mass (LSTM) index-Z score (A), appendicular LSTM-Z score (B), HAZ-adjusted appendicular LSTM (C), and fat mass index-Z score (FMI-Z) (D) by age group. R-square values were determined by mixed effects regression for each age group (Ages 5 to 9.9 y, n = 2130 observations; Ages 10–14.9 y, n = 3920 observations; Ages 15–19.9 y, n = 3069 observations). Appendicular LSTM index-Z score had a modest association with height-for-age Z-score at younger ages but not for 15- to 20-y-olds. Appendicular LSTM-Z score had a very strong association with HAZ at all ages, providing the rationale for the HAZ adjustment equations. FMI-Z score was positively associated with HAZ in younger children and negatively associated with HAZ in older children, but the associations were modest.
Several secondary outcomes are not indices and do not adjust for stature. The association of HAZ with Z-scores for appendicular LSTM (R2 = 0.61), total LSTM (R2 = 0.65), and subtotal LSTM (R2 = 0.65) were strong. Figure 3 illustrates the modest associations of HAZ with appendicular LSTM Index-Z (Figure 3A) and FMI-Z (Figure 3D) compared with appendicular LSTM-Z (Figure 3B). For leg LSTM, the overall association was relatively weak (R2 = 0.06), but stronger for 10- to 15-y-olds (R2 = 0.13). For FM, the overall association was moderate (R2 = 0.23) but significantly stronger for 5–10 (R2 = 0.36) than older children. Recognizing that the associations of shorter compared with larger stature for these secondary outcomes are age-dependent, we used sex-specific regression analysis to estimate the predicted association of HAZ for each whole year age category (e.g., 10.0–10.9 y) for total LSTM, subtotal LSTM, appendicular LSTM, leg LSTM, and FM. Figure 3C illustrates the absence of association between HAZ-adjusted appendicular LSTM-Z and HAZ. The HAZ adjustment equations are provided in Supplementary Tables 11–15. Each HAZ prediction equation has the format:
(as shown in Equation 3 in Methods), where a is the intercept and b is the slope. An HAZ-adjusted Z-score is calculated as the original Z-score minus the HAZ predicted Z-score (Equation 4 in Methods). The HAZ-adjusted Z-score indicates the degree to which the original Z-score may be affected by shorter or taller stature.
An example of calculations for appendicular LSTM-Z score and the use of the adjustment equations is as follows for an 11.5-y-old boy with 9.67 kg of appendicular LSTM and HAZ of −1.82.
| Appendicular LSTM-Z = [((X/M)L) – 1]/L•S |
| Appendicular LSTM-Z = [((9.67/13.66)-0.046)-1]/-0.046 x 0.17 =–1.99 |
| HAZ Predicted Appendicular LSTM-Z = a + b•HAZ |
| HAZ Predicted Appendicular LSTM-Z =-0.34 + (-1.82∗0.941) =–1.90 |
| HAZ-adjusted Appendicular LSTM-Z = Appendicular LSTM-Z - HAZ Predicted Appendicular LSTM-Z |
| HAZ-adjusted Appendicular LSTM-Z = −1.99 – (−1.90) = −0.09 |
Subgroup differences in body composition distributions
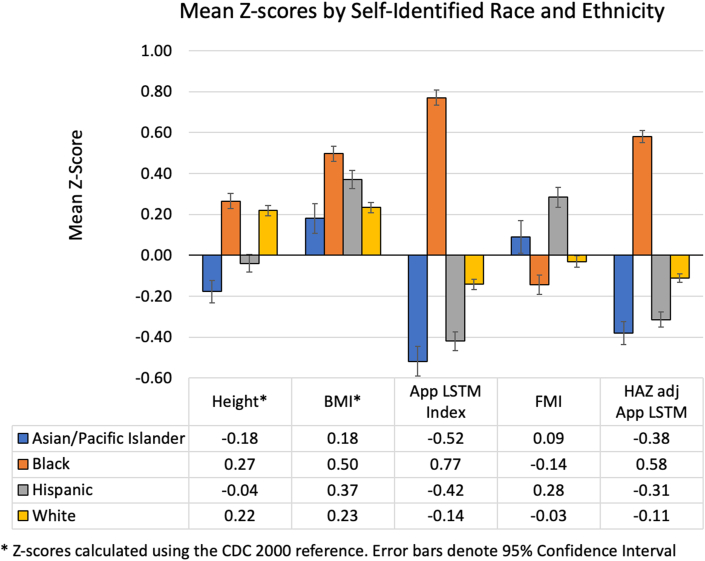
We evaluated differences in HAZ, BMIZ, appendicular LSTM Index-Z, FMI-Z, and HAZ-adjusted appendicular LSTM-Z between the 4 largest subgroups based on self-identified race (Asian/Pacific Islander, Black, and White) and Hispanic ethnicity. Means for each subgroup are shown in Figure 4, illustrating moderate differences in HAZ and BMIZ and large differences between subgroups in body composition outcomes. For example, the mean appendicular LSTM Index-Z for the Asian/Pacific Islander subgroup was −0.52 ± 0.93, and for the Non-Hispanic Black subgroup, it was 0.77 ± 0.87. These differences were paralleled by differences between means for HAZ, so we also compared group means for HAZ-adjusted appendicular LSTM-Z. Subgroup differences were less extreme (i.e., closer to zero) but remained quite different; for example, the Asian/Pacific Islander subgroup had a mean HAZ-adjusted appendicular LSTM-Z of −0.38 ± 0.71 whereas the Black subgroup mean was 0.58 ± 0.70. We used mixed effects regression analysis (accounting for multiple observations per person) and confirmed that most subgroup means were significantly different, even after adjustment for height and BMI Z-scores.
FIGURE 4.
Mean Z-scores by self-identified race and ethnicity groups for height-for-age, BMI, appendicular lean soft tissue mass index and HAZ-adjusted appendicular lean soft tissue mass. Note that height and BMI Z-scores were calculated using the CDC 2000 reference [4]. CDC, Centers for Disease Control and Prevention; HAZ, height-for-age Z-score.
Given the large differences in appendicular LSTM Index-Z between the Black and other subgroups, and to be consistent with the BMD reference ranges previously published for this cohort, we generated age- and sex-specific reference ranges for the Black subgroup and for all other subgroups combined. These values are provided for all primary and secondary body composition outcomes as abbreviated tables with HAZ adjustment equations (Supplementary Tables 16–24) and extended tables with ranges for decimal years (Supplementary Tables 25–33). There were insufficient numbers to generate separate reference ranges for the Hispanic and Asian/Pacific Islander subgroups. Nevertheless, subgroup differences were attenuated when compared using these group-specific reference ranges as shown in Supplementary Figure 1, but most remained statistically significant.
Discussion
DXA is commonly used in body composition assessment in children [27,28]. We showed excellent precision of body composition values (%CV range 0.70–1.96 overall) that was comparable to the precision for bone mineral density (%CV range 0.85–1.65 overall) [29]. Among the youngest children, ages 6–9.9 y, precision was close to that of older children (0.90%–2.50% compared with 0.52%–1.85%). For growing children, there are substantial age- and sex-related differences in body composition, necessitating the use of age- and sex-specific reference ranges for interpretation of body composition values. Previously published reference ranges using NHANES data (collected 1999–2004) were based on a cohort with a high prevalence of obesity (20%) and excluded children aged <8 y[11]. In addition, reference ranges for an expanded set of body composition phenotypes are needed because of the growing interest in measures of skeletal muscle, which is highly relevant to bone mineral accrual and physical activity research. Here, we present pediatric body composition reference ranges that meet these needs.
Our primary outcomes were appendicular LSTM Index and FMI. These indices largely account for the effects of linear growth on these body compartments. Many investigators have used total body fat percentage (%BF, FM as a percentage of total body mass) as an indicator for body composition and to identify individuals living with obesity. We did not use this measure for several reasons. First, %BF is relatively well-preserved under semistarvation conditions, so it is not a good indicator of nutritional status or energy reserves [30]. Second, as individuals gain excess weight, they gain both fat and lean tissue, so %BF may not reflect the absolute size of the adipose tissue compartment.
Appendicular LSTM (sometimes referred to as skeletal lean tissue mass) has garnered increasing interest as a measure of sarcopenia in adults [31] and skeletal muscle deficits in children [32]. Total body fat-free mass (body mass that excludes adipose tissue) and LSTM (excludes bone mass) include organ tissue, whereas appendicular LSTM, which is only in the arms and legs, is largely muscle tissue. Muscle mass is often of interest as it is more sensitive to changes in physical activity and loading. Appendicular muscle mass is particularly important for studies of bone health because of the “functional muscle-bone unit,” i.e., the response of bone to the mechanical forces of weight-bearing loads [33]. Appendicular LSTM is more strongly associated with bone outcomes than total body LSTM in healthy children. In an early report, Goulding et al. [34] demonstrated in 5-y-olds that appendicular LSTM Index was more strongly associated with total body bone mineral content than total body LSTM Index. Similar findings were reported more recently for a large sample of 5- to 19-y-old Chinese children [35]. Appendicular LSTM outcomes may be even more relevant as a measure of skeletal muscle deficits in children with chronic health conditions. For example, Summer et al. [32] demonstrated the loss in appendicular LSTM Index in boys with Duchenne’s muscular dystrophy, and Guo et al. [36] reported more pronounced deficits in appendicular LSTM Index (compared to total LSTM Index) in survivors of neuroblastoma. Of note, we included reference ranges for total LSTM Index and subtotal LSTM Index in supplementary tables because appendicular LSTM may not be available to all clinicians or investigators.
We used appendicular LSTM Index-Z as the primary outcome because it largely accounts for the association of height with appendicular LSTM. We demonstrated some residual association of appendicular LSTM Index-Z with HAZ in youth under age 15 y. As an alternative, we provide HAZ adjustment equations for appendicular LSTM and other body composition compartments (FM, total LSTM, subtotal LSTM) that completely account for the effects of smaller or taller status on these body composition outcomes. The difference between the age-based (unadjusted) Z-score and the HAZ-adjusted Z-score reflects the degree to which the age-based Z-score may be influenced by stature. For example, for a 10-y-old with short stature and an appendicular LSTM-Z score of–2 and an HAZ-adjusted appendicular LSTM-Z score of–1, the interpretation is that a large portion (1 SD) of their low appendicular LSTM relative to same age-sex peers is due to short stature, yet their appendicular LSTM remains lower than average. An important caveat is that this approach represents a statistical adjustment and may not accurately represent the effects of short or large stature for children outside the range of HAZ in the BMDCS cohort.
Previous studies have used DXA data from the NHANES to develop reference ranges for both Lunar and Hologic devices [11,37]. Weber et al. [11] reported reference ranges using data from 5195 males and 3766 females. Our study includes over 9800 data points on a sample of ∼2000 individuals. Unlike the NHANES sample, our study sample was not a strategic random sample of the general population. We enrolled healthy children who met enrollment criteria from 5 geographically diverse clinical centers in the United States. Our sample had fewer children with obesity (defined as BMI ≥95th percentile for age and sex) (6%) compared to the NHANES sample reported by Weber et al. [11] (20%). Comparison of our reference ranges with those of NHANES showed remarkable agreement in the curves at and below the median. However, the upper end of the NHANES reference range, at +2 SD, has values that are much greater than those for the BMDCS cohort. This is consistent with the difference between these samples in obesity prevalence. The implication of this difference is that children with greater amounts of body fat will have a lower Z-scores using the NHANES reference compared with the BMDCS reference. For example, in our cohort, an 8.5-y-old male with a BMI Z-score of 2.1 had a BMDCS FMI-Z score of 2.1 (98th percentile), yet their NHANES Z-score was 1.1 (87th percentile). Therefore, using the BMDCS reference range provides information that is more consistent with the CDC 2000 BMI charts [23] used to categorize overweight and obesity in children.
It is important to note that the body composition values reported here were obtained on Hologic DXA devices using Hologic Discovery 12.3 software at baseline and Apex 2.1 thereafter. Our reference ranges should only be applied to body composition results obtained from Hologic scanners using a Hologic software version that employs a similar algorithm for estimating body composition. For example, versions of Hologic software since 12.1 have increased accuracy in body composition estimates for children <40 kg [9]. In addition, we chose to use body composition values that were not adjusted using the “NHANES body composition analysis” option. This option reduces estimated lean mass and increases estimated fat mass. However, this adjustment was based on studies of adults 19–82 y of age, with a mean BMI of 27 (range 16–44 kg/m2). The mean BMI in our cohort was 20.5 (interquartile range: 17.4–22.9). Measurements obtained with the NHANES body composition analysis option can be converted so that they are comparable to the values used to generate our reference ranges using the following equations:
| Equation 5 |
| Equation 6 |
Differences between self-identified race and ethnicity groups in BMI and body composition have been reported previously [11,[38], [39], [40]]. Consistent with our study, Black children have greater lean mass and Hispanic children have greater fat mass than White children. The underlying mechanisms for these group differences are unknown. We acknowledge that self-identified race and ethnicity groups are social constructs, and environmental, social, behavioral, and nutritional factors contribute to these observations of group differences in body composition. In addition, these constructs overlap with population ancestry, and differences in gene frequencies may contribute to body composition patterns. Our cohort is not a nationally representative sample, nor did we use sample weights to try to emulate the race and ethnicity distribution of the population in the United States. Twenty-two percent of our cohort was Black, and 17 percent was Hispanic, compared to 14% and 19%, respectively, in the US population in 2021 [41]. Similar to the population in United States, 7% percent of the cohort self-identified as Asian/Pacific Islander, but we do not have more specific information on region of origin. Nevertheless, we report differences in our subgroups to provide a frame of reference for expected differences between major race and ethnicity groups in the context of low levels of obesity and overall good health. The sample sizes were not sufficient to create separate reference ranges for all subgroups. However, we created separate supplementary curves for Black and non-Black subgroups for consistency with our previously published bone density reference ranges [21]. According to the recommendations for pediatric bone health assessment, body composition measures are important to aid in the interpretation of bone density measurements in children at risk of malnutrition or muscle deficits [16]. The curves for Black and non-Black children fill an important gap in providing body composition reference ranges that parallel bone density reference curves for implementing this recommendation.
Our study has numerous strengths. The sample was large, geographically and ethnically diverse, and enrolled children in good health with lower levels of obesity than has been seen in other cohorts such as NHANES. As such, the use of these curves will not minimize the magnitude of excessive adiposity. We provided reference curves for appendicular LSTM Index, which is a good index of skeletal muscle, and is highly relevant for evaluating benefits of physical activity interventions, effects of diminished weight-bearing ability, nutritional status, and bone mineral density. In addition, we provided reference ranges for other body composition outcomes that may be relevant in some contexts, such as when subregion DXA values are not available for calculation of appendicular lean mass or when muscle mass in the legs is of particular interest in patients with ambulation issues. Finally, we provide a method for adjusting body composition measures for shorter or taller stature—a useful method when evaluating patients with delayed or advanced maturation and/or short stature.
The primary limitation of our study was that the cohort was a sample of convenience and was not fully representative of the US population. We were not able to develop reference ranges for all race and ethnicity groups because of their smaller sample size. All DXA scans were acquired on Hologic devices and centrally analyzed using standardized, proprietary software. For DXA measurements obtained on devices from GE Lunar, standardization equations should be applied before comparison with these reference ranges [42]. However, care should be taken to assure that the body composition acquisition and analysis algorithms are consistent with the Hologic software used to create these reference ranges and standardization equations for measurements obtained on other manufacturer’s devices.
In sum, we provide robust body composition reference ranges for healthy children aged 5–20 y of age. These reference ranges fill a gap in the literature for assessment of nutritional status, muscle deficits, and excess adiposity, for interpretation of bone health, and for monitoring the effects of physical activity. Consistent with other studies, we demonstrated large differences in body composition between self-identified race and ethnicity groups. The observed differences provide a frame of reference for these group differences in a cohort with low levels of obesity.
Acknowledgments
We are deeply indebted to the children and their families who participated in the BMDCS and the staff who contributed to data collection.
Author contributions
The authors’ responsibilities were as follows – KKW, BSZ, JAS, JML, SEO, HJK: designed the original research project, developed the overall research plan, provided study oversight and collected the data; JAS: was responsible for the centralized analysis of DXA scans; BSZ, JM, AK, HJK: participated in the analysis of data and design of statistical analyses; BSZ, HJK: wrote the paper with critical feedback from SFAG, JAS, JM, KKW, AK; BSZ: has primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript.
Conflicts of interest
BSZ, HJK, JML, SFAG, JM, AK, and KKW report no conflicts of interest. JAS declares the following:
-
-
Investigator Initiated Study from Hologic in the past 2 y.
-
-
Investigator Initiated Study from GE in the past 2 y.
-
-
Patents pending with Hologic regarding the measure of DXA multicompartment body composition.
None of the above are directly related to this study or this analysis.
Funding
This work was funded by the National Institute of Child Health and Human Development (NICHD) contracts NO1-HD-1-3228, -3329, -3330, -3331, -3332, and -3333, and grant R01 HD100406, and the Clinical and Translational Science Award Grants 5-MO1-RR-000240 and UL1 RR-026314. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Data described in the manuscript and the code book are available upon request from the NICHD DASH website (dash.nichd.nih.gov) pending application and approval. The analytic code will be made available upon request pending application and approval by the corresponding author.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.08.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wang Z., Ying Z., Bosy-Westphal A., Zhang J., Heller M., Later W., et al. Evaluation of specific metabolic rates of major organs and tissues: comparison between men and women. Am. J. Hum. Biol. 2011;23(3):333–338. doi: 10.1002/ajhb.21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zemel B. In: Human Growth and Development. Cameron N., Schell L., editors. Academic Press; Amsterdam: 2022. Body composition during growth and development; pp. 517–545. [Google Scholar]
- 3.Purcell S.A., Kok D.E., Ketterl T., Garcia M.B., Joffe L., Brown J.C., et al. Pharmacokinetics of cancer therapeutics and energy balance: the role of diet intake, energy expenditure, and body composition. J. Natl. Cancer Inst. Monogr. 2023;2023(61):3–11. doi: 10.1093/jncimonographs/lgad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson P.A., Rosner G.L., Matthay K.K., Moore T.B., Bomgaars L.R., Ellis K.J., et al. Impact of body composition on pharmacokinetics of doxorubicin in children: a Glaser Pediatric Research Network study. Cancer Chemother. Pharmacol. 2009;64(2):243–251. doi: 10.1007/s00280-008-0854-z. [DOI] [PubMed] [Google Scholar]
- 5.Dienemann T., Ziolkowski S.L., Bender S., Goral S., Long J., Baker J.F., et al. Changes in body composition, muscle strength, and fat distribution following kidney transplantation. Am. J. Kidney Dis. 2021;78(6):816–825. doi: 10.1053/j.ajkd.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tram N.K., Chou T.H., Janse S.A., Bobbey A.J., Audino A.N., Onofrey J.A., et al. Deep learning of image-derived measures of body composition in pediatric, adolescent, and young adult lymphoma: association with late treatment effects. Eur. Radiol. 2023;33(9):6599–6607. doi: 10.1007/s00330-023-09587-z. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill K.N., Aubrey E., Howe L.D., Stergiakouli E., Rodriguez S., Kearney P.M., et al. Mitochondrial DNA haplogroups and trajectories of cardiometabolic risk factors during childhood and adolescence: a prospective cohort study. PLOS ONE. 2023;18(4) doi: 10.1371/journal.pone.0284226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Gonzalez D., Wells J.C.K., Parra-Carriedo A., Bilbao G., Mendez M., Clark P. Body composition assessment in Mexican children and adolescents. Part 1: comparisons between skinfold-thickness, dual X-ray absorptiometry, air-displacement plethysmography, deuterium oxide dilution, and magnetic resonance imaging with the 4-C model. Nutrients. 2022;14(5):1073. doi: 10.3390/nu14051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shypailo R.J., Butte N.F., Ellis K.J. DXA: can it be used as a criterion reference for body fat measurements in children? Obesity (Silver Spring) 2008;16(2):457–462. doi: 10.1038/oby.2007.81. [DOI] [PubMed] [Google Scholar]
- 10.Rudnev S., Burns J.S., Williams P.L., Lee M.M., Korrick S.A., Denisova T., et al. Comparison of bioimpedance body composition in young adults in the Russian Children’s Study. Clin. Nutr. ESPEN. 2020;35:153–161. doi: 10.1016/j.clnesp.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber D.R., Moore R.H., Leonard M.B., Zemel B.S. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am. J. Clin. Nutr. 2013;98(1):49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden C.L., Flegal K.M. Changes in terminology for childhood overweight and obesity. Natl. Health Stat. Rep. 2010;(25):1–5. [PubMed] [Google Scholar]
- 13.Basha M.A., Aboelnour N.H., Aly S.M., Kamel F.A.H. Impact of Kinect-based virtual reality training on physical fitness and quality of life in severely burned children: a monocentric randomized controlled trial. Ann. Phys. Rehabil. Med. 2022;65(1):101471. doi: 10.1016/j.rehab.2020.101471. [DOI] [PubMed] [Google Scholar]
- 14.Sherlock S.P., Palmer J., Wagner K.R., Abdel-Hamid H.Z., Tian C., Mah J.K., et al. Dual-energy X-ray absorptiometry measures of lean body mass as a biomarker for progression in boys with Duchenne muscular dystrophy. Sci. Rep. 2022;12(1):18762. doi: 10.1038/s41598-022-23072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree N.J., Arabi A., Bachrach L.K., Fewtrell M., El-Hajj Fuleihan G., Kecskemethy H.H., et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014;17(2):225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Gordon C.M., Bachrach L.K., Carpenter T.O., Crabtree N., El-Hajj Fuleihan G., Kutilek S., et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J. Clin. Densitom. 2008;11(1):43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Ogden C.L., Kuczmarski R.J., Flegal K.M., Mei Z., Guo S., Wei R., et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Kalkwarf H.J., Shepherd J.A., Hans D., Gonzalez Rodriguez E., Kindler J.M., Lappe J.M., et al. Trabecular bone score reference values for children and adolescents according to age, sex, and ancestry. J. Bone Miner. Res. 2022;37(4):776–785. doi: 10.1002/jbmr.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindler J.M., Kalkwarf H.J., Lappe J.M., Gilsanz V., Oberfield S., Shepherd J.A., et al. Pediatric reference ranges for ultradistal radius bone density: results from the Bone Mineral Density in Childhood Study. J. Clin. Endocrinol. Metab. 2020;105(10):e3529–e3539. doi: 10.1210/clinem/dgaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kindler J.M., Lappe J.M., Gilsanz V., Oberfield S., Shepherd J.A., Kelly A., et al. Lumbar spine bone mineral apparent density in children: results from the Bone Mineral Density in Childhood Study. J. Clin. Endocrinol. Metab. 2019;104(4):1283–1292. doi: 10.1210/jc.2018-01693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zemel B.S., Kalkwarf H.J., Gilsanz V., Lappe J.M., Oberfield S., Shepherd J.A., et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J. Clin. Endocrinol. Metab. 2011;96(10):3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalkwarf H.J., Zemel B.S., Gilsanz V., Lappe J.M., Horlick M., Oberfield S., et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J. Clin. Endocrinol. Metab. 2007;92(6):2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski R.J., Ogden C.L., Grummer-Strawn L.M., Flegal K.M., Guo S.S., Wei R., et al. CDC growth charts: United States. Adv. Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 24.Schoeller D.A., Tylavsky F.A., Baer D.J., Chumlea W.C., Earthman C.P., Fuerst T., et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am. J. Clin. Nutr. 2005;81(5):1018–1025. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- 25.Cole T.J., Green P.J. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat. Med. 1992;11(10):1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 26.Zemel B.S., Leonard M.B., Kelly A., Lappe J.M., Gilsanz V., Oberfield S., et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J. Clin. Endocrinol. Metab. 2010;95(3):1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasserman H., O’Donnell J.M., Gordon C.M. Use of dual energy X-ray absorptiometry in pediatric patients. Bone. 2017;104:84–90. doi: 10.1016/j.bone.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohman T.G., Going S.B. Body composition assessment for development of an international growth standard for preadolescent and adolescent children. Food Nutr. Bull. 2006;27(4 Suppl Growth Standard):S314–S325. doi: 10.1177/15648265060274S512. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd J.A., Wang L., Fan B., Gilsanz V., Kalkwarf H.J., Lappe J., et al. Optimal monitoring time interval between DXA measures in children. J. Bone Miner. Res. 2011;26(11):2745–2752. doi: 10.1002/jbmr.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.VanItallie T.B., Yang M.U., Heymsfield S.B., Funk R.C., Boileau R.A. Height-normalized indices of the body's fat-free mass and fat mass: potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990;52(6):953–959. doi: 10.1093/ajcn/52.6.953. [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyère O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summer S.S., Wong B.L., Rutter M.M., Horn P.S., Tian C., Rybalsky I., et al. Age-related changes in appendicular lean mass in males with Duchenne muscular dystrophy: a retrospective review. Muscle Nerve. 2021;63(2):231–238. doi: 10.1002/mus.27107. [DOI] [PubMed] [Google Scholar]
- 33.Schoenau E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J. Musculoskelet. Neuronal Interact. 2005;5(3):232–238. [PubMed] [Google Scholar]
- 34.Goulding A., Taylor R.W., Grant A.M., Jones S., Taylor B.J., Williams S.M. Relationships of appendicular LMI and total body LMI to bone mass and physical activity levels in a birth cohort of New Zealand five-year olds. Bone. 2009;45(3):455–459. doi: 10.1016/j.bone.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Guo B., Wu Q., Gong J., Xiao Z., Tang Y., Shang J., et al. Relationships between the lean mass index and bone mass and reference values of muscular status in healthy Chinese children and adolescents. J. Bone Miner. Metab. 2016;34(6):703–713. doi: 10.1007/s00774-015-0725-8. [DOI] [PubMed] [Google Scholar]
- 36.Guo M., Zemel B.S., Hawkes C.P., Long J., Kelly A., Leonard M.B., et al. Sarcopenia and preserved bone mineral density in paediatric survivors of high-risk neuroblastoma with growth failure. J. Cachexia Sarcopenia Muscle. 2021;12(4):1024–1033. doi: 10.1002/jcsm.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duran I., Martakis K., Rehberg M., Stark C., Schafmeyer L., Schönau E. Reference centiles for the evaluation of nutritional status in children using body fat percentage, fat mass and lean body mass index. J. Clin. Densitom. 2020;23(3):349–363. doi: 10.1016/j.jocd.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Shypailo R.J., Wong W.W. Fat and fat-free mass index references in children and young adults: assessments along racial and ethnic lines. Am. J. Clin. Nutr. 2020;112(3):566–575. doi: 10.1093/ajcn/nqaa128. [DOI] [PubMed] [Google Scholar]
- 39.Martin C.B., Stierman B., Yanovski J.A., Hales C.M., Sarafrazi N., Ogden C.L. Body fat differences among US youth aged 8-19 by race and Hispanic origin, 2011-2018, Pediatr. Obes. 2022;17(7) doi: 10.1111/ijpo.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freedman D.S., Wang J., Thornton J.C., Mei Z., Pierson R.N., Jr., Dietz W.H., et al. Racial/ethnic differences in body fatness among children and adolescents. Obesity (Silver Spring) 2008;16(5):1105–1111. doi: 10.1038/oby.2008.30. [DOI] [PubMed] [Google Scholar]
- 41.United States Census Bureau Quick Facts [Internet]. [cited 29 December, 2022]. Available from https://www.census.gov/quickfacts/fact/table/US/PST045221.
- 42.Shepherd J.A., Fan B., Lu Y., Wu X.P., Wacker W.K., Ergun D.L., et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J. Bone Miner. Res. 2012;27(10):2208–2216. doi: 10.1002/jbmr.1654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript and the code book are available upon request from the NICHD DASH website (dash.nichd.nih.gov) pending application and approval. The analytic code will be made available upon request pending application and approval by the corresponding author.