Abstract
目的
探讨血脂各项指标及血脂异常不同临床分类与糖尿病肾病(diabetic kidney disease, DKD)的关系,并比较血脂异常不同临床分类对DKD的预测价值。
方法
连续纳入2020年10月–2021年10月重庆医科大学附属永川医院和重庆医科大学附属第一医院收治的2型糖尿病(type 2 diabetes mellitus, T2DM)患者356例作为研究对象,按照是否合并DKD分为DKD组(n=126)和单纯T2DM组(n=230),并选取同期健康体检者250例作为对照组,测定3组的血压、血脂、血糖、肾功能等指标,采用非条件logistic回归模型分析血脂异常不同临床分类对DKD的影响,进一步构建ROC曲线,并计算曲线下面积(area under the curve, AUC),分析血脂异常不同临床分类对DKD的预测价值。
结果
DKD组和单纯T2DM组患者的舒张压(diastolic blood pressure, DBP)、收缩压(systolic blood pressure, SBP)、总胆固醇(total cholesterol, TC)、三酰甘油(triacylglycerol, TG)、低密度脂蛋白胆固醇(low-density lipoprotein cholesterol, LDL-C)、血清肌酐(serum creatinine, Scr)、尿酸(uric acid, UA)和糖化血红蛋白A1c(glycosylated hemoglobin A1c, HbA1c)均分别高于对照组,而两组的高密度脂蛋白胆固醇(high-density lipoprotein cholesterol, HDL-C)则分别低于对照组,差异均有统计学意义(均P<0.05);DKD组患者的T2DM病程、DBP、SBP、TC、TG、Scr、UA和HbA1c均高于单纯T2DM组,差异均有统计学意义(均P<0.05)。在校正T2DM病程、DBP、SBP、Scr、UA和HbA1c的影响后,结果显示:TC(OR=1.426,95%CI:1.088~1.868)和TG(OR=1.404,95%CI:1.075~1.833)是DKD发病的独立危险因素;高胆固醇血症(OR=1.817,95%CI:1.040~3.177)和混合型高脂血症(OR=2.148,95%CI:1.110~4.159)均为DKD的独立危险因素(均P<0.05)。高胆固醇血症的AUC(95%CI)为0.789(0.729~0.871),混合型高脂血症AUC(95%CI)为0.671(0.579~0.760);高胆固醇血症对DKD的诊断预测价值更大。
结论
血脂水平中,TC和TG是DKD的独立危险因素,血脂异常临床分类中,高胆固醇血症和混合型高脂血症是DKD的独立危险因素;高胆固醇血症可以作为T2DM患者中DKD筛查的预测因子,适合在门诊患者中进行筛查推广。
Keywords: 糖尿病肾病, 2型糖尿病, 血脂异常, 相关性, 预测价值
Abstract
Objective
To explore the relationship between blood lipid indicators and different clinical classifications of dyslipidemia and diabetic kidney disease (DKD) and to compare the value of different clinical classifications of dyslipidemia for predicting DKD.
Methods
Continuously enrollment of subjects was conducted at the First Affiliated Hospital of Chongqing Medical University and the Yongchuan Hospital of Chongqing Medical University between October 2020 and October 2021. A total of 356 type 2 diabetes mellitus (T2DM) patients admitted to the two hospitals were enrolled. They were divided into DKD group (n=126) and simple T2DM group (n=230) according to whether their T2DM was combined with DKD. In addition, 250 healthy individuals undergoing physical examination during the same period were enrolled for the control group. The blood pressure, blood lipid, blood glucose, and the kidney function indicators of the three groups were measured. The effects of different classifications of dyslipidemia on DKD were analyzed with unconditional logistic regression models, the receiver operating characteristic (ROC) curve was constructed, the area under the curve (AUC) of ROC was calculated, and the value of different classifications of dyslipidemia for predicting DKD was analyzed.
Results
The diastolic blood pressure (DBP), systolic blood pressure (SBP), total cholesterol (TC), triacylglycerol (TG), low-density lipoprotein cholesterol (LDL-C), serum creatinine (Scr), uric acid (UA), and glycosylated hemoglobin A1c (HbA1c) of the DKD group and the simple T2DM group were significantly higher than those of the control group, while the high-density lipoprotein cholesterol (HDL-C) levels of the DKD group and the simple T2DM group were lower than that of the control group (all P<0.05). The disease course of T2DM, DBP, SBP, TC, TG, Scr, UA and HbA1c of the DKD group were significantly higher than those of the T2DM group (all P<0.05). After adjusting for the effects of T2DM disease course, DBP, SBP, Scr, UA and HbA1c, the results showed that TC (OR=1.426, 95%CI: 1.088-1.868) and TG (OR=1.404, 95%CI: 1.075-1.833) were independent risk factors for DKD, and that hypercholesterolemia (OR=1.817, 95%CI: 1.040-3.177) and mixed hyperlipidemia (OR=2.148, 95%CI: 1.110-4.159) were independent risk factors for DKD (all P<0.05). The AUC (95%CI) of hypercholesterolemia was 0.789 (0.729-0.871). The AUC (95%CI) of mixed hyperlipidemia was 0.671 (0.579-0.760). Hypercholesterolemia showed better predictive value for the diagnosis and prediction of DKD.
Conclusion
Among the blood lipid indicators, TC and TG are independent risk factors of DKD. In the clinical classifications of dyslipidemia, hypercholesterolemia and mixed hyperlipidemia are independent risk factors of DKD. Hypercholesterolemia can be used as a predictor to screen for DKD among T2DM patients and is well suited for extensive application in outpatient screening.
Keywords: Diabetic kidney disease, Type 2 diabetes mellitus, Dyslipidemia, Correlation, Predictive value
糖尿病肾病(diabetic kidney disease, DKD)是一类由糖尿病引起的慢性肾脏疾病[1-2],主要表现为持续性尿蛋白排泄率增加、肾小球滤过率进行性下降。DKD是2型糖尿病(type 2 diabetes mellitus, T2DM)的常见微血管并发症,同时也是导致终末期肾病(end-stage renal disease, ESRD)的重要原因[3-4],给社会和家庭带来了沉重的疾病负担和经济负担[5],因此,探讨DKD的致病因素对疾病的早期诊断、预防和延缓DKD向ESRD进展具有重要意义[6]。DKD的发病机制目前尚未完全清楚,但已有研究表明[7-8],血脂异常等多种因素参与了DKD的发生发展过程,高甘油三酯血症患者由于血液黏稠度增加、毛细血管中的红细胞聚集等原因,引起血管微循环灌流减少,与此同时,高胆固醇血症会进一步加重缺血及再灌注和其他炎性反应对毛细血管的刺激,最终促进和加速DKD的发生和进展。但目前关于血脂异常不同临床分类与DKD的关系及血脂异常不同临床分类对DKD的预测价值研究,国内外还鲜有报道。为此,本研究对比分析主要血脂指标在DKD、T2DM及健康对照人群的差异以及血脂异常不同临床分类与DKD的相关性,以探讨血脂异常不同临床分类对DKD的预测价值,以期为DKD的临床早期诊断提供相关参考依据。
1. 资料与方法
1.1. 临床资料
回顾性连续纳入2020年10月–2021年10月重庆医科大学附属永川医院和重庆医科大学附属第一医院肾病风湿科、肾内科收治的T2DM患者和同期的健康体检者。纳入标准:①年龄18~65岁,性别不限;②DKD的诊断标准参照2019年《中国糖尿病肾脏疾病防治指南》[9],由糖尿病引起的慢性肾病,且估算肾小球滤过率(estimate glomerular filtration rate, eGFR)<60 mL/(min·1.73 m2)和(或)尿白蛋白肌酐比(urine albumin-to-creatinine ratio, UACR)>30 mg/g持续时间>3个月;③T2DM的诊断标准参照《中国2型糖尿病防治指南(2020年版)》[10],典型糖尿病症状加随机血糖≥11.1 mmol/L或空腹血糖≥7.0 mmol/L或口服葡萄糖耐量试验2 h血糖≥11.1 mmol/L或糖化血红蛋白(glycated hemoglobin A1c, HbA1c)≥6.5%,对无典型糖尿病症状者,应改日复查诊断。排除标准:1型糖尿病患者,继发性糖尿病患者,糖尿病急性并发症患者(糖尿病酮症、高血糖高渗状态等),恶性肿瘤患者,肾脏原发性疾病患者,患有自身免疫性疾病患者,合并各种急性感染、甲状腺疾病、严重心肺及肝脏病变患者,使用激素治疗患者,精神异常等不能配合完成研究患者。按照纳入、排除标准,共纳入356例T2DM患者,按照是否合并DKD分为DKD组(n=126)和单纯T2DM组(n=230),并随机选取同期健康体检者250例作为对照组。全部研究对象均签署知情同意书,本研究通过了重庆医科大学附属永川医院医学伦理委员会批准( 2021年科伦审14号)。
1.2. 研究方法
1.2.1. 临床资料收集
通过医院病案管理系统收集研究对象的一般人口学资料及临床资料,主要包括性别、年龄、体质量指数(body mass index, BMI)、血压、吸烟饮酒情况、糖尿病病程、既往疾病史(高血压、冠心病等)、糖尿病家族史等。
1.2.2. 实验室指标检测
全部研究对象空腹8~12 h后的次日清晨8:00–9:00采静脉血5 mL,采用全自动生化分析系统(ARCHITECT c16000)测定其血脂指标,包括:总胆固醇(total cholesterol, TC)、三酰甘油(triacylglycerol, TG)、高密度脂蛋白胆固醇(high-density lipoprotein cholesterol, HDL-C)和低密度脂蛋白胆固醇(low-density lipoprotein cholesterol, LDL-C),通过葡萄糖氧化酶法测定静脉血浆葡萄糖水平,通过免疫荧光法(TOSOH自动酶免疫测定分析仪AIA-2000ST)测量静脉血浆胰岛素水平,通过高效液相色谱仪(BIO-RAD VARIANT 2, USA)测定静脉血浆糖化血红蛋白(glycosylated hemoglobin A1c, HbA1c)水平。
1.2.3. 血脂异常诊断及临床分类标准
参照《中国成人血脂异常防治指南(2016年修订版)》[11]《血脂异常基层诊疗指南(实践版·2019)》[12]关于血脂异常诊断及临床分类标准分为:①高胆固醇血症:单纯TC升高(TC≥5.2 mmol/L,TG<1.7 mmol/L),②高甘油三酯血症:单纯TG升高(TG≥1.7 mmol/L,TC<5.2 mmol/L),③混合型高脂血症:TC和TG均升高(TC≥5.2 mmol/L,TG≥1.7 mmol/L),④低HDL-C血症:HDL-C偏低(HDL-C<1.0 mmol/L)。
1.3. 统计学方法
对符合或近似符合正态分布的定量资料采用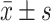 进行描述,多组间比较采用单因素分差分析(one-way ANOVA),进一步的两两比较采用LSD-t法,两组间比较采用两独立样本的t检验;对于非正态分布的定量资料采用中位数(四分位间距)进行描述,组间比较采用Kruskal-Wallis H检验;定性资料采用百分比或构成比进行描述,组间比较采用χ2检验。分别采用多因素非条件logistic回归模型分析血脂水平和血脂异常不同临床分类与DKD发病的关系,纳入单因素分析有统计学意义的一般资料及实验室指标作为协变量。预测价值评价运用R-3.5.2 Medition软件绘制ROC曲线,采用Z检验比较血脂异常不同临床分类的ROC曲线下面积(area under the curve, AUC)的差异。P<0.05为差异有统计学意义。
进行描述,多组间比较采用单因素分差分析(one-way ANOVA),进一步的两两比较采用LSD-t法,两组间比较采用两独立样本的t检验;对于非正态分布的定量资料采用中位数(四分位间距)进行描述,组间比较采用Kruskal-Wallis H检验;定性资料采用百分比或构成比进行描述,组间比较采用χ2检验。分别采用多因素非条件logistic回归模型分析血脂水平和血脂异常不同临床分类与DKD发病的关系,纳入单因素分析有统计学意义的一般资料及实验室指标作为协变量。预测价值评价运用R-3.5.2 Medition软件绘制ROC曲线,采用Z检验比较血脂异常不同临床分类的ROC曲线下面积(area under the curve, AUC)的差异。P<0.05为差异有统计学意义。
2. 结果
2.1. 3组一般资料及实验室指标比较
舒张压(diastolic blood pressure, DBP)、收缩压(systolic blood pressure, SBP)、T2DM病程、TC、TG、LDL-C、HDL-C、血清肌酐(serum creatinine, Scr)、血尿酸(uric acid, UA)和HbA1c在3组间差异有统计学意义(均P<0.05)。进一步组间两两比较发现:DKD组和单纯T2DM组患者的DBP、SBP、TC、TG、LDL-C、Scr、UA和HbA1c均分别高于对照组,而两组的HDL-C则分别低于对照组,差异均有统计学意义(均P<0.05);DKD组患者的T2DM病程、DBP、SBP、TC、TG、Scr、UA和HbA1c均高于单纯T2DM组,差异均有统计学意义(均P<0.05)。见表1。
表 1. Comparison of the general information and laboratory indicators of the three groups.
3组一般资料及实验室指标比较
| Variable | DKD group (n=126) |
T2DM group (n=230) |
Control group (n=250) | χ2/F/t | P |
| BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; T2DM: type 2 diabetes; TC: total cholesterol; TG: triacylglycerol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; Scr: serum creatinine; BUN: blood urea nitrogen; UA: uric acid; HbA1c: glycated hemoglobin A1c. * P<0.05, vs. control group; # P<0.05, vs. T2DM group. 1 mmHg=0.133 kPa. | |||||
| Sex/case (%) | 0.671 | 0.715 | |||
| Male | 75 (59.52) | 132 (57.39) | 138 (55.20) | ||
| Female | 51 (40.48) | 98 (42.61) | 112 (44.80) | ||
Age/yr., 
|
55.94±9.49 | 55.32±9.45 | 53.65±10.00 | 2.348 | 0.096 |
BMI/(kg/m2), 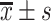
|
23.05±4.62 | 23.68±4.78 | 23.71±4.80 | 0.924 | 0.398 |
DBP/mmHg, 
|
80.96±10.40*, # | 77.65±10.42* | 73.54±8.16 | 27.294 | <0.001 |
SBP/mmHg, 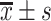
|
137.21±17.33*, # | 131.15±15.03* | 124.91±13.83 | 29.297 | <0.001 |
T2DM course/year, 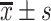
|
9.19±3.34# | 4.99±2.06 | 0 | −16.411 | <0.001 |
| Smoking/case (%) | 57 (45.24) | 101 (43.91) | 100 (40.00) | 1.212 | 0.546 |
| Drinking/case (%) | 37 (29.37) | 60 (26.09) | 53 (21.20) | 3.353 | 0.187 |
TC/(mmol/L), 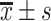
|
5.40±1.07*, # | 5.05±0.91* | 4.58±0.99 | 31.584 | <0.001 |
TG/(mmol/L), 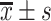
|
2.21±1.11*, # | 1.97±1.10* | 1.27±0.81 | 48.277 | <0.001 |
LDL-C/(mmol/L), 
|
3.12±0.89* | 3.06±0.92* | 2.77±0.88 | 9.162 | <0.001 |
HDL-C/(mmol/L), 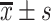
|
1.22±0.45* | 1.25±0.50* | 1.35±0.24 | 5.234 | 0.006 |
Scr/(μmol/L), 
|
109.23±21.22*, # | 105.90±20.88* | 95.02±17.78 | 28.551 | <0.001 |
BUN/(μmol/L), 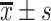
|
5.91±2.85 | 6.04±2.80 | 5.68±2.87 | 0.997 | 0.370 |
UA/(μmol/L), 
|
303.71±49.62*, # | 288.19±40.92* | 278.93±44.14 | 13.221 | <0.001 |
HbA1c/%, 
|
8.89±1.08*, # | 7.81±1.09* | 5.46±0.99 | 547.363 | <0.001 |
2.2. 血脂水平与DKD发病危险因素的非条件logistic回归分析
在T2DM患者中,以有无DKD作为因变量(Y,1=DKD,0=单纯T2DM),分别以TC、TG、LDL-C、HDL-C作为自变量,采用向前逐步回归法筛选自变量(Forward:LR;α入=0.05,α出=0.10),校正T2DM病程、DBP、SBP、Scr、UA和HbA1c的影响后,结果显示:TC(OR=1.426)和TG(OR=1.404)是DKD发病的独立危险因素。见表2。
表 2. Unconditional logistic regression of blood lipid levels and risk factors for DKD.
血脂水平与DKD发病危险因素的非条件logistic回归分析
| Variable | Model 1 | Model 2 | |||||
| β | OR (95% CI) | P | β | OR (95% CI) | P | ||
| β: partial regression coefficient; OR: odds ratio; CI: confidence interval. Model 1 is uncalibrated; model 2 is adjusted for the effect of T2DM disease course, DBP, SBP, Scr, UA, and HbA1c. | |||||||
| TC | 0.863 | 2.370 (1.820-3.086) | <0.001 | 0.355 | 1.426 (1.088-1.868) | 0.010 | |
| TG | 0.372 | 1.450 (1.143-1.839) | 0.002 | 0.339 | 1.404 (1.075-1.833) | 0.013 | |
| LDL-C | 0.054 | 1.056 (0.825-1.352) | 0.666 | 0.071 | 1.073 (0.809-1.425) | 0.624 | |
| HDL-C | −0.132 | 0.876 (0.556-1.391) | 0.570 | −0.033 | 0.968 (0.573-1.634) | 0.903 | |
2.3. 血脂异常不同临床分类与DKD发病危险因素的非条件logistic回归分析
在T2DM患者中,血脂正常121例、高胆固醇血症157例、高甘油三酯血症133例、混合型高脂血症195例、低HDL-C血症124例。以有无DKD作为因变量(Y,1=DKD,0=单纯T2DM),以不同血脂异常的临床分类(高胆固醇血症/血脂正常、高甘油三酯血症/血脂正常、混合型高脂血症/血脂正常、低HDL-C血症/血脂正常)作为自变量,并纳入T2DM病程、DBP、SBP、Scr、UA、HbA1c作为校正因素进行非条件logistic回归分析,结果显示:高胆固醇血症(OR=1.817)和混合型高脂血症(OR=2.148)均为DKD的独立危险因素(均P<0.05)。见表3。
表 3. Unconditional logistic regression of different clinical classifications of dyslipidemia and risk factors for DKD.
血脂异常不同临床分类与DKD发病危险因素的非条件logistic回归分析
| Variable | Model 1 | Model 2 | |||||
| β | OR (95% CI) | P | β | OR (95% CI) | P | ||
| The notes are the same as those for Table 2. | |||||||
| Normal blood lipids | - | Reference | - | - | Reference | - | |
| Hypercholesterolemia | 0.737 | 2.090 (1.086-4.022) | 0.027 | 0.597 | 1.817 (1.040-3.177) | 0.036 | |
| Hypertriglyceridemia | 0.143 | 1.154 (0.592-2.247) | 0.674 | 0.398 | 1.488 (0.803-2.758) | 0.206 | |
| Mixed hyperlipidemia | 0.870 | 2.387 (1.829-3.114) | 0.001 | 0.765 | 2.148 (1.110-4.159) | 0.023 | |
| Hypo HDL-C anemia | -0.195 | 0.823 (0.495-1.368) | 0.453 | -0.161 | 0.851 (0.527-1.375) | 0.511 | |
2.4. 血脂异常不同临床分类对DKD预测价值的ROC曲线分析
在T2DM患者中,分别以DKD的独立危险因素高胆固醇血症和混合型高脂血症进行ROC曲线分析,结果显示:高胆固醇血症的AUC(95%CI)为0.789(0.729~0.871),混合型高脂血症AUC(95%CI)为0.671(0.579~0.760),高胆固醇血症对DKD的诊断预测价值更大。见图1。
图 1.
ROC curve for the diagnosis of DKD in patients with hypercholesterolemia and mixed hyperlipidemia
高胆固醇血症和混合型高脂血症诊断DKD的ROC曲线
3. 讨论
DKD是糖尿病引起的慢性肾脏病,有约25%~40%的糖尿病患者会合并DKD[13],全球约有30%~50%的ESRD患者由DKD所导致[14],在我国DKD已经成为中老年ESRD患者的首要病因[15],早期DKD的肾脏病理学改变尚有逆转可能,如果可以在早期发现诊断DKD并进行及时的干预,可延缓甚至阻止DKD进展为ESRD[16]。然而DKD发病较隐匿,早期症状不典型,因此,探索临床上易获取、简单、经济的DKD诊断方法,对DKD的早期诊断及干预,延缓和阻止DKD进展为ESRD具有重要的临床意义。
目前关于DKD的发病机制尚未完全阐明,近年来,已有大量研究证实高脂血症是DKD的独立危险因素,血脂水平异常与肾功能下降密切相关[17-18]。本研究还发现,DKD患者和单纯T2DM患者的血压、血脂、血糖、肾功能指标均高于健康对照人群,进一步分析发现,DKD患者的T2DM病程、血压、血脂、血糖、肾功能指标均明显高于单纯T2DM组。由于T2DM患者存在胰岛素抵抗、胰岛素敏感性降低等现象,因此,DKD患者易出现血脂异常,而血脂异常会进一步对肾功能造成损伤。研究发现对DKD合并血脂异常患者采取降脂治疗后可以延缓肾病进展[19]。因此,对于血脂异常的DKD患者,除了进行积极的药物治疗延缓其ESRD进程外,降血脂治疗也有助于延缓肾脏病进展,降低肾功能的下降速度。
本研究进一步采用非条件logistic回归模型对血脂水平(TC、TG、LDL-C、HDL-C)和血脂异常不同临床分类(高胆固醇血症、高甘油三酯血症、混合型高脂血症、低HDL-C血症)与DKD的相关性进行分析,结果发现,在校正混杂因素的影响后,血脂水平中,TC(OR=1.426)和TG(OR=1.404)是DKD的独立危险因素,血脂异常临床分类中,高胆固醇血症(OR=1.817)和混合型高脂血症(OR=2.148)是DKD的独立危险因素。DKD患者普遍存在血脂异常,DKD患者脂代谢异常主要表现为,TC升高、TG升高、HDL-C降低;高脂血症(主要包括高胆固醇血症、混合型高脂血症)可损伤血管,破坏内皮细胞,影响其屏障功能。这也印证了本研究中TC、TG升高及高胆固醇血症、混合型高脂血症是DKD患者危险因素这一结论。因此,《糖尿病肾脏疾病临床诊疗中国指南》[1]也建议,对于DKD合并血脂异常患者,需要进行降脂治疗及生活方式干预,并在初期每1~3个月进行1次空腹血脂(TC、TG、LDL-C、HDL-C)检测。
上述研究结果说明血脂异常,血脂异常不同临床分类中的高胆固醇血症、混合型高脂血症是DKD的独立危险因素。为了进一步验证高胆固醇血症与混合型高脂血症对DKD的诊断预测价值,本研究进行了ROC曲线分析,结果显示高胆固醇血症对DKD的诊断预测价值更大,提示在T2DM患者中,当发现高胆固醇血症患者时,需要更加关注患者的肾脏功能,进一步完善相关检查,以期早期发现和早期干预DKD[20-21]。
综上所述,血脂水平中,TC和TG是DKD的独立危险因素,血脂异常临床分类中,高胆固醇血症和混合型高脂血症是DKD的独立危险因素;高胆固醇血症可以作为T2DM患者中DKD筛查的预测因子,且血脂指标简单、经济、易获取,适合在门诊患者中进行筛查推广。但本研究结论还需进一步进行多中心、大样本、前瞻性的队列研究来进一步加以证实。
* * *
作者贡献声明 胡煜琳负责论文构思、数据审编、正式分析、调查研究和初稿写作,杜晓刚经费获取、提供资源和审读与编辑写作。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿,并同意对工作的所有方面负责。
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
重庆市永川区自然科学基金(No.Ycstc2018nb0226)和重庆医科大学附属永川医院院内课题(No.YJLC201711)资助
Contributor Information
煜琳 胡 (Yulin HU), Email: Huyulin_430@163.com.
晓刚 杜 (Xiaogang DU), Email: dxgcxm@163.com.
References
- 1.中华医学会肾脏病学分会专家组 糖尿病肾脏疾病临床诊疗中国指南. 中华肾脏病杂志. 2021;37(3):255–304. doi: 10.3760/cma.j.cn441217-20201125-00041. [DOI] [Google Scholar]
- 2.EDER S, LEIERER J, KERSCHBAUM J, et al Guidelines and clinical practice at the primary level of healthcare in patients with type 2 diabetes mellitus with and without kidney disease in five European countries. Diab Vasc Dis Res. 2019;16(1):47–56. doi: 10.1177/1479164118795559. [DOI] [PubMed] [Google Scholar]
- 3.中华医学会肾脏病学分会专家组 终末期糖尿病肾脏病肾替代治疗的中国指南. 中华肾脏病杂志. 2022;38(1):62–75. doi: 10.3760/cma.j.cn441217-20210322-00012. [DOI] [Google Scholar]
- 4.SAGOO M K, GNUDI L Diabetic nephropathy: an overview. Methods Mol Biol. 2020;2067:3–7. doi: 10.1007/978-1-4939-9841-8_1. [DOI] [PubMed] [Google Scholar]
- 5.SUGRUE D M, WARD T, RAI S, et al Economic modelling of chronic kidney disease: a systematic literature review to inform conceptual model design. J Pharmacoeconomics. 2019;37(12):1451–1468. doi: 10.1007/s40273-019-00835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.UEKI K, SASAKO T, OKAZAKI Y, et al Multifactorial intervention has a significant effect on diabetic kidney disease in patients with type 2 diabetes. Kidney Int. 2021;99(1):256–266. doi: 10.1016/j.kint.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 7.OPAZO-RÍOS L, MAS S, MARÍN-ROYO G, et al Lipotoxicity and diabetic nephropathy: novel mechanistic insights and therapeutic opportunities. Int J Mol Sci. 2020;21(7):2632. doi: 10.3390/ijms21072632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EID S, SAS K M, ABCOUWER S F, et al New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539–1549. doi: 10.1007/s00125-019-4959-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.中华医学会糖尿病学分会微血管并发症学组 中国糖尿病肾脏疾病防治临床指南. 中华糖尿病杂志. 2019;11(1):15–28. doi: 10.3760/cma.j.issn.1674-5809.2019.01.004. [DOI] [Google Scholar]
- 10.中华医学会糖尿病学分会 中国2型糖尿病防治指南(2020年版) 中华糖尿病杂志. 2021;13(4):315–409. doi: 10.3760/cma.j.cn115791-20210221-00095. [DOI] [Google Scholar]
- 11.中国成人血脂异常防治指南修订联合委员会 中国成人血脂异常防治指南(2016年修订版) 中华全科医师杂志. 2017;16(1):15–35. doi: 10.3760/cma.j.issn.1671-7368.2017.01.006. [DOI] [Google Scholar]
- 12.中华医学会, 中华医学会杂志社, 中华医学会全科医学分会, 等 血脂异常基层诊疗指南(实践版·2019) 中华全科医师杂志. 2019;18(5):417–421. doi: 10.3760/cma.j.issn.1671-7368.2019.05.004. [DOI] [Google Scholar]
- 13.AFKARIAN M, ZELNICK L R, HALL Y N, et al Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA. 2016;316(6):602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RUIZ-ORTEGA M, RODRIGUES-DIEZ R R, LAVOZ C, et al Special issue "Diabetic nephropathy: diagnosis, prevention and treatment". J Clin Med. 2020;9(3):813. doi: 10.3390/jcm9030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HU R, QUAN S, WANG Y, et al Spectrum of biopsy proven renal diseases in Central China: a 10-year retrospective study based on 34, 630 cases. Sci Rep. 2020;10(1):10994. doi: 10.1038/s41598-020-67910-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LASSÉN E, DAEHN I S Molecular mechanisms in early diabetic kidney disease: glomerular endothelial cell dysfunction. Int J Mol Sci. 2020;21(24):9456. doi: 10.3390/ijms21249456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LYTVYN Y, BJORNSTAD P, Van RAALTE D H, et al The new biology of diabetic kidney disease-mechanisms and therapeutic implications. Endocr Rev. 2020;41(2):202–231. doi: 10.1210/endrev/bnz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DEFRONZO R A, REEVES W B, AWAD A S Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17(5):319–334. doi: 10.1038/s41581-021-00393-8. [DOI] [PubMed] [Google Scholar]
- 19.AKHTAR M, TAHA N M, NAUMAN A, et al Diabetic kidney disease: past and present. Adv Anat Pathol. 2020;27(2):87–97. doi: 10.1097/PAP.0000000000000257. [DOI] [PubMed] [Google Scholar]
- 20.YAMAZAKI T, MIMURA I, TANAKA T, et al Treatment of diabetic kidney disease: current and future. Diabetes Metab J. 2021;45(1):11–26. doi: 10.4093/dmj.2020.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.PÉREZ-MORALES R E, Del PINO M D, VALDIVIELSO J M, et al Inflammation in diabetic kidney disease. Nephron. 2019;143(1):12–16. doi: 10.1159/000493278. [DOI] [PubMed] [Google Scholar]



