Abstract
目的
本研究旨在探讨橙皮素(hesperetin, Hes)能否通过调控沉默信息调节因子1(silent information regulator 1, SIRT1)/核转录因子E2相关因子2(nuclear transcription factor E2-related factor 2, NRF2)信号减轻氧化应激改善阿霉素(doxorubicin, DOX)诱导的H9c2心肌细胞毒性。
方法
采用DOX诱导的H9c2心肌细胞毒性模型,随机分为4组:对照组(Control)、DOX处理组(DOX)、Hes加DOX处理组(DOX+Hes)以及Hes加SIRT1抑制剂EX527联合DOX处理组(DOX+Hes+EX527)。光镜下观察细胞形态,CCK-8检测各组细胞活力,流式细胞术检测各组细胞凋亡率,DCFH-DA染色观察各组ROS水平,ELISA试剂盒检测乳酸脱氢酶(lactic dehydrogenase, LDH)、超氧化物歧化酶(superoxide dismutase, SOD)、过氧化氢酶(catalase, CAT)和SIRT1活性以及丙二醛(malondialdehyde, MDA)含量,Western blot检测裂解的caspase-3(cleaved caspase-3)、细胞色素c(cytochrome c)、SIRT1、Ac-FOXO1、NRF2和血红素加氧酶1(heme oxygenase 1, HO-1)的表达。
结果
和Control组相比,DOX组细胞形态肿胀,密度减小,细胞活力降低,培养基中LDH活性增加(P<0.01);细胞凋亡增多,cleaved caspase-3和cytochrome c表达增加(P<0.01);CAT和SOD活性降低,MDA含量和ROS水平增加(P<0.01);SIRT1、NRF2和HO-1表达以及SIRT1活性降低,Ac-FOXO1表达增加(P<0.01);与DOX组相比,DOX+Hes组细胞形态改善,密度和细胞活力增加,培养基中LDH活性降低(P<0.01);细胞凋亡减少,cleaved caspase-3和cytochrome c表达降低(P<0.01);CAT和SOD活性升高,MDA含量和ROS水平降低(P<0.01);SIRT1、NRF2和HO-1表达以及SIRT1活性增加,Ac-FOXO1表达降低(P<0.01);而与DOX+Hes组相比,EX527阻断Hes对DOX诱导的H9c2细胞损伤、氧化应激和SIRT1/NRF2信号的作用。
结论
Hes可以通过调控SIRT1/NRF2信号抑制氧化应激和细胞凋亡减轻DOX诱导的H9c2心肌细胞毒性。
Keywords: 橙皮素, 氧化应激, SIRT1/NRF2, H9c2细胞, 阿霉素
Abstract
Objective
To investigate whether hesperetin (Hes) alleviates doxorubicin (DOX)-induced cardiomyocytotoxicity by reducing oxidative stress via regulating silent information regulator 1 (SIRT1)/nuclear transcription factor E2-related factor 2 (NRF2) signaling in H9c2 cells.
Methods
H9c2 cells were treated with DOX to establish the cardiotoxicity model and were randomly assigned to four groups, a control group (Control) and three treatment groups, receiving respectively DOX (the DOX group), Hes+DOX (the DOX+Hes group), and Hes+SIRT1 inhibitor EX527+DOX (the DOX+Hes+EX527 group). Cellular morphology was observed by the light microscope. Cell viability was evaluated by CCK-8. DOX-induced apoptosis in H9c2 cells was examined by flow cytometry. The levels of reactive oxygen species (ROS) in the H9c2 cells of the four groups were determied with 2'-7'-dichlorodihydrofluorescein diacetate (DCFH-DA) staining. The activities of lactate dehydrogenase (LDH), superoxide dismutase (SOD), catalase (CAT), and SIRT1 as well as the malondialdehyde (MDA) content were measured using ELISA kits. The expressions of cleaved caspase-3, cytochrome c, SIRT1, Ac-FOXO1, NRF2, and heme oxygenase 1 (HO-1) were determined by Western blot.
Results
Compared with the Control group, the DOX group showed swollen cellular morphology, decreased cell density and viability, and increased LDH activity in the medium (P<0.01); both apoptosis and the expression of cleaved caspase-3 and cytochrome c increased (P<0.01); the activities of CAT and SOD decreased while the contents of MDA and ROS increased (P<0.01); the expression of SIRT1, NRF2, and HO-1 decreased, the activity of SIRT1 decreased, and the expression of Ac-FOXO1 increased (P<0.01). Compared with the DOX group, the DOX+Hes group showed improved cellular morphology, increased cell density and viability, and decreased LDH activity in the medium (P<0.01); the apoptosis and the expression of cleaved caspase-3 and cytochrome c decreased (P<0.01); the activities of CAT and SOD increased while the levels of MDA and ROS decreased (P<0.01); the expression of SIRT1, NRF2, and HO-1 increased, the activity of SIRT1 increased, and the expression of Ac-FOXO1 decreased (P<0.01). Comparison of the findings for the DOX+Hes group and the DOX+Hes+EX527 group showed that EX527 could block the protective effects of Hes against DOX-induced cell injury, oxidative stress, and SIRT1/NRF2 signaling.
Conclusion
Hes inhibits oxidative stress and apoptosis via regulating SIRT1/NRF2 signaling, thereby reducing DOX-induced cardiotoxicity in H9c2 cells.
Keywords: Hesperetin, Oxidative stress, SIRT1/NRF2, H9c2 cells, Doxorubicin
尽管癌症和心血管疾病的检测手段和治疗方法均有了长足的进步,但二者仍是我国乃至全世界高发病率和死亡率的主要原因[1]。蒽环类药物如阿霉素(doxorubicin, DOX)是临床上广泛使用的广谱抗肿瘤药物,在多种实体瘤或非实体瘤的治疗中发挥重要作用[2-3]。然而,DOX时间和剂量依赖的心脏毒性会导致患者左心室功能障碍甚至出现心力衰竭等严重毒副作用,这极大地限制了其临床应用[4]。近年来,关于DOX导致心脏毒性的病理机制研究成为多学科研究的热点。已有研究表明,DOX可能通过活性氧簇、拓扑异构酶2β、Ca2+稳态失衡、炎症反应等因素导致心脏毒性[5-7],但具体机制仍有待进一步阐明。
橙皮素(hesperetin, Hes)是芸香科柑橘属植物果实中的一种重要生物活性成分[8]。作为天然的黄酮类化合物,Hes具有广泛的药理学特性,包括抗炎、抗氧化、抗凋亡等作用,在肿瘤和心脑血管疾病的防治中发挥重要作用[9-11]。有研究表明,Hes可通过激活PI3K/Akt通路抑制心肌细胞凋亡,减轻氧化应激和炎症反应改善心肌细胞缺氧/复氧损伤[12]。同时,Hes对DOX引起的心脏纤维化也具有一定的保护作用[13]。沉默信息调节因子1(silent information regulator 1, SIRT1)是去乙酰化酶家族研究最为广泛的成员,与细胞的多种生理过程如凋亡、炎症和氧化应激等密切相关[14-15]。SIRT1能调节多种转录因子如核转录因子E2相关因子2(nuclear transcription factor E2-related factor 2, NRF2),而NRF2是胞内重要的抗氧化应激因子,参与胞内活性氧(reactive oxygen species, ROS)含量的调节[16-17]。本研究采用DOX诱导的H9c2心肌细胞毒性模型,探讨Hes能否通过调节SIRT1/NRF2信号发挥抗氧化应激作用改善DOX诱导的心脏毒性。
1. 材料和方法
1.1. 实验材料
H9c2细胞购自中国上海天成科技有限公司;EX527购自Selleck公司;裂解的caspase-3(cleaved caspase-3)、细胞色素c(cytochrome c)、SIRT1、Ac-FOXO1、NRF2、血红素加氧酶1(heme oxygenase 1, HO-1)和GAPDH抗体购自武汉三鹰生物技术有限公司;BCA蛋白定量试剂盒购自ThermoFisher Scientific公司;RIPA裂解液购自碧云天生物技术有限公司;TUNEL染色试剂盒购自Roche公司;CCK-8试剂盒、过氧化氢酶(catalase, CAT)活性、超氧化物歧化酶(superoxide dismutase, SOD)活性、SIRT1活性、丙二醛(malondialdehyde, MDA)含量和乳酸脱氢酶(lactate dehydrogenase, LDH)活性检测试剂盒购自碧云天生物技术有限公司;辣根过氧化物酶标记的山羊抗兔和山羊抗鼠抗体购自武汉谷歌生物技术有限公司。
1.2. 细胞培养和模型建立
H9c2细胞用含有10%胎牛血清和青霉素/链霉素溶液的DMEM培养基在37 ℃恒温培养箱(体积分数21%O2及体积分数5%CO2)中培养。用1 μmol/L的DOX处理H9c2细胞24 h模拟心脏毒性模型,再随机分为4组:对照组(Control)、DOX处理组(DOX)、Hes加DOX处理组(DOX+Hes)以及Hes加SIRT1抑制剂EX527联合DOX处理组(DOX+Hes+EX527)。Control组采用正常培养基培养;DOX组用1 μmol/L的DOX处理24 h;DOX+Hes组H9c2细胞先用10 μmol/L的Hes预处理24 h,再用1 μmol/L的DOX处理24 h;DOX+Hes+EX527组H9c2细胞先用10 μmol/L的EX527预处理1 h,再用Hes处理24 h,最后用1 μmol/L的DOX处理24 h。根据文献确定DOX、Hes和EX527的浓度[18-20]。
1.3. H9c2细胞活力检测
取4组细胞,将培养的H9c2细胞接种在96孔板中。严格按照CCK-8说明书的步骤测定各组细胞存活率,通过酶标仪检测各组样品的光密度。各组处理结束后将CCK-8溶液加入10 μL的每个孔中,并在37 ℃下在避光孵育2 h后检测。使用微板读取器分光光度法读取450 nm处的吸光度(A450)值。细胞活力检测正比于A450值。然后以对照组的细胞活力的均数为100%,计算其他组相对于对照组的细胞活力。
1.4. ELISA检测H9c2细胞LDH活性和流式细胞术检测H9c2细胞凋亡
将H9c2细胞2×105 mL-1的密度均匀种在6孔板中,待正常贴壁生长后,根据不同分组培养处理,按照LDH检测试剂盒步骤测定收集的4组细胞培养液上清中LDH的活性。使用酶标仪测量每个孔中A450读数,根据说明书计算各组LDH活性。LDH活性正比于A450值。
1.5. 流式细胞术检测H9c2细胞凋亡
取4组细胞,用PBS小心洗涤收集各组细胞,离心弃上清并保留沉淀,用正常培养基重悬细胞,每组细胞用500 μL结合缓冲液、5 μL PI和5 μL Annexin Ⅴ-FITC室温下孵育10 min,流式细胞仪检测各组细胞凋亡程度。
1.6. 免疫印迹法检测H9c2细胞蛋白表达
将H9c2细胞培养于60 mm细胞培养皿中,根据分组处理后,弃上清,PBS清洗。用RIPA裂解液将细胞重悬,在冰上裂解20 min,并于4 ℃ 12000×g离心20 min,取上清,BCA定量。采用聚丙烯酰胺凝胶100 V分离蛋白,湿转法将凝胶上的蛋白转至PVDF膜90 min;将膜置于5%脱脂奶粉室温封闭2 h,将膜放入抗cleaved caspase-3(1∶1000)、抗cytochrome c(1∶1000)、抗SIRT1(1∶1000)、抗Ac-FOXO1(1∶1000)、抗NRF2(1∶1000)、抗HO-1(1∶1000)和抗GAPDH抗体(1∶5000)中,4 ℃摇床过夜;用含有0.1%吐温20的TBS(TBST)洗膜后,加入HRP标记的二抗室温下孵育2 h,Bio-Rad化学发光系统检测,并用Image Lab软件进行灰度值计算。以内参蛋白的灰度值为1,计算目的条带相对内参蛋白的灰度值,为目的蛋白的相对表达量。然后以对照组的蛋白相对表达量的均数为100%,计算其他组相对于对照组目的蛋白的表达量。
1.7. 免疫荧光染色检测ROS含量的变化
取4组细胞,用PBS洗涤3次,并加入含有10 μmol/L浓度DCFH-DA染液的无血清培养基,在培养箱中避光孵育30 min,随后用无血清培养基洗涤3次,激光共聚焦显微镜下拍摄图像并保存。
1.8. ELISA检测CAT活性、SOD活性、MDA含量和SIRT1活性
取4组细胞,用预冷的PBS小心洗涤3次,加入裂解液并在冰上充分裂解20 min,4 ℃ 12000×g离心10 min,取上清,用BCA法进行蛋白浓度检测。按照CAT活性检测试剂盒、SOD活性检测试剂盒、MDA检测试剂盒以及SIRT1活性检测试剂盒说明书的要求,将反应液和待测样品充分混匀,采用酶标仪测定各组样品吸光值并根据相应公式转换为各组的活力或含量值。
1.9. 统计学方法
数据采用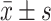 表示,多组间比较采用单因素方差分析(One-way ANOVA),再用Bonferroni法比较两组间差异,P<0.05为差异有统计学意义。
表示,多组间比较采用单因素方差分析(One-way ANOVA),再用Bonferroni法比较两组间差异,P<0.05为差异有统计学意义。
2. 结果
2.1. Hes改善DOX引起的H9c2细胞损伤
如图1显示,和Control组相比,DOX组细胞密度减小,细胞形态明显皱缩,细胞活力降低(P<0.01),培养基中LDH活性增加(P<0.01);与DOX组相比,DOX+Hes组细胞密度增加,细胞形态改善,细胞活力增加(P<0.01),培养基中LDH活性降低(P<0.01);而与DOX+Hes组相比,DOX+Hes+EX527组细胞密度减小,细胞形态皱缩,细胞活力降低(P<0.01),培养基中LDH活性增加(P<0.01)。这些结果说明Hes具有改善DOX引起的H9c2细胞损伤的作用,而这一作用与SIRT1信号有关。
图 1.

The alleviative effect of Hes on DOX-induced H9c2 cell injury
Hes改善DOX引起的H9c2细胞损伤
n=6. ** P<0.01, vs. Control group; ## P<0.01, vs. DOX group; && P<0.01, vs. DOX+Hes group.
2.2. Hes减轻DOX引起的H9c2细胞凋亡的作用
如图2,与Control组相比,流式细胞术和Western blot结果显示,DOX组细胞凋亡增多,凋亡相关蛋白cleaved caspase-3和cytochrome c表达增加(P<0.01);与DOX组相比,DOX+Hes组细胞凋亡减少,凋亡相关蛋白cleaved caspase-3和cytochrome c表达降低(P<0.01);而与DOX+Hes组相比,DOX+Hes+EX527组细胞凋亡增多,凋亡相关蛋白cleaved caspase-3和cytochrome c表达增加(P<0.01)。这些结果说明Hes能够改善DOX引起的H9c2细胞凋亡,而这一作用与SIRT1信号有关。
图 2.

The alleviative effect of Hes on DOX-induced apoptosis in H9c2 cells
Hes改善DOX引起的H9c2细胞凋亡
A, Flow cytometry; B, Western blot. n=6. ** P<0.01, vs. Control group; ## P<0.01, vs. DOX group; && P<0.01, vs. DOX+Hes group.
2.3. Hes减轻DOX引起的H9c2细胞氧化应激损伤
如图3,与Control组相比,ELSIA和DCFH-DA染色结果显示,DOX组CAT和SOD活性降低,MDA含量和ROS水平增加(P<0.01);与DOX组相比,DOX+Hes组CAT和SOD活性增加,MDA含量和ROS水平降低(P<0.01);而与DOX+Hes组相比,DOX+Hes+EX527组CAT和SOD活性降低,MDA含量和ROS水平增加(P<0.01)。这些结果说明Hes能够改善DOX引起的H9c2细胞氧化应激损伤,其作用与SIRT1信号有关。
图 3.

The alleviative effect of Hes on DOX-induced oxidative stress in H9c2 cells
Hes改善DOX引起的H9c2细胞氧化应激
CAT: catalase; SOD: superoxide dismutase; MDA: malondialdehyde. A, Control group; B, DOX group; C, DOX+Hes group; D, DOX+Hes+EX527 group. n=6. ** P<0.01, vs. A group; ## P<0.01, vs. B group; && P<0.01, vs. C group.
2.4. Hes改善DOX引起的SIRT1/NRF2信号抑制
如图4和图5,与Control组相比,Western blot结果显示,DOX组SIRT1、NRF2和HO-1表达降低,Ac-FOXO1表达增加(P<0.01),SIRT1活性降低(P<0.01);与DOX组相比,DOX+Hes组SIRT1、NRF2和HO-1表达增加,Ac-FOXO1表达降低(P<0.01),SIRT1活性增加(P<0.01);而与DOX+Hes组相比,DOX+Hes+EX527组SIRT1、NRF2和HO-1表达降低,Ac-FOXO1表达增加(P<0.01),SIRT1活性降低(P<0.01)。这些结果表明Hes能够改善DOX引起的SIRT1/NRF2信号抑制。
图 4.
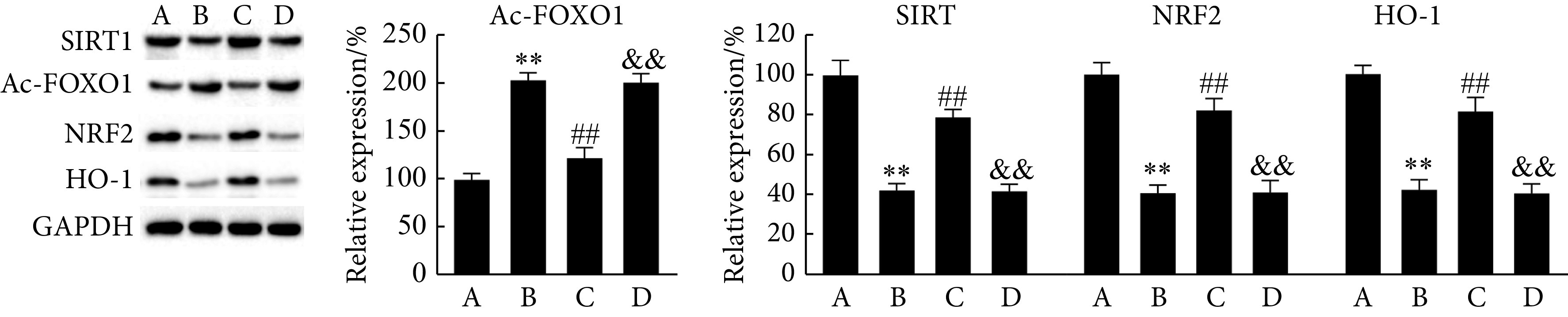
Western blot was performed to determine the alleviative effect of Hes on DOX-induced SIRT1/NRF2 signaling in H9c2 cells
Western blot检测Hes改善DOX引起的H9c2细胞SIRT1/NRF2信号
A, Control group; B, DOX group; C, DOX+Hes group; D, DOX+Hes+EX527 group. n=6, ** P<0.01, vs. A group; ## P<0.01, vs. B group; && P<0.01, vs. C group.
图 5.
ELISA assay was performed to determine the alleviative effect of Hes on DOX-induced SIRT1 activity in H9c2 cells
ELISA检测Hes改善DOX引起的H9c2细胞SIRT1活性变化
A, Control group; B, DOX group; C, DOX+Hes group; D, DOX+Hes+EX527 group. n=6. ** P<0.01, vs. A group; ## P<0.01, vs. B group; && P<0.01, vs. C group.
3. 讨论
DOX是一种有效的蒽环类抗肿瘤药物,用于多种恶性肿瘤如的化疗[2]。然而,DOX在临床的使用过程中往往会受到严重不良反应的限制,尤其是其剂量依赖地导致患者出现左室收缩功能障碍、心律失常、扩张型心肌病和心力衰竭等心脏毒副作用[3, 21]。已有临床研究表明,当DOX的使用剂量从400 mg/m2增加到700 mg/m2时,患者发生心脏毒性的概率从5%增加到48%[22]。即使没有明显的临床症状,仍有30%~35%的癌症患者心脏生物标志物明显增加[22]。如何防治DOX引起的心脏毒性减轻其毒副作用成为临床关注的重点。本研究发现,Hes可以通过激活SIRT1/NRF2信号改善DOX引起的心肌细胞损伤、细胞凋亡和氧化应激,这一结果提示Hes是临床上防治DOX引起的心脏毒性的潜在治疗药物。
有研究表明,Hes对DOX导致的小鼠心脏纤维化具有保护作用[13]。另外,Hes可以改善DOX诱导的大鼠心肌细胞氧化应激和DNA损伤[8]。众所周知,DOX导致心脏毒性的一个重要方面是引起心肌细胞不可逆性损伤并死亡,而心肌细胞是高度分化的终末细胞,无法进行细胞分裂,其基本丧失再生能力[23]。因此,心肌死亡后并不能通过细胞增殖弥补缺失的细胞数量。DOX处理H9c2细胞引起的细胞损伤和凋亡可以在体外条件下模拟心脏毒性作用[24]。本实验结果显示,DOX会导致H9c2细胞活力降低,培养基中LDH活性增加,细胞凋亡增多;Hes干预后能明显提高H9c2细胞活力,降低LDH活性和细胞凋亡。而EX527能阻断Hes对DOX引起的H9c2细胞损伤的改善作用。
Hes具有的抗炎、抗血小板聚集、抗氧化、舒张血管等药理学特性,在心血管系统中发挥着重要作用[11]。有研究表明,Hes可通过抑制心肌细胞凋亡,减轻氧化应激和炎症改善心肌细胞缺氧/复氧损伤[12]。然而,Hes是否能够通过减轻氧化应激改善DOX引起的心脏毒性还不清楚。作为转录因子,NRF2是胞内一种重要的抗氧化因子,通过调节下游抗氧化基因如HO-1和醌氧化还原酶的表达来抑制氧化应激[17]。激活的NRF2可以与ARE结合,启动抗氧化基因的转录过程。CAT和SOD是胞内重要的抗氧化酶,参与氧自由基的清除,而MDA是脂质过氧化的重要代谢产物,过多的累积会导致细胞毒性[10]。氧化应激是DOX诱导的心脏毒性的主要因素之一[6]。本实验结果表明,DOX能明显增加ROS和MDA含量,降低CAT和SOD活性,与此同时,NRF2和HO-1的表达也明显降低,这说明DOX加重H9c2细胞氧化应激损伤;而Hes可明显降低ROS和MDA含量,提高CAT和SOD活性,并增加NRF2和HO-1的表达,这些结果证明Hes在DOX引起的心肌细胞氧化应激损伤中发挥保护作用。
SIRT1是一类NAD+依赖的组蛋白去乙酰化酶,是细胞内重要的调节因子,在细胞代谢、凋亡、炎症反应和氧化应激等多种细胞生理或病理过程中发挥保护作用[14-15]。Hes能否通过SIRT1信号在DOX导致的心脏毒性中发挥保护作用目前还未见报道,其具体机制仍不清楚。在心血管系统中,SIRT1可以通过FOXOs、NRF2、核因子-κB、PGC1-α或p53等下游信号分子发挥作用[15, 25]。研究发现,白藜芦醇可上调SIRT1表达抑制DOX诱导的H9c2细胞损伤[26];另外,SIRT1在褪黑素抗DOX所致心肌细胞氧化应激损伤中发挥重要作用[27]。本实验结果显示,Hes能够上调DOX引起的SIRT1表达和活性抑制,降低Ac-FOXO1的表达,同时,增加NRF2和HO-1的表达;而EX527能阻断Hes对SIRT1及其底物Ac-FOXO1以及NRF2和HO-1表达的影响。这些结果说明Hes可通过激活SIRT1/NRF2信号降低氧化应激损伤改善DOX引起的H9c2细胞心脏毒性。
综上所述,本实验证明Hes在H9c2细胞心脏毒性模型中具有保护作用,并证实Hes可通过调控SIRT1/NRF2信号降低氧化应激损伤改善H9c2细胞心脏毒性。本实验为临床应用Hes防治抗肿瘤药物引起的心脏毒性提供了实验依据和理论支持。由于本实验只采用了H9c2细胞,在今后的研究中将增加动物实验,进一步明确Hes调控SIRT1、NRF2的表达与心肌毒性之间的直接关系。
* * *
作者贡献声明 李新华、闫爱丽和李汾负责调查研究,李新华和朱娟霞负责文章的构思,常晋瑞和李汾负责数据审编和正式分析,李新华和朱娟霞负责审读与编辑写作。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿,并同意对工作的所有方面负责。
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
陕西省教育厅专项科研计划项目(No. 20JK0883、No. 20JS135)资助
References
- 1.KARLSTAEDT A, MOSLEHI J, De BOER R A Cardio-onco-metabolism: metabolic remodelling in cardiovascular disease and cancer. Nat Rev Cardiol. 2022;19(6):414–425. doi: 10.1038/s41569-022-00698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SHANBHAG S, AMBINDERR F Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi: 10.3322/caac.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LI Y, LIN R, PENG X, et al The role of mitochondrial quality control in anthracycline-induced cardiotoxicity: from bench to bedside. Oxid Med Cell Longev. 2022;2022:3659278. doi: 10.1155/2022/3659278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EFENTAKIS P, VARELA A, CHAVDOULA E, et al Levosimendan prevents doxorubicin-induced cardiotoxicity in time- and dose-dependent manner: implications for inotropy. Cardiovasc Res. 2020;116(3):576–591. doi: 10.1093/cvr/cvz163. [DOI] [PubMed] [Google Scholar]
- 5.WENNINGMANN N, KNAPP M, ANDE A, et al Insights into doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Mol Pharmacol. 2019;96(2):219–232. doi: 10.1124/mol.119.115725. [DOI] [PubMed] [Google Scholar]
- 6.KONG C Y, GUO Z, SONG P, et al Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int J Biol Sci. 2022;18(2):760–770. doi: 10.7150/ijbs.65258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KAVIYARASI R, ABILASH V G, TIRUPATHI PICHIAH P B, et al Molecular mechanism of doxorubicin-induced cardiomyopathy--an update. Eur J Pharmacol. 2018;818:241–253. doi: 10.1016/j.ejphar.2017.10.043. [DOI] [PubMed] [Google Scholar]
- 8.ROOHBAKHSH A, PARHIZ H, SOLTANI F, et al Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 9.CHEN X, WEI W, LI Y, et al Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem Biol Interact. 2019;308:269–278. doi: 10.1016/j.cbi.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 10.LIU P, LI J, LIU M, et al Hesperetin modulates the Sirt1/Nrf2 signaling pathway in counteracting myocardial ischemia through suppression of oxidative stress, inflammation, and apoptosis. Biomed Pharmacother. 2021;139:111552. doi: 10.1016/j.biopha.2021.111552. [DOI] [PubMed] [Google Scholar]
- 11.PARHIZ H, ROOHBAKHSH A, SOLTANI F, et al Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 12.HE S, WANG X, ZHONG Y, et al Hesperetin post-treatment prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating PI3K/Akt signaling pathway. Biomed Pharmacother. 2017;91:1106–1112. doi: 10.1016/j.biopha.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 13.王敏, 孙祖玥 橙皮素对阿霉素心脏纤维化的保护作用. 中国医药指南. 2019;17(25):22–23. doi: 10.15912/j.cnki.gocm.2019.25.014. [DOI] [Google Scholar]
- 14.SINGH V, UBAID S Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43(5):1589–1598. doi: 10.1007/s10753-020-01242-9. [DOI] [PubMed] [Google Scholar]
- 15.XU Y, ZHANG Y, LIANG H, et al Coumestrol mitigates retinal cell inflammation, apoptosis, and oxidative stress in a rat model of diabetic retinopathy via activation of SIRT1. Aging (Albany NY) 2021;13(4):5342–5357. doi: 10.18632/aging.202467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KASAI S, SHIMIZU S, TATARA Y, et al Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;10(2):320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CADENAS S ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 18.LIU D, MA Z, DI S, et al AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic Biol Med. 2018;129:59–72. doi: 10.1016/j.freeradbiomed.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 19.VELUSAMY P, MOHAN T, RAVID B, et al Targeting the Nrf2/ARE signalling pathway to mitigate isoproterenol-induced cardiac hypertrophy: plausible role of hesperetin in redox homeostasis. Oxid Med Cell Longev. 2020;2020:9568278. doi: 10.1155/2020/9568278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WU Y Z, ZHAN G L, WU Z X, et al Berberine ameliorates doxorubicin-induced cardiotoxicity via a SIRT1/p66Shc-mediated pathway. Oxid Med Cell Longev. 2019;2019:2150394. doi: 10.1155/2019/2150394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.SHEIBANI M, AZIZI Y, SHAYAN M, et al Doxorubicin-induced cardiotoxicity: an overview on pre-clinical therapeutic approaches. Cardiovasc Toxicol. 2022;22(4):292–310. doi: 10.1007/s12012-022-09721-1. [DOI] [PubMed] [Google Scholar]
- 22.SCHIRONE L, D'AMBROSIO L, FORTE M, et al Mitochondria and doxorubicin-induced cardiomyopathy: a complex interplay. Cells. 2022;11(13):2000. doi: 10.3390/cells11132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NAKAMURA M, SADOSHIMA J Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 24.DALLONS M, ALPAN E, SCHEPKENS C, et al GPR91 receptor mediates protection against doxorubicin-induced cardiotoxicity without altering its anticancer efficacy. An in vitro study on H9C2 cardiomyoblasts and breast cancer-derived MCF-7 cells. Cells. 2020;9(10):2177. doi: 10.3390/cells9102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.TINKOVA A, NGUYENT T, SANTAMARIA A, et al Sirtuins as molecular targets, mediators, and protective agents in metal-induced toxicity. Arch Toxicol. 2021;95(7):2263–2278. doi: 10.1007/s00204-021-03048-6. [DOI] [PubMed] [Google Scholar]
- 26.夏洪娟, 王延鹏, 朱伟, 等 白藜芦醇通过上调SIRT1抑制阿霉素诱导的H9c2细胞损伤. 中国药理学通报. 2014;30(2):220–224. doi: 10.3969/j.issn.1001-1978.2014.02.017. [DOI] [Google Scholar]
- 27.楚瀚杰, 鲍家银 沉默信息调控因子1介导褪黑素抗阿霉素致心肌细胞氧化应激损伤的机制研究. 中国分子心脏病学杂志. 2022;20(3):3412–3416. doi: 10.16563/j.cnki.1671-6272.2020.06.015. [DOI] [Google Scholar]



