Abstract
目的
总25(OH)D〔total 25(OH)D, t-25(OH)D〕是传统评价机体维生素D(VitD)的标志物,它包括25(OH)D2和25(OH)D3及C3-差向异构体-25(OH)D3〔C3-epimers-25(OH)D3, C3-epi〕。本研究分析血清VitD代谢物与糖尿病肾脏疾病(diabetic kidney disease, DKD)肾功能损伤的关系。
方法
共计339例研究对象,其中表观健康人群(healthy control, HC)114例、无肾小球滤过功能障碍的2型糖尿病患者(diabetes mellitus, DM)74例和DKD患者151例。采用估算肾小球滤过率(estimated glomerular filtration rate, eGFR)与尿白蛋白/肌酐比值(urine albumin to creatinine ratio, UACR)联合评估方法将DKD组分为4个亚组:stage 2〔DM合并慢性肾脏病(chronic kidney disease, CKD)2期〕、stage 3(DM合并CKD3期)、stage 4(DM合并CKD4期)、stage 5(DM合并CKD5期)。使用超高效液相色谱-串联质谱法(ultra performance liquid chromatography-tandem mass spectrometry, UPLC-MS/MS)检测研究对象25(OH)D2、25(OH)D3以及C3-epi水平,并计算活性VitD3 〔the activity level of 25(OH)D3, AVitD3〕、t-25(OH)D浓度,25(OH)D2/25(OH)D3比值,C3-epi/t-25(OH)D比值,C3-epi/AVitD3比值。
结果
DKD组25(OH)D3、t-25(OH)D、AVitD3低于DM组和HC组(P均<0.05);DKD组C3-epi/t-25(OH)D比值、C3-epi/AVitD3比值高于HC组(P均<0.05)。stage 5亚组25(OH)D3、t-25(OH)D、AVitD3和C3-epi低于stage 2和stage 3亚组(P均<0.05);stage 4亚组25(OH)D3、t-25(OH)D、C3-epi低于stage 3亚组(P均<0.05);stage 4亚组25(OH)D3、t-25(OH)D、AVitD3水平低于stage 2亚组(P均<0.05)。
结论
UPLC-MS/MS法能用于准确评估DKD患者VitD营养状况。DKD患者血清t-25(OH)D、25(OH)D3、AVitD3水平降低,且均随着CKD分期增加而降低。C3-epi与25(OH)D3变化趋势并不一致。
Keywords: 维生素D, 糖尿病肾脏疾病, C3-差向异构体-25(OH)D3
Abstract
Objective
Total 25(OH)D (t-25[OH]D), a marker traditionally used in the assessment of vitamin D (VitD) in the human body, includes 25(OH)D2, 25(OH)D3, and C3-epimers-25(OH)D3 (C3-epi). In this study, we analyzed the relationship between serum VitD metabolites and renal impairment in patients with diabetic kidney disease (DKD).
Methods
We covered, in the study, 339 subjects, including 114 otherwise healthy controls (HC), 74 type 2 diabetes mellitus (DM) patients with no glomerular filtration dysfunction, and 151 DKD patients. According to the results of combined evaluation of estimated glomerular filtration rate (eGFR) and urine albumin-to-creatinine ratio (UACR), the DKD patients were further divided into four subgroups, stage 2 subgroup of patients of DM combined with stage-2 chronic kidney disease (CKD2), stage 3 subgroup of patients of DM combined with CKD3, stage 4 subgroup of patients of DM combined with CKD4, and stage 5 subgroup of patients of DM combined with CKD5. The levels of 25(OH)D2, 25(OH)D3, and C3-epi were measured by ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS), and the activity level of 25(OH)3 (AVitD3), t-25(OH)D concentration, 25(OH)D2/25(OH)D3 ratio, C3-epi/t-25(OH)D ratio, and C3-epi/AVitD3 ratio were calculated.
Results
The levels of 25(OH)D3, t-25(OH)D, and AVitD3 were lower in the DKD group than those in the DM and HC groups (all P<0.05). C3-epi/t-25(OH)D ratio and C3-epi/AVitD3 ratio were higher in the DKD group than those in the HC group (all P<0.05). The levels of 25(OH)D3, t-25(OH)D, AVitD3, and C3-epi were lower in the stage 5 subgroup than those in the stage 2 and stage 3 subgroups (all P<0.05). The levels of 25(OH)D3, t-25(OH)D, and C3-epi were lower in the stage 4 subgroup than those in the stage 3 subgroup (all P<0.05). The 25(OH)D3, t-25(OH)D, and AVitD3 levels were lower in the stage 4 subgroup than those in the stage 2 subgroup (all P<0.05).
Conclusions
UPLC-MS/MS can be used to perform accurate evaluation of VitD nutritional status in DKD patients. DKD patients have decreased levels of serum t-25(OH)D, 25(OH)D3, and AVitD3, all of which progressively decrease along with the rise in CKD staging. The trend of C3-epi and 25(OH)D3 changes were not consistent.
Keywords: Vitamin D, Diabetic kidney disease, C3-epimer-25(OH)D3
糖尿病肾脏疾病(diabetic kidney disease, DKD)作为最常见的糖尿病(diabetes mellitus, DM)微血管病并发症,在DM人群中的发病率高达20%~40%,也是引起终末期肾病(end-stage kidney disease, ESKD)的主要病因[1]。近年来研究发现,维生素D(vitmin D, VitD)在机体内除了调节钙磷代谢外,还与心血管疾病[2]、癌症[3]、自身免疫性疾病[4]和慢性肾脏疾病[5-6]等许多非骨骼性疾病的发生发展密切相关。已有研究证实,慢性肾脏病(chronic kidney disease, CKD)患者普遍存在VitD缺乏或不足[7-8],且其肾功能损伤的进展与VitD缺乏的程度相关[9]。同时研究[10]表明VitD及其类似物通过抵抗肾脏炎性介质的表达和释放可以发挥肾脏保护作用。
VitD是一种类固醇激素,已发现50多种结构相似的VitD代谢物[11]。其中总25(OH)D〔total 25(OH)D, t-25(OH)D〕含量最高,是传统评价机体VitD的标志物,它包括25(OH)D2和25(OH)D3及C3-差向异构体-25(OH)D3〔C3-epimers-25(OH)D3, C3-epi〕。C3-epi无生物活性,25(OH)D3活性是25(OH)D2活性的2~3倍[12]。因此,为了更准确地反映受试者体内VitD贮存,一些研究者[9]去除C3-epi后将25(OH)D2折算为25(OH)D3,计算生物可利用VitD,即活性AVitD3,AVitD3值为25(OH)D2测值的 1/3与25(OH)D3测值的总和。
目前绝大多数临床实验室使用免疫学方法检测血清中t-25(OH)D该法虽简便快速,但无法检测样本中不同VitD代谢物水平,导致不同国家和/或地区判断VitD贮存的标准不相一致[13]。随着检测技术的发展 UPLC-MS/MS技术成为国际上公认的VitD检测“金标准”[14],该方法可检测受试者体内不同的VitD代谢物水平,因此能够准确评估人体内VitD的营养状况以及疾病状态下VitD代谢物的变化情况(如C3-epi水平)。本实验用UPLC-MS/MS技术检测受试者VitD代谢物组分,精确评估其体内VitD贮存状况,以及探究肾功能指标对不同VitD代谢物组分的影响,为DKD患者血清VitD代谢物与肾功能损伤可能存在的关系提供实验室检测依据。
1. 对象与方法
1.1. 研究对象
回顾性收集2020年12月–2022年2月成都市第二人民医院就诊的DKD患者,男性89例、女性62例,年龄范围30~90(66.0±11.9)岁;无肾小球滤过功能障碍的DM患者,男性42例、女性32例,年龄范围33~89(62.4±12.1)岁。纳入同期来我院体检血糖、肝功能、肾功能指标正常的健康对照(HC组),男性71例、女性43例,年龄范围23~80(62.9±13.8)岁。纳入标准:DKD组患者符合美国糖尿病协会(American Diabetes Association, ADA)关于DM之微血管并发症诊断标准[15]和改善全球肾脏疾病预后指南(Kidney disease: Improving Global Outcomes, KDIGO)[16]标准,估算肾小球滤过率(estimated glomerular filtration rate, eGFR)低于60 mL/(min·1.73 m2)和/或有肾功能损伤伴UACR≥30 mg/g;据KDIGO指南[16],采用eGFR与UACR联合评估方法将DKD患者分为4个亚组:Stage 2亚组,即DM合并CKD2期〔eGFR取值60~89 mL/(min·1.73 m2)且UACR≥30 mg/g〕;Stage 3亚组,即DM合并CKD3期〔eGFR取值30~59 mL/(min·1.73 m2)〕;Stage 4亚组,即DM合并CKD4期〔eGFR取值15~29 mL/(min·1.73 m2)〕;Stage 5亚组,即DM合并CKD5期〔eGFR低于15 mL/(min·1.73 m2)〕。无肾小球滤过功能障碍的DM组患者符合ADA关于DM诊断标准,其eGFR高于60 mL/(min·1.73 m2)且UACR<30 mg/g。排除标准:DM病史不足5年者、非DM所致肾脏疾病者,以及采血前1周内有生长抑素类药物或血管活性药物或影响VitD水平的药物服用史以及接受钙补充、维生素D补充治疗者。本研究经成都市第二人民医院医学伦理委员会审查批准,批准号2020193。
1.2. 样本采集
血液样本:受试者禁食过夜,于清晨(8:00~10:00)用2支含分离胶/纤维蛋白酶促凝剂的静脉采血管和用1支含EDTA-K2抗凝剂的静脉采血管(CDRICH®,成都)采集受试者空腹静脉血共3支,采血量至刻度。含促凝剂的血液样本,静置30 min后,以3000 r/min离心10 min,分离出血清。一支于2 h内完成尿素(urea)、肌酐(creatinine, Crea)、尿酸(uric acid, UA)、胱抑素C(cystatin C, CysC)、空腹血糖(fasting plasma glucose, FPG)、白蛋白(albumin, Alb)检测;另一支用于VitD代谢物检测,当不能立即检测时,将血清用移液器吸入1.5 mL洁净离心管中,在−80 ℃冰箱冻存,2 d内完成检测。EDTA-K2抗凝样本用于糖化血红蛋白(glycosylated hemoglobin A1c, HbA1c)测定,2 h内完成。
尿液样本:嘱受试者用一次性无菌尿杯,于血液样本采集完成后30 min内留取清洁中段尿约10.0 mL,将尿液转移至一次性离心管,以3000 r/min离心5 min,上清液用于尿肌酐和尿白蛋白(urine albumin,uAlb)检测。留尿后2 h内完成测定。
1.3. 样本检测
VitD代谢物:将受试者样本使用JasperTM HPLC(岛津,日本)液相串联AB SCIEX Triple QuadTM 4500MD(ABI,美国)质谱仪进行检测。采用配套软件Analyst®MD软件(版本号:1.6.3)和MultiquantTM MD软件(版本号:3.0.2)对色谱图的输出以及相应的数据进行处理。
生化指标:Urea用尿素酶-谷氨酸脱氢酶法、Crea用肌氨酸氧化酶法、CysC用胶乳免疫比浊法、UA用尿酸酶法、uAlb用改良透射免疫比浊法、Alb用溴甲酚绿法和FPG用己糖激酶法检测,所有项目均采用LABOSPECT 008AS型全自动生化分析仪(日立,日本)进行测定,试剂盒均购自四川迈克生物科技股份有限公司。HbA1c用高效液相色谱法,用HA-8180自动糖化血红蛋白分析仪(爱科来,日本)检测。eGFR的计算方法采用Creatinine-Cystatin C联合方程(CKD-EPI 2012)[17]。
1.4. VitD营养状况评估方法
依据慢性肾脏疾病骨代谢和疾病临床实践(K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease)[18]指南将t-25(OH)D<15 ng/mL划分为缺乏,15~30 ng/mL划分为不足,>30 ng/mL划分为充足;t-25(OH)D值为25(OH)D3与25(OH)D2测值的总和。
1.5. 统计学方法
连续正态分布变量用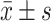 表示。多组间比较采用单因素方差分析,进一步两两比较用LSD-t检验。偏态分布变量用中位数(P25, P75)表示。多组间比较采用多个独立样本Kruskal-Wallis非参数检验,组间两两比较采用Bonferroni法,并采用校正后的P值来确定是否有显著性差异。VitD代谢物在各组间比较时采用协方差分析,以校正年龄、性别、糖尿病病程、血清白蛋白和血糖的影响。计数资料以例数和百分数表示,组间比较采用χ2检验。采用Spearman相关性分析,分析所有DKD患者血清VitD代谢物水平与肾功能指标间的关系。P<0.05为差异有统计学意义。
表示。多组间比较采用单因素方差分析,进一步两两比较用LSD-t检验。偏态分布变量用中位数(P25, P75)表示。多组间比较采用多个独立样本Kruskal-Wallis非参数检验,组间两两比较采用Bonferroni法,并采用校正后的P值来确定是否有显著性差异。VitD代谢物在各组间比较时采用协方差分析,以校正年龄、性别、糖尿病病程、血清白蛋白和血糖的影响。计数资料以例数和百分数表示,组间比较采用χ2检验。采用Spearman相关性分析,分析所有DKD患者血清VitD代谢物水平与肾功能指标间的关系。P<0.05为差异有统计学意义。
2. 结果
2.1. 受试者人口学特征及实验室观察指标
见表1,正态性检验结果显示,除年龄外,受试者所观察的实验室指标均呈偏态分布。HC组、DM组和DKD组的性别比、年龄、VitD营养状况分类比率差异无统计学意义。本次纳入的研究对象VitD营养状况普遍偏低,DKD组中36.5%研究对象VitD缺乏,仅有9.9%充足。
表 1. General information and laboratory observation indicators of the subjects.
受试者的一般资料和实验室观察指标
| Indicator | HC group (n=114) | DM group (n=74) | DKD group (n=151) | P |
| HbA1c: glycosylated hemoglobin A1c; FPG: fasting plasma glucose; Alb: albumin; C3-epi: C3-epimers-25(OH)D3; t-25(OH)D: total 25(OH)D; AVitD3: the activity level of 25(OH)D3; CysC: cystatin C; eGFR: estimated glomerular filtration rate; UACR: urine albumin to creatinine ratio; Crea: creatinine; UA: uric acid. a,a* P<0.05, vs. HC group; b,b* P<0.05, vs. DM group. a*, b* are P values after adjustment for age, sex, serum albumin, and glucose. | ||||
| (Male/female)/case | 71/43 | 42/32 | 89/62 | 0.684 |
Age/yr., 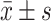
|
62.9±13.8 | 62.4±12.1 | 66.0±11.9 | 0.057 |
| HbA1c/%, median (P25, P75) | 5.5 (5.3, 5.7) | 7.40 (6.50, 8.80)a | 7.50 (6.50, 9.40)a | <0.001 |
| FPG/(mmol/L), median (P25, P75) | 5.20 (4.91, 5.55) | 7.76 (6.05, 9.70)a | 7.83 (6.00, 10.10)a | <0.001 |
| Alb/(g/L), median (P25, P75) | 41.00 (39.00, 43.00) | 41.00 (39.10, 43.50) | 40.50 (36.00, 44.30) | 0.369 |
| C3-epi/(ng/mL), median (P25, P75) | 1.32 (1.05, 1.90) | 1.56 (1.03, 2.03) | 1.32 (0.97, 1.77) | 0.195 |
| 25(OH)D2/(ng/mL), median (P25, P75) | 0.95 (0.62, 1.67) | 0.92 (0.55, 1.24) | 0.82 (0.52, 1.45) | 0.390 |
| 25(OH)D3/(ng/mL), median (P25, P75) | 20.28 (14.76, 23.69) | 19.85 (13.82, 25.30) | 16.91 (12.00, 22.34)a*, b* | 0.001 |
| t-25(OH)D/(ng/mL), median (P25, P75) | 21.26 (16.04, 25.20) | 21.81 (14.67, 26.43) | 18.35 (13.04, 23.65)a, b | 0.008 |
| t-25(OH)D states | 0.056 | |||
| Deficiency | 20.4% (23/114) | 26.7% (20/74) | 36.5% (55/151) | |
| Insufficiency | 68.1% (78/114) | 58.7% (43/74) | 53.6% (81/151) | |
| Sufficiency | 11.5% (13/114) | 14.6% (11/74) | 9.9% (15/151) | |
| 25(OH)D2/25(OH)D3 (median [P25, P75]) | 0.06 (0.04, 0.08) | 0.04 (0.03, 0.07) | 0.05 (0.03, 0.11) | 0.121 |
| AVitD3/(ng/mL), median (P25, P75) | 20.68 (15.35, 24.14) | 21.24 (13.98, 25.55) | 17.21 (12.35, 22.56)a, b | 0.002 |
| C3-epi/t-25(OH)D (median [P25, P75]) | 0.07 (0.06, 0.08) | 0.08 (0.06, 0.09) | 0.08 (0.06, 0.10)a | 0.012 |
| C3-epi/AVitD3 (median [P25, P75]) | 0.07 (0.06, 0.09) | 0.08 (0.07, 0.09) | 0.08 (0.07, 0.10)a | 0.007 |
| CysC/(mg/L), median (P25, P75) | 0.81 (0.71, 0.94) | 0.93 (0.76, 1.05) | 1.38 (1.09, 2.13)a, b | <0.001 |
| eGFR/(mL/[min·1.73m2]), median (P25, P75) | 96.00 (83.00, 109.00) | 84.00 (75.00, 102.00) | 58.00 (38.00, 72.00)a, b | <0.001 |
| UACR/(mg/g), median (P25, P75) | 6.90 (3.80, 14.55) | 6.50 (3.70, 11.90) | 126.00 (44.60, 901.40)a, b | <0.001 |
| Urea/(mmol/L), median (P25, P75) | 4.60 (3.65, 5.50) | 5.60 (4.70, 6.71)a, | 7.40 (5.58, 11.40)a, b | <0.001 |
| Crea/(μmol/L), median (P25, P75) | 61.30 (53.40, 74.00) | 62.00 (51.00, 77.00) | 94.60 (66.00, 151.0)a, b | <0.001 |
| UA/(μmol/L), median (P25, P75) | 324.00 (257.00, 361.50) | 311.00 (244.00, 386.00) | 358.00 (294.25, 445.0)a, b | <0.001 |
糖代谢指标方面,DKD组和DM组HbA1c和FPG均高于HC组,差异有统计学意义(P均<0.001)。
肾功能指标方面,DKD组CysC、UACR、Urea、Crea、UA均高于DM组和HC组(P均<0.001),DKD组eGFR(P<0.001)低于DM组和HC组,差异有统计学意义。
DKD组在25(OH)D3、t-25(OH)D、AVitD3均低于DM组和HC组(P均<0.05),DKD组的C3-epi/t-25(OH)D比值和C3-epi/AVitD3比值高于HC组(P均<0.05),差异有统计学意义;3个组在C3-epi、25(OH)D2、25(OH)D2/25(OH)D3比值均差异无统计学意义。
2.2. DKD不同亚组间实验室观察指标
见表2、图1。DKD亚组间血清25(OH)D3水平差异有统计学意义(P<0.05)。根据年龄、性别、糖尿病病程、血清白蛋白、血糖进行校正后两两比较,stage 5亚组25(OH)D3水平低于stage 2和stage 3亚组(P均<0.05);stage 4亚组25(OH)D3水平低于stage 2和stage 3亚组(P均<0.05)。
表 2. Laboratory observation indicators of subgroups of different stages of DKD.
DKD各亚组间实验室观察指标
| Indicator | Stage 2 (n=60) | Stage 3 (n=61) | Stage 4 (n=18) | Stage 5 (n=12) | P |
| The abbreviations are explained in the note to Table 1. a P<0.05, a* P<0.05, vs. stage 2; b P<0.05, b* P<0.05, vs. stage 3. a*, b* are P values after adjustment for age, sex, duration of diabetes, serum albumin, and glucose. | |||||
| (Male/female)/case | 31/29 | 34/27 | 14/4 | 9/3 | 0.143 |
Age/yr., 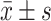
|
63.23±10.78 | 66.71±13.41 | 70.16±11.53 | 69.83±5.52 | 0.073 |
| Course of disease/year, median (P25, P75) | 9.50 (6.00, 15.00) | 10.00 (9.00, 15.50) | 15.00 (10.00, 17.00) | 16.00 (10.00, 20.00)a | 0.004 |
| HbA1c/%, median (P25, P75) | 8.35 (6.93, 10.8) | 7.30 (6.30, 9.15) | 6, 45 (5.78, 8.38)a | 8.35 (6.73, 10.00) | 0.004 |
| FPG/(mmol/L), median (P25, P75) | 7.92 (6.23, 10.48) | 7.60 (5.95, 9.97) | 6.27 (5.77, 8.32) | 10.54 (7.16, 15.20) | 0.070 |
| Alb/(g/L), median (P25, P75) | 42.30 (38.48, 45.00) | 40.00 (35.00, 44.00) | 38.10 (33.00, 42.90) | 36.65 (31.90, 40.53)a | 0.008 |
| C3-epi/(ng/mL), median (P25, P75) | 1.40 (1.10, 1.78) | 1.44 (1.08, 2.06) | 1.04 (0.92, 1.35)b* | 0.85 (0.69, 1.19)a*, b* | 0.001 |
| 25(OH)D2/(ng/mL), median (P25, P75) | 0.83 (0.56, 1.37) | 0.96 (0.53, 2.09) | 0.68 (0.48, 1.01) | 0.81 (0.38, 1.19) | 0.275 |
| 25(OH)D3/(ng/mL), median (P25, P75) | 18.22 (13.20, 22.68) | 17.79 (11.87, 23.50) | 12.87 (10.09, 16.27)a*, b* | 10.64 (7.30, 15.63)a*, b* | 0.001 |
| t-25(OH)D/(ng/mL), median (P25, P75) | 19.36 (15.08, 23.65) | 19.44 (13.17, 26.59) | 13.60 (10.56, 17.08)a, b | 11.84 (7.92, 16.77)a, b | <0.001 |
| 25(OH)D2/25(OH)D3 (median [P25, P75]) | 0.05 (0.03, 0.88) | 0.07 (0.03, 0.16) | 0.55 (0.04, 0.85) | 0.65 (0.05, 0.10) | 0.628 |
| C3-epi/t-25(OH)D (median [P25, P75]) | 0.08 (0.06, 0.09) | 0.07 (0.06, 0.10) | 0.09 (0.07, 0.10) | 0.06 (0.05, 0.09) | 0.701 |
| C3-epi/AVitD3 (median [P25, P75]) | 0.08 (0.07, 0.10) | 0.08 (0.07, 0.10) | 0.09 (0.08, 0.11) | 0.09 (0.63, 0.11) | 0.641 |
| AVitD3/(ng/mL), median (P25, P75) | 18.72 (13.61, 22.91) | 18.21 (12.35, 24.70) | 13.13 (10.24, 16.50)a | 11.13 (7.51, 15.98)a, b | <0.001 |
图 1.

Comparison of VitD metabolites levels between subgroups of different stages of DKD
DKD各亚组间VitD代谢物水平比较
* P<0.05, ** P<0.01.
DKD各亚组间血清C3-epi水平差异有统计学意义(P<0.05)。根据年龄、性别、糖尿病病程、血清白蛋白、血糖进行校正后两两比较,stage 5亚组的C3-epi水平低于stage 2和stage 3亚组(P均<0.05);stage 4亚组的C3-epi水平低于stage 3亚组(P<0.05)。
DKD各亚组间血清AVitD3、t-25(OH)D水平差异有统计学意义(P<0.05)。stage 5亚组的AVitD3、t-25(OH)D水平低于stage 2和stage 3亚组(P均<0.05);stage 4亚组的AVitD3水平低于stage 2亚组(P<0.05),t-25(OH)D水平低于stage 2和stage 3亚组(P均<0.05)。
DKD各亚组间血清25(OH)D2水平、25(OH)D2/25(OH)D3比值、C3-epi/t-25(OH)D比值和C3-epi/AVitD3比值均差异无统计学意义。
2.3. DKD患者血清VitD代谢物与观察指标间的关系
见表3。DKD患者血清25(OH)D2水平除了与UACR负相关(P<0.05)外,与其他肾功能指标不相关;25(OH)D2/25(OH)D3比值与所有肾功能指标不相关;t-25(OH)D、25(OH)D3、AVitD3和C3-epi水平与肾功能指标(Urea、Crea、CysC、UACR)呈负相关性,与eGFR呈正相关性(均P<0.05),与UA均不相关。
表 3. Correlation between serum VitD metabolites and observational indicators in DKD patients.
DKD患者血清VitD代谢物与观察指标间的关系
| Indicator | C3-epi | 25(OH)D2 | 25(OH)D3 | t-25(OH)D | 25(OH)D2/25(OH)D3 | AVitD3 | |||||||||||
| r | P | r | P | r | P | r | P | r | P | r | P | ||||||
| The units of each indicator are as follows, 25(OH)D3, t-25(OH)D, 25(OH)D2, C3-epi, and AVitD3: ng/mL; CysC: mg/L; eGFR: mL/(min·1.73 m2); UACR: mg/g; Urea: mmol/L; Crea: μmol/L; UA: μmol/L. | |||||||||||||||||
| CysC | −0.206 | 0.011 | −0.058 | 0.476 | −0.239 | 0.003 | −0.237 | 0.003 | 0.098 | 0.232 | −0.238 | 0.003 | |||||
| eGFR | 0.229 | 0.005 | 0.063 | 0.439 | 0.275 | 0.001 | 0.273 | 0.001 | −0.110 | 0.180 | 0.275 | 0.001 | |||||
| UACR | −0.283 | <0.001 | −0.222 | 0.006 | −0.285 | <0.001 | −0.330 | <0.001 | −0.057 | 0.487 | −0.300 | <0.001 | |||||
| Urea | −0.210 | 0.010 | −0.089 | 0.275 | −0.182 | 0.026 | −0.197 | 0.015 | 0.049 | 0.552 | −0.192 | 0.018 | |||||
| Crea | −0.218 | 0.007 | −0.052 | 0.523 | −0.225 | 0.006 | −0.223 | 0.006 | 0.090 | 0.269 | −0.221 | 0.006 | |||||
| UA | −0.014 | 0.869 | −0.103 | 0.207 | −0.064 | 0.435 | −0.108 | 0.188 | −0.030 | 0.713 | −0.085 | 0.299 | |||||
3. 讨论
肾脏是参与合成VitD激素代谢物的主要器官,VitD缺乏是慢性肾脏病的一个共同特征。本研究采用UPLC-MS/MS技术检测DKD患者体内25(OH)D3、25(OH)D2和C3-epi水平,并与HC组和DM组比较,发现DKD组的25(OH)D3水平低于对照两组,C3-epi水平和25(OH)D2水平三组间无明显差异。同时本研究发现C3-epi/t-25(OH)D比值、C3-epi/AVitD3比值方面,DKD组均高于HC组,提示DKD患者体内可能会发生代偿性的C3-epi合成,DKD患者VitD代谢物的比值可能是一个很重要的观察指标。本研究推断C3-epi/t-25(OH)D比值、C3-epi/AVitD3比值可能是预测DKD发生的指标。C3-epi不仅几乎没有生物活性或活性较低而且不同年龄和患病状态下人体内C3-epi浓度差异也较大。目前对婴幼儿体内C3-epi研究较多,一些研究人员观察到新生儿或婴儿(<1岁)中C3-epi的比例高于成人,大约占VitD的8.7%~61.1%[19],随着婴儿年龄的增长,他们的差向异构体水平开始下降,并在整个成年生活中大致保持不变。本研究纳入的DKD患者C3-epi/AVitD3比值中位数0.08,最高达0.19。目前C3-epi与DKD之间仍然没有明确的因果关系,因此C3-epi水平对DKD的潜在影响机制需要进一步阐明。
本研究通过比较DKD各亚组患者的VitD代谢物水平以及与肾功能生化指标的相关关系推断,25(OH)D3水平可能与DKD患者肾功能水平甚至疾病的进展密切相关。DKD患者25(OH)D3血清水平降低可能有两方面原因:首先,随着患者肾纤维化,肾近端管状细胞中1α-羟化酶肾内数量和活性均降低,导致25(OH)D3体内转化减少。研究[20]发现随着肾小球滤过率下降,CKD患者往往伴随着贫血、高钾血症、代谢性酸中毒和矿物质骨疾病等严重并发症的发生。早期患者循环中升高的成纤维细胞生长因子(FGF-23)可能靶向残余肾单位,FGF-23降低血磷水平的同时会抑制肾小管1α-羟化酶活性从而影响VitD的羟基化[21]。其次,还可能与蛋白尿损失有关系。研究[19]发现,在循环中约85%~90%的VitD与特殊的载体即维生素D结合蛋白(vitamin D-binding protein, DBP)紧密结合,尿液中VitD-DBP复合物的损失可能与肾小管的损伤有关。
t-25(OH)D在血液循环中含量最高,半衰期较长,常常被用来判断人体VitD营养状态的指标。本研究采用UPLC-MS/MS技术消除了通常的免疫方法不能排除的异构体干扰,精确检测了t-25(OH)D值。研究[22]表明阳光照射、气候、地理位置以及包括年龄、生活方式和皮肤色素沉着等一般因素都会影响体内VitD的生成。本研究发现DKD组t-25(OH)D值小于15 ng/mL即VitD缺乏的比例高达36.5%,同时还发现本研究地区(成都)除了DKD人群以外,健康人群VitD缺乏的比例也较高。
然而,本研究仍有一定的局限性。VitD的合成受饮食、季节等外在影响较大,本次研究仅仅是成都地区的水平,可能结果有片面性和地域差异。另外由于缺乏远期随访以及个体间的VitD易受肝功能、肾功能、性类固醇等激素以及遗传背景等多种混杂因素的影响,本次研究的VitD代谢物水平的临床价值有一定的局限性,需要后续进行长期、大样本、多中心的临床评估。
总之,本研究采用UPLC-MS/MS检测25(OH)D2和25(OH)D3和C3-epi水平,准确评价了DKD患者体内VitD的存储状态,还分析了不同的代谢物以及他们的比值与DKD患者肾功能的关系。本研究结果显示,25(OH)D3缺乏在DKD患者中很常见,并且不同VitD代谢物与DKD患者肾脏疾病进展有较高风险的独立相关。
* * *
作者贡献声明 杨文玉和朱艳负责数据审编,夏芹芹和俸家富负责论文构思、正式分析、研究方法、可视化、初稿写作和审读与编辑写作,俸家富负责经费获取、提供资源和监督指导,夏芹芹、杨文玉、朱艳负责调查研究和研究项目管理。所有作者已经同意将文章提交给本刊,且对将要发表的版本进行最终定稿并同意对工作的所有方面负责。
利益冲突 所有作者均声明不存在利益冲突
Funding Statement
四川省科学技术厅计划项目(No. 2021YJ0239)资助
Contributor Information
芹芹 夏 (Qinqin XIA), Email: 119944046@qq.com.
家富 俸 (Jiafu FENG), Email: jiafufengacad@foxmail.com.
References
- 1.PERSSON F, ROSSING P Diagnosis of diabetic kidney disease: state of the art and future perspective. Kidney Int Suppl (2011) 2018;8(1):2–7. doi: 10.1016/j.kisu.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.KAUL S, MANIKINDA J Role of vitamin D in cerebrovascular disease. Ann Indian Acad Neurol. 2021;24(2):142–145. doi: 10.4103/aian.AIAN_928_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CARLBERG C, MUÑOZ A An update on vitamin D signaling and cancer. Semin Cancer Biol. 2022;79:217–230. doi: 10.1016/j.semcancer.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 4.ISMAILOVA A, WHITE J H Vitamin D, infections and immunity. Rev Endocr Metab Disord. 2022;23(2):265–277. doi: 10.1007/s11154-021-09679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.冯世栋, 田秀娟, 王妍, 等 维生素D对终末期肾病患者心血管保护研究进展. 新医学. 2018;49(12):863–867. doi: 10.3969/j.issn.0253-9802.2018.12.004. [DOI] [Google Scholar]
- 6.XIE S, HUANG L, CAO W, et al Association between serum 25-hydroxyvitamin D and diabetic kidney disease in Chinese patients with type 2 diabetes. PLoS One. 2019;14(4):e0214728. doi: 10.1371/journal.pone.0214728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PILZ S, IODICE S, ZITTERMANN A, et al Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58(3):374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 8.WETMORE J B, KIMBER C, MAHNKEN J D, et al Cholecalciferol v.ergocalciferol for 25-hydroxyvitamin D (25(OH)D) repletion in chronic kidney disease: a randomized clinical trial. Br J Nutr. 2016;116(12):2074–2081. doi: 10.1017/S000711451600427X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LI Y M, FENG Q, JIANG W Q, et al Evaluation of vitamin D storage in patients with chronic kidney disease: detection of serum vitamin D metabolites using high performance liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol. 2021;210:105860. doi: 10.1016/j.jsbmb.2021.105860. [DOI] [PubMed] [Google Scholar]
- 10.AGARWAL R Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol. 2009;4(9):1523–1528. doi: 10.2215/CJN.02010309. [DOI] [PubMed] [Google Scholar]
- 11.MÁČOVÁ L, BIČÍKOVÁ M Vitamin D: current challenges between the laboratory and clinical practice. Nutrients. 2021;13(6):1758. doi: 10.3390/nu13061758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.HEANEY R P, RECKER R R, GROTE J, et al Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011;96(3):E447–E452. doi: 10.1210/jc.2010-2230. [DOI] [PubMed] [Google Scholar]
- 13.STOKES C S, LAMMERT F, VOLMER D A Analytical methods for quantification of vitamin D and implications for research and clinical practice. Anticancer Res. 2018;38(2):1137–1144. doi: 10.21873/anticanres.12332. [DOI] [PubMed] [Google Scholar]
- 14.VOLMER D A, MENDES L R, STOKES C S Analysis of vitamin D metabolic markers by mass spectrometry: current techniques, limitations of the "gold standard" method, and anticipated future directions. Mass Spectrom Rev. 2015;34(1):2–23. doi: 10.1002/mas.21408. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Microvascular complications and foot care: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S135–S151. doi: 10.2337/dc20-S011. [DOI] [PubMed] [Google Scholar]
- 16.STEVENS P E, LEVIN A Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 17.INKER L A, SCHMID C H, TIGHIOUART H, et al Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.GARABED E, ADEERA L, NATHAN W L K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):1–201. doi: 10.1016/S0272-6386(03)00905-3. [DOI] [PubMed] [Google Scholar]
- 19.Al-ZOHILY B, Al-MENHALI A, GARIBALLA S, et al Epimers of vitamin D: a review. Int J Mol Sci. 2020;21(2):470. doi: 10.3390/ijms21020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HU L, NAPOLETANO A, PROVENZANO M, et al Mineral bone disorders in kidney disease patients: the ever-current topic. Int J Mol Sci. 2022;23(20):12223. doi: 10.3390/ijms232012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LARSSON T, NISBETH U, LJUNGGREN O, et al Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 22.李铮, 韩睿 从纬度差异到分子机制: 维生素D与糖尿病. 重庆医科大学学报. 2018;43(6):808–813. doi: 10.13406/j.cnki.cyxb.001531. [DOI] [Google Scholar]


