Abstract
Summary
Proton pump inhibitors (PPIs) are associated with increased risk of osteoporotic fracture; however, the mechanism is unclear. PPI users taking calcium supplements were more likely to have hyperparathyroidism compared to non-users (OR 1.56, CI 1.08–2.23, p = 0.018). This highlights the importance of monitoring PPI use, especially in older adults.
Purpose
Proton pump inhibitors (PPIs) are associated with increased risk of osteoporotic fracture. Hyperparathyroidism may be implicated, but few studies have considered this relationship. This study evaluated the relationship between PPI use and hyperparathyroidism in older adults.
Methods
Participants were from the TUDA study, a large cross-sectional cohort of older Irish adults. Participants with an estimated glomerular filtration rate (eGFR) < 30 ml/min and serum calcium > 2.5 mmol/l were excluded to avoid hyperparathyroidism due to chronic renal disease and primary hyperparathyroidism. Hyperparathyroidism was defined as a parathyroid hormone (PTH) > 65 pg/ml. Multivariate regression models were used to analyse the relationship between PPI use and hyperparathyroidism.
Results
A total of 4139 participants met the inclusion criteria, of whom 37.8% (n = 1563) were taking PPI medication. PPI use was identified in 41.4% of calcium supplement users and 35.4% of non-calcium supplement users. Overall, compared to non-users of PPIs, those taking PPIs were older (74.8 vs 72.9 years, p < 0.001) and had a higher prevalence of hyperparathyroidism (17.8 vs 11.0%, p < 0.001). In those taking calcium supplements (but not in non-users), PPI use was significantly associated with hyperparathyroidism (OR 1.56, CI 1.08–2.23, p = 0.018) after adjusting for age, sex, body mass index, serum vitamin D, eGFR, timed-up-and-go, dairy intake, medications, and comorbidities.
Discussion
The results are consistent with the hypothesis of PPIs reducing calcium absorption, leading to a rise in PTH which could mediate increased fracture risk. No relationship of PPI use with hyperparathyroidism was observed in non-users of calcium supplements, possibly owing to lower dietary calcium intake. These results highlight the importance of monitoring PPI use, especially in older adults at risk of fracture.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-023-06867-8.
Keywords: Calcium absorption, Hyperparathyroidism, Osteoporosis, Proton pump inhibitor
Introduction
In recent years, greater attention has been paid to the potential adverse effects of prolonged use of proton pump inhibitors (PPIs) [1]. Across the world, PPIs are among the most commonly prescribed medications, as well as being available over the counter [2]. They are used to suppress gastric acid, but frequently, they are prescribed for dubious indications and are often continued for longer than necessary. PPI use is especially common in older adults and contributes to inappropriate polypharmacy [3].
Osteoporosis is a major health concern in the ageing world population because of its enormous impact on morbidity, mortality, and health economics [4]. Hip fractures are associated with a 22% mortality at 1 year [5]. Approximately 1 in 2 women and 1 in 5 men aged 50 years or older will experience an osteoporotic fracture in their remaining lifetime. The incidence of hip fracture rises exponentially with age, particularly among women 60 years or older and men 70 years or older [6]. The link between PPIs and fracture risk has been clearly described [7–13]. A meta-analysis of 33 studies pooling data from 2,714,502 individuals found a 28% increased risk of fracture in PPI users compared to non-users [14]. However, there is conflicting evidence on the relationship between PPIs and bone mineral density (BMD) [15–19].
Parathyroid hormone (PTH) plays a pivotal role in calcium homeostasis and bone metabolism. PTH maintains serum calcium concentration by releasing calcium from the skeleton through bone resorption, renal tubular calcium reabsorption, and activating vitamin D. PTH has major effects on bone turnover and remodelling [20]. Hyperparathyroidism causes excessive bone remodelling, with bone resorption outpacing formation leading to lower BMD, altered microarchitecture and increased fracture risk [21].
The mechanisms by which PPIs contribute to fracture risk are currently unknown, but a number of ideas have been postulated including inhibition of gastric acid secretion and reduced absorption of calcium and other nutrients (e.g., B vitamins) which may be important for bone health and hypergastrinaemia [8, 9, 22]. However, only a few small studies have previously explored the relationship between PPIs and serum PTH [23–25]. Therefore, the aim of this study was to evaluate the potential relationship between PPIs use and hyperparathyroidism in older adults. It was hypothesised that older adults treated with PPI drugs would be more likely to have hyperparathyroidism than non-PPI users.
Methodology
Study population and design
This study involved the analysis of data from the Trinity-Ulster-Department of Agriculture (TUDA) study (clinicaltrials.gov identifier: NCT02664584)—a large cross-sectional study of 5186 community-dwelling older Irish adults. A detailed description of the study population and recruitment has been previously described [26, 27]. Briefly, the inclusion criteria for the TUDA study were born on the island of Ireland, aged ≥ 60 years, and without an existing diagnosis of dementia. Study participants were recruited between 2008 and 2012.
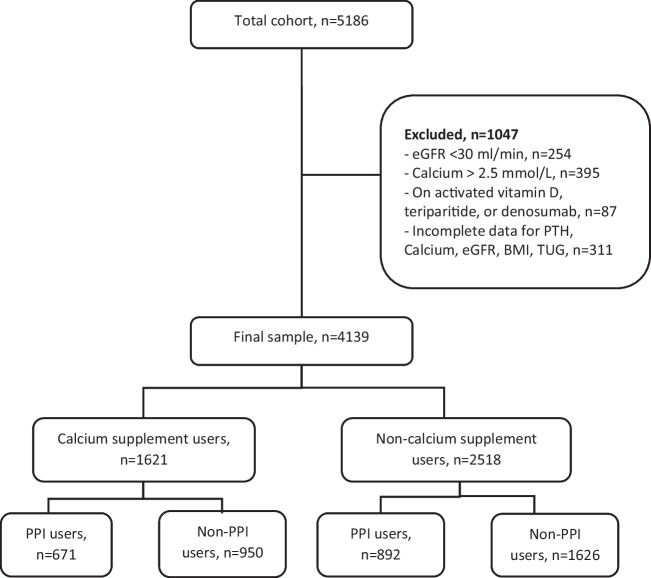
For the purposes of this investigation, patients with an estimated glomerular filtration rate (eGFR) < 30 ml/min and serum calcium > 2.5 mmol/l were excluded to avoid hyperparathyroidism due to chronic renal disease and primary hyperparathyroidism. We also excluded patients on denosumab and teriparatide and those on activated forms of vitamin D, i.e., alfacalcidiol and calcitriol. Participants with incomplete or missing data were also excluded (see Fig. 1). Participants who were taking PPIs (prescribed by their doctor) at the time of study recruitment constituted PPI users.
Fig. 1.
Flow diagram of study participants
Health and lifestyle assessments
All participants underwent a single assessment lasting about 90 min and included an interview recording self-reported information (demographic details, medical and psychosocial history, nutrient supplement usage, current medications, smoking, and alcohol history). Comorbidities described were based on self-report. Body mass index (BMI) and Timed-Up-and-Go (TUG) (a marker of frailty and functional status, which measures the time required to get up from a seated position, walk three metres and return to that position) were recorded. The MMSE (Mini Mental State Examination) was used as a measure of cognitive function. Dietary information on specific foods was collected using a short food frequency questionnaire. The total daily dairy intake was calculated by combining the calculated daily servings of all milk, yogurt, and cheese components for each participant as reported elsewhere [28].
Blood sampling and laboratory analysis
All participants provided a non-fasting (50 ml) blood sample. All samples were processed within 4 h of collection. Analysis for routine clinical blood biochemistry profile was performed at the time of blood collection in participating hospital laboratories. Blood aliquots for PTH and 25-hydroxyvitamin D (25(OH)D) were stored at – 70 °C until batch analysis. Intact PTH was measured at St. James’s Hospital, Dublin, using an electrochemiluminescence immunoassay (ECLIA) (Modular E170, Roche Diagnostics, Dublin, Ireland) with an inter-assay CV of < 2.9% and an assay measurement range of 1.2–5000 pg/ml. Hyperparathyroidism was defined as a PTH > 65 pg/ml. Serum 25(OH)D concentrations were quantified using LC–MS/MS (API 4000; AB SCIEX; Chromsystems GmbH) with an inter-assay CV of < 5.7% (detection range 7.5–624 nmol/l) [18, 19]. Renal function tests (creatinine) and calcium were analysed using a Roche Cobas c701 (Roche 8000 modular system) with an inter-assay CV < 5%. Glomerular filtration rate (eGFR) was estimated using the Cockcroft-Gault equation.
Statistical analysis
Statistical analysis was performed using JMP Student Edition 17.0 (SAS Institute Inc.). Data were assessed for normality by visual inspection of Q-Q plots and measurement of skewness and kurtosis. Descriptive statistics included means and standard deviations for normally distributed variables and, medians and interquartile ranges for non-normally distributed variables. Differences in population characteristics according to PPI status were explored with independent t-tests or Chi-squared test as appropriate. Statistical significance was accepted with a p value < 0.05.
Multivariate logistic regression models were used to assess the relationship between PPI use and hyperparathyroidism (PTH > 65 ng/ml) adjusting for relevant covariates including factors and medications which have a known or theoretical relationship with hyperparathyroidism [29–31]. Usual criteria for logistic regression analysis were applied including non-collinearity of independent variables and linear relationship between continuous independent variables and log of outcome (logit). Where non-linearity was detected, the knotted spline function was applied to the independent variable [32]. In determining the number of parameters to include in regression models, we used the “one in ten rule”, i.e., one independent variable per ten events [33].
The majority (93%) of patients on calcium supplements were also on concurrent vitamin D supplements, with calcium supplement use and serum 25(OH)D being co-correlated. Therefore, the analysis was dichotomised into users and non-users of calcium supplements. This approach also allowed us to explore for potential differences in the relationship between PPIs and serum PTH by calcium supplement use, as PPI-induced gastric acid suppression causing calcium malabsorption is a proposed mechanism to explain their adverse effects on bone.
Model 1 included basic demographic covariates: age, sex, and factors well-known to influence serum PTH (serum 25 hydroxyvitamin D and eGFR). In model 2, we further adjusted for other factors that may influence PTH: BMI, TUG, and total dairy intake. In model 3, we included medications (e.g., thiazides, loop diuretics, bisphosphonates, and glucocorticoids) that could theoretically affect PTH. Finally, model 4 included comorbidities that differed significantly between PPI and non-PPI users.
We also performed an analysis of the relationship between PPI use and hyperparathyroidism (dichotomised by calcium supplement users) in a sub-sample of participants who were not taking thiazide or loop diuretics (adjusting with covariates in the above 3 models). Furthermore, in a sub-analysis, we looked at the prevalence of hyperparathyroidism by PPI use in those with eGFR > 60 ml/min and assessed whether PPI’s predicted the same.
Finally, we examined the prevalence of hyperparathyroidism by high- and low-dose PPI use and whether it predicted an increased likelihood in multivariate regression (both in users and non-users of calcium supplements) Participants on ≥ 40 mg/day of omeprazole, pantoprazole, or esomeprazole, ≥ 30 mg/day of lansoprazole, or ≥ 20 mg/day or rabeprazole were considered to be on a “high-dose” PPI. The potential effect of high-dose PPI versus no PPI use on hyperparathyroidism was also similarly explored. In these sub-analyses, we adjusted for covariates in the above 3 models due to smaller sample sizes.
Results
There were 4139 participants in the final analysis (see Fig. 1). Of these, 1621 (39.2%) were on calcium supplementation. PPI use was identified in 41.4% of calcium supplement users and 35.4% of non-calcium supplement users. The PPIs used included omeprazole, pantoprazole, lansoprazole, esomeprazole, and rabeprazole. Nearly all (98%) were taking a PPI for ≥ 6 months. The dose of PPI was unavailable for 51 participants. Excluding those, 52.9% (800/1512) were on a high-dose PPI with the remainder on a low-dose PPI.
Table 1 illustrates the characteristics of PPI users and non-users, dichotomised by calcium supplementation. PPI users were older than non-PPI users in both calcium supplement users (mean age 76.2 vs 73.9 years, p < 0.001) and non-calcium supplement users (73.9 vs 72.3 years, p < 0.001). There was a lower proportion of females in PPI users in both those on calcium supplements (77.8 vs. 83.3%, p = 0.006) and not on supplements (58.8% vs 63.3%, p = 0.008). In both groups, PPI users had a higher BMI (p < 0.001), were physically frailer with a longer TUG (p < 0.001), and had more comorbidities including type 2 diabetes mellitus (p < 0.001) and vascular disease and heart failure (p < 0.001). Hypertension was also more common in PPI users on calcium supplements (p < 0.001), but not in non-users of calcium supplements (p = 0.377). MMSE scores were statistically lower in PPI users in both groups (p < 0.001) though the median MMSE was the same.
Table 1.
Characteristics of PPI users and non-PPI users according to calcium supplement use
| Calcium supplement users | Non-calcium supplement users | |||||
|---|---|---|---|---|---|---|
| PPI users (n = 671) | Non-PPI users (n = 950) | p value* | PPI users (n = 892) | Non-PPI users (n = 1626) | p value* | |
| General | ||||||
| Age (mean ± SD years) | 76.2 ± 0.3 | 73.9 ± 0.3 | < 0.001 | 73.9 ± 0.25 | 72.3 ± 1.19 | < 0.001 |
| Sex (% female) | 77.8 | 83.3 | 0.006 | 58.8 | 63.3 | 0.008 |
| BMI (mean ± SD kg/m2) | 27.4 ± 0.2 | 26.27 ± 0.2 | < 0.001 | 29.6 ± 0.2 | 28.7 ± 0.1 | < 0.001 |
| Comorbidities | ||||||
| Vascular disease (%) b | 38.2 | 23.2 | < 0.001 | 45.6 | 29.1 | < 0.001 |
| Type 2 diabetes mellitus (%) c | 14.1 | 7.6 | < 0.001 | 22.5 | 16.8 | < 0.001 |
| Hypertension (%) | 65.0 | 52.8 | < 0.001 | 83.9 | 82.5 | 0.377 |
| Heart failure (%) | 8.4 | 4.1 | < 0.001 | 7.4 | 3.1 | < 0.001 |
| Biophysical and cognitive function | ||||||
| MMSE (median (IQR)) a | 28 (26–29) | 28 (26–29) | < 0.001 | 28 (26–29) | 28 (26–29) | < 0.001 |
| TUG (median (IQR) seconds) a | 15 (9–23) | 10 (8–17) | < 0.001 | 11.6 (8.6–18) | 9.7 (8–14) | < 0.001 |
| Dietary and lifestyle | ||||||
| Dairy intake (mean ± SD portion/day) | 1.31 ± 0.03 | 1.22 ± 0.03 | 0.037 | 1.10 | 1.09 | 0.63 |
| Vitamin D supplementation (%) | 97.5 | 97.7 | 0.781 | 17.9 | 20.6 | 0.11 |
| Current smoking (%) | 10.9 | 12.6 | 0.281 | 12.6 | 10.9 | 0.218 |
| Current alcohol consumption (%) | 54.8 | 62.0 | 0.003 | 54.1 | 59.4 | 0.010 |
| Medications | ||||||
| Bisphosphonate use (%) | 46.9 | 43.2 | 0.136 | 7.5 | 5.0 | 0.010 |
| Current oral glucocorticoid use (%) | 8.1 | 2.8 | < 0.001 | 1.3 | 0.9 | 0.338 |
| Thiazide diuretic (%) | 13.0 | 14.1 | 0.510 | 24.9 | 29.8 | 0.008 |
| Loop diuretic (%) | 21.2 | 9.2 | < 0.001 | 17.2 | 9.4 | < 0.001 |
| Blood results | ||||||
| Calcium (mean ± SD mmol/l) | 2.29 ± 0.00 | 2.31 ± 0.00 | < 0.001 | 2.29 ± 0.00 | 2.30 ± 0.00 | 0.011 |
| Phosphate (mean ± SD mmol/l) | 1.01 ± 0.01 | 1.01 ± 0.01 | 0.680 | 1.02 ± 0.01 | 1.02 ± 0.00 | 0.776 |
| PTH (median (IQR) pg/ml) a | 39.1 (29.8–52.5) | 33.6 (52.5–45.5) | < 0.001 | 44.7 (34.0–60.8) | 40.1 (30.9–52.6) | < 0.001 |
| PTH > 65 pg/ml (%) | 13.9 | 7.1 | < 0.001 | 20.7 | 13.4 | < 0.001 |
| 25(OH)D (mean ± SD nmol/l) | 75.5 ± 1.1 | 77.6 ± 0.9 | 0.150 | 45.2 ± 0.8 | 47.6 ± 0.6 | 0.021 |
| < 30 nmol/l (%) | 6.7 | 5.7 | 0.257 | 31.7 | 25.6 | 0.002 |
| 30–50 nmol/l (%) | 12.3 | 12.3 | 32.2 | 35.3 | ||
| 51–75 nmol/l (%) | 30.2 | 26.6 | 24.9 | 24.3 | ||
| > 75 nmol/l (%) | 50.8 | 55.3 | 11.1 | 14.7 | ||
| eGFR (mean ± SD ml/min) | 62.4 ± 0.8 | 67.6 ± 0.7 | < 0.001 | 69.3 ± 0.8 | 74.2 ± 0.6 | < 0.001 |
IQR, interquartile range; SD, standard deviation
*p value calculated χ2 for categorical variables, t-test for normally distributed continuous variables, Wilcoxon rank for non-normally distributed continuous variables. aNon-normally distributed continuous variable—other continuous variables are normally distributed. aComposite of ischaemic heart disease, previous stroke or TIA, peripheral artery disease. bComposite of self-report, consumption of diabetic medications, HBA1c ≥ 48 mmol/l
The overall dairy intake (mean daily portion of milk, cheese, yogurt) in the sample was low at 1.15 portions per day. Among those on calcium supplements, PPI users versus non-users had a slightly higher dairy intake (1.31 vs 1.22, p = 0.037), but there was no difference in non-supplement users. The mean daily dairy intake was also higher in users versus non-users of calcium supplements (1.26 vs 1.06, p < 0.001).
Comparing medications, there was no difference in bisphosphonate use between PPI users and non-users in those taking calcium supplements (46.9 vs 43.2%, p = 0.136). In those not on calcium supplements, bisphosphonate use was much less frequent, but more common in PPI users compared to non-users (7.5 vs. 5.0%, p < 0.010). Glucocorticoids and loop diuretics were more frequent in PPI users versus non-PPI users in both those taking and not taking calcium supplements (p < 0.001). Conversely, in those taking calcium supplements, thiazide diuretics were less common in PPI users versus non-PPI users (24.9 vs 29.8%, p = 0.008).
As regards blood results, PPI users had a marginally lower serum calcium in both users of calcium supplements (2.29 vs 2.31 mmol/l, p < 0.011) and non-users of calcium supplements (2.29 vs 2.30 mmol/l, p = 0.011). Serum 25(OH)D was slightly lower in PPI users versus non-PPI users in those not taking calcium supplements (45.2 vs 47.6 nmol/l, p = 0.021). Kidney function (eGFR) was lower in PPI users versus non-PPI users in both those on calcium supplements (62.4 vs 67.6 ml/min, p < 0.001) and not on calcium supplements (69.3 vs 74.2 ml/min, p < 0.001).
Overall, hyperparathyroidism was more common in PPI users versus non-PPI users (17.8 vs 11.0%, p < 0.001) including in those taking calcium supplements (13.9 vs 7.1%, p < 0.0001) and not taking calcium supplements (20.7 vs 13.4%, p < 0.0001).When comparing PPI users taking calcium supplements versus no calcium supplements, there was a lower prevalence of hyperparathyroidism (13.9.% vs 20.7%, p = 0.001).
Table 2 shows the results of multivariate logistic regression analysis exploring the relationship between PPIs and hyperparathyroidism. Model 1 demonstrates a significant association between PPI use and hyperparathyroidism in patients both on calcium supplements (OR 1.85, CI 1.31—2.62, p = 0.001) and not on calcium supplements (OR 1.43, CI 1.13—1.81, p = 0.003). The association became insignificant in non-calcium supplement users in model 2. However, in calcium supplement users, it remains significant in models 2, 3, and 4 with a 56% higher risk of hyperparathyroidism identified after full adjustment (OR 1.56, CI 1.08–2.23, p = 0.018).
Table 2.
Relationship between Hyperparathyroidism and PPI use (dichotomised by calcium supplement use)
| Calcium supplement use (Yes, n = 1621), (no, n = 2518) |
β coefficient | Odds ratio* | 95% confidence interval | p value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model 1 | On calcium | 0.31 | 1.85 | 1.31 | 2.62 | 0.001 |
| Not on calcium | 0.18 | 1.43 | 1.13 | 1.81 | 0.003 | |
| Model 2 | On calcium | 0.25 | 1.66 | 1.16 | 2.37 | 0.006 |
| Not on calcium | 0.08 | 1.16 | 0.91 | 1.48 | 0.229 | |
| Model 3 | On calcium | 0.22 | 1.55 | 1.07 | 2.23 | 0.019 |
| Not on calcium | 0.06 | 1.13 | 0.88 | 1.46 | 0.183 | |
| Model 4 | On calcium | 0.24 | 1.56 | 1.08 | 2.23 | 0.018 |
| Not on calcium | 0.06 | 1.12 | 0.87 | 1.44 | 0.397 | |
*Reference group: PPI non-users
Model 1: adjusted for age, sex, serum 25(OH)Dr, eGFRa
Model 2: model 1 and BMI, timed up and goa, daily dairy intake
Model 3: model 2 and thiazides, loop diuretics, bisphosphonates, current glucocorticoid use
Model 4: model 3 and vascular disease (ischaemic heart disease, stroke/TIA, peripheral artery disease), hypertension, diabetes, and heart failure
aKnotted spline function applied due to non-linearity between this variable and the logit of hyperparathyroidism
*PTH > 65 (n = 160 in calcium supplements users and n = 402 in non-calcium supplement users)
After excluding participants taking thiazides and loop diuretics, we found that users of calcium supplements on PPIs versus no PPIs had a higher prevalence of hyperparathyroidism (10.8 vs 5.3%, p < 0.001) (see supplemental Table 1). This increased risk of hyperparathyroidism remained significant after multivariate adjustment (p ≤ 0.013) in users of calcium supplements but not in non-users of calcium supplements (see Table 3). In a sub-analysis of participants with an eGFR > 60 ml/min, PPI users compared to non-PPI users also had a higher prevalence of hyperparathyroidism both in calcium supplement users (9.0 vs 3.8%, p < 0.001) and non-users (15.3 vs 9.6%, p < 0.001) and the relationship remained significant after initial adjustment for age, sex, vitamin D, and eGFR. In non-users of calcium supplements where adjustment for further covariates was possible, the association became insignificant (see supplemental Table 2).
Table 3.
Relationship between hyperparathyroidism and PPI use (dichotomised by calcium supplement use, excluding those on thiazide and loop diuretics)
| Calcium supplement use (Yes, n = 1179), (no, n = 1522) |
β coefficient | Odds ratio* | 95% confidence interval | p value | ||
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Model 1 | On calcium | 0.32 | 1.89 | 1.19 | 2.98 | 0.007 |
| Not on calcium | 0.22 | 1.54 | 1.10 | 2.16 | 0.011 | |
| Model 2 | On calcium | 0.29 | 1.80 | 1.13 | 2.86 | 0.013 |
| Not on calcium | 0.12 | 1.28 | 0.91 | 1.82 | 0.161 | |
| Model 3a | On calcium | 0.29 | 1.80 | 1.13 | 2.87 | 0.013 |
| Not on calcium | 0.12 | 1.28 | 0.90 | 1.81 | 0.166 | |
| Model 3b | On calcium | 0.30 | 1.82 | 1.14 | 2.90 | 0.012 |
| Not on calcium | 0.13 | 1.28 | 0.91 | 1.82 | 0.159 | |
*Reference group: PPI non-users
Model 1: adjusted for age, sex, serum 25(OH)Da, eGFRa
Model 2: model 1 and BMI, timed up and goa, daily dairy intake
Model 3a: model 2 and bisphosphonates
Model 3b: model 2 and current glucocorticoid use
aKnotted spline function applied due to non-linearity between the logit of hyperparathyroidism and dependant variable
*PTH > 65 (n = 87 in calcium supplements users and n = 179 in non-calcium supplement users)
Lastly, we found no difference in the risk of hyperparathyroidism between high-dose and low-dose PPI users in those taking calcium supplements (OR 1.19, CI 0.73–1.96, p = 0.485) or not taking calcium supplements (OR 1.10, CI 0.74–1.63, p = 0.634), (see supplemental Table 3). When we limited our analysis to high-dose PPI versus no PPI, there was a significant association between PPI use and hyperparathyroidism in users of calcium supplements (OR 1.54, CI 1.01–2.36, p = 0.049) but not in non-users of calcium supplements (OR 1.22, CI 0.89–1.66, p = 0.213) (see supplemental Table 4).
Discussion
In this study of over 4000 older adults, PPI use was associated with an increased risk of hyperparathyroidism in individuals taking calcium supplements. Several meta-analyses have demonstrated an association between PPIs and increased fracture risk with various mechanisms proposed [7, 10, 14]. Specifically, PPIs may reduce calcium absorption from the gut, which may result in a compensatory rise in PTH, and this could be a possible mechanism by which fracture risk may be mediated.
PPIs act by irreversibly blocking the hydrogen proton pump of gastric parietal cells, leading to suppression of gastric acid production. Consequently, PPIs are very effective in reducing pain from indigestion and heartburn [34]. However, gastric acid is necessary for adequate absorption of calcium and other nutrients [35]. Before it can be absorbed, calcium must be in solution in an ionised form, which is facilitated by the acidic environment of the stomach [36]. By suppressing gastric acid production, PPIs reduce the fractional absorption of calcium, resulting in secondary hyperparathyroidism. Hypochlorhydria has also long been associated with osteoporosis and fracture risk. Even before the advent of PPIs, patients post-gastrectomy and vagotomy have been noted to have lower BMD and increased risk of fracture risk [35]. While there are many confounding and contributory factors in these patients such as weight loss and post-operative malabsorption, hypochlorhydria may be important.
We found that the relationship between hyperparathyroidism and PPI use was significant only in those on calcium supplements. The majority of dietary calcium in the Irish diet is derived from dairy products [28]. In this study, the mean dairy intake was low which may explain why a significant relationship between hyperparathyroidism and PPI use was only seen in those on calcium supplements; i.e., dietary calcium intake may have been too low for the absolute reduction in calcium absorption mediated by PPIs to result in significant changes in PTH levels. Calcium supplements do appear to suppress PTH in PPI users as well as non-PPI users though our results suggest this is significantly blunted by PPI use.
Calcium carbonate is the most commonly used calcium supplement, and reduction of its absorption due to PPI-induced hypochlorhydria is well-described [35]. However, the extent to which hypochlorhydria inhibits other calcium supplements is less clear [37], and the effect of PPIs on dietary calcium absorption is more complex [11, 38, 39]. It was notable that PPI users in this study had a significantly lower serum calcium which may be due to reduced absorption. Reduced calcium absorption, itself, is linked to osteoporosis and vertebral and hip fractures [40, 41]. Importantly, older patients are also less able to increase calcium absorption efficiency to compensate for inadequate dietary calcium intake [42]. We did not find a significant relationship between high and low PPI dose and risk of hyperparathyroidism. However, the high- and low-dose classification of different forms of PPI may not accurately correlate with the potencies of these agents. The analysis may also have been underpowered to detect differences in serum PTH between PPI groups. Of interest, higher dose PPI use has been associated with vitamin B12 deficiency (through suppression of gastric acid secretion) [27], while supplementation of B12-deficient individuals with B vitamins may increase BMD [22]. However, no study has shown any association between hyperparathyroidism and serum B vitamin levels.
In the literature to date, there are only a few small studies which have described the relationship between PPIs and hyperparathyroidism. A 2015 study performed a retrospective review of 80 patients (40 on PPI and 40 not on a PPI) and examined for between group differences in serum PTH concentrations [25]. Half of each group were taking a bisphosphonate. Higher PTH were found in PPI compared to non-PPI users, irrespective of bisphosphonates use, though there was no adjustment for potential confounders or record of calcium supplement use. A Japanese study of 19 patients aged 67 ± 13 years with gastric ulcer treated with omeprazole demonstrated a 28% increase in PTH which was associated with an increase in serum bone turnover markers [43]. On the other hand, a small study of 12 healthy young men treated with oral rabeprazole demonstrated no clinically relevant change in serum PTH concentrations [23] though the study was of very short duration (2 weeks). Two other studies examined fractional calcium absorption and changes in serum PTH using the dual stable isotope method after 14 days of PPI exposure in 12 young adults [44] and 30 days of PPI exposure in twenty-one post-menopausal women [39]. While no significant reduction in calcium absorption or increase in PTH was found in either study, PPI exposure was short.
Another mechanism by which PPIs are thought to increase serum PTH is through increased gastrin secretion [9]. In response to acid suppression, increased gastrin secretion occurs which may lead to increased PTH concentrations independent of calcium absorption. In a study of chickens, an infusion of gastrin increased the weight of the parathyroid glands and was coupled to reduced femur density [45]. The same mechanism may be responsible for osteopenia in young rats on long-term omeprazole [46]. Evidence on the relationship between hypergastrinemia and hyperparathyroidism in humans is more heterogenous [23, 43, 47]. Although we did not measure gastrin in this study, the absence of a significant increase in hyperparathyroidism in PPI users not on calcium supplements does not support this theory.
There is also some evidence that PPIs may blunt the antifracture effect of bisphosphonates in older adults in a dose-dependent manner and may even result in a greater PPI-associated increase in fracture risk than PPIs alone [12, 48]. In this regard, secondary hyperparathyroidism has been found to reduce the BMD response to bisphosphonates and denosumab and could be a mechanism by which PPIs blunt their effects [49, 50].
Strengths and limitations of the study
The major strengths of this study include its large size and well-characterised population. We were also able to adjust for a wide range of potential confounders including comorbidities and medications. In previous studies, questions over associations between PPIs and potential adverse effects have been questioned as users of PPIs had higher levels of comorbidity that were not controlled for. Over 98% of PPI users had been on the medication for over 6 months, a duration long enough to demonstrate the effects of chronic PPI therapy on serum PTH. A further strength included the high validity of the PTH and vitamin D assays used.
The major limitation though is its cross-sectional design, and hence, we cannot infer causality between PPIs and hyperparathyroidism. While we excluded participants with hypercalcaemia to avoid those with primary hyperparathyroidism, there may have been a small proportion of participants with normocalcaemic primary hyperparathyroidism. However, given the low prevalence of this condition in similar populations (0.4%) [13], we expect this number too small to significantly impact our results.
We did not have an accurate measure of calcium intake (a significant determinant of serum PTH concentrations) using a validated food frequency questionnaire. However, we did record dairy intake which is the predominant source of calcium in the Irish diet. Information on the medical indication for prescribing PPI was not available, and thus, we could not adjust for this as a potential confounder in our regression models. A further limitation includes the lack of serum gastrin results. PPIs increase gastrin concentrations which are suspected to stimulate PTH secretion and may be an important mechanism of PPIs effect on bone.
Finally, we did not assess whether the increased risk of hyperparathyroidism was associated with lower BMD or translated into a greater fracture risk. A substantial proportion of the cohort (35%) did not have bone density measured, and a large proportion was recruited from a bone health clinic and on treatment or previously on treatment, for osteoporosis which would be a significant confounding factor. Changes in BMD occur at a much slower rate than biochemical change, and while the vast majority were on PPIs for more than 6 months, we had no specific information on the exact duration of use. Furthermore, secondary hyperparathyroidism is known to cause microarchitectural changes in bone that is not captured by DXA measurement of BMD [51].
Indeed, the influence of PPIs on bone mineral density is controversial. Several cross-sectional studies have described lower BMD in PPI users [16–18]. However, a longitudinal study of 4512 Canadian adults found no significant change in BMD at 10 years [52], and in the Women’s Health Initiative, there was only a very small decline in BMD [19]. Despite this, numerous studies and meta-analyses have clearly identified a higher risk of fractures with PPIs independent of confounding variables [7–14], and some of this effect on fracture risk is likely independent of BMD.
This is the largest study to date examining hyperparathyroidism as a potential mechanism by which PPIs exert their adverse effect on bone. Our results are consistent with the hypothesis that PPIs reduce calcium absorption, leading to secondary hyperparathyroidism which may increase fracture risk. No relationship between PPI use and hyperparathyroidism was observed in non-users of calcium supplements, possibly owing to them having much lower dietary calcium intake. Given the prevalence of PPI usage, it is important to be aware of this potential adverse effect. Findings illustrate the importance of monitoring PPI use, especially in older adults at higher risk of osteoporotic fracture. Deprescribing of these medications when no longer indicated should be encouraged.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Open Access funding provided by the IReL Consortium The authors acknowledge funding from the Mercer’s Institute for Research on Ageing, the Irish Department of Agriculture, Food and the Marine and the Health Research Board (under its Food Institutional Research Measure), and the Northern Ireland Department for Employment and Learning (under its strengthening the all-Ireland research base initiative).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
Ethical approval was granted from the Office for Research Ethics Committees Northern Ireland (ORECNI; ref. 08/NIR03/113), with corresponding approval from the Northern and Western Health and Social Care Trusts in Northern Ireland, and the Research Ethics Committee of St James’s Hospital and the Adelaide and Meath Hospital, Dublin. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendment. All participants provided written informed consent at the time of recruitment.
Conflict of interest
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Daniels B, Pearson SA, Buckley NA, Bruno C, Zoega H. Long-term use of proton-pump inhibitors: whole-of-population patterns in Australia 2013–2016. Therap Adv Gastroenterol. 2020 doi: 10.1177/1756284820913743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19–24. doi: 10.1016/j.ejim.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Borgström F, Karlsson L, Ortsäter G, et al. Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos. 2020 doi: 10.1007/S11657-020-0706-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture - a systematic review. World J Orthop. 2019;10:166. doi: 10.5312/wjo.v10.i3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM, Cooper C. The epidemiology of osteoporosis. Br Med Bull. 2020;133:105–117. doi: 10.1093/bmb/ldaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou B, Huang Y, Li H, Sun W, Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 8.Briganti SI, Naciu AM, Tabacco G, Cesareo R, Napoli N, Trimboli P, Castellana M, Manfrini S, Palermo A. Proton pump inhibitors and fractures in adults: a critical appraisal and review of the literature. Int J Endocrinol. 2021 doi: 10.1155/2021/8902367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thong BKS, Ima-Nirwana S, Chin KY. Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health. 2019 doi: 10.3390/IJERPH16091571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poly TN, Islam MM, Yang HC, Wu CC, Li YCJ. Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int. 2019;30:103–114. doi: 10.1007/s00198-018-4788-y. [DOI] [PubMed] [Google Scholar]
- 11.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJH, Youn K, Choi NK, Lee JJH, Kang D, Song HJ, Park BJ. A population-based case-control study: proton pump inhibition and risk of hip fracture by use of bisphosphonate. J Gastroenterol. 2013;48:1016–1022. doi: 10.1007/s00535-012-0722-9. [DOI] [PubMed] [Google Scholar]
- 13.Leontiadis GI, Moayyedi P (2014) Proton pump inhibitors and risk of bone fractures. Curr Treat Options Gastroenterol 12:414–423 [DOI] [PubMed]
- 14.Nassar Y, Richter S. Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. J Bone Metab. 2018;25:141. doi: 10.11005/jbm.2018.25.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based from the Canadian multicentre osteoporosis study (CaMos) Am J Gastroenterol. 2012;107:1361. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattahi MR, Niknam R, Shams M, Anushiravani A, Taghavi SA, Omrani GR, Mahmoudi L. The association between prolonged proton pump inhibitors use and bone mineral density. Risk Manag Healthc Policy. 2019;12:349. doi: 10.2147/RMHP.S223118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arj A, Razavi Zade M, Yavari M, Akbari H, Zamani B, Asemi Z (2016) Proton pump inhibitors use and change in bone mineral density. Int J Rheum Dis 19:864–868 [DOI] [PubMed]
- 18.Bahtiri E, Islami H, Hoxha R, Qorraj-Bytyqi H, Rexhepi S, Hoti K, Thaçi K, Thaçi S, Karakulak Ç. Esomeprazole use is independently associated with significant reduction of BMD: 1-year prospective comparative safety study of four proton pump inhibitors. J Bone Miner Metab. 2016;34:571–579. doi: 10.1007/s00774-015-0699-6. [DOI] [PubMed] [Google Scholar]
- 19.Gray SL, Lacroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, Chen Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 22.Clements M, Heffernan M, Ward M, et al. A 2-year randomized controlled trial with low-dose B-vitamin supplementation shows benefits on bone mineral density in adults with lower B12 status. J Bone Miner Res. 2022;37:2443–2455. doi: 10.1002/jbmr.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dammann HG, Burkhardt F, Wolf N (1999) The effects of oral rabeprazole on endocrine and gastric secretory function in healthy volunteers. Aliment Pharmacol Ther 13:1195–1203 [DOI] [PubMed]
- 24.Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K+-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 25.Hinson AM, Wilkerson BM, Rothman-Fitts I, Riggs AT, Stack BC, Bodenner DL. Hyperparathyroidism associated with long-term proton pump inhibitors independent of concurrent bisphosphonate therapy in elderly adults. J Am Geriatr Soc. 2015;63:2070–2073. doi: 10.1111/jgs.13661. [DOI] [PubMed] [Google Scholar]
- 26.McCarroll K, Beirne A, Casey M, et al. Determinants of 25-hydroxyvitamin D in older Irish adults. Age Ageing. 2015;44:847–853. doi: 10.1093/ageing/afv090. [DOI] [PubMed] [Google Scholar]
- 27.Porter KM, Hoey L, Hughes CF, et al. Associations of atrophic gastritis and proton-pump inhibitor drug use with vitamin B-12 status, and the impact of fortified foods, in older adults. Am J Clin Nutr. 2021;114:1286–1294. doi: 10.1093/ajcn/nqab193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laird E, Casey MC, Ward M, Hoey L, Hughes CF, McCarroll K, Cunningham C, Strain JJ, McNulty H, Molloy AM (2017) Dairy intakes in older irish adults and effects on vitamin micronutrient status: data from the TUDA study. Journal of Nutrition, Health and Aging 21:954–961 [DOI] [PubMed]
- 29.Corapi KM, McMahon GM, Wenger JB, Seifter JL, Bhan I. Association of loop diuretic use with higher parathyroid hormone levels in patients with normal renal function. JAMA Intern Med. 2015;175:137. doi: 10.1001/jamainternmed.2014.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaker JL, Wermers RA. The eucalcemic patient with elevated parathyroid hormone levels. J Endocr Soc. 2023 doi: 10.1210/jendso/bvad013/7005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jorde R, Saleh F, Figenschau Y, Kamycheva E, Haug E, Sundsfjord J. Serum parathyroid hormone (PTH) levels in smokers and non-smokers. The fifth Tromsø study. Eur J Endocrinol. 2005;152:39–45. doi: 10.1530/eje.1.01816. [DOI] [PubMed] [Google Scholar]
- 32.Schuster NA, Rijnhart JJM, Twisk JWR, Heymans MW. Modeling non-linear relationships in epidemiological data: the application and interpretation of spline models. Front Epidemiol. 2022;2:29. doi: 10.3389/fepid.2022.975380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstem AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/S0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 34.Stedman CAM, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–978. doi: 10.1046/j.1365-2036.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- 35.Kopic S, Geibel JP. Gastric acid, calcium absorption, and their impact on bone health. Physiol Rev. 2013;93:189–268. doi: 10.1152/physrev.00015.2012. [DOI] [PubMed] [Google Scholar]
- 36.Shkembi B, Huppertz T. Calcium absorption from food products: food matrix effects. Nutrients. 2022;14:180. doi: 10.3390/nu14010180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118:778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Graziani G, Como G, Badalamenti S, Finazzi S, Malesci A, Gallieni M, Brancaccio D, Ponticelli C. Effect of gastric acid secretion on intestinal phosphate and calcium absorption in normal subjects. Nephrol Dial Transplant. 1995;10:1376–1380. [PubMed] [Google Scholar]
- 39.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Ziegler TE, Penniston KL, Alvig AL, Shafer MM. Do proton pump inhibitors decrease calcium absorption? J Bone Miner Res. 2010;25:2786. doi: 10.1002/jbmr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensrud KE, Duong T, Cauley JA, Heaney RP, Wolf RL, Harris E, Cummings SR. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;132:345–353. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 41.Nordin BEC, O’Loughlin PD, Need AG, Horowitz M, Morris HA. Radiocalcium absorption is reduced in postmenopausal women with vertebral and most types of peripheral fractures. Osteoporos Int. 2004;15:27–31. doi: 10.1007/s00198-003-1493-1. [DOI] [PubMed] [Google Scholar]
- 42.Nordin BEC. Calcium and osteoporosis. Nutrition. 1997;13:664–686. doi: 10.1016/S0899-9007(97)83011-0. [DOI] [PubMed] [Google Scholar]
- 43.Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K(+)-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 44.Wright MJ, Sullivan RR, Gaffney-Stomberg E, Caseria DM, O’Brien KO, Proctor DD, Simpson CA, Kerstetter JE, Insogna KL. Inhibiting gastric acid production does not affect intestinal calcium absorption in young, healthy individuals: a randomized, crossover, controlled clinical trial. J Bone Miner Res. 2010;25:2205. doi: 10.1002/jbmr.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gagnemo-Persson R, Håkanson R, Sundler F, Persson P (1994) Growth of the parathyroid glands in omeprazole-treated chickens. Scand J Gastroenterol 29:493–497 [DOI] [PubMed]
- 46.Grimelius L, Johansson H, Lundqvist G, Olazabal A, Polak JH, Pearse AGE (1977) The parathyroid glands in experimentally induced hypergastrinemia in the rat. Scand J Gastroenterol 12:739–744 [DOI] [PubMed]
- 47.Selking Ö, Borch K, Johansson H, Ljunghall S, Wide L (1982) Evaluation of parathyroid function in patients with hypergastrinaemia and pernicious anaemia. Ups J Med Sci 87:215–222 [DOI] [PubMed]
- 48.Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med. 2011;171:998–1004. doi: 10.1001/archinternmed.2011.20. [DOI] [PubMed] [Google Scholar]
- 49.Barone A, Giusti A, Pioli G, Girasole G, Razzano M, Pizzonia M, Palummeri E, Bianchi G. Secondary hyperparathyroidism due to hypovitaminosis D affects bone mineral density response to alendronate in elderly women with osteoporosis: a randomized controlled trial. J Am Geriatr Soc. 2007;55:752–757. doi: 10.1111/j.1532-5415.2007.01161.x. [DOI] [PubMed] [Google Scholar]
- 50.Mosali P, Bernard L, Wajed J, Mohamed Z, Ewang M, Moore A, Fogelman I, Hampson G. Vitamin D status and parathyroid hormone concentrations influence the skeletal response to zoledronate and denosumab. Calcif Tissue Int. 2014;94:553–559. doi: 10.1007/s00223-014-9840-0. [DOI] [PubMed] [Google Scholar]
- 51.Kužma M, Jackuliak P, Killinger Z, Payer J. Parathyroid hormone-related changes of bone structure. Physiol Res. 2021;70:S3. doi: 10.33549/physiolres.934779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012;107:1361–1369. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.