Abstract
Abstract
This prospectively registered systematic review and meta-analysis examines whether exercise (EX) training has an additive effect to osteoanabolic and/or antiresorptive pharmacological therapy (PT) in people with osteoporosis on bone mineral density (BMD), bone turnover markers (BTMs), fracture healing, and fractures. Four databases (inception to 6 May 2022), 5 trial registries, and reference lists were searched. Included were randomized controlled trials comparing the effect of EX + PT vs. PT with regard to BMD, BTM, fracture healing, and fractures. Risk of bias was assessed using the Cochrane RoB2 and certainty of evidence by the GRADE approach. Random-effects meta-analysis with Hartung-Knapp-Sidik-Jonkman adjustment was used to estimate standardized mean differences and 95% confidence intervals. Out of 2593 records, five RCTs with 530 participants were included. Meta-analysis showed with very low certainty evidence and wide confidence intervals that EX + PT compared to PT had larger effect sizes for BMD at 12 months at the hip (SMD [95%CI]: 0.18 [− 1.71; 2.06], n = 3 studies), tibia (0.25 [− 4.85; 5.34], n = 2), lumbar spine (0.20 [− 1.15; 1.55], n = 4), and forearm (0.05 [− 0.35; 0.46], n = 3), but not femoral neck (− 0.03 [− 1.80; 1.75], n = 3). Furthermore, no improvement was revealed for BTM such as bone ALP (− 0.68 [− 5.88; 4.53], n = 3), PINP (− 0.74 [− 10.42; 8.93], n = 2), and CTX-I (− 0.69 [− 9.61; 8.23], n = 2), but with very wide confidence intervals. Three potentially relevant ongoing trials were identified via registries. No data were found for fracture healing or fracture outcomes. It remains unclear whether EX has an additive impact to PT in people with osteoporosis. High-quality, adequately powered, targetted RCTs are required.
Protocol Registration
PROSPERO CRD42022336132.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-023-06829-0.
Keywords: Exercise training, People with osteoporosis, Pharmacological therapy
Introduction
Exercise (EX) and adequate physical activity is recommended by clinical practice guidelines worldwide as part of the management of osteopenia and osteoporosis [1]. Thereby, osteopenia is considered an intermediate stage between normal bone mineral density (BMD) and osteoporosis [2]. In 2017, 21 million US citizens aged over 50 [3] and 32 million European people across all ages in 2019 had an osteoporosis diagnosis [4]. Women are more affected than men [3, 4]. Direct medical costs of osteoporosis in the USA were predicted to be more than $16.9 billion in 2005 with estimated annually costs amounting to 25.3 billion dollars by 2025 [5]. In Europe, an economic burden of €56.9 billion was attributed to osteoporosis. Of these, 64%, 33%, and 3% were fracture, long-term disability, and pharmacological intervention costs [4]. According to the World Health Organization (WHO) [2], osteoporosis is defined as a progressive systematic skeletal disease characterized by decreased bone mass and micro-architectural deterioration of bone tissue or the presence of a fragility fracture. These fragility fractures are the fourth most common cause of chronic disease in Europe (EU) and occur mainly in the spine, pelvis, and femur. A loss of just 10% of hip bone mass can increase the risk of hip fractures by a factor of 2.5 [6]. It is estimated that 33% women and 20% men ≥ 50 years old worldwide will experience osteoporotic fractures during their lifetime [7–9]. In addition, women aged 65 years old with a previously diagnosed vertebral fracture are expected to have higher risk (one out of four women) of additional fractures within 5 years [10]. The risk for fractures can be reduced to one in eight with preventative treatment [10].
Several strategies exist to reduce fracture risk. Two recognized strategies are exercise and pharmacological therapies (PTs) [11]. To date, several PTs have shown efficacy for the prevention and treatment of osteoporosis and are used worldwide [12]. Corresponding drugs can be divided into antiresorptive and osteoanabolic agents. Bisphosphonates such as alendronate, ibandronate, risedronate, and zoledronic acid are the most frequently used antiresorptive agents [13]. Older, less frequently prescribed bisphosphonates (e.g., clodronate, etidronate) differ in their mode of action (nitrogen-containing bisphosphonates), appropriateness (administration), and clinical evidence, based on the effects on bone health parameters (such as dual-energy X-ray absorptiometry) [13, 14]. Additionally, denosumab (antiresorptive agent), teriparatide (osteoanabolic agent), and romosozumab (osteoanabolic agent) are often used for the treatment of osteoporosis [11, 14]. The choice of drug depends on patient and provider preference, comorbidities, side effects, costs, and the fracture risk level [15].
Previous studies have shown that bones respond positively to mechanical loading by muscle and ground reaction forces [16, 17]. As a result, exercise can improve bone density, muscle mass, and strength regardless of age [15, 16, 18]. Higher muscle forces maintain bone mass and promote bone formation [19]. Bone metabolic markers typically adapt to bone-anabolic exercise with an increase in bone formation markers and minimal or no reduction on bone resorption markers [20]. Exercise also promotes self-confidence and coordination, hence contributes to fall prevention and reduces the risk of fractures [21]. To date, meta-analyses have been published for isolated PT efficacy [22, 23] and effect of EX on osteoporosis [24, 25]. Recently, combined effects of EX + PT have also been considered. The meta-analysis by Zhao et al. [26] addresses the effect of combining different antiresorptives and EX on bone mineral density (BMD) in the population of postmenopausal women. Zhang et al. [27] investigate the effect of bisphosphonates, hormone replacement therapies (HRTs), and various exercise types in people with decreased bone mass on BMD. Although these previous studies have already provided information on the effectiveness of medications and EX interventions in osteoporosis, these did not permit specific commentary on whether EX has an additive effect to PT in osteoporosis due to their selection of patient groups, interventions, and/or outcomes. For practical reasons, the effect of all frequently used medications without any limitation in exercise mode and patient population with increased fracture risk needs to be investigated.
The aim of the present study was to conduct a systematic review and meta-analysis to investigate whether EX + PT has an additive effect over PT alone on BMD, bone turnover marker (BTM), fracture healing, and fractures in patients with increased fracture risk due to osteopenia or osteoporosis.
Methods
The review was prospectively registered with PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022336132). Data and statistical codes used were uploaded on the Open Science Framework (https://osf.io/yjhvz/?view_only=7f2dae1632c64ce1909ab391b34d2a6e). The systematic review was conducted and reported in accordance with 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA2020; Supplement 1) [28].
Eligibility criteria
Inclusion criteria followed the Participants, Interventions, Comparators, Outcomes, and Study (PICOS) design framework [29]. Studies that did not meet the following criteria were excluded.
Population
The population group of interest was patients with increased risk of fracture due to osteoporosis or osteopenia (T-score < − 1 SD) [2]. There were no restrictions on age, sex, gender, or race.
Intervention
Involved the addition of exercise to pharmacological therapy. Exercise had to contain the following aspects: planned, structured, repetitive, and targeted [30]. The aim of exercise is the improvement or attainment of physical fitness components [30] (e.g., aerobic, walking, cycling, jogging, resistance, strengthening, endurance exercises, whole body vibration). The combination of different types of exercises did not lead to exclusion. Co-interventions in form of advisory and educational services were allowed (e.g., calls, web calls, education material in form of booklets). Other co-interventions that have a direct impact to bodily physiology such as nutrition other than vitamin D, cholecalciferol, or calcium to compensate potential deficiencies were excluded.
Pharmacological therapies included the following [12, 14] (Supplement 2): alendronate, risedronate, ibandronate, zoledronate, denosumab, teriparatide, and romosozumab.
Comparators
Received pharmacological therapy only without any exercise training (see Supplement 2 for details of included therapies). Supplementation with vitamin D, cholecalciferol, and/or calcium alone was not considered as an included pharmacological therapy. In the case that participants received different medications, decisions for inclusion/exclusion were made based on the most frequently used medication. One person could not act as both an intervention and a control group participant (e.g., crossover RCTs).
Outcomes
Included BMD (areal and volumetric), BTM, fracture healing (radiologically evaluated), and fractures. No bone strength measures were included (see Supplement 3 for further details and priority for extraction).
Study
Included studies were required to be randomized controlled trials (RCTs; parallel, cluster, or cross-over designs) published in German or English as a full peer-reviewed journal publication (i.e., grey literature including theses and conference abstracts were excluded). There were no restrictions regarding the date of publication.
Information sources and search strategy
An electronic database search of MEDLINE (via PubMed), EMBASE (via Ovid), CINAHL (via EBSCO Host), and CENTRAL (via the Cochrane Library) was conducted using keywords, Medical Subject Headings (MeSH) terms, as well as related text words. Searches were performed from the database inception to May 6, 2022. The full search strategy is contained in Supplement 4. Unpublished and ongoing trials were searched via International Standard Randomized Controlled Trial Number (https://www.isrctn.com/), US National Institutes of Health (https://clinicaltrials.gov/), EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/), German Clinical Trials Register (https://www.drks.de/), and Australian New Zealand Clinical Trials Register (https://www.anzctr.org.au/). In addition, the reference lists of prior relevant systematic reviews were screened for additional studies. Forward and backward citation tracking of included studies was performed via Web of Science Core Collection. Hand searches were performed for older bisphosphonates (see Supplement 5).
Selection process
Two independent assessors (from a pool of three screeners: AKS, NKA, EAC) screened each record against predefined inclusion criteria based on title and abstract and subsequently full text. Covidence (www.covidence.org) was used for the screening process. Duplicate records were detected and removed via the Covidence software. Disagreements were resolved through discussions between reviewers and, if necessary, an adjudicator (NLM) and a senior member of the research team (DLB, PJO, BB) were consulted.
Data collection process and data extraction
Data was extracted independently by two extractors (from a pool of three extractors: AKS, NKA, EAC) using GoogleSheets. Differences and extraction errors for the measures of spread and/or impact of studies reporting were identified by a reviewer (AKS) and were resolved through discussions between reviewers and, if necessary, an adjudicator (NLM) and a senior member of the research team (DLB, PJO, BB) were consulted. Prior to commencing data extraction, this method was piloted on ten studies chosen at random and the results were collated by one team member (AKS). In the custom GoogleSheets for extraction, comment bubbles contained detailed information for the extractors as to what data to extract for each parameter and information on how to address unclear information in publications. The following study information were extracted: publication information (author, year, title, journal, funding), study design, study demographics (e.g., age, sex, number of participants), medication interventions (e.g., category, dose, and frequency), exercise interventions (e.g., types, session and overall intervention duration, intensity, and frequency), measurement time points, and outcomes (e.g., BMD, BTM). If multiple follow-ups existed within each timeframe, we extracted the follow-up data closest to 12 months for BMD outcomes and end of intervention if exercise period deviated. For BTM outcome, data closest to 3 months and end of intervention were extracted (see Supplement 3 for further information).
Data for the main outcomes were extracted as number of participants, mean, and standard deviation (SD) where possible. In case these were reported using other measures of center and spread (e.g., median and interquartile range reported instead), we used standard equations to convert the data [31]. All applied data handling methods are available for each study and outcome in Supplement 6. Where data was presented in a figure only, ImageJ (https://imagej.nih.gov/ij/) was used to extract the values by measuring the length of the axes in pixels followed by the length of the relevant data of interest [32]. If it was not possible to extract the required data, information was requested from the corresponding author with a minimum of three times over a 4-week period.
Study risk of bias assessment, reporting bias assessment, and certainty assessment
The Cochrane Collaboration Risk of Bias Tool 2 (RoB2) was used to examine potential bias from the randomisation process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results for randomized trials [33]. An overall risk of bias judgement was made for BMD and BTM outcomes.
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the certainty of the evidence [34]. Risk of bias, imprecision, inconsistency, indirectness, and publication bias are considered in the GRADE approach to rate the certainty [34]. Further details on the criteria used for GRADE are available in Supplement 7. Due to the exclusive presence of RCTs, a high level of confidence in the evidence was assumed with a possibility of downgrade after the assessment. Two independent assessors (AKS, EAC) assessed risk of bias and GRADE. Conflicts were resolved by discussion and, if necessary, consultation of an adjudicator (NLM).
Synthesis methods and effect measures
All statistical analyses and forest plots were completed in R version 4.2.1. (https://www.r-project.org/) and the R packages meta [35], metafor [36], and metadat [37].
As all outcomes of interest were continuous, but could be measured on different scales, standardized mean difference (SMD) with Hedges correction (Hedges’ g) was used as the effect estimate [38]. Random-effects meta-analysis was used. To estimate the 95% confidence intervals (95%CI), the Hartung-Knapp-Sidik-Jonkman adjustment with ad hoc correction was used [39, 41]. Statistical significance was set as an alpha of ≤ 0.05.
Heterogeneity was assessed by I2 statistic via the reported 95% prediction intervals (95%PI) where possible. The heterogeneity parameter τ (tau) was estimated via restricted maximum likelihood (REML) estimation. Funnel plots and the Egger’s test modified by Pustejovsky [42] to assess the risk of publication bias and small study effects are only recommended if at least 10 studies were available [43]. Furthermore, this threshold also applies for meta-regression and subgroups analysis [43]. Accordingly, these statistical approaches would only be used if ≥ 10 studies were included. In order to test the robustness of the results, sensitivity analyses exploring the role of outliers and influential trials were included [44].
Results
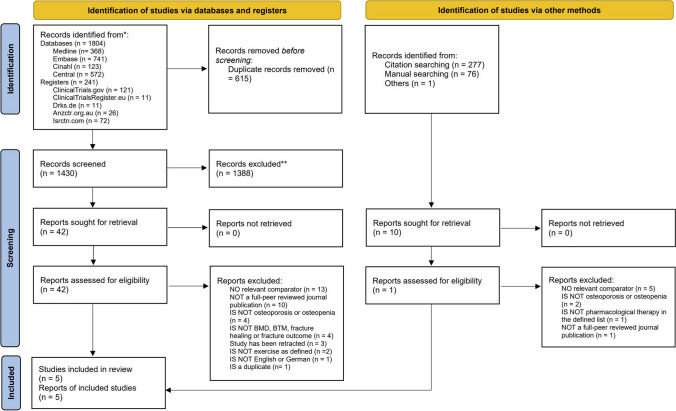
A total of 2593 records were identified. After removing duplicates, screening titles, and abstracts of all remaining reports, 52 full text articles were assessed for eligibility (Fig. 1). Five records from five RCTs [45, 49] were included. Literature sources and reasons for exclusion of ineligible studies of studies at full-text stage are reported in Supplement 8. Additionally, three potentially relevant ongoing trials were found through registry search but were not included due to pending trial completion (Supplement 9). Three author groups [45, 47, 48] were contacted to request missing data and two [45, 47] responded (see Supplement 6).
Fig. 1.
PRISMA flow chart
Study characteristics
The details of the five included RCTs [45–48] are shown in Table 1 and Supplement 10. The sample size of each record varied from 35 to 249 participants with a total sample of 530 participants with a mean age from 46.6 to 81 years old. The length of interventions in included studies ranged from 6 to 24 months.
Table 1.
Characteristics of included studies
| Intervention group/control group | Intervention group | Control group | Co-intervention | Intervention period | Measurement time points | Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (year) | Population group | Age (mean) (EX + PT; PT) | Sample size n (EX + PT;PT) | PT (dose, frequency) | EX | Frequency | Types, session, intensity | PT (dose, frequency) | Type | (months) | (months) | |
| Edwards et al. (2018) | Spinal cord injury | 46.6 (15.4); 47.6 (16.3) | 61 (20;20) |
TRP (20 µg DLY) |
Vibration | DLY | 10 min | TRP (20 µg DLY) | CH | 12, 24 | Baseline, 3, 6, 9, 12, 18, 24 |
aBMD (femoral neck, femur, hip, spine, tibia); BTM: bone ALP, CTX-I, PINP |
| Fu and Fan (2021) | Type 2 diabetes osteoporosis |
63.73 (5.64); 62.04 (5.33) |
117 (63;54) |
ALN (70 mg WKLY) |
Individual rehabilitation therapy | 2–3 times DLY | 60–90 min |
ALN (70 mg WKLY) |
Vit D, CA, health education |
6 | Baseline, 6 | aBMD (femoral neck, spine); BTM: bone ALP, OC, TRACP |
| Jepsen et al. (2019) | Postmenopausal osteoporotic women | 69 (5); 69 (8) | 35 (17;18) |
TRP (20 µg DLY) |
Whole body vibration | Three days WKLY | Rest ratio 1:1 min, including six minutes of vibration | TRP (20 µg DLY) | Vit D, CA | 12 | Baseline, 3, 6, 12 |
aBMD (hip, spine); vBMD (radius, tibia); BTM: CTX-I, PINP |
| Nawrat Szoltysik et al. (2018) | Osteoporosis and osteopenia | 81 (NA)1 | 68 (24;44) | ALN (70 mg WKLY) | Modified Sinaki exercises and Nordic walking | 4 days WKLY | 70% of individual max heart rate, walking speed: 2–3 km/h | ALN (70 mg WKLY) | Vit D, CA | 12 | Baseline, 12 | aBMD (forearm) |
| Waltman et al. (2010) | Postmenopausal breast cancer survivors | 58.69 (7.5)2 | 249 (125; 124) | RIS (35 mg WKLY) | Strength/weight training | Twice WKLY | 30–45 min, 8–12 repetitions, progressive increase of weights | RIS (35 mg WKLY) | Vit D, CA | 12, 24 | 12, 24 |
aBMD (femoral neck, hip, spine, radius); BTM: bone ALP, NTX-I |
aBMD areal bone mineral density, ALN alendronate, bone ALP bone-specific alkaline phosphatase, BTM bone turnover marker, CA calcium, CH cholecalciferol, CTX-I C-terminal crosslinking telopeptide of type I collagen, DLY daily, Dmab denosumab, EX exercise training, IB ibandronate, km/h kilometers per hour, min minutes, µg microgram, mg milligram, NTX-I N-terminal crosslinking telopeptide of type I collagen, NA not available, OC osteocalcin, PINP procollagen type I N propeptide, PT pharmacological therapy, RIS risedronate, TRACP tartrate-resistant acid phosphatase, TRP teriparatide, vBMD volumetric bone mineral density, Vit D vitamin D, WKLY weekly, ZA zoledronic acid
1Only an overall mean age was available. No statement of standard deviation was given
2Only an overall mean age was available
Population
Two studies included both males and females [45, 49], whereas three studies [46–48] investigated females only. The participant groups consisted of chronic spinal cord injury with low bone mass [45], postmenopausal osteoporotic women [46], postmenopausal breast cancer survivors [48], women with osteoporosis or osteopenia [47], and patients with type 2 diabetic osteoporosis [49].
Intervention/comparator
Three of the included trials had two groups (PT alone, EX + PT) [46, 48, 49], one study had three groups (PT alone, EX alone, EX + PT) [45], and one study had four groups (PT alone, EX + PT, EX + PT, EX combined + PT) [47]. For trials with more than two groups, only data from PT alone and EX + PT/EX combined + PT group were extracted.
As pharmacological therapy, teriparatide was used in two trials [45, 46], two studies used alendronate [47, 49], and one study used risedronate [48]. As exercise intervention, vibration was used in two trials [45, 46], strength/weight training [48], Sinaki combined with Nordic walking [47], and individual exercise rehabilitation therapy [49] was each used in one study. As co-intervention, cholecalciferol was used in one study [45], vitamin D in three studies [46, 48], and calcium with health education in combination with diabetes treatment in one study [49].
Outcome
All studies [45–49] assessed BMD as an outcome, whereas only four studies [45, 46, 48, 49] assessed BTM outcomes. None of the included studies assessed radiological evaluated fracture healing or fractures.
Study
All five included studies were parallel arm RCTs with patients being the unit of randomization. Three studies received no/non-profit funding [45, 46, 48] and two studies gave no information about funding [47, 49] (see Supplement 10). The primary hypothesis of three studies [45, 46, 48] was to investigate EX + PT vs. PT alone, whereas one study evaluated the influence of different exercises on osteoporosis [47] and another one the influence of aerobic exercise on type 2 diabetic osteoporosis [49].
Risk of bias and GRADE assessment
A summary of the risk of bias assessment for each study is shown in Supplement 11. Summary risk of bias plots for BMD and BTM outcomes and a detailed risk of bias assessment for each included study and outcome were created (Supplement 11).
For the five trials assessing BMD outcome, 80% had a high risk of bias and 20% some concerns overall. There was low risk of bias for randomization process (40%), deviations from intended interventions (60%), measurement of outcome (40%), and selection of reported results (20%). Missing outcome data was rated as having high risk of bias in 80% of included studies.
For the four trials assessing BTM outcome, 75% had a high and 25% a low risk of bias overall. There was a low risk of bias for randomization process (50%), deviations from intended interventions (75%), missing outcome data (25%), measurement of outcome (50%), and selection of reported results (25%). The certainty of evidence was rated as very low for all study outcomes (Supplement 12). The main reasons for downgrading were risk of bias, inconsistency, and imprecision. Publication bias was assessed by funnel plots (Supplement 13) in addition to criteria used (Supplement 7).
Data handling
Three trials had the mean and SD extracted directly [45, 46, 49]. For one study [47], the mean and standard error were extracted from a figure. Standard error was converted to SD. One study [48] had mean and SD as percentage change from baseline data. Due to missing data and non-responsiveness of the authors, no transformation of the data was possible. Two trials [45, 47] required pooling of groups due to multiple groups and various recruitment sites in both intervention and comparator groups.
Meta-analysis of bone mineral density (BMD)
The meta-analytic findings of all BMD related meta-analyses are presented in Table 2.
Table 2.
Effect sizes of BMD outcomes
| Region | Timepoint | Intervention | Comparator | Studies | N | Hedges’ g [95% CI] | P value | I2 | Prediction interval1 |
|---|---|---|---|---|---|---|---|---|---|
| Hip | 12 months | EX + PT | PT | 3 | 294 | 0.1782 [− 1.7086; 2.0649] | P = 0.7239 | 90.3% | [− 10.6389; 10.9952] |
| Hip | End intervention | EX + PT | PT | 2 | 237 | 0.5501 [− 7.0765; 8.1767] | P = 0.5277 | 79.0% | NA |
| Femoral neck | 12 months | EX + PT | PT | 3 | 337 | − 0.0265 [− 1.8050; 1.7520] | P = 0.9547 | 93.9% | [− 10.3243; 10.2704] |
| Femoral neck | End intervention | EX + PT | PT | 2 | 239 | 0.1321 [− 7.3403; 7.6044] | P = 0.8594 | 80.2% | NA |
| Tibia | 12 months | EX + PT | PT | 2 | 61 | 0.2469 [− 4.8459; 5.3396] | P = 0.6486 | 57.8% | NA |
| Spine | 12 months | EX + PT | PT | 4 | 412 | 0.1976 [− 1.1535; 1.5487] | P = 0.6734 | 90.2% | [− 3.7200; 4.1151] |
| Spine | End intervention | EX + PT | PT | 2 | 237 | 0.8528 [− 5.0231; 6.7287] | P = 0.3163 | 57.6% | NA |
| Forearm | 12 months | EX + PT | PT | 3 | 313 | 0.0537 [− 0.3505; 0.4580] | P = 0.6251 | 0% | [− 1.4159; 1.5234] |
EX exercise training, PT pharmacological therapy, 95%CI 95% confidence intervals, NA not available
1The prediction interval indicates the heterogeneity in the data and the range of potential values that could be possible in future studies
Meta-analysis of hip bone mineral density (BMD)
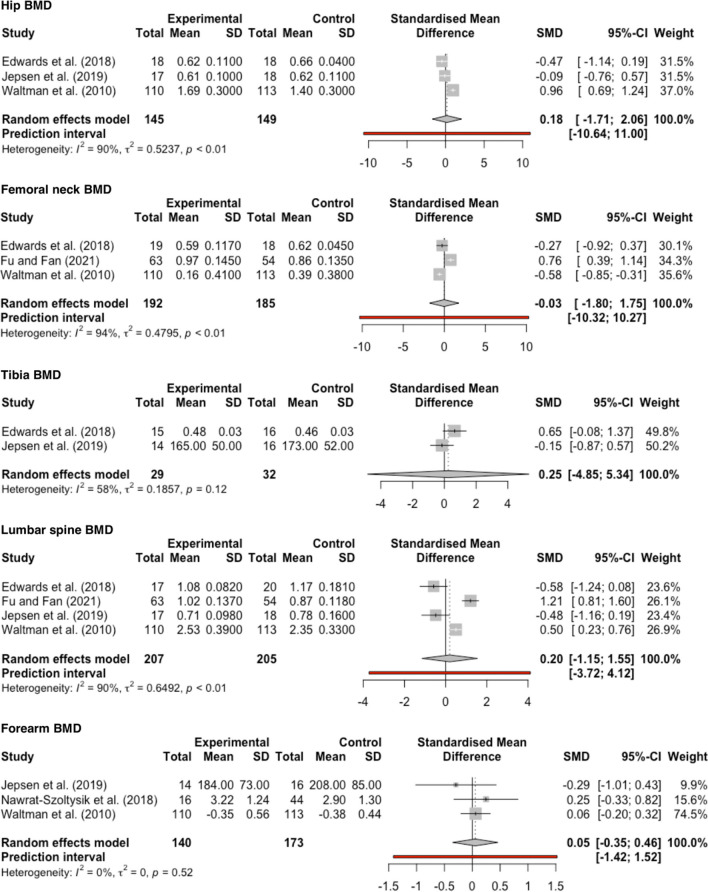
At 12 months, three trials [45, 46, 48] involving 294 participants and at end intervention two trials [45, 48] with 237 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that hip aBMD increased at 12 months (SMD [95%CI]: 0.18 [− 1.71; 2.06]; P = 0.72; I2 = 90.3%, GRADE: very low) (Fig. 2) and at end intervention (0.55 [− 7.08; 8.18]; P = 0.53; I2 = 79.0%, GRADE: very low) (Supplement 14).
Fig. 2.
Forest plot of intervention effect on BMD at 12 months. The data are shown as pooled SMD with 95%CI for changes in experimental (EX + PT) and control (PT) groups. The prediction interval for tibia BMD could not be calculated because there were only two studies included in the meta-analysis
Meta-analysis of femoral neck bone mineral density (BMD)
At 12 months, three trials [45, 48, 49] involving 377 participants and at end intervention two trials [45, 48] involving 239 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that femoral neck aBMD decreased at 12 months (− 0.03 [− 1.80; 1.75]; P = 0.95; I2 = 93.9%, GRADE: very low) (Fig. 2) and increased at end intervention (0.13; [− 7.34; 7.60]; P = 0.86; I2 = 80.2%, GRADE: very low) (Supplement 14).
Meta-analysis of tibia bone mineral density (BMD)
At 12 months, two trials [45, 46] involving 61 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that tibia BMD increased (0.25 [− 4.85; 5.34]; P = 0.65; I2 = 57.8%, GRADE: very low) (Fig. 2).
Meta-analysis of lumbar spine bone mineral density (BMD)
At 12 months, four trials [45, 46, 48, 49] involving 412 participants and at end intervention two trials [45, 48] involving 237 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that lumbar spine aBMD increased at 12 months (0.20 [− 1.15; 1.55]; P = 0.67; I2 = 90.2%, GRADE: very low) (Fig. 2) and at end intervention (0.85 [− 5.02; 6.73]; P = 0.32; I2 = 57.6%, GRADE: very low) (Supplement 14).
Meta-analysis of forearm bone mineral density (BMD)
At 12 months, three trials [46–48] involving 313 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that forearm BMD increased (0.05 [− 0.35; 0.46]; P = 0.63; I2 = 0%, GRADE: very low) (Fig. 2).
Meta-analysis of bone turnover markers (BTM)
The meta-analytic findings of all BMT related meta-analyses are presented in Table 3.
Table 3.
Effect sizes of BTM outcomes
| Time point | Intervention | Comparator | Studies | N | Hedges’ g [95%CI] | P value | I2 | Prediction interval1 | |
|---|---|---|---|---|---|---|---|---|---|
| Bone ALP | End intervention | EX + PT | PT | 3 | 379 | − 0.6758 [− 5.8782; 4.5266] | P = 0.6325 | 96.7% | [− 30.9090; 29.5574] |
| PINP | 3 months | EX + PT | PT | 2 | 73 | − 0.7434 [− 10.4164; 8.9297] | P = 0.5076 | 89.1% | NA |
| PINP | End intervention | EX + PT | PT | 2 | 73 | − 0.3927 [− 10.9450; 10.1595] | P = 0.7188 | 91.1% | NA |
| CTX-I | 3 months | EX + PT | PT | 2 | 73 | − 0.6890 [− 9.6091; 8.2310] | P = 0.5059 | 87.4% | NA |
| CTX-I | End intervention | EX + PT | PT | 2 | 73 | − 0.0405 [− 7.0467; 6.9657] | P = 0.9533 | 81.1% | NA |
EX exercise training, PT pharmacological therapy, 95%CI 95% confidence intervals, NA not available
1The prediction interval indicates the heterogeneity in the data and the range of potential values that could be possible in future studies
Meta-analysis of bone ALP (bone-specific alkaline phosphatase)
At end intervention, three trials [45, 48, 49] involving 379 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that bone ALP decreased (− 0.68 [− 5.88; 4.53]; P = 0.63; I2 = 96.7%, GRADE: very low) (Supplement 14).
Meta-analysis of PINP (procollagen type I N propeptide)
At 3 months and end intervention, two trials [45, 46] involving 73 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that PINP decreased at both three months (− 0.74 [− 10.42; 8.93]; P = 0.51; I2 = 89.1%, GRADE: very low) and end intervention (− 0.39 [− 10.94; 10.16]; P = 0.72; I2 = 91.1%, GRADE: very low) (Supplement 14).
Meta-analysis of CTX-I (C-terminal crosslinking telopeptide of type I collagen)
At 3 months and end intervention, two trials [45, 46] involving 73 participants compared the effect of EX + PT with PT alone. Meta-analysis showed that CTX-I decreased at both 3 months (− 0.69 [− 9.61; 8.23]; P = 0.51; I2 = 87.4%, GRADE: very low) and end intervention (− 0.04 [− 7.05; 6.97]; P = 0.95; I2 = 81.1%; GRADE: very low) (Supplement 14).
Results of sensitivity analysis
A sensitivity analysis exploring the role of outliers and influential trials was conducted. When the trial from Waltman et al. [48] was excluded, as the results were only given as percentage change data and could not be transformed, results of the meta-analyses changed to a weaker impact of exercise with some results reversed results into negative findings and therefore were no longer effective. Further details of the sensitivity analysis can be seen in Supplement 15.
Results of subgroup analysis
A subgroup analysis was not possible for any outcome as the number of studies included was too low (n ≤ 10 studies) [50].
Results of meta-regression
A meta-regression was not possible for any outcome as the number of studies included was too low (n ≤ 10 studies) [50].
Protocol additions since PROSPERO registration
We decided to add a search for unpublished and ongoing trials in five trial registries and to search for potentially relevant studies with older bisphosphonates by performing an additional hand search in all databases used. No other protocol deviations occurred.
Discussion
This systematic review and meta-analysis found with very low certainty of evidence that while changes in BMD typically favored exercise added to pharmacological therapy, the effects were not statistically significant. Additionally, the different modes of action from anabolic agents (teriparatide) and antiresorptive agents (bisphosphonates) can be seen in Fig. 2. Anabolic agents stimulate bone formation and increase BMD [51], whereas antiresorptive agents inhibit bone resorption and preserve BMD [52]. BTMs were lowered at all timepoints, but confidence intervals were very wide and evidence certainty very low. No results were available for fracture healing or fracture. There was marked heterogeneity, both clinically and in the individual study outcomes and one study [48] appeared to be influential in the analysis.
Results in the context of other evidence
Previous systematic reviews and meta-analyses have largely focused on specific populations [19, 53, 54], pharmacological therapies [53, 55, 56], and/or types of exercises [57, 58, 58, 59]. The meta-analysis by Yan et al. (2021) [60] has proceeded in a comparable manner, as they also compare EX + PT versus PT alone. They also included different types of exercises with focus on BMD, BTM, and pain. No further details were given regarding the pharmacological therapy used. Their meta-analysis showed significant improvements for BMD, but no improvements for BTMs. The reason of different results may occur because they included more studies (n = 20) and therefore involved more participants (n = 1824). Additionally, any activity to enhance and improve physical health with intervention periods less than 6 months were included. Furthermore, they searched in Chinese databases and included reports only published in Chinese except of one German article. In comparison to the existing literature, our current study focusses on the comparison of exercises instead of routine activities, with currently prescribed pharmacological therapies. In addition, we included only studies that could reach clinically based results due to their duration of intervention (see Supplement 3).
Implications of the results for practice, policy, and future research
Recent clinical practice guidelines [61–63] give very similar recommendations in recommending exercise training in combination to pharmacological therapy for treatment of osteoporosis. On the basis of the findings of the current study, we cannot yet recommend additions to clinical practice guidelines that exercise can have an additive effect to pharmacological therapies. While it seems logical to prescribe both exercise and pharmacological therapy to patients with osteoporosis, the certainty of evidence is very low and heterogeneity very high, precluding a possible recommendation.
High-quality RCTs are required to examine the additive effect of exercise to antiresorptive and/or osteoanabolic medication. Specifically, future RCTs should be adequately powered and provide sufficient and useful data quality for a meta-analysis. In terms of participants, future studies should focus primarily on primary osteopenia/osteoporosis. Also, it is possible a greater benefit of exercise may be seen in less active individuals; this should be considered in study design and recruitment. Sex, age, race, treatment history, and comorbidities may moderate treatment effects and should be considered in study design and/or statistical analysis. In terms of the exercise intervention, high-impact exercise and high-intensity progressive resistance training have been shown to have with osteoanabolic effects [64]. Future primary RCTs could and should focus on these interventions, but also consider that these forms of exercise may not suit all populations and other forms of exercise and physical activity (e.g., aerobic dance) should also be evaluated. The frequency of exercise sessions and duration of exercise should be considered, and we recommend adhering to current guideline recommendations [64]. In terms of the pharmacological intervention, it should be investigated if the efficacy of currently in several guidelines suggested sequential therapies using an osteoanabolic agent first followed by an antiresorptive for individuals with very high fracture risk can be enhanced by exercise. For outcome measures, BMD is often taken as a surrogate marker of bone fracture risk, and is a more feasible (e.g., total hip or femoral neck BMD) outcome to assess than long-term fracture rates, even if the latter is the ultimate goal of osteoporosis management. Furthermore, the method of measuring BMD (DXA, QCT, pQCT, and/or QUS) should be considered versus their advantages and limitations. Further, in terms of study design, 9 months of intervention is a conservatively set time period to measure detectable changes in BMD via, for example, DXA [65]. Finally, while it is difficult to blind participants to exercise, the assessors of the bone outcomes must be blinded to the group allocation of the participants.
Strengths and limitations
This study has some limitations. Nearly all study outcomes had a high risk of bias. The lack of blinding participants to exercise treatment may overestimate the intervention effects. Due to the small number of included studies (n = 5), it was not reasonable to perform subgroup analysis or to assess the publication bias and meta-regression by using a statistical approach. A further limitation is the small population in the respective studies indicating further demand on studies [66]. Regarding the BMD outcome, we only included aBMD and vBMD to examine the effect of intervention on bone mineral density. This leads to limited findings [67]. Besides exercise and pharmacological therapies, BTM also depends on various other controllable factors such as nutrition, as well as non-controllable factors such as genetic predisposition [68]. The influenceable factors might also depend on social-economical aspects [69, 70]. These factors should be considered while interpreting the final results and deriving measurements according to the results.
The strength of the current study was a systematic review approach, which is the highest level of evidence. Additionally, the Hartung-Knapp-Sidik-Jonkman adjustment was used. This is important to implement in meta-analyses that include less than five trials [39–41]. The role of outliers in sensitivity analysis was explored to examine the robustness of the results. The addition of searches of trial registries and backward/forward citation tracking strengthened the search process.
Conclusion
While the mean effect sizes typically favored additional exercise to pharmacological treatment in improving BMD and lowering BTMs, confidence and prediction intervals were very wide and evidence certainty was very low. No results were found for fracture healing and fractures. Even though the study compiles all available information in the field of the research question, no explicit recommendation for additional exercise is possible. Targeted RCTs that are adequately powered, targeting primary osteopenia/osteoporosis with an osteoanabolic exercise program and are subsequently well reported are needed to derive more definitive recommendations.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- aBMD
Areal bone mineral density
- Bone ALP
Bone-specific alkaline phosphatase
- BMD
Bone mineral density
- BTM
Bone turnover marker
- vBMD
Volumetric bone mineral density
- CENTRAL
Cochrane Central Register of Controlled Trials
- CI
Confidence interval
- CINAHL
Cumulative Index to Nursing and Allied Health Literature
- CTX-I
C-terminal crosslinking telopeptide of type I collagen
- DXA
Dual-energy X-ray absorptiometry
- EMBASE
Excerpta Medica dataBASE
- EU
Europe
- EX
Exercise
- FRAX
Fracture Risk Assessment Tool
- GRADE
Grading of Recommendations, Assessment, Development and Evaluation
- HRT
Hormone replacement therapy
- ITT
Intention-to-treat
- MEDLINE
Medical Literature Analysis and Retrieval System Online
- NTX-I
N-terminal crosslinking telopeptide of type I collagen
- OC
Osteocalcin
- OP
Osteoporosis
- PI
Prediction interval
- PICOS
Population, Intervention, Comparison, Outcome, Study Design
- PICP
Procollagen type I C propeptide
- PINP
Procollagen type I N propeptide
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PT
Pharmacological therapy
- PubMed
Public Medicine
- pQCT
Peripheral quantitative computed tomography
- QCT
Quantitative computed tomography
- QUS
Quantitative ultrasound
- RANKL
Receptor activator of NF-κB
- REML
Restricted maximum likelihood
- RCT
Randomized controlled trial
- RESP
Respectively
- SERMS
Selective estrogen receptor modulators
- SMD
Standardized mean difference
- TBS
Trabecular bone score
- TRACP
Tartrate-resistant acid phosphatase
- VFA
Vertebral fracture assessment
- WHO
World Health Organization
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was received for the conduct of the study.
Data availability
Included manuscript and appendix are tables with source data and links to online data repositories with statistical code and source data are presented in the manuscript.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
Conflicts of interest
AKS, EAC, NKA, PJO, and NLM declare that they have no actual or potential conflict of interest. DLB has given paid presentations on the role of exercise in osteoporosis (funded by not-for-profit educational organizations). BB has received research support, consultancy fees, and/or honoraria from AbbVie, Amgen, Biogen, GE/Lunar, Janssen, Galapagos, Gilead, Medimaps, MSD, Sanofi Genzyme, Theramex, and UCB. UM has received honorary for scientific presentation and Ad Boards from Ag Novos, Lilly, Amgen, UCB, Theramex, Alexion, Kyowa Kirin, and Medi.
Footnotes
Key points
This systematic review and meta-analysis examined whether exercise training has an additive effect to pharmacological therapy in improving bone-related outcomes in people with osteoporosis. Mean effect sizes favored additional exercise training with large confidence intervals and low certainty of evidence. High-quality, adequately powered, and well-reported RCTs are required.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/2/2024
ORCID ID for Nitin Kumar Arora:is updated to https://orcid.org/0000-0002-8841-2549.
Contributor Information
Ann-Kathrin Schumm, Email: ann-kathrin.schumm@gmx.de.
Daniel L. Belavy, Email: belavy@gmail.com, Email: daniel.belavy@hs-gesundheit.de
References
- 1.Cheung AM, Giangregorio L. Mechanical stimuli and bone health: what is the evidence? Curr Opin Rheumatol. 2012;24:561–566. doi: 10.1097/BOR.0b013e3283570238. [DOI] [PubMed] [Google Scholar]
- 2.Kanis J, Оn behalf of the World Health Organization Scientific Group (2007) Assessment of osteoporosis at the primary health-care level." World Health Organization Collaborating Centre for Metabolic Bone Diseases.–University of Sheffield.–UK. Technical Report (2007)
- 3.Sarafrazi N, Wambogo EA, Shepherd JA. Osteoporosis or low bone mass in older adults: United States, 2017–2018. Natl Cent Health Stat. 2021 doi: 10.15620/cdc:103477. [DOI] [PubMed] [Google Scholar]
- 4.Kanis J, Norton N, Harvey NC, et al. SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos. 2021;16:82. doi: 10.1007/s11657-020-00871-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burge R, Dawson-Hughes B, Solomon DH, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 6.Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 7.Curtis EM, van der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988–2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanis J, Johnell O, Oden A et al (2000) Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int 11:669–674 [DOI] [PubMed]
- 9.Melton LJ, Atkinson EJ, O’Connor MK, et al. Bone density and fracture risk in men. J Bone Miner Res. 1998;13:1915–1923. doi: 10.1359/jbmr.1998.13.12.1915. [DOI] [PubMed] [Google Scholar]
- 10.Kaptoge S, Armbrecht G, Felsenberg D, et al. When should the doctor order a spine X-ray? Identifying vertebral fractures for osteoporosis care: results from the European Prospective Osteoporosis Study (EPOS) J Bone Miner Res. 2004;19:1982–1993. doi: 10.1359/jbmr.040901. [DOI] [PubMed] [Google Scholar]
- 11.LeBoff MS, Greenspan SL, Insogna KL et al (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int Published Online First. 10.1007/s00198-021-05900-y [DOI] [PMC free article] [PubMed]
- 12.Obermayer-Pietsch B, Fössl I, Dimai HP. Langfristige Therapiekonzepte bei Osteoporose. Internist. 2021;62:474–485. doi: 10.1007/s00108-021-00993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giusti A, Papapoulos SE (2018) Bisphosphonates for postmenopausal osteoporosis. In: Bilezikian JP, Bouillon R, Clemens T, et al. eds. Primer on the metabolic bone diseases and disorders of mineral metabolism. Wiley. 10.1002/9781119266594
- 14.Pavone V, Testa G, Giardina SMC, et al. Pharmacological therapy of osteoporosis: a systematic current review of literature. Front Pharmacol. 2017;8:803. doi: 10.3389/fphar.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compston J, Cooper A, Gregson C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 12:1–24 [DOI] [PMC free article] [PubMed]
- 16.Marini S, Barone G, Masini A, et al. The effect of physical activity on bone biomarkers in people with osteoporosis: a systematic review. Front Endocrinol. 2020;11:585689. doi: 10.3389/fendo.2020.585689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohammad Rahimi GhR, Niyazi A, Alaee S. The effect of exercise training on osteocalcin, adipocytokines, and insulin resistance: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2021;32:213–224. doi: 10.1007/s00198-020-05592-w. [DOI] [PubMed] [Google Scholar]
- 18.Yokota H, Leong DJ, Sun HB. Mechanical loading: bone remodeling and cartilage maintenance. Curr Osteoporos Rep. 2011;9:237–242. doi: 10.1007/s11914-011-0067-y. [DOI] [PubMed] [Google Scholar]
- 19.Howe TE, Shea B, Dawson LJ et al (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev Published Online First. 10.1002/14651858.CD000333.pub2 [DOI] [PubMed]
- 20.Dolan E, Varley I, Ackerman KE, et al. The bone metabolic response to exercise and nutrition. Exerc Sport Sci Rev. 2020;48:49–58. doi: 10.1249/JES.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 21.Girgis CM. Integrated therapies for osteoporosis and sarcopenia: from signaling pathways to clinical trials. Calcif Tissue Int. 2015;96:243–255. doi: 10.1007/s00223-015-9956-x. [DOI] [PubMed] [Google Scholar]
- 22.Ding L-L, Wen F, Wang H, et al. Osteoporosis drugs for prevention of clinical fracture in white postmenopausal women: a network meta-analysis of survival data. Osteoporos Int. 2020;31:961–971. doi: 10.1007/s00198-019-05183-4. [DOI] [PubMed] [Google Scholar]
- 23.Saito T, Sterbenz JM, Malay S, et al. Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporos Int. 2017;28:3289–3300. doi: 10.1007/s00198-017-4175-0. [DOI] [PubMed] [Google Scholar]
- 24.Ponzano M, Rodrigues IB, Hosseini Z, et al. Progressive resistance training for improving health-related outcomes in people at risk of fracture: a systematic review and meta-analysis of randomized controlled trials. Phys Ther. 2021;101:pzaa221. doi: 10.1093/ptj/pzaa221. [DOI] [PubMed] [Google Scholar]
- 25.Varahra A, Rodrigues IB, MacDermid JC, et al. Exercise to improve functional outcomes in persons with osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2018;29:265–286. doi: 10.1007/s00198-017-4339-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhao R, Xu Z, Zhao M. Antiresorptive agents increase the effects of exercise on preventing postmenopausal bone loss in women: a meta-analysis. PLOS One. 2015;10:e0116729. doi: 10.1371/journal.pone.0116729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Gao R, Cao P, et al. Additive effects of antiresorptive agents and exercise on lumbar spine bone mineral density in adults with low bone mass: a meta-analysis. Osteoporos Int. 2014;25:1585–1594. doi: 10.1007/s00198-014-2644-2. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Shamseer L, Clarke M et al (2015) Preferred Reporting Items for Systematic Review and Meta-Analysis protocols (PRISMA-P) 2015 statement. Syst Rev 9 [DOI] [PMC free article] [PubMed]
- 29.Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed]
- 30.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- 31.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vucic K, Jelicic Kadic A, Puljak L. Survey of Cochrane protocols found methods for data extraction from figures not mentioned or unclear. J Clin Epidemiol. 2015;68:1161–1164. doi: 10.1016/j.jclinepi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Sterne JAC, Savović J, Page MJ et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ l4898. 10.1136/bmj.l4898 [DOI] [PubMed]
- 34.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–94. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viechtbauer W (2010) Conducting meta-analyses in R with the metafor package. J Stat Softw 36. 10.18637/jss.v036.i03
- 37.White T, Noble D, Senior A et al (2022) _metadat: meta-analysis datasets_. R pacage version 1.2–0. https://CRAN.R-project.org/package=metadat>
- 38.White IR, Thomas J. Standardized mean differences in individually-randomized and cluster-randomized trials, with applications to meta-analysis. Clin Trials. 2005;2:141–151. doi: 10.1191/1740774505cn081oa. [DOI] [PubMed] [Google Scholar]
- 39.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20:3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 40.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Röver C, Knapp G, Friede T. Hartung-Knapp-Sidik-Jonkman approach and its modification for random-effects meta-analysis with few studies. BMC Med Res Methodol. 2015;15:99. doi: 10.1186/s12874-015-0091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pustejovsky JE, Rodgers MA. Testing for funnel plot asymmetry of standardized mean differences. Res Synth Methods. 2019;10:57–71. doi: 10.1002/jrsm.1332. [DOI] [PubMed] [Google Scholar]
- 43.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002–d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 44.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–25. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 45.Edwards WB, Simonian N, Haider IT, et al. Effects of teriparatide and vibration on bone mass and bone strength in people with bone loss and spinal cord injury: a randomized, controlled trial. J Bone Miner Res. 2018;33:1729–1740. doi: 10.1002/jbmr.3525. [DOI] [PubMed] [Google Scholar]
- 46.Jepsen DB, Ryg J, Hansen S, et al. The combined effect of Parathyroid hormone (1–34) and whole-body Vibration exercise in the treatment of postmenopausal OSteoporosis (PaVOS study): a randomized controlled trial. Osteoporos Int. 2019;30:1827–1836. doi: 10.1007/s00198-019-05029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nawrat-Szołtysik A, Polak A, Małecki A, et al. Effect of physical activity on the sequelae of osteoporosis in female residents of residential care facilities. Adv Clin Exp Med. 2018;27:633–42. doi: 10.17219/acem/68381. [DOI] [PubMed] [Google Scholar]
- 48.Waltman NL, Twiss JJ, Ott CD, et al. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. 2010;21:1361–1369. doi: 10.1007/s00198-009-1083-y. [DOI] [PubMed] [Google Scholar]
- 49.Fu W, Fan J (2021) Intervention effect of exercise rehabilitation therapy on patients with type 2 diabetic osteoporosis. 9 [PMC free article] [PubMed]
- 50.Deeks J, Higgins J, Altman D (2022) (editors) Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins J, Thomas J, Chandler J, et al., eds. Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022). Cochrane. Available from www.training.cochrane.org/handbook
- 51.Recker RR, Kimmel DB, Dempster D, et al. Issues in modern bone histomorphometry. Bone. 2011;49:955–964. doi: 10.1016/j.bone.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou H, Dempster DW (2021) Lessons from bone histomorphometry on the mechanisms of action of osteoporosis drugs. In: Marcus and Feldman’s Osteoporosis. Elsevier 1835–63. 10.1016/B978-0-12-813073-5.00081-2
- 53.Wu Y, Wang F, Zhang Z. The efficacy and safety of bisphosphonate analogs for treatment of osteoporosis after spinal cord injury: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2021;32:1117–1127. doi: 10.1007/s00198-020-05807-0. [DOI] [PubMed] [Google Scholar]
- 54.Nayak S, Greenspan SL. Osteoporosis treatment efficacy for men: a systematic review and meta-analysis. J Am Geriatr Soc. 2017;65:490–495. doi: 10.1111/jgs.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Díez-Pérez A, Marin F, Eriksen EF, et al. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone. 2019;120:1–8. doi: 10.1016/j.bone.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Sun Y, Li Y, Li J, et al. Efficacy of the combination of teriparatide and denosumab in the treatment of postmenopausal osteoporosis: a meta-analysis. Front Pharmacol. 2022;13:888208. doi: 10.3389/fphar.2022.888208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jepsen DB, Thomsen K, Hansen S, et al. Effect of whole-body vibration exercise in preventing falls and fractures: a systematic review and meta-analysis. BMJ Open. 2017;7:e018342. doi: 10.1136/bmjopen-2017-018342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dionello CF, Morel DS, Moreira-Marconi E et al (2016) Effects of whole body vibration exercises on bone mineral density of women with postmenopausal osteoporosis without medications: novel findings and literature review. 11 [PMC free article] [PubMed]
- 59.Shojaa M, von Stengel S, Kohl M et al (2020) Effects of dynamic resistance exercise on bone mineral density in postmenopausal women: a systematic review and meta-analysis with special emphasis on exercise parameters. Osteoporos Int 31:1427–1444. 10.1007/s00198-020-05441-w [DOI] [PMC free article] [PubMed]
- 60.Yan Y, Tan B, Fu F, et al. Exercise vs conventional treatment for treatment of primary osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Orthop Surg. 2021;13:1474–1487. doi: 10.1111/os.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanis JA, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.The Royal Australian College of General Practitioners and Osteoporosis Australia (2017) Osteoporosis prevention, diagnosis and management in postmenopausal women and men over 50 years of age. 2nd edn. East Melbourne: : Vic: RACGP
- 63.Compston J, Cooper A, Cooper C et al (2017) UK clinical guideline for the prevention and treatment of osteoporosis. Archives of Osteoporosis 12:1–24 [DOI] [PMC free article] [PubMed]
- 64.Beck BR, Daly RM, Singh MAF, et al. Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J Sci Med Sport. 2017;20:438–445. doi: 10.1016/j.jsams.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 65.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3:S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sterne JAC, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 11 [DOI] [PubMed]
- 67.Polidoulis I, Beyene J, Cheung AM (2012) The effect of exercise on pQCT parameters of bone structure and strength in postmenopausal women—a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 23:39–51. 10.1007/s00198-011-1734-7 [DOI] [PubMed]
- 68.Delmas PD, Eastell R, Garnero P, et al. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000;11:S2–17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 69.del C Navarro M, Saavedra P, Jódar E, et al. Osteoporosis and metabolic syndrome according to socio-economic status, contribution of PTH, vitamin D and body weight: the Canarian Osteoporosis Poverty Study (COPS) Clin Endocrinol (Oxf) 2013;78:681–6. doi: 10.1111/cen.12051. [DOI] [PubMed] [Google Scholar]
- 70.Brennan SL, Pasco JA, Urquhart DM, et al. The association between socioeconomic status and osteoporotic fracture in population-based adults: a systematic review. Osteoporos Int. 2009;20:1487–1497. doi: 10.1007/s00198-008-0822-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Included manuscript and appendix are tables with source data and links to online data repositories with statistical code and source data are presented in the manuscript.