Abstract
Objective
To synthesise the evidence on the effects of physical activity on symptoms of depression, anxiety and psychological distress in adult populations.
Design
Umbrella review.
Data sources
Twelve electronic databases were searched for eligible studies published from inception to 1 January 2022.
Eligibility criteria for selecting studies
Systematic reviews with meta-analyses of randomised controlled trials designed to increase physical activity in an adult population and that assessed depression, anxiety or psychological distress were eligible. Study selection was undertaken in duplicate by two independent reviewers.
Results
Ninety-seven reviews (1039 trials and 128 119 participants) were included. Populations included healthy adults, people with mental health disorders and people with various chronic diseases. Most reviews (n=77) had a critically low A MeaSurement Tool to Assess systematic Reviews score. Physical activity had medium effects on depression (median effect size=−0.43, IQR=−0.66 to –0.27), anxiety (median effect size=−0.42, IQR=−0.66 to –0.26) and psychological distress (effect size=−0.60, 95% CI −0.78 to –0.42), compared with usual care across all populations. The largest benefits were seen in people with depression, HIV and kidney disease, in pregnant and postpartum women, and in healthy individuals. Higher intensity physical activity was associated with greater improvements in symptoms. Effectiveness of physical activity interventions diminished with longer duration interventions.
Conclusion and relevance
Physical activity is highly beneficial for improving symptoms of depression, anxiety and distress across a wide range of adult populations, including the general population, people with diagnosed mental health disorders and people with chronic disease. Physical activity should be a mainstay approach in the management of depression, anxiety and psychological distress.
PROSPERO registration number
CRD42021292710.
Keywords: depression; anxiety; physical activity; exercise; stress, physiological
Introduction
Mental health disorders are among the leading causes of the global health-related burden, with substantial individual and societal costs.1 2 In 2019, one in eight people (970 million) worldwide were affected by a mental health disorder3 and almost one in two (44%) will experience a mental health disorder in their lifetime.4 The annual global costs of mental health disorders have been estimated at $2.5 trillion (USD), which is projected to increase to $6 trillion (USD) by 2030.5 Depression is the leading cause of mental health-related disease burden,6 while anxiety is the most prevalent mental health disorder.3 Additionally, the COVID-19 pandemic has been associated with increased rates of psychological distress, with prevalence ranging between 35% and 38% worldwide.7–9
The role of lifestyle management approaches, such as exercise, sleep hygiene and a healthy diet, varies between clinical practice guidelines in different countries. In US clinical guidelines,10 psychotherapy or pharmacotherapy is recommended as the initial treatment approaches, with lifestyle approaches considered as ‘complementary alternative treatments’ where psychotherapy and pharmacotherapy are ‘ineffective or unacceptable’. In other countries such as Australia, lifestyle management is recommended as the first-line treatment approach,11 12 though in practice, pharmacotherapy is often provided first.
There have been hundreds of research trials examining the effects of physical activity (PA) on depression, anxiety and psychological distress, many of which suggest that PA may have similar effects to psychotherapy and pharmacotherapy (and with numerous advantages over psychotherapy and pharmacotherapy, in terms of cost, side-effects and ancillary health benefits).13–18 Despite the evidence for the benefits of PA, it has not been widely adopted therapeutically. Patient resistance, the difficulty of prescribing and monitoring PA in clinical settings, as well as the huge volume of largely incommensurable studies, have probably impeded a wider take-up in practice.13 14 17
Meta-reviews are systematic reviews of systematic reviews, offering a way of synthesising a vast evidence base. While there have been several meta-reviews of PA for depression, anxiety and psychological distress,17 19–24 they have focused on specific population subgroups, particular conditions (eg, depression only) or on particular forms of PA. We set out to undertake the most comprehensive synthesis to date of evidence regarding the effects of all modes of PA on symptoms of depression, anxiety and psychological distress in adult populations.
Methods
Protocol and registration
The protocol for this systematic umbrella review was prospectively registered on PROSPERO and results are reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)25 guidelines.
Selection criteria and search strategy
The population, intervention, comparison, outcomes and study type (PICOS) framework was used to develop the inclusion criteria as follows: population: any adult population (aged ≥18 years); intervention: interventions designed to increase PA. The following definition of PA was used: ‘any bodily movement produced by the contraction of skeletal muscles that results in a substantial increase in caloric requirements over resting energy expenditure’.26 Reviews were eligible irrespective of PA modality, supervision, delivery (eg, in-person or online) or dose (frequency, intensity and duration). Reviews were ineligible if they included any randomised control trials (RCTs) of non-PA interventions, if PA was combined with another intervention (eg, diet) or if they evaluated single bouts of acute exercise. Comparator: reviews were eligible if ≥75% of the included RCTs involved either usual care, waitlist, nothing an equal attention intervention or a lower/lesser PA intervention (eg, a supervised exercise intervention vs printed PA materials). During study selection, it became apparent that the comparator inclusion/exclusion criteria needed elaboration. After careful consideration and discussion, we decided to exclude reviews where >25% of component RCTs compared PA to pharmaceutical interventions or compared two types of equal dose exercise (eg, resistance vs aerobic exercise) without a non-PA comparison, since the inclusion of such reviews would limit our ability to evaluate the effectiveness of PA per se. Outcomes: any self-report or clinician-rated assessment of depression, anxiety or psychological distress symptoms. Study type: systematic reviews with meta-analyses of RCTs only, which included meta-analyses of the outcomes of interest.
Twelve databases were searched (CINAHL, Cochrane, Embase, MEDLINE, Emcare, ProQuest Health and Medical Complete, ProQuest Nursing and Allied Health Source, PsycINFO, Scopus, Sport Discus, EBSCOhost and Web of Science) using subject heading, keyword and Medical Subject Headings (MeSH) term searches for ‘systematic review’, ‘meta-analysis’, ‘physical activity’, ‘exercise’, ‘anxiety’, ‘depression’ and ‘psychological distress’ (see online supplemental eTable 1 for the full search strategy). Database searches were limited to peer-reviewed journal articles published in English language from inception to 1 January 2022.
bjsports-2022-106195supp001.pdf (2.1MB, pdf)
Data management and extraction
Search results were imported into EndNote V.x9 (Clarivate, Philadelphia) where duplicates were removed, then exported into Covidence (Veritas Health Innovation, Melbourne, Australia). Title/abstract and full-text screening, data extraction and risk of bias scoring were completed in duplicate by two independent reviewers (BS and AM, AW, CEMS, DD, EE, EO, KS, RC, RV or TF), with disagreements resolved by team discussion.
Data were extracted in duplicate by two independent reviewers (BS and AM, AW, CEMS, DD, EE, EO, KS, RC, RV or TF) using a standardised extraction form,27 28 and discrepancies were resolved by team discussion. The risk of bias of the included reviews was assessed by two independent reviewers (BS and AM, AW, CEMS, DD, EE, EO, KS, RC, RV or TF) in duplicate using the A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) tool.29 The AMSTAR-2 tool involves 16 items, with each item scored as yes, partial yes or no. Seven items are considered ‘critical’ and nine ‘non-critical’.29 The critical domains are protocol registration, adequacy of search strategy, justification for excluding individual studies, risk of bias assessment, appropriateness of meta-analysis methods, use of risk of bias during interpretation and assessment of publication bias. Reviews were rated as ‘high confidence’ (0 critical weakness and <3 non-critical weaknesses), ‘moderate’ (one critical weakness and <3 non-critical weaknesses), ‘low’ (>1 critical weakness and <3 non-critical weaknesses) or ‘critically low’ (>1 critical weakness and ≥3 non-critical weaknesses).29
Umbrella review synthesis methods
The overlap in component RCTs that were included across all eligible reviews was assessed using the Corrected Covered Area (CCA) method.30 A CCA of 100% indicates that every review included in our umbrella review comprised the same component RCTs, while a CCA of 0% indicates that every review in our umbrella review included entirely unique RCTs. The following cut-offs were used to quantify the CCA: 0%–5%=‘slight overlap’; 6%–10%=‘moderate’; 11%–15%=‘high’ and >15%=‘very high’ overlap.30 Publication bias was assessed by creating a funnel plot and observing the presence of asymmetries or missing sections.31
Meta-analysis results from each review were presented using forest plots. Separate forest plots were created for meta-analyses reporting standardised (eg, standardised mean difference, SMD) and unstandardised effect sizes (eg, mean difference). For meta-analyses that reported standardised effect sizes, we undertook subgroup analyses for clinical status and intervention characteristics. Meta-analysis results were summarised using medians and IQRs
The Oxford Centre for Evidence-Based Medicine levels of evidence and grades for recommendations32 were used to classify the overall level of evidence as grade A: consistent level 1 studies (ie, systematic reviews of RCTs or individual RCTs); B: consistent level 2 (ie, systematic reviews of cohort studies or individual cohort studies) or level 3 studies (ie, systematic reviews of case–control studies or individual case–control studies) or extrapolations from level 1 studies; C: level 4 studies (ie, case series) or extrapolations from level 2 or 3 studies or D: level 5 (ie, expert opinion without explicit critical appraisal) evidence or troublingly inconsistent or inconclusive studies of any level.32
Results
Of the 1280 records identified, 97 were eligible. They included 1039 unique (component) RCTs and the CCA was 0.6%, indicating slight overlap (see online supplemental eFigure 1 for PRISMA flowchart, including reasons for exclusions). Evaluation of funnel plots indicated no evidence of publication bias (online supplemental eFigure 2).
An overview of all reviews’ characteristics is shown in online supplemental eTable 2. There was a total of >128 119 participants (n=133 did not report the number of participants). Mean participant age ranged from 29 to 86 (median=55) years, and most reviews (n=83, 86%) involved female and male participants. An overview of all populations and PA modalities is shown in table 1. Fifteen reviews specifically involved individuals with depression33–41 and three involved individuals with anxiety.42–44 Most reviews involved various PA modes (n=70) and most (n=77) had a critically low AMSTAR-2 score (low: n=10; high: n=10, online supplemental eTable 3).
Table 1.
Overview of all populations, conditions and physical activity modes of the included reviews
| Population or condition | Number of reviews |
| Cancer | n=2745–62 98–106 |
| Adults with depression | n=1163 64 66–73 75 |
| Dementia | n=5107–111 |
| Older adults | n=533–37 |
| Chronic obstructive pulmonary disease | n=478 112–114 |
| Multiple sclerosis | n=481 115–117 |
| Pregnant or postnatal or post-partum women | n=438–41 |
| Adults with various chronic diseases | n=3118–120 |
| Apparently healthy adults | n=342–44 |
| Anxiety disorders | n=384 85 121 |
| Cardiovascular disease | n=380 122 123 |
| Older adults with depression | n=463 65 74 124 |
| Renal disease | n=386 125 126 |
| Stroke | n=321 127 128 |
| Arthritis and rheumatic diseases | n=282 129 130 |
| Cognitive impairment | n=277 131 |
| HIV/AIDS | n=276 132 |
| Post-traumatic stress disorder | n=2133 134 |
| Various mental health disorders | n=283 135 |
| Fatigue | n=1136 |
| Knee osteoarthritis | n=1129 |
| Neurological disorders | n=1137 |
| Schizophrenia | n=1138 |
| Substance use disorder | n=179 |
| Physical activity modality | Number of reviews |
| Mixed-mode exercise, not including mind-body exercise (eg, Yoga, Tai Chi or Qigong) | n=3713 21 38 41 47 50 52–55 58 59 66–70 73–75 81 84 86 100 101 103 106 108–110 112 121 123 125 126 132 133 |
| Mixed-mode exercise, including mind-body exercises (eg, Yoga, Tai Chi or Qigong) | n=3333–37 40 42 49 51 56 57 60 61 63 72 76–80 98 99 102 104 107 115–117 124 129 130 134 137 |
| Yoga | n=739 45 46 62 127 135 138 |
| Aerobic exercise | n=643 71 82 83 85 122 |
| Tai Chi | n=665 113 118 128 131 136 |
| Qigong | n=3104 114 120 |
| Resistance exercise | n=364 111 119 |
| Dance | n=244 48 |
Meta-analysis results: depression
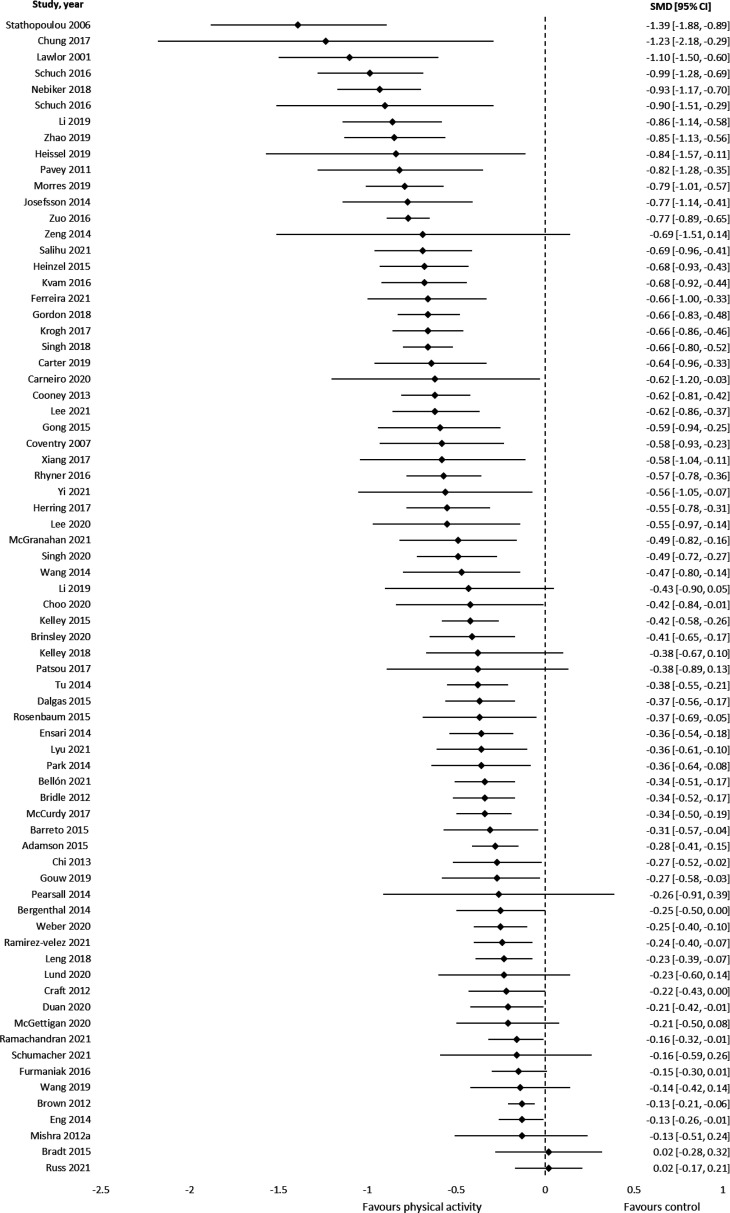
Results from 72 meta-analyses based on SMD (n=875 component RCTs, >62 040 participants) showed a medium effect in favour of PA for reducing depression and depressive symptoms (median SMD=−0.43, IQR=−0.66 to –0.27, figure 1).
Figure 1.
Results of meta-analyses that assessed symptoms of depression using standardised mean differences (negative values represent a reduction in symptoms).
MD effect size for each instrument was: profile of mood states: −7.68 (1 review), Beck Depression Inventory: −5.53 (IQR=−6.24 to –4.81), The Edinburgh Postnatal Depression Scale: −2.97 (IQR=−3.49 to –2.44), self-rating scale: −3.99 (one review), Brief Symptom Inventory 18: −3.02 (one review), Centre for Epidemiological Studies Depression: −0.36 (IQR=−1.25 to 0.02), Montgomery-Asberg Depression Rating Scale: −1.80 and Hospital Anxiety and Depression Scale: −1.26 (IQR=−1.41 to –1.18, online supplemental eFigure 3 and online supplemental eTable 4).
Grade of recommendation: (A) Consistent level 1 studies.
Anxiety
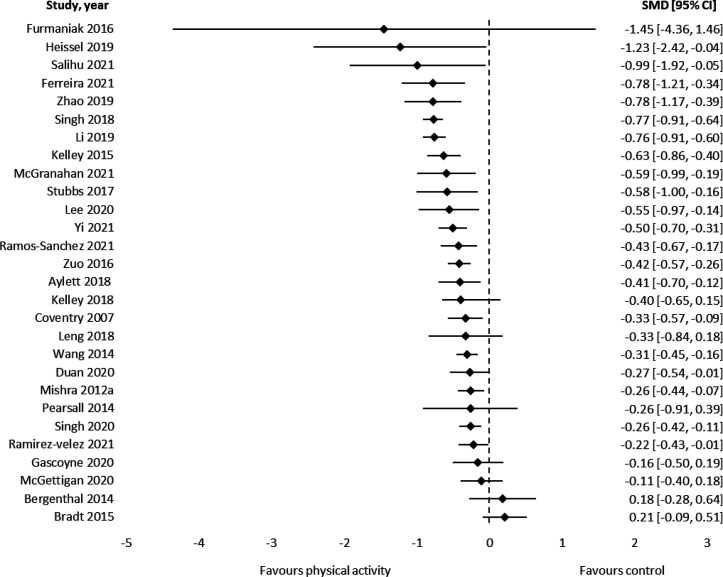
Results from 28 meta-analyses using SMD (171 component RCTs, >10 952 participants) showed a medium effect of PA for reducing anxiety (median SMD=−0.42, IQR=−0.66 to –0.26, figure 2).
Figure 2.
Results of meta-analyses that assessed symptoms of anxiety using standardised mean differences (negative values represent a reduction in symptoms).
MD effect sizes for each instrument were: The State-Trait Anxiety Inventory: −3.61 (IQR=−6.01 to –1.66), Brief Symptom Inventory-18: −5.45 (1 review), Self-rating scale: −4.57 (1 review), Hospital Anxiety and Depression Scale: −1.26 (IQR=−1.26 to –0.79, online supplemental eTable 4 and online supplemental eFigure 5).
Grade of recommendation: (A) Consistent level 1 studies.
Psychological distress
One systematic review45 reported SMD results for psychological distress (six component RCTs, 508 participants), while another systematic review46 reported MD results (one component RCT, 39 participants). Results showed a medium effect in favour of PA, compared with usual care (SMD=−0.60, 95% CI −0.78 to –0.42). For MD, findings showed no significant effect (MD=−0.30, 95% CI −5.55, 4.95, one review, one component RCT, 39 participants).
Grade of recommendation: (B) Consistent level 2 or 3 studies or extrapolations from level 1 studies.
Subgroup analyses: clinical status
Depression
Seventeen reviews provided data on patients with cancer,45 47–62 and 16 on people with depression or depressive symptoms.10 33 39 63–75 PA was effective in reducing depressive symptoms across all conditions (median SMD range: –0.85 (kidney disease), –0.16 (cardiovascular disease)). The largest effects were found in kidney disease, HIV, chronic obstructive pulmonary disease, generally healthy adults and individuals diagnosed with depression (table 2).
Table 2.
Summary data on the effects of physical activity interventions on depression for a range of clinical conditions, including the number of reviews, studies and participants covered; and the 25th percentile, median and 75th percentile for standardised mean differences
| Condition | Reviews | RCTs* | Participants† | Standardised mean difference | ||
| 25th percentile | Median | 75th percentile | ||||
| Arthritis | 4 | 55 | 3713 | –0.73 | –0.40 | –0.31 |
| Cancer | 17 | 196 | >14 616 | –0.46 | –0.22 | –0.15 |
| Blood | 2 | 5 | 315 | –0.25 | –0.24 | –0.22 |
| Breast | 8 | 84 | 7113 | –0.61 | –0.47 | –0.17 |
| Colorectal | 3 | 8 | 575 | –0.23 | –0.21 | –0.11 |
| Lung | 1 | 6 | 695 | –0.49 | ||
| Lymphoma | 1 | 2 | 161 | –0.35 | ||
| Non-specific | 5 | 85 | >5543 | –0.22 | –0.21 | –0.13 |
| Prostate | 2 | 4 | 214 | –0.20 | –0.18 | –0.16 |
| Chronic disease | 3 | 18 | >994 | –0.38 | –0.27 | –0.27 |
| Chronic obstructive pulmonary disease | 2 | 16 | 1175 | –0.86 | –0.72 | –0.58 |
| Cardiovascular disease | 5 | 54 | 6681 | –0.37 | –0.16 | –0.14 |
| Stroke | 2 | 19 | 1580 | –0.36 | –0.25 | –0.13 |
| Heart disease | 3 | 26 | 4435 | –0.33 | –0.16 | –0.15 |
| Dementia | 4 | 37 | >3470 | –0.37 | –0.27 | –0.11 |
| Diagnosed | 3 | 16 | >881 | –0.41 | –0.31 | –0.06 |
| Cognitive impairment | 1 | 21 | 2589 | –0.23 | ||
| Depression | 15 | 299 | >13 318 | –0.82 | –0.62 | –0.33 |
| Diagnosed | 14 | 273 | >12 953 | –0.96 | –0.66 | –0.38 |
| Symptoms | 3 | 26 | >365 | –0.74 | –0.56 | –0.34 |
| General population | 9 | 208 | >14 145 | –0.69 | –0.62 | –0.35 |
| Adults | 6 | 99 | >9016 | –0.82 | –0.69 | –0.66 |
| Older adults | 3 | 75 | >4862 | –0.52 | –0.36 | –0.28 |
| HIV/AIDS | 1 | 9 | 395 | –0.84 | ||
| Kidney disease | 3 | 11 | 567 | –1.14 | –0.85 | −0.71 |
| Mental health disorders | 6 | 39 | 2083 | –0.49 | –0.44 | –0.37 |
| Fatigue | 1 | 7 | 415 | –0.58 | ||
| Non-specific | 1 | 13 | 362 | –0.41 | ||
| Post-traumatic stress disorder | 2 | 7 | 332 | –0.49 | –0.43 | –0.37 |
| Schizophrenia | 1 | 3 | 94 | –0.26 | ||
| Substance abuse | 1 | 9 | 610 | –0.47 | ||
| Neurological disease | 4 | 62 | 2901 | –0.46 | –0.37 | –0.32 |
| Multiple sclerosis | 3 | 39 | 1577 | –0.51 | –0.37 | –0.36 |
| Non-specific | 1 | 23 | 1324 | –0.28 | ||
| Pregnancy/postpartum | 3 | 43 | 3364 | –0.63 | –0.59 | –0.40 |
| Pregnant | 1 | 10 | 609 | –0.59 | ||
| Postpartum | 2 | 33 | 2755 | –0.69 | –0.49 | –0.34 |
*Reviews may have included overlapping randomised controlled trials.
†Randomised controlled trials may have included overlapping participants.
RCTs, randomised controlled trials.
Anxiety
PA was generally effective for reducing anxiety across disease conditions, with median SMDs ranging from –1.23 (HIV) to –0.16 (multiple sclerosis). However, the evidence base was limited except for cancer and anxiety disorders (table 3).
Table 3.
Summary data on the effects of physical activity interventions on anxiety for a range of clinical conditions, including the number of reviews, studies and participants covered; and the 25th percentile, median and 75th percentile for standardised mean differences
| Condition | Reviews | RCTs* | Participants† | Standardised mean difference | ||
| 25th percentile | Median | 75th percentile | ||||
| Arthritis | 2 | 29 | 1859 | –0.63 | –0.52 | –0.40 |
| Cancer | 12 | 72 | 4955 | –0.50 | –0.24 | 0.06 |
| Blood | 1 | 3 | 249 | 0.18 | ||
| Breast | 7 | 46 | 2862 | –0.72 | –0.53 | 0.44 |
| Colorectal | 1 | 4 | 98 | –0.11 | ||
| Lung | 1 | 6 | 695 | –0.26 | ||
| Non-specific | 2 | 13 | 951 | –0.21 | –0.05 | 0.17 |
| Chronis obstructive pulmonary disease | 2 | 14 | 1067 | –0.76 | –0.55 | –0.33 |
| General population | 2 | 7 | 2781 | –0.99 | –0.85 | –0.70 |
| HIV/AIDS | 1 | 5 | 185 | –1.23 | ||
| Kidney disease | 2 | 4 | 246 | –0.78 | –0.78 | –0.78 |
| Mental disorders | 6 | 40 | >2133 | –0.59 | –0.43 | –0.34 |
| Anxiety | 3 | 25 | >1197 | –0.54 | –0.43 | –0.41 |
| Cognitive impairment | 1 | 3 | 210 | –0.33 | ||
| Post-traumatic stress disorder | 2 | 9 | >101 | –0.59 | –0.44 | –0.30 |
| Schizophrenia | 1 | 3 | 94 | –0.26 | ||
| Substance abuse disorder | 1 | 7 | 741 | –0.31 | ||
| Multiple sclerosis | 1 | 4 | 133 | –0.16 | ||
*Reviews may have included overlapping randomised controlled trials.
†Randomised controlled trials may have included overlapping participants.
RCTs, randomised controlled trials.
Exercise mode
Depression
Eighteen reviews33 34 37 39 42 51 57 58 60 61 72–74 76–80 provided analyses by exercise mode (310 component RCTs, >14 496 participants, online supplemental eFigure 6). All modes were effective, and median effect sizes (SMDs) were similar across modes: –0.64 (IQR=–0.86 to–0.19) for strength-based interventions (nine reviews); –0.47 (IQR=–0.64 to–0.29) for mixed-mode interventions (12 reviews); –0.46 (IQR=–0.77 to–0.33) for stretching, yoga and other mind–body modalities (11 reviews) and –0.45 (IQR=–0.79 to–0.37) for aerobic exercise (15 reviews).
Anxiety
Fifteen reviews44 45 48 51 58 60 61 78 79 81–86 reported analyses by exercise mode (115 component RCTs, >5451 participants, online supplemental eFigure 7). All modes were effective, with median SMDs of –0.23 (IQR=–0.37 to –0.08) for strength-based interventions (two reviews); –0.35 (IQR=–0.86 to –0.23) for mixed modes (four reviews); –0.42 (IQR=–0.78 to –0.16) for stretching, yoga, and other mind-body modalities (seven reviews) and –0.29 (IQR=–0.54, –0.16) for aerobic exercise (six reviews).
Exercise intensity
Depression
Five reviews21 42 58 73 74 reported analyses by exercise intensity (63 component RCTs, >2776 participants, online supplemental eFigure 8). Low, moderate and high-intensity exercise interventions had a median SMD of –0.22 (IQR=–0.50 to –0.12), –0.56 (IQR=–1.03 to –0.33) and –0.70 (IQR=–1.25 to –0.24), respectively.
Anxiety
Two reviews58 84 reported analyses by exercise intensity (23 component RCTs, online supplemental eFigure 9). All intensities were effective. The single review for low-intensity exercise had a median SMD of –0.26; the one for moderate-intensity exercise –0.47, and the two for high-intensity exercise –0.44 (IQR=–0.49 to –0.13).
Intervention duration
Depression
Twelve reviews38 42 56 57 60 61 65 68 69 78 80 reported analyses by intervention duration (166 component RCTs, 15 669 participants, online supplemental eFigure 10). All durations were effective, but effectiveness declined as intervention duration increased. The median SMDs for short (≤12 weeks, 12 reviews), medium (12–23 weeks, 11 reviews) and long duration (≥24 weeks, 4 reviews) interventions were –0.84 (IQR=–1.50 to –0.48), –0.46 (IQR=–0.53 to –0.25) and –0.28 (IQR=–1.15 to –0.17), respectively.
Anxiety
Four reviews56 60 61 78 reported analyses by intervention duration (38 component RCTs, 2325 participants, online supplemental eFigure 11). Median SMDs for short (12 weeks) and median-duration (12–23 weeks) interventions were –0.55 (IQR=–0.83 to –0.27) and –0.47 (IQR=–0.72 to –0.08), respectively. The single review reporting on longer interventions (≥24 weeks) reported a median SMD of –0.15.
Weekly duration
Depression
Four reviews42 44 57 58 presented analyses by weekly session duration (68 component RCTs, >5016 participants, online supplemental eFigure 12). The median SMD for ≤150 min/week and >150 min/week was –0.58 (IQR=–0.77 to –0.30) and –0.29 (IQR=–0.40 to –0.07), respectively.
Anxiety
One review58 provided analyses by weekly session duration (17 component RCTs, online supplemental eFigure 13). The median SMDs for <150 min/week and ≥150 min/week were –1.23 and –0.99, respectively.
Session frequency
Depression
Three reviews42 76 78 (36 component RCTs, >232 participants) reported on session frequency. High-frequency (5–7 sessions per week), moderate-frequency (4–5 per week) and low-frequency (<4 per week) interventions had a median SMD of –0.76 (IQR=–1.20 to –0.32), –1.12 (–1.39 to –0.85) and –0.47 (IQR=–0.59 to–0.35), respectively (online supplemental eFigure 14).
Anxiety
One review78 compared session frequency, with SMDs of –0.50, –0.96 and –0.52 for 2–3, 4–5 and 6–7 session per week, respectively (online supplemental eFigure 13).
Session duration
Depression
Three reviews42 50 78 presented analyses on session duration (online supplemental content 17). Long (≥60 min, SMD=–0.57, IQR –0.85 to –0.35) and medium (30–60 min, SMD=–0.60, IQR –0.78 to –0.41) session durations had similar benefits. The sole study of short sessions (<30 min) had a SMD of 0.01 (online supplemental eFigure 15).
Anxiety
One review78 reported on the effects of session duration (online supplemental content 16). There was no difference between long (SMD=–0.63) and short (SMD=–0.83) sessions (online supplemental eFigure 13).
Discussion
This is the first ever study to compile the extensive base of evidence regarding the effects of PA on depression, anxiety and psychological distress. We identified 97 systematic reviews, reporting the findings of 1039 unique RCTs, involving 128 119 participants. Findings suggest that PA interventions are effective in improving symptoms of depression and anxiety. Improvements were observed across all clinical populations, though the magnitude of effect varied across different clinical populations. The greatest benefits were seen in people with depression, pregnant and postpartum women, apparently healthy individuals and individuals diagnosed with HIV or kidney disease. All PA modes were effective, and higher intensity exercise was associated with greater improvements for depression and anxiety. Longer duration interventions had smaller effects compared with short and mid-duration, though the longest duration interventions still had positive effects.
PA was effective at reducing depression and anxiety across all clinical conditions, though the magnitude of the benefit varied between clinical groups. The larger effect sizes observed in clinical populations may reflect that these populations experience above-average symptoms of depression and anxiety and have low PA levels, and, therefore, have a greater scope for improvement compared with non-clinical populations.17
All PA modes were beneficial, including aerobic, resistance, mixed-mode exercise and yoga. It is likely that the beneficial effects of PA on depression and anxiety are due to a combination of various psychological, neurophysiological and social mechanisms.87 Different modes of PA stimulate different physiological88 and psychosocial effects,88–90 and this was supported by our findings (eg, resistance exercise had the largest effects on depression, while Yoga and other mind–body exercises were most effective for reducing anxiety). Furthermore, our findings showed that moderate-intensity and high-intensity PA modes were more effective than lower intensities. PA improves depression though various neuromolecular mechanisms including increased expression of neurotrophic factors, increased availability of serotonin and norepinephrine, regulation of hypothalamic–pituitary–adrenal axis activity and reduced systemic inflammation.91 92 Therefore, low-intensity PA may be insufficient for stimulating the neurological and hormonal changes that are associated with larger improvements in depression and anxiety.87 Overall, our findings add further support to public health guidelines, which recommend multimodal, moderate and vigorous intensity PA.
Our findings that longer duration interventions were less effective than shorter interventions may seem counter intuitive. It is possible that this finding reflects a decline in adherence with longer interventions. Furthermore, due to a lack of blinding of participants in PA trials, participants may have expected to have improved symptoms. It is possible that after experiencing short-term improvements in depression or anxiety, the expectancy effect may diminish over longer periods of time. An alternative explanation is that the longer interventions might not provide sufficient progression of PA dose, leading to a reduction in their effectiveness. Furthermore, it was somewhat surprising that smaller weekly duration interventions demonstrated larger effects than higher weekly duration. This is the opposite to the dose–benefit relationship observed for exercise and physical health outcomes.93 It is possible that shorter duration interventions are easier for participants to comply with, whereas longer weekly duration interventions are more burdensome and that may be impacting the psychological benefits. It is a useful message that interventions do not need to provide high doses of PA for improvements in depression.
The key strength of this study was that it is the first umbrella review to evaluate the effects of all types of PA on depression, anxiety and psychological distress in all adult populations. We included only the highest level of evidence: meta-analyses of RCTs and applied stringent criteria regarding the design of the component RCTs to ensure that effects could be confidently attributed to PA rather than other intervention components. Additionally, there was only slight overlap in the component RCTs, increasing our confidence in the findings.
A limitation of the review is that most evidence focused on mild-to-moderate depression, with fewer reviews addressing anxiety and psychological distress, preventing us from reaching firm conclusions in the subgroup analyses for these outcomes. Furthermore, most (n=77) of the included reviews were rated as ‘critically low’, based on the AMSTAR-2 quality rating.
Clinical implications
PA is effective for managing symptoms of depression and anxiety across numerous populations, including the general population, people with mental illnesses and various other clinical populations. While the benefit of exercise for depression and anxiety is generally recognised, it is often overlooked in the management of these conditions. Furthermore, many people with depression and anxiety have comorbidities, and PA is beneficial for their mental health and disease management. This underscores the need for PA to be a mainstay approach for managing depression and anxiety.
All modes of PA are effective, with moderate-to-high intensities more effective than low intensity. Larger benefits are achieved from shorter interventions, which has health service delivery cost implications–suggesting that benefits can be obtained following short-term interventions, and intensive long-term interventions are not necessarily required to achieve therapeutic benefit. The effect size reductions in symptoms of depression (−0.43) and anxiety (−0.42) are comparable to or slightly greater than the effects observed for psychotherapy and pharmacotherapy (SMD range=−0.22 to −0.37).94–97 Future research to understand the relative effectiveness of PA compared with (and in combination with) other treatments is needed to confirm these findings.
In conclusion, PA is effective for improving depression and anxiety across a very wide range of populations. All PA modes are effective, and higher intensity is associated with greater benefit. The findings from this umbrella review underscore the need for PA, including structured exercise interventions, as a mainstay approach for managing depression and anxiety.
What is already known.
Previous research trials suggest that physical activity may have similar effects to psychotherapy and pharmacotherapy for patients with depression, anxiety or psychological distress.
Studies have evaluated different forms of physical activity, in varying dosages, in different population subgroups, and using different comparator groups, making it difficult for clinicians to understand the body of evidence for physical activity in the management of mental health disorders.
What are the new findings.
Results showed that physical activity is effective for reducing mild-to-moderate symptoms of depression, anxiety and psychological distress (median effect size range=−0.42 to –0.60), compared with usual care across all populations.
Our findings underscore the important role of physical activity in the management of mild-to-moderate symptoms of depression, anxiety and psychological distress.
Footnotes
Twitter: @bensinghphd
Correction notice: This article has been corrected since it published Online First. The article type has been changed to systematic review.
Contributors: BS, TO and CM conceived the idea for the review. BS, RC, DD, RV, AW, KS, EOC, TF, EE, AM and CEMS conducted search, study selection, data extraction and quality assessment. BS, TO and CM drafted the initial manuscript. RC, DD, RV, AW, KS, EOC, TF, EE, AM and CEMS contributed to writing the manuscript. All authors reviewed and approved the final manuscript.
Funding: DD is supported by the Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship APP1162166 and by the Centre of Research Excellence in Driving Global Investment in Adolescent Health funded by NHMRC APP1171981. AM is supported by the Centre of Research Excellence in Driving Global Investment in Adolescent Health funded by NHMRC APP1171981. Dr Maher is supported by a Medical Research Future Fund Emerging Leader Grant (GNT1193862).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. World Health Organization . Depression and other common mental disorders: global health estimates. licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Santomauro DF, Mantilla Herrera AM, Shadid J. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021;398:1700–12. 10.1016/S0140-6736(21)02143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Institute for Health Metrics and Evaluation . Global health data exchange 2019. University of Washington, 2022. [Google Scholar]
- 4. Australian Bureau of Statistics . National study of mental health and wellbeing, 2021-2011. Australian Government, 2022. Available: url:%20https://www.abs.gov.au/statistics/health/mental-health/national-study-mental-health-and-wellbeing/latest-release [Google Scholar]
- 5. Marquez PV, Saxena S. Making mental health a global priority. Cerebrum 2016;2016:cer-10-16. [PMC free article] [PubMed] [Google Scholar]
- 6. Patel V, Chisholm D, Parikh R, et al. Addressing the burden of mental, neurological, and substance use disorders: key messages from disease control priorities, 3rd edition. Lancet 2016;387:1672–85. 10.1016/S0140-6736(15)00390-6 [DOI] [PubMed] [Google Scholar]
- 7. Necho M, Tsehay M, Birkie M, et al. Prevalence of anxiety, depression, and psychological distress among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Int J Soc Psychiatry 2021;67:892–906. 10.1177/00207640211003121 [DOI] [PubMed] [Google Scholar]
- 8. Qiu J, Shen B, Zhao M, et al. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: implications and policy recommendations. Gen Psychiatr 2020;33:e100213. 10.1136/gpsych-2020-100213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaix B, Delamon G, Guillemassé A, et al. Psychological distress during the COVID-19 pandemic in France: a national assessment of at-risk populations. Gen Psychiatr 2020;33:e100349. 10.1136/gpsych-2020-100349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Physhological Association . Clinical practice guideline for the treatment of depression across three age cohorts. Washington: American Psychological Association, 2019. [Google Scholar]
- 11. Australian Government Productivity Commission . Productivity commission inquiry report: mental health. Canberra, Australia, 2020. [Google Scholar]
- 12. Malhi GS, Bell E, Bassett D, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry 2021;55:7–117. 10.1177/0004867420979353 [DOI] [PubMed] [Google Scholar]
- 13. Kvam S, Kleppe CL, Nordhus IH, et al. Exercise as a treatment for depression: a meta-analysis. J Affect Disord 2016;202:67–86. 10.1016/j.jad.2016.03.063 [DOI] [PubMed] [Google Scholar]
- 14. Schuch FB, Stubbs B. The role of exercise in preventing and treating depression. Curr Sports Med Rep 2019;18:299–304. 10.1249/JSR.0000000000000620 [DOI] [PubMed] [Google Scholar]
- 15. Schuch FB, Vancampfort D, Firth J, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry 2018;175:631–48. 10.1176/appi.ajp.2018.17111194 [DOI] [PubMed] [Google Scholar]
- 16. Gianfredi V, Blandi L, Cacitti S, et al. Depression and objectively measured physical activity: a systematic review and meta-analysis. Int J Environ Res Public Health 2020;17:3738–45. 10.3390/ijerph17103738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rebar AL, Stanton R, Geard D, et al. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev 2015;9:366–78. 10.1080/17437199.2015.1022901 [DOI] [PubMed] [Google Scholar]
- 18. Schuch FB, Stubbs B, Meyer J, et al. Physical activity protects from incident anxiety: a meta-analysis of prospective cohort studies. Depress Anxiety 2019;36:846–58. 10.1002/da.22915 [DOI] [PubMed] [Google Scholar]
- 19. Narita Z, Inagawa T, Stickley A, et al. Physical activity for diabetes-related depression: a systematic review and meta-analysis. J Psychiatr Res 2019;113:100–7. 10.1016/j.jpsychires.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 20. Ramírez-Vélez R, Zambom-Ferraresi F, García-Hermoso A, et al. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta-analysis. Cancers (Basel) 2021;13:264–72. 10.3390/cancers13020264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eng JJ, Reime B. Exercise for depressive symptoms in stroke patients: a systematic review and meta-analysis. Clin Rehabil 2014;28:731–9. 10.1177/0269215514523631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martland R, Mondelli V, Gaughran F, et al. Can high-intensity interval training improve physical and mental health outcomes? A meta-review of 33 systematic reviews across the lifespan. J Sports Sci 2020;38:430–69. 10.1080/02640414.2019.1706829 [DOI] [PubMed] [Google Scholar]
- 23. Bigarella LG, Ballotin VR, Mazurkiewicz LF, et al. Exercise for depression and depressive symptoms in older adults: an umbrella review of systematic reviews and meta-analyses. Aging Ment Health 2022;26:1503–13. 10.1080/13607863.2021.1951660 [DOI] [PubMed] [Google Scholar]
- 24. Catalan-Matamoros D, Gomez-Conesa A, Stubbs B, et al. Exercise improves depressive symptoms in older adults: an umbrella review of systematic reviews and meta-analyses. Psychiatry Res 2016;244:202–9. 10.1016/j.psychres.2016.07.028 [DOI] [PubMed] [Google Scholar]
- 25. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 27. Hutchesson MJ, Gough C, Müller AM, et al. EHealth interventions targeting nutrition, physical activity, sedentary behavior, or obesity in adults: a scoping review of systematic reviews. Obes Rev 2021;22:e13295. 10.1111/obr.13295 [DOI] [PubMed] [Google Scholar]
- 28. Kracht CL, Hutchesson M, Ahmed M, et al. E‐ & mHealth interventions targeting nutrition, physical activity, sedentary behavior, and/or obesity among children: a scoping review of systematic reviews and meta‐analyses. Obesity Reviews 2021;22:12. 10.1111/obr.13331 Available: https://onlinelibrary.wiley.com/toc/1467789x/22/12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pieper D, Antoine S-L, Mathes T, et al. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol 2014;67:368–75. 10.1016/j.jclinepi.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oxford Centre for Evidence-Based Medicine . Levels of evidence (March 2009). University of Oxford, 2022. [Google Scholar]
- 33. Rhyner KT, Watts A. Exercise and depressive symptoms in older adults: a systematic meta-analytic review. J Aging Phys Act 2016;24:234–46. 10.1123/japa.2015-0146 [DOI] [PubMed] [Google Scholar]
- 34. Heinzel S, Lawrence JB, Kallies G, et al. Using exercise to fight depression in older adults: A systematic review and meta-analysis. GeroPsych: The Journal of Gerontopsychology and Geriatric Psychiatry 2015;28:149–62. [Google Scholar]
- 35. Miller KJ, Areerob P, Hennessy D, et al. Aerobic, resistance, and mind-body exercise are equivalent to mitigate symptoms of depression in older adults: a systematic review and network meta-analysis of randomised controlled trials. F1000Res 2020;9:1325. 10.12688/f1000research.27123.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park SH, Han KS, Kang CB. Effects of exercise programs on depressive symptoms, quality of life, and self-esteem in older people: a systematic review of randomized controlled trials. Appl Nurs Res 2014;27:219–26. 10.1016/j.apnr.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 37. Weber M, Schnorr T, Morat M, et al. Effects of mind-body interventions involving meditative movements on quality of life, depressive symptoms, fear of falling and sleep quality in older adults: a systematic review with meta-analysis. Int J Environ Res Public Health 2020;17:6556–63. 10.3390/ijerph17186556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carter T, Bastounis A, Guo B, et al. The effectiveness of exercise-based interventions for preventing or treating postpartum depression: a systematic review and meta-analysis. Arch Womens Ment Health 2019;22:37–53. 10.1007/s00737-018-0869-3 [DOI] [PubMed] [Google Scholar]
- 39. Gong H, Ni C, Shen X, et al. Yoga for prenatal depression: a systematic review and meta-analysis. BMC Psychiatry 2015;15:14. 10.1186/s12888-015-0393-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCurdy AP, Boulé NG, Sivak A, et al. Effects of exercise on mild-to-moderate depressive symptoms in the postpartum period: a meta-analysis. Obstet Gynecol 2017;129:1087–97. 10.1097/AOG.0000000000002053 [DOI] [PubMed] [Google Scholar]
- 41. Pentland V, Spilsbury S, Biswas A, et al. Does walking reduce postpartum depressive symptoms? A systematic review and meta-analysis of randomized controlled trials. J Womens Health (Larchmt) 2022;31:555–63. 10.1089/jwh.2021.0296 [DOI] [PubMed] [Google Scholar]
- 42. Bellón JÁ, Conejo-Cerón S, Sánchez-Calderón A, et al. Effectiveness of exercise-based interventions in reducing depressive symptoms in people without clinical depression: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2021;219:578–87. 10.1192/bjp.2021.5 [DOI] [PubMed] [Google Scholar]
- 43. Pavey TG, Taylor AH, Fox KR, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ 2011;343:d6462. 10.1136/bmj.d6462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salihu D, Kwan RYC, Wong EML. The effect of dancing interventions on depression symptoms, anxiety, and stress in adults without musculoskeletal disorders: an integrative review and meta-analysis. Complement Ther Clin Pract 2021;45:101467. 10.1016/j.ctcp.2021.101467 [DOI] [PubMed] [Google Scholar]
- 45. Zuo X-L, Li Q, Gao F, et al. Effects of yoga on negative emotions in patients with breast cancer: a meta-analysis of randomized controlled trials. International Journal of Nursing Sciences 2016;3:299–306. 10.1016/j.ijnss.2016.07.009 [DOI] [Google Scholar]
- 46. Felbel S, Meerpohl JJ, Monsef I, et al. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev 2014;2014:CD010146. 10.1002/14651858.CD010146.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bergenthal N, Will A, Streckmann F, et al. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev 2014:CD009075. 10.1002/14651858.CD009075.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bradt J, Shim M, Goodill SW. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev 2015;1:CD007103. 10.1002/14651858.CD007103.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS ONE 2012;7:e30955. 10.1371/journal.pone.0030955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Craft LL, Vaniterson EH, Helenowski IB, et al. Exercise effects on depressive symptoms in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21:3–19. 10.1158/1055-9965.EPI-11-0634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Duan L, Xu Y, Li M. Effects of mind-body exercise in cancer survivors: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2020;2020:7607161. 10.1155/2020/7607161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev 2016;9:CD005001. 10.1002/14651858.CD005001.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee J, Lee MG. Effects of exercise interventions on breast cancer patients during adjuvant therapy: a systematic review and meta-analysis of randomized controlled trials. Cancer Nurs 2020;43:115–25. 10.1097/NCC.0000000000000682 [DOI] [PubMed] [Google Scholar]
- 54. Lund CM, Dolin TG, Mikkelsen MK, et al. Effect of exercise on physical function and psychological well-being in older patients with colorectal cancer receiving chemotherapy-A systematic review. Clin Colorectal Cancer 2020;19:e243–57. 10.1016/j.clcc.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 55. McGettigan M, Cardwell CR, Cantwell MM, et al. Physical activity interventions for disease-related physical and mental health during and following treatment in people with non-advanced colorectal cancer. Cochrane Database Syst Rev 2020;5:CD012864. 10.1002/14651858.CD012864.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mishra SI, Scherer RW, Geigle PM, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev 2012;2012:CD007566. 10.1002/14651858.CD007566.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Patsou ED, Alexias GD, Anagnostopoulos FG, et al. Effects of physical activity on depressive symptoms during breast cancer survivorship: a meta-analysis of randomised control trials. ESMO Open 2017;2:e000271. 10.1136/esmoopen-2017-000271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ramírez-Vélez R, Zambom-Ferraresi F, García-Hermoso A, et al. Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta-analysis. Cancers (Basel) 2021;13:264. 10.3390/cancers13020264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schumacher O, Luo H, Taaffe DR, et al. Effects of exercise during radiation therapy on physical function and treatment-related side effects in men with prostate cancer: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 2021;111:716–31. 10.1016/j.ijrobp.2021.06.034 [DOI] [PubMed] [Google Scholar]
- 60. Singh B, Spence R, Steele ML, et al. Exercise for individuals with lung cancer: a systematic review and meta-analysis of adverse events, feasibility, and effectiveness. Semin Oncol Nurs 2020;36:151076. 10.1016/j.soncn.2020.151076 [DOI] [PubMed] [Google Scholar]
- 61. Singh B, Spence RR, Steele ML, et al. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil 2018;99:2621–36. 10.1016/j.apmr.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 62. Yi L-J, Tian X, Jin Y-F, et al. Effects of yoga on health-related quality, physical health and psychological health in women with breast cancer receiving chemotherapy: a systematic review and meta-analysis. Ann Palliat Med 2021;10:1961–75. 10.21037/apm-20-1484 [DOI] [PubMed] [Google Scholar]
- 63. Bridle C, Spanjers K, Patel S, et al. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2012;201:180–5. 10.1192/bjp.bp.111.095174 [DOI] [PubMed] [Google Scholar]
- 64. Carneiro L, Afonso J, Ramirez-Campillo R, et al. The effects of exclusively resistance training-based supervised programs in people with depression: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health 2020;17:6715. 10.3390/ijerph17186715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chi I, Jordan-Marsh M, Guo M, et al. Tai chi and reduction of depressive symptoms for older adults: a meta-analysis of randomized trials. Geriatr Gerontol Int 2013;13:3–12. 10.1111/j.1447-0594.2012.00882.x [DOI] [PubMed] [Google Scholar]
- 66. Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev 2013;2013:CD004366. 10.1002/14651858.CD004366.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Josefsson T, Lindwall M, Archer T. Physical exercise intervention in depressive disorders: meta-analysis and systematic review. Scand J Med Sci Sports 2014;24:259–72. 10.1111/sms.12050 [DOI] [PubMed] [Google Scholar]
- 68. Krogh J, Hjorthøj C, Speyer H, et al. Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open 2017;7:e014820. 10.1136/bmjopen-2016-014820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ 2001;322:763–7. 10.1136/bmj.322.7289.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee J, Gierc M, Vila-Rodriguez F, et al. Efficacy of exercise combined with standard treatment for depression compared to standard treatment alone: a systematic review and meta-analysis of randomized controlled trials. J Affect Disord 2021;295:1494–511. 10.1016/j.jad.2021.09.043 [DOI] [PubMed] [Google Scholar]
- 71. Morres ID, Hatzigeorgiadis A, Stathi A, et al. Aerobic exercise for adult patients with major depressive disorder in mental health services: a systematic review and meta-analysis. Depress Anxiety 2019;36:39–53. 10.1002/da.22842 [DOI] [PubMed] [Google Scholar]
- 72. Nebiker L, Lichtenstein E, Minghetti A, et al. Moderating effects of exercise duration and intensity in neuromuscular vs. endurance exercise interventions for the treatment of depression: A meta-analytical review. Front Psychiatry 2018;9:305. 10.3389/fpsyt.2018.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schuch FB, Vancampfort D, Richards J, et al. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res 2016;77:42–51. 10.1016/j.jpsychires.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 74. Schuch FB, Vancampfort D, Rosenbaum S, et al. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Braz J Psychiatry 2016;38:247–54. 10.1590/1516-4446-2016-1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stathopoulou G, Powers MB, Berry AC, et al. Exercise interventions for mental health: a quantitative and qualitative review. Clinical Psychology: Science and Practice 2006;13:179–93. 10.1111/j.1468-2850.2006.00021.x [DOI] [Google Scholar]
- 76. Heissel A, Zech P, Rapp MA, et al. Effects of exercise on depression and anxiety in persons living with HIV: a meta-analysis. J Psychosom Res 2019;126:109823. 10.1016/j.jpsychores.2019.109823 [DOI] [PubMed] [Google Scholar]
- 77. Leng M, Liang B, Zhou H, et al. Effects of physical exercise on depressive symptoms in patients with cognitive impairment: a systematic review and meta-analysis. J Nerv Ment Dis 2018;206:809–23. 10.1097/NMD.0000000000000887 [DOI] [PubMed] [Google Scholar]
- 78. Li Z, Liu S, Wang L, et al. Mind-body exercise for anxiety and depression in COPD patients: a systematic review and meta-analysis. Int J Environ Res Public Health 2019;17:22–32. 10.3390/ijerph17010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang D, Wang Y, Wang Y, et al. Impact of physical exercise on substance use disorders: a meta-analysis. PLoS ONE 2014;9:e110728. 10.1371/journal.pone.0110728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tu R-H, Zeng Z-Y, Zhong G-Q, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail 2014;16:749–57. 10.1002/ejhf.101 [DOI] [PubMed] [Google Scholar]
- 81. Gascoyne C, Karahalios A, Demaneuf T, et al. Effect of exercise interventions on anxiety in people with multiple sclerosis: a systematic review and meta-analysis. Int J MS Care 2020;22:103–9. 10.7224/1537-2073.2019-009R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kelley GA, Kelley KS, Callahan LF. Community-deliverable exercise and anxiety in adults with arthritis and other rheumatic diseases: a systematic review with meta-analysis of randomised controlled trials. BMJ Open 2018;8:e019138. 10.1136/bmjopen-2017-019138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pearsall R, Smith DJ, Pelosi A, et al. Exercise therapy in adults with serious mental illness: a systematic review and meta-analysis. BMC Psychiatry 2014;14:117. 10.1186/1471-244X-14-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ramos-Sanchez CP, Schuch FB, Seedat S, et al. The anxiolytic effects of exercise for people with anxiety and related disorders: an update of the available meta-analytic evidence. Psychiatry Res 2021;302:114046. 10.1016/j.psychres.2021.114046 [DOI] [PubMed] [Google Scholar]
- 85. Stubbs B, Vancampfort D, Rosenbaum S, et al. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: a meta-analysis. Psychiatry Res 2017;249:102–8. 10.1016/j.psychres.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 86. Zhao Q-G, Zhang H-R, Wen X, et al. Exercise interventions on patients with end-stage renal disease: a systematic review. Clin Rehabil 2019;33:147–56. 10.1177/0269215518817083 [DOI] [PubMed] [Google Scholar]
- 87. Arent SM, Walker AJ, Arent MA. The effects of exercise on anxiety and depression. In: Handbook of Sport Psychology. 2020: 872–90. [Google Scholar]
- 88. Rivera-Brown AM, Frontera WR. Principles of exercise physiology: responses to acute exercise and long-term adaptations to training. PM R 2012;4:797–804. 10.1016/j.pmrj.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 89. Carin-Levy G, Kendall M, Young A, et al. The psychosocial effects of exercise and relaxation classes for persons surviving a stroke. Can J Occup Ther 2009;76:73–80. 10.1177/000841740907600204 [DOI] [PubMed] [Google Scholar]
- 90. Lesser IA, Nienhuis CP, Belanger L. Active by nature: exploring cancer survivors’ exercise barriers, facilitators, preferences, and psychosocial benefits of engaging in outdoor physical activity. Support Care Cancer 2021;29:4095–103. 10.1007/s00520-020-05938-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gujral S, Aizenstein H, Reynolds CF, et al. Exercise effects on depression: possible neural mechanisms. Gen Hosp Psychiatry 2017;49:2–10. 10.1016/j.genhosppsych.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ernst C, Olson AK, Pinel JPJ, et al. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- 93. Geidl W, Schlesinger S, Mino E, et al. Dose-response relationship between physical activity and mortality in adults with noncommunicable diseases: a systematic review and meta-analysis of prospective observational studies. Int J Behav Nutr Phys Act 2020;17:109. 10.1186/s12966-020-01007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Twomey C, O’Reilly G, Byrne M. Effectiveness of cognitive behavioural therapy for anxiety and depression in primary care: a meta-analysis. Fam Pract 2015;32:3–15. 10.1093/fampra/cmu060 [DOI] [PubMed] [Google Scholar]
- 95. Carpenter JK, Andrews LA, Witcraft SM, et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety 2018;35:502–14. 10.1002/da.22728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hieronymus F, Emilsson JF, Nilsson S, et al. Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Mol Psychiatry 2016;21:523–30. 10.1038/mp.2015.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Focus (Am Psychiatr Publ) 2018;16:420–9. 10.1176/appi.focus.16407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Fong DYT, Ho JWC, Hui BPH, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ 2012;344:e70. 10.1136/bmj.e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu L, He X, Feng L. Exercise on quality of life and cancer-related fatigue for lymphoma survivors: a systematic review and meta-analysis. Support Care Cancer 2019;27:4069–82. 10.1007/s00520-019-04983-y [DOI] [PubMed] [Google Scholar]
- 100. Peddle-McIntyre CJ, Singh F, Thomas R, et al. Exercise training for advanced lung cancer. Cochrane Database Syst Rev 2019;2:CD012685. 10.1002/14651858.CD012685.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. van Haren IEPM, Timmerman H, Potting CM, et al. Physical exercise for patients undergoing hematopoietic stem cell transplantation: systematic review and meta-analyses of randomized controlled trials. Phys Ther 2013;93:514–28. 10.2522/ptj.20120181 [DOI] [PubMed] [Google Scholar]
- 102. Vashistha V, Singh B, Kaur S, et al. The effects of exercise on fatigue, quality of life, and psychological function for men with prostate cancer: systematic review and meta-analyses. Eur Urol Focus 2016;2:284–95. 10.1016/j.euf.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 103. Zeng J, Wu J, Tang C, et al. Effects of exercise during or postchemotherapy in cancer patients: a systematic review and meta-analysis. Worldviews Evid Based Nurs 2019;16:92–101. 10.1111/wvn.12341 [DOI] [PubMed] [Google Scholar]
- 104. Zeng Y, Luo T, Xie H, et al. Health benefits of qigong or tai chi for cancer patients: a systematic review and meta-analyses. Complement Ther Med 2014;22:173–86. 10.1016/j.ctim.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 105. Zeng Y, Xie X, Cheng ASK. Qigong or tai chi in cancer care: an updated systematic review and meta-analysis. Curr Oncol Rep 2019;21:48. 10.1007/s11912-019-0786-2 [DOI] [PubMed] [Google Scholar]
- 106. Zhou L, Chen Q, Zhang J. Effect of exercise on fatigue in patients with lung cancer: a systematic review and meta-analysis of randomized trials. J Palliat Med 2021;24:932–43. 10.1089/jpm.2020.0504 [DOI] [PubMed] [Google Scholar]
- 107. Barreto P de S, Demougeot L, Pillard F, et al. Exercise training for managing behavioral and psychological symptoms in people with dementia: a systematic review and meta-analysis. Ageing Res Rev 2015;24:274–85. 10.1016/j.arr.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 108. de Almeida SIL, Gomes da Silva M, Marques ASP de D. Home-based physical activity programs for people with dementia: systematic review and meta-analysis. Gerontologist 2020;60:600–8. 10.1093/geront/gnz176 [DOI] [PubMed] [Google Scholar]
- 109. Forbes D, Forbes S, Morgan DG, et al. Physical activity programs for persons with dementia. Cochrane Database Syst Rev 2008:CD006489. 10.1002/14651858.CD006489.pub2 [DOI] [PubMed] [Google Scholar]
- 110. Li X, Guo R, Wei Z, et al. Effectiveness of exercise programs on patients with dementia: a systematic review and meta-analysis of randomized controlled trials. Biomed Res Int 2019;2019:2308475. 10.1155/2019/2308475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Russ J, Weyh C, Pilat C. High-Intensity exercise programs in people with dementia — a systematic review and meta-analysis. Ger J Exerc Sport Res 2021;51:4–16. 10.1007/s12662-020-00688-1 [DOI] [Google Scholar]
- 112. Coventry PA, Hind D. Comprehensive pulmonary rehabilitation for anxiety and depression in adults with chronic obstructive pulmonary disease: systematic review and meta-analysis. J Psychosom Res 2007;63:551–65. 10.1016/j.jpsychores.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 113. Guo C, Xiang G, Xie L, et al. Effects of tai chi training on the physical and mental health status in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Thorac Dis 2020;12:504–21. 10.21037/jtd.2020.01.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu J, Zhang Y, Du W, et al. Effect of qigong on self-rating depression and anxiety scale scores of COPD patients. Medicine 2019;98:e15776. 10.1097/MD.0000000000015776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dalgas U, Stenager E, Sloth M, et al. The effect of exercise on depressive symptoms in multiple sclerosis based on a meta-analysis and critical review of the literature. Eur J Neurol 2015;22:443–e34. 10.1111/ene.12576 [DOI] [PubMed] [Google Scholar]
- 116. Ensari I, Motl RW, Pilutti LA. Exercise training improves depressive symptoms in people with multiple sclerosis: results of a meta-analysis. J Psychosom Res 2014;76:465–71. 10.1016/j.jpsychores.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 117. Herring MP, Fleming KM, Hayes SP, et al. Moderators of exercise effects on depressive symptoms in multiple sclerosis: a meta-regression. Am J Prev Med 2017;53:508–18. 10.1016/j.amepre.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 118. Choo YT, Jiang Y, Hong J, et al. Effectiveness of tai chi on quality of life, depressive symptoms and physical function among community-dwelling older adults with chronic disease: a systematic review and meta-analysis. Int J Nurs Stud 2020;111:103737. 10.1016/j.ijnurstu.2020.103737 [DOI] [PubMed] [Google Scholar]
- 119. Gordon BR, McDowell CP, Hallgren M, et al. Association of efficacy of resistance exercise training with depressive symptoms: meta-analysis and meta-regression analysis of randomized clinical trials. JAMA Psychiatry 2018;75:566–76. 10.1001/jamapsychiatry.2018.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gouw VXH, Jiang Y, Seah B, et al. Effectiveness of internal qigong on quality of life, depressive symptoms and self-efficacy among community-dwelling older adults with chronic disease: a systematic review and meta-analysis. Int J Nurs Stud 2019;99:103378. 10.1016/j.ijnurstu.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 121. Aylett E, Small N, Bower P. Exercise in the treatment of clinical anxiety in general practice-a systematic review and meta-analysis. BMC Health Serv Res 2018;18:559. 10.1186/s12913-018-3313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ramachandran HJ, Jiang Y, Tam W, et al. Effectiveness of home-based cardiac telerehabilitation as an alternative to phase 2 cardiac rehabilitation of coronary heart disease: a systematic review and meta-analysis. Eur J Prev Cardiol 2021;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wang Z, Peng X, Li K, et al. Effects of combined aerobic and resistance training in patients with heart failure: a meta-analysis of randomized, controlled trials. Nurs Health Sci 2019;21:148–56. 10.1111/nhs.12593 [DOI] [PubMed] [Google Scholar]
- 124. Miller KJ, Gonçalves-Bradley DC, Areerob P, et al. Comparative effectiveness of three exercise types to treat clinical depression in older adults: a systematic review and network meta-analysis of randomised controlled trials. Ageing Res Rev 2020;58:100999. 10.1016/j.arr.2019.100999 [DOI] [PubMed] [Google Scholar]
- 125. Chung YC, Yeh ML, Liu YM. Effects of intradialytic exercise on the physical function, depression and quality of life for haemodialysis patients: a systematic review and meta-analysis of randomised controlled trials. J Clin Nurs 2017;26:1801–13. 10.1111/jocn.13514 [DOI] [PubMed] [Google Scholar]
- 126. Ferreira TL, Ribeiro HS, Ribeiro ALA, et al. Exercise interventions improve depression and anxiety in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol 2021;53:925–33. 10.1007/s11255-020-02612-w [DOI] [PubMed] [Google Scholar]
- 127. Lawrence M, Celestino Junior FT, Matozinho HH, et al. Yoga for stroke rehabilitation. Cochrane Database Syst Rev 2017;2017:12. 10.1002/14651858.CD011483.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lyu D, Wang J, Yang F, et al. Effect of tai chi on post-stroke non-motor disorders: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil 2021;35:26–38. 10.1177/0269215520951020 [DOI] [PubMed] [Google Scholar]
- 129. Hall M, Dobson F, Van Ginckel A, et al. Comparative effectiveness of exercise programs for psychological well-being in knee osteoarthritis: A systematic review and network meta-analysis. Semin Arthritis Rheum 2021;51:1023–32. 10.1016/j.semarthrit.2021.07.007 [DOI] [PubMed] [Google Scholar]
- 130. Kelley GA, Kelley KS, Hootman JM. Effects of exercise on depression in adults with arthritis: a systematic review with meta-analysis of randomized controlled trials. Arthritis Res Ther 2015;17:21. 10.1186/s13075-015-0533-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang Q, Hu J, Wei L, et al. Effects of traditional Chinese exercise on cognitive and psychological outcomes in older adults with mild cognitive impairment. Medicine (Baltimore) 2019;98:e14581. 10.1097/MD.0000000000014581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Nixon S, O’Brien K, Glazier RH, et al. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev 2005:CD001796. 10.1002/14651858.CD001796.pub2 [DOI] [PubMed] [Google Scholar]
- 133. McGranahan MJ, O’Connor PJ. Exercise training effects on sleep quality and symptoms of anxiety and depression in post-traumatic stress disorder: a systematic review and meta-analysis of randomized control trials. Mental Health and Physical Activity 2021;20:100385. 10.1016/j.mhpa.2021.100385 [DOI] [Google Scholar]
- 134. Rosenbaum S, Vancampfort D, Steel Z, et al. Physical activity in the treatment of post-traumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res 2015;230:130–6. 10.1016/j.psychres.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 135. Brinsley J, Schuch F, Lederman O, et al. Effects of yoga on depressive symptoms in people with mental disorders: a systematic review and meta-analysis. Br J Sports Med 2021;55:992–1000. 10.1136/bjsports-2019-101242 [DOI] [PubMed] [Google Scholar]
- 136. Xiang Y, Lu L, Chen X, et al. Does tai chi relieve fatigue? A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2017;12:e0174872. 10.1371/journal.pone.0174872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil 2015;96:1329–38. 10.1016/j.apmr.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 138. Broderick J, Knowles A, Chadwick J, et al. Yoga versus standard care for schizophrenia. Cochrane Database Syst Rev 2015;2015:CD010554. 10.1002/14651858.CD010554.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bjsports-2022-106195supp001.pdf (2.1MB, pdf)