Abstract
Objectives
Many patients with rheumatoid arthritis (RA) require treatment with tumour necrosis factor inhibitor (TNFi) to reach remission. It is debated whether tapering of TNFi to discontinuation should be considered in sustained remission. The aim of ARCTIC REWIND TNFi was to assess the effect of tapering TNFi to withdrawal compared with stable treatment on the risk of disease activity flares in patients with RA in remission ≥1 year.
Methods
This randomised, open-label, non-inferiority trial was undertaken at nine Norwegian rheumatology departments. Patients with RA in remission ≥12 months on stable TNFi therapy were allocated by computer-based block-randomisation to tapering to discontinuation of TNFi or stable TNFi. Conventional synthetic disease-modifying antirheumatic co-medication was unchanged. The primary endpoint was disease flare during the 12-month study period (non-inferiority margin 20%), assessed in the per-protocol population.
Results
Between June 2013 and January 2019, 99 patients were enrolled and 92 received the allocated treatment strategy. Eighty-four patients were included in the per-protocol population. In the tapering TNFi group, 27/43 (63%) experienced a flare during 12 months, compared with 2/41 (5%) in the stable TNFi group; risk difference (95% CI) 58% (42% to 74%). The tapering strategy was not non-inferior to continued stable treatment. The number of total/serious adverse events was 49/3 in the tapering group, 57/2 in the stable group.
Conclusion
In patients with RA in remission for more than 1 year while using TNFi, an increase in flare rate was reported in those who tapered TNFi to discontinuation. However, most regained remission after reinstatement of full-dose treatment.
Trial registration numbers
EudraCT: 2012-005275-14 and clinicaltrials.gov: NCT01881308.
Keywords: treatment; tumor necrosis factor inhibitors; arthritis, rheumatoid
WHAT IS ALREADY KNOWN ON THIS TOPIC
The treatment goal for most patients with rheumatoid arthritis (RA) is to reach and sustain remission, with prevention of structural joint damage and disability.
Several studies have assessed tapering or stopping of tumour necrosis factor inhibitor (TNFi) in patients with RA in low disease activity or who fulfil remission criteria based on Disease Activity Score calculated with 28 joints.
There are less data available for the clinically relevant group of patients who have been in prolonged remission and who do not show clinical signs of synovitis.
WHAT THIS STUDY ADDS
The ARCTIC REWIND Trial was conducted in patients with RA where remission had been sustained for at least 1 year on stable medication, and the patient had no swollen joints at inclusion.
Despite the stringent inclusion criteria, there was a large increase in frequency of disease activity flares in patients tapering TNFi to discontinuation versus continuation of stable TNFi dose.
The response to reinstatement of the initial TNFi dose was mostly good without any difference in radiographic joint damage progression.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Current European Alliance of Associations for Rheumatology recommendations for management and American College of Rheumatology guidelines for treatment of RA outline that tapering of biologic disease modifying antirheumatic drugs could be considered in patients with RA in sustained remission.
Our findings show that the risk of experiencing a flare if TNFi is tapered to discontinuation is significant even in prolonged remission, but that most patients regain remission when the initial dose is started again.
The results may support shared treatment decisions between clinicians and the growing group of patients with RA who attain sustained remission using a TNFi.
Introduction
Rheumatoid arthritis (RA) is an inflammatory disease, and treatment commonly involves tumour necrosis factor inhibitors (TNFis).1 The best clinical approach to treating patients with RA who have been in long-term remission using these medications has not been established.2–4
According to current recommendations, patients with RA will be treated with methotrexate monotherapy after disease onset. In cases of insufficient response or failure to reach remission, therapy will be escalated, typically with the addition of a TNFi.3 4 Such treat-to-target strategies have contributed to a marked increase in the proportion of patients with RA reaching remission, that is, near absence of disease symptoms, but often results in treatment with a combination of more than one disease modifying antirheumatic drug (DMARD).
Several studies have assessed tapering or stopping of TNFi in patients with RA in low disease activity or who fulfil the least stringent remission criteria based on Disease Activity Score calculated with 28 joints (DAS28). However, according to the European Alliance of Associations for Rheumatology (EULAR) treatment recommendations DMARDs should not be tapered unless the patient has been in a state of stringent remission for at least 6 months, and there is less evidence regarding this clinically relevant group of patients.4–7 In studies when patients were not required to be in remission before reducing the TNFi dose, tapering of TNFi increased the risk of experiencing a disease worsening, especially when tapering TNFi to withdrawal.8–11 If patients were treated with a combination of methotrexate and TNFi at disease onset, without failing methotrexate monotherapy first, results indicate that dose reduction or withdrawal of TNFi can be effective.12–15 Data on stepwise lengthening of the TNFi administration intervals among patients with RA in remission and low disease activity (LDA) are mixed.16 17
The primary objective of this study was to compare the effect of tapering and withdrawal of TNFi versus continuing stable TNFi on the risk of flares among patients with RA in sustained clinical remission for at least a year.
Methods
Study design
This randomised, open-label non-inferiority trial was designed to evaluate the effect of tapering of TNFi on disease flares in patients with RA who had been in sustained remission for at least 1 year. The ARCTIC REWIND (REmission in Rheumatoid Arthritis - Assessing WIthrawal of Disease-modifying Antirheumatic Drugs in a Non-inferiority Design) study consisted of two randomised clinical trials, assessing tapering of TNFi (the current study) and tapering of conventional synthetic DMARDs (csDMARDs),18 19 respectively. Enrolment of patients took place at nine hospital-based rheumatology practices in Norway (online supplemental table 1). The study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. The Regional Ethical Committee and the Norwegian Medicines Agency approved the protocol (online supplemental file 1) and patient consent.
ard-2023-224476supp001.pdf (491.6KB, pdf)
Patients
Participants were 18–80 years, fulfilled the American College of Rheumatology (ACR)/EULAR classification criteria for RA, had been in sustained remission for ≥12 months, and were in remission at inclusion with no swollen joints and fulfilment of the Disease Activity Score (DAS) remission criteria. DAS is a disease activity composite measure (range 0–1020), which incorporates evaluation of swelling in 44 joints, the Ritchie Articular Index, erythrocyte sedimentation rate (ESR) and patient global assessment of disease activity on a Visual Analogue Scale. Higher scores indicate more disease activity, remission is defined by DAS<1.6. For inclusion into the study, treatment with standard dosages of TNFi had to be unchanged in the last 12 months, with stable csDMARD co-medication (online supplemental appendix 1). The initial protocol required symptom duration less than 5 years; this was removed in a protocol update. The reasons included that some patients could not be included due to difficulties in determining symptom duration, and that the protocol update increased the number of patients eligible for enrolment. All patients provided a written informed consent before inclusion.
Randomisation
Patients were randomly assigned 1:1 to either continued stable TNFi treatment or to tapering of TNFi treatment (computer-based block randomisation with a block size of 4 and stratification for study centre). Inclusion and randomisation of patients were performed by study nurses and investigators at the individual study sites. The allocated treatment group was revealed in the electronic case report form (Viedoc V.3, Uppsala, Sweden) after randomisation.
Procedures
If a patient was randomised to tapering, the TNFi was reduced to half dose for 4 months, and withdrawn at the 4-month visit if the patient was still in remission. Any co-medication with csDMARDs was kept stable throughout the study. According to the protocol, patients with flares in the tapering group restarted their baseline TNFi treatment, while flares in the stable group were managed according to current recommendations. If a patient regained remission after a flare, no further attempts were made to taper medication. Patients were examined every 4 months, and additional visits scheduled within a week if the patient experienced increasing symptoms.
Outcomes
The primary efficacy endpoint was the proportion of patients with a disease flare between baseline and 12 months, defined as a combination of DAS >1.6 (remission cut point), an increase in DAS score ≥0.6 points from the previous visit (measurement error for DAS),21 and at least two swollen joints on examination of 44 joints at the same visit, with all components having to be present to fulfil the definition. A patient could also be considered to have a flare if the patient and investigator agreed that a clinically significant flare had occurred. In a sensitivity analysis, we assessed the primary outcome according to fulfilment of the definition solely based on DAS and swollen joint count. Secondary endpoints for disease activity included changes and area under the curve (0–12 months) for the composite indices DAS, Simplified Disease Activity Index (SDAI, range 0–86), Clinical Disease Activity Index (CDAI; range 0–76) and DAS28 (range 0–9.4). For all disease activity composite indices, higher scores reflect higher disease activity. Disease activity remission was defined according to cut-offs for DAS, SDAI (<3.3),22 CDAI (<2.8)22 as well as ACR/EULAR Boolean remission (combination of ≤1 swollen joints, ≤1 tender joints, patient global assessment of disease activity ≤1 (0–10 scale) and C reactive protein (CRP, mg/dL) ≤1).22 Individual measures of disease activity included tender joints (Ritchie Articular Index, range 0–78),23 swollen joints (0–44), patient and physician global assessment of disease activity (0–100), ESR (mm/hour) and CRP (mg/L). Joint pain and fatigue were recorded by VAS (0–100), and physical function by the Patient-Reported Outcomes Measurement Information 20-item short form (transformed to a T-score with mean 50 and SD of 10). Additionally, the following questionnaires were completed; The EuroQol-5 Dimension, the Rheumatoid Arthritis Impact of Disease and the 36-Item Short-Form Survey. Prednisolone use and intra-articular injections were recorded consecutively.
Ultrasound examinations (baseline and 12 months) were performed by a 32-joint scoring system,24 with joints scored 0–3 for grey scale (total score 0–96) and power Doppler (total score 0–96).24 Radiographs of hands and feet (baseline and 12 months, known chronological order) were scored by two readers blinded for clinical information and treatment according to the van der Heijde modified Sharp Score (vdHSS).25 The smallest detectable change (SDC) was calculated to be 1.38, calculations made with an SD of the differences between change-scores of two reading sessions.26 Progression of joint damage was defined as a change of ≥1 unit per year (average score of the readers), sensitivity analysis with cut-offs of ≥0.5, ≥2 and ≥5 units per year, as well as the SDC.
Response to treatment was assessed by ACR 20/50/70/90 response, EULAR good and moderate response, the US Food and Drug Administration major clinical response,27 as well as disease activity measures in patients who experienced a flare. Safety was evaluated at each visit by assessment of clinical and laboratory adverse events, coded by the Medical Dictionary for Regulatory Activities (V.21.1E).
Data on work productivity and MRI were not yet available.
Statistical analyses
A sample size of 126 patients (63 in each group) was determined assuming a common flare rate of 20% (estimate based on data from the Norwegian Antirehumatic Drug Register, NOR-DMARD),28 with 80% power to conclude the non-inferiority of tapering. The non-inferiority margin was set to a difference in flare risk of 20%, and non-inferiority would be concluded on if the 95% CI could exclude a difference of this magnitude. After adjustments for a potential dropout rate of up to 20%, the aim was to include 80 patients in each group. The non-inferiority margin was based on discussions with clinicians, researchers and health economists regarding the upper limit of increased flare rate that would be acceptable when tapering TNFi treatment, guided by the Food and Drug Administration guidance document for non-inferiority clinical trials.29
The main analyses were predefined in the statistical analysis plan signed prior to the database lock (online supplemental file 1). If non-inferiority could not be shown it was prespecified to assess if there were more statistically significant flares in one of the groups compared with the other. Baseline characteristics were described by n (%), median (IQR) or mean (SD). The inferiority null hypothesis was tested in the per-protocol population. The primary analysis was performed by mixed-effects logistic regression, with any disease flare during the 12-month follow-up as response, and treatment group as fixed factor. Randomisation was stratified by centre, and thus was included as a random effect in the model. The difference in flare rate was estimated as the marginal probability of flare under tapered therapy minus that of stable therapy, with 95% CI estimated by the delta method. A sensitivity analysis was performed in the population initiating treatment, and repeated in patients with csDMARD co-medication in a post hoc sensitivity analysis. In post hoc analyses we repeated the analysis in the restricted definition of the primary outcome and for the first 4-month period, corresponding to the treatment period for half-dose TNFi.
Mixed-effects logistic regression was used to assess dichotomous secondary outcomes, and linear mixed models (adjusted for the baseline value) were used for continuous outcomes. For outcomes assessed repeatedly, fixed factors for treatment group, time and their interaction were included, in addition to centre and patient-level random effects. Examination of model residuals were used for model validity checks. The results for analyses of secondary endpoints should be interpreted as exploratory, given the potential for type 1 errors due to multiple comparisons. The variables included in the primary endpoint were fully monitored during the study. All analyses were performed in Stata v14.0 (StataCorp). The trial was registered in EudraCT with identification number: 2012-005275-14, and in clinicaltrials.gov with identification number NCT01881308.
Role of the funding source
ARCTIC REWIND was an investigator-initiated trial, fully funded by governmental research grants as outlined under Acknowledgements. The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Patient and public involvement
Patient representatives were involved in the interpretation of the results.
Results
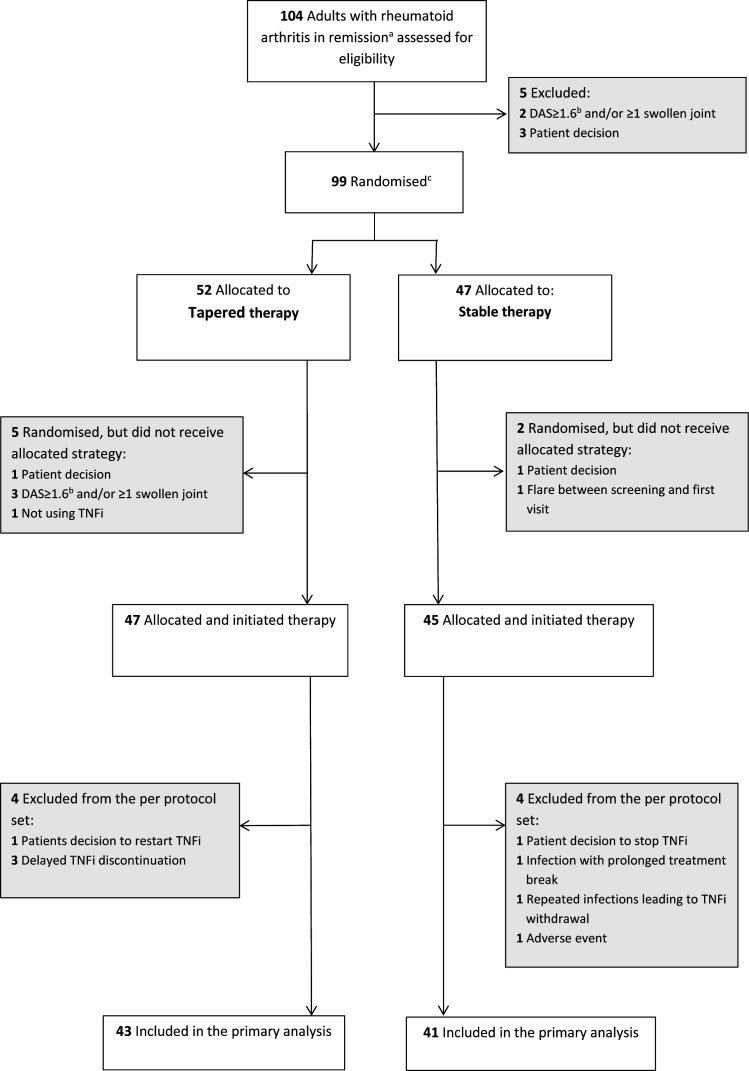
During the period from 17 June 2013 to 4 January 2019, 99 patients were randomly assigned to the two groups. Forty-seven patients tapered TNFi treatment and 45 received stable TNFi treatment (figure 1). Four patients in each group were excluded from the per-protocol data set due to major protocol violations. Baseline characteristics were overall well balanced, but with more patients on infliximab in the tapering group (table 1). Mean DAS was 0.8 (0.3) in the tapering group and 0.9 (0.4) in the stable group, 45/47 (96%) and 34/45 (76%) were in ACR/EULAR SDAI remission, while ultrasound power Doppler was absent in 44/47 (94%) and 42/44 (96%) of patients, respectively. In the tapering TNFi group, 42/47 (89%) received csDMARD co-medication, compared with 41/45 (91%) in the stable TNFi group, predominantly methotrexate monotherapy. In methotrexate users, the mean (SD) baseline dose was 16.3 (5.2) mg/week in the tapering group and 17.8 (5.3) mg/week in the stable group.
Figure 1.
Flow of patients in the ARCTIC REWIND TNFi Study. aRemission according to established criteria should be documented for at least 12 months. bDAS is a composite measure of disease activity with scores ranging from 0 to 10, higher scores indicate more disease activity, remission defined as <1.6. cStratified by study site. DAS, Disease Activity Score. TNFi, tumour necrosis factor inhibitor.
Table 1.
Baseline characteristics of the study population*
| Characteristic | Tapering TNFi (n=47) |
Stable TNFi (n=45) |
| Age, years, mean (SD) | 57.6 (12.6) | 57.4 (10.7) |
| Female, no (%) | 25 (53%) | 30 (67%) |
| Time since first swollen joint, years, mean (SD) | 11.9 (6.9) | 10.0 (7.2) |
| Positive for anticitrullinated peptide antibodies, no (%) | 36 (77%) | 35 (78%) |
| Positive for rheumatoid factor, no (%) | 32 (68%) | 28 (62%) |
| Body mass index (kg/m2), mean (SD) | 25.9 (3.4) | 25.8 (5.0) |
| Current smoker, no (%) | 8 (17%) | 10 (22%) |
| Measures of disease activity | ||
| Disease Activity Score†, mean (SD) | 0.8 (0.3) | 0.9 (0.4) |
| Simplified Disease Activity Index‡, mean (SD) | 1.1 (1.0) | 1.9 (2.3) |
| Simplified Disease Activity Index‡remission, no (%) | 45 (96%) | 34 (76%) |
| ACR/EULAR remission§, no (%) | 38 (81%) | 30 (67%) |
| Swollen joint count¶, mean (SD) | 0.0 (0.0) | 0.0 (0.0) |
| Tender joint count (Ritchie Articular Index)**, mean (SD) | 0.1 (0.2) | 0.2 (0.5) |
| ESR, mm/hour††, normal value <17 mm/hour in women and <12 mm/hour in men, median (IQR) | 7.0 (5.0–14.0) | 8.0 (5.0–15.0) |
| CRP, mg/dL‡‡, normal value <0.4 mg/dL, median (IQR) | 0.1 (0.1–0.3) | 0.1 (0.1–0.2) |
| Patient’s global assessment (0–100)§§, median (IQR) | 3.0 (1.0–12.0) | 2.0 (1.0–12.0) |
| Physician’s global assessment (0–100)§§, median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–2.0) |
| Functional outcomes | ||
| PROMIS Physical Function¶¶, median (IQR) | 52.6 (49.0–62.5) | 51.2 (44.2–62.5) |
| Fatigue Visual Analogue Scale (0–100 mm)***, median (IQR) | 7.0 (1.0–22.0) | 3.0 (0.0–31.0) |
| Pain Visual Analogue Scale (0–100 mm)***, median (IQR) | 4.0 (2.0–11.0) | 3.0 (1.0–11.0) |
| Radiographic joint damage | ||
| Total van der Heijde modified Sharp Score†††, median (IQR) | 6.5 (1.5–12.0) | 5.0 (1.5–13.5) |
| van der Heijde Sharp Erosion, median (IQR) | 2.5 (1.0–5.0) | 2.0 (0.5–5.0) |
| van der Heijde Sharp Joint Space Narrowing, median (IQR) | 2.5 (0.5–7.0) | 1.0 (0.0–10.5) |
| Ultrasound outcomes‡‡‡ | ||
| Total power Doppler Signal Score, median (IQR) | 0.0 (0.0– 0.0) | 0.0 (0.0–0.0) |
| Total Grey Scale Score, median (IQR) | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) |
| No power Doppler signal in any joint | 44 (94%) | 42 (96%) |
| Medication | ||
| Etanercept, no (%) | 20 (43%) | 20 (44%) |
| Certolizumab pegol, no (%) | 14 (30%) | 15 (33%) |
| Golimumab, no (%) | 1 (2%) | 4 (9%) |
| Infliximab, no (%) | 9 (19%) | 0 (0%) |
| Adalimumab, no (%) | 3 (6%) | 6 (13%) |
| Co-medication with csDMARDs, no (%) | 42 (89%) | 41 (91%) |
| Co-medication with methotrexate, no (%) | 38 (81%) | 38 (84%) |
*As allocated and initiated treatment. Seven patients who were randomised but did not have verified initiation of treatment were excluded, five from the tapering group and two from the stable group.
†Disease Activity Score (DAS, range 0–10) includes a 44 swollen joint count (SJC44), assessment of tender joints by Ritchie Articular Index (RAI), the ESR and patient’s global assessment (PGA) of disease activity on a VAS 0–100 mm. It is calculated as follows: DAS=0.54*square root(RAI) + 0.065*(SJC44+0.33*Ln(ESR) + 0.0072*PGA. Remission is defined as any value below 1.6, low disease activity 1.6–2.4, moderate disease activity >2.4–3.7, and high disease activity >3.7, thus higher scores indicating more disease activity.
‡Simplified Disease Activity Index (SDAI, range 0–86) includes a 28 swollen (SJC28) and tender joint count (TJC28), CRP, PGA and the physician’s global assessment of disease activity (PhGA) on a VAS 0–100 mm. It is calculated as follows: SDAI=TJC28 + SJC28 + PGA/10+PhGA/10+CRP. Remission is defined as a score ≤3.3, with higher scores indicating more disease activity.
§ACR/EULAR remission is defined as tender joint count ≤1 and swollen joint count ≤1 and CRP ≤1 mg/dL and patient global assessment ≤10 (on a 0–100 scale).
¶The swollen joint count is the number of swollen joints out of 44 joints assessed.
**The tender joint count is performed by the Ritchie Articular Index assessing tenderness of 26 joint regions, the index ranges 0–3 for individual measures and the sum 0–78 overall, with higher scores indicating more tenderness.
††At the time of baseline visit, normal values may vary among laboratories.
‡‡At the time of baseline visit, normal values may vary among laboratories. To convert CRP to mg/L (SI unit), multiply by 10.
§§The patient’s and physician’s global assessments are self-reported and physician-reported, respectively, overall assessments of disease with use of a VAS that ranges 0–100 mm, with higher scores indicating more severe disease.
¶¶PROMIS 20-item short form range 0–100, with scores lower than 50 indicating disability worse than average.
***Fatigue and joint pain are self-reported with use of a VAS ranging 0–100 mm, with higher scores indicating more severe fatigue.
†††van der Heijde modified Sharp scoring method assesses erosions in 16 joints of each hand (range 0–5 for each joint) and 6 joints of each foot (range 0–10 per joint), and joint space narrowing in 15 joints for each hand, as well as six joints for each foot (range 0–4 per joint). This gives scores for erosions on a scale of 0–280 and joint-space narrowing on a scale of 0–168, thus the total van der Heijde Sharp score range is 0–448 with higher scores indicating greater joint damage.
‡‡‡Two aspects of synovitis can be assessed by ultrasound; morphology and quantity using grey scale and synovial vascularity using power Doppler. The ultrasound examination was performed using 0–3 semiquantitative scoring systems for both grey scale and power Doppler in 32 joints.
ACR, American College of Rheumatology; CRP, C reactive protein; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS, Disease Activity Score; ESR, erythrocyte sedimentation rate; EULAR, European Alliance of Associations for Rheumatology; PROMIS, Patient-reported Outcomes Measurement Information Score; TNFi, tumour necrosis factor inhibitor; VAS, Visual Analogue Scale.
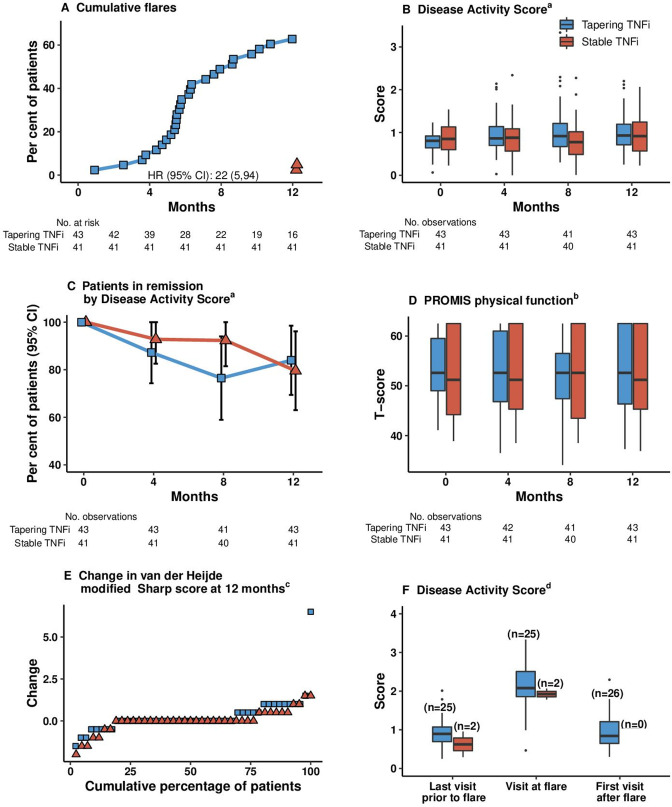
Twenty-seven of 43 (63%) patients treated by tapered TNFi therapy experienced a flare during the 12-month follow-up, compared with 2/41 (5%) in the stable TNFi treatment group, risk difference 58% (95% CI 42% to 74%); p value for comparison <0.0001 (figure 2, figure 3A). The lower limit of the CI exceeded the non-inferiority margin, and non-inferiority was not shown.
Figure 2.
Flare rate (primary outcome) in tapered versus stable TNFi treatment flare was defined as a combination of Disease Activity Score (DAS) above the cut-off for remission (1.6), a change in DAS of at least 0.6, and at least two swollen joints or that both the treating physician and the patient agreed that a clinically significant flare had occurred. The dotted, vertical line represents the non-inferiority margin. aThe primary analysis was performed in all randomised patients meeting the study entry criteria and with no protocol deviations affecting the treatment efficacy (defined as failure to follow the treatment regimen or withdrawal from the study). bSeven patients who were randomised but did not have verified initiation of treatment are excluded (five from the tapering group and two from the stable group). cAnalysis performed in patients within the primary analysis population who used methotrexate co-medication. csDMARD, conventional synthetic disease-modifying antirheumatic drug; TNFi, tumour necrosis factor inhibitor.
Figure 3.
Secondary endpoints. Analysed in the primary analysis population, defined as all randomised patients meeting the study entry criteria and with no protocol deviations affecting the treatment efficacy. Patients were followed up for a median (IQR) of 364 days (358–378 days) in the tapered group and 366 days (357–378 days) in the stable group. Variables are displayed based on clinical relevance. Boxes mark first and third quartiles, the band inside the box is the second quartile (the median), while the whiskers indicate the highest and lowest values within 1.5 × the IQR. Dots denote individual patients (outliers).a Disease Activity Score (DAS, range 0–10) includes a 44 swollen joint count assessment of tender joints by the Ritchie Articular Index, the erythrocyte sedimentation rate (ESR) and patient’s global assessment of disease activity on a VAS 0–100 mm. Remission is defined as any value below 1.6, low disease activity 1.6–2.4, moderate disease activity >2.4–3.7 and high disease activity >3.7, thus higher scores indicating more disease activity. bPROMIS assesses the ability to perform basic and instrumental activities of daily living. The total score is translated into a T score with a mean (SD) of 50 (10). A score of 50 equals the average for the general US population. cThe van der Heijde–modified Sharp scoring method assesses erosions in 16 joints of each hand and 6 joints of each foot, and the erosions are given a score of 1 to 5. Joint space narrowing is assessed in 15 joints for each hand and 6 joints for each foot. This gives scores for erosions on a scale of 0–280 and joint space narrowing on a scale of 0–168, thus the total van der Heijde–modified Sharp score ranges from 0 to 448, with higher scores indicating greater joint damage. A good radiographic outcome is commonly defined as no progression. dDisease Activity Score at the visit before a flare occurred, at the flare visit, and at visits after flare in the half-dose arm in those with all components available to calculate DAS. A flare could be recorded both at regular visits and at additional visits. PROMIS, Patient-Reported Outcomes Measurement Information 20-item Short Form Physical Function; TNFi, tumour necrosis factor inhibitor; VAS Visual Analogue Scale.
At 12 months, 37/42 (88%) patients in the tapering group and 34/40 (85%) patients in the stable group were in DAS remission (figure 3B and C). Composite DAS, physical function and the number of patients in remission according to different criteria were similar in the two groups at 12 months (online supplemental table 2, figure 3D). The mean (SE) DAS at time of flare was 2.2 (0.8) in the tapering group and 1.9 (0.2) in the stable group (figure 3F), corresponding to low disease activity. Flares occurred in patients treated with all types of TNFi (online supplemental table 3). In patients with at least one visit postflare, 23/26 (88%) in the tapering group were in DAS remission at the subsequent visit (online supplemental table 4). At the time of flare, patients in the tapering group had higher median CRP and swollen joint count than at the visit before and after flare (online supplemental figure 1).
The mean (SD) increases in total van der Heijde modified Sharp Score was 0.3 (1.2) units in the tapering group, compared with 0.1 (0.6) in the stable group (online supplemental table 2), difference (95% CI) 0.2 (−0.2 to 0.6) units. In the tapering TNFi group, 34/42 (81%) had no progression of radiographic joint damage, compared with 36/40 (90%) of patients in the stable TNFi group, risk difference −9% (95% CI −24% to 6%, figure 3E). Of the patients with flare and radiographic data available, 7/29 (24%) had progression of radiographic joint damage, compared with 5/53 (9%) without a flare, value of p=0.10 for comparison between the groups. The mean (SD) change in vdHSS in patients with flare was 0.44 (1.29), compared with −0.01 (0.61) in those without a flare, value of p=0.03. The median (IQR) change in vdHSS in patients with flare was 0 (0.0–0.5), compared with 0 (0.0–0.0) in those without a flare, value of p for comparison =0.04. At 12 months, 35 (85%) patients in the tapering group and 35 (90%) in the stable group had no power Doppler signal in any joint, risk difference −4% (−19% to 10%, online supplemental table 2).
In the tapering group 49 adverse events were reported, and 57 in the stable group (table 2). Three (6%) of the patients in the tapering group experienced a serious adverse event (including one serious viral infection), versus two (4%) in the stable group. No malignancies or deaths were reported. One of the adverse events in the stable group led to study discontinuation. The most frequently reported adverse event were upper respiratory tract infections (5 in the tapering group, 14 in the stable group).
Table 2.
Adverse events from month 0 to month 12
| Tapering TNFi (n=47) |
Stable TNFi (n=45) |
|
| Adverse events* | ||
| Upper respiratory tract infections, no | 5 | 14 |
| Diarrhoea, no | 3 | 0 |
| Fracture, no | 3 | 0 |
| Influenza, no | 0 | 3 |
| Urinary tract infection (including pyelonephritis), no | 3 | 1 |
| Patients with adverse events | ||
| 1, no (%) | 10 (21) | 16 (36) |
| ≥2, no (%) | 13 (28) | 15 (33) |
| Adverse events | ||
| Serious†‡, no | 3 | 2 |
| Leading to study discontinuation§, no | 0 | 1 |
| Total, no | 49 | 57 |
*Adverse events occurring with a frequency of 3 or more in at least one of the groups are listed. Additionally, there were three events total of iridocyclitis and joint pain; two events total of abdominal pain, back pain, conjunctivitis, dyspepsia, herpes zoster, hypercholesterolaemia, infections not otherwise specified, liver enzyme elevation, palpitations, skin reaction, upper respiratory tract symptoms and wound infection; one event each of angina pectoris, ankle oedema, ankle sprain, atrioventricular block third degree, borrelia burgdorferi, bursitis, cardiac pacemaker insertion, carotid artery calcification, chest pain, chronic obstructive lung disease, colon polypectomy, depression, fall with syncope, forgetfulness, gastrointestinal bleeding, heart attack, haematuria, incontinence, insect sting allergy, leucopenia, liver cirrhosis, menstruation irregular, nausea, night sweat, nocturia, osteoporosis, otitis media, periodontitis, pneumonia, postoperative infection, pruritus cutaneous, rheumatoid vasculitis, shoulder operation, Sjogren’s syndrome, stomach influenza, syrinx, rupture of tendon, skin biopsy, tinnitus, tiredness, transient cerebral ischaemia, tooth infection and urinary tract pain.
†The serious adverse events were one case of heart attack, one viral infection and one case of fall with syncope in the tapering group; one atrioventricular block third degree and one case of rheumatoid vasculitis in the stable group. The term serious adverse event included any untoward medical occurrence that resulted in death, was immediately life-threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, was a congenital abnormality or birth defect, or was an important medical event that could jeopardise the subject or could require medical intervention to prevent one of the outcomes listed above.
‡There were no cancers, and none of the adverse events led to death.
§Rheumatoid vasculitis.
TNFi, tumour necrosis factor inhibitor.
In a sensitivity analysis of the primary outcome assessing patients who were randomised and initiated treatment, similar results were found as for the main analysis (figure 2). This also applied to the results from a post hoc sensitivity analysis among patients receiving co-medication with csDMARDs.
In post hoc analyses, a higher risk of flare was observed in the tapering group also for the flare definition based solely on DAS and swollen joint counts, with 14/43 (33%) of patients in the tapering group having a flare according to this definition, compared with 1/41 (2%) in the stable group, risk difference 30% (95% CI 15% to 45%) (online supplemental figure 2). During the first 4 months of the follow-up period, when patients in the tapering group received half-dose TNFi, 5/43 (12%) in the tapering group flared, compared with none of the patients in the stable group. Thus, 5 of the total 27 (19%) flares recorded in the tapering to withdrawal group occurred when patients received half-dose TNFi. In the tapering group, 26/27 (96%) adjusted DMARD medication following the flare, compared with 1/2 (50%) in the stable group. Among patients with available data at 12 months, 25 (60%) in the tapering group were using TNFi, compared with 40 (100%) in the stable group. Flare occurred after a mean (SD) time of 192 (76) days in the tapering group and 371 (1) days in the stable group.
Discussion
Among patients with RA in long-standing remission without clinical synovitis at inclusion, significantly higher flare rates during 1 year follow-up were observed in those tapering TNFi to discontinuation, compared with patients continuing stable TNFi treatment. Tapered TNFi therapy was not non-inferior to continued stable TNFi treatment. Patients responded well to reinstated treatment, and remission rates in the two study arms were comparable at 12 months.
According to current treatment recommendations, dose reduction should only be considered if the patient has reached the treatment target (specified as ACR/EULAR remission in the EULAR recommendations), for at least 6 months,3 as also suggested by the results from a post hoc analysis of three etanercept tapering studies.4 30 Patients participating in our study had been in sustained remission for at least 12 months, and clinical and ultrasonographic examination revealed no signs of inflammatory activity, with 81% of those randomised to tapering fulfilling the ACR/EULAR Boolean remission criteria at time of inclusion. Thus, an outcome of non-inferiority of the tapering strategy was perceived realistic. However, an unexpectedly large discrepancy in flare rates between the two study groups was observed despite the low level of disease activity.
The opportunity to compare flare rates between studies is limited by the application of different inclusion criteria, tapering strategies and flare definitions.31 In clinical trials assessing patients with RA in remission for ≥3 months who continue csDMARDs, tapering of TNFi to half dosages led to disease worsening in 36%–41% of patients,32 33 while 45%–80% experienced a flare within a maximum of 12 months after withdrawal.33–35 The flare rate observed in the tapering group of the current study was thus in the same range as in studies with somewhat less stringent inclusion criteria, while the flare rate in the stable treatment group is much lower than what has been observed in previous studies, resulting in the large risk difference.6
As csDMARDs were kept unchanged in all patients on combination therapy in our study, we do not know whether reduction of these drugs would have been more successful than tapering of the TNFi first. Two studies have compared withdrawal of either csDMARD or TNFi in sustained RA remission on combination therapy; the double-blinded SEAM-RA (Study of Etanercept and Methotrexate in Combination or as Monotherapy in Subjects With Rheumatoid Arthritis) trial, assessing patients in SDAI remission for 24 weeks, showed that significantly more patients continuing combination therapy (53%) or etanercept monotherapy (50%) maintained remission compared with methotrexate monotherapy (29%),36 while the order of tapering did not affect flare rates in the TARA (TApering strategies in Rheumatoid Arthritis) trial.37
Previous studies have supported that reduction of TNFi to half dose has limited effect on flare rates.7 Although the ARCTIC REWIND Trial did not aim to study the isolated effect of tapering TNFi to half dose, explorative data from the first 4 months of the trial indicate an increased flare rate also on half-dose TNFi. The trend of some increase in radiographic joint damage in patients experiencing a flare supports previous data from the PRESERVE (A Randomized, Double-Blind Study Comparing the Safety & Efficacy of Once-Weekly Etanercept 50 mg, Etanercept 25 mg, & Placebo in Combination With Methotrexate in Subjects With Active Rheumatoid Arthritis) trial, where patients with flare had more structural progression, in addition to poorer clinical and functional outcomes.38 This was found across treatment groups (50 mg etanercept weekly in combination with methotrexate, 25 mg etanercept weekly in combination with methotrexate or placebo with methotrexate).38
Our results demonstrate excellent long-term outcomes in patients with RA who have reached remission on TNFi treatment, and show that most patients need continuous TNF inhibition to maintain remission. We also provide data which show very good response to the clinically relevant situation of reinstatement of the initial TNFi treatment in patients who tapered TNFi treatment and flared, with no observed significant difference in radiographic joint damage or functional outcomes at 12 months. Both information about increased flare risk and the possibility of regaining control of the disease are important aspects to address in shared decision making,39 as tapering of TNFi should be decided by the patient and clinician based on the total situation of the patient.40 In patients tapering treatment, the control regimen should take into account the potential of disease activity flare, with an aim to capture and treat significant flares at an early stage. There is a need for further research to personalise medicine within this patient group.
Our study had limitations. First, the open label design might influence the evaluation of flares. Study personnel were continuously instructed about the importance of recording flares in a similar manner in both groups. This pragmatic approach mirrors clinical care where patients know what treatment they are receiving. Second, a clinically significant flare could be recorded without fulfilment of the formal definition. This option was intended to capture increases in disease activity not assessed by the joint count, but could be vulnerable to expectation bias. The increase in CRP, swollen joint count and ultrasound power Doppler at time of flare supports that the flares recorded represented a true increase in inflammation. The findings are strengthened by the consistency in sensitivity analyses restricting the primary outcome to the definition based on DAS and swollen joint count. Third, the patient recruitment was closed before the target number had been reached due to a lower inclusion rate than anticipated, but the conclusions that could be drawn based on the data are still clear (online supplemental appendix 2). Fourth, heterogeneity is present in treatment regimens, but analyses did not highlight large discrepancies in flare rates between the drugs. Fifth, fulfilment of ACR/EULAR Boolean remission criteria was not necessary for inclusion into the study. To ensure that patients did not have significant disease activity in the feet, we required remission according to DAS (based on 44 joints, including feet) at inclusion, in addition to no swollen joints.
In conclusion, tapering TNFi therapy to discontinuation in prolonged RA remission on stable medication was not non-inferior to continuation of stable therapy on the risk of flares. Stable treatment with TNFi was associated with a very low risk of flare, while significantly more flares were observed when tapering of TNFi to discontinuation. However, in those experiencing a flare when tapering TNFi therapy, most regained remission on reinstated full-dose treatment.
Acknowledgments
The authors thank the patients who participated in the ARCTIC REWIND Study for the time and effort they have invested in the project. The authors also thank the investigators, study nurses, patient partners and medical staff at the study centres for their contribution to study. The authors thank Camilla Fongen (PT, MSc) for her contribution to the organisation of the study and the data collection, Department for Immunology and Transfusion medicine at Oslo University Hospital for analyses of serological markers, as well as the funding sources.
Footnotes
Handling editor: Josef S Smolen
Contributors: SL, ABA and EAH designed the study; recruited and enrolled participants; collected, analysed, and interpreted data and wrote the report. NPS collected, analysed and interpreted data, and wrote the report. JS was the trial statistician, analysed and interpreted data, and wrote the report. ICO designed the study, analysed and interpreted data, and reviewed the manuscript. HF, CS, TMM, CAH, GB, ÅL, IMH, HH, EM and TU recruited and enrolled patients, interpreted data, and reviewed the manuscript. DHS, DvdH and TKK designed the study, interpreted data, and reviewed the manuscript. SL is guarantor and accepts full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish.
Funding: Research Council of Norway and South-Eastern Norway Regional Health Authority.
Competing interests: SL, NPS, JS and EM report grants from The Research Council of Norway and from The South-Eastern Norway Regional Health Authority during the conduct of the study. ABA reports personal fees from AbbVie, personal fees from Eli Lilly, personal fees from Novartis, personal fees from Pfizer, outside the submitted work. ICO, HF, CS, ÅL, IMH have nothing to disclose. CAH reports personal fees from Novartis, outside the submitted work. GB reports personal fees from Novartis, personal fees from UCB, outside the submitted work. HH reports personal fees from Novartis, outside the submitted work. TU reports personal fees from Galapagos, personal fees from Lilly, personal fees from Novartis, personal fees from Pfizer, personal fees from UCB, outside the submitted work. DHS reports that Abbvie donated drugs for a trial, that Amgen donated drugs for a trial, grants from Corrona, grants from Janssen, grants from Pfizer, grants from Roche/Genentech, outside the submitted work. DvdH reports personal fees from AbbVie, personal fees from Amgen, personal fees from Astellas, personal fees from AstraZeneca, personal fees from BMS, personal fees from Boehringer Ingelheim, personal fees from Celgene, personal fees from Cyxone, personal fees from Daichii, personal fees from Eisai, personal fees from Eli Lilly, personal fees from Galapagos, personal fees from Gilead, personal fees from GSK, personal fees from Janssen, personal fees from Merck, personal fees from Novartis, personal fees from Pfizer, personal fees from Regeneron, personal fees from Roche, personal fees from Sanofi, personal fees from Takeda, personal fees from UCB Pharma, outside the submitted work; and is Director Imaging Rheumatology BV. TKK reports grants and personal fees from AbbVie, personal fees from Biogen, personal fees from Celltrion, personal fees from Egis, personal fees from Lilly, grants and personal fees from MSD, personal fees from Mylan, personal fees from Hikma, grants and personal fees from Novartis, personal fees from Oktal, personal fees from Orion Pharma, grants and personal fees from Pfizer, personal fees from Roche, personal fees from Sandoz, personal fees from Sanofi, grants and personal fees from UCB, grants from BMS, outside the submitted work. EAH reports grants from The Research Council of Norway, grants from The South-Eastern Norway Regional Health Authority, during the conduct of the study; personal fees from Pfizer, personal fees from AbbVie, personal fees from Janssen-Cilag, personal fees from Gilead, personal fees from UCB Pharma, personal fees from Celgene, personal fees from Lilly, personal fees from Roche, outside the submitted work.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. A de-identified patient data set can be made available to researchers upon reasonable request and in adherence with applicable laws and regulations. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses, and will have to be approved by the ARCTIC REWIND project group.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by REK Sør-Øst2012/2285/REK sør-øst. Participants gave informed consent to participate in the study before taking part.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2. Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis 2016;75:1428–37. 10.1136/annrheumdis-2016-209201 [DOI] [PubMed] [Google Scholar]
- 3. Fraenkel L, Bathon JM, England BR, et al. American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2021;73:924–39. 10.1002/acr.24596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 5. Uhrenholt L, Christensen R, Dinesen WKH, et al. Risk of flare after tapering or withdrawal of biologic/targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis or axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2022;61:3107–22. 10.1093/rheumatology/keab902 [DOI] [PubMed] [Google Scholar]
- 6. Bergstra SA, Sepriano A, Kerschbaumer A, et al. Efficacy, duration of use and safety of glucocorticoids: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2023;82:81–94. 10.1136/ard-2022-223358 [DOI] [PubMed] [Google Scholar]
- 7. Verhoef LM, van den Bemt BJ, van der Maas A, et al. Down-titration and discontinuation strategies of tumour necrosis factor-blocking agents for rheumatoid arthritis in patients with low disease activity. Cochrane Database Syst Rev 2019;5:CD010455. 10.1002/14651858.CD010455.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Vollenhoven RF, Østergaard M, Leirisalo-Repo M, et al. Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis. Ann Rheum Dis 2016;75:52–8. 10.1136/annrheumdis-2014-205726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghiti Moghadam M, Vonkeman HE, Ten Klooster PM, et al. Stopping tumor necrosis factor inhibitor treatment in patients with established rheumatoid arthritis in remission or with stable low disease activity: a pragmatic multicenter, open-label randomized controlled trial. Arthritis Rheumatol 2016;68:1810–7. 10.1002/art.39626 [DOI] [PubMed] [Google Scholar]
- 10. Ibrahim F, Lorente-Cánovas B, Doré CJ, et al. Optimizing treatment with tumour necrosis factor inhibitors in rheumatoid arthritis-a proof of principle and exploratory trial: is dose tapering practical in good responders Rheumatology (Oxford) 2017;56:2004–14. 10.1093/rheumatology/kex315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of Etanercept after treatment with Etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. The Lancet 2013;381:918–29. 10.1016/S0140-6736(12)61811-X [DOI] [PubMed] [Google Scholar]
- 12. Smolen JS, Emery P, Fleischmann R, et al. Adjustment of therapy in rheumatoid arthritis on the basis of achievement of stable low disease activity with adalimumab plus methotrexate or methotrexate alone: the randomised controlled OPTIMA trial. Lancet 2014;383:321–32. 10.1016/S0140-6736(13)61751-1 [DOI] [PubMed] [Google Scholar]
- 13. Emery P, Hammoudeh M, FitzGerald O, et al. Sustained remission with etanercept tapering in early rheumatoid arthritis. N Engl J Med 2014;371:1781–92. 10.1056/NEJMoa1316133 [DOI] [PubMed] [Google Scholar]
- 14. Weinblatt ME, Bingham CO, Burmester G-R, et al. A phase III study evaluating continuation, tapering, and withdrawal of Certolizumab Pegol after one year of therapy in patients with early rheumatoid arthritis. Arthritis Rheumatol 2017;69:1937–48. 10.1002/art.40196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van den Broek M, Klarenbeek NB, Dirven L, et al. Discontinuation of Infliximab and potential predictors of persistent low disease activity in patients with early rheumatoid arthritis and disease activity score-steered therapy: subanalysis of the best study. Annals of the Rheumatic Diseases 2011;70:1389–94. 10.1136/ard.2010.147751 [DOI] [PubMed] [Google Scholar]
- 16. van Herwaarden N, van der Maas A, Minten MJM, et al. Disease activity guided dose reduction and withdrawal of adalimumab or etanercept compared with usual care in rheumatoid arthritis: open label, randomised controlled, non-inferiority trial. BMJ 2015;350:h1389. 10.1136/bmj.h1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fautrel B, Pham T, Alfaiate T, et al. Step-down strategy of spacing TNF-blocker injections for established rheumatoid arthritis in remission: results of the multicentre non-inferiority randomised open-label controlled trial (STRASS: spacing of TNF-blocker injections in rheumatoid arthritis study). Ann Rheum Dis 2016;75:59–67. 10.1136/annrheumdis-2014-206696 [DOI] [PubMed] [Google Scholar]
- 18. Lillegraven S, Paulshus Sundlisæter N, Aga A-B, et al. Effect of half-dose vs stable-dose conventional synthetic disease-modifying antirheumatic drugs on disease flares in patients with rheumatoid arthritis in remission: the ARCTIC REWIND randomized clinical trial. JAMA 2021;325:1755–64. 10.1001/jama.2021.4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lillegraven S, Paulshus Sundlisæter N, Aga A-B, et al. Discontinuation of conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and excellent disease control. JAMA 2023;329:1024–6. 10.1001/jama.2023.0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Heijde DM, van ’t Hof MA, van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Annals of the Rheumatic Diseases 1990;49:916–20. 10.1136/ard.49.11.916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Gestel AM, Prevoo ML, van ’t Hof MA, et al. Development and validation of the European league against rheumatism response criteria for rheumatoid arthritis. comparison with the preliminary American college of rheumatology and the world health organization/International League against rheumatism criteria. Arthritis Rheum 1996;39:34–40. 10.1002/art.1780390105 [DOI] [PubMed] [Google Scholar]
- 22. Felson DT, Smolen JS, Wells G, et al. American college of rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011;63:573–86. 10.1002/art.30129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ritchie DM, Boyle JA, McInnes JM, et al. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. QJMed 1968;37:393–406. [PubMed] [Google Scholar]
- 24. Hammer HB, Bolton-King P, Bakkeheim V, et al. Examination of intra and interrater reliability with a new ultrasonographic reference atlas for scoring of synovitis in patients with rheumatoid arthritis. Ann Rheum Dis 2011;70:1995–8. 10.1136/ard.2011.152926 [DOI] [PubMed] [Google Scholar]
- 25. van der Heijde D. How to read radiographs according to the sharp/Van der Heijde method. J Rheumatol 1999;26:743–5. [PubMed] [Google Scholar]
- 26. Bruynesteyn K, Boers M, Kostense P. Deciding on progression of joint damage in paired films of individual patients: smallest detectable difference or change. Annals of the Rheumatic Diseases 2005;64:179–82. 10.1136/ard.2003.018457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. U. S. Department of Heath and Human Services, Food and Drug Administration guidance for industry : Clinical development programs for drugs devices, and biological products for the treatment of rheumatoid arthritis (RA). 1999.
- 28. Kvien TK, Lie E, et al. A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol 2005;23(5 Suppl 39):S188–94. [PubMed] [Google Scholar]
- 29. U.S.Department of Health and Human Services Food and Drug Administration . Non-inferiority clinical trials to establish effectiveness - guidance for industry; 2016.
- 30. Tanaka Y, Smolen JS, Jones H, et al. The effect of deep or sustained remission on maintenance of remission after dose reduction or withdrawal of etanercept in patients with rheumatoid arthritis. Arthritis Res Ther 2019;21. 10.1186/s13075-019-1937-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Maas A, Lie E, Christensen R, et al. Construct and criterion validity of several proposed Das28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis 2013;72:1800–5. 10.1136/annrheumdis-2012-202281 [DOI] [PubMed] [Google Scholar]
- 32. Bertrand D, Stouten V, De Cock D, et al. Tapering of etanercept is feasible in patients with rheumatoid arthritis in sustained remission: a pragmatic randomized controlled trial. Scand J Rheumatol 2022;51:470–80. 10.1080/03009742.2021.1955467 [DOI] [PubMed] [Google Scholar]
- 33. Emery P, Burmester GR, Naredo E, et al. Adalimumab dose tapering in patients with rheumatoid arthritis who are in long-standing clinical remission: results of the phase IV PREDICTRA study. Ann Rheum Dis 2020;79:1023–30. 10.1136/annrheumdis-2020-217246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatzidionysiou K, Turesson C, Teleman A, et al. A multicentre, randomised, controlled, open-label pilot study on the feasibility of discontinuation of adalimumab in established patients with rheumatoid arthritis in stable clinical remission. RMD Open 2016;2:e000133. 10.1136/rmdopen-2015-000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamanaka H, Nagaoka S, Lee S-K, et al. Discontinuation of etanercept after achievement of sustained remission in patients with rheumatoid arthritis who initially had moderate disease activity-results from the ENCOURAGE study, a prospective, International, multicenter randomized study. Modern Rheumatology 2016;26:651–61. 10.3109/14397595.2015.1123349 [DOI] [PubMed] [Google Scholar]
- 36. Curtis JR, Emery P, Karis E, et al. Etanercept or methotrexate withdrawal in rheumatoid arthritis patients in sustained remission. Arthritis Rheumatol 2021;73:759–68. 10.1002/art.41589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Mulligen E, Weel AE, Hazes JM, et al. Tapering towards DMARD-free remission in established rheumatoid arthritis: 2-year results of the TARA trial. Ann Rheum Dis 2020;79:1174–81. 10.1136/annrheumdis-2020-217485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smolen JS, Pedersen R, Jones H, et al. Impact of flare on radiographic progression after etanercept continuation, tapering or withdrawal in patients with rheumatoid arthritis. Rheumatology (Oxford) 2020;59:153–64. 10.1093/rheumatology/kez224 [DOI] [PubMed] [Google Scholar]
- 39. Verhoef LM, Selten EMH, Vriezekolk JE, et al. The patient perspective on biologic DMARD dose reduction in rheumatoid arthritis: a mixed methods study. Rheumatology (Oxford) 2018;57:1947–55. 10.1093/rheumatology/key205 [DOI] [PubMed] [Google Scholar]
- 40. Chan SJ, Stamp LK, Liebergreen N, et al. Tapering biologic therapy for rheumatoid arthritis: a qualitative study of patient perspectives. Patient 2020;13:225–34. 10.1007/s40271-019-00403-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2023-224476supp001.pdf (491.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. A de-identified patient data set can be made available to researchers upon reasonable request and in adherence with applicable laws and regulations. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses, and will have to be approved by the ARCTIC REWIND project group.