Abstract
Vegetative propagation of potatoes makes it possible for potato viruses to be transmitted through tubers. Potato virus A (PVA) is one of these viruses, which belongs to the Potyvirus genus in the Potyviridae family. Potato tuber yield can be reduced by 30–40% by PVA alone. Losses can be further exacerbated by potato virus X and/or potato virus Y infection. PVA is transmitted primarily by several species of aphids in non-persistent manner. With the aim of resolving this problem, we developed one-step reverse transcription-recombinase polymerase amplification (RT-RPA), a highly sensitive and cost-effective method for detecting PVA in both potato tubers and leaves. Detection and amplification are performed using isothermal conditions in this method. There was good amplification of the coat protein gene in PVA with all three primers tested. To conduct this study, a primer set that can amplify specific 185 base pair (bp) product was selected. PVA detection was optimized by 30-min amplification reactions, which showed no cross-reactivity with other potato viruses. A simple heating block or water bath was used to amplify PVA product using RT-RPA at a temperature range of 38–42 °C. In comparison to conventional reverse transcription-polymerase chain reaction (RT-PCR), the newly developed RT-RPA protocol exhibited high sensitivity for both potato leaves and tuber tissues. Using cellular paper-based simple RNA extraction procedure, the virus was detected in leaf samples as efficiently as purified total RNA. We also found that combining LiCl-based RNA precipitation with cellular paper discs allowed us to successfully optimize RNA extraction for one-step RT-RPA for detecting PVA in tubers. Tests using this simplified one-step RT-RPA method were successfully applied to 300 samples of both leaves and tubers from various potato cultivars. In our knowledge, this is the first report of an RT-RPA assay utilizing simple RNA obtained from either cellular disc paper or LiCl coupled with cellular disc paper to detect PVA. As a result, this method was equally sensitive and specific for detecting PVA in potatoes. The developed RT-RPA assay is more versatile, durable, and do not require highly purified RNA templates, thus providing an effective alternative to RT-PCR assays for screening of germplasm, certifying planting materials, breeding for virus resistance, and real-time monitoring of PVA.
Keywords: PVA, Simple RNA extract, Detection, Sensitivity, Potato, DAS-ELISA, Isothermal, RT-PCR, RT-RPA, Specificity
Introduction
Potato (Solanum tuberosum L.) is a very valuable and frequently grown commodity all over the world. It is a member of the family Solanaceae and comes in at number four in terms of production, behind only maize, rice, and wheat, respectively (Sundaresha et al. 2022; Lal et al. 2022). Its short growing season and flexibility in planting and harvesting periods makes it a valuable crop that can be grown in a variety of intensive-cropping systems without putting an excessive amount of pressure on limited land and water resources (Jeevalatha et al. 2016; Kumar et al. 2022a, c, 2023; Chikh-Ali and Karasev 2023). China is the greatest potato grower in the world, followed by India, the Russian Federation, and Ukraine. In 2021, global potato output surpassed 376 million metric tons, which were met from over 17 million hectares of land (Naga et al. 2021c; Kumar et al. 2021a, 2022a; Chikh-Ali and Karasev 2023). In 2022, India produced 53.58 million metric tons of potatoes across an area of 2.20 million hectares, with an average yield of 24.35 t/ha (Kumar et al. 2022c, 2023). There is a growing need for potatoes in the world as a result of population pressure; yet, the crop faces various obstacles that have an impact on its production and yield potential. These concerns include both biotic and abiotic problems. Some of the biotic challenges include viral, bacterial, fungal, and nematode diseases (Wang et al. 2011; Tiwari et al. 2021a, 2022; Chikh-Ali and Karasev 2023).
The potato crop is susceptible to infection by more than 50 different viruses, 7 of which are considered to be the major potato viruses: tomato leaf curl New Delhi virus (ToLCNDV), potato virus Y (PVY), potato virus S (PVS), potato leafroll virus (PLRV), potato virus M (PVM), potato virus A (PVA), and potato virus X (PVX). These viruses cause a huge risk to potato production across the world, including in India, and have the potential to result in enormous economic damage (Kumar et al. 2021a, 2022b, 2023; Chikh-Ali and Karasev 2023). To prevent diseases in potato, it is important to plant healthy and virus-free seed potatoes, which can be achieved through rigorous screening of seed tubers and vegetative planting materials. For the production of mini tubers, meristem tissue culture-based planting material is mostly used. Under field conditions, these mini tubers are propagated as seed potatoes in two generations/seasons. The seed potatoes are tested for the presence of viruses at each stage of propagation, and further certified as virus free (Kumar et al. 2022c; Chikh-Ali and Karasev 2023).
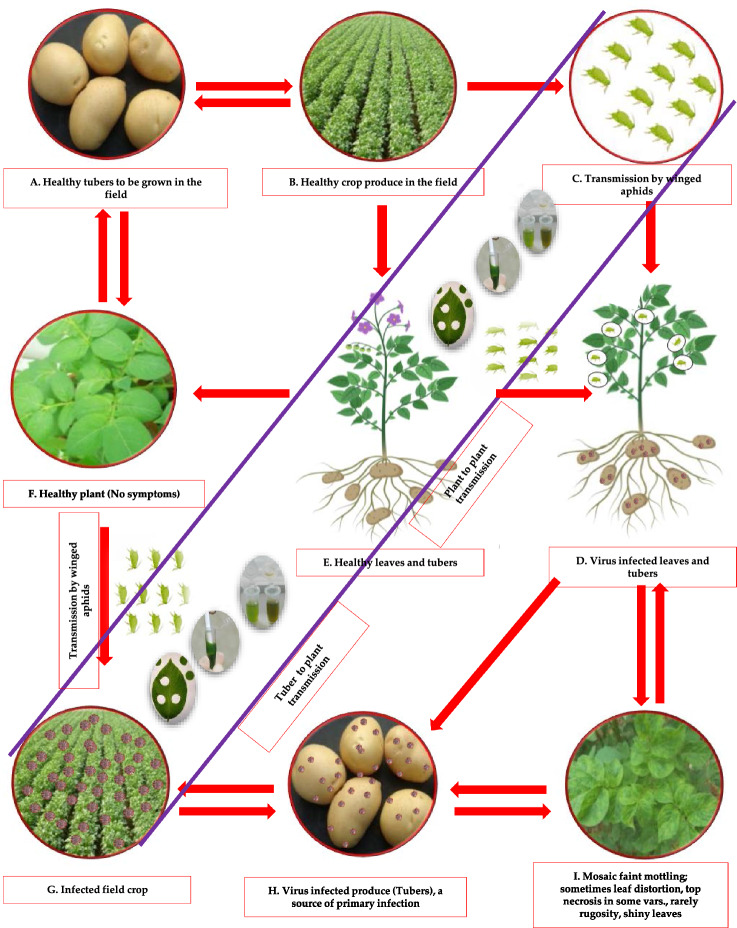
Potato virus A (PVA) is a member of the genus Potyvirus of the family Potyviridae and has positive-sense single-stranded RNA and is serologically linked to PVY. PVA can reduce potato tuber yield by 30-40% alone, but coinfection with PVX and/or PVY can increase losses. PVA poses a severe risk to potato crops all over the world and has the potential to significantly reduce crop yields. The genome of PVA is a 9.5-kb messenger-polarity ssRNA with a virus-encoded protein (VPg) covalently bonded to the 5′ end and a 3′-poly(A) tail (Paul Khurana 2006; Raigond et al. 2020b, 2022). Its virion particles are flexuous filaments measuring 730 x 11 nm. Infected plants feature shiny leaves, vein mottling, and an open growth habit. This is likely since PVA typically causes mild symptoms in potato plants, such as mosaic patterns on the foliage and a yield reduction, rather than crop loss or severe damage. However, it is frequently coupled with PVX to produce the effect known as "potato crinkle." PVA is primarily transmitted through infected potato tubers or vegetative propagation of plant material. In some species of plants, "true" or botanical seeds are used for propagation. In the case of potatoes, however, seed tubers and clonal multiplication are used for propagation, resulting in the progressive accumulation of viruses that can degrade seed quality. This occurrence is commonly known as “seed degeneration (Fig. 1)”. Healthy seed potato production typically depends on certified seed production schemes due to the risk of seed degeneration and virus transmission (Paul Khurana 2006; Kreuze et al. 2020; Kumar et al. 2022c; Jeevalatha et al. 2021). These initiatives involve the production of pathogen-free potato seeds under strict guidelines and the implementation of quality control measures to reduce the risk of virus transmission (Singh et al. 2014; Kumar et al. 2017b; Jeevalatha et al. 2021; Naga et al. 2021a, b) .
Fig. 1.
Dissemination of PVA under field conditions (primary and secondary infection symptoms). A Virus-free tubers to be grown in the field, B healthy crop produce in the field from healthy tubers in first season, C transmission of PVA by winged aphids under field conditions, D virus-infected plant (leaves and tubers) produce in the field, E virus-free plant (leaves and tubers) produce in the field if aphids are not transmitted or not present in the field, F if virus-free plant (leaves and tubers) produce in the field, then no symptoms of the virus, G if virus-infected plant (leaves and tubers) produce in the field, then winged aphids will spread the virus to other plants, and symptoms of the virus will be seen, H if virus transmitted in the field, then produced tubers will be virus infected, I the typical symptoms of the virus on leaves may be seen under field conditions and will be the source of secondary infection
PVA has been reliably identified in potatoes using a number of diagnostic methods in the last few decades, including symptomatological studies during field inspections and on indicator hosts, electron microscopy, and the double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) (Kumar et al. 2014; Meena et al. 2017; Raigond et al. 2020b). In these approaches, PVA was not detected in dormant tubers because they were inefficient, insensitive, and time-consuming. As diagnostic tools have improved, stackholders and technical persons have been able to use reverse transcription-polymerase chain reaction (RT-PCR), multiplex RT-PCR, immunocapture RT-PCR, and real-time RT-PCR to detect PVA much more effectively (Raigond et al. 2020b; Fuentes et al. 2021). Even though these methods are very sensitive and specific, they can be hard to use, difficult, and expensive too. They also need well-equipped labs and technical staff with a lot of training.
With the introduction of a new isothermal amplification method, i.e., recombinase polymerase amplification (RPA) for the detection of several plant viruses (Piepenburg et al. 2006; Donoso and Valenzuela 2018; Jiao et al. 2020), it also could be useful for detecting PVA in potatoes quickly and accurately, which would allow for early intervention and control measures. Using oligonucleotide primers along with a recombinase enzyme, RPA forms a protein filament using nucleic acid. By scanning for homologous sequences in the target DNA, this filament amplifies the expected sequences under low temperature conditions. A gel electrophoresis can be used to visualize the resulting products, and they can also be directly sequenced like PCR products. LAMP produces multimeric products, so this is not possible (Kapoor et al. 2017; Kumar et al. 2023). RPA produces results in as little as 10–30 min and does not require a thermal cycler for initial denaturation of the double-stranded DNA (dsDNA) target. This method can detect as low as ten copies of the virus in a given reaction, demonstrating its great sensitivity and specificity. It can also be used with isolated RNA in reverse transcription (RT)-RPA. For field-based applications, RPA offers advantages due to the requirement for minimal sample preparation and execution at a constant low temperature (37–42° C). Additionally, RPA is cost-effective, portable, and instrument simplification adds value in field-based diagnostics. It has been demonstrated that the RPA test is just as sensitive as RT-PCR and ELISA when it comes to detecting PVA in potato plants. The RPA test is convenient in the sense that it does not call for heat cycling and can be carried out at water bath or incubator or room temperature (Ju et al. 2019; Mohandas and Bhat 2020; Kumar et al. 2022a, b). This makes it one of the most significant advantages of the procedure. Additionally, the RPA test is inexpensive and does not require any particular equipment or training, which makes it perfect for low-resource situations as it is both cost-effective and convenient.
As a point-of-care diagnostic tool, RPA is ideally suited for monitoring quarantine services and plant clinics. Implementing it in on-site testing programs can greatly contribute to eradicating diseased plants, thereby reducing the risk of disease spread. DNA and RNA viruses that possess DNA or RNA as their genetic material have been successfully detected by RPA. There are several examples of tomato mottle virus, bean golden yellow mosaic virus, tomato yellow leaf curl virus, banana bunchy top virus, piper yellow mottle virus, yam mosaic virus, rose rosette virus, maize chlorotic mottle virus, cucumber green mottle mosaic virus, cucumber mosaic virus in bananas, potato virus Y, potato mop-top virus, cardamom vein clearing virus, ginger chlorotic fleck-associated virus, potato virus S, potato virus X, and potato leafroll virus. Using RPA in these cases demonstrates its ability to detect and control a wide range of plant viruses rapidly and accurately (Babu et al. 2017; Kapoor et al. 2017; Babujee et al. 2019; Jiao et al. 2019, 2020; Srivastava et al. 2019; Mohandas and Bhat 2020; Qin et al. 2021; Kumar et al. 2022a).
Potato leaves and tubers have been extracted using several simple nucleic acid extraction methods (Hwang et al. 2013; Mekuria et al. 2014; Zou et al. 2017; Silva et al. 2018; Choudhary et al. 2019; Onozuka et al. 2020; Kumar et al. 2021b). We developed a simple RNA extract-based reverse transcription-recombinase polymerase amplification assay (RT-RPA) for detecting PVA at low cost. The improved cellular filter paper-based RNA preparation method has previously been reported to be simple, fast, and inexpensive, allowing the RNA solution to be eluted from the disc and preserved (Onozuka et al. 2020; Kumar et al. 2021b). PVA detection in potato leaves can be achieved effectively and efficiently using the modified disc paper-based method. However, it could not be able to detect PVA in tubers. The presence of polyphenols and polysaccharides in potato tubers may inhibit the isolation of RNA from them, which may reduce the sensitivity of PVA detection by RT-PCR (Kumar et al. 2022a, b, 2023). Our study also evaluated the effect of lithium chloride (LiCl) in conjugation with improved cellular disc paper-based RNA preparation from potato tubers to improve RNA yield and quality. Finally, we developed a simple RNA extract-based high-throughput RT-RPA assay to detect PVA from leaf and tuber samples and compared it to DAS-ELISA and RT-PCR tests for sensitivity.
Materials and methods
Pure virus culture and planting materials
Virus-infected potato plants and tubers and pure virus cultures used in this study were sourced from the virus pure culture facility of ICAR-Central Potato Research Institute, Shimla, Himachal Pradesh, India. To confirm the presence of PVA infection in the samples, two different techniques were employed: DAS-ELISA (double antibody sandwich enzyme-linked immunosorbent assay) and RT-PCR (reverse transcription polymerase chain reaction). These tests were carried out to ensure that the samples used in the study were indeed infected with PVA. To establish a healthy control for comparison, virus-free tissue culture-raised microplants or minitubers were utilized. These healthy plants were free from PVA infection and served as a reference for evaluating the effects and characteristics of the virus-infected plants. Negative controls were also included in the study to rule out any false-positive results or contamination. In these negative controls, PCR-grade water was used instead of a template during the experimental procedures. Moreover, potato plants infected with other viruses were maintained for the purpose of assessing the specificity of the study, including tomato leaf curl New Delhi virus (ToLCNDV), potato virus Y (PVY), potato virus S (PVS), potato virus X (PVX), potato virus M (PVM), and potato leafroll virus (PLRV). Commercially available positive control of another potyvirus i.e., potato virus V (PVV) was also tested to rule out any cross-reactivity between potyvirus infect potato. These infected plants/positive controls were used in specificity tests to ensure that the observations and results obtained were specific to PVA and not influenced by other viral infections.
RNA extraction, synthesis of cDNA, and RT-PCR reaction
With the SpectrumTM Plant Total RNA kit (Sigma-Aldrich, Missouri, USA), total RNA was extracted from plant materials (leaves and tubers). In this study, RNA extracts were prepared from 25 mg of tuber tissue ground and dissolved in 1 ml of extraction buffer #2 (containing approximately 100-400 ng/µl of RNA) or from 2 µl of cellular disc paper-based RNA extract. The first-strand cDNA was synthesized from 2 μl of RNA as template with Revert aidTM First-strand cDNA synthesis kit (Fermentas, Thermo Fisher Scientific, USA). Through RT-PCR, specific primers and protocols were used to confirm the presence of PVA infection (Meena et al. 2017). Amplification was conducted targeting 560 bp of PVA coat protein (CP) gene. The RT-PCR was performed using a GeneAmp 9700 PCR system from Applied Biosystems. The Verso 1-Step RT-PCR kit was used to compare the sensitivity of one-step RT-RPA with RT-PCR using Thermo Prime Taq (Thermo Fisher Scientific, USA) reagents. Previously published primer sequences and annealing temperatures were used (Meena et al. 2017). The newly designed RPA primers were used in a one-step RT-PCR under similar conditions as the two-step RT-PCR. A 50-µl reaction volume was prepared for the one-step RT-PCR with 1 µl of Verso enzyme mix, 25 µl of 2X 1-step PCR ReddyMix, 2.5 µl of RT enhancer, 10 µM forward and reverse primers, and 1.0 µl of RNA (100-500ng/ µl). cDNA synthesis takes place at 50 °C for 15 min after 2 min of inactivation at 95 °C. We then ran 35 cycles of amplification: denaturation for 20 s at 95 °C, annealing for 30 s at 58 °C, and extension for 1 min at 72 °C. For 5 min, 72 °C was used as the final extension temperature. The amplified products were visualized by electrophoresis in a 1% (w/v) agarose gel stained with ethidium bromide solution in 1× TAE buffer, running at 100 volts for 1 h alongside a DNA ladder under UV light. Using a Thermo ScientificTM NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA), yield and quality of cDNA and RNA were assessed.
Designing and synthesis of primers
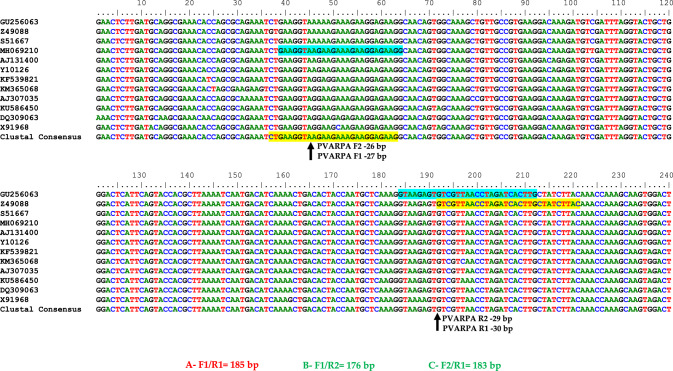
For the one-step RT-RPA assay targeting PVA, three primer sets were designed using Primer3plus software, following the instructions provided by TwistAmp® reaction kit (https://www.twistdx.co.uk). In this design, conserved regions of the coat protein gene sequences of PVA were used, which are available in the GenBank database at the National Center for Biotechnology Information (NCBI). In the design, the sequence accession numbers were GU256063, Z49088, S51667, MH069210, AJ131400, Y10126, KF539821, KM365068, AJ307035, KU586450, DQ309063 andX91968. Figure 2 illustrates the positions of the primers. To identify conserved nucleotide sequences, sequence alignments were performed using the Bio-Edit Software online. Using the Basic Local Alignment Search Tool (BLAST) with Blastn, an in silico analysis was conducted to ensure primer specificity.
Fig. 2.
An illustration of the primer positions within the coat protein gene of potato virus A (PVA)
Primers were synthesized from Imperial Life Sciences (P) Limited, Haryana, India. RT-RPA assay optimization was conducted using three primer sets (A, B, and C). The primer set A (PVARPAF1/R1) was selected for specific detection of PVA and was also used for subsequent studies on specificity and sensitivity. Table 1 provides a list of all primers used in this study, along with annealing temperatures, GC content (%), and expected sizes of PVA amplification products.
Table 1.
Primers used in this study to optimize one-step reverse transcription-recombinase polymerase amplification (RT-RPA) for detection of potato virus A (PVA)
| Target virus | Primer set No | Primers | Polarity | Sequence 5ʹ–3ʹ | Bases | Target position on coat protein gene | Position on accession number in NCBI GeneBank (AJ131400.1) | GC (%) | Tm (°C) | Amplicon size (bp) |
|---|---|---|---|---|---|---|---|---|---|---|
| PVA | A | PVARPAF1 | Sense | CTGAAGGTAAGAAGAAAGAAGGAGAAG | 27 | 37–63 | 8572–8756 | 40.7 | 60.7 | 185a |
| PVARPAR1 | Antisense | GTAAGATAGCAAGTGATCTAGGTTAACGAC | 30 | 192–221 | 40 | 61.3 | ||||
| B | PVARPAF1 | Sense | CTGAAGGTAAGAAGAAAGAAGGAGAAG | 27 | 37–63 | 8572–8747 | 40.7 | 60.7 | 176 | |
| PVARPAR2 | Antisense | CAAGTGATCTAGGTTAACGACACTCTTAC | 29 | 184–212 | 41.4 | 61.7 | ||||
| C | PVARPAF2 | Sense | GAAGGTAAGAAGAAAGAAGGAGAAGG | 26 | 39–64 | 8574–8756 | 42.3 | 60.6 | 183 | |
| PVARPAR1 | Antisense | GTAAGATAGCAAGTGATCTAGGTTAACGAC | 30 | 192–221 | 40 | 61.3 |
aFor optimization and validation of one-step RT-RPA, the best primer pair was selected
Selection of primers for PVA detection and optimization of RT-RPA
To identify the optimal primer pair for PVA detection, RT-PCR was performed in two steps (Meena et al. 2017). cDNA was used as a template in the assay, and four incubation temperatures (56, 58, 60, and 62 °C) were tested for all three primer sets to determine the optimal primer pair for RT-RPA under thermal cycler. In primer set A (PVARPAF1/R1), 185 bp was expected, in primer set B (PVARPAF1/R2), 176 bp was expected, while in primer set C (PVARPAF2/R1), 183 bp was expected. In addition, three incubation temperatures (38, 40, and 42 °C) were tested under the heating block and water bath using cDNA in two steps and RNA in one step as a template and primer set A (PVARPAF1/R1).
RT-RPA reactions were performed under isothermal conditions using two types of equipment: a heating block (Model-Wealtec HB-2, Wealtec Corp., USA) and a water bath (Model-Hoefer RCB 500, Hoefer Scientific Instruments, California, USA). Before placing the sample tubes in the holding unit, the temperature in the heating block was set and maintained at 40 °C. Each 0.2 ml sample tube was placed into the heat block's holding unit, which could accommodate 88 tubes. In the water bath, sterile distilled water was added to achieve the required final volume, and the temperature was maintained at 40 °C. Using a floatable polyfoam tube holder, the sample tubes (0.2–0.5 ml) were placed on the surface of the water bath after the desired temperature was reached. RT-RPA was performed using the TwistAmp® basic kit from TwistDx. Rehydration buffer and nuclease-free water were used to rehydrate the freeze-dried reaction pellets. A cost-effective assay was achieved by dividing the reaction mixture equally between five fresh PCR tubes, with 7.54 µl of reaction mix in each tube, resulting in five individual 10-µl reactions. In each PCR tube, primers (forward and reverse) were added at a concentration of 10 pmol, and 0.5 µl per primer was used. For each tube, 1 µl of cDNA template was added. A 14 mM magnesium acetate solution (per 10 µl reaction) was added to initiate the RT-RPA reaction. A 4-min incubation at 40 °C was performed on the reaction mixtures. A short time after incubation, the samples were removed from the heating block/water bath/thermal cycler, mixed by pipetting, spun, and then placed back into the respective heating block/water bath/thermal cycler for further incubation, which lasted for at least 30 min. After the tubes had been subjected to a thermal step at 65 °C for 10 min using the thermal cycler/heating block/water bath, the RT-RPA reaction was verified. The RT-RPA samples were then electrophoresed on a 2.5% agarose gel.
Optimization of PVA detection by one-step RT-RPA
With the goal of developing a robust method, we optimized RT-RPA by including the following components in the reaction mix: 0.5 μl of reverse transcriptase (200 U/μl), 0.5 μl of RNase inhibitor (20 U/μl), and RNA as a template. Each reaction volume was 11 μl, using either 1 μl of total RNA (approximately 150–200 ng/μl) or 1 μl of cellular disc paper-based RNA extract (prepared by grinding 25 mg of leaf tissues in 1 ml of extraction buffer #2). As described in Sect. “Selection of primers for PVA detection and optimization of RT-RPA”, the remaining components of the reaction mix were similar to those of the two-step RT-RPA protocol. The one-step RT-RPA was conducted at different incubation temperatures (38, 40, and 42 °C) with RNA as the template to speed up the cDNA synthesis and primer set A (PVARPAF1/R1) amplification steps in the two-step RT-RPA. We chose 40 °C for further experiments based on the results. Further, we standardized the incubation times (10, 20, 30, 40, and 50 min) for one-step and two-step RT-RPA reactions using a thermal cycler and a heating block/water bath. For our subsequent studies, we determined a 30-min incubation time would be suitable for both one-step and two-step RT-RPA. The one-step RT-RPA assay was also optimized by testing various magnesium acetate concentrations (12, 14, 16, 18, and 20 mM). To ensure consistency and reliability, these experiments were repeated 3–4 times. To evaluate non-specific amplification in the RT-RPA, a healthy control was included with the template and any undesired amplification was monitored.
Optimization of RNA extraction method and RT-RPA for detection of PVA in tuber
Four recently reported simple RNA extraction methods (Zou et al. 2017; Silva et al. 2018; Onozuka et al. 2020; Kumar et al. 2023) were compared with the Spectrum™ Plant Total RNA kit from Sigma-Aldrich, Missouri, USA. The aim was to optimize a cost-effective and efficient RT-RPA detection method for potato virus A (PVA) in potato leaves and tubers. Among the four extraction methods, a modified cellular disc paper-based RNA preparation method (Onozuka et al. 2020) proved highly effective in identifying PVA in potato leaves but not in tubers. In this method, a filter paper disc was placed in a tube, and solutions were added and removed, with RNA eluted in RNase-free water. However, high-quality RNA extraction from tubers was challenging due to high levels of polyphenols and polysaccharides. To improve RNA yield and quality from tubers, the addition of 8 M lithium chloride (LiCl) was investigated in combination with the modified cellular disc paper-based RNA preparation method (Fig. 3). In the cellular disc paper-based RNA extraction buffer, LiCl was added along with other components. The modified method, coupled with LiCl treatment, was then used for RT-RPA detection of PVA, along with purified RNA obtained from the SpectrumTM Plant Total RNA kit.
Fig. 3.
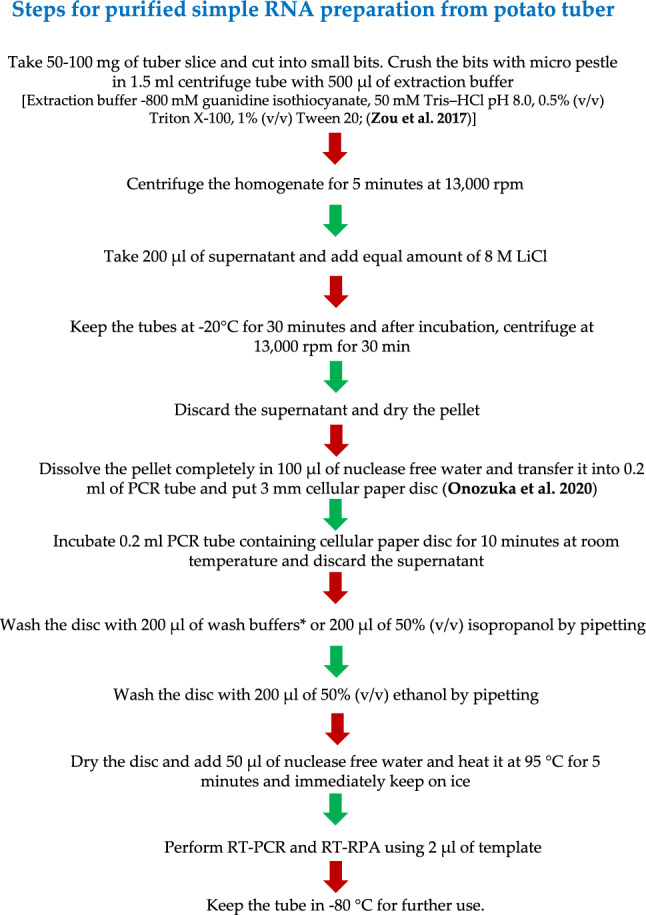
Flow chart of simple RNA extraction preparation by coupling of lithium chloride (LiCl)-based RNA precipitation and cellular paper disc methods from potato tuber for potato virus A (PVA) detection
PVA detection by one-step RT-PCR/RT-RPA in leaves and tubers: sensitivity and specificity analysis
One-step RT-PCR/RPA results may be affected by RNA concentration and purity. To determine the sensitivity of the one-step RT-RPA method, we performed a serial dilution of 1 μg total RNA from potato leaves and tubers ten times (10–0 to 10–8). As a similar procedure, cellular disc paper-based RNA extracts were diluted by ten folds (10–0 to 10–8 dilutions) using 25 mg leaf tissues in 1 ml of extraction buffer #2. 1 μl of each dilution was used as template for the RT-RPA and RT-PCR assays. At the lowest concentration of RNA at which a detection limit can be established, a positive result is obtained. RT-RPA assays for PVA were performed against samples infected with ToLCNDV, PLRV, PVY, PVS, PVX, PVV, and PVM along with negative control to test the specificity of the primers. In both RT-RPA and RT-PCR, detection limits were confirmed by electrophoresis on 2.5% and 1.0% agarose gels.
Confirmation of specific genes by cloning, elution, and sequencing
To analyze the specificity of the best selected RT-RPA primer pair and identification of RT-RPA product, electrophoresis DNA were eluted using a GeneJET™ Gel Extraction kit (Fermentas, Thermo Fisher Scientific, USA), and the amplicons were cloned into Escherichia coli JM107 by following the sticky-end cloning protocol using the cloneJET PCR cloning kit (Fermentas, Thermo Fisher Scientific, USA). The colony PCR was performed to identify the cloned product, and the M13 forward and reverse primers were used for sequencing of the amplified product (Applied Biosystems-Hitachi, USA). We then screened these sequences using BLAST (http://www.ncbi.nlm.nih.govt/blast) to find similar sequences in the NCBI nucleotide database (http://www.ncbi.nlm.nih.govt/blast/).
Natural infection samples were validated with one-step RT-PCR and DAS-ELISA using one-step RT-RPA
For validation of the optimized one-step RT-RPA method, 300 PVA suspected field samples from the most popular potato varieties were collected (150 leaves and 150 tubers from the same plant). The samples were collected from the different states of the India viz., Bihar, Himachal Pradesh, Haryana, West Bengal, Madhya Pradesh, Punjab, Uttar Pradesh and Meghalaya during the year 2019–2022. A virus-free tissue-culture-raised mother plant/minituber was also used as healthy control. RNA of these samples were extracted and stored at − 80 °C until used. Validation also included stored tubers harvested from infected potato plants. The results of one-step RT-RPA were confirmed using dormant tubers (without sprouts) as well as DAS-ELISA and one-step RT-PCR (Tables 2 and 3). DAS-ELISA was performed using earlier described procedures (Kumar et al. 2017a). A sample was considered positive if the optical density increased by three times the mean background level. For each ELISA plate, mean background levels were enumerated by scaling at least two wells containing all reagents except sap extract.
Table 2.
An analysis of the one-step reverse transcription-polymerase chain reaction (RT-PCR) and reverse transcription-recombinase polymerase amplification (RT-RPA) in potato samples (leaves and tubers) from different states of India to compare and validate the presence of potato virus A (PVA) in the samples
| Field Sample No | One-step RT-PCR (Meena et al. 2017) | One-step RT-PCR with primer pair A (PVARPAF1/R1) | One-step RT-RPA with primer pair A (PVARPAF1/R1) under heating block/ water bath | One-step RT-RPA with primer pair A (PVARPAF1/R1) under heating block/ water bath | ||||
|---|---|---|---|---|---|---|---|---|
| Purified RNA | Purified RNA | Purified RNA | Simple RNA without LiCl | Simple RNA with LiCl | ||||
| Leaf | Tuber | Leaf | Tuber | Leaf | Tuber | Leaf | Tuber | |
| 1 | + | + | + | + | − | + | − | + |
| 2 | + | + | + | + | + | + | + | + |
| 3 | + | + | − | + | − | + | − | + |
| 4 | + | + | + | + | + | − | + | − |
| 5 | + | + | + | + | + | + | + | + |
| 6 | + | + | + | + | + | + | + | + |
| 7 | + | + | + | − | + | − | + | − |
| 8 | + | + | + | + | − | − | − | − |
| 9 | + | + | + | + | + | + | + | + |
| 10 | + | + | + | + | + | + | + | + |
| 11 | + | + | + | + | + | + | + | + |
| 12 | + | + | + | + | + | + | + | + |
| 13 | − | + | − | + | − | + | − | + |
| 14 | + | + | + | + | + | + | + | + |
| 15 | + | + | + | + | + | + | + | + |
| PC | + | + | + | + | + | + | + | + |
| HC | − | − | − | − | − | − | − | − |
| NC | − | − | − | − | − | − | − | − |
PC known positive leaf/tuber sample of potato, HC healthy potato leaf/tuber sample, NC negative (water) control, + PVA positive; − PVA negative
Table 3.
A comparative study of double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA), reverse transcription-polymerase chain reaction (RT-PCR), and reverse transcription-recombinase polymerase amplification (RT-RPA) methods for the detection of potato virus A (PVA) in field-collected suspected plant parts collected across agroecological zones in different states of India (including both the leaves and tubers of the same plant)
| Sample No | Location/state | Cultivar/variety | DAS-ELISA | One-step RT-PCR (Meena et al. 2017) | One-step RT-RPA with primer pair A (PVARPAF1/R1) under heating block/water bath | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Purified RNA | Purified RNA | Simple RNA without LiCl | Simple RNA with LiCl | ||||||||
| Leaves | Dormant tubers | Sprouted tubers | Leaves | Dormant tubers | Leaves | Dormant tubers | Leaves | Dormant tubers | |||
| 1 | Gujarat | Laddy Rosetta | − | − | − | − | − | − | − | − | − |
| 2 | Gujarat | Kufri Pukhraj | + | − | + | + | + | + | + | + | + |
| 3 | Gujarat | Kufri Jyoti | + | − | − | + | + | + | + | + | + |
| 4 | Gujarat | Kufri Sindhuri | − | − | − | − | − | − | − | − | − |
| 5 | Punjab | Kufri Pukhraj | − | − | − | − | − | + | − | + | − |
| 6 | Punjab | Kufri Badshah | − | − | − | + | + | + | + | + | + |
| 7 | Punjab | Laddy Rosetta | − | − | − | − | − | − | − | − | − |
| 8 | Punjab | Kufri Ashoka | + | − | − | + | + | + | + | + | + |
| 9 | Haryana | Kufri Chandramukhi | + | − | − | + | + | + | + | + | + |
| 10 | Haryana | Kufri Jyoti | + | − | + | + | + | + | + | + | + |
| 11 | Haryana | Kufri Bahar | − | − | − | − | − | − | − | − | − |
| 12 | Haryana | Kufri Jawahar | − | − | − | + | + | + | + | + | + |
| 13 | Himachal Pradesh | Kufri Jyoti | − | − | − | − | − | − | − | − | − |
| 14 | Himachal Pradesh | Kufri Himalini | − | − | − | − | − | − | − | − | − |
| 15 | Himachal Pradesh | Kufri Chandramukhi | + | − | + | + | + | + | + | + | + |
| 16 | Himachal Pradesh | Kufri Shailja | + | − | − | + | + | + | + | + | + |
| 17 | Uttar Pradesh | Kufri Pukhraj | − | − | − | + | − | + | + | + | + |
| 18 | Uttar Pradesh | Kufri Chipsona 1 | + | − | + | + | + | + | + | + | + |
| 19 | Uttar Pradesh | Kufri Chipsona 2 | − | − | − | + | − | + | + | + | + |
| 20 | Uttar Pradesh | Kufri Bahar | + | − | − | + | − | + | + | + | + |
| 21 | Bihar | Kufri Sindhuri | + | − | − | + | + | + | + | + | + |
| 22 | Bihar | Kufri Chandramukhi | − | − | − | + | - | + | + | + | + |
| 23 | Bihar | Kufri Anand | + | − | + | + | + | + | + | + | + |
| 24 | Bihar | Kufri Sindhuri | − | − | − | − | − | − | − | − | − |
| 25 | Madhya Pradesh | Kufri Pukhraj | + | − | + | + | + | + | + | + | + |
| 26 | Madhya Pradesh | Kufri Lauvkar | − | − | − | + | + | + | + | + | + |
| 27 | Madhya Pradesh | Kufri Chandramukhi | + | − | − | + | + | + | + | + | + |
| 28 | Madhya Pradesh | Kufri Surya | − | − | − | − | − | − | − | − | − |
| 29 | West Bengal | Kufri Jyoti | + | − | − | + | + | + | + | + | + |
| 30 | West Bengal | Kufri Chandramukhi | − | − | − | − | − | − | − | − | − |
| 31 | West Bengal | Kufri Pukhraj | + | − | − | + | + | + | + | + | + |
| 32 | West Bengal | Kufri Ashoka | + | − | + | + | + | + | + | + | + |
| 33 | Meghalaya | Kufri Megha | − | − | − | − | − | − | − | − | − |
| 34 | Meghalaya | Kufri Giriraj | + | − | + | + | + | + | + | + | + |
| 35 | Meghalaya | Kufri Jyoti | − | − | − | − | − | − | − | − | − |
| Total | 18 | 0 | 08 | 23 | 19 | 25 | 23 | 25 | 23 | ||
+ PVA positive; − PVA negative
Results
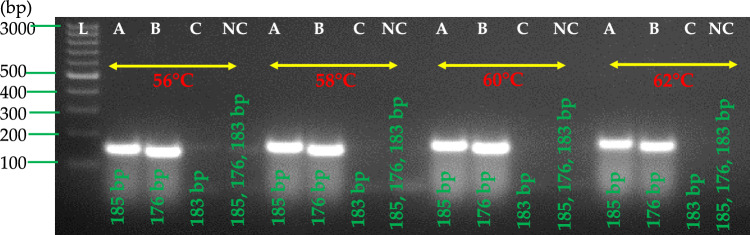
Three primer sets (A- PVARPAF1/R1, B- PVARPAF1/R2, C- PVARPAF2/R1) were designed based on aligned conserved regions of full coat protein gene sequences available in the GenBank of PVA. Figure 2 illustrates the positions of primer pairs. RT-RPA primers were designed according to the manufacturer's (TwistAmp® Basic kit) guidelines. For the development of a successful RT-RPA method, the manufacturer recommends a temperature range of 37–42 °C. With purified total RNA of a PVA-infected potato leaf sample, cDNA was synthesized to evaluate the efficiency of all three designed primer pairs (A, B, and C). A thermal cycler was used to perform the two-step RT-PCR at a range of temperatures (56–62 °C). All temperatures tested detected PVA, as shown in Fig. 4. In leaf samples infected with PVA, RT-PCR amplified fragments of 185 base pairs (PVARPAF1/R1), 176 bases pairs (PVARPAF1/R2), and 183 bases pairs (PVARPAF2/R1). This primer set (PVARPAF1/R1) was chosen because it has a smaller amplicon, is reproducible, specific, and produces sharp amplification at (56–62 °C). The primer was used for RT-RPA optimization in all subsequent studies (Table 1). All the primers had 40.0–42.3% GC content according to manufacturer recommendations (Table 1).
Fig. 4.
PVA detection by RT-PCR using three newly designed primer pairs (A–C). In lanes L-1 Kb or 100 bp ladder, primer set A (PVARPAF1/R1) was expected to give a product of 185 bp; primer set B (PVARPAF1/R2) was expected to produce a product of 176 bp; primer set C (PVARPAF2/R1) was expected to produce a product of 183 bp; lane NC is a negative (water) control
One-step RT-RPA optimization with a selected primer for the detection of PVA
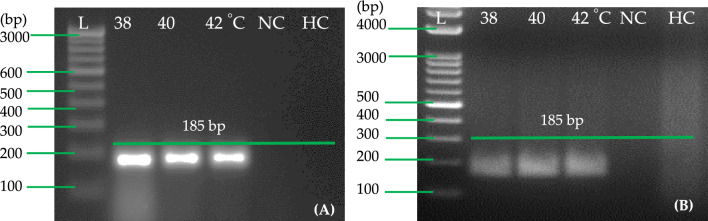
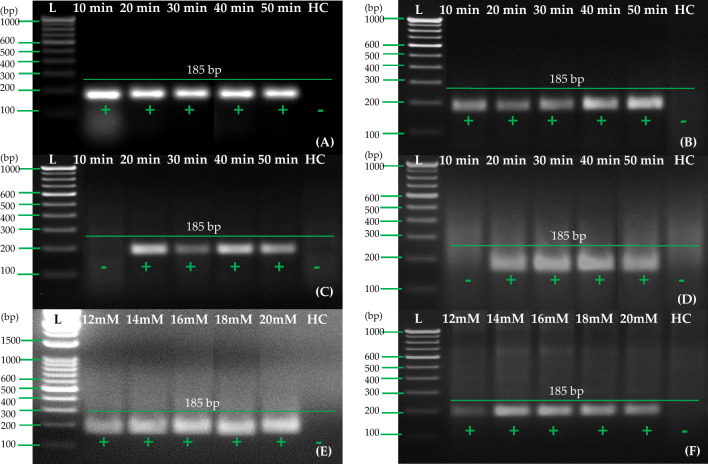
We evaluated the selected primer pair A by performing RT-RPA reactions in a water bath and heating block for 30 min to assess the temperature requirements. Three temperatures (38, 40, and 42 °C) were used for RT-RPA in two steps (Fig. 5A) and one step (Fig. 5B). All three temperatures tested under the heating block/water bath worked efficiently for one-step/two-step RT-RPA when cDNA/RNA samples were used. Negative and healthy controls did not exhibit any amplification. Through two-step RT-RPA using cDNA and one-step RT-RPA using RNA, accessible amplification of PVA by RT-RPA can be achieved within 30 min at a temperature of 38–42 °C.
Fig. 5.
The optimization of the RT-RPA reaction for the detection of PVA with primer pair A (PVARPAF1/R1). A with primer set A; RNA extracted from potato leaf sample of PVA (RNA as template; B with primer set A; lane NC (A and B)—negative control with primer set A; lane HC (A and B)—healthy control with primer set A
The next step involved selecting 40 °C for further RT-RPA experiments and evaluating the incubation time (10, 20, 30, 40, and 50 min) in one-step RT-RPA reactions as well as two-step RT-RPA reactions. The results of these experiments revealed that a 10-min incubation time (Fig. 6A and B) was sufficient to detect PVA when using two-step RT-RPA in both the thermal cycler and the heating block/water bath incubation conditions. In both incubation conditions (thermal cycler and heating block/water bath), the incubation time of 30 min (Fig. 6C and D) was better with one-step RT-RPA. RT-RPA detected PVA within 10 min using cDNA as a template, while one-step RT-RPA took between 20 and 30 min to detect PVA. Toward the optimization of the one-step RT-RPA method, an incubation time of 30 min was used for the further experiments. Afterward, the optimal magnesium acetate concentration for the one-step RT-RPA assay was determined by standardized values of magnesium acetate. We used five different concentrations (12, 14, 16, 18, and 20 mM) to test the effects of each concentration. There was a significant variation in the amount of magnesium acetate that was required to optimize one-step RT-RPA, ranging from 12 to 20 mM (Fig. 6E and F). However, 14 mM was selected for better performance in a water bath/heating block condition compared with 12–20 mM magnesium acetate. We repeated all of the assays three or four times to ensure accuracy. We also used a healthy control to check whether any non-specific amplification had occurred in the RT-RPA as well.
Fig. 6.
The optimization of incubation period and magnesium acetate concentrations in the RT-RPA reaction for the detection of PVA using primer pair A (PVARPAF1/R1). In A, cDNA is used as a template in a thermal cycler. In B, cDNA is used as template in the water bath/heating block. In C, RNA is used as a template, under a thermal cycler. D Using RNA as a template and a water bath/heating block. E RNA was used as a template for determining the optimum concentration of magnesium acetate, under thermal cycler. F RNA was used as a template for determining the optimum concentration of magnesium acetate, under water bath/heating block. Lane L—1 Kb or 100 bp ladder, lane HC healthy control (cDNA/RNA)
Evaluation and optimization of RNA extraction methods
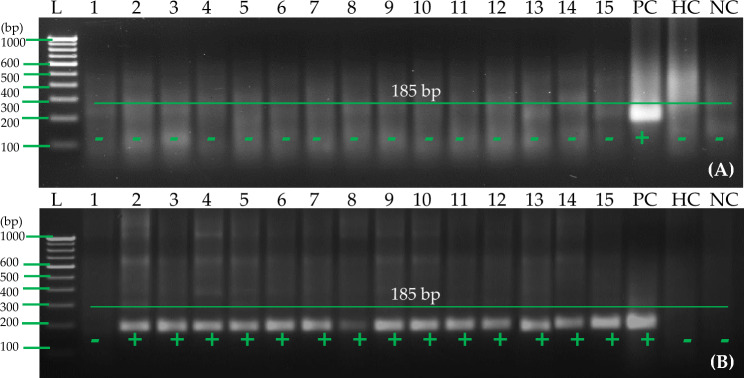
Despite the fact that there are many simple RNA extraction methods available, it was necessary to analyze and compare four different methods with pure total RNA using RT-PCR using selected primer set A to develop a cost-effective and efficient one-step RT-RPA. A cellular disc paper-based RNA extract was able to successfully detect PVA in leaves out of four RNA extracts tested. There was, however, no evidence that this method could detect PVA in potato tubers (Fig. 7A). Hence, further experiments were conducted to see if the cellular disc paper-based RNA preparation previously reported by us could be used to enhance RNA production and quality from potato tubers. In Fig. 7B, lithium chloride (LiCl) coupled with cellular disc paper-based RNA preparation method increases the amount and quality of RNA from potato tubers. We compared the results to those of commercially available RNA purified from kits for the detection of PVA using RT-PCR. After LiCl was added to other components of an RNA extraction method using cellular disc paper, PVA was successfully detected from the tuber. For further experiments, potato tuber RNA was prepared using 8 M LiCl and cellular disc paper. This modified method was used with purified RNA obtained from the Spectrum™ Plant Total RNA kit to detect PVA using RT-PCR and RT-RPA.
Fig. 7.
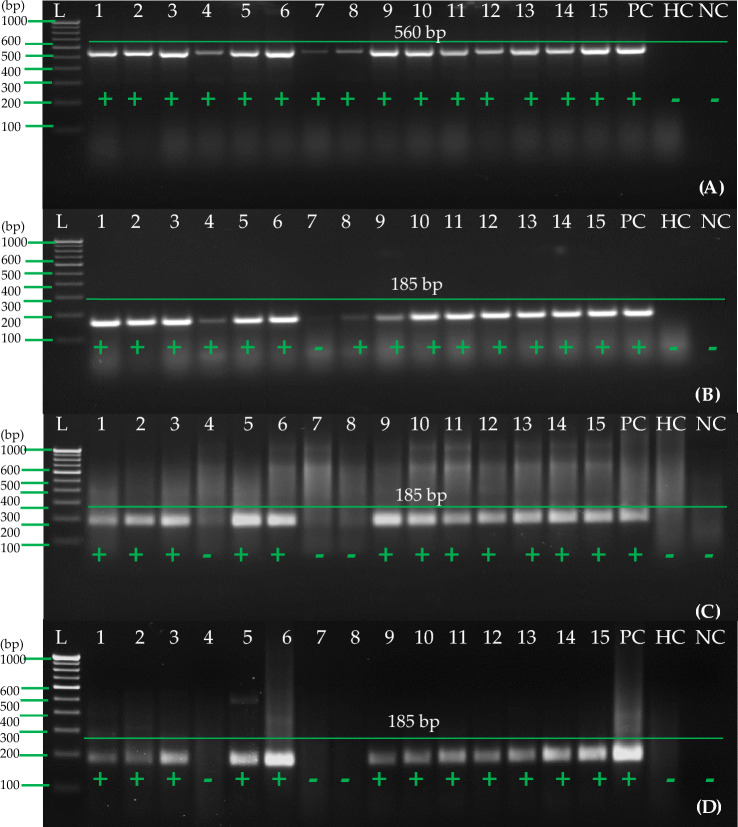
Detection of potato virus A (PVA) from tubers with RT-RPA using primer pair A (PVARPAF1/R1) A RT-RPA reactions were performed at 40 °C with 2 μl of cellular disc paper-based RNA preparation (25 mg tuber tissues ground in 1 ml of extraction buffer #2). B 2 μl of cellular disc paper-based RNA preparation (25 mg tuber tissues ground in 1 ml of extraction buffer #2) coupled with 8 M LiCl was used for the RT-RPA reactions at 40 °C. Lane L—1 Kb or 100 bp ladder; lanes 1–15—cellular disc paper-based RNA extract of 15 PVA-infected tuber samples. PC positive control (purified total RNA of one infected leaf sample), lane HC healthy control (purified total RNA of one healthy leaf sample), lane NC negative control (water used as a template)
Sensitivity and specificity analysis for the specific detection of PVA in leaves and tubers
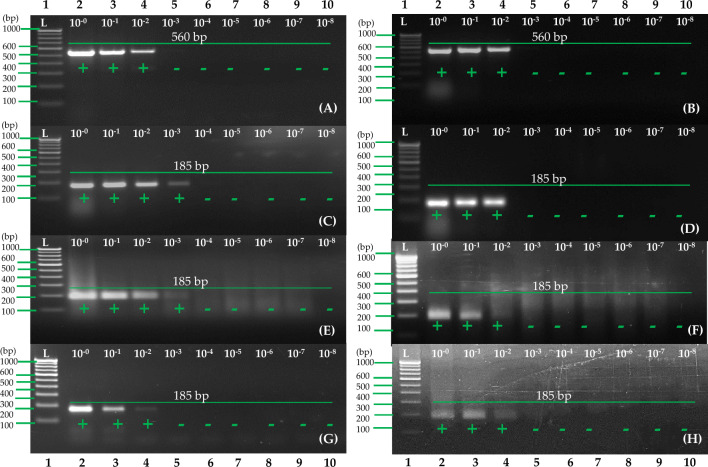
One-step RT-RPA has been evaluated for its sensitivity by serial dilutions of 1 µg total RNA from potato leaves and tubers by ten folds (10–0 to 10–8 dilutions). This was compared to previously published RT-PCR (Meena et al. 2017). A sensitivity test was also performed using dilutions of RNA extract from a cellular disc paper-based RNA preparation method, consisting of 10–0 to 10–8 dilutions of RNA extract from 1 μl of the extract. As a template, 1 μl of dilution of simple RNA extract was taken for use as a template for the one-step RT-RPA procedure. In this study, electrophoresis tests on 2.5 and 1.0% agarose gels were used to determine the detection limits of the one-step RT-RPA and RT-PCR methods. The results indicated that when total RNA dilution was evaluated with selected primer set A and compared to earlier reported primers (Meena et al. 2017), primer set A had ten times higher sensitivity in the leaves and equally high sensitivity in the tubers (Fig. 8A–D). When comparing simple RNA extract with purified RNA dilutions, there were no differences in RT-RPA sensitivity. Based on the results (Fig. 8E–H), the detection limit of one-step RT-RPA using simple RNA extract was the same as that of RT-RPA and RT-PCR using purified RNA. PVA detection using RT-RPA mediated by simple RNA extracts was highly effective and robust since sensitivity was equally high. In addition, the specificity of the primers was tested using one-step RT-RPA assays for PVA, ToLCNDV, PLRV, PVY, PVS, PVV, PVX, and PVM infected samples with healthy controls. In this study, only PVA was detected by primer set A, and no cross-reaction was observed with other potato viruses (Fig. 9).
Fig. 8.
Potato virus A (PVA) samples were used to compare sensitivity of one-step RT-PCR and RT-RPA. A Performed one-step RT-PCR following eralier reported primers (Meena et al. 2017) and purified RNA (leaf), B performed one-step RT-PCR following eralier reported primers (Meena et al. 2017) and purified RNA (tuber), C performed one-step RT-PCR with primer pair A and purified RNA (leaf), D performed one-step RT-PCR with primer pair A and purified RNA (tuber), E performed one-step RT-RPA with primer pair A and purified RNA (leaf), F performed one-step RT-RPA with primer pair A and purified RNA (tuber), G performed one-step RT-RPA with primer pair A and cellular disc paper-based RNA extract (leaf), H performed one-step RT-RPA with primer pair A and 8 M LiCl with cellular disc paper-based RNA extract (tuber). Lane L—1 Kb or 100 bp ladder; Lanes 2 to10—RT-RPA/RT-PCR products obtained with total RNA dilutions from 10–0–10–8 (A–F) and cellular disc-based RNA preparation (G, H)
Fig. 9.
Specificity analysis of selected primer pair for the detection of PVA by one-step RT-RPA. Lane L—100 bp ladder; other lanes—RT-RPA products obtained with total RNA of PVA, PLRV, PVYO, PVM, PVS, PVV, PVX, and ToLCNDV; lane NC negative (water) control
Confirmation of specific genes by cloning, elution, sequencing, and blast analysis
To confirm the amplicons obtained from the gel, the target fragments were excised, cloned, and sequenced to verify their identity. In this study, the amplicons showed 100% sequence similarity with the PVA sequence. Thus, the results provided by this study proved that the RT-RPA assay was a highly accurate method for detecting PVA. With the aid of the BLASTn program, an in silico analysis of the designed RT-RPA primers was performed to assess their specificity. Based on an in silico analysis, it was determined that the coat protein gene of PVA (Y11421, S51667, MT521082, and MT502364) had 100% identity (query coverage 100%). Under this study, no matches were detected in terms of specificity between this virus and the others, including those which were taken for specificity analysis under this study (Fig. 9).
Natural infection samples were validated with one-step RT-PCR and DAS-ELISA using one-step RT-RPA
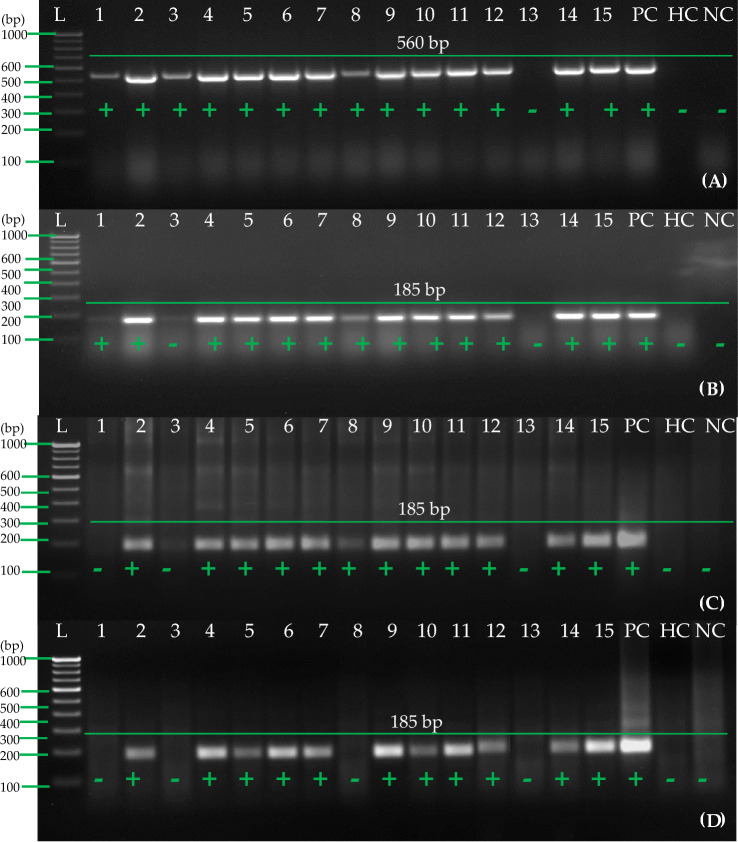
The goal of this study was to test the reliability of our optimized one-step RT-RPA method by taking a total of 300 suspected PVA samples in the field from leading potato cultivars from different states of India (150 leaves and 150 tubers from the same plant). As a healthy control, tissue-culture-raised mother plants and minitubers, which were free from viruses, were used. After the 300 samples were examined using the DAS-ELISA method, further validation was done using the one-step RT-PCR method and the one-step RT-RPA method. From 150 samples of leaves (Table 2), a total of 15 samples were randomly selected where 14 samples were detected as positive by RT-PCR, using primers which have been previously reported (Meena et al. 2017) (Fig. 10A). As with the one-step RT-PCR, 13 samples were found to be positive while 11 samples were seen to be positive when using purified RNA for the RT-RPA with RPA primer set A (Fig. 10B and C). Similarly, when tissue-based RNA extracts were used as templates for one-step RT-RPA using primer set A, the same results were seen (Fig. 10D).
Fig. 10.
Detection of PVA in field samples of potato (leaves) by one-step RT-RPA and RT-PCR. A One-step RT-PCR was carried out following earlier reported primers (Meena et al. 2017) and purified RNA, B One-step RT-PCR was carried out with primer pair A and purified RNA, C One-step RT-RPA was carried out with primer pair A and purified RNA, D One-step RT-RPA was carried out with primer pair A and cellular disc paper-based RNA extract. Lane L-1 Kb or 100 bp ladder, Lane 1–15 shows different samples of potato leaves, PC known positive leaf sample of potato, HC healthy potato leaf sample, NC negative (water) control
The results of the one-step RT-PCR analysis of 15 randomly selected tuber samples from 150 samples of tubers (both dormant and stored) for this study showed that all 15 samples tested positive for RNA (Fig. 11A). Furthermore, 14 samples were found positive in one-step RT-PCR (Fig. 11B) while 12 samples were found positive in RT-RPA using purified RNA with primer set A (Fig. 11C). When cellular disk paper-based RNA extract coupled with 8 M LiCl was used as a template for one-step RT-RPA using primer set A (Fig. 11D), 12 samples were identified in both the one-step RT-RPA and the RT-RPA with primer set A (Table 2). There was also a significant variation among samples regarding the intensity of the amplicon (185 bp) of the PVA amplicon. There was a significant difference in the exposure to virus in plants/tubers that could be correlated with the sharpness of the amplifications from the infected plants/tubers.
Fig. 11.
Detection of PVA in field samples of potato (tubers) by one-step RT-RPA and RT-PCR. A One-step RT-PCR was carried out following earlier reported primers (Meena et al. 2017) and purified RNA, B One-step RT-PCR was carried out with primer pair A and purified RNA, C One-step RT-RPA was carried out with primer pair A and purified RNA, D One-step RT-RPA was carried out with primer pair A and cellular disc paper-based RNA extract with 8 M LiCl. Lane L-1Kb or 100 bp ladder, Lane 1–15 shows different samples of potato tubers, PC- known positive tuber sample of potato, HC-healthy potato tuber sample, NC-Negative (water) control
We have compared the sensitivity and specificity of three different methods of detection, namely DAS-ELISA, one-step RT-PCR, and one-step RT-RPA, all of which utilize purified RNA and cellular disk paper-based RNA extracts to detect PVA levels from leaves, dormant tubers, and sprouted tubers (in case of DAS-ELISA) (Table 3). There were a total of 35 samples collected in this activity, representing each state and cultivar differently. DAS-ELISA was conducted on 35 samples of leaves, sprouted tubers, and dormant tubers. Among them, PVA was detected in 18 samples and in 8 samples of sprouted tubers. On the other hand, a one-step RT-PCR that was performed on purified RNA from leaves and dormant tubers was adjudged to be positive for PVA in 23 leaves samples and 19 dormant tuber samples. The results showed that, using 3 types of RNA templates to make the RT-RPA in leaves and dormant tubers, a total of 25 and 23 samples, respectively, were found to be positive (Table 3). The results of these experiments indicated that improved single-step RT-RPA might be a robust, highly specific, and sensitive test method and could be used by labs with limited resources for routine screening of potato germplasms, field samples, and virus indexing on a large scale.
Discussion
To prevent viral infections from spreading, it is crucial to detect viruses early on in planting materials. Potato virus A (PVA) is a particularly damaging and notorious virus that primarily affects potatoes through mechanical transmission (Naga et al. 2021a, b). This study developed a robust RT-RPA method, which is an effective tool for testing PVA at large scales in a healthy seed production system. It is particularly important to apply the developed RT-RPA in potato tubers since PVA primarily spreads through seed tubers. A number of diagnostic methods have been available in the past for detecting PVA in potatoes, including DAS-ELISA, conventional RT-PCR, multiplex RT-PCR, and real-time RT-PCR (Kumar et al. 2014, 2020; Meena et al. 2017; Raigond et al. 2020b). Nevertheless, these methods require complex laboratory equipment and require a lot of time. PVA has also been detected using RT-LAMP (reverse transcription loop-mediated amplification) methods based on isothermal reactions. The assay is more expensive because it requires purified RNA templates, higher temperatures, and longer incubation times, as well as 4–6 primers for amplification (Babujee et al. 2019; Zeng et al. 2019; Raigond et al. 2019; Mohandas and Bhat 2020; Kumar et al. 2022a). In contrast, the one-step RT-RPA method used in this study allows for rapid and simple isothermal amplification without the need for a thermal cycler in the laboratory. It takes 30 min to complete the entire process. Interestingly, the one-step RT-RPA method used here exhibited the same sensitivity as the RT-PCR method previously reported. In addition, by adding LiCl precipitation to the extraction buffer, we developed a cost-effective RNA preparation method using cellular disc paper for dormant tubers. Based on RT-RPA detection from leaves, the sensitivity and specificity achieved were comparable. A rapid and cost-effective solution for detecting PVA in potatoes can be achieved using the developed one-step RT-RPA method. In the context of seed production systems, it is highly suitable for large-scale testing due to its simplicity, short assay time, and minimal equipment requirements (Singh et al. 2014; Silva et al. 2018; Raigond et al. 2019; Naga et al. 2020; Shah et al. 2020; Tiwari et al. 2021b). With the use of paper-based cellular disc RNA extracts and an optimized extraction method for dormant tubers, the technique becomes even more practical and affordable.
CP (coat protein) gene of PVA was targeted in this study to ensure specific and sensitive detection. Virus-infected potato plant cells contain a high abundance of this gene. In addition, reverse transcription is highly favored due to CP's location at the 3'-end of PVA RNA (Paul Khurana 2006; Agindotan et al. 2007; Fuentes et al. 2021). It was critical to determine the specificity and sensitivity of the primers when developing a successful one-step RT-RPA assay. In RT-RPA assays, primers are designed similarly to those used in RT-PCR, but they are longer in length in RT-RPA. The specificity and sensitivity of the test were optimized by evaluating multiple primers. The efficiency of RT-RPA may be affected by a variety of factors, including poorly designed primers, RNA quality and quantity, and annealing temperature. RT-RPA conditions also require consideration of factors such as primer lengths and GC compositions (Raigond et al. 2020a; Kumar et al. 2022c, a). In designing primers, we considered these factors as well as information from the NCBI database regarding PVA's genetic diversity. It was possible to design primers with a broad geographic range based on this information. Both one-step RT-PCR and one-step RT-RPA were employed to evaluate the selected primer set, referred to as primer set A, under thermal conditions with a heating block or water bath to assess its sensitivity and specificity. The RT-RPA assay for detecting PVA was designed to be accurate and reliable by considering these factors and evaluating primers rigorously.
It has been shown that RT-RPA exhibits selective advantages over RT-PCR or ELISA because of a constant temperature range of 25–42 °C along with a short reaction time (Piepenburg et al. 2006; Donoso and Valenzuela 2018; Ivan Magrina Lobato 2020; Jiao et al. 2020; Mohandas and Bhat 2020; Kumar et al. 2021b). A one-step RT-PCR required more than 90 min to complete amplification cycles (normally, 35 cycles) before gel documentation could be carried out in one-step RT-PCR. In the same way, a minimum of 2 days is needed for conducting a DAS-ELISA test. The thermal cycler is essential in most cases when RT-RCR assay is used, since cDNA synthesis is usually required for RT-RPA assays. As a result of the temperature necessity for the RT-RPA reaction, the assay can be carried out in poorly equipped laboratories near potato fields due to the flexibility of the RT-RPA reaction.
It has been demonstrated that cellular disc paper-based RNA extract, as well as the three simple methods of RNA extraction (Zou et al. 2017; Nouayti et al. 2018; Silva et al. 2018; Onozuka et al. 2020), have proven to be the most effective method of extracting RNA for detection of PVA in leaves. In spite of the fact that the method was unable to detect PVA in tubers, this hurdle was also overcome by enhancing RNA yield and quality by amending the cellular disc paper-based RNA preparation method with LiCl. Moreover, our findings are in agreement with those of previous studies which showed that LiCl-based extraction protocols yielded two to three times more amounts of RNA than those of water-based extraction protocols.
The purpose of the present study was to develop a one-step RT-RPA using RNA extract that was capable of amplifying the desired amplicon (185 bp) at a temperature of 40 °C at an isothermal incubation temperature. According to this, it has been demonstrated that accurate detection can be obtained using only simple heating blocks and water baths, which decreases the cost of the assay as already emphasized (Srivastava et al. 2019; Mohandas and Bhat 2020; Naveen and Bhat 2020). There is a reduction in the cost of the one-step RT-RPA due to the use of simple plant extracts as templates and the short period of time it takes to obtain results. The one-step RT-RPA is completed in under 30 min. One of the main advantages of the one-step RT-RPA that is used in this method is that the samples can be prepared in a single reaction tube, so there are no separate steps of cDNA synthesis required, and there are no additional chemicals to be used except for RT and RI for cDNA synthesis. In addition to the lower cost of using this method of detection, there is also a significant reduction in the volume of the reaction (11 μl instead of 50 μl) without compromising the assay's sensitivity. In spite of the fact that RT-RPA reagents are initially more expensive than RT-PCR reagents, in the end, RT-RPA allows you to conduct the test at a much lower cost than RT-PCR and RT-LAMP, as RT-PCR requires purified RNA, which can be obtained using commercial kit products. This one-step RT-RPA is more suitable for applications in low-resource laboratories that have a large number of samples in need of testing than those that lack the resources to prepare RNA and maintain a temperature of 40 °C for the detection of PVA due to its combination of simple RNA preparation and 40 °C temperature requirements. Recent studies have demonstrated that RT-RPA is successful under simple incubation conditions as demonstrated by recent findings (Mekuria et al. 2014; Babujee et al. 2019; Zeng et al. 2019; Chi et al. 2020; Naveen and Bhat 2020; Kumar et al. 2021b, 2022a).
This study demonstrated that the one-step RT-RPA assay developed in this study was able to provide highly compatible results when compared with conventional DAS-ELISA and RT-PCR methods when used for 300 field samples (150 leaf samples and 150 tuber samples). It has been reported that the number of positive samples through one-step RT-RPA detection compared to RT-PCR detection was almost similar in leaves and tuber samples, respectively. There is a possibility that the inability of DAS-ELISA to detect PVA in dormant tubers could be due to the virus concentration being below the sensitivity of DAS-ELISA or uneven distribution of virus within the dormant tubers themselves. There is a possibility that the PVA multiplies rapidly in the germinating sprouts after the dormancy has been broken, and accumulates within them. As a result, it is not hard to speculate that the detection of PVA by DAS-ELISA in 18 samples of sprouted tubers might be due to this factor, while none of the samples were detected positive by DAS-ELISA using dormant tubers. It was demonstrated by means of the one-step RT-RPA mediated testing of the infected samples in comparison with RT-PCR that RT-RPA provides equal sensitivity and greater specificity than RT-PCR. Furthermore, a detailed comparative analysis of three detection methods (DAS-ELISA, one-step RT-PCR (Meena et al. 2017), and one-step RT-RPA) utilizing purified RNA and cellular disk paper-based RNA extracts showed that the one-step RT-RPA method was equally sensitive to detect leaves, dormant tubers, and sprouts (Table 2). RT-RPA has been reported to be as sensitive as or more sensitive than RT-PCR in detecting plant RNA viruses (Londoño et al. 2016; Srivastava et al. 2019; Ivan Magrina Lobato 2020; Wang et al. 2020) when they are detected in combination with RT-PCR. To detect PVA effectively and robustly using simple RNA extracts, optimizing one-step RT-RPA appears to be a preferable alternative to two-step RT-RPA.
Conclusion
The end result of this study is the development of a user-friendly, fast, highly sensitive, and specific one-step RT-RPA assay that can be used for the identification of PVA in leaf tissue and tuber tissue samples. One-step RT-RPA was able to demonstrate high durability to plant inhibitors (polysaccharides and polyphenols) while requiring the least amount of sample preparation and with improved specificity and sensitivity compared to two-step RT-RPA. An RNA extraction can be performed using only a very small quantity of simple RNA extracted from fresh or stored samples. Purified RNA is not necessary for the procedure to be performed. This method can be used to index tissue-cultured plants, to check the viability of viral-free seed certification programs, as well as to screen germplasm for resistance to PVA. It has the potential to be a strong alternative to RT-PCR in this regard. Our study is the first to report the detection of PVA by one-step reverse transcription-polymerase chain reaction by simple leaf RNA extracts and tuber RNA extracts using simple RNA extracts.
Acknowledgements
Authors would like to acknowledge the Director of ICAR-Central Potato Research Institute, Shimla for their constant support and guidance.
Author contributions
Conceptualization and design, R.K. (Ravinder Kumar), P.K., and R.K.T.; interpretation of data and original draft preparation, M.K.L., R.K.T., H.K., and B.S.; analysis and interpretation of data and writing, R.K. (Rakesh Kumar) and V.S.; performed analysis, interpretation of data and resources, revised draft manuscript and supervision, R.K. (Ravinder Kumar), M.K.L., B.S., and R.K.T. All authors have read and agreed to the published version of the manuscript.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Rahul Kumar Tiwari, Email: rahultiwari226@gmail.com.
Milan Kumar Lal, Email: milan2925@gmail.com.
References
- Agindotan BO, Shiel PJ, Berger PH. Simultaneous detection of potato viruses, PLRV, PVA, PVX and PVY from dormant potato tubers by TaqMan®real-time RT-PCR. J Virol Methods. 2007;142:1–9. doi: 10.1016/j.jviromet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Babu B, Washburn BK, Miller SH, et al. A rapid assay for detection of Rose rosette virus using reverse transcription-recombinase polymerase amplification using multiple gene targets. J Virol Methods. 2017;240:78–84. doi: 10.1016/j.jviromet.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Babujee L, Witherell RA, Mikami K, et al. Optimization of an isothermal recombinase polymerase amplification method for real-time detection of Potato virus Y O and N types in potato. J Virol Methods. 2019;267:16–21. doi: 10.1016/j.jviromet.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Chi YK, Zhao W, Di YM, et al. Evaluation of recombinase polymerase amplification assay for detecting meloidogyne javanica. Plant Dis. 2020;104:801–807. doi: 10.1094/PDIS-07-19-1473-RE. [DOI] [PubMed] [Google Scholar]
- Chikh-Ali M, Karasev AV. Virus diseases of potato and their control. Potato Prod Worldw. 2023 doi: 10.1016/B978-0-12-822925-5.00008-6. [DOI] [Google Scholar]
- Choudhary P, Das S, Chakdar H, et al. Rapid high throughput template preparation (rHTTP) method: a novel cost effective method of direct PCR for a wide range of plants. BMC Biotechnol. 2019;19:1–10. doi: 10.1186/s12896-019-0560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso A, Valenzuela S. In-field molecular diagnosis of plant pathogens: recent trends and future perspectives. Plant Pathol. 2018;67:1451–1461. doi: 10.1111/PPA.12859. [DOI] [Google Scholar]
- Fuentes S, Gibbs AJ, Adams IP, et al. Potato virus a isolates from three continents: their biological properties, phylogenetics, and prehistory. Phytopathology. 2021;111:217–226. doi: 10.1094/PHYTO-08-20-0354-FI. [DOI] [PubMed] [Google Scholar]
- Hwang H, Bae S-C, Lee S, et al. A rapid and simple genotyping method for various plants by Direct-PCR. Plant Breed Biotechnol. 2013;1:290–297. doi: 10.9787/pbb.2013.1.3.290. [DOI] [Google Scholar]
- Ivan Magrina Lobato CKO Recombinase polymerase amplification: basics, applications and recent advances. Trends Anal Chem. 2020;98(2018):19–35. doi: 10.1016/j.trac.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevalatha A, Kaundal P, Kumar R, et al. Analysis of the coat protein gene of Indian Potato virus X isolates for identification of strain groups and determination of the complete genome sequence of two isolates. Eur J Plant Pathol. 2016;145:447–458. doi: 10.1007/s10658-016-0858-1. [DOI] [Google Scholar]
- Jeevalatha A, Vanishree G, Siddappa S, et al. Molecular characterization and infectivity analysis of tomato leaf curl New Delhi virus isolates infecting potato. 3 Biotech. 2021;11:4. doi: 10.1007/S13205-021-02752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Jiang J, Wu Y, Xia Z. Rapid detection of Cucumber green mottle mosaic virus in watermelon through a recombinase polymerase amplification assay. J Virol Methods. 2019;270:146–149. doi: 10.1016/j.jviromet.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Xu C, Li J, et al. Characterization and a RT-RPA assay for rapid detection of Chilli Veinal mottle virus (ChiVMV) in tobacco. Virol J. 2020;17:1–9. doi: 10.1186/s12985-020-01299-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y, Lin Y, Yang G, et al. Development of recombinase polymerase amplification assay for rapid detection of Meloidogyne incognita, M. javanica, M. arenaria, and M. enterolobii. Eur J Plant Pathol. 2019;155:1155–1163. doi: 10.1007/s10658-019-01844-6. [DOI] [Google Scholar]
- Kapoor R, Srivastava N, Kumar S, et al. Development of a recombinase polymerase amplification assay for the diagnosis of banana bunchy top virus in different banana cultivars. Adv Virol. 2017;162:2791–2796. doi: 10.1007/s00705-017-3399-9. [DOI] [PubMed] [Google Scholar]
- Kreuze JF, Souza-Dias JAC, Jeevalatha A, Figueira AR, Valkonen JPT, Jones RAC. Viral diseases in potato. In: Campos H, Ortiz O, editors. The potato crop. Cham: Springer; 2020. [Google Scholar]
- Kumar R, Jeevalatha A, Raigond B, et al. A multiplex reverse transcription PCR protocol for simultaneous detection of four potato viruses in potato plants and dorm. Int J Innov Hortic. 2014;3:22–29. [Google Scholar]
- Kumar R, Jeevalatha A, Baswaraj R, et al. A multiplex RT-PCR assay for simultaneous detection of five viruses in potato. J Plant Pathol. 2017;99(1):37–45. doi: 10.4454/jpp.v99i1.3824. [DOI] [Google Scholar]
- Kumar R, Jeevalatha A, Raigond B. Viral diseases and their management in seed potato production. In: Panday NK, Singh DK, Singh BP, Jeevalatha A, Baswaraj R, Singh B, editors. Advances in quality potato production and post-harvest management. Udaipur: Agrotech Publishing Academy; 2017. pp. 181–189. [Google Scholar]
- Kumar R, Jeevalatha A, Raigond B, Tiwari RK. Viral and viroid diseases of potato and their management. In: Singh AK, Chakrabarti SK, Singh B, Sharma J, Dua VK, editors. Potato science & technology for sub tropics. Ist. New Delhi: New India Publishing Agency; 2020. pp. 267–292. [Google Scholar]
- Kumar R, Tiwari RK, Jeevalatha A, et al. Potato apical leaf curl disease: current status and perspectives on a disease caused by tomato leaf curl New Delhi virus. J Plant Dis Prot. 2021;128:897–911. doi: 10.1007/s41348-021-00463-w. [DOI] [Google Scholar]
- Kumar RR, Kaundal P, Tiwari RK, et al. Rapid and sensitive detection of potato virus X by one-step reverse transcription-recombinase polymerase amplification method in potato leaves and dormant tubers. Mol Cell Probes. 2021;58:101743. doi: 10.1016/j.mcp.2021.101743. [DOI] [PubMed] [Google Scholar]
- Kumar R, Kaundal P, Tiwari RK, et al. Establishment of a one-step reverse transcription recombinase polymerase amplification assay for the detection of potato virus S Establishment of a one-step reverse transcription recombinase polymerase amplification assay for the detection of potato virus. J Virol Methods. 2022;307:114568. doi: 10.1016/j.jviromet.2022.114568. [DOI] [PubMed] [Google Scholar]
- Kumar R, Kaundal P, Tiwari RK, et al. Establishment of a one-step reverse transcription recombinase polymerase amplification assay for the detection of potato virus S. J Virol Methods. 2022;307:114568. doi: 10.1016/J.JVIROMET.2022.114568. [DOI] [PubMed] [Google Scholar]
- Kumar R, Tiwari RK, Sundaresha S, Kaundal P. Potato viruses and their management. Springer; 2022. [Google Scholar]
- Kumar R, Kaundal P, Tiwari RK, et al. Development of reverse transcription recombinase polymerase amplification (RT-RPA): a methodology for quick diagnosis of potato leafroll viral disease in potato development of reverse transcription recombinase polymerase amplification (RT-RPA): a meth. Int J Mol Sci. 2023 doi: 10.3390/ijms24032511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal MK, Tiwari RK, Kumar A, et al. Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants. 2022;11:2857. doi: 10.3390/PLANTS11212857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londoño MA, Harmon CL, Polston JE. Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virol J. 2016;13:1–9. doi: 10.1186/s12985-016-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena P, Kumar R, Raigond B, Jeevalatha A. Simultaneous detection of potato viruses A and M using CP gene specific primers in an optimized duplex RT-PCR. J Pharmacogn Phytochem. 2017;6:1635–1640. [Google Scholar]
- Mekuria TA, Zhang S, Eastwell KC. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. J Virol Methods. 2014;205:24–30. doi: 10.1016/j.jviromet.2014.04.015. [DOI] [PubMed] [Google Scholar]
- Mohandas A, Bhat AI. Recombinase polymerase amplification assay for the detection of piper yellow mottle virus infecting black pepper. Virus Dis. 2020;31:38–44. doi: 10.1007/s13337-019-00566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naga KC, Buckseth T, Subhash S, et al. Transmission efficiency of potato leaf roll virus (Plrv) by potato aphid Aulacorthum solani and green peach aphid Myzus persicae. Indian J Entomol. 2020;82:68–71. doi: 10.5958/0974-8172.2020.00017.6. [DOI] [Google Scholar]
- Naga KC, Shivaramu S, Bhatnagar A, Tiwari RK. Influence of host plants on virus acquisition and endosymbionts of whitefly Bemisia tabaci (Gennadius) Indian J Entomol. 2021 doi: 10.5958/0974-8172.2020.00206.0. [DOI] [Google Scholar]
- Naga KC, Siddappa S, Kumar R, et al. A new record of Asia II 5 genetic group of Bemisia tabaci (Gennadius) in the major potato growing areas of India and its relationship with tomato leaf curl New Delhi virus infecting potato. 3 Biotech. 2021;11:421. doi: 10.1007/s13205-021-02966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naga KC, Subhash S, Bhatnagar A, et al. Influence of host plants on virus acquisition and endosymbionts of whitefly Bemisia tabaci (Gennadius) Indian J Entomol. 2021 doi: 10.5958/0974-8172.2020.00206.0. [DOI] [Google Scholar]
- Naveen KP, Bhat AI. Reverse transcriptase loop-mediated isothermal amplification and reverse transcriptase recombinase amplification assays for rapid and sensitive detection of cardamom vein clearing virus. 3 Biotech. 2020;10:1–9. doi: 10.1007/s13205-020-02238-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouayti F, Tahiri A, Madani I, et al. Comparison of RNA extraction methods for the detection of BNYVV rhizomania virus from roots of sugar beet. Comptes Rendus Biol. 2018;341:343–348. doi: 10.1016/j.crvi.2018.06.003. [DOI] [PubMed] [Google Scholar]
- Onozuka N, Ohki T, Oka N, Maoka T. Detection of four major potato viruses in Japan using a simple RNA preparation and one-step multiplex RT-PCR. J Gen Plant Pathol. 2020;86:290–299. doi: 10.1007/s10327-020-00923-5. [DOI] [Google Scholar]
- Paul Khurana SM. Potato Viruses and their Management. Dis Fruits Veg. 2006;II:389–440. doi: 10.1007/1-4020-2607-2_11. [DOI] [Google Scholar]
- Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4:1115–1121. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Yin Z, Shen D, et al. Development of a recombinase polymerase amplification combined with lateral flow dipstick assay for rapid and sensitive detection of bean common mosaic virus. Phytopathol Res. 2021 doi: 10.1186/s42483-021-00080-3. [DOI] [Google Scholar]
- Raigond B, Verma A, Roach S, et al. One-step reverse transcription loop-mediated isothermal amplification: a simple, sensitive and rapid assay for detection of potato virus X in potato leaves and tubers. Indian Phytopathol. 2019;72:321–328. doi: 10.1007/s42360-019-00147-4. [DOI] [Google Scholar]
- Raigond B, Jeevalatha A, Kumar R, Verma G. Virus detection and diagnosis. In: Chakrabarti SK, Bhardwaj V, editors. Potato in sub-tropics: a saga of success, international books & periodical supply service. DELHI (India): Publisher of Scientific Books; 2020. pp. 83–96. [Google Scholar]
- Raigond B, Verma A, Pathania S, et al. Development of a reverse transcription loop-mediated isothermal amplification for detection of potato virus a in potato and in insect vector aphids. Crop Prot. 2020;137:105296. doi: 10.1016/j.cropro.2020.105296. [DOI] [Google Scholar]
- Raigond B, Verma G, Kumar R, Tiwari RK. Serological and molecular diagnosis of potato viruses: an overview. Springer; 2022. [Google Scholar]
- Shah MA, Kumar R, Kaundal P, Sharma S. Prevalence of natural infection of potato viruses in weeds and other crops. Springer; 2020. [Google Scholar]
- Silva G, Oyekanmi J, Nkere CK, et al. Rapid detection of potyviruses from crude plant extracts. Anal Biochem. 2018;546:17–22. doi: 10.1016/j.ab.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh BP, Raigond B, Sridhar J et al (2014) Potato seed production systems in India. In: Conference: national seminar on emerging problems of potato, held on 1–2 November 2014 at CPRI, Shimla, P-125
- Srivastava N, Kapoor R, Kumar R, et al. Rapid diagnosis of Cucumber mosaic virus in banana plants using a fluorescence-based real-time isothermal reverse transcription-recombinase polymerase amplification assay. J Virol Methods. 2019;270:52–58. doi: 10.1016/j.jviromet.2019.04.024. [DOI] [PubMed] [Google Scholar]
- Sundaresha S, Jeevalatha A, Kumar R, Sood S. RNA interference: a versatile tool to augment plant protection strategies in potato. Springer; 2022. [Google Scholar]
- Tiwari RK, Kumar R, Naga KC, Sagar V. Recent advancements in integrated management of potato diseases. In: More SJ, Giri NA, Suresh KJ, Visalakshi CC, Tadigiri S, editors. Recent advances in root and tuber crops. New Delhi: Brillion Publishing; 2021. pp. 185–203. [Google Scholar]
- Tiwari RK, Sharma S, Kumar R, et al. Morpho-molecular identification of potato silver scurf caused by Helminthosporium solani and standardization of spore inundation technique. Potato J. 2021;48:59–66. [Google Scholar]
- Tiwari RK, Bashyal BM, Shanmugam V, et al. First report of dry rot of potato caused by Fusarium proliferatum in India. J Plant Dis Prot. 2022;129:173–179. doi: 10.1007/s41348-021-00556-6. [DOI] [Google Scholar]
- Wang B, Ma Y, Zhang Z, et al. Potato viruses in China. Crop Prot. 2011;30:1117–1123. doi: 10.1016/j.cropro.2011.04.001. [DOI] [Google Scholar]
- Wang Y, Chen R, Nie X, et al. Rapid and sensitive detection of potato virus Y by isothermal reverse transcription-recombinase polymerase amplification assay in potato. Mol Cell Probes. 2020;50:101505. doi: 10.1016/j.mcp.2019.101505. [DOI] [PubMed] [Google Scholar]
- Zeng R, Luo J, Gao S, et al. Rapid detection of Cucumber green mottle mosaic virus by reverse transcription recombinase polymerase amplification. Mol Cell Probes. 2019;43:84–85. doi: 10.1016/j.mcp.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Zou Y, Mason MG, Wang Y, et al. Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol. 2017;15:1–22. doi: 10.1371/journal.pbio.2003916. [DOI] [PMC free article] [PubMed] [Google Scholar]