Summary
Background
People with complex symptomatology but unclear diagnosis presenting to a centre for rare diseases (CRD) may present with mental (co-)morbidity. We hypothesised that combining an expert in somatic medicine with a mental health specialist working in tandem will improve the diagnostic outcome.
Methods
Patients aged 12 years and older who presented to one of the 11 participating German CRDs with an unknown diagnosis were recruited into this prospective cohort trial with a two-phase cohort design. From October 1, 2018 to September 30, 2019, participants were allocated to standard care (SC, N = 684), and from October 1, 2019 to January 31, 2021 to innovative care (IC, N = 695). The cohorts consisted mainly of adult participants with only a minority of children included (N = 67). IC included the involvement of a mental health specialist in all aspects of care (e.g., assessing medical records, clinic visits, telehealth care, and case conferences). Clinicaltrials.gov identifier: NCT03563677.
Findings
The proportion of patients with diagnoses established within 12 months after the first visit to the CRD explaining the entire symptomatology (primary outcome) was 19% (N = 131 of 672) in the SC and 42% (N = 286 of 686) in the IC cohort (OR adjusted for centre effects 3.45 [95% CrI: 1.99–5.65]). The difference was mainly due to a higher prevalence of mental disorders and non-rare somatic diseases in the IC cohort. The median time to explaining diagnoses was one month shorter with IC (95% CrI: 1–2), and significantly more patients could be referred to local regular care in the IC (27.5%; N = 181 of 659) compared to the SC (12.3%; N = 81 of 658) cohort (OR adjusted for centre effects 2.70 [95% CrI: 2.02–3.60]). At 12-month follow-up, patient satisfaction with care was significantly higher in the IC compared to the SC cohort, while quality of life was not different between cohorts.
Interpretation
Our findings suggested that including a mental health specialist in the entire evaluation process of CRDs for undiagnosed adolescents and adults should become an integral part of the assessment of individuals with a suspected rare disease.
Funding
The study was funded by the Global Innovation Fund from the Joint Federal Committee in Germany (Innovationsfonds des Gemeinsamen Bundesausschusses), grant number 01NVF17031.
Keywords: Rare diseases, Diagnostic services, Mental health, Patient care team, Medically unexplained symptoms
Research in context.
Evidence before this study
A search on PubMed conducted on March 21, 2018, with no restrictions to language or article type, using the terms “rare disease” AND [(“undiagnosed” AND “multidisciplinary or interdisciplinary”) OR (“multidisciplinary_diagnosis” or interdisciplinary_diagnosis”)] yielded 30 articles with no relevant information on diagnostic success of interdisciplinary undiagnosed disease programs. A repeated analysis on March 8, 2023 retrieved 95 publications with some providing such information. These studies have highlighted that projects aiming to improve the diagnostic process in rare diseases using reviews of medical records, examination of patients as well as next generation sequencing have been able to clinically and genetically establish diagnoses in about 18–35% of people investigated for a complex symptomatology.
Added value of this study
This multi-centre cohort study shows that a mental health specialist working hand-in-hand with an experienced physician during the entire diagnostic process from chart review through clinical and diagnostic evaluation improves diagnostic outcome in adolescents and adults with an unclear diagnosis examined at a centre for rare diseases. The proportion of patients with a conclusive diagnosis or a combination of diagnoses explaining the entire symptomatic spectrum presented was more than twice as high in the innovative approach (42%) compared to standard care (19%), with mental disorders contributing in almost 30% of cases. In our study, the innovative approach resulted in an exclusion of a rare disease with high confidence in about 30% of the people evaluated. Time to diagnosis was reduced by one month, and the number of people successfully referred to regular care was doubled. Patient satisfaction with care was significantly higher with innovative care.
Implications of all the available evidence
Our results underline the importance of a combination of an expert in somatic medicine and a mental health specialist working in tandem for the diagnostic workup of all adolescents and adults presenting to a centre for rare diseases with an unclear diagnosis. The institutions and health insurances involved in the study will continue this novel and unique care. Inclusion of the innovative care into regular care will be promoted in Germany with the support of the German umbrella organisation Alliance for Chronic Rare Diseases Germany (ACHSE) e.V., representing more than 120 individual patient organisations.
Introduction
About 3–6% of the population or an estimated 300 million people worldwide are affected by one of the approximately 7000–10,000 rare diseases (RDs).1, 2, 3, 4 These conditions often manifest with non-specific symptoms and involve several organ systems.1 The time span from the first symptoms to the conclusive diagnosis often extends over several years.5
To improve the diagnostic process and outcome for people with persistent symptoms of unclear origin and a suspected RD, several undiagnosed disease programmes offering expert clinical evaluation, in-depth phenotyping, and whole exome or genome sequencing with innovative analyses were established. In a recent report from the National Institute of Health Undiagnosed Disease Network on 791 mainly paediatric participants, definitive diagnoses could be established in 231 (29%) individuals.6 In a German study involving ten centres for RDs (CRD), definitive diagnoses were made in 30% of 5652 patients, 3619 of whom were children and adolescents.7
The complex symptomatology of individuals presenting to a CRD for diagnostic work-up frequently includes psychopathological symptoms or even mental disorders.7,8 In fact, psychopathological symptoms are part of the clinical presentation of some RDs,9 may develop in the frequently long and tedious course of searching for a diagnosis,10 may independently co-occur with a (rare) disease or may even mimic a RD. Finally, a RD may be misdiagnosed as mental disorder, delaying diagnosis as well as appropriate treatment.11
We hypothesised that involving a mental health specialist in the entire evaluation process of people with a suspected RD would increase the proportion of people with one or more diagnoses explaining the entire symptomatology (primary outcome). Secondary outcomes included the time to explaining diagnoses, the proportion of participants successfully transitioning to regular care, health-related quality of life (HRQoL), and patient satisfaction with care.
Methods
Study design
ZSE-DUO is a prospective, controlled trial with a two-phase cohort design conducted in 11 CRDs in Germany (clinicaltrials.gov identifier: NCT03563677). A detailed description of the methodology is available in the online Supplementary Material and the published study protocol.12 The original study protocol is available at https://www.ukw.de/fileadmin/uk/zese/ZSE-DUO_Studienprotokoll_V1.3_04SEP2020.pdf.
Ethics committee approval
All ethics committees of the participating CRDs located at the university hospitals in Aachen, Bochum, Frankfurt, Hannover, Magdeburg/Halle, Mainz, Münster, Regensburg, Tübingen, Ulm and Würzburg, and of the institutions involved in data analysis (University Medical Center Hamburg-Eppendorf, Hannover Medical School, and University of Würzburg) approved the project. Written informed consent was obtained from all participants and guardians, where applicable; all minors gave assent.
Patient and public involvement
The German umbrella patient organisation Alliance for Chronic Rare Diseases (ACHSE) e.V., representing more than 120 individual patient organisations, was deeply involved in the planning and conduct of the trial, and is represented by Christine Mundlos among the authors. Inclusion of the innovative care into regular care will be promoted in Germany with the support of ACHSE e.V.
Participants
Individuals aged 12 years or older who were referred by their treating physician for further diagnostic evaluation of a suspected RD to one of the participating CRDs were invited to participate in the trial. Referring physicians were required to provide a medical summary, including reasons for suspecting a RD. Additional inclusion criteria were: 1) first contact with any of the participating CRDs, 2) attending the CRD's outpatient clinic for undiagnosed cases, and 3) providing written informed consent for study participation. Referrals were excluded from participation if medical records available to the CRD were incomplete or one or more disease(s) had previously been diagnosed, explaining the entire symptomatic spectrum presented. Furthermore, only patients insured by statutory health insurances covering about 90% of the German population were included.
Standard care (SC) and innovative care (IC)
The standard care (SC) cohort was recruited between October 1, 2018 and September 30, 2019. Once all required medical documents were available, a summary document of the medical information was produced, and a multidisciplinary team discussed the case. If the team concluded that no diagnoses covering the symptomatic spectrum were evident, the patient was invited to participate in the trial and to attend the CRD outpatient clinic for undiagnosed cases. There, participants were seen by a physician with expertise in RDs and a specialisation in a ‘somatic’ medical discipline who was guiding the diagnostic process. Diagnoses were generally made with the input and advice of experts from other disciplines located at the same university hospital (e.g., geneticists, neurologists, rheumatologists, etc.) who participated in regular case conferences or bilateral consultations. The referring physician and the participant received a letter summarising the findings and proposing future care.
The innovative care (IC) cohort, recruited between October 1, 2019 and January 31, 2021, received the same care as the SC group augmented by additional components. The major innovation was the inclusion of a mental health specialist (physician with specialisation in psychiatry or psychosomatic medicine) in all aspects of the care process. For children and adolescents, a child psychiatrist was part of the expert pair. The mental health specialist reviewed the participant's medical information upon admission to the CRD, evaluated the participant in the outpatient clinic in addition to the ‘somatic’ specialist, and was involved in all decisions and actions taken. Mental disorders were diagnosed through extensive clinical evaluation using the diagnostic interview for mental disorders — the Mini-DIPS Open Access.13 Depending on their findings, the mental health specialist could offer up to ten face-to-face or teleconsultation sessions with the participant for further evaluation or to bridge the time to local mental health care. Nationwide case conferences among participating CRDs were conducted as additional component.
Procedures
We assessed socio-demographic data, signs, and symptoms according to the human phenotype ontology (HPO)14 and the past medical history, including all prior confirmed diagnoses. Sex was reported by participants and physicians, with no differences between assessments. At baseline and 12-month follow-up, patients indicated their HRQoL on the visual analogue scale of the EQ-5D-5L,15 which ranges from 0 (worst imaginable health state) to 100 (best imaginable health state). Additionally, at 12-month follow-up, patients completed an established German questionnaire assessing satisfaction with care (ZUF-8).16 The questionnaire comprises eight questions, each rated from 1 to 4 (total score 8–32). Of note, at 12-month follow-up the visual analogue scale was available in 1005 (73.5%) participants and the ZUF-8 in 947 (69.3%) participants.
Outcomes
Primary outcome
The primary outcome of the study was the proportion of patients with one or more diagnoses explaining their entire symptomatic spectrum. This outcome was assessed 12 months after the first visit to the CRD. Physicians were asked to give their best judgment on whether all the previous and new diagnoses made during the intervention period fully explained the entire symptomatology (referred to as explaining diagnoses in this publication). In addition, they recorded each new diagnosis made during the intervention period and indicated whether it was a RD, a mental disorder, or a non-rare somatic disease.
Secondary outcomes
-
1)
Time to diagnosis. Time to diagnosis was defined as the period between the initial visit to the CRD and the time the explaining diagnoses was made (in months).
-
2)
Transition to regular care. The success of the transition to regular care was defined as having attended a treatment appointment in a regular care setting following CRD recommendations after at least one new diagnosis had been established. The number of patients successfully transferred was related to the total number of participants in the respective cohort.
-
3)
Change in HRQoL from baseline to 12-month follow-up and satisfaction with care at 12-month follow-up.
Sample size calculation
We hypothesised that the IC would increase the proportion of patients with one or more diagnoses established during the work-up in the CRD, explaining the entire symptomatic spectrum of the patient (explaining diagnoses) from 30% with SC to 40% with IC. Assuming a 20% dropout rate, a sample size of 682 patients in each group, was calculated to detect the above difference with a probability of a type 1 error <0.05 and a power of ≥0.8.
Statistical analysis
Descriptive data are presented as numbers/proportions for nominal or ordinal variables and median/interquartile range (IQR) for continuous variables. For the primary outcome “explaining diagnoses”, a mixed logistic regression model including a fixed study group effect along with random centre effects and random period effects nested within centres was employed. In further steps, the basic model was extended by adding demographic characteristics (e.g., sex, age, education), and interaction terms between these characteristics and the cohorts. The interaction effect for SC/IC∗age was excluded due to multicollinearity with SC/IC. Main effects are provided as odds ratios (OR) with 95% credibility intervals (95% CrI).
For baseline data and secondary outcomes, differences between groups were tested using the χ2 test (with Yates' continuity correction for 2 x 2 tables), Fisher's exact tests, or Mann–Whitney U-tests, and Hodges–Lehman median difference according to the distribution of the variables. For the secondary outcome “transition to regular care”, a logistic regression was calculated. Participants with missing data were excluded from the respective analyses. Statistical significance was assumed at p < 0.05 (two-sided test).
The statistical analyses were performed in SAS, Stata 15.1, and SPSS 27. Detailed information is available in the online supplement.
Role of the funding source
The Innovation Fund of the Joint Federal Committee of the Federal Republic of Germany funded the study, grant number 01NVF17031. The Committee did not have any role in design and conduct of the study, analysis and interpretation of the data, preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
Participants
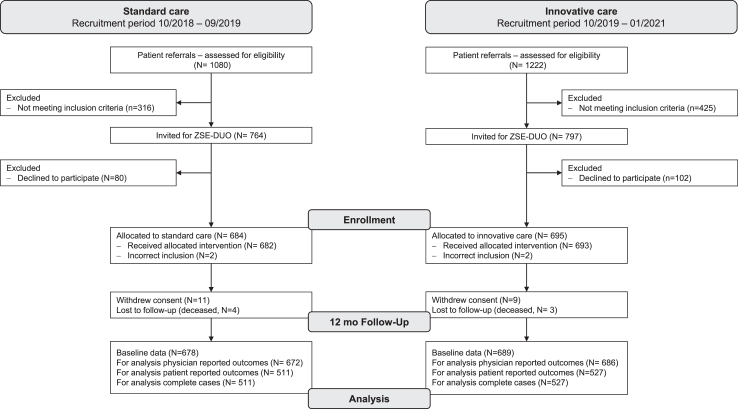
Fig. 1 depicts the flow of patients through the study. We enrolled 1379 patients, of whom only 21 did not reach the 12-month follow-up. Thus, the sample size estimated to address the primary outcome of the study was reached.
Fig. 1.
Flow diagram for the ZSE-DUO trial based on the reporting of observational studies in epidemiology (STROBE) checklist for cohort studies.
Patients who declined to participate (N = 182) did not differ from patients who were enrolled with regards to age, sex distribution, and duration of symptoms (Table S1).
Participants' characteristics at baseline are summarised in Tables S1, S2 and S5. Participants were predominantly female (N = 827, 60.5%), the median age was 46 years (IQR 32–57), and only 67 (4.9%) children and adolescents participated. The SC and IC cohorts differed in the number of children and adolescents and in post-secondary education (Table 1). Tables S3 and S4 in the online supplement provide participants’ characteristics separately for female and male participants and children/adolescents and adults. There were no significant differences in baseline characteristics between the SC and the IC cohorts within these subgroups, except for HQoL in the paediatric group (Table S4). There were subtle differences in HPO-coded symptoms between the SC and IC cohorts (Table S5). The high burden of disease was exemplified by a low HRQoL (median 50, IQR 30–70, on a VAS from 0 to 100) and a high proportion of participants with a formally acknowledged disability (N = 504, 36.9%). In total, 500 (36.6%) participants had been evaluated by a mental health specialist during the 12 months prior to the first visit to the CRD, with no differences between SC and IC, neither in the entire cohorts nor in the female, male, child/adolescent, or adult subsamples (Tables S2–S4).
Table 1.
Participants' characteristics at baseline.
| Standard care cohort (N = 678) | Innovative care cohort (N = 689) | p valuea | |
|---|---|---|---|
| Age (years) [median, IQR] | 46 (32–56) | 46 (32–57) | 0.86 |
| Children/adolescents (≥12 to <18 years) [n, %] | 42 (6.2%) | 25 (3.6%) | 0.038 |
| Female sex [n, %] | 404 (59.6%) | 423 (61.4%) | 0.61 |
| Migratory background | 0.61 | ||
| No migratory background [n, %] | 474 (69.9%) | 495 (71.8%) | |
| Migratory background [n, %] | 185 (27.3%) | 180 (26.1%) | |
| Declined to answer [n, %] | 12 (1.8%) | 10 (1.5%) | |
| Missing [n, %] | 7 (1.0%) | 4 (0.6%) | |
| Highest school education | 0.14 | ||
| No graduation [n, %] | 55 (8.1%) | 38 (5.5%) | |
| Lower secondary (ISCED 2)b [n, %] | 362 (53.4%) | 364 (52.8%) | |
| Upper secondary (ISCED 3)b [n, %] | 246 (36.3%) | 267 (38.8%) | |
| Other educational degree [n, %] | 3 (0.4%) | 5 (0.7%) | |
| Missing [n, %] | 12 (1.8%) | 15 (2.2%) | |
| Highest post-secondary education | 0.026 | ||
| Currently enrolled in secondary/tertiary/vocational educationd [n, %] | 92 (13.6%) | 67 (9.7%) | |
| No tertiary/vocational education, not currently enrolled [n, %] | 48 (7.1%) | 50 (7.3%) | |
| Vocational qualification (ISCED 4)b [n, %] | 329 (48.5%) | 315 (45.7%) | |
| Bachelor's/postgraduate degree (ISCED 5–8)b,e [n, %] | 188 (27.7%) | 234 (34.0%) | |
| Other tertiary/vocational degree [n, %] | 1 (0.1%) | 3 (0.4%) | |
| Declined to answer [n, %] | 20 (2.9%) | 20 (2.9%) | |
| Missing [n, %] | 0 (0.0%) | 0 (0.0%) | |
| Employment status | 0.070 | ||
| Full-time [n, %] | 205 (30.2%) | 231 (33.5%) | |
| Part-time or less [n, %] | 89 (13.1%) | 109 (15.8%) | |
| Unemployed [n, %] | 73 (10.8%) | 60 (8.7%) | |
| Retired due to disability [n, %] | 91 (13.4%) | 67 (9.7%) | |
| Outside of the labour force for other Reasons [n, %] | 171 (25.2%) | 163 (23.7%) | |
| Declined to answer [n, %] | 49 (7.2%) | 59 (8.6%) | |
| Missing [n, %] | 0 (0.0%) | 0 (0.0%) | |
| Duration of main symptom (years) [median. IQR] | 6 (3–15) | 6 (3–15) | 0.61 |
| Missing [n, %] | 2 (0.3%) | 7 (1.0%) | |
| Number of HPO codes per patient [median, IQR] | 6 (3–9) | 5 (3–8) | 0.61 |
| Missing [n. %] | 6 (0.9%) | 4 (0.6%) | |
| Disability formally acknowledged [n. %]c | 249 (36.7%) | 255 (37.0%) | 1.00 |
| Information not provided [n. %] | 19 (2.8%) | 17 (2.5%) | |
| HRQoL: EQ-5D-5L VAS [median. IQR] | 50 (30–70) | 50 (30–66) | 0.90 |
| Missing [n, %] | 0 (0.0%) | 1 (0.1%) |
Abbreviations: HPO = human phenotype ontology, HRQoL = health-related quality of life, VAS = visual analogue scale (0–100).
Chi-square test for categorical variables with Yates' continuity correction for 2 × 2 tables or non-parametric test (Mann–Whitney U-test) for numerical variables.
ISCED = International Standard Classification of Education 2011.
Reported by patients.
p = 0.033.
p = 0.015 when comparing SC and IC cohorts using bivariate Chi-square test with Yates' continuity correction for 2 × 2 tables.
Genetic testing was performed in 180/672 (26.8%) patients in the SC cohort and 228/686 (33.2%) patients in the IC cohort. In total, 291 new diagnoses could be established in the SC cohort and 1158 in the IC cohort (Table S6). Figure S3 and Tables S19–S22 provide information on patients with at least one confirmed newly established diagnosis. A RD diagnosis was made (explanatory or not) in 67/672 patients (10.0%) of the SC and 94 of 686 participants (13.7%) of the IC cohort (Figure S1). The percentage of participants with at least one newly established mental disorder was 4.2% (N = 28) in the CG and 50.4% (N = 346) in the IG (Figure S3). The most common mental disorders diagnosed were from the spectrum of anxiety disorders (total of 404 diagnoses) followed by affective disorders (total of 144 diagnoses) (Table S6). The percentage of participants with at least one newly established non-rare diseases was 18.9% (N = 127) in the CG and 30.5% (N = 209) in the IC (Figure S3). In both groups the most common non-rare diseases were ‘disorders of the nervous system’ followed by ‘diseases of the musculoskeletal system and connective tissue’, and ‘endocrine, nutritional and metabolic diseases’ (Table S6).
Primary outcome
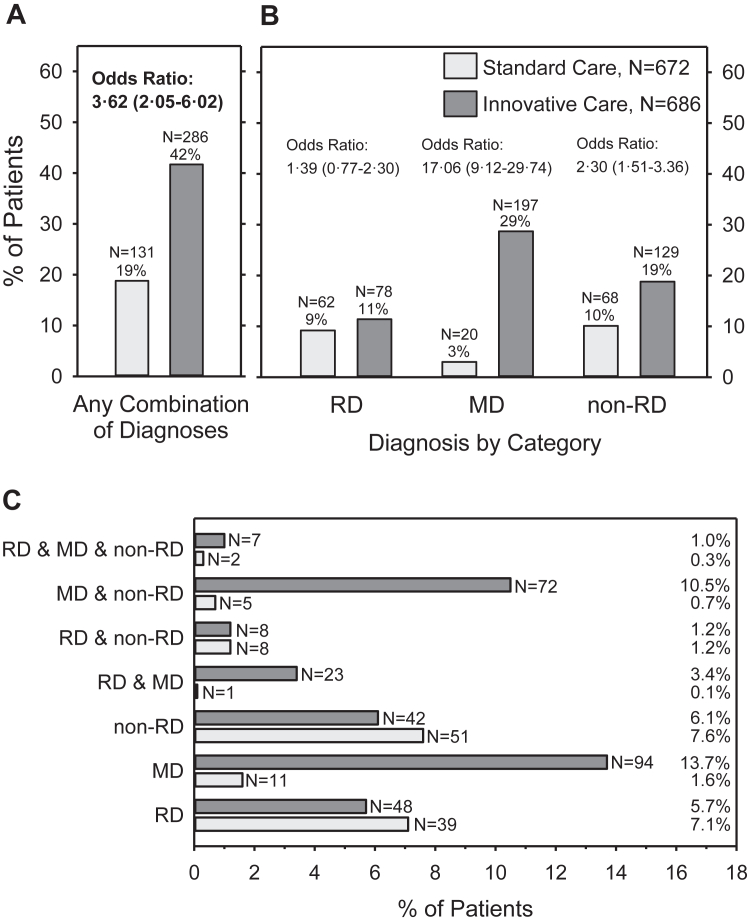
The analysis of the primary outcome included 1358 participants, N = 672 in the SC cohort and N = 686 in the IC cohort (Fig. 1). Compared to SC, IC resulted in a higher proportion of participants with one or more explaining diagnoses (Fig. 2A). A Bayesian mixed logistic regression controlling for the variance between the 11 CRDs resulted in an odds ratio (OR) of 3.45 (95% CrI: 1.99–5.65), favouring IC. The effect was confirmed in two further models with additional adjustments for sex, age, and education (OR = 3.62, 95% CrI: 2.05–6.02) and with additional interaction terms (OR = 4.11, 95% CrI: 2.16–7.22) (Table S7). The model with adjustments for sex, age, and education showed the best fit.
Fig. 2.
Proportion of patients in the standard care and the innovative care cohorts for whom one or more diagnoses were confirmed during the evaluation process that explained the entire symptomatic spectrum presented by the patient (explaining diagnoses). A) Patients with any combination of newly established diagnoses (primary outcome). B) Patients clustered by diagnostic category. Please note, some participant received diagnoses from more than one diagnostic category and are included in each applicable category. C) Combinations of diagnostic categories explaining the full symptomatic spectrum RD = rare disease, MD = mental disorder, non-RD = non-rare somatic disease Main effects are presented as odds ratios with 95% credibility intervals based on basic statistical models.
Fig. 2B shows the proportion of participants with explaining diagnoses in the SC and IC cohorts separately for each of the three diagnostic categories. Fig. 2C depicts all possible combinations of diagnostic categories explaining the entire symptomatology. There was no difference in the proportion of participants diagnosed with a RD between cohorts (OR 1.31, 95% CrI: 0.73–2.13). However, significantly more participants were diagnosed with mental disorders (OR 16.98, 95% CrI: 9.14–29.78) and non-rare, somatic conditions (OR 2.26, 95% CrI: 1.50–3.27) in the IC cohort compared to the SC cohort (Tables S8–S10). Of note, in 67 of 672 participants (10.0%) of the SC cohort and 208 of 686 participants (30.3%) of the IC cohort, explaining diagnoses were established which did not include a RD diagnosis.
The above differences between the SC and IC cohorts were also evident when analysing the female and male participants, and the adult participants separately (Table S11, S12 and S14). The logistic regression model revealed a significant interaction effect between sex and care group with respect to mental disorders only (Table S9) with a larger difference between SC and IC in (partially) explaining mental disorders in female (SC: N = 8, 2.0% vs. IC: N = 125, 29.9%) compared to male participants (SC: N = 11, 4.1% vs. IC: N = 71, 26.5%; Tables S11 and S12). No differences were found in the proportion of explaining diagnoses between SC and IC in children/adolescents (Table S13). However, comparable to the adults, there were significantly more mental disorders in the IC than the SC paediatric group contributing to explaining the entire symptomatology (Table S13).
Secondary outcomes
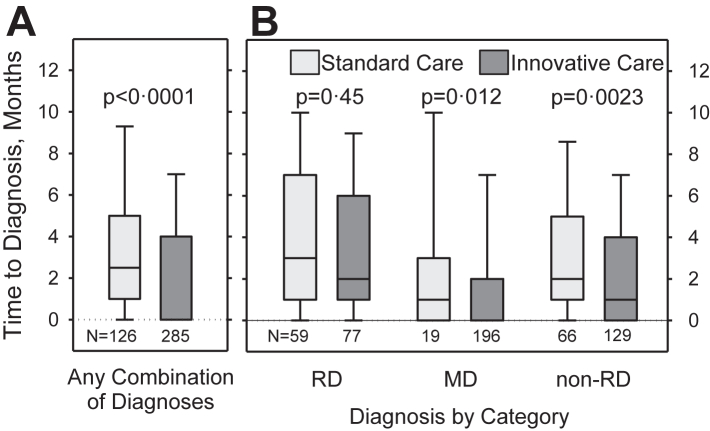
Time to explaining diagnoses was significantly shorter in the IC compared to the SC cohort (median difference one month [95% confidence interval (95% CI): 1–2]) (Fig. 3A, Tables S15 and S16). While no significant difference between SC and IC was observed between participants diagnosed with a RD, participants diagnosed with a mental disorder and/or a non-rare health condition received explaining diagnoses faster with IC than with SC (both median difference one month [95% CI: 1–2]) (Fig. 3B, Table S15).
Fig. 3.
Time between first visit to the CRD and newly established explaining diagnoses in the standard care and innovative care cohorts. A) Patients with any combination of newly established and explaining diagnoses. B) Patients clustered by diagnostic category. Please note, some participants received diagnoses from more than one diagnostic category and are included in each applicable category. RD = rare disease, MD = mental disorder, non-RD = non-rare somatic disease. Boxplots show 10th, 25th, 50th, 75th, and 90th centiles. Differences between groups were tested using Mann–Whitney U-tests.
The proportion of participants with at least one newly diagnosed condition who were successfully referred to (local) standard care was significantly higher in the IC cohort (181 of 659 participants with valid information, 27.5%) than in the SC cohort (81 of 658 participants, 12.3%, p < 0.001; OR 2.70 [95% CI: 2.02–3.60]) (Tables S25 and S26). However, there was no difference between IC and SC when the number of successfully referred participants was related to the number of participants with any newly established diagnoses (IC: 126/374 = 33.7%, SC: 56/152 = 36.8%, p = 0.93) (Table S27). More detailed analyses are available in the online supplement (Tables S17, S18, S25, and S27).
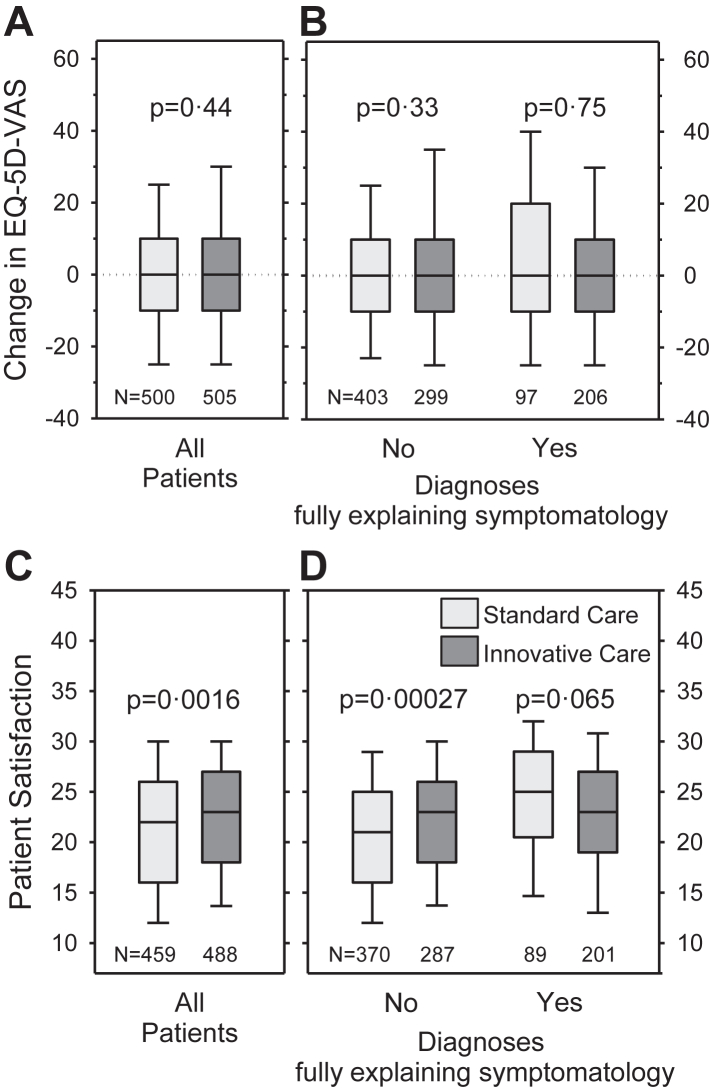
HRQoL was not different between groups at baseline (Table 1) and did not change over time from baseline to 12-month follow-up in either cohort (Fig. 4A), irrespective of whether explaining diagnoses could be established or not (Fig. 4B).
Fig. 4.
Patient-reported outcomes. A and B) Change in health-related quality of life as indicated on the EQ-5D visual analogue scale at baseline and 12-month follow-up in the standard care and the innovative care cohorts (A: entire sample; B: separately for patients with and without explaining diagnoses). C and D) Patient satisfaction with care at 12-month follow-up in the standard care and the innovative care cohorts (C: entire sample; D: separately for participants with and without explaining diagnoses). Boxplots show 10th, 25th, 50th, 75th, and 90th centiles. Differences between groups were tested using Mann–Whitney U-tests.
Satisfaction with care assessed at 12-month follow-up was significantly higher in the IC compared to the SC cohort (median difference: one [95% CI: 1–2]) (Fig. 4C). Participants without explaining diagnoses were more satisfied with care in the IC than in the SC cohort (median difference: two [95% CI: 1–3]) (Fig. 4D). Detailed additional information is available in the online supplement (Figures S1, S2, S5, and S6; Tables S28 and S29).
Discussion
Our study suggested that in people with symptoms of unclear origin seen at a CRD, the involvement of a mental health expert in all parts of the evaluation process considerably increased the probability of establishing diagnoses explaining their entire symptomatic spectrum. While IC did not result in more RD diagnoses contributing to explaining diagnoses compared to SC, there was a higher proportion of mental disorders and non-RDs in the IC cohort. This was true for female and male participants alike. In the small paediatric sample (N = 67, 4.9%), differences between SC and IC were only evident with respect to mental disorders favouring IC. Compared with SC, IC accelerated the time to diagnosis and allowed the transition of more patients to local regular care. Additionally, patient satisfaction with care was significantly higher in the IC cohort compared to the SC cohort.
In evaluating selected patients with an undiagnosed condition despite thorough prior evaluation, next-generation sequencing has been a focus in other projects.17,18 Extensive genetic testing may establish a diagnosis in about 25% of cases.17,18 Clinical diagnoses are less frequently established, leaving about 65% of patients evaluated by the US Undiagnosed Disease Network without a diagnosis.17
In the predominantly adult population of the current project, a RD was diagnosed in 10% and 14% of the SC and IC cohort, respectively. In other programmes for undiagnosed diseases, higher proportions have been reported.6,7 However, these programmes focused predominantly on children. In a recent study most inherited RDs were diagnosed in children, whereas a more diverse picture appeared in adults, with non-RDs and mental health conditions representing about 40% of all established diagnoses.7
Because mental disorders and non-RDs explained the entire symptomatology in about 30% of patients in the IC cohort, a RD could be excluded with high confidence in these cases. Thus, the innovative approach in our project is an important addition to current clinical and genomic concepts for people with undiagnosed conditions. To our knowledge, our approach with a somatic expert and a mental health specialist working hand-in-hand during the entire diagnostic process in people with symptoms of unclear origin is novel.
Current mental disorders were more frequently diagnosed in the IC (50%) than in the SC cohort (4%). In 13.7% of the IC cohort a mental disorder was the sole explanation for the entire symptomatology. In an additional 14.9%, a mental disorder contributed to the explaining diagnoses. In contrast, the point prevalence in the SC cohort who only had access to a mental health specialist via referral was exceptionally low but comparable to that observed by Rillig et al.,7 who reported that 4.3% (N = 88) of 2033 adults presenting to CRDs in Germany met the criteria for a mental disorder. In a meta-analysis of 37 studies comprising 24 different RDs, the pooled prevalence estimates for current affective disorders were 21.2% and for current anxiety disorders 39.6%.19 However, this is the first study systematically assessing current mental disorders using a state-of-the-art approach in an unselected sample of patients referred to a CRD. Thus, we are confident that the frequency and type of mental diagnoses are reliable and valid. Unfortunately, due to the cross-sectional nature of the study, we cannot discern if mental disorders were separate and entirely independent diagnoses or, in some way, causally linked to RDs or non-RDs.
The point prevalence of mental disorders of 50% in the IC cohort is markedly higher compared to general population samples. The most recent study using an interview-based approach in a nationally representative sample of the German population reported a 12-month prevalence rate of mental disorders of 27.8% in the 5303 participants aged 18–79 years.20 Similarly, results from the 2019 National Survey on Drug Use and Health in the US found that 24.5% of 50,731 individuals aged 18 years or older met the criteria for a mental disorder.21
Interestingly, with IC also significantly more explaining non-rare somatic diseases were diagnosed. Possibly, the interactions and interdisciplinary case discussions between the two experienced physicians working in tandem during the entire diagnostic process stimulated a more intense evaluation of all medical aspects. The added diagnostic value of interdisciplinary collaboration has previously been shown for various health conditions.22,23
In a German multi-centre study time to diagnoses was reported to be 76 days.24 In our study time to explaining diagnosis was shorter in the IC than the SC cohort (medians zero and 2.5 months, respectively), even though the process was more complex requiring repeated internal discussion and despite the COVID pandemic reducing the availability of examinations. This might have been due to 1) the high prevalence of mental disorders that can be diagnosed clinically and 2) a close cooperation between the somatic and mental health experts reducing referral times.
HRQoL at baseline was much lower in the participants of the ZSE-DUO study compared with general European population samples and did not change during the follow-up period.25,26 The poor HRQoL in both the SC and the IC cohorts is in line with other reports on people with chronic health conditions and multimorbidity.27 At first, the lack of improvements in HRQoL over 12 months seems surprising as many new diagnoses could be established during the study, especially in the IC cohort. However, mental disorders are associated with a reduced HRQoL, adequate treatment is not readily available (e.g., psychotherapy), and frequently does not have an immediate effect. Furthermore, RDs are typically affecting multiple organ systems and require complex treatment with often limited effectiveness. Thus, a diagnosis may result in anxiety, worries, and resistance.19 Furthermore, in population samples HRQoL decreased during COVID-19 lockdown.28 A stable HRQoL might thus be seen as success.
HRQoL and satisfaction with care are not synonymous concepts. Even though HRQoL did not differ between cohorts and did not change over time, the median values of the satisfaction with care score at the 12-month follow-up were significantly higher in the IC compared to the SC cohort. It is tempting to speculate that the evaluation by a mental health expert increased patient satisfaction with care, specifically in participants who did not receive explaining diagnoses. Additionally, satisfaction with care was higher in the IC cohort despite more mental disorders being diagnosed.
When planning ZSE-DUO, a cohort design was chosen instead of a randomised controlled design, which is considered the gold standard for therapeutic clinical trials.29 However, with a randomised controlled study, the risk of contamination of the SC cohort by components of the intervention was considered too high, given the high psychological strain and distress of people with unclear diagnoses.30 In other words, including a mental health specialist in the team while performing standard care would have likely lead to involving this expert also in the care of SC participants. The physicians who were involved in guiding the diagnostic process were also responsible for the assessment of the main outcome “diagnoses explaining the entire symptomatic spectrum”. This outcome is prone to some degree of subjectivity which introduces a risk for bias. Additionally, the Hawthorne effect of awareness of the study being conducted by the physicians guiding the diagnostic process might have introduced detection and performance bias. To reduce bias we developed SOPs at the start of the project to harmonize and standardize diagnostic procedures within and across centres during the entire conduct of the study. Blinding of CRD team members, who were responsible for the assessment of the primary outcome, would have been ideal, but was impossible due to the nature of the intervention. Likewise, a cluster-randomised design was not feasible due to the low number of CRDs in our study. Results from unblinded trials should always be interpreted with caution since they tend to overestimate treatment effects.
The sequential study design of the current study might have allowed for a higher diagnostic yield in the second cohort due to a ‘learning curve’ in CRD staff. However, all CRDs involved in the project had several years of experience in evaluating people with unclear diagnoses long before study initiation, and collaboration with experts from different disciplines was well established. Nevertheless, as there was a change in staff over the study period in some centres, we cannot fully exclude a relevant acquisition of skills and knowledge in the CRDs over time. It might even be argued that the increase in diagnoses of non-RD observed with IC was based on such a learning effect. However, there was no such increase in the rate of RD diagnoses. The benefit observed with IC in diagnosing more non-RD, in our opinion, was mainly based on the introduction of an additional specialist who not only made diagnoses of mental disorders but also added medical expertise in general and stimulated case discussions.
The prevalence of (non) RD and mental disorders will likely depend on the characteristics of the investigated cohort (e.g., age, pre-selection of participants). It might be argued that sample recruitment differed between SC and IC cohorts. However, in ZSE-DUO, only people who were 1) referred by their physician and 2) selected by a multidisciplinary team in the CRD for further evaluation were invited to participate. The cohorts were well matched in most characteristics, and statistical analyses included adjusted models to account for the small but significant differences between cohorts. The reported effects of IC were also visible in these adjusted models.
The COVID-19 pandemic led to lockdowns during the IC period of ZSE-DUO in 2020 and 2021 and, thus, interfered with recruitment, study conduct, and diagnostic procedures. Nevertheless, there were significantly more cases with a newly diagnosed somatic disease and no decline in cases with a RD diagnosis in the IC cohort compared to the SC cohort recruited in 2018 and 2019. In France, a nearly 50% decrease in newly diagnosed cases with a RD was reported in 2020 compared to 2019.31
This study is the first to introduce diagnoses that explain the entire symptomatology of participants. Other studies report definitive diagnoses but do not state if the diagnoses are explaining the entire symptomatic spectrum, thereby leaving open whether additional diagnoses are possible or even likely. Additionally, we employed wide inclusion criteria to improve the external validity (generalizability to the affected population) of our results. The large sample size and multi-centre design further strengthen the findings of the study.
The results suggest that people referred to a CRD may have a high (co)morbidity with mental disorders that strongly contribute to explaining their symptomatology, either alone or in conjunction with a (non) RD. Thus, a mental health specialist should be an integral part of the interdisciplinary team in CRDs evaluating patients with complex symptomatology of unclear origin. Future inclusion of the IC into regular care will be promoted with the support of the German umbrella organisation Alliance for Chronic Rare Diseases Germany (ACHSE) e.V.
Contributors
HH, JD, CZ, MdZ, CS, CK, KH, JQ, CM, JBS, MoB, HC, K-MD, JH, AH-W, PH, FJ, ThL, KM, MaMo, AP-M, OlR, LS, KU, TW, SZ developed the concept of the study. HH, JD, CZ, MdZ, CS, CK, KH, JQ, JBS, TL, AB, KS, OT, FR, HoG, MB, HaG, CM were responsible for funding acquisition. KH, CK, A-ML, JQ, SW, HH, MdZ, JD, OT were responsible for the methodology. LP, LiB, KH, CK, A-ML, JQ, SW, HH, AB-M, LeB, FB, KD-J, JE, AG, CG, AH, SH, BH, JaK, LBa, HeK, ACL, ToM, IMdS, MaMü, SM, L-SP, KrS, AnS, US, AlS, SeS, StS, SV, SaW, KaZa, KaZe, DZ were responsible for project administration, data curation, and validation. AB, AD, CS, CZ, FR, GH, HaG, HH, HoG, JBS, JD-H, JD, KS, LaB, LP, LZ, MB, MdZ, OT, SS, TL, TM, VB, FA, CB, MdG, VG, EG, LeH, IH, MeH, JuH, LaH, CI, K-TK, BK, JuK, PL, ThM, MaNi, MaNo, SO, CP-D, CR, SiS, MS, TS, MV, DV, CV, BW were involved in clinical investigation. KH, A-ML, LiB, CK, SW, HH, JQ, JuF conducted the formal analysis and visualisation. HH and MdZ wrote the original draft with support from CK, A-ML, KH, and SW. All authors reviewed and edited the manuscript. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication. HH takes responsibility for the overall content as guarantor. He accepts full responsibility for the finished work and the conduct of the study. HH, AML, LB, LP, KH, and SW have verified the underlying data.
Data sharing statement
Qualifying researchers who wish to access our data should submit a proposal with a valuable research question to the corresponding author. Proposals will be assessed by a committee formed from the trial management group, including senior epidemiological and clinical representatives. Available data collected for the study include de-identified individual participant data and a data dictionary.
Declaration of interests
HH, MB and TM report funding from the Bavarian State Ministry of Science and the Arts paid to their institution to support projects on rare diseases unrelated to ZSE-DUO. HH also reports unrelated funding to his institution by the Federal Ministry of Education and Research, and honoraria from Springer Verlag, Takeda Pharma GmbH and Chiesi GmbH. JD reports funding to his institution from the German Research Association, German Secretary of Education and Research, and the Innovation Fund for several unrelated projects. GH's institution has received funding from the Innovation Fund for another project. OT's institution has received funding from the European Regional Development Fund, EU Horizon 2020, Leibniz Association, and the Federal Ministry of Education and Research. OT has received royalties as an author. CZ reports funding to her institution by the Federal Ministry of Education and Research and the European Union, and voluntary work for the advisory board of the severe chronic neutropenia international registry. All other authors declare no competing interests.
Acknowledgements
The authors thank the following contributors for their valuable support and contributions: Federica Akkaya (FA), Christine Babka (CB), Lisa Bannert (LBa), Anja Bärsch-Michelmann (AB-M), Leonie Böhm (LeB), Folke Brinkmann (FB), Monika Bullinger (MoB), Holger Cario (HC), Moritz de Greck (MdG), Klaus-Michael Debatin (K-MD), Katrin Dillmann-Jehn (KD-J), Jutta Eymann (JE), Julia Frisch (JuF), Anja Glode (AG), Vega Gödecke (VG), Corinna Grasemann (CG), Eva Grauer (EG), Astrid Haas (AH), Lea Haisch (LeH), Isabell Heinrich (IH), Melissa Held (MeH), Julia Hennermann (JH), Stephan Herpertz (SH), Anne Herrmann-Werner (AH-W), Julian Hett (JuH), Peter Heuschmann (PH), Bettina Hilbig (BH), Laura Holthöfer (LaH), Christiane Imhof (CI), Florian Junne (FJ), Jan Kassubek (JaK), Kevin-Thomas Koschitzki (K-TK), Heike Krassort (HeK), Birgit Kropff (BK), Julia Kuhn (JuK), Philipp Latzko (PL), Thomas Loew (ThL), Albert C. Ludolph (ACL), Torsten Meyer (ToM), Isabell Meyer dos Santos (IMdS), Klaus Mohnike (KM), Martina Monninger (MaMo), Martin Mücke (MaMü), Susanne Müller (SM), Thomas Musacchio (ThM), Margret Nießen (MaNi), Mariel Nöhre (MaNo), Stephan Ott (SO), Andrea Petermann-Meyer (AP-M), Christina Pfeifer-Duck (CP-D), Lea-Sophie Piduhn (L-SP), Carina Rampp (CR), Olaf Rieß (OlR), Kristina Schaubert (KrS), Annika Schmidt (AnS), Simone Schneider (SiS), Ludger Schoels (LS), Martina Schwalba (MS), Udo Selig (US), Alexandra Sroka (AlS), Toni Steinbüchel (TS), Sebastian Stösser (SeS), Steffi Suchant (StS), Kathrin Ungethüm (KU), Matthias Vogel (MV), Daniela Volk (DV), Christoph Vollmuth (CV), Solange Volnov (SV), Thomas O. F. Wagner (TW), Sabrina Walter (SaW), Bodo Warrings (BW), Kamil Zajt (KaZa), Karola Zenker (KaZe), David Zhang (DZ), Stephan Zipfel (SZ).The study was funded by the Innovation Fund of the Joint Federal Committee of the Federal Republic of Germany, grant number 01NVF17031.
Footnotes
Translation: For the German translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102260.
Contributor Information
Helge Hebestreit, Email: hebestreit@uni-wuerzburg.de.
ZSE-DUO Working Group:
Federica Akkaya, Christine Babka, Lisa Bannert, Anja Bärsch-Michelmann, Leonie Böhm, Folke Brinkmann, Monika Bullinger, Holger Cario, Moritz de Greck, Klaus-Michael Debatin, Katrin Dillmann-Jehn, Jutta Eymann, Julia Frisch, Anja Glode, Vega Gödecke, Corinna Grasemann, Eva Grauer, Astrid Haas, Lea Haisch, Isabell, Heinrich, Melissa Held, Julia Hennermann, Stephan Herpertz, Anne Herrmann-Werner, Julian Hett, Peter Heuschmann, Bettina Hilbig, Laura Holthöfer, Christiane Imhof, Florian Junne, Jan Kassubek, Kevin-Thomas Koschitzki, Heike Krassort, Birgit Kropff, Julia Kuhn, Philipp Latzko, Thomas Loew, Albert C. Ludolph, Torsten Meyer, Isabell Meyer dos Santos, Klaus Mohnike, Martina Monninger, Martin Mücke, Susanne Müller, Thomas Musacchio, Margret Nießen, Mariel Nöhre, Stephan Ott, Andrea Petermann-Meyer, Christina Pfeifer-Duck, Lea-Sophie Piduhn, Carina Rampp, Olaf Rieß, Kristina Schaubert, Annika Schmidt, Simone Schneider, Ludger Schoels, Martina Schwalba, Udo Selig, Alexandra Sroka, Toni Steinbüchel, Sebastian Stösser, Steffi Suchant, Kathrin Ungethüm, Matthias Vogel, Daniela Volk, Christoph Vollmuth, Solange Volnov, Thomas O.F. Wagner, Sabrina Walter, Bodo Warrings, Kamil Zajt, Karola Zenker, David Zhang, Stephan Zipfel, Helge Hebestreit, Anne-Marie Lapstich, Lilly Brandstetter, Christian Krauth, Jürgen Deckert, Kirsten Haas, Lisa Pfister, Stefanie Witt, Christopher Schippers, Jan Dieris-Hirche, Tim Maisch, Oliver Tüscher, Lavinia Aurelia Bârlescu, Alexandra Berger, Mark Berneburg, Vanessa Britz, Anna Deibele, Holm Graeßner, Harald Gündel, Gereon Heuft, Thomas Lücke, Christine Mundlos, Julia Hannah Quitmann, Frank Rutsch, Katharina Schubert, Jörg B. Schulz, Susann Schweiger, Cornelia Zeidler, Lena Margarete Zeltner, and Martina de Zwaan
Appendix A. Supplementary data
References
- 1.Ferreira C.R. The burden of rare diseases. Am J Med Genet A. 2019;179:885–892. doi: 10.1002/ajmg.a.61124. [DOI] [PubMed] [Google Scholar]
- 2.Davies S.C. Annual report of the chief medical officer 2016, generation genome london. 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/631043/CMO_annual_report_generation_genome.pdf Available at:
- 3.Nguengang Wakap S., Lambert D.M., Olry A., et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet. 2020;28:165–173. doi: 10.1038/s41431-019-0508-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations General Assembly Addressing the challenges of persons living with a rare disease and their families. 2021. https://www.rarediseasesinternational.org/wp-content/uploads/2022/01/Final-UN-Text-UN-Resolution-on-Persons-Living-with-a-Rare-Disease-and-their-Families.pdf Available at:
- 5.Isono M., Kokado M., Kato K. Why does it take so long for rare disease patients to get an accurate diagnosis?-A qualitative investigation of patient experiences of hereditary angioedema. PLoS One. 2022;17 doi: 10.1371/journal.pone.0265847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoch K., Esteves C., Bican A., et al. Clinical sites of the Undiagnosed Diseases Network: unique contributions to genomic medicine and science. Genet Med. 2021;23:259–271. doi: 10.1038/s41436-020-00984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rillig F., Grüters A., Schramm C., Krude H. The interdisciplinary diagnosis of rare diseases. Dtsch Arztebl Int. 2022;119:469–475. doi: 10.3238/arztebl.m2022.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolb-Niemann B., Kruse J. [Importance of psychosomatic medicine for people with rare diseases] Internist. 2019;60:638–643. doi: 10.1007/s00108-019-0613-8. [DOI] [PubMed] [Google Scholar]
- 9.McDonald-McGinn D.M., Sullivan K.E., Marino B., et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. 2015;1 doi: 10.1038/nrdp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunn R. “It's not all in my head!” - the complex relationship between rare diseases and mental health problems. Orphanet J Rare Dis. 2017;12:29. doi: 10.1186/s13023-017-0591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schippers C., Volk D., de Zwaan M., et al. [ZSE-DUO - dual guidance structure at the centre for rare diseases] Inn Med. 2022;63:791–797. doi: 10.1007/s00108-022-01350-8. [DOI] [PubMed] [Google Scholar]
- 12.Hebestreit H., Zeidler C., Schippers C., et al. Dual guidance structure for evaluation of patients with unclear diagnosis in centers for rare diseases (ZSE-DUO): study protocol for a controlled multi-center cohort study. Orphanet J Rare Dis. 2022;17:47. doi: 10.1186/s13023-022-02176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margraf J., Cwik J.C. 2017. Mini-DIPS open access: diagnostisches Kurzinterview bei psychischen Störungen. [Mini-DIPS open access: diagnostic interview for mental disorders]. Bochum: forschungs-und Behandlungszentrum für psychische Gesundheit, Ruhr-Universität Bochum. [Google Scholar]
- 14.Köhler S., Gargano M., Matentzoglu N., et al. The human phenotype ontology in 2021. Nucleic Acids Res. 2021;49:D1207–D1217. doi: 10.1093/nar/gkaa1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng Y.S., Kohlmann T., Janssen M.F., Buchholz I. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30:647–673. doi: 10.1007/s11136-020-02688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt J., Lamprecht F., Wittmann W.W. [Satisfaction with inpatient management. Development of a questionnaire and initial validity studies] Psychother Psychosom Med Psychol. 1989;39:248–255. [PubMed] [Google Scholar]
- 17.Splinter K., Adams D.R., Bacino C.A., et al. Effect of genetic diagnosis on patients with previously undiagnosed disease. N Engl J Med. 2018;379:2131–2139. doi: 10.1056/NEJMoa1714458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.100,000 Genomes Project Pilot Investigators. Smedley D., Smith K.R., Martin A., et al. 100,000 Genomes pilot on rare-disease diagnosis in health care - preliminary report. N Engl J Med. 2021;385:1868–1880. doi: 10.1056/NEJMoa2035790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhlenbusch N., Swaydan J., Höller A., Löwe B., Depping M.K. Affective and anxiety disorders in patients with different rare chronic diseases: a systematic review and meta-analysis. Psychol Med. 2021;51:1–11. doi: 10.1017/S0033291721003792. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi F., Höfler M., Siegert J., et al. Twelve-month prevalence, comorbidity and correlates of mental disorders in Germany: the mental health module of the German health interview and examination survey for adults (DEGS1-MH.) Int J Methods Psychiatr Res. 2014;23:304–319. doi: 10.1002/mpr.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Substance Abuse and Mental Health Services Administration (SAMHSA), U.S. Department of Health and Human Services (HHS) website Key substance use and mental health indicators in the United States: results from the 2019 national survey on Drug use and health. 2020. https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR1PDFW090120.pdf Available at:
- 22.Pillay B., Wootten A.C., Crowe H., et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. doi: 10.1016/j.ctrv.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Haj-Mirzaian A., Patel B.N., Fishman E.K., Zaheer A. Value of multidisciplinary collaboration in acute and chronic pancreatitis. Abdom Radiol (NY) 2020;45:1458–1467. doi: 10.1007/s00261-019-02320-9. [DOI] [PubMed] [Google Scholar]
- 24.Wainwright K., Baumgarten S., Bostanci I., et al. TRANSLATE-NAMSE - evaluation report to the innovation fund. 2022. https://innovationsfonds.g-ba.de/downloads/beschluss-dokumente/159/2022-04-01_TRANSLATE-NAMSE_Evaluationsbericht.pdf page 54–55. Available at:
- 25.Janssen M.F., Szende A., Cabases J., Ramos-Goñi J.M., Vilagut G., König H.H. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20:205–216. doi: 10.1007/s10198-018-0955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grochtdreis T., Dams J., König H.H., Konnopka A. Health-related quality of life measured with the EQ-5D-5L: estimation of normative index values based on a representative German population sample and value set. Eur J Health Econ. 2019;20:933–944. doi: 10.1007/s10198-019-01054-1. [DOI] [PubMed] [Google Scholar]
- 27.Peters M., Kelly L., Potter C.M., et al. Quality of life and burden of morbidity in primary care users with multimorbidity. Patient Relat Outcome Meas. 2018;9 doi: 10.2147/PROM.S148358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauz E., Walther L., Junker S., et al. Time trends in mental health indicators in Germany's adult population before and during the COVID-19 pandemic. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1065938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bothwell L.E., Greene J.A., Podolsky S.H., Jones D.S. Assessing the gold standard--Lessons from the history of RCTs. N Engl J Med. 2016;374:2175–2181. doi: 10.1056/NEJMms1604593. [DOI] [PubMed] [Google Scholar]
- 30.Witt S., Kristensen K., Blömeke J., et al. [Quality of Life and experienced distress of patients suspected of having a rare (chronic) health condition - initial findings from the ZSE-DUO study] Psychother Psychosom Med Psychol. 2023;73:9–15. doi: 10.1055/a-1814-3998. [DOI] [PubMed] [Google Scholar]
- 31.Soussand L., Kuchenbuch M., Messiaen C., Sandrin A., Jannot A.S., Nabbout R. Impact of the COVID-19 pandemic on the care of rare and undiagnosed diseases patients in France: a longitudinal population-based study. Orphanet J Rare Dis. 2022;17:430. doi: 10.1186/s13023-022-02580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.