Abstract
In this work, we investigated in detail the upconversion properties of several types of nanoparticles, including NaYF4:5%Yb3+/30%Mn2+, NaYF4:40%Mn2+/x%Yb3+ (x% = 1, 5, 10, 20, 30, and 40), NaYF4:2%Er3+/x%Mn2+ (x% = 20, 30, 40, 50, 60, and 70), NaYF4:40%Mn2+/x%Er3+ (x% = 1, 2, 5, and 10), and NaYF4:40%Mn2+/1%Yb3+/x%Er3+ (x% = 0, 2, 5, and 10). We studied their upconversion emission under 980 nm excitation in both pulsed and continuous wave modes at different synthesis temperatures. The nanoparticles were characterized using transmission electron microscopy (TEM), X-ray diffraction (XRD), and photoluminescence (PL) spectroscopy. The doping of Yb3+ and Mn2+ ions resulted in the nanoparticles assuming cubic and hexagonal crystal structures. The emission intensity increased (106.4 (a.u.*103) to 334.4(a.u.*103)) with increasing synthesis temperature from 120 to 140 °C, while a sharp decrease was observed when the synthesis temperature was increased to 200 °C. The gradual decrease in peak intensity with increasing Mn2+ concentration from 20 to 70% was attributed to energy transfer from Mn2+ to Yb3+. In NaYF4:Mn2+/Yb3+/Er3+ UCNPs, increasing the Er3+ concentration from 0 to 10% led to the disappearance of the blue, orange, and green emission bands. The intense upconversion luminescence pattern with high spatial resolution indicates excellent potential for applications in displays, biological sensors, photodetectors, and solar energy converters.
Subject terms: Materials science, Nanoscience and technology, Optics and photonics
Introduction
Upconversion nanoparticles (UCNPs) possess a range of useful properties, such as low auto-fluorescence from cells, high penetration depth of light, high sensitivity for detection, sharp emission bandwidth, and large anti-Stokes shifts1–7, which make them valuable for applications in biological, therapeutic, photonics, and other areas8–11. UCNPs typically consist of two types of lanthanide (Ln) dopants, sensitizers and activators, immersed in an inorganic host lattice12. Rare-earth (RE) ion-doped UCNPs have attracted increasing interest due to their broad potential applications and unique upconversion (UC) luminescence properties. Specifically, RE-doped UCNPs can convert spectral energy in the infrared, visible, or UV ranges13–15.
Sodium yttrium fluoride (NaYF4) is one of the most efficient host materials for red, green, and blue UC phosphors16. Nanoparticles of NaYF4 exist in two structures: hexagonal and cubic, with the hexagonal form typically prepared by a high-energy consumption method at relatively high temperatures and the cubic form prepared by hydrothermal and solvothermal methods at relatively low temperatures17. NaYF4 has often been applied as a doping host for Ln sensitizers and activators due to its low energy modes (< 400/cm)18. Among the Er3+(2H11/2/4S3/2), Tm3+(3F2/3/3H4), Pr3+(3P1/3P0), Eu3+(5D1/5D0), Dy3+(4I15/2/4F9/2), and Nd3+(4F5/2/4F3/2) ions that often employed, Er3+-doped NaYF4 is recognized as one of the most stable UCNPs upon near-infrared light (NIR) excitations19–26. More recently, the incorporation of divalent manganese (Mn2+) ions has been recognized to decrease the short-wavelength green emission and increase the long-wavelength red emission due to energy transfer between the RE (often Er3+) and Mn2+ ions27. The Mn2+ emission bands, depending on the host surroundings, reside in the region of about 460–700 nm28. Previous studies have demonstrated that doping NaYF4 with Mn2+ ions can lead to strong red fluorescence in UCNPs, making them useful for in vivo imaging and drug delivery29.
In this paper, we aim to shed further light on this mechanism by investigating the upconversion properties of several types of nanoparticles, including NaYF4:5%Yb3+/30%Mn2+ at different synthesis temperatures, NaYF4:40%Mn2+/x%Yb3+ (x% = 1, 5, 10, 20, 30, and 40), NaYF4:2%Er3+/x%Mn2+ (x% = 20, 30, 40, 50, 60, and 70), NaYF4:40%Mn2+/x%Er3+ (x% = 1, 2, 5, and 10), and NaYF4:40%Mn2+/1%Yb3+/x%Er3+ (x% = 0, 2, 5, and 10) nanoparticles under 980 nm excitation in both pulsed and continuous wave modes. We characterized these nanoparticles using transmission electron microscopy (TEM), X-ray diffraction (XRD), and photoluminescence (PL) spectroscopy.
Previous studies have demonstrated that the doping of Mn2+ can decrease the non-radiative transition probability, thus improving the intensity of UCNP emission30–32. Additionally, among UC materials, α-phase NaYF4 is reported as one of the most efficient hosts for enhancing near-infrared (NIR) to single-red band up-conversion when tri-doped with Yb3+/Er3+/Mn2+ ions33. It has also been demonstrated that other nanosystems, including NaLuF4:Yb3+/Er3+/Mn2+, MnF2:Yb3+/Er3+, and KMnF3:Yb3+/Er3+, are effective emitters34.
In our study, we investigated the upconversion properties of several types of UCNPs to better understand the energy transfer mechanisms among Yb3+/Mn2+, Mn2+/Er3+, and Mn2+/Er3+/Yb3+ ions. We found that NaYF4 is an efficient host material for red, green, and blue UC phosphors with cubic and hexagonal structures. Er3+-doped NaYF4 is recognized as one of the most stable UCNPs upon NIR excitations, while Mn2+-doped NaYF4 can lead to strong red fluorescence in UCNPs. Our results suggest that the doping of Mn2+ can decrease the non-radiative transition probability, thus improving the intensity of UCNP emission.
Overall, our findings help to shed further light on the energy transfer mechanisms in UCNPs and highlight the potential applications of these materials in various fields, such as biological sensing, photonics, and solar energy conversion.
Experiment
Materials
Yttrium (III) chloride hexahydrate (YCl3∙6H2O, 99.99%), Erbium (III) chloride hexahydrate (ErCl3∙6H2O, 99.99%), Ytterbium (III) chloride hexahydrate (YbCl3∙6H2O, 99.99%), Manganese (II) chloride hexahydrate (MnCl2∙6H2O, 99.99%), Sodium hydroxide (NaOH), oleic acid (OA), ammonium fluoride (NH4F), ethanol (pure: 99.9), and cyclohexane were purchased from Sigma-Aldrich company. All chemicals were used as received without further purification.
Nanoparticle synthesis
Synthesis of NaYF4:5%Yb3+/30%Mn2+ nanoparticles
Yb3+ and Mn2+ co-doped NaYF4 nanoparticles were synthesized using a well-established hydrothermal method. NaOH (0.2 M, 1.2 ml) was added to a 50 ml centrifuge tube and stirred for 10 min. A mixture of ethanol (5 ml) and OA (5 ml) was added to NaOH and magnetically stirred for 15 min at room temperature until the solution became uniform and clear. A Ln solution was added to the centrifuge tube (Ln in total is 0.4 mmol): YCl3 (0.26 mmol), YbCl3 (0.02 mmol) and MnCl3 (0.12 mmol). The solution was kept under magnetic stirring for 15 min. Subsequently, 59.26 mg of NH4F dissolved in 1.6 ml of deionized water was added to the solution. The final solution was stirred magnetically for 1 h at a temperature of 40 °C. Thereafter, the solution was transferred into a Teflon (PTFE) reactor and sealed with Argon (Ar) gas. The PTFE reactor was inserted into an autoclave and heated at 120 °C, 140 °C, 160 °C, 180 °C, and 200 °C for 8 h. After 8 h, the autoclave was emptied, and the material in the PTFE reactor was cooled to room temperature. The solution was precipitated with ethanol (20 ml), collected by centrifugation (temperature: room temperature, time: 5 min, spin speed: 7500 rpm) washed several times with ethanol (10 ml), and dispersed in cyclohexane (10 ml) for further characterization. Further details of the synthesis of nanoparticles are given in the supplementary file.
Characterization
The size, shape, structure, and morphological characterization of the as-prepared UCNPs were characterized using a transmission electron microscope (TEM, JEOL, JEM-1400). The optical absorption spectrum in the wavelength range of 300–1000 nm was measured using an Edinburgh FS5 spectrophotometer. UC luminescence (UCL) spectra and decay curves were obtained using an Edinburgh FS5 spectrophotometer equipped with a 980 nm diode laser, operating in pulsed and continuous-wave (CW) mode. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 1 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device.
Results and discussion
Structure and morphology characterization
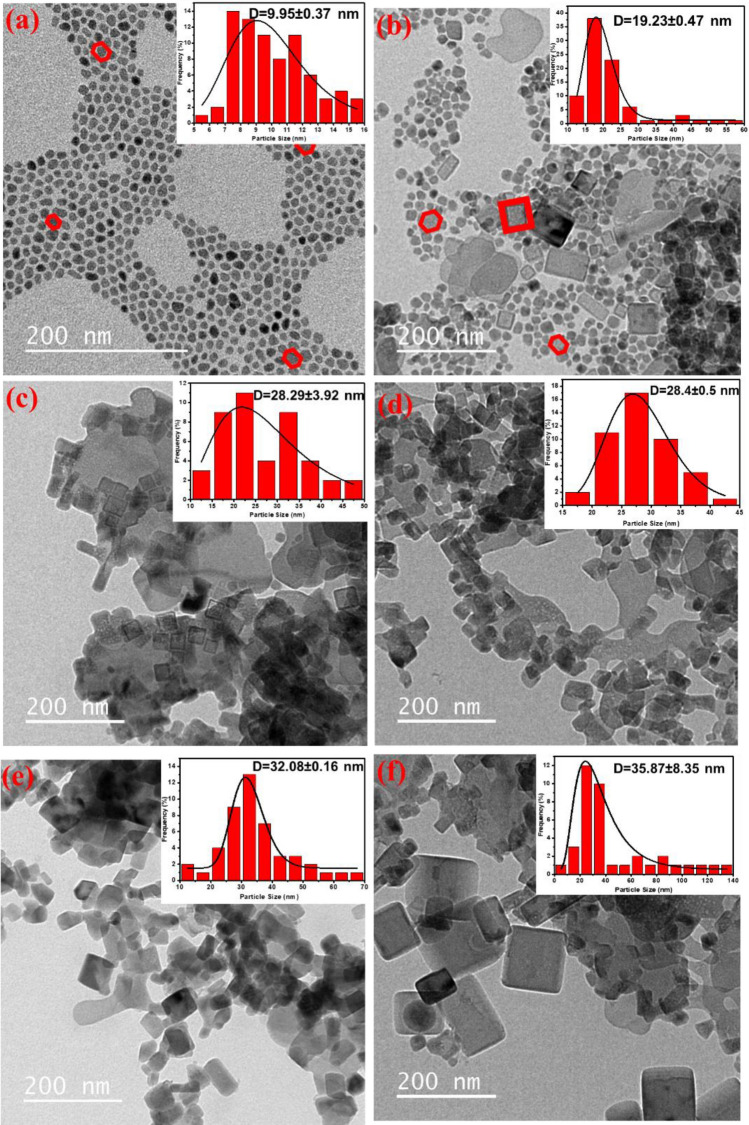
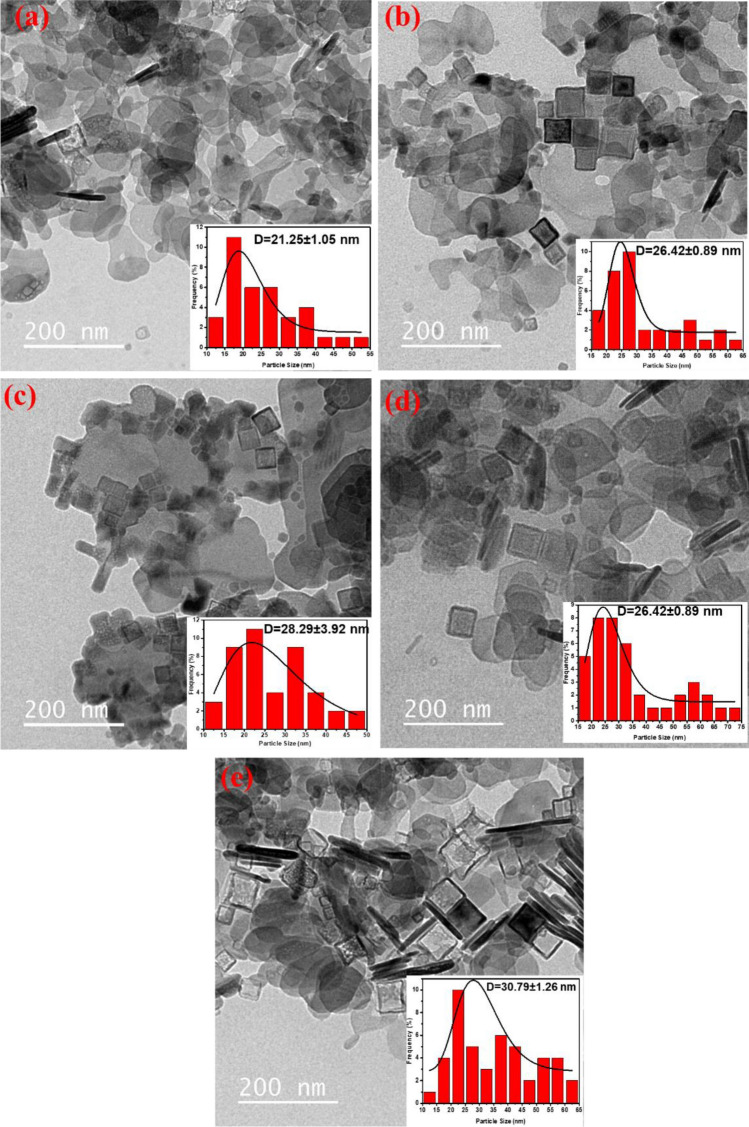
TEM was used to characterize the morphologies of the as-prepared UCNPs. Figure 1 displays TEM images of non-doped NaYF4 UCNPs and NaYF4: 30% Mn2+, 5%Yb3+ UCNPs at various synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C, and 200 °C). The non-doped NaYF4 UCNP crystal structure appeared completely hexagonal, while with the addition of Yb3+ and Mn2+ ions as dopants, the nanoparticles exhibited both cubic and hexagonal crystal structures. The average width of the nanoparticles was determined using the Digimizer software (version 4. 1. 1. 0, MedCalc software) (Fig. 1), and only a minor dispersion of the nanoparticles’ diameters was observed. To estimate the mean diameter of the nanoparticles, the obtained data were fitted with the Log-Normal distribution equation35.
| 1 |
| 2 |
| 3 |
Figure 1.
TEM images of the as-synthesized (a) NaYF4, (b) 30%Mn 5%Yb 120 °C, (c) 30%Mn 5%Yb 140 °C, (d) 30%Mn 5%Yb 160 °C, (e) 30%Mn 5%Yb 180 °C, and (f) 30%Mn 5%Yb 200 °C. The analyzed samples were in solution form.
Equations (1–3) were used to fit the data and obtain the fitting parameters D0 and σ. Additionally, the standard deviation (σD) and mean diameter of the nanoparticles <D> were calculated using the results obtained from the data fitting. As shown in Fig. 1, the diameter of the nanoparticles increased from 19 to 36 nm as the temperature increased from 120 to 200 °C.
The TEM micrographs in Fig. 2 reveal that the NaYF4:5%Yb3+/x%Mn2+ (x% = 30, 40, 50, 60, 70) nanoparticles are polyhedral in shape with a uniform size. The average diameter of the NaYF4:5%Yb3+/x%Mn2+ (x% = 30, 40, 50, 60, and 70) nanoparticles was found to be 28 nm. These results suggest that the crystal size of the nanoparticles increases and undergoes continuous and regular changes with the increase in Mn2+ content. As Mn2+ concentration increases from 30 to 70%, the number and size of the nanoparticles increases, as calculated using Eq. (2). The data demonstrate that the size of the nanoparticles increases from 21 to 30 nm as the Mn2+ concentration increases from 30 to 70%. Figure 2 also shows the appearance of several hexagonal hollow nanoparticles as Mn2+ concentration increases up to 70%. These observations are consistent with earlier reports36,37.
Figure 2.
TEM images of NaYF4: Mn2+, Yb3+ nanocrystals doped with (a) 30%, (b) 40%, (c) 50%, (d) 60% and (d) 70% Mn2+ ions. The analyzed samples were in solution form.
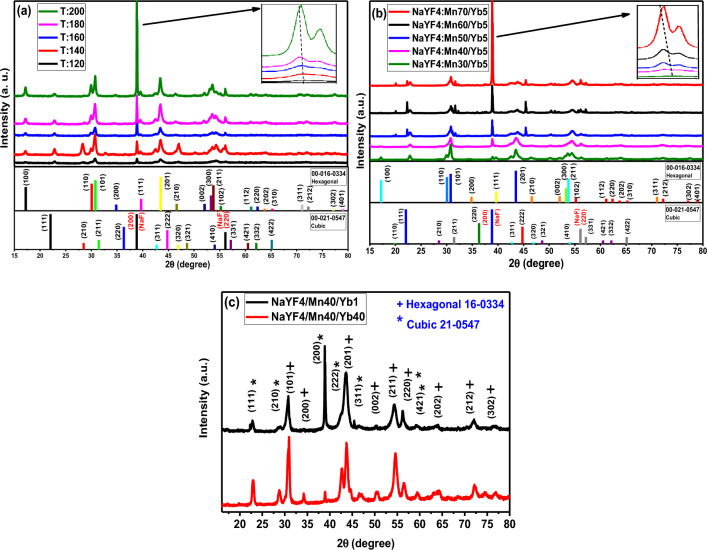
The crystal structure and purity of NaYF4:5%Yb3+/30%Mn2+ UCNPs were studied by analyzing the powder X-ray diffraction (XRD) patterns at different synthesis temperatures. Figure 3a displays XRD patterns of NaYF4:5%Yb3+/30%Mn2+ UCNPs at different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C, 200 °C). The XRD patterns of samples prepared at different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C, 200 °C) indicate a mixed-phase NaYF4 crystal, in agreement with standard patterns of cubic (JCPDS: 00-021-0547) and hexagonal (JCPDS: 00-016-0334). The results demonstrate that increasing the synthesis temperature from 120 to 200 °C leads to an increase in the cubic NaYF4 phase peaks and crystallinity, which is consistent with the TEM images. Additionally, the zoomed image (Fig. 3a) shows that when the synthesis temperature is increased to 200 °C, the diffraction peak (111) slightly shifts to the lower-angle side, which can be attributed to the substitution of Y3+ ions by smaller Mn2+ ions in the host lattice36. Figure 3b shows the XRD patterns of NaYF4:5%Yb3+/x%Mn2+ (x% = 30, 40, 50, 60, 70) UCNPs. The XRD patterns indicate a mixed-phase NaYF4 crystal and are consistent with the standard patterns of cubic (JCPDS: 00-021-0547) and hexagonal (JCPDS: 00-016-0334) phases. When the concentration of Mn2+ is increased from 30 to 40%, the XRD pattern shows a cubic NaYF4 phase. However, when the concentration of Mn2+ is further increased from 40 to 70%, the hexagonal phase reappears. Also, by increasing the concentration of Mn2+ ions from 40 to 70%, the cubic phase peaks increase. Additionally, increasing the concentration of Mn2+ ions from 40 to 70% leads to an increase in cubic phase peaks, which is consistent with the TEM images. The zoomed image (Fig. 3b) shows that with an increase in the concentration of Mn2+ ions, the diffraction peak (111) slightly shifts to the lower angle side due to the substitution of Y3+ ions by smaller Mn2+ ions in the host lattice36. Figure 3c displays the XRD patterns of NaYF4 UCNPs at different Yb3+ concentrations (NaYF4:40%Mn2+/1%Yb3+ and NaYF4:40%Mn2+/40%Yb3+). The XRD patterns indicate a mixed-phase NaYF4 crystal, consistent with standard patterns of hexagonal (JCPDS: 00-016-0334) and cubic (JCPDS: 00-021-0547) phases. The results show that increasing the Yb3+ concentration from 1 to 40% leads to a decrease in crystallinity. Moreover, with an increase in Yb3+ concentration, the cubic NaYF4 phase increases while the hexagonal phase decreases. Therefore, the NaYF4:40%Mn2+/1%Yb3+ sample was chosen as the main sample.
Figure 3.
XRD patterns of (a) NaYF4: 5%Yb3+/30%Mn2+ UCNPs at different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C, 200 °C), (b) NaYF4: 5%Yb3+/x%Mn2+ (x% = 30, 40, 50, 60, 70%) UCNPs, (c) NaYF4 UCNPs at different Yb3+ concentration (NaYF4:40%Mn2+ /1%Yb3+ and NaYF4:40%Mn2+ /40%Yb3+). The analyzed samples were in solution form.
In summary, XRD is a powerful technique that allows for the analysis of crystal structure and purity. The XRD patterns of NaYF4: 5%Yb3+/30%Mn2+ UCNPs, NaYF4: 5%Yb3+/x%Mn2+ (x% = 30, 40, 50, 60, 70) UCNPs, and NaYF4 UCNPs at different Yb3+ concentrations were analyzed, and the results indicate mixed-phase NaYF4 crystals with standard patterns of cubic and hexagonal phases. The crystallinity and phase composition were found to be dependent on temperature and dopant concentration, which is consistent with the TEM images.
Figure 4 displays XRD patterns of NaYF4 and NaYF4 UCNPs at different Er3+ concentrations (NaYF4:1%Yb3+/40%Mn2+/2%Er3+ and NaYF4:1%Yb3+/40%Mn2+/10% Er3+). The XRD pattern of the UCNPs can be assigned to a mixed-phase NaYF4 crystal with the standard pattern of hexagonal (JCPDS: 00-016-0334) and cubic (JCPDS: 00-021-0547). No significant additional diffraction peaks were detected, even when the Er3+ feeding content was as high as 10 mol%. The results indicate that the crystallinity decreased with increasing Er3+ concentration from 2 to 10%. Additionally, with an increased Er3+ concentration, the XRD peak intensity increases in the cubic and hexagonal phases. Consequently, the NaYF4:1%Yb3+/40%Mn2+/10% Er3+ UCNP sample was chosen as the primary sample.
Figure 4.
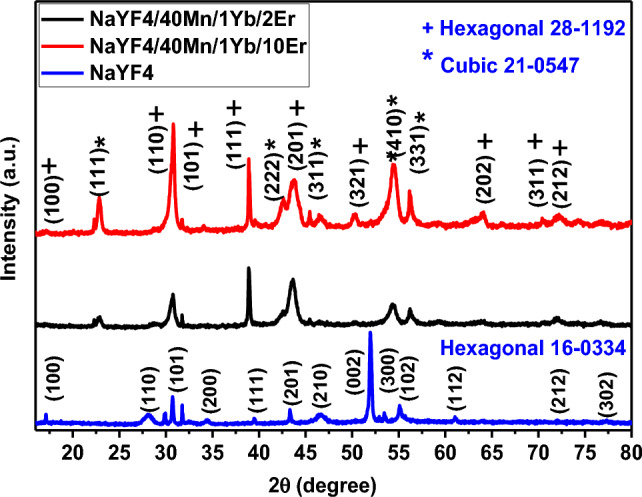
XRD patterns of NaYF4, NaYF4:1%Yb3+/40%Mn2+/2%Er3+ and NaYF4:1%Yb3+/40%Mn2+/10% Er3+ UCNPs. The analyzed samples were in solution form.
Determination of optimum synthesis temperature for NaYF4: 5% Yb3+/30% Mn2+ nanoparticles
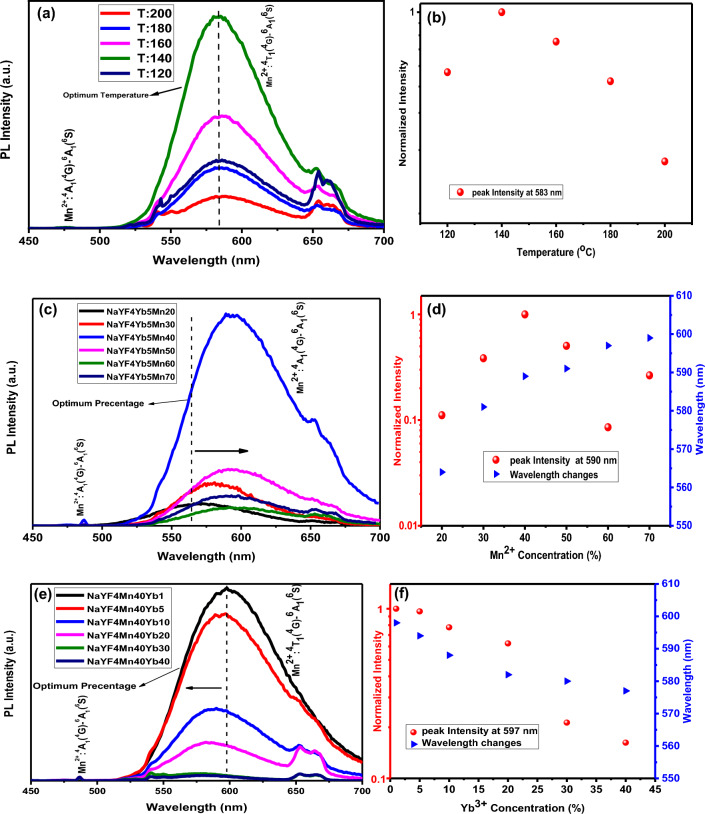
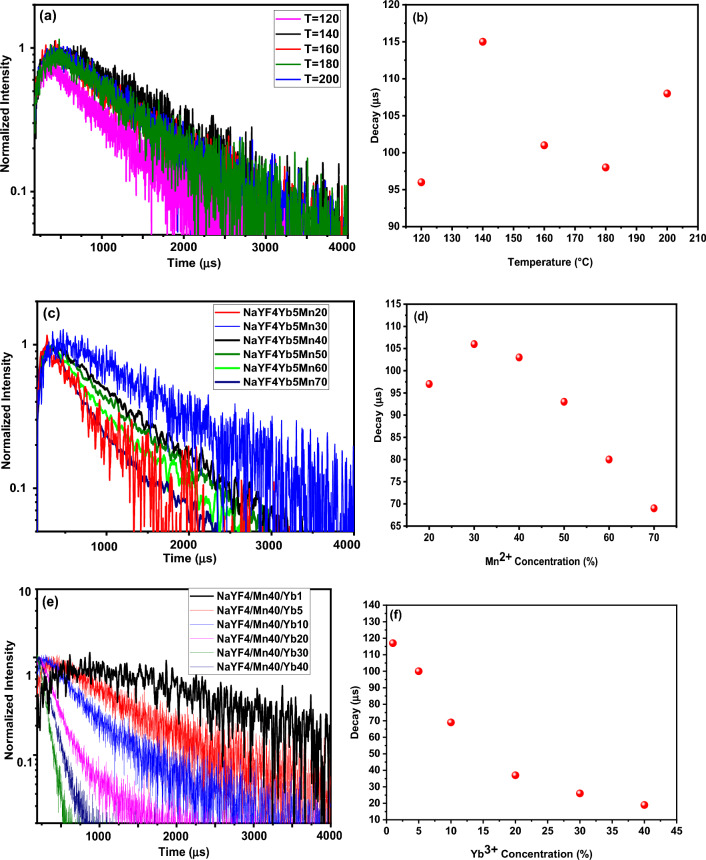
The UCL spectra of the different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C, 200 °C) of NaYF4: 5% Yb3+/30% Mn2+ nanoparticles were measured using CW 980 nm excitation. Figure 5a displays the UCL emission spectra of these nanoparticles, revealing a relatively strong emission band at 583 nm and a weaker emission band at 575 nm under 980 nm excitation, corresponding to Mn2+: 4A1 (4G) → 6A1 (6S) and Mn2+: 4T1 (4G) → 6A1 (6S) respectively, as shown in Fig. 5a. Our objective was to determine the optimal synthesis temperature for subsequent experiments, and thus, the relationship between reaction temperature and emission intensity was crucial. As depicted in Fig. 5a, the emission intensity increased as the synthesis temperature increased from 120 to 140 °C, but significantly decreased as the synthesis temperature increased to 200 °C. Figure 5b illustrates the log intensity different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C, 200 °C) nanoparticles, indicating that the synthesis temperature of 140 °C resulted in the highest emission intensity among all samples, which was considered the optimum synthesis temperature. These observations were attributed to the shrinking host lattice and the decreased non-radiative relaxation processes, consistent with a previous study38.
Figure 5.
(a) UCL emission spectra of NaYF4:5%Yb3+/30%Mn2+ nanoparticles with different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C and 200 °C) and (b) log intensity versus synthesis temperature under 980 nm CW excitation. (c) UCL emission spectra of NaYF4:5%Yb3+/x%Mn2+ (x% = 20, 30, 40, 50, 60 and 70%) nanoparticles and (d) log intensity versus Mn2+ concentration under 980 nm CW excitation. (e) UCL emission spectra of NaYF4:40% Mn2+/x%Yb3+ (x% = 1, 5, 10, 20, 30 and 40%) nanoparticles and (f) log intensity versus Yb3+ concentration under 980 nm CW excitation. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 1 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device.
Optical properties of NaYF4: Yb3+/Mn2+ under 980 nm excitation
Figure 5c and e present the UCL yellow emission spectra of NaYF4: Yb3+/Mn2+ nanoparticles at their optimum synthesis temperature (140 °C). The UCL spectra were measured using CW 980 nm excitation, revealing a visible area to yellow UCL emission. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 1 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device. The UCL spectrum consisted of a weak emission peak at 487 nm, corresponding to the d–d transition [Mn2+: 4A1 (4G) → 6A1 (6S)] and a broadband emission peak at approximately 590 nm, corresponding to Mn2+: 4T1 (4G) → 6A1 (6S). Figure 5c illustrates the concentration-dependent UCL emission of NaYF4: 5Yb3+/x%Mn2+ (x% = 20, 30, 40, 50, 60 and 70), with the optimal doping concentration of Mn2+ determined to be x = 40%. Figure 5d displays the log intensity versus Mn2+ concentration, indicating an increase in emission intensity as the Mn2+ percentage increased from 20 to 40%, followed by a sharp decrease as the Mn2+ percentage increased to 70%. The gradual decrease in peak intensity with increasing Mn2+ concentration was attributed to energy transfer from Mn2+ to Yb3+39. Additionally, with increasing Mn2+ concentration, the peak position shifted from 563 nm (yellow) to 593 nm (orange), confirming this energy transfer. Figure 5e shows the PL intensity versus Yb3+ concentration, indicating a decrease in emission intensity as the Yb3+ percentage increased from 1 to 40%, with the optimal doping concentration of Yb3+ determined to be x = 1%33. The decrease in peak intensity with increasing Yb3+ concentration was attributed to an energy transfer from Yb3+ to Mn2+39. Furthermore, with increasing Yb3+ concentration, the peak position shifted from 599 nm (orange) to 573 nm (yellow), confirming this energy transfer (Fig. 5f). Visible photons from the excited Yb3+-Mn2+ pairs were released via the sequential ground-state absorption (GSA, |2F7/2, 6A1(6S) > →|2F5/2, 6A1(6S)>) and excited-state absorption (ESA, |2F5/2, 6A1(6S) > →|2F7/2, 4T1(6S)>) processes.
Luminescence decay time of NaYF4: Yb3+/Mn2+ nanoparticles under 980 nm excitation
To provide further evidence for the role that temperature plays in enhancing UC emission, decay curves of the UCNPs are presented in Fig. 6a–f. Decay curves were fitted with the following formula proposed by Nakazawa40:
where τm is the effective decay time constant, and I(t) is the intensity at time t.
Figure 6.
(a) Lifetimes spectra of NaYF4:5%Yb3+/30%Mn2+ nanoparticles with different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C and 200 °C) and (b) amount of decay under 980 nm pulsed excitation. (c) Lifetimes spectra of NaYF4:5%Yb3+/x%Mn2+ (x% = 20,30, 40, 50, 60 and 70) nanoparticles, (d) amount of decay under 980 nm pulsed excitation. (e) Lifetimes spectra of NaYF4:40% Mn2+/x%Yb3+ (x% = 1, 5, 10, 20, 30 and 40) nanoparticles, (f) amount of decay under 980 nm pulsed excitation. The power density and spot diameter of the used 980-nm laser are 10 W/cm2 and 3.0 mm, respectively. Luminescence decay times were measured in the red wavelength region. The analyzed samples were in solution form.
Figure 6 displays the UC luminescence decay curves of (a) NaYF4:5%Yb3+/30%Mn2+ nanoparticles at different synthesis temperatures (120 °C, 140 °C, 160 °C, 180 °C and 200 °C), (b) the amount of decay under 980 nm pulsed excitation, (c) NaYF4:5%Yb3+/x% Mn2+ (x% = 20, 30, 40, 50, 60 and 70) nanoparticles decay, (d) the amount of decay under 980 nm pulsed excitation, (e) NaYF4:40% Mn2+/x%Yb3+ (x% = 1, 5, 10, 20, 30 and 40) nanoparticles decay, and (f) the amount of decay under 980 nm pulsed excitation. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 10 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device.
Figures 6a and b show that the luminescence lifetimes increase from 96 to 115 µs as the synthesis temperature rises from 120 to 140 °C, while as the synthesis temperature increases from 140 to 200 °C, the luminescence lifetimes decrease. Therefore, the NaYF4:5%Yb3+/30%Mn2+ nanoparticles with a synthesis temperature of 140 °C, showing the longest luminescence lifetime, were selected as the main sample. As previously demonstrated, a synthesis temperature of 140 °C is the optimal synthesis temperature for producing samples. Figure 6c and d reveal that by increasing the Mn2+ concentration from 20 to 70%, the luminescence lifetimes of the Yb3+: 2F5/2 level first increase and then decrease significantly. The luminescence lifetimes for Yb3+ were found to decrease monotonically with increasing Mn2+ concentration, providing evidence for efficient energy transfer from Yb3+ to Mn2+ ions37. These time-decay measurements are consistent with the UCL emission spectra. Figure 6e and f illustrate that by increasing Yb3+ concentration from 1 to 40%, the luminescence lifetimes of the Yb3+: 2F5/2 level decrease significantly from 116 to 15 µs. The luminescence lifetimes for NaYF4: 40%Mn2+/x%Yb3+ (x% = 1, 5, 10, 20, 30 and 40) UCNPs were found to decrease monotonically with increasing Yb3+ concentration, providing evidence for efficient energy transfer from Yb3+ to Mn2+ ions37. Our main objective was to find a combination of Yb3+ and Mn2+ that had the longest luminescence lifetime. Therefore, a sample of NaYF4: 40%Mn2+/1% Yb3+ was selected as the main sample and used in the subsequent steps. These results are in good agreement with the UCL emission spectra and observed structural properties.
Optical properties of NaYF4:Er3+/Mn2+ nanoparticles under 980 nm excitation
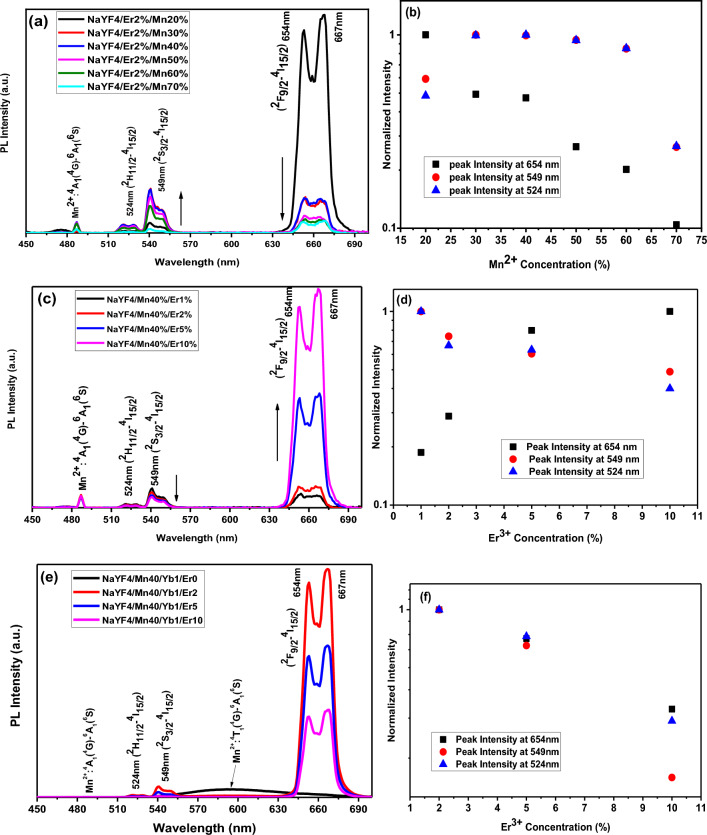
To investigate the energy transfer between Mn2+ and Er3+ and the luminescence properties of NaYF4: Er3+/Mn2+, the UCL emission spectra of synthetic samples were measured under CW 980 nm excitation. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 1 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device. Figure 7a displays the UCL emission spectra of NaYF4: 5%Er3+/x%Mn2+ (x% = 20, 30, 40, 50, 60 and 70) nanoparticles. The UCL spectra consist of four emission bands centered at 486 nm (blue), 524 (green), 549 nm (green), and 654 nm (red), corresponding to the transitions of 4A1 (4G) → 6A1 (6S), 2H11/2 → 4I15/2, 2S3/2 → 4I15/2 and 2F9/2 → 4I15/2, respectively. The UCL spectra in Fig. 7a demonstrate that the green and blue emissions increase when the Mn2+ concentration increases to 40%, but then decrease when it increases to 70%. Additionally, the red emission decreases when the Mn2+ concentration increases from 20 to 70%41. These results suggest that increasing the Mn2+ concentration leads to a continuous decrease in the emission intensity of Er3+, indicating the possibility of energy transfer from Er3+ ions to Mn2+42.
Figure 7.
(a) UCL emission spectra of NaYF4: 40% Mn2+/x%Er3+ (x% = 1, 2, 5 and 10) nanoparticles and (b) log intensity versus Er3+ concentration under 980 nm CW excitation. (c) UCL emission spectra of NaYF4: 2% Er3+/x%Mn2+ (x% = 20, 30, 40, 50, 60 and 70) nanoparticles (d) log intensity versus Mn2+ concentration under 980 nm CW excitation. (e) UCL emission spectra of NaYF4: 1% Yb3+/40% Mn2+/x%Er3+ (x% = 0, 2, 5, and 10) nanoparticles (f) log intensity versus Er3+ concentration under 980 nm CW excitation. NaYF4:Yb3+/Mn2+ nanoparticles emitted relatively strong emission band at 575 nm. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 1 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device.
To gain further insight into the mechanism of Mn2+ doped NaYF4: 2%Er3+ nanoparticles, the log intensity versus Mn2+ concentration for 524 nm, 549 nm, and 654 nm was calculated and presented in Fig. 7b. The green emission intensity increases as the Mn2+ concentration increases from 20 to 40%, but then decreases as it increases to 70%. Moreover, the red emission intensity decreases as the Mn2+ concentration increases from 20 to 70%, confirming the role of Mn2+ in enhancing the green and blue emission and suppressing the red emission in the NaYF4: Er3+/Mn2+ system41.
Figure 7c shows the UCL emission spectra of the NaYF4: 40Mn2+/x%Er3+ (x% = 1, 2, 5 and 10) nanoparticles. The spectra consist of four emission bands centered at 486 nm (blue), 524 nm (green), 549 nm (green), and 654 nm (red), corresponding to the transitions of 4A1 (4G) → 6A1 (6S), 2H11/2 → 4I15/2, 2S3/2 → 4I15/2 and 2F9/2 → 4I15/2, respectively. The results indicate that as the Er3+ concentration increases from 1 to 10%, the green and blue emissions decrease, while the red emission increases41. This finding suggests that increasing the Er3+ concentration leads to a continuous decrease in the emission intensity of Mn2+, indicating the possibility of energy transfer from Mn2+ ions to Er3+42.
To gain a deeper understanding of the mechanism of Er3+ doped NaYF4: 40%Mn2+ nanoparticles, the log intensity versus Er3+ concentration for 524 nm, 549 nm, and 654 nm was calculated and presented in Fig. 7d. The results show that as the Er3+ concentration increases from 1 to 10%, the green UC emission bands at 524 nm (Er3+:2H11/2–4I15/2) and 549 nm (Er3+:4S3/2–4I15/2) and blue UC emission bands at 486 nm (Mn2+: 4A1 (4G) → 6A1) decrease, while the red UC emission bands at 654 nm (Er3+: 2F9/2 → 4I15/2) increase, confirming the role of Er3+ in reducing the green and blue emission and enhancing the red emission in NaYF4: Er3+/Mn2+ systems41. First of all, the possible energy transfer from Er3+ to Mn2+–Yb3+ dimer, which contributes to the emission intensity as 524 and 549 nm decreased, can be explained by the following mechanisms: 4S3/2 (Er3+) 2F7/2, 6A1 (Mn2+–Yb3+ dimer) 4I15/2 (Er3+) 2F7/2, 4T1 (Mn2+–Yb3+ dimer). Secondly, the increased luminescent centers lead the emission intensity at 657 nm (Er3+: 4F9/2–4I15/2) may be energy transfer from Mn2+–Yb3+ dimer to Er3+ ions. The mechanism of energy transfer from Mn2+–Yb3+ dimer to Er3+ ions was proposed as follows: ∣2F7/2, 4T1〉 (Mn2+–Yb3+ dimer) + 4I15/2 (Er3+)–4F9/2 (Er3+) + ∣2F7/2, 6A1〉 (Mn2+–Yb3+ dimer), so the energy transfer bridge was constituted by the energy transfer process between Er3+ ion and the Mn2+–Yb3+ dimer.
To further investigate the impact of Er3+ doping on the upconversion luminescence (UCL) properties of Yb3+/Er3+/Mn2+ triply-doped NaYF4 nanoparticles, the emission intensity versus wavelength of NaYF4: 1%Yb3+/40%Mn2+/x%Er3+ (x% = 0, 2, 5, and 10) nanoparticles at the optimum synthesis temperature is presented in Fig. 7e. When excited at 980 nm, five UCL bands at 486 nm, 524 nm, 549 nm, 595 nm, and 654 nm are detected, which are attributed to the 4A1 (4G) → 6A1 (6S), 2H11/2 → 4I15/2, 2S3/2 → 4I15/2, 4T1 (4G) → 6A1 (6S) and 2F9/2 → 4I15/2 transitions, respectively. The results show that as the Er3+ concentration increases from 0 to 10%, all emission bands, blue, orange, and green, disappear for the NaYF4:Mn2+/Yb3+/Er3+ nanoparticles. This single-band UCL emission can be attributed to a non-radiative energy transfer from the 4S3/2 and 2H11/2 levels of Er3+ to the 4T1 and 4A1 levels of Mn2+43. The disappearance of green emissions with increasing Er3+ concentration suggests an efficient exchange energy transfer process between Mn2+ and Er3+ ions, which is attributed to their proximity and nearly perfect overlap of energy levels in the host lattices33,44–46. Additionally, it is noteworthy that the red emission intensity gradually decreases with the increase in Er3+ concentration due to concentration quenching between neighboring Er3+ ions43.
In Fig. 7c, the luminescence of sample NaYF4: 10%Er3+, 40% Mn2+ is the highest. But when the three dopants, namely; manganese, yttrium and ytterbium are added to NaYF4, sample NaYF4: 1% Yb3+/40% Mn2+/2%Er3+ is the highest, so we consider sample NaYF4: 1% Yb3+/40% Mn2+/2%Er3+ as the optimal sample (Fig. 7e).
To better comprehend the UCL mechanism, the log intensity versus Er3+ concentration for NaYF4: 1%Yb3+/40%Mn2+/x%Er3+ (x% = 0, 2, 5, 10) nanoparticles under CW 980 nm excitation is calculated and presented in Fig. 7f. The results show that as the Er3+ concentration increases, the green emission disappears and the red emission intensity decreases.
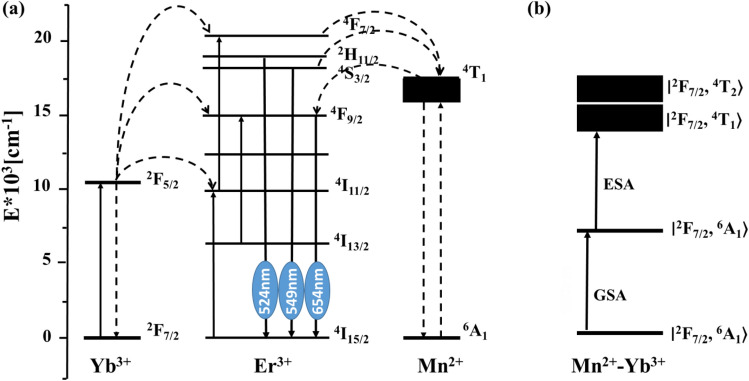
To further investigate the effect of Mn2+ on the upconversion luminescence (UCL) properties of Yb3+/Mn2+/Er3+ triply-doped NaYF4 nanoparticles, a diagram of energy levels and corresponding energy transfer mechanisms is presented in Fig. 8a. When Mn2+ ions are introduced into NaYF4: Yb3+/Er3+, a new energy transfer process between Er3+ and Mn2+ is induced under the excitation of 980 nm CW laser. This process leads to a decrease in the radiative transition rate of Er3+:H11/2 and Er3+: S3/2 levels to the ground state, while the population density of Mn2+:4T1 increases due to the resonance energy transfer. Subsequently, a back-energy transfer from 4T1 of Mn2+ to the 4F7/2 level of Er3+ leads to an enhancement in the red emission. Here, the direct multi-phonon relaxation process and the indirect energy transfer process of (F5/2(Yb3+), 4I13/2(Er3+)) → (2F7/2(Yb3+), 7I9/2(Er3+)) are expected to have a minor contribution to the population of 4S3/2 energy level in Er3+ ions. It is noteworthy that the Er3+: F9/2 lifetime is shorter than that of Mn2+:4T1, which explains why no orange luminescence is observed corresponding to the Mn2+: 4T1 → 6A1 transition. A similar mechanism has been discussed in NaYF4 by Zhangyu Huang et al.34. The Schematic of energy level diagrams of Mn2+–Yb3+ dimer is shown in Fig. 8b. For the Mn2+–Yb3+ dimer, the sensitization through the Mn2+–Yb3+ dimer complex entails both ground state absorption (GSA) and excited state absorption (ESA). The Mn2+–Yb3+ dimer ground state is represented by ∣2F7/2, 6A1〉, the intermediate excited state in the NIR by ∣2F5/2, 6A1〉, and the relevant higher excited states by ∣2F7/2, 4T1〉 and ∣2F7/2, 4T2〉.
Figure 8.
(a) Schematic of energy level diagrams of NaYF4:Yb3+ /Mn2+/Er3+ and the proposed mechanism of the UC process under the excitation of 980 nm. (b) Schematic of energy level diagrams of Mn2+–Yb3+ dimer.
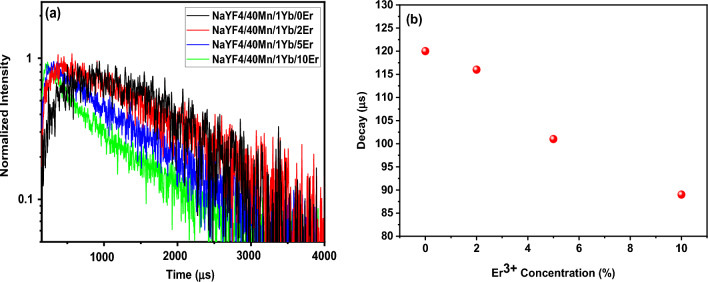
Luminescence decay time of NaYF4: 1% Yb3+/40% Mn2+/x%Er3+ (x% = 0, 2, 5, and 10) nanoparticles under 980 nm excitation
To provide further evidence of the role played by Er+3 in enhancing UC emission, Fig. 9a and b presents the decay curves of the UCNPs. The pulse duration for the impact mode was 3 min, the power density of the 980 nm laser was 10 W/cm2, and the spot width was 3 mm. 1000 µl of the solution was poured into the cuvette and the cuvette was placed inside the measuring device. Figure 9a shows the lifetime spectra of NaYF4: 1% Yb3+/40% Mn2+/x% Er3+ (x% = 0, 2, 5, and 10) nanoparticles, while Fig. 9b shows the amount of decay under 980 nm pulsed excitation. The luminescence lifetimes of NaYF4: Yb3+/Mn2+/Er3+ were found to decrease monotonically with increasing Er3+ concentration. This provides evidence for efficient energy transfer from Yb3+ to Mn2+ ions38. The decrease in lifetime is likely due to a competitive effect between the energy transfer of Er3+ and Mn2+ ions and the decrease in radiative transition probability resulting from increased local symmetry after Er3+ doping. The former prolongs the lifetime, while the latter decreases it.
Figure 9.
(a) Lifetimes spectra of NaYF4: 1% Yb3+/40% Mn2+/x%Er3+ (x% = 0, 2, 5, and 10) nanoparticles, (b) amount of decay under 980 nm pulsed excitation. The power density and spot diameter of the used 980-nm laser are 10 W/cm2 and 3.0 mm, respectively. Luminescence decay times were measured in the red wavelength region. The analyzed samples were in solution form.
Conclusions
In this study, we investigated the UC energy transfer mechanism of Yb3+/Mn2+/Er3+ tri-doped uniform cubic NaYF4 nanoparticles under 980 nm excitation in pulsed or continuous-wave modes. Firstly, we synthesized NaYF4:5%Yb3+/30%Mn2+ nanoparticles at different synthesis temperatures, and the optimal synthesis temperature of 140 °C was selected for subsequent experiments. Next, we studied NaYF4:5%Yb3+ nanoparticles with varying x%Mn2+ (x% = 20, 30, 40, 50, 60, 70) concentration and NaYF4:40% Mn2+ nanoparticles with varying x%Yb3+ (x% = 1, 5, 10, 20, 30, 40) concentration. Finally, we synthesized NaYF4:40% Mn2+/1%Yb3+ nanoparticles with different x%Er3+ (x% = 0, 2, 5, 10) concentrations by selecting the optimal Mn2+ and Yb3+ concentrations. The UCL spectra of NaYF4: 1% Yb3+/40% Mn2+/x%Er3+ (x% = 0, 2, 5, and 10) nanoparticles exhibited four emission bands centered at 486 nm (blue), 524 nm (green), 549 nm (green), and 654 nm (red), corresponding to the transitions of 4A1 (4G) → 6A1 (6S), 2H11/2 → 4I15/2, 2S3/2 → 4I15/2, and 2F9/2 → 4I15/2, respectively. As the concentration of Er3+ increased from 1 to 10 mol%, the green and blue emission intensities decreased, while the red emission intensity increased. This confirms the role of Er3+ in enhancing green and blue emission and suppressing red emission in NaYF4:Er3+/Mn2+ systems. These findings introduce the application of Mn2+ in Yb3+–Er3+ codoped NaYF4 UCNPs in color modulation, temperature sensing, and optical heating. The data presented in this study may provide useful information for further development of Ln-doped nanoparticles for these applications.
Supplementary Information
Acknowledgements
The authors thank Dr. Haichun Liu and Dr. Lucia Labrador Paez for their valuable advice.
Author contributions
R.Z.M. and H.A. wrote the main manuscript text. R.Z.M. collaborated in laboratory work. H.R.D. and M.H.E. have determined the issue. H.R.D. cooperated in choosing the topic and writing the article. All authors reviewed the manuscript.
Data availability
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Reza Zarei Moghadam, Email: r.zarei1991@gmail.com.
Mohammad Hossein Ehsani, Email: ehsani@semnan.ac.ir.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-44947-1.
References
- 1.Bloembergen N. Solid state infrared quantum counters. Phys. Rev. Lett. 1959;2:84–85. doi: 10.1103/PhysRevLett.2.84. [DOI] [Google Scholar]
- 2.Auzel F. Compteur quantique par transfert d’énergie entre deux ions de terres rares dans un tungstate mixte et dans un verre. CR Acad. Sci. Paris. 1966;262:1016–1019. [Google Scholar]
- 3.Van Uitert LG, Johnson LF. Energy transfer between rare-earth ions. J. Chem. Phys. 1966;44:3514–3522. doi: 10.1063/1.1727258. [DOI] [Google Scholar]
- 4.Heer S, Kompe K, Gudel HU, Haase M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide-doped NaYF4 nanocrystals. Adv. Mater. 2004;16:2102–2105. doi: 10.1002/adma.200400772. [DOI] [Google Scholar]
- 5.Suyver JF, Aebischer A, Biner D, Gerner P, Grimm J, Heer S, Kramer KW, Reinhard C, Gudel HU. Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion. Opt. Mater. 2005;27:1111–1130. doi: 10.1016/j.optmat.2004.10.021. [DOI] [Google Scholar]
- 6.Haase M, Schafer H. Upconverting nanoparticles. Angew. Chem Int. Ed. 2011;50:5808–5829. doi: 10.1002/anie.201005159. [DOI] [PubMed] [Google Scholar]
- 7.Shan J, Ju Y. A single-step synthesis and the kinetic mechanism for monodisperse and hexagonal-phase NaYF4: Yb, Er upconversion nanophosphors. Nanotechnology. 2009;20:11969. doi: 10.1088/0957-4484/20/27/275603. [DOI] [PubMed] [Google Scholar]
- 8.Dou QQ, Idris NM, Zhang Y. Sandwich-structured upconversion nanoparticles with tunable color for multiplexed cell labeling. Biomaterials. 2013;34:1722–1731. doi: 10.1016/j.biomaterials.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Han Y, Lim CS, Lu YH, Wang J, Xu J, Chen HY, Zhang C, Hong MH, Liu XG. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature. 2010;463:1061–1065. doi: 10.1038/nature08777. [DOI] [PubMed] [Google Scholar]
- 10.Priyam A, Idris NM, Zhang Y. Gold nanoshell coated NaYF4 nanoparticles for simultaneously enhanced upconversion fluorescence and darkfield imaging. J. Mater. Chem. 2012;22:960–965. doi: 10.1039/C1JM14040J. [DOI] [Google Scholar]
- 11.He E, Zheng H, Gao W, Tu Y, Lu Y, Tian H, Li G. Enhancement and regulation of fluorescence emission from NaYF4: Yb3+, Er3+ nanocrystals by codoping Mn2+ ions. J. Nanosci. Nanotechnol. 2014;14:4139–4146. doi: 10.1166/jnn.2014.8040. [DOI] [PubMed] [Google Scholar]
- 12.Auzel F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004;104:139–173. doi: 10.1021/cr020357g. [DOI] [PubMed] [Google Scholar]
- 13.Li ZQ, Zhang Y. Facile synthesis of lanthanide nanoparticles with paramagnetic, down-and up-conversion properties. Nanoscale. 2010;2:1240–1243. doi: 10.1039/c0nr00073f. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Huang K, Valiev RR, Zhan Q, Zhang Y, Ågren H. Photon upconversion kinetic nanosystems and their optical response. Laser Photon. Rev. 2017;12:1700144. doi: 10.1002/lpor.201700144. [DOI] [Google Scholar]
- 15.He J, Zheng W, Ligmajer F, Chan C-F, Bao Z, Wong K-L, Chen X, Hao J, Dai J, Yu S-F, Lei DY. Plasmonic enhancement and polarization dependence of nonlinear upconversion emissions from single gold nanorod@ SiO2@ CaF2: Yb3+, Er3+ hybrid core–shell–satellite nanostructures. Light Sci. Appl. 2017;6:16217–16217. doi: 10.1038/lsa.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu XF, Li M, Xie MY, Chen LD, Li Y, Wang QQ. Victims and vectors: Highly pathogenic avian influenza H5N1 and the ecology of wild birds. Nano Res. 2010;3:51–73. doi: 10.1007/s12274-010-1008-2. [DOI] [Google Scholar]
- 17.Zhang Q, Zhang QM. Synthesis and photoluminescent properties of α-NaYF4: Nd/α-NaYF4 core/shell nanostructure with enhanced near infrared (NIR) emission. Mater. Lett. 2009;63:376–378. doi: 10.1016/j.matlet.2008.10.064. [DOI] [Google Scholar]
- 18.Liu Y, Tu D, Zhu H, Chen X. Lanthanide-doped luminescent nanoprobes: Controlled synthesis, optical spectroscopy, and bioapplications. Chem. Soc. Rev. 2013;42:6924–6958. doi: 10.1039/c3cs60060b. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Li Y, Zhang Y, Gu H, Chen W. Rare earth compound nanowires: Synthesis, properties and applications. Rev. Nanosci. Nanotech. 2014;3:1–19. doi: 10.1166/rnn.2014.1041. [DOI] [Google Scholar]
- 20.Wang X, Wang Y, Bu Y, Yan X, Wang J, Cai P, Seo HJ. Influence of doping and excitation powers on optical thermometry in Yb3+-Er3+ doped CaWO4. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep43383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rakov N, Maciel GS. Three-photon upconversion and optical thermometry characterization of Er3+: Yb3+ co-doped yttrium silicate powders. Sens. Actuator. B. 2012;164:96–100. doi: 10.1016/j.snb.2012.01.070. [DOI] [Google Scholar]
- 22.Wang X, Liu Q, Cai P, Wang J, Qin L, Vu T, Seo HJ. Excitation powder dependent optical temperature behavior of Er3+ doped transparent Sr0.69 La0.31 F2.31 glass ceramics. Opt. Express. 2016;24:17792–17804. doi: 10.1364/OE.24.017792. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, Zhao H, Zhang Z, Cao W. highly sensitive optical thermometry through thermally enhanced near infrared emissions from Nd3+/Yb3+ codoped oxyfluoride glass ceramic. Sens. Actuator. B. 2013;178:520–524. doi: 10.1016/j.snb.2012.12.050. [DOI] [Google Scholar]
- 24.Li L, Guo C, Jiang S, Agrawal DK, Li T. Green up-conversion luminescence of Yb3+-Er3+ co-doped CaLa2 ZnO5 for optically temperature sensing. RSC Adv. 2014;4:6391–6396. doi: 10.1039/c3ra47264g. [DOI] [Google Scholar]
- 25.Wang X, Liu Q, Bu Y, Liu CS, Liu T, Yan X. Optical temperature sensing of rare-earth ion doped phosphors. RSC Adv. 2015;5:86219–86236. doi: 10.1039/C5RA16986K. [DOI] [Google Scholar]
- 26.Min Q, Bian W, Qi Y, Lu W, Yu X, Xu X, Qiu J. Temperature sensing based on the up-conversion emission of Tm3+ in a single KLuF4 microcrystal. J. Alloys Compd. 2017;728:1037–1042. doi: 10.1016/j.jallcom.2017.09.050. [DOI] [Google Scholar]
- 27.Qiang Q, Wang Y. Enhanced optical temperature sensing and upconversion emissions based on the Mn2+ codoped NaGdF4: Yb3+, Ho3+ nanophosphor. New J Chem. 2019;43:5011–5019. doi: 10.1039/C8NJ05079A. [DOI] [Google Scholar]
- 28.Dan HK, Zhou D, Wang R, Jiao Q, Yang Z, Song Z, Yu X, Qiu J. Effect of Mn2+ ions on the enhancement red upconversion emission of Mn2+/Er3+/Yb3+ tri-doped in transparent glass-ceramics. Opt Laser Technol. 2014;64:264–268. doi: 10.1016/j.optlastec.2014.05.002. [DOI] [Google Scholar]
- 29.Tian G, Gu ZJ, Zhou LJ, Yin WY, Liu XX, Yan L, Jin S, Ren WL, Xing GM, Li SJ, Zhao YL. Mn2+ dopant-controlled synthesis of NaYF4: Yb/Er upconversion nanoparticles for in vivo imaging and drug delivery. Adv. Mater. 2012;24:1226–1231. doi: 10.1002/adma.201104741. [DOI] [PubMed] [Google Scholar]
- 30.Ye S, Song EH, Zhang QY. Transition metal-involved photon upconversion. Adv. Sci. 2016;3:1600302. doi: 10.1002/advs.201600302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dan HK, Zhou DC, Wang RF, Jiao Q, Yang ZW, Song ZG, Yu X, Qiu JB. Effect of Mn2+ ions on the enhancement red upconversion emission of Mn2+/Er3+/Yb3+ tri-doped in transparent glass-ceramics. Opt. Laser Technol. 2014;64:264–268. doi: 10.1016/j.optlastec.2014.05.002. [DOI] [Google Scholar]
- 32.Zeng SJ, Yi ZG, Lu W, Qian C, Wang HB, Rao L, Zeng TM, Liu HR, Liu HJ, Fei B, Hao JH. Simultaneous realization of phase/size manipulation upconversion luminescence enhancement, and blood vessel imaging in multifunctional nanoprobes through transtion metal Mn2+ doping. Adv. Funct. Mater. 2014;24:4051–4059. doi: 10.1002/adfm.201304270. [DOI] [Google Scholar]
- 33.MacKenzie LE, Alvarez-Ruiz D, Pal R. Low-temperature open-air synthesis of PVP-coated NaYF4: Yb, Er, Mn upconversion nanoparticles with strong red emission. R. Soc. Open Sci. 2022;9:211508. doi: 10.1098/rsos.211508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Z, Gao H, Mao Y. Understanding the effect of Mn2+ on Yb3+/Er3+ upconversion and obtaining a maximum upconversion fluorescence enhancement in inert-core/active-shell/inert-shell structures. RSC Adv. 2016;6:83321–83327. doi: 10.1039/C6RA10969A. [DOI] [Google Scholar]
- 35.Gholizadeh M, Zarei MR, Mohammadi AA, Ehsani MH, Rezagholipour DH. "Design and fabrication of MgF2 single-layer antireflection coating by glancing angle deposition. Mater. Res. Inno. 2020;24:442–446. doi: 10.1080/14328917.2020.1723991. [DOI] [Google Scholar]
- 36.Qiang Q, Wang Y. Effect of Mn2+ on upconversion emission, thermal sensing and optical heater behavior of Yb3+ - Er3+ Codoped NaGdF4 nanophosphors. Front. Chem. 2019;7:425. doi: 10.3389/fchem.2019.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prorok K, Olk M, Skowicki M, Kowalczyk A, Kotulska A, Lipiński T, Bednarkiewicz A. Near-infrared excited luminescence and in vitro imaging of HeLa cells by using Mn2+ enhanced Tb3+ and Yb3+ cooperative upconversion in NaYF4 nanocrystals. Nanoscale Adv. 2019;1:3463–3473. doi: 10.1039/C9NA00336C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song E, Han X, Zhou Y, Wei Y, Jiang XF, Ye S, Zhou B, Xia Z, Zhang Q. Long-lived photon upconversion phosphorescence in RbCaF3: Mn2+, Yb3+ and the dynamic color separation effect. Iscience. 2019;19:597–606. doi: 10.1016/j.isci.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao G, Wondraczek L. Near-infrared down-conversion in Mn2+–Yb3+ co-doped Zn2 GeO4. J. Mater. Chem. C. 2013;1:1952–1958. doi: 10.1039/c3tc00803g. [DOI] [Google Scholar]
- 40.Nakazawa E, Shionoya S, Yen WM. Phosphor Handbook. CRC Press; 1999. p. 102. [Google Scholar]
- 41.Wang R, Yuan M, Zhang Ch, Wang H, Xu X. Tunable multicolor and enhanced red emission of monodisperse CaF2: Yb3+/Ho3+ microspheres via Mn2+ doping. Opt. Mater. 2018;79:403–407. doi: 10.1016/j.optmat.2018.03.048. [DOI] [Google Scholar]
- 42.Cheng C, Zeng N, Jiao Q, Zhang X, Liu X. Tunable upconversion white photoemission in Yb3+/Mn2+/Tm3+ tri-doped transparent glass ceramics. Opt. Mater. 2020;100:109718. doi: 10.1016/j.optmat.2020.109718. [DOI] [Google Scholar]
- 43.Bai Z, Lin H, Johnson J, Rong Gui SC, Imakita K, Montazami R, Fujii M, Hashemi N. The single-band red upconversion luminescence from morphology and size controllable Er3+/Yb3+ doped MnF2 nanostructures. J. Mater. Chem. C. 2014;2:1736–1741. doi: 10.1039/c3tc32143f. [DOI] [Google Scholar]
- 44.Zhang Y, Lin JD, Vijayaragavan V, Bhakoo KK, Tan TTY. Tuning sub-10 nm single-phase NaMnF3 nanocrystals as ultrasensitive hosts for pure intense fluorescence and excellent T1 magnetic resonance imaging. Chem. Commun. 2012;48:10322–10324. doi: 10.1039/c2cc34858f. [DOI] [PubMed] [Google Scholar]
- 45.Szczeszak A, Grzyb T, Nowaczyk G, Ekner-Grzyb A. Emission colour changes in the CaF2 sub-microspheres doped with Yb3+, Er3+ and Mn2+ ions. J. Alloys Compd. 2020;817:152718. doi: 10.1016/j.jallcom.2019.152718. [DOI] [Google Scholar]
- 46.Yuan M, Wang R, Zhang C, Yang Z, Yang X, Han K, Ye J, Wang H, Xu X. Revisiting the enhanced red upconversion emission from a single β-NaYF4:Yb/Er microcrystal by doping with Mn2+ Ions. Nanoscale Res. Lett. 2019;14:103. doi: 10.1186/s11671-019-2931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.