Summary
Methanogenesis allows methanogenic archaea to generate cellular energy for their growth while producing methane. Thermophilic hydrogenotrophic species of the genus Methanothermobacter have been recognized as robust biocatalysts for a circular carbon economy and are already applied in power-to-gas technology with biomethanation, which is a platform to store renewable energy and utilize captured carbon dioxide. Here, we generated curated genome-scale metabolic reconstructions for three Methanothermobacter strains and investigated differences in the growth performance of these same strains in chemostat bioreactor experiments with hydrogen and carbon dioxide or formate as substrates. Using an integrated systems biology approach, we identified differences in formate anabolism between the strains and revealed that formate anabolism influences the diversion of carbon between biomass and methane. This finding, together with the omics datasets and the metabolic models we generated, can be implemented for biotechnological applications of Methanothermobacter in power-to-gas technology, and as a perspective, for value-added chemical production.
Subject areas: Genetic engineering, Systems biology, Proteomics
Graphical abstract

Highlights
-
•
Curated genome-scale reconstructions for thermophilic methanogens are presented
-
•
Replicate chemostat bioreactors result in reproducible systems-biology datasets
-
•
Genetically engineered strain has stable performance in chemostat bioreactors
-
•
Formate anabolism is hypothesized to influence carbon distribution between products
Genetic engineering; Systems biology; Proteomics
Introduction
Solutions are needed to mitigate the devastating effects of greenhouse gas emissions, primarily carbon dioxide (CO2), and defossilize the energy and industrial sectors. Societies must efficiently implement: (1) renewable electric power to replace fossil sources; and (2) the use of the emitted CO2 as a feedstock for the production of commodities within a circular carbon economy. Power-to-gas technologies can convert excess renewable electric power into dioxygen (O2) and molecular hydrogen (H2) via water electrolysis. Thus, power-to-gas systems can provide H2 to store renewable electric energy but the hydrogen storage and transportation infrastructure is not well-established.1,2 Legal regulatory frameworks typically limit the injection of H2 into the established natural gas grid to under 10% v/v due to the different physical properties of H2 and natural gas, which result in limited infrastructure and material compatibility.1,3,4 Instead, methane (CH4), the main constituent of natural gas, can be injected into the existing natural gas grid infrastructure to replace fossil natural gas without limitations for storage, distribution, and consumption.5,6
In power-to-gas technology, CH4 can be derived from an additional methanation step in which the H2 is combined with CO2 to produce CH4 and water (Equation 1).
| (Equation 1) |
The methanation step in power-to-gas can be performed thermochemically via the hydrocarbon-forming Sabatier process at high temperatures (>200°C) and pressures (>1 MPa) with metal catalysts (e.g., iron, nickel, cobalt, ruthenium), which are sensitive to gas impurities.7,8,9,10 Alternatively, the methanation step can be performed biologically with microbes as biocatalysts. Different groups of hydrogenotrophic methanogenic archaea (methanogens) natively metabolize CO2 and H2, producing CH4 (Equation 1),6,9,11 and are therefore being investigated as biocatalysts for the methanation step in power-to-gas technology.12,13 Many methanogens exhibit low growth rates and biomass yields, which limits their potential for large-scale industrial processes. An exception to this are thermophilic methanogenic species of the genus Methanothermobacter, which readily grow in minimal salt media without any complex compounds. Their thermophilic growth temperature coupled with the exothermic methanogenic process results in lower temperature-control demands at industrial scales,14 explaining the success of Methanothermobacter as production strains in large-scale industrial processes.13,15,16,17 As an example, the company Electrochaea GmbH is creating an industrial-scale biotechnology platform with Methanothermobacter.
A systematic understanding of the biocatalyst’s metabolic capacities is required to advance this biotechnology platform further. Previous studies expanded knowledge on the core metabolism of the genus Methanothermobacter, which includes methanogenesis.18 Exemplary is an extensive comparative genome study between Methanothermobacter thermautotrophicus ΔH (formerly Methanobacterium thermoautotrophicum ΔH) and Methanothermobacter marburgensis Marburg (formerly Methanobacterium thermoautotrophicum Marburg).19 However, only limited studies have looked at Methanothermobacter strains systematically in chemostat bioreactors and at the transcriptomic or proteomic level.20,21,22,23,24,25,26 In fact, more than 500 hypothetical proteins and many pathways have not yet been resolved.27 Some strains, such as Methanothermobacter thermautotrophicus Z-245 (formerly Methanobacterium thermoformicicum Z-24518), can utilize formic acid (or the deprotonated form, formate) as a sole growth substrate (Equation 2) in addition to CO2 and H2 due to the presence of a catabolic formate dehydrogenase.23
| (Equation 2) |
Formic acid is derived from the (electro)chemical reduction of CO2 and has the advantage of being in the liquid phase. Therefore, in the context of a circular carbon economy, formic acid can serve as a potential intermediate storage molecule in converting CO2 into valuable chemicals, with formatotrophic (i.e., formic acid-utilizing) microbes as vital biocatalysts.28
A promising approach to systematically predict phenotypes and identify potential bottlenecks in microbial metabolisms is through mathematical analyses of their potential metabolic networks.29 Genome-scale network reconstructions represent the theoretical metabolic capacities of a microbe. A genome-scale metabolic model (GEM) is a mathematically constrained reconstruction. These mathematical models enable in-silico investigations, including investigating the effects of gene deletions and insertions for metabolic engineering purposes. Thousands of reconstructions and GEMs have been assembled across all three domains of life; however, archaea remain underrepresented. Only 127 of 6239 (2%) are archaeal GEMs.30 Of those, only ten models have been manually curated, which is a procedure that leads to higher-quality models.30 The applications of GEMs and related models have been extensively reviewed, covering methods that are based on GEMs from flux balance analysis to kinetic modeling and machine learning.31,32,33,34,35,36
To further optimize methanogens for large-scale applications, metabolic engineering of these biocatalysts and an in-depth understanding of their metabolism is required. However, well-developed genetic tools exist for only a couple of mesophilic methanogens, namely different species of the genus Methanosarcina and Methanococcus maripaludis.37,38 In previous work, we generated a genetic system for M. thermautotrophicus ΔH,39,40 which allows genetically modifying this strain. To use Methanothermobacter strains in more versatile biotechnological applications, such as chemical production, it is necessary to further expand the available systems biology datasets and methodologies. Here, we conduct a multi-disciplinary systematic approach to assess differences in three species of the genus Methanothermobacter: M. thermautotrophicus ΔH, M. marburgensis Marburg, and M. thermautotrophicus Z-245. With this approach, we reveal differences in the formate metabolism of closely related strains, including a genetically engineered strain.40 We observed that these differences alter the diversion of carbon between biomass and CH4 production in the three strains. Our observations could, in future work, be exploited to implement metabolic engineering strategies for chemical production with species of the genus Methanothermobacter.
Results
Updated genome sequences provide the basis for high-quality genome-scale metabolic reconstructions
De-novo sequencing and assembly of the genomes of M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg with long-read sequencing technology provided high-quality sequences (first release of the M. thermautotrophicus Z-245 genome sequence; Table 1; Data S1 - Table S1; STAR Methods). Our sequencing approach allowed us to deduce the specific methylation pattern of the three strains, which could be important for future genetic-engineering efforts (supplemental information - Note S1; Data S1 - Table S2). In our genome annotation, we assigned COG functional categories to 1501/1796, 1505/1804, and 1440/1730 genes for M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg, respectively (Data S1 - Table S3). We also highlighted differences to the old annotations (supplemental information - Note S2; Data S1 - Table S4; STAR Methods).
Table 1.
Summary of sequenced genomes
| Strain | Plasmid | Length (bps) | GC % | Gene Count | CDS | Previous Sequence Accessions | Difference with Old Sequence | Similarities with Old Sequence |
|---|---|---|---|---|---|---|---|---|
| M. thermautotrophicus ΔH | N/A | 1,751,429 | 49.56 | 1844 | 1796 |
NC_000916.1 Smith et al.62 |
448 SNa 247 IBb 91 O2Nc 92 N2Od |
1733 CDS |
| M. thermautotrophicus Z-245 | pFZ1 | 1,758,798 11,015e | 49.46 42.51e |
1840 12e |
1792 12e |
GCA_013330715.1f Rinke et al.63 | N/A | N/A |
| M. marburgensis Marburg | pM2001 (pMTBMA4) | 1,634,705 4,441e |
48.65 45.40e |
1774 5e |
1725 5e |
NC_014408.1 Liesegang et al.64 | 68 SN 30 IB 25 O2N 66 N2O |
1675 CDS |
Based on the updated genome sequences, we constructed a pan-genome-scale metabolic reconstruction (pan-model) that integrates the metabolic capabilities of all three strains (STAR Methods). The pan-model reflects 29.3% (526/1796, M. thermautotrophicus ΔH), 28.8% (520/1804, M. thermautotrophicus Z-245), and 29.4% (509/1730, M. marburgensis Marburg) of the protein-coding genes. Therefore, our pan-model is comparable to published methanogen reconstructions.41 Based on the pan-model, we derived strain-specific GEMs for each of the three strains (iMTD22IC, iMTZ22IC, iMMM22IC), which we used for further modeling to explain observed differences between the three strains as described below (Data S3).
Chemostat bioreactors reveal differences in CH4 and biomass production rates
We operated chemostat bioreactors with the three Methanothermobacter strains and analyzed the generated data to compare their metabolism under steady-state growth conditions (Figures 1 and S1A; STAR Methods). Compared to both M. thermautotrophicus Z-245 and M. marburgensis Marburg, M. thermautotrophicus ΔH had a significantly higher consumption rate for H2 (1.59-fold and 1.72-fold, respectively) and CO2 (1.69-fold and 1.72-fold, respectively), as well as a higher rate of CH4 production (1.62-fold and 1.82-fold; Figures 1A and 1B; Table 2; Data S1 - Table S5; Data S3; STAR Methods). However, M. marburgensis Marburg had a significantly higher biomass concentration (1.36-fold) than the other two strains (Figures 1A and 1B; Table 2; Data S1 - Table S5; Data S3). We assured that this difference was not due to an air intrusion (that led to higher O2 levels) into one of the M. marburgensis Marburg bioreactors (supplemental information - Note S3; Data S3; Figure S2). The divergence resulted in differences in the normalized distribution of products, with the highest CH4-to-biomass ratio of 96.4 ± 0.57 for M. thermautotrophicus ΔH and the lowest of 93.5 ± 0.98 for M. marburgensis Marburg (Figure 1C).
Figure 1.
Fermentation data from the first experiment in chemostat bioreactors with M. thermautotrophicus ΔH (ΔH), M. thermautotrophicus Z-245 (ZZ), and M. marburgensis Marburg (MM)
(A) Gas consumption (H2 and CO2 uptake), and CH4 and biomass production data from quadruplicate (N = 4, ΔH and MM) and triplicate (N = 3, ZZ) bioreactors for the fermentation period of 7 days (mean ±1 standard deviation). For ZZ, we discarded one replicate due to a pump malfunction in this bioreactor and wash-out of the cells. Data for further analyses (transcriptomics, proteomics) were taken on day seven, as indicated by arrows.
(B) Average gas consumption (H2 and CO2 uptake) and CH4 and biomass production data during a steady-state period (days 4–7). For statistical analysis in pairwise comparisons with t-test, data points without suspected gross measurement error (red circles) were included, data points with suspected gross measurement error (gray circles) were excluded (STAR Methods).
(C) Average normalized product distribution (mean ±1 standard deviation), including statistical analysis by ANOVA (n = 11 (ΔH), n = 10 (ZZ), n = 16 (MM); STAR Methods). ΔH, M. thermautotrophicus ΔH; ZZ, M. thermautotrophicus Z-245; MM, M. marburgensis Marburg: F, F value; Fcrit, F critical value.
Table 2.
Summary of the gas fermentation data for the four strains used in the first and second chemostat bioreactor experiments
| First Fermentation Experiment – Period 2 (H2 and CO2)a | |||
|---|---|---|---|
| ΔHb | ZZc | MMd | |
| Biomass (gCDW) | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.01 |
| H2 Uptake Rates (mmol/gCDW/h) | 234.36 ± 40.76 | 147.24 ± 23.88 | 136.12 ± 12.00 |
| CO2 Uptake Rates (mmol/gCDW/h) | 58.18 ± 11.49 | 34.36 ± 6.98 | 33.80 ± 3.78 |
| CH4 Production Rates (mmol/gCDW/h) | 52.50 ± 8.53 | 32.34 ± 3.92 | 28.83 ± 4.93 |
| Second Fermentation Experiment (H2 and CO2 or Formate)e | |||
|---|---|---|---|
| ΔHHf | ΔHFg | ZZFh | |
| Biomass (gCDW) | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.02 |
| H2 Uptake Rates (mmol/gCDW/h) | 127.31 ± 28.18 | N/A | N/A |
| H2 Production Rates (mmol/gCDW/h) | N/A | 0.84 ± 0.34 | 0.55 ± 0.28 |
| CO2 Uptake Rates (mmol/gCDW/h) | 29.16 ± 3.91 | N/A | N/A |
| CO2 Production Rates (mmol/gCDW/h) | N/A | 31.87 ± 11.12 | 34.29 ± 9.77 |
| CH4 Production Rates (mmol/gCDW/h) | 30.53 ± 3.97 | 9.69 ± 3.62 | 10.55 ± 3.18 |
The standard deviation (±) was calculated based on the entire population.
Values can be found in Data S3 and represent mean values and standard deviations calculated from values without gross measurement errors (STAR Methods).
ΔH, M. thermautotrophicus ΔH.
ZZ, M. thermautotrophicus Z-245.
MM, M. marburgensis Marburg.
Values can be found in Data S6 and represent mean values and standard deviations calculated from values without gross measurement errors (STAR Methods).
ΔHH, M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 grown on H2 and CO2.
ΔHF, M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 grown on Na-formate.
ZZF, M. thermautotrophicus Z-245 grown on Na-formate.
Multi-omics analysis under steady-state bioreactor growth conditions reveals different gene-expression patterns
To clarify why the three closely related strains displayed significant differences in the growth behavior under identical growth conditions (Figure 1), we performed transcriptomic and proteomic analyses during steady state for all replicate bioreactors (Data S4; STAR Methods). For each strain, we obtained highly reproducible transcriptomic and proteomic data across replicates as well as a high coverage of the transcriptome (94.18–99.77%) and proteome (78.29–79.82%) (Table 3 Individual analyses).
Table 3.
Metrics of the transcriptomics and proteomics for the individual analyses and the differential expression and differential abundance analyses for the three strains
| Omics | Metric | ΔHa | ZZb | MMc |
|---|---|---|---|---|
| Individual analyses | ||||
| Transcriptomics | Million reads | 26.3 (±2.0) | 27.4 (±0.8) | 26.0 (±1.1) |
| Number of CDSs found (%) | 1697/1796 (94.48%) | 1699/1804 (94.18%) | 1726/1730 (99.77%) | |
| Average CVdof all genes | 35.03 | 33.07 | 26.60 | |
| Percent of genes with CVdvalues ≤ 20% | 44.12 | 39.61 | 26.83 | |
| Proteomics | Number of proteins found (%) | 1406/1796 (78.29%) | 1440/1804 (79.82%) | 1374/1730 (79.42%) |
| Average CVeof all proteins | 11.37 | 18.73 | 15.51 | |
| Percent of proteins with CVevalues ≤ 20%8 | 88.35 | 66.48 | 77.72 | |
| Omics | Metric | MM vs. ΔH | ZZ vs. ΔH | ZZ vs. MM |
|---|---|---|---|---|
| Differential analyses | ||||
| Transcriptomics | DEfgenes (range of log2FC) | 1482 (−13.1 to 17.8) | 816 (−17.0 to 13.8) | 1498 (−14.1 to 16.7) |
| DE genes with |log2FC| ≥ 2 | 975 | 265 | 1001 | |
| DE genes that map to a reaction in the GEM | 469 | 249 | 484 | |
| Proteomics | DAgproteins (range of log2FC) | 121 (−6.6 to 6.6) | 91 (−6.6 to 6.6) | 93 (−6.0 to 5.9) |
| DA proteins with |log2FC| ≥ 2 | 103 | 59 | 35 | |
| DA proteins that map to a reaction in the GEM | 43 | 17 | 38 | |
| Transcriptomics + Proteomics | Fraction of DA proteins that were also DE genes | 110/121 (91%) | 45/91 (50%) | 90/102 (88%) |
Statistical significance was determined by an adjusted p-value ≤0.05. The standard deviation (±) was calculated based on the entire population.
ΔH, M. thermautotrophicus ΔH.
ZZ, M. thermautotrophicus Z-245.
MM, M. marburgensis Marburg.
Coefficient of variance (CV) values for the transcriptomics data were calculated using transcripts per million (TPM) values and can be found in Sheets 1–3 of Data S4 and Figure S6B.
Coefficient of variance (CV) values for the proteomics data were calculated using iBAQ values and can be found in Sheets 7–9 of Data S4 and Figure S6B.
DE, differential expression.
DA, differential abundance.
To compare the omics datasets between strains, we merged the genome-derived transcriptome and proteome of the three strains into pan-transcriptome and pan-proteome databases (creating reference genes, termed “gene groups,” Data S4; STAR Methods). Merging allowed us to perform differential expression analyses. We found many differentially expressed genes in the pan-transcriptome but much fewer differentially abundant proteins in the pan-proteome comparison, and in both omics sets, fewer differences between M. thermautotrophicus ΔH and M. thermautotrophicus Z-245 than to M. marburgensis Marburg, all of which is consistent with their phylogeny (Figures S3C–S3G; Table 3 – Differential analyses; supplemental information - Note S4; Data S1 - Table S6; Data S4).18 Because the quality of both the transcriptomic and proteomic data was high (supplemental information - Note S4), we hypothesized that each strain may have evolved different transcriptional regulation patterns leading to a very similar outcome on the proteome level to maximize growth under thermodynamically limited conditions.
A closer look at the differential expression patterns of methanogenesis – the core metabolic feature of methanogens – did not provide any clear explanation for the observed differences in CH4 production between the strains (supplemental information - Note S5). Next, we investigated reactions that branch off from methanogenesis toward biomass growth and further downstream in the anabolism but still did not find explanations for the observed differences in growth behavior (supplemental information - Note S6). Thus, we integrated the omics and fermentation data into the GEMs to better understand these differences.
Integration of experimental data into GEMs results in solutions for flux balance analyses
We constrained the GEMs in flux balance analysis simulations with the steady-state CO2 and H2 consumption rates and CH4 and biomass production rates from the bioreactor experiment; these rates were corrected for experimental errors to obtain gross-measurement-error-adjusted values (Data S5 [Simulations 25–28, 37–40, and 49–52]; STAR Methods). In these simulations, the GEMs found solutions with a 0.1%, 0.1%, and 0.5% deviation of the reaction flux bounds from the calculated fluxes for M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg, respectively (Data S5). The maximum fluxes of the non-growth-associated maintenance energy (reaction ATPM) varied between the three strains (Data S1 - Table S7).
We further constrained the GEMs with transcriptomics and proteomics using the GIMME algorithm,42 which resulted in reduced GEMs. We performed a set of flux balance analysis simulations comparing the unconstrained and reduced GEMs (Data S5 [transcriptomics: Simulations 29–32, 41–44, and 53–56; proteomics: Simulations 33–36, 37–40, and 57–60]; supplemental information - Note S7; STAR Methods). This led us to detect differences in the formate anabolism pathway among the three strains, as detailed below.
Flux balance analyses reveal differences in formate anabolism
Among the three investigated Methanothermobacter strains, only M. thermautotrophicus Z-245 is able to use formate for methanogenesis, and thus as the sole growth substrate.23 However, in all three Methanothermobacter strains, formate is a crucial metabolite for anabolism and has to be synthesized during growth with CO2 and H2.43,44,45 This is because formate is an important intermediate for de-novo purine biosynthesis.19,46
Formate for biomass could result from the activity of a formate dehydrogenase (Fdh), which, in the case of methanogens, interconverts CO2 into formate with concomitant reduction of cofactor F420.47 The use of formate as a growth substrate in M. thermautotrophicus Z-245 depends on the presence of a Fdh catalyzing the reverse reaction.23 Although M. thermautotrophicus ΔH encodes an Fdh (MTH1140/ISG35_05415 and MTH1139/ISG35_05410, gene groups 967 and 968; Data S4), this strain is not able to utilize formate as a growth substrate.18 Instead, the Fdh of gene groups 967 and 968 was previously hypothesized to be the relevant enzyme for the synthesis of formate for biomass growth.19 In spite of this, reducing the GEM with proteomic data eliminated the corresponding FDHf420 reaction in the flux balance analysis simulation because Fdh was not detected via proteomics (Figure 2; Data S5).
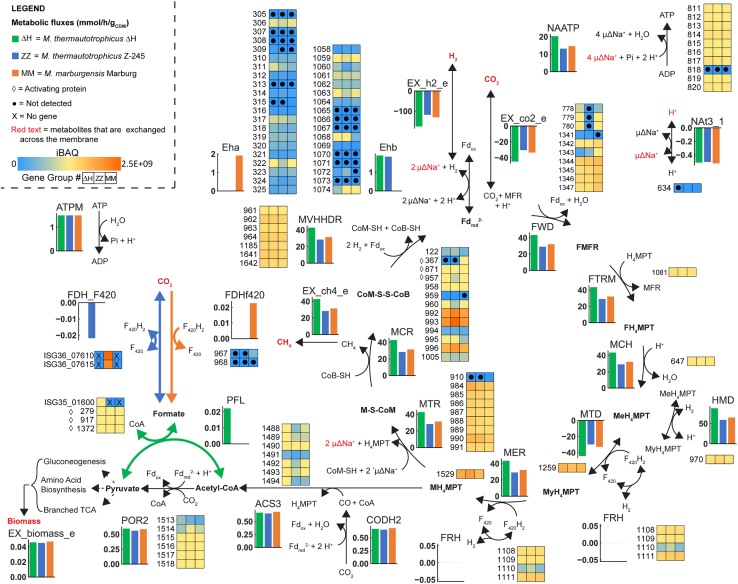
Figure 2.
Wolfe Cycle adapted from Thauer61 with reactions involved in the energy metabolism
Fluxes are from the proteomics reduced model (using intensity based absolute quantification (iBAQ) values), constrained with experimental data that was adjusted for gross measurement error. iBAQ values are given for proteins. Gene group is used as ID for the omics. For the PFL reaction, only M. thermautotrophicus ΔH has the gene; thus, the gene ID is used (ISG35_01600). For the FDH_F420, only M. thermautotrophicus Z-245 has the formate dehydrogenase cassette; thus, the gene IDs are used (ISG36_07610 and ISG36_07615). Red text refers to metabolites that are exchanged across the membrane. Microbes: ΔH, M. thermautotrophicus ΔH; MM, M. marburgensis Marburg; ZZ, M. thermautotrophicus Z-245. Compounds: CH4, methane; CO, carbon monoxide; CoA, Coenzyme A; CoB, coenzyme B; CoM, coenzyme M; CoM-S-S-CoB, CoM-CoB heterodisulfide; CO2, carbon dioxide; F, formyl; Fdox/rd, ferredoxin oxidized/reduced; H2, hydrogen; H+, proton; H4MPT, tetrahydromethanopterin; M, methyl; Me, methenyl; MFR, methanofuran; My, methylene; Na+, sodium ion. Reactions/Enzymes: ATPM, ATP maintenance (pseudo reaction); CODHr2, CO dehydrogenase/acetyl-CoA synthase; Eha/Ehb, energy converting hydrogenases; EX_biomass_e, biomass exchange (pseudo reaction); EX_ch4_e, CH4 exchange (pseudo reaction); EX_co2_e, CO2 exchange (pseudo reaction); EX_h2_e, H2 exchange (pseudo reaction); FDHf420, F420-dependent formate dehydrogenase; FDH_F420, F420-dependent formate dehydrogenase cassette; FRH, F420-reducing hydrogenase; FTRM, FMFR/H4MPT formyltransferase; FWD, FMFR dehydrogenase (tungsten- and molybdenum-dependent isozymes); HMD, MeH4MPT hydrogenase; MCH, MeH4MPT cyclohydrolase; MCR, MCoM reductase (I and II); MER, MyH4MPT reductase; MTD, MyH4MPT dehydrogenase; MTR, MH4MPT/CoM methyltransferase; MVHHDR, F420-non-reducing hydrogenase with the heterodisulfide reductase; NAATP, ATP synthase; Nat3_1, Na+/H+ antiporter; PFL, pyruvate formate-lyase; POR2, pyruvate synthase. Other: ◊, activating protein; •, not detected; X, no gene. For an extended figure see Figure S4.
In contrast, the model constrained with the proteomic data suggested a formate acetyltransferase/pyruvate-formate lyase (Pfl) (PFL reaction) as the preferred enzyme for the production of formate (Figure 2; Data S5) in M. thermautotrophicus ΔH (MTH346/ISG35_1600). This enzyme catalyzes the CoA-dependent reversible conversion of pyruvate to formate and was previously identified in M. thermautotrophicus ΔH.19,48 This observation implied that M. thermautotrophicus ΔH primarily produces formate for biomass growth via Pfl. This hypothesis will need to be confirmed experimentally to corroborate the proteomics-derived evidence for the lack of the Fdh enzyme.
Neither the genome of M. marburgensis Marburg19,48 nor M. thermautotrophicus Z-245 encodes a Pfl (supplemental information - Note S8). However, in contrast to both M. thermautotrophicus ΔH and M. marburgensis Marburg, M. thermautotrophicus Z-245 has two Fdhs. The first Fdh in M. thermautotrophicus Z-245 is homologous to the Fdh found in M. thermautotrophicus ΔH (gene groups 967 and 968). The second Fdh is encoded in the operon fdhCAB (i.e., fdhcassette, ISG36_07620, ISG36_07615, ISG36_07610). Importantly, the operon includes an additional putative formate transporter (FdhC), and was previously reported to be responsible for the utilization of formate as the sole growth substrate in M. thermautotrophicus Z-245.23 Accordingly, the expression of the Fdhcassette is regulated by the relative abundance of substrates, namely, formate, CO2 and H2.23
Based on these previous reports and because we fed the bioreactors with H2, we had anticipated that the Fdhcassette would not be expressed (or found at low abundance) in our conditions. Unexpectedly, we found high absolute levels of the Fdhcassette in our transcriptome and proteome (Figures 2 and S4; Data S2). By contrast, the first Fdh was less abundant in our transcriptome and not detected in the proteome (Data S2). This indicated that in the presence of H2 and CO2 as sole growth substrates M. thermautotrophicus Z-245 uses the Fdhcassette to produce formate from H2 and CO2 for biomass growth. Indeed, our flux balance analysis simulations with M. thermautotrophicus Z-245 primarily predicted the use of the FDH_F420 (Fdhcassette) in the direction of formate production rather than FDHf420 (the aforementioned first Fdh) (Figures 2 and S4; Data S5). Future research will need to confirm the absence of the first Fdh and activity of the Fdhcassette in the direction of formate production in vivo.
While the genome of M. marburgensis Marburg also encodes the first Fdh (MTBMA_c15220/ISG34_07515 and MTBMA_c15230/ISG34_07520, gene groups 967 and 968), it neither encodes the Pfl nor the Fdhcassette (Data S4). Thus, formate for biomass production is likely only produced from the first Fdh (gene groups 967 and 968). This was also hypothesized previously, and this hypothesis was supported by a formate-auxotrophic strain of M. marburgensis Marburg, which did not exhibit Fdh activity anymore.49 All flux balance analysis simulations (including the omics-constrained ones) predicted the occurrence of the first Fdh reaction, that is the FDHf420 reaction (Data S5). The two subunits were upregulated in the transcriptome by 2.4–4.9 log2 fold change compared to the other two strains (Figure S4; Data S4). However, we found only one subunit (FdhA) in the proteome with low abundance (gene group 967: 0.000132%). This was three orders of magnitude lower than the homologous subunit of the Fdhcassette in M. thermautotrophicus Z-245 (FdhB: 0.254%, FdhA: 0.191%, FdhC: 0.126%) (Figure 2; Data S4). These findings raise questions about how M. marburgensis Marburg produced formate for biomass growth as discussed below.
Genetically engineered M. thermautotrophicus ΔH can stably grow on formate in chemostat bioreactors
The finding that formate anabolism might be distinct in all three strains was unexpected and we set out to further assess formate metabolism with a second chemostat bioreactor experiment. We investigated the growth behavior of a genetically engineered M. thermautotrophicus ΔH strain encoding the Fdhcassette from M. thermautotrophicus Z-245 on a plasmid (M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245).40 M. thermautotrophicus Z-245 is able to use formate as the sole growth substrate via the Fdhcassette reaction40 but presumably also produces formate for biomass growth by the reverse activity of the Fdhcassette. Instead, M. thermautotrophicus ΔH uses Pfl for this purpose, as indicated by our omics data. Here we operated chemostat bioreactors that were fed with 355 ± 5 mM sodium formate instead of H2 and CO2 with M. thermautotrophicus Z-245 and M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 (Figure 3A; STAR Methods). Additionally, for M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245, we operated also bioreactors with H2 and CO2 to allow a comparison to the first chemostat bioreactor experiment (Table 2; Figures 3A and S1B; STAR Methods). With sodium formate, these two strains neither showed significant differences in sodium formate uptake rates nor in CH4, CO2, and biomass production rates (Table 2; Figure 3B; Data S6), confirming that the episomal expression of the Fdhcassette enables M. thermautotrophicus ΔH to grow on formate as M. thermautotrophicus Z-245 does. During growth on H2 and CO2, the M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 exhibited a growth behavior more similar to M. thermautotrophicus Z-245 and M. marburgensis Marburg compared to wild-type M. thermautotrophicus ΔH (Table 2; Figure S5). These results indicate that, under the tested fermentation conditions, the reverse activity of the Fdhcassette enables M. thermautotrophicus ΔH to more efficiently form formate for biomass growth than by the sole activity of Pfl. The differences in growth behavior between the genetically engineered M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 strain and the wild-type M. thermautotrophicus ΔH might have implications for biotechnological applications, as discussed below. Notably, the plasmid was maintained in the M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 culture in the absence of antibiotics (Figures S1B and S1C). While we cannot completely rule out that only a subpopulation of the modified strain maintained the plasmid without antibiotic selection, this finding further supports the applicability of the genetic system for bioprocessing.
Figure 3.
Fermentation data from the second experiment in chemostat bioreactors with M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 (ΔH) and M. thermautotrophicus Z-245 (ZZ)
(A) Gas consumption (H2 and CO2 uptake) or sodium formate consumption, and CH4 and biomass production data from quadruplicate bioreactors for the fermentation period of 11 (ΔHH) and 17 (ΔHF and ZZF) days (mean ±1 standard deviation). Formate uptake rates were only determined once steady state was reached and all formate provided was consumed.
(B) Average sodium formate consumption and H2, CO2, CH4, and biomass production data during the steady-state period (days 13–17) for ΔHF (N = 3) and ZZF (N = 4). For statistical analysis in pairwise comparisons with t-test, data points without suspected gross measurement error (red circles) were included, and data points with suspected gross measurement error (gray circles) were excluded (STAR Methods).
(C) Average normalized product distribution (mean ±1 standard deviation), including a statistical analysis by ANOVA for ΔHF (N = 3) and ZZF (N = 4) (STAR Methods). CH4, methane; CO2, carbon dioxide; H2, molecular hydrogen; ΔH, M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245; ZZ, M. thermautotrophicus Z-245; F, sodium formate as substrate; H, H2 and CO2 as substrates; F, F value; Fcrit, F critical value.
Discussion
Although methanogenesis is well studied, there are still knowledge gaps surrounding methanogenic metabolism. In particular, understanding the metabolic strengths and limitations of different strains is necessary to select the most appropriate strain for a given biotechnological application, such as optimization of CH4 production or engineering strains to produce value-added products. In this study, we systematically compared M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg to identify differences in their metabolism.
Under the conditions of our bioreactor experiments, M. thermautotrophicus ΔH had a higher specific CH4 production rate than the other strains, while M. marburgensis Marburg reached higher biomass production rates. From our modeling results (with the maximization of the ATPM reaction), we deduced that M. thermautotrophicus ΔH has the highest and M. marburgensis Marburg has the lowest non-growth-associated maintenance energy (Data S1 - Table S7). The non-growth-associated maintenance energy represents the dissipation of ATP (as no storage compounds are known).50 The higher the non-growth-associated maintenance energy, the more CH4 (and ATP) must be produced per biomass unit. Based on our multi-omics analysis we propose that differences in anabolism could explain this observation, while further investigations are required to confirm this hypothesis. Our data indicate that the three Methanothermobacter strains might use three different enzymes to produce formate for biomass growth, such as for purine biosynthesis.43,44,45 From an ecological perspective, the observed differences in formate anabolism might have an impact on the adaptation to different ecological niches in which, for example, a higher CH4-production rate over biomass yield would provide a selective benefit to M. thermautotrophicus ΔH. Furthermore, as ATP is the primary energy currency in the cell, these findings are important to optimize the use of Methanothermobacter strains as cell factories by metabolic engineering in the future.51
Intriguingly, while M. marburgensis Marburg reached higher biomass production rates (Figure 1C), none of the putative enzymes for formate anabolism were abundant in our proteomics analyses. If the low-abundant Fdh is responsible for formate production in M. marburgensis Marburg, questions on the kinetic properties of this enzyme remain. Alternatively, formate could be produced via other, yet unconsidered pathways, for example, through a hydrogen-dependent CO2 reductase (HDCR)-like activity.52 While formate is not an intermediate in CO2 reduction during methanogenesis, a weak formate dehydrogenase activity has been ascribed to the formylmethanofuran dehydrogenases (Fmd/Fwd).53 Recently, a large enzyme complex was shown to be involved in the electron-bifurcating step of CO2 reduction without the release of ferredoxin.54 This enzyme complex may provide the formate that is required for anabolism by an apparent HDCR-like activity.
Understanding the metabolism of a microbe is especially important to guide its rewiring for biotechnological purposes. Here, we demonstrated the long-term ability of the strain M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245, encoding the catabolic Fdhcassette from M. thermautotrophicus Z-245 on a plasmid, to grow on formate in chemostat bioreactors. Introducing the Fdhcassette into M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 resulted in a growth behavior that is more similar to wildtype M. thermautotrophicus Z-245 than to M. thermautotrophicus ΔH. This finding supports our hypothesis that, indeed, the different solutions for the three methanogens to produce formate in anabolism have an impact on the CH4-to-biomass ratios. The CH4-to-biomass ratio is an essential parameter in bioprocessing (it should be high for CH4 production but low for chemical production). These observations have implications to design strategies for the redirection of carbon from CH4 to other high-value products with metabolic engineering, although further investigation is needed (e.g., with biochemical investigations of the enzyme systems). The ability to combine the GEM with the genetic system will be a considerable step toward chemical production with thermophilic methanogens because the use of validated GEMs will enable phenotypes to be predicted. In summary, our combined methods lay the foundation for archaeal biotechnology to optimize existing power-to-gas processes or enable the production of value-added chemicals by metabolic engineering of microbes of the genus Methanothermobacter.
Limitations of the study
Based on our findings, M. thermautotrophicus ΔH appears the most suitable among the three investigated strains for industrially relevant power-to-gas systems due to its superior kinetics and CH4 specificity. However, it is essential to consider the fermentation conditions of our study. We applied a volume gas per volume bioreactor per minute (vvm) of 0.08, while others used a much higher vvm of up to 2.01.13 It must be further noted that the composition of the growth medium was not optimized for any of the three strains, to avoid a bias toward one of the strains. Whether the superior behavior of M. thermautotrophicus ΔH would hold at the higher vvm and with strain-optimized specific media was not evaluated here and should be considered.
Our modeling approach is based on assumptions of steady state and considers static fluxes in the metabolism. Many additional factors need to be considered, such as enzyme kinetics and reaction capacities, which may result in bottlenecks in certain pathways. Those considerations were not included in the modeling approach that we applied here, in part due to limited data on enzyme kinetics for the Methanothermobacter strains. However, our manually curated genome-scale reconstruction is the prerequisite for follow-up modeling approaches including but not limited to: (1) integrating transcriptomics and proteomics with alternative algorithms and methods, such as iMAT55,56 or GIMMEp;57 (2) model constraining with additional types of data, such as enzyme kinetic data (e.g., kinetome58) and 13C fluxomics;59 and (3) generating superimposed models, such as metabolic and expression models (ME-models).60
Our multi-disciplinary systematic approach led us to hypothesize that the three investigated Methanothermobacter strains produce formate for biomass growth in distinct ways. By including the genetically engineered M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 strain, we further support this hypothesis. However, future experiments will need to confirm our observations and hypotheses, for example, by measuring the involved intracellular metabolites or biochemically assaying the enzymatic reactions.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| Methanothermobacter thermautotrophicus ΔH | DSMZ | DSM 1053 |
| Methanothermobacter thermautotrophicus Z-245 | DSMZ | DSM 3720 |
| Methanothermobacter marburgensis Marburg | DSMZ | DSM 2133 |
| Methanothermobacter thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 | Bastian Molitor, Fink et al.40 | https://doi.org/10.1128/mBio.02766-21 |
| Chemicals, peptides, and recombinant proteins | ||
| Anti Foam SE-15 | Sigma-Aldrich | A8582 |
| ROTI®Phenol/Chloroform/Isoamyl alcohol | Carl Roth | Cat# A156.2 |
| Vacuum grease Dow Corning® | VWR International GmbH | Cat# 44224.KT |
| Chloroform/Isoamyl alcohol (24:1) | VWR International GmbH | Cat# SERA39554.01 |
| SYBR™ Safe DNA Gel Stain | Thermo Fischer Scientific | Cat# S33102 |
| RNAlater™ Stabilization Solution | Thermo Fischer Scientific | Cat# AM7021 |
| Critical commercial assays | ||
| Genomic DNA 165 kb Kit | Agilent Technologies | Cat# FP-1002-0275 |
| Qubit® dsDNA HS Assay Kit | Thermo Fischer Scientific | Cat# Q32851 |
| SMRTbell® Express Template Preparation Kit | Pacific Biosciences | PN 101-397-100 Version 3 |
| Thermo Scientific™ Phire Plant Direct PCR Master Mix | Thermo Fischer Scientific | Cat# F160S |
| RNA Clean & Concentrator Kit | Zymo Research | Cat# R1019 |
| Agilent RNA 6000 Nano Kit | Agilent Technologies | Cat# 5067-1511 |
| TruSeq™ Stranded Total RNA Kit | Illumina | Cat# 20020597 |
| Illumina Ribo-Zero Plus rRNA Depletion Kit | Illumina | Cat# 20037135 |
| Deposited data | ||
| Supplemental data sets Data S1, S3–S7 | This manuscript | https://github.com/isacasini/Casini_GEM_2023 |
| Raw and analyzed genome sequencing data | This manuscript | NCBI Bioproject: PRJNA674001 |
| Raw and analyzed RNA sequencing data | This manuscript | GEO: GSE218145 |
| Raw and analyzed Proteomics data | This manuscript | PRIDE: PXD039574 |
| GEM modeling related files M. thermautotrophicus ΔH | This manuscript | Biomodels: MODEL2211290001 |
| GEM modeling related files M. thermautotrophicus Z-245 | This manuscript | Biomodels: MODEL2211290002 |
| GEM modeling related files M. marburgensis Marburg | This manuscript | Biomodels: MODEL2211290003 |
| Experimental models: Organisms/strains | ||
| M. thermautotrophicus ΔH genome sequence | NCBI, Smith et al.62 | NC_000916.1 |
| M. marburgensis Marburg genome sequence | NCBI, Liesegang et al.64 | NC_014408.1 |
| Oligonucleotides | ||
| Mth_RS05590_selec_FW2_opt | This manuscript | M. thermautotrophicus ΔH Forward |
| Mth_RS05590_selec_RV_2_opt | This manuscript | M. thermautotrophicus ΔH Reverse |
| Seq_specific_MtZ_pFZ1_FW | This manuscript | M. thermautotrophicus Z-245 Forward |
| Seq_specific_MtZ_pFZ1_RV | This manuscript | M. thermautotrophicus Z-245 Reverse |
| Primer_pME2001_XhoI_FW | Bastian Molitor, Fink et al.40 | M. marburgensis Marburg Forward |
| pME2001_Rev_1440_1000Xho | Bastian Molitor, Fink et al.40 | M. marburgensis Marburg Reverse |
| Software and algorithms | ||
| Canu Versions 1.7 & 2.0 | Koren et al.115 |
https://github.com/marbl/canu; RRID: SCR_015880 |
| Smrttools Version 9.0.0.92188 | Pacific Biosciences | https://downloads.pacbcloud.com/public/software/installers/smrtlink_9.0.0.92188.zip |
| Samtools Versions 1.6 & 1.11 | Li et al.116,Danecek et al.117 | http://www.htslib.org/ |
| Blast via Genome Workbench 3.5 | Kuznetsov and Bollin118 | https://ftp.ncbi.nlm.nih.gov/toolbox/gbench/ver-3.5.0/ |
| Biopython Version 1.77 | Cock et al.120 | http://biopython.org/DIST/biopython-1.77.zip |
| MotifMaker Version 0.3.1 | Li et al.119 | https://github.com/bioinfomaticsCSU/MultiMotifMaker |
| Smrtlink Version 10.2.0.133434 | Pacific Biosciences | https://downloads.pacbcloud.com/public/software/installers/smrtlink_10.2.1.143962.zip |
| BaseModFunction.v2.1.R | Pacific Biosciences | https://github.com/PacificBiosciences/Bioinformatics-Training/tree/master/basemods (link no longer works) |
| Basic Local Alignment Search Tool (BLASTp+) Version 2.10.0 | Camacho et al.121,Altschul et al.122,Altschul et al.123 | https://ftp.ncbi.nlm.nih.gov/blast/executables/blast+/2.10.0/ |
| cdd2cog from bac-genomics-scripts Version v0.2 | Leimbach125 | https://github.com/aleimba/bac-genomics-scripts |
| Microsoft® Excel® Version 2202 | Microsoft 365 MSO | https://www.office.com/ |
| cxcalc from MarvinSketch Version 18.8.0 | ChemAxon | https://www.chemaxon.com |
| MEMOTE Version 0.13.0 | Lieven et al.74 | https://github.com/opencobra/memote |
| multiTFA | Mahamkali et al.104 | https://github.com/biosustain/multitfa |
| Gurobi Optimizer v.9.0.1 | Gurobi Optimization LLC114 | https://www.gurobi.com/downloads/gurobi-software/ |
| COBRApy version 0.22.1 | Ebrahim et al.107 | https://github.com/opencobra/cobrapy |
| Escher | King et al.76 | https://escher.github.io/#/ |
| Python 3.6.13 | Python Software Foundation | https://www.python.org/downloads/release/python-3613/ |
| Researchpy package Version 0.3.2 | Bryant130 | https://github.com/researchpy/researchpy |
| Illumina bcl2fastq Version 2.20 | Illumina | https://emea.support.illumina.com/downloads/bcl2fastq-conversion-software-v2-20.html |
| BBMap Version 38.93 | Bushnell131 | https://anaconda.org/bioconda/bbmap/files?sort=uploader.name&sort_order=desc&version=38.93 |
| FastX Toolkit Version 0.0.14 | Gordon and Hannon132 | http://hannonlab.cshl.edu/fastx_toolkit/download.html |
| BWA-MEM Version 0.7.17 | Li133 | https://github.com/lh3/bwa |
| Subread package Version 2.0.1 | Liao et al.134 | https://github.com/ShiLab-Bioinformatics/subread |
| Salmon Version 1.5.2 | Patro et al.135 | https://github.com/COMBINE-lab/salmon/releases |
| DESeq2 Version 1.32.0 | Love et al.136 | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| ConsensusDE Version 1.10.0 | Waardenberg and Field137 | https://anaconda.org/bioconda/bioconductor-consensusde/files?version= |
| Snakemake Version 6.8.0 | Mölder et al.138 | https://github.com/snakemake/snakemake |
| Proteome Discoverer Software Version 2.5.0.400 | Thermo Fisher Scientific | https://www.thermofisher.com/de/en/home/industrial/mass-spectrometry/liquid-chromatography-mass-spectrometry-lc-ms/lc-ms-software/multi-omics-data-analysis/proteome-discoverer-software.html |
| Rapid Peptides Generator Version 1.2.4 | Maillet112 | https://gitlab.pasteur.fr/nmaillet/rpg |
| GIMME | Becker and Palsson42 | https://github.com/opencobra/cobratoolbox/tree/master/src/dataIntegration/transcriptomics/GIMME |
| COBRA Toolbox v.3.1 | Heirendt et al.113 | https://github.com/opencobra/cobratoolbox |
| MATLAB Version R2018b | The MathWorks, Inc. | https://de.mathworks.com/products/matlab.html |
| Other | ||
| UNIlab Pro Eco glovebox | MBraun | Not listed |
| Centrifuge 5920 R | Eppendorf | Cat# 5948000010 |
| Vortex-Genie® 2 | VWR International GmbH | Cat# 444-0873P |
| Centrifuge 5424 | Eppendorf | No longer sold |
| ThermoMixer® C | Eppendorf | Cat# 5382000015 |
| Femto Pulse System 1.0.0.32 | Agilent | Cat# M5330AA |
| Qubit® 2.0 Fluorometer | Invitrogen | No longer sold |
| Sequel I System | Pacific Biosciences | No longer sold |
| BioXplorer 100 bioreactor & WinISO version 2.3.149.1 software | H.E.L | https://helgroup.com/products/bioreactors/bioxplorer-100/ |
| pH sensors | I&L Biosystems GmbH | Cat# Z001013510 |
| ORP sensors | I&L Biosystems GmbH | Cat# Z061013510 |
| Red-y smart min - mass-flow controller | Vögtlin | https://www.voegtlin.com/massedurchflussmesser-und-massedurchflussregler/red-y-smart-series/ |
| Bronkhorst EL-Flow® Prestige mass flow controllers | Bronkhorst Deutschland Nord GmbH | https://www.bronkhorst.com/int/products/gas-flow/el-flow-prestige/ |
| MilliGascounter MGC-1 V3.4 PMMA | Dr.-Ing. RITTER Apparatebau GmbH & Co. KG | https://www.ritter.de/en/milligascounter-mgc-1-pmma/ |
| FiveEasy™ Plus pH/mV Benchtop meter | Mettler-Toledo GmbH | Cat#: 30266627 |
| Micro pH electrode LE422 | Mettler-Toledo GmbH | Cat#: 30089747 |
| Mastercycler® pro S | Eppendorf | No longer sold |
| Gel Doc™ XR+ visualizer | Bio-Rad | https://www.bio-rad.com/de-de/product/gel-doc-xr-gel-documentation-system?ID=O494WJE8Z |
| 490 Micro Gas Chromatograph | Agilent | No longer sold (G3581-90001) |
| High-Pressure Liquid Chromatographer (HPLC) Nexera Series SIL-40C | Shimadzu Europa | https://www.ssi.shimadzu.com/products/liquid-chromatography/hplcuhplc/nexera-series/index.html |
| Multifuge X3R with the rotor TX-1000 | Fischer Scientific | No longer sold |
| Lysing Matrix B | MP Biomedicals Germany GmbH | Cat# 116911050-CF |
| FastPrep-24TM 5G Bead-Beater | MP Biomedicals Germany GmbH | Cat# 116005500 |
| 2100 Agilent Bioanalyzer | Agilent Technologies | Cat# G2939BA |
| NovaSeq™ 6000 | Illumina | https://emea.illumina.com/systems/sequencing-platforms/novaseq.html |
| Easy-nLC™ 1200 | Thermo Fisher Scientific | Cat# LC140 |
| Orbitrap Exploris™ 480 Mass Spectrometer | Thermo Fisher Scientific | Cat# BRE725539 |
Resource availability
Lead contact
Further information and request for resources should be directed to Bastian Molitor, bastian.molitor@uni-tuebingen.de.
Materials availability
This study did not generate new unique materials.
Experimental model and subject details
Microbial strains
M. thermautotrophicus ΔH (DSM 1053), M. thermautotrophicus Z-245 (DSM 3720), and M. marburgensis Marburg (DSM 2133) were obtained from the DSMZ (Braunschweig, Germany). M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 was previously generated in our lab.40
Growth media
Preparation of batch and continuous media for bioreactor runs was adjusted from the mineral media of Balch et al.77 and Martin et al.15 The mineral medium contained (per liter): nitrilotriacetic acid (NTA), 0.096 g; trisodium nitrilotriacetate, 0.275 g; sodium chloride, 0.675 g; di-potassium hydrogen phosphate, 0.255 g; ammonium chloride, 2.006 g; magnesium chloride hexahydrate, 0.12 g; calcium chloride dihydrate, 0.090 g; potassium di-hydrogen phosphate, 0.345; ammonium nickel sulfate, 1.5 mL (0.2% w/v); iron(II) chloride tetrahydrate, 1.5 mL (0.2% w/v); resazurin indicator solution, 4 mL (0.025% w/v); and trace element solution, 1.5 mL. The trace element solution was prepared 10-fold as stated by Balch et al.77 with minor modifications, and contained (per liter): nitrilotriacetic acid (NTA), 2.0 g; magnesium sulfate heptahydrate, 30.0 g; manganese(II) sulfate, 5.0 g; sodium chloride, 10.0 g; iron(II) sulfate heptahydrate, 1.0 g; cobalt(II) chloride hexahydrate, 1.8 g; calcium chloride dihydrate, 1.0 g; zinc sulfate heptahydrate, 1.8 g; copper sulfate pentahydrate, 0.1 g; aluminum potassium sulfate dodecahydrate, 0.18 g; boric acid, 0.1 g; sodium molybdate dihydrate, 0.1 g; ammonium nickel(II) sulfate hexahydrate, 2.8 g; sodium tungstate dihydrate, 0.1; and sodium selenate, 0.1 g. The pH of the trace element solution was adjusted to 6.0 with 5 M potassium hydroxide. In all the continuous media, 0.02 mL/L of Anti Foam SE-15 (Sigma-Aldrich, Merck, Darmstadt, Germany) was supplemented. For growth with sodium formate, serum bottles for batch cultivation were sparged with N2/CO2 (80/20 %, v/v), and 100 mM sodium formate was added after autoclaving. Further, the serum bottles were supplemented with 0.001 mM sodium selenate and 0.01 mM sodium molybdate dihydrate. For growth with sodium formate, the continuous media contained 355 ± 5 mM sodium formate and was supplemented with 0.0015 mM sodium selenate and 0.015 mM sodium molybdate dihydrate (final pH was 5.85). A concentrated sodium formate solution was prepared and sterilely added to the continuous media after autoclaving, sparging, and reducing the media. All media were prepared with Millipore water (18.2 MΩ·cm). The medium was autoclaved either in Schott bottles with butyl septa with Masterflex® L/S Norprene Food-Grade Tubing, L/S 14 tubing (Cole-Parmer GmbH, Wertheim, Germany) or directly in the bioreactor vessels.
For anaerobic handling of media and materials the atmosphere of the anaerobic chamber (UniLab Pro Eco, MBraun, Garching, Germany) contained 95% v/v N2 and 5% v/v CO2.
Growth conditions
Cultivations in serum bottles for all strains were essentially as described in Fink et al.40 Continuous fermentations were carried out in the BioXplorer 100 bioreactor platform controlled with the WinISO version 2.3.149.1 software (H.E.L, London, England). Each bioreactor was equipped with temperature, pH (part number Z001013510), and ORP (part number Z061013510) sensors (I&L Biosystems GmbH, Königswinter, Germany); a 0.15 μm sparging stone; a magnetic coupled stirring system; three peristaltic pumps for media feed-in, base feed-in (1 N NaOH was used for pH control), and effluent-out; a mass-flow controller (Red-y smart min; Vögtlin, Muttenz, Switzerland) to control the inlet gas flow rate; a condenser for the exhaust-gas line; and a separate sampling and inoculum port (fitted with a rubber butyl stopper). The bioreactors were fitted with Masterflex® L/S Norprene Food-Grade Tubing, L/S 14 (Cole-Parmer GmbH, Wertheim-Mondfel, Germany) except for the gas inlet lines, which were fitted with Masterflex® C-Flex ULTRA tubing, L/S 16 (Cole Parmer, Wertheim-Mondfel, Germany). The upstream gas mixture was set using Bronkhorst EL-Flow® Prestige mass flow controllers (Bronkhorst Deutschland Nord GmbH, Kamen, Germany) and mixed in a doubled-ended cylinder (Swagelok® Stuttgart, Reutlingen, Germany). The exhaust gas flow rate was measured offline using a MilliGascounter MGC-1 V3.4 PMMA (Dr.-Ing. RITTER Apparatebau GmbH & Co. KG, Bochum, Germany). The pH and ORP sensors, the pumps, and the MFCs were calibrated before each experiment. The bioreactors were filled with the mineral medium and autoclaved for one hour at 121°C. Afterward, the bioreactors were connected to the bioreactor platform, and the temperature was set to 65°C with agitation at 700 rpm.
For the first bioreactor experiment, the bioreactors were then sparged through sterile filters Minisart® HY (0.2 μm pore size; Sartorius AG, Göttingen, Germany) for two hours with H2/CO2 (80/20% v/v) at a gas flow rate of 10 mL/min. Before inoculation, the mineral medium was reduced with sterile anaerobic L-cysteine-HCl (0.5 g/L) and disodium sulfide nonahydrate (0.3 g/L), and the pH control was set to 7.3. Each bioreactor was inoculated with either 4 mL (M. thermautotrophicus ΔH and M. thermautotrophicus Z-245) or 3.6 mL (M. marburgensis Marburg) of preculture grown in serum bottles to an OD600 of 0.35-0.36. The bioreactors were operated in batch mode for one day until an OD600 of approximately 1.00 was reached, at which point continuous mode was started with a medium feed at a dilution rate of 0.83 d-1. The first continuous operating period was conducted for approximately 12 days, after which the bioreactors were emptied except for about 3-5 mL, which were used as inoculum for a second period with a starting OD600 of 0.20-0.30. When an OD600 of approximately 1.00 was reached, the bioreactors were switched into continuous mode with a medium feed at a dilution rate of 1.11 d-1. Steady-state was reached after three hydraulic retention times (HRT),78 which was 2.7 days in our setup. After an additional 3 HRTs (day 6.8), the transcriptomics, proteomics, and gram cell-dry weight determination samples were taken.
For the second bioreactor experiment, the bioreactor setup was the same as in the first experiment, except for the following adjustments for bioreactors fed with sodium formate: 1) pH adjusted using 1 N HCl instead of 1 N NaOH; and 2) sparged with N2/CO2 (80/20 %, v/v) rather than H2/CO2 (80/20 %, v/v). Each bioreactor was inoculated with 6 mL (M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 and M. thermautotrophicus Z-245) of preculture grown in serum bottles (OD600 ∼0.2). The bioreactors were operated in batch mode until an OD600 of approximately 1.00 for bioreactors grown on H2 and CO2 and 0.10-0.15 for bioreactors grown on sodium formate was reached. At this point, the continuous mode was started with a media feed at a dilution rate of ∼1.00 d-1 (this rate was achieved by ramping over several days for the bioreactors grown on sodium formate). After maintaining steady-state for three HRTs (10.6 days for H2/CO2 and 16.8 days for sodium formate), samples for transcriptomics, proteomics, and gram cell-dry weight determination were taken.
Daily samples of OD600, pH, exhaust gas flow rate, and inlet and exhaust gas composition were taken during both experiments and periods. For the liquid culture samples, 1 mL of dead volume was first removed, and then 1 mL was used for OD600 and pH measurements. To measure pH, the samples were maintained at 65°C (ThermoMixer® C 460-0223, Eppendorf, Hamburg, Germany) and measured within 1 min of taking the sample (FiveEasy™ Plus pH/mV Benchtop meter with the micro pH electrode LE422 [Mettler-Toledo GmbH, Gießen, Germany] calibrated at 25°C and set to 65°C).
Method details
Genome sequencing
M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg were grown in mineral medium overnight. For sequencing, we used strains directly from the DSMZ without excessive subcultivation. The entire biomass (50 mL) was collected by centrifugation at 3170 × g and room temperature for 20 min (5920 R Eppendorf, Hamburg, Germany), and the genomic DNA was extracted using a phenol-chloroform extraction method. For this, to the biomass pellet, 500 μL of cetyltrimethylammonium bromide buffer (CTAB, made according to Cold Spring Harbor Protocols,79 however, without the Polyvinylpyrrolidone) was added, and the pellet was resuspended. The mixture was transferred to sterile 2 mL bead beating tubes (containing 500 μL of 0.1 μm BeadBeater® zirconia beads, Carl Roth, Karlsruhe, Germany) and vortexed (Vortex-Genie® 2, VWR International GmbH, Darmstadt, Germany) at 2700 min-1 for 5 s on, then 1 s off, repeatedly for 1 min, after which the tube was placed on ice. 500 μL of ROTI®Phenol/Chloroform/Isoamyl alcohol (ratio of 25:24:1, Carl Roth, Karlsruhe, Germany) was added, the tube inverted, and then centrifuged at 4°C and 16000 × g for 10 min (5424, Eppendorf, Hamburg, Germany). The top layer was transferred (using wide orifice pipette tips) to a phase lock tube, which was prepared ahead by adding 2 mm3 of vacuum grease Dow Corning® (VWR International GmbH, Darmstadt, Germany) into 2 mL tubes, which were centrifuged until 9391 × g was reached, and then autoclaved to sterilize. Another 500 μL of ROTI®Phenol/Chloroform/Isoamyl alcohol was added, the tube was inverted to mix, and centrifugation was performed as in the previous step. The supernatant was transferred to new tubes, 500 μL of Chloroform/Isoamyl alcohol (24:1, VWR International GmbH, Darmstadt, Germany) was added, the tube was inverted to mix, and centrifugation was performed as in the previous step. The supernatant was added to a fresh tube, and the gDNA was precipitated by adding 0.1 volumes of cold 3 M sodium acetate and 2 volumes of ice-cold absolute ethanol, inverting the tube to mix, and then incubating it overnight at -20°C. The following day, the tube was centrifuged again at 4°C and 16000 × g for 10 min, the supernatant was removed, and the gDNA was washed with 300 μL of ice-cold 70% v/v ethanol. Centrifugation was performed as in the previous step, the supernatant was removed, and the tube was air-dried for approximately 1.5 h at 50°C in a ThermoMixer® C (Eppendorf, Hamburg, Germany). The pellet was resuspended with 44 μL elution buffer (10 mM Tris·Cl, pH 8.5, and nuclease-free water [New England Biolabs, Ipswich, United States]) and allowed to rest for an hour to resolve at room temperature. To remove RNA, 1 μL of Bovine Ribonuclease A (VWR International GmbH, Darmstadt, Germany) was added, and the tube was allowed to rest for 30 min at room temperature. The quality of the gDNA was then checked using the Femto Pulse System (1.0.0.32, Agilent, Santa Clara, United States) according to the manufacturer’s instructions, using the Genomic DNA 165 kb Kit (Agilent, Santa Clara, United States) and a 70 min separation time. The quantity of double-stranded DNA was measured with the Qubit® 2.0 Fluorometer (Invitrogen, Carlsbad, CA, USA) using the Qubit® dsDNA HS Assay Kit (Thermo Fischer Scientific, Dreieich, Germany). Library preparation was conducted with the SMRTbell® Express Template Preparation Kit (PN 101-397-100 Version 3, January 2018, Pacific Biosciences, Menlo Park, United States) as specified in the manufacturer’s instructions. The genomes were then sequenced using the Sequel I System (Pacific Biosciences, Menlo Park, United States).
Genome-scale metabolic model reconstruction
A pan-model for M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg, which included all reactions from the three strains, was built in Microsoft® Excel® (Microsoft 365 MSO, Version 2202, Washington, United States), following the protocol established by Thiele and Palsson.35 This pan-model consists of 618 reactions (including 46 exchange reactions, 56 transport reactions, and seven biomass-associated reactions, with eleven transport and eleven exchange reactions that act as pseudo reactions for orphan metabolites to refer to compounds that are either only produced or consumed), 555 metabolites, and 545 genes (Data S2). The pan-model used genome sequences, assemblies, and annotations as its backbone. Reactions and pathways were added based on data from KEGG,80 ModelSEED,81 UniProt,82 Brenda,83 BioCyc,84 MetaCyc,85 BIGG,86 and NCBI.87 Metabolite protonation was determined (for pH 7.0) using the command line tool cxcalc and MarvinSketch 18.8.0, ChemAxon (https://www.chemaxon.com, accessed in 2018).
When possible, reactions and genes were verified with literature. Genes for which no genus-specific evidence was found were BLAST searched to genes of species with stronger evidence. These BLAST results are specified in the comment section of the reconstruction. Over 790 references were cited for which the microbe and type of evidence were recorded using Evidence & Conclusion Ontology (categories: biochemical, genetic, physiological, sequence, modeling, and no data), which were then used to determine the confidence of each reaction for each strain.35 Published GEMs from the following strains were used to gap-fill and validate pathways in the Methanothermobacter pan-model: 1) Methanosarcina acetivorans: iVS941,88 iMB745,89 iMAC868a,90 iST807;91 2) Methanosarcina barkeri: iAF692,92 iMG746;93 3) Methanospirillum hungatei: iMhu428;94 4) Methanococcus maripaludis S2: iMM518;95,96 5) Methanobrevibacter smithii: iMsi385;97 6) Methanocaldococcus jannaschii: iTS436.98
The pan-model was converted to three strain-specific GEMs written in SBML Level 3 Version 1,99 including the extension packages for flux balance constraints (fbc) version 2100 and groups,101 and verified in MEMOTE 0.13.0.74 We followed recent recommendations for best practices, including for the GEM nomenclature (iMTD22IC, iMTZ22IC, iMMM22IC).102 For verification in MEMOTE, the GEMs were constrained with Conditions 1, 8, and 15 for M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg, respectively (Data S2 and S5). Each model scored 85% (Data S2).74 The GEMs aimed to be MIRIAM-compliant, including metabolite and reaction annotations with Compact Identifiers103 for various databases (gene annotations were not available given the newly annotated genomes; however, the old gene annotations can be found in Data S2).
The directionality of the reactions was determined using thermodynamics-based flux variability analysis (TFVA)104 and the ModelSEED database.105 For the TFVA method, a sink reaction (reversible uptake and production possible) was added to each metabolite in the GEMs. A core model was then generated using the tmodel function with the following constraints for cytosol and extracellular: 1) pH (7.6, 7.3); 2) ionic strength (0.1, 0); 3) temperature (338.15 K, 338.15 K); and 4) membrane potentials ([0,150], [-150, 0] for [cytosol, extracellular]). The minimum and maximum flux values were found with Gurobi as the solver, using the box or univariate method.104 If the flux values spanned zero, the reaction bounds were set to (-1000, 1000). If both the minimum and maximum flux values were less than zero, the reaction bounds were set to (-1000, 0). Lastly, if both the minimum and maximum flux values were greater than zero, the reaction bounds were set to (0, 1000). If directionality could not be determined with either TFVA or the ModelSEED database, directionality was set to reversible, except for reactions that caused loops with ATP (e.g., with ATP losing a phosphate group,35 or between redox carriers).106
Seven constrain conditions per strain were designed that depict relevant cultivation conditions: Conditions 1-7, Conditions 8-14, and Conditions 15-21 for M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg, respectively (Data S5). Flux balance analysis simulations with maximization of biomass exchange (EX_biomass_e) as the objective function and these different conditions were performed to verify the ability of the three strain-specific GEMs to grow under these cultivation conditions (Data S5 [Simulations 1-24]). The flux balance analysis simulations were run using COBRApy version 0.22.1 (Data S5 and S7).107 We produced metabolic pathway maps to visualize the GEMs with Escher (Data S2).76
Biomass and maintenance energy determination
The biomass composition for the three strains in the GEMs is assumed to be the same (Data S2). Briefly, the fraction for each of the molecules that make up the biomass was found by averaging those from previously published methanogen GEMs41 and incorporating empirically found data for M. thermautotrophicus ΔH (reaction ID, BIOMASS_X, where X is ΔH, ZZ, or MM; Data S2). The elemental composition of the biomass, CH1.681O0.418N0.222S0.004 (molecular weight of 23.502 g/mol) was taken from Duboc et al.108 Growth-associated maintenance (GAM) energy is included in the biomass macromolecule synthesis reactions. Non-growth-associated ATP maintenance costs (NGAM) are represented using the ATP hydrolysis reaction (ATPM).
Cross-contamination check
The bioreactors were checked for cross-contamination by polymerase chain reaction (PCR) using the Thermo Scientific™ Phire Plant Direct PCR Master Mix (Thermo Fischer Scientific, Dreieich, Germany) and custom primers (Data S1 - Table S9). Cells were lysed by boiling 300 μL of the bioreactor culture (with an approximate OD600 of 1.00) at 100°C for 10 min (ThermoMixer® C 460-0223, Eppendorf, Hamburg, Germany); 1 μL was directly taken as a template for the PCR reaction. Primers were used at a 10 μM concentration. The PCR was carried out for 28 cycles in a Mastercycler® pro S (Eppendorf, Hamburg, Germany). The results of the reactions were visualized with gel electrophoresis (1% w/v agarose and SYBR™ Safe DNA Gel Stain (Thermo Fischer Scientific, Dreieich, Germany) and Gel Doc™ XR+ visualizer (Bio-Rad, Feldkirchen, Germany).
Fermentation gas analysis
A 490 Micro Gas Chromatograph (microGC; Agilent, Santa Clara, United States) fitted with a multi-valve port system (Teckso GmbH, Neukirchen-Vluyn, Germany) was used to analyze the inlet and outlet gas compositions. The microGC was equipped with two columns, the Molecular Sieve 5A PLOT 0.25 mm, 10 m (Agilent, Santa Clara, United States) that used Argon as a carrier gas to measure H2, O2, N2, CH4, and CO, and the PoraPLOT Q PLOT, 0.25 mm, 10 m (Agilent, Santa Clara, United States) that used Helium as a carrier gas to measure CO2, N2 (combined with O2), and H2S. The microGC was calibrated before the run using six and four calibration levels for experiments 1 and 2, respectively (Data S1 - Table S10). Each level was sampled for four replicates, and the average was taken as the calibration point. The total method lasted 180 s with a sample time of 20 s. The injector and sample line temperatures were set at 110°C, and column temperatures and pressures at 60°C and 150 kPa, respectively.
Biomass concentration analysis
The biomass correlation coefficient (K in g/L/OD600), as defined in Valgepea et al.,109 was found by sampling 60 mL from each bioreactor (6 Falcon™ tubes of 10 mL) at the end of each steady-state period. The samples were centrifuged, the supernatant removed, and two Falcon™ tubes combined with 0.5 mL of Millipore water in pre-weighed glass vials (548-0028; VWR International GmbH, Darmstadt, Germany), resulting in three technical replicates per bioreactor. The vials were dried at 200 mbar (absolute pressure) and 80°C for three days, and the weight of the biomass was recorded. The slope of the measured biomass weight to the corresponding OD600 was taken and divided by the volume (0.02 L) to give the following K values (g/L/OD600): First experiment: M. thermautotrophicus ΔH, 0.32; M. thermautotrophicus Z-245, 0.31; and M. marburgensis Marburg, 0.28. Second experiment: M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 (H2 and CO2), 0.31; M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 (sodium formate), 0.89; and M. thermautotrophicus Z-245 (sodium formate), 0.95.
Sodium formate concentration measurements
Sodium formate concentrations were analyzed via high-pressure liquid chromatography (HPLC) (SIL-40C, Shimadzu Europa, Duisburg, Germany) system that was equipped with an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad, CA, USA) and a refractive index detector (RID-20A). A 5 mM sulfuric acid solution was used as the eluent, with a flow rate of 0.6 mL min−1 and a sample run time of 30-60 min. The oven temperature was set to 60°C, while the sample rack of the attached autosampler to 4°C. For HPLC sample preparation, all culture samples (0.5 mL ± 0.1 mL) were filtered using 0.22 μm filters (ROTILABO® PVDF, 13 mm, Carl Roth, Karlsruhe, Germany). Sodium formate calibration curves were prepared with concentrations ranging from 0.5-10 mM and 20-400 mM.
RNA sample preparation
When the steady state was reached, four technical replicates of 9 mL (n=4) of bioreactor sample were placed into 5 mL of prechilled (overnight at 4°C) RNAlater™ Stabilization Solution (Thermo Fischer Scientific, Dreieich, Germany). The samples were stored overnight at 4°C and then frozen at -20°C until RNA isolation. The samples were thawed on ice and centrifuged at 4°C and 4100 × g for 10 min (Multifuge X3R TX-1000, Fischer Scientific, USA), the supernatant was discarded, and the samples were resuspended in 800 μL of RNase-free water. The 800 μL were mixed with 950 μL of saturated phenol (Sigma-Aldrich, Merck, Germany) and 115 μL of a lysis solution in Lysing Matrix B (MP Biomedicals Germany GmbH, Eschwege, Germany). The lysis solution contained sodium acetate (20 mM pH 5.2), sodium dodecyl sulfate (SDS; 0.5 % v/v), ethylenediaminetetraacetic acid (EDTA; 1 mM), and DNase/RNase-free distilled water (DI; Invitrogen, Thermo Fischer Scientific, Germany). The cells were homogenized for one cycle (5 x 40 s at 6 m/s and 20 s off) in a FastPrep-24™ 5G bead-beater (MP Biomedicals Germany GmbH, Eschwege, Germany) and then centrifuged at room temperature and 21130 × g for 10 min (5424 Eppendorf, Hamburg, Germany). The top layer was placed into 600 μL of phenol-chloroform-isoamyl alcohol (ROTI® Aqua-P/C/I, Carl Roth, Karlsruhe, Germany) and centrifuged as in the previous step. This step was repeated twice. The top layer was added to 1.2 mL of ethanol (undenatured absolute, SERVA Electrophoresis GmbH, Heidelberg, Germany) and left overnight at -80°C to precipitate the RNA. The following day, the tubes were centrifuged at 4°C and 21130 × g for 10 min, and then the supernatant was removed. The RNA pellet was washed with 1 mL of 75% v/v ethanol solution and centrifuged as in the previous step. The supernatant was pipetted off, and the pellet was resuspended with 53 μL of DNase/RNase-free distilled water. The RNA was cleaned (which included DNA depletion) and concentrated using the RNA Clean & Concentrator Kit (Zymo Research, Irvine, CA, United States) according to the manufacturer’s instructions. The cleaning and concentrating protocols were repeated four times. The quantity (6-16 μg) and quality (RNA integrity index > 9) of the purified RNA were measured with a 2100 Agilent Bioanalyzer (Agilent Technologies, Santa Clara, United States) using the Agilent RNA 6000 Nano Kit (Agilent Technologies, Santa Clara, United States) before freezing the samples at -80°C until sequencing.
RNA sequencing
The library preparation was performed using 100 ng of RNA and the TruSeq™ Stranded Total RNA Kit with Ribo-Zero™ Plus (Illumina, San Diego, United States). Pair-ended sequencing was performed using the NovaSeq™ 6000 with the Flow Cell Type 2 x 100 bp (Illumina, San Diego, United States). Demultiplexing of the sequences was performed with Illumina bcl2fastq (2.20) software. The RNA sequencing was performed by CeGaT GmbH (Tübingen, Germany).
Protein sample preparation
The proteomics sampling and sample preparation methods from Valgepea et al.50 were used with the following modifications. Screw-cap 2-mL tubes with lysing matrix B (MP Biomedicals Germany GmbH, Eschwege, Germany) were used in the homogenizer (FastPrep-24™ 5G, MP Biomedicals Germany GmbH, Eschwege, Germany). For cell lysis, the one cycle of bead beating as described for transcriptomics sample preparation was repeated three times. The following protein precipitation method from the Proteome Center Tübingen (PCT) at the University of Tübingen was applied. The supernatant was transferred to a 15-mL Falcon™ tube with 4 mL and 0.5 mL of ice-cold 100% acetone and 100% methanol, respectively, and precipitated overnight at -20°C. The following day, the tubes were centrifuged at 2200 × g and 4°C for 20 min (Multifuge X3R TX-1000, Thermo Fischer Scientific, Waltham, United States). The supernatant was removed, and the pellet was washed with 1 mL of ice-cold acetone (80/20% v/v in water) and centrifuged as in the previous step. The supernatant was removed, and the protein pellet was left to air dry on ice for 15 min before freezing at -20°C.
Protein measurement
Pellets were resuspended in a denaturation buffer (6 M urea, 2 M thiourea in 10 mM Tris pH 8.0), and 20 μg of protein were subjected to tryptic in-solution digestion (0.2 μg trypsin). The samples were run for an LC-MS/MS analysis on an Easy-nLC™ 1200 (Thermo Fisher Scientific, Dreieich, Germany) coupled to an Orbitrap Exploris™ 480 mass spectrometer (Thermo Fisher Scientific, Dreieich, Germany). The LC-MS analysis was performed as described previously in Fagbadebo et al.,110 with the exception of the duration and gradient of the peptide elution. Solvent A was 0.1% formic acid, and solvent B was 80% acetonitrile in 0.1% formic acid.110 The LC-MS was operated at 40°C.110 The peptides were eluted from 0-113 min with a linear gradient from 10% to 33% of solvent B at a flow rate of 200 nL/min. Then, from 113-116 minutes, the gradient increased from 33% to 50% of solvent B at 200 nL/min. To wash the remaining peptides from the column, from minutes 116-119, the gradient increased from 50% to 90% of solvent B with a gradually increasing flow rate from 200 nL/min to 500 nL/min. Lastly, the column was then washed from 119-127 min with 90% solvent B at 500 nL/min.
Pan-genome differential expression database
With the M. thermautotrophicus ΔH genes as a reference, an intersection of homologous genes/proteins based on the best BLASTp+ hits from M. thermautotrophicus Z-245 and M. marburgensis Marburg was used (Equation 3). M. thermautotrophicus ΔH and M. thermautotrophicus Z-245 had 81 additional homologous genes/proteins, which were not considered when M. marburgensis Marburg was being analyzed, while M. thermautotrophicus ΔH and M. marburgensis Marburg had 11. This pan-genome of homologous gene groups can be found in Data S4.
| (Equation 3) |
A protein FASTA file that represented the homologous clusters was required to perform relative proteomics across different species. Each homologous cluster FASTA entry needs to account for genetic heterogeneity that leads to variation in protein sequences across strains. Therefore, peptide sequences from the other strains had to be added to the gene-group sets.111 An in-silico tryptic digestion of the homologous protein FASTA sequences for M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg was performed using Rapid Peptides Generator version 1.2.4 to retrieve all peptides for each strain.112 A new enzyme was defined to cleave after lysine (K) or arginine (R) except if proline (P) follows, with the cleaving rule as (K or R,) with the exception (K or R,)(P). Then, using the M. thermautotrophicus ΔH protein FASTA sequence as a reference, unique peptides (with a length greater than six amino acids) from homologous proteins of M. thermautotrophicus Z-245 and M. marburgensis Marburg that were not already in the M. thermautotrophicus ΔH protein were inserted prior to the C-terminal peptide (Data S7). If M. thermautotrophicus Z-245 and M. marburgensis Marburg shared a C-terminus different from the one of M. thermautotrophicus ΔH, then that C-terminus was additionally amended as the C-terminus for the new combined protein, otherwise the C-terminus from M. thermautotrophicus ΔH was kept. This new protein FASTA file was then used for the proteomics analysis (Section 1.21).
Integrating fermentation data in the GEMs
Experimental fermentation data (Conditions 22-24) were used to constrain the models for the flux balance analysis simulations (with biomass maximization as the objective function) to validate the three strain-specific GEMs (Data S5 and S7). The data did not include time points with suspected gross measurement error and was adjusted with the maximum likelihood estimates.
Reduced experimentally-constrained GEMs were created by applying the GIMME42 algorithm with the transcriptomics and proteomics data (thresholds set at the lower quartile). This step was accomplished in the COBRA Toolbox v.3.1113 using MATLAB (R2018b) and the Gurobi Optimizer v.9.0.1.114 Also in the COBRA Toolbox, flux balance analysis simulations (with and without loops allowed) were run on the resulting reduced GEMs, using either the maximization of biomass exchange (EX_biomass_e) or the maximization of ATP dissipation (ATPM) as the objective function (Data S5 and S7).
Visualization
The graphical abstract was created with Biorender.
Quantification and statistical analysis
Genome assembly
De novo genome assembly (including the plasmid when applicable) was conducted, and methylation patterns were deciphered from the PacBio sequencing results. The utilized pipeline included seven main aspects: 1) contamination control by DIAMOND alignment against the NCBI-nr database to confirm the absence of any non-Methanothermobacter reads; 2) assembly by Canu115 (which includes error correction, trimming, and assembly); 3) first polishing by BlastR, samtools,116,117 and Arrow; 4) circularizing by Blast118 and BioPython; 5) second polishing by BlastR, samtools,116,117 and Arrow; and 6) methylation patterns prediction by ipdSummary, MotifMaker,119 and BaseModFunctions. The final annotation was performed by National Center for Biotechnology Information (NCBI) upon genome sequence submission. Details on the applied programs can be found in Data S1 - Table S8.
Genome comparisons
Protein FASTA files were generated using the GenBank files from NCBI and the BioPython version 1.77.120 Genome-wide comparisons were performed with protein Basic Local Alignment Search Tool (BLASTp+) version 2.10.0 locally.121,122,123 The new M. thermautotrophicus ΔH and M. marburgensis Marburg sequences were compared to the previously published ones, accession numbers NC_000916.1 62 and NC_014408.1,64 respectively. The alignments were performed twice, both times with an expectation value (e-value) cut-off of 10E-30. The first time, the new sequences were set as the queries and the old sequences as the subjects. The second time the query and subject were reversed. The protein hit with the highest bit score was taken as the matching protein (and gene). For homologous gene comparisons (which were used for the pan-genome and pan-model construction), the e-value cut-off was set to 0.001. The following pairwise comparisons were performed with the following query and subject pairs: 1) M. thermautotrophicus ΔH and M. marburgensis Marburg; 2) M. thermautotrophicus ΔH and M. thermautotrophicus Z-245; and 3) M. marburgensis Marburg and M. thermautotrophicus Z-245. The protein hit with the highest bit score was taken as the homologous protein (and gene). The three comparisons were merged with M. thermautotrophicus ΔH as the backbone via the LOOKUP function in Excel®. Some supplementary gene-wise annotations were completed with the online BLASTp tool.124
COG functional annotation
The clusters of orthologous genes (COG) functional annotation was performed on the protein FASTA file for each strain with the script cdd2cog v0.2 from the bac-genomics-scripts according to the author’s instructions.125 A modification to the code, as suggested in issue #14 (https://github.com/aleimba/bac-genomics-scripts/issues/14, accessed on 12/2021), was applied to allow the use of the COG2020 database.126
Carbon and electron balance calculations
For the first experiment, initial carbon balances using molar flow rates (, mmol/h) were calculated according to conservation of mass (Equation 4) using the ideal gas law (Equation 5) with the following assumptions:
-
•
The ideal gas constant of 8.3144 L·kPa/K/mol
-
•
A temperature (T) of 25°C (298.15 K)
-
•
A pressure (P) of 1 atm (101.325 kPa).
| (Equation 4) |
| (Equation 5) |
The volumetric flow rate (, L/h) was found by multiplying the gas concentration (the fraction measured by the microGC) by the measured gas flow rate. This was done for the CO2 of the inlet gas (CO2,gas_in), and the CO2 (CO2,gas_out) and CH4 (CH4,gas_out) of the outlet gas. In addition, Henry’s law was used to account for the soluble CO2 (CO2,aq_out) that was removed from the bioreactors in the effluent. To find the quantities of soluble bicarbonate and carbonate in the medium, the acid dissociation constants (and pKa) were found using the equations provided by Prieto and Millero127 (Data S3). The salinity of the medium (4.565 ppt), required for the equations in Prieto and Millero,127 was estimated by summing the amount of cations and anions of the salts that were added to the medium. The estimated concentrations of solubilized and removed carbon species were subtracted from the calculated gas uptake values. Lastly, to calculate the biomass concentration in the bioreactor (biomassaq_out), the biomass correlation coefficient (K in g/L/OD600) was divided by the measured bioreactor OD600 and volume. The resulting gCDW was divided by the molecular weight (23.502 g/mol)108 and multiplied by the measured dilution rate of the bioreactors (h-1). Given inconsistencies in carbon balances in our measurements, a gross measurement error analysis was performed following the macroscopic balance method described in Wang and Stephanopoulos128 (Data S7). In brief, first, the probable gross measurement error was identified via statistical hypothesis testing with a chi-square test. Second, maximum likelihood estimates were calculated (dropping the data points with gross measurement error) to find corrected values for the gas and biomass data.
For the second experiment, on H2 and CO2, the carbon balances were performed as described above with an adjusted salinity value of 4.568 ppt rather than 4.565 ppt. However, given more considerable inconsistencies in carbon and electron balances with the raw data for bioreactor runs on sodium formate, a different method was applied in this case. No sodium formate was detected in the liquid media during steady state. Thus, we assumed that the microbes consumed the entire sodium formate from the feed in the bioreactor (Na-formatein). However, the quantity of 1 M HCl solution added to the bioreactors for pH control was not insignificant (unlike the amount of NaOH added for fermentations on H2 and CO2). The feed rate of the pH control pumps was determined, and a dilution factor was calculated. Approximately 21.88 ± 2.93% of the liquid feed into the bioreactors was due to the acid feed, thus lowering the effective sodium formate feed concentration to 291.38 ± 10.55 mM (Data S1 - Table S11). Further, the estimation of dissolved carbon species is very sensitive to salinity. The increased salt concentrations from the sodium formate (new value estimated at 17.80 ± 0.44 ppt) were insufficient for closing both carbon and electron balances. It is also noted that variation in pH was more frequent due to the production of NaOH with sodium formate consumption, and at times, there was a noticeable shift in the online pH probe measurement that required a manual offset after offline pH measurements. Furthermore, the dissolved CO2 values are sensitive to changes in pH. Thus, to solve for the quantities of produced CH4 and CO2, Equations 6 and 7, which represent the carbon and electron (using degree of reduction) balances, were solved. The biomass coefficient in Equation 7 was calculated assuming a molecular formula of CH1.681O0.481N0.222, as described in Michael and Kargi.129 H2 gas was detected in the outlet gas stream (H2,gas_out), and thus it was also included in Equation 7.
| (Equation 6) |
| (Equation 7) |
Normalized product distribution
Product distributions were calculated using CH4 and biomass data (mmol/h) for bioreactors and time points that did not have suspected gross measurement errors (STAR Methods – carbon and electron balance calculations). The quantity of CH4 or biomass was divided by the sum of the CH4 and biomass and then multiplied by 100, resulting in the normalized product distribution percentage. For fermentations on sodium formate, the CO2 production was also accounted for in the products. An analysis of variance (ANOVA) test was conducted in Excel® using the ANOVA:Single Factor (ɑ = 0.05) function of the Data Analysis Add-in to analyze statistically significant differences between the product distribution ratios of the three different strains (first experiment). Further, product distribution ratios were compared for: 1) M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 and M. thermautotrophicus Z-245 grown on sodium formate (second experiment); and 2) product distribution ratios of M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg grown on H2/CO2 (from the first experiment) with M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 grown on H2/CO2 (from the second experiment).
Interspecies comparison
For the first experiment, all gas and biomass data points without suspected gross measurement error were used for the comparative analysis (independent t-tests) between the three strains, except for one bioreactor replicate for M. thermautotrophicus Z-245 that experienced a pump malfunction and wash-out of bioreactor broth during the fermentation.
For the second bioreactor experiment, one replicate of M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 grown on sodium formate was discarded because of a mixer malfunction, and thus, the gas and biomass data points were not included in the t-tests. The rest of the gas and biomass data points were used for the comparative analysis between M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 and M. thermautotrophicus Z-245 grown on sodium formate. The second set of t-tests was conducted between M. thermautotrophicus ΔH, M. thermautotrophicus Z-245, and M. marburgensis Marburg grown on H2/CO2 from the first experiment with M. thermautotrophicus ΔH pMVS1111A:PhmtB-fdhZ-245 grown on H2/CO2 from the second experiment.
The t-tests (two-tailed and confidence intervals of 95%) were carried out in Python 3.6.13 using the Researchpy package Version 0.3.2 and the ttest function.130 Three-way comparisons were performed in Excel® using the ANOVA:Single Factor (ɑ = 0.05) function of the Data Analysis Add-in. The maximum likelihood estimates of the measurements for time points without gross measurement error were calculated,128 which resulted in closed carbon balances for those time points (assuming 90% confidence intervals for the test function). These adjusted values were then averaged across time points for each strain and used to constrain the GEMs (Data S3).
Raw RNA sequencing data analysis
Sequencing reads were quality filtered and trimmed with BBMap version 38.93131 and FastX Toolkit version 0.0.14132 with specific parameters for paired-end reads. Reads from each bioreactor culture were mapped to the reference genomes of M. thermautotrophicus ΔH (CP064324), M. thermautotrophicus Z-245 (CP064336 and CP064337), and M. marburgensis Marburg (CP069376 and CP069377) using BWA-MEM version 0.7.17.133 Mapped reads were assigned to genomic features using the FeatureCounts program from the Subread package version 2.0.1.134 Additionally, an alignment-free quantification of gene features was performed using Salmon version 1.5.2.135 Pairwise differential expression analysis was performed on the Salmon counts using DESeq2 version 1.32.0136 with the homologous genes present in the pan-genome database (STAR Methods - pan-genome differential expression database) and ConsensusDE version 1.10.0.137 The adjusted P-value cut-off threshold was set to 0.05. This process was orchestrated using Snakemake version 6.8.0138 to achieve reproducibility (Data S7). The output files from Salmon were used to analyze each strain individually, and only genes with at least two non-zero transcript values were considered. The averages of each gene were then used.
Protein analysis with proteome Discoverer
Raw data-dependent acquisition (DDA) data from LC-MS/MS analysis was performed using Thermo Fisher Proteome Discoverer software (version 2.5.0.400, Thermo Fisher Scientific, Scoresby, Australia). Files were processed using two different methods: 1) for intensity-based absolute quantification (iBAQ) analysis, each strain was processed individually through the precursor label-free quantification workflow with their corresponding FASTA files and then converted to iBAQ as described in Schwanhäusser et al.139; and 2) for differential expression analysis, all files were processed together using the custom pan-genome FASTA file (STAR Methods - pan-genome differential expression database). Both methods used the same workflows and only differed in their choice of FASTA file. Briefly, spectra were searched against the respective databases with carbamidomethylation set as a fixed modification and up to three methionine oxidations. Acetylation, methionine loss, and acetylation after methionine loss at the N-terminus were also permitted. The mass tolerance of precursors and fragments were 10 ppm and 0.02 Da, respectively. The minimum and maximum peptide lengths were six and 144 amino acids, respectively. Two missed cleavages were allowed per peptide. Proteins were filtered to a 1% FDR cut-off threshold and for an adjusted P-Value of 0.05. For quantification, only peptides unique to a protein group were used. All other parameters were kept at their default settings.
Eha/Ehb ratio determination
The ratio of the energy-converting hydrogenases Eha and Ehb subunits was found by summing the abundances (either transcripts or relative protein abundances) of the subunits detected for each energy-converting hydrogenase and dividing those sums by the number of subunits found. This was done to normalize against subunits that were not found (the stoichiometry between all subunits was considered as equimolar based on current knowledge from the literature).140 The normalized abundance of Eha was then divided by that of Ehb. This ratio was then averaged across the replicates (bioreactor samples) of each strain. An ANOVA test was conducted in Excel® using the ANOVA:Single Factor (ɑ = 0.05) function of the Data Analysis Addin to test if there was a statistically significant difference between the Eha/Ehb ratios for the three different strains (Data S4).
Methanogenesis relative abundances
Gap-filled relative abundances were found for the different proteins (or complexes) involved in the Wolfe cycle of methanogenesis. The iBAQ values of each subunit were summed and divided by the number of detected subunits (the maximum number of subunits missing per strain per protein complex was one). This sum was then multiplied by the theoretical number of subunits that should be found (effectively assigning the non-detected subunit the average iBAQ value for the complex) to produce the entire protein complex normalized iBAQ value. This value was then divided by the sum of all iBAQ values in the proteome and multiplied by 100, resulting in relative (normalized) protein abundance percentages (Data S4). The stoichiometry between all subunits was considered as equimolar based on current knowledge from the literature.140
Acknowledgments
The authors would like to thank Luis Antoniotti and Jürgen Barth from the Max Planck Institute for Biology Tübingen workshop for their help with modifying the bioreactor system. The authors would like to thank Lucas Mühling, Nils Rohbohm, and Andrés Ortíz Ardilla for helpful input and support with experimentation, and Nicolai Kreitli for support with bioreactor experiments. The authors would like to acknowledge the assistance of the Genome Center of the Max Planck Institute for Biology Tübingen, particularly Christa Lanz, for their assistance in the PacBio sequencing, and the Proteome Center Tübingen (PCT) for their support with the proteomics. The authors would like to acknowledge the fruitful conversations with Theresa Ahrens and Doris Hafenbradl from Electrochaea GmbH, Planegg, Germany. The authors would like to thank Libera Lo Presti from the Cluster of Excellence – CMFI at the University of Tübingen for language editing and proofreading the manuscript. This work was supported by the Humboldt Foundation in the framework of the Humboldt professorship, which was awarded to LTA, and by the Max Planck Institute for Biology Tübingen (REL and LTA). IC would like to acknowledge support from the German Academic Exchange Service (DAAD) through the DAAD Kurzzeitstipendien für Doktoranden. EM acknowledges support from the Australian Research Council for the Center of Excellence in Synthetic Biology.
Author contributions
I.C.: Methodology, Software, Formal Analysis, Investigation, Data Curation, Writing - Original Draft & Review & Editing, Visualization, Project Administration, Funding acquisition; T.M.: Methodology, Software, Formal Analysis, Writing – Review and Editing; S.E-E.: Methodology, Investigation, Writing – Review and Editing; G.G.L.: Software, Formal Analysis, Writing – Review and Editing; D.E.: Formal Analysis, Investigation, Writing - Review and Editing; C.F.: Investigation, Writing – Review and Editing; S.B.: Investigation, Writing – Review and Editing; N.D.Y.: Methodology, Writing – Review and Editing; L.A.: Writing - Review and Editing; D.H.H.: Supervision, Writing – Review and Editing, Funding Acquisition; A.D.: Software, Writing – Review and Editing, Funding Acquisition; R.E.L.: Resources, Supervision, Writing – Review and Editing, Funding Acquisition; E.M.: Methodology, Resources, Writing – Review and Editing, Funding Acquisition; L.T.A.: Conceptualization, Resources, Writing – Review and Editing, Supervision, Project administration, Funding Acquisition; B.M.: Conceptualization, Writing – Original Draft, Review and Editing, Supervision, Project administration, Funding acquisition.
Declaration of interests
The authors declare no conflict of interest.
Published: September 22, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.108016.
Supplemental information
Data and code availability
-
•
Supplemental tables can be found in Data_S1.xlsx. The genome sequences, annotations, and methylation patterns were deposited to NCBI65 and can be found under Bioproject ID PRJNA674001 (Data S1 - Table S1). The transcriptomic data (gene expression data) discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus66 and are accessible through GEO Series accession number GSE218145 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE218145). The mass spectrometry proteomic DDA data were deposited to the ProteomeXchange Consortium via the PRIDE partner repository67,68,69 with the dataset identifier PXD039574. The modeling-related files (Data S2) were deposited in an Open Modeling EXchange format (OMEX)70 to BioModels71,72 and assigned the identifiers MODEL2211290001 (M. thermautotrophicus ΔH), MODEL2211290002 (M. thermautotrophicus Z-245), and MODEL2211290003 (M. marburgensis Marburg). The pan-model is provided as an Excel® model (.xlsx, densely commented). The GEMs for each strain are provided in the following formats: .xml (SBML73), .json, and .mat. Additional files include: the benchmarking reports (.html) from MEMOTE,74 the FROG analysis files (.omex) from fbc_curation,75 and the map files for visualization (.json) with Escher.76 Further, the data used to constrain the models are provided as supplemental data. This includes: the gas fermentation data (as Excel® files, Data S3 and S6) and the individual strain and differential expression analyses data for both the transcriptomics and proteomics (as an Excel® file, Data S4). Data S1 and S3–S6 are available on GitHub at https://github.com/isacasini/Casini_2023_GEM.
-
•
Additional original code (scripts and programs) are found in Data S7. Data S7 is available on GitHub at https://github.com/isacasini/Casini_2023_GEM.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Zhao Y., McDonell V., Samuelsen S. Assessment of the combustion performance of a room furnace operating on pipeline natural gas mixed with simulated biogas or hydrogen. Int. J. Hydrogen Energy. 2020;45:11368–11379. [Google Scholar]
- 2.Reuß M., Grube T., Robinius M., Stolten D. A hydrogen supply chain with spatial resolution: Comparative analysis of infrastructure technologies in Germany. Appl. Energy. 2019;247:438–453. [Google Scholar]
- 3.Melaina M.W., Antonia O., Penev M. National Renewable Energy Laboratory; 2013. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues. NREL/TP-5600-51995. [Google Scholar]
- 4.Hall J.E., Hooker P., Jeffrey K.E. Gas detection of hydrogen/natural gas blends in the gas industry. Int. J. Hydrogen Energy. 2021;46:12555–12565. [Google Scholar]
- 5.Schiebahn S., Grube T., Robinius M., Tietze V., Kumar B., Stolten D. Power to gas: Technological overview, systems analysis and economic assessment for a case study in Germany. Int. J. Hydrogen Energy. 2015;40:4285–4294. [Google Scholar]
- 6.Guerra L., Rossi S., Rodrigues J., Gomes J., Puna J., Santos M.T. Methane production by a combined Sabatier reaction/water electrolysis process. J. Environ. Chem. Eng. 2018;6:671–676. [Google Scholar]
- 7.Müller K., Städter M., Rachow F., Hoffmannbeck D., Schmeißer D. Sabatier-based CO2-methanation by catalytic conversion. Environ. Earth Sci. 2013;70:3771–3778. [Google Scholar]
- 8.Dry M.E. The fischer–tropsch process: 1950–2000. Catal. Today. 2002;71:227–241. [Google Scholar]
- 9.Leonzio G. Process analysis of biological Sabatier reaction for bio-methane production. Chem. Eng. J. 2016;290:490–498. [Google Scholar]
- 10.Van Der Laan G.P., Beenackers A.A.C.M. Kinetics and selectivity of the Fischer–Tropsch synthesis: a literature review. Catal. Rev. 1999;41:255–318. [Google Scholar]
- 11.Rachbauer L., Beyer R., Bochmann G., Fuchs W. Characteristics of adapted hydrogenotrophic community during biomethanation. Sci. Total Environ. 2017;595:912–919. doi: 10.1016/j.scitotenv.2017.03.074. [DOI] [PubMed] [Google Scholar]
- 12.Thauer R.K., Kaster A.-K., Seedorf H., Buckel W., Hedderich R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008;6:579–591. doi: 10.1038/nrmicro1931. [DOI] [PubMed] [Google Scholar]
- 13.Pfeifer K., Ergal İ., Koller M., Basen M., Schuster B., Rittmann S.K.M.R. Archaea biotechnology. Biotechnol. Adv. 2021;47:107668. doi: 10.1016/j.biotechadv.2020.107668. [DOI] [PubMed] [Google Scholar]
- 14.Rusmanis D., O’Shea R., Wall D.M., Murphy J.D. Biological hydrogen methanation systems–an overview of design and efficiency. Bioengineered. 2019;10:604–634. doi: 10.1080/21655979.2019.1684607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin M.R., Fornero J.J., Stark R., Mets L., Angenent L.T. A Single-Culture Bioprocess of Methanothermobacter thermautotrophicus to Upgrade Digester Biogas by CO2-to-CH4 Conversion with H2. Archaea. 2013;2013:157529. doi: 10.1155/2013/157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaul A., Böllmann A., Thema M., Kalb L., Stöckl R., Huber H., Sterner M., Bellack A. Combining a robust thermophilic methanogen and packing material with high liquid hold-up to optimize biological methanation in trickle-bed reactors. Bioresour. Technol. 2022;345:126524. doi: 10.1016/j.biortech.2021.126524. [DOI] [PubMed] [Google Scholar]
- 17.Thema M., Weidlich T., Kaul A., Böllmann A., Huber H., Bellack A., Karl J., Sterner M. Optimized biological CO2-methanation with a pure culture of thermophilic methanogenic archaea in a trickle-bed reactor. Bioresour. Technol. 2021;333:125135. doi: 10.1016/j.biortech.2021.125135. [DOI] [PubMed] [Google Scholar]
- 18.Wasserfallen A., Nölling J., Pfister P., Reeve J., Conway de Macario E. Phylogenetic analysis of 18 thermophilic Methanobacterium isolates supports the proposals to create a new genus, Methanothermobacter gen. nov., and to reclassify several isolates in three species, Methanothermobacter thermautotrophicus comb. nov., Methanothermobacter wolfeii comb. nov., and Methanothermobacter marburgensis sp. nov. Int. J. Syst. Evol. Microbiol. 2000;50 Pt 1:43–53. doi: 10.1099/00207713-50-1-43. [DOI] [PubMed] [Google Scholar]
- 19.Kaster A.-K., Goenrich M., Seedorf H., Liesegang H., Wollherr A., Gottschalk G., Thauer R.K. More than 200 Genes Required for Methane Formation from H2 and CO2 and Energy Conservation Are Present in Methanothermobacter marburgensis and Methanothermobacter thermautotrophicus. Archaea. 2011;2011:973848. doi: 10.1155/2011/973848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Poorter L.M.I., Geerts W.G., Theuvenet A.P.R., Keltjens J.T. Bioenergetics of the formyl-methanofuran dehydrogenase and heterodisulfide reductase reactions in Methanothermobacter thermautotrophicus. Eur. J. Biochem. 2003;270:66–75. doi: 10.1046/j.1432-1033.2003.03362.x. [DOI] [PubMed] [Google Scholar]
- 21.de Poorter L.M.I., Geerts W.J., Keltjens J.T. Coupling of Methanothermobacter thermautotrophicus methane formation and growth in fed-batch and continuous cultures under different H2 gassing regimens. Appl. Environ. Microbiol. 2007;73:740–749. doi: 10.1128/AEM.01885-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afting C., Kremmer E., Brucker C., Hochheimer A., Thauer R.K. Regulation of the synthesis of H2-forming methylenetetrahydromethanopterin dehydrogenase (Hmd) and of HmdII and HmdIII in Methanothermobacter marburgensis. Arch. Microbiol. 2000;174:225–232. doi: 10.1007/s002030000197. [DOI] [PubMed] [Google Scholar]
- 23.Nölling J., Reeve J.N. Growth-and substrate-dependent transcription of the formate dehydrogenase (fdhCAB) operon in Methanobacterium thermoformicicum Z-245. J. Bacteriol. 1997;179:899–908. doi: 10.1128/jb.179.3.899-908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato S., Kosaka T., Watanabe K. Comparative transcriptome analysis of responses of Methanothermobacter thermautotrophicus to different environmental stimuli. Environ. Microbiol. 2008;10:893–905. doi: 10.1111/j.1462-2920.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu C., Mao L., Zheng X., Yuan J., Hu B., Cai Y., Xie H., Peng X., Ding X. Comparative proteomic analysis of Methanothermobacter thermautotrophicus reveals methane formation from H2 and CO2 under different temperature conditions. MicrobiologyOpen. 2019;8:e00715. doi: 10.1002/mbo3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diender M., Pereira R., Wessels H.J.C.T., Stams A.J.M., Sousa D.Z. Proteomic Analysis of the Hydrogen and Carbon Monoxide Metabolism of Methanothermobacter marburgensis. Front. Microbiol. 2016;7:1049. doi: 10.3389/fmicb.2016.01049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PATRIC Methanothermobacter thermautotrophicus Str. ΔH. 2014. https://www.patricbrc.org/view/Genome/187420.15
- 28.Yishai O., Lindner S.N., Gonzalez de la Cruz J., Tenenboim H., Bar-Even A. The formate bio-economy. Curr. Opin. Chem. Biol. 2016;35:1–9. doi: 10.1016/j.cbpa.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Feierabend M., Renz A., Zelle E., Nöh K., Wiechert W., Dräger A. High-Quality Genome-Scale Reconstruction of Corynebacterium glutamicum ATCC 13032. Front. Microbiol. 2021;12:750206. doi: 10.3389/fmicb.2021.750206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu C., Kim G.B., Kim W.J., Kim H.U., Lee S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019;20:121. doi: 10.1186/s13059-019-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suthers P.F., Foster C.J., Sarkar D., Wang L., Maranas C.D. Recent advances in constraint and machine learning-based metabolic modeling by leveraging stoichiometric balances, thermodynamic feasibility and kinetic law formalisms. Metab. Eng. 2021;63:13–33. doi: 10.1016/j.ymben.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Lu H., Li F., Sánchez B.J., Zhu Z., Li G., Domenzain I., Marcišauskas S., Anton P.M., Lappa D., Lieven C. A consensus S. cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-11581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu H., Kerkhoven E.J., Nielsen J. Multiscale models quantifying yeast physiology: towards a whole-cell model. Trends Biotechnol. 2022;40:291–305. doi: 10.1016/j.tibtech.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Mendoza S.N., Olivier B.G., Molenaar D., Teusink B. A systematic assessment of current genome-scale metabolic reconstruction tools. Genome Biol. 2019;20:158. doi: 10.1186/s13059-019-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiele I., Palsson B.Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 2010;5:93–121. doi: 10.1038/nprot.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baumler D.J., Peplinski R.G., Reed J.L., Glasner J.D., Perna N.T. The evolution of metabolic networks of E. coli. BMC Syst. Biol. 2011;5:182. doi: 10.1186/1752-0509-5-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Enzmann F., Mayer F., Rother M., Holtmann D. Methanogens: biochemical background and biotechnological applications. Amb. Express. 2018;8:1. doi: 10.1186/s13568-017-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farkas J.A., Picking J.W., Santangelo T.J. Genetic techniques for the archaea. Annu. Rev. Genet. 2013;47:539–561. doi: 10.1146/annurev-genet-111212-133225. [DOI] [PubMed] [Google Scholar]
- 39.Fink C., Martinez-Cano G., Shuster J., Panzera A., Angenent L.T., Molitor B. The targeted deletion of genes responsible for expression of the Mth60 fimbriae leads to loss of cell-cell connections in M. thermautotrophicus ΔH. bioRxiv. 2022 doi: 10.1101/2022.05.13.491833. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink C., Beblawy S., Enkerlin A.M., Mühling L., Angenent L.T., Molitor B. A shuttle-vector system allows heterologous gene expression in the thermophilic methanogen Methanothermobacter thermautotrophicus ΔH. mBio. 2021;12:e0276621. doi: 10.1128/mBio.02766-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thor S., Peterson J.R., Luthey-Schulten Z. Genome-scale Metabolic Modeling of Archaea Lends Insight into Diversity of Metabolic Function. Archaea. 2017;2017:9763848. doi: 10.1155/2017/9763848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker S.A., Palsson B.Ø. Context-specific metabolic networks are consistent with experiments. PLoS Comput. Biol. 2008;4:e1000082. doi: 10.1371/journal.pcbi.1000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White R.H. Purine biosynthesis in the domain Archaea without folates or modified folates. J. Bacteriol. 1997;179:3374–3377. doi: 10.1128/jb.179.10.3374-3377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei Y., Li B., Prakash D., Ferry J.G., Elliott S.J., Stubbe J. A ferredoxin disulfide reductase delivers electrons to the Methanosarcina barkeri class III ribonucleotide reductase. Biochemistry. 2015;54:7019–7028. doi: 10.1021/acs.biochem.5b01092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown A.M., Hoopes S.L., White R.H., Sarisky C.A. Purine biosynthesis in archaea: variations on a theme. Biol. Direct. 2011;6:63. doi: 10.1186/1745-6150-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchenau B., Thauer R.K. Tetrahydrofolate-specific enzymes in Methanosarcina barkeri and growth dependence of this methanogenic archaeon on folic acid or p-aminobenzoic acid. Arch. Microbiol. 2004;182:313–325. doi: 10.1007/s00203-004-0714-0. [DOI] [PubMed] [Google Scholar]
- 47.Schauer N.L., Ferry J.G. Properties of formate dehydrogenase in Methanobacterium formicicum. J. Bacteriol. 1982;150:1–7. doi: 10.1128/jb.150.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawers G., Watson G. A glycyl radical solution: oxygen-dependent interconversion of pyruvate formate-lyase. Mol. Microbiol. 1998;29:945–954. doi: 10.1046/j.1365-2958.1998.00941.x. [DOI] [PubMed] [Google Scholar]
- 49.Tanner R.S., McInerney M.J., Nagle D.P. Formate auxotroph of Methanobacterium thermoautotrophicum Marburg. J. Bacteriol. 1989;171:6534–6538. doi: 10.1128/jb.171.12.6534-6538.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valgepea K., de Souza Pinto Lemgruber R., Abdalla T., Binos S., Takemori N., Takemori A., Tanaka Y., Tappel R., Köpke M., Simpson S.D., et al. H2 drives metabolic rearrangements in gas-fermenting Clostridium autoethanogenum. Biotechnol. Biofuels. 2018;11:55. doi: 10.1186/s13068-018-1052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y., Nielsen J. Energy metabolism controls phenotypes by protein efficiency and allocation. Proc. Natl. Acad. Sci. USA. 2019;116:17592–17597. doi: 10.1073/pnas.1906569116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuchmann K., Müller V. Direct and reversible hydrogenation of CO2 to formate by a bacterial carbon dioxide reductase. Science. 2013;342:1382–1385. doi: 10.1126/science.1244758. [DOI] [PubMed] [Google Scholar]
- 53.Bertram P.A., Karrasch M., Schmitz R.A., Böcher R., Albracht S.P., Thauer R.K. Formylmethanofuran dehydrogenases from methanogenic Archaea Substrate specificity, EPR properties and reversible inactivation by cyanide of the molybdenum or tungsten iron-sulfur proteins. Eur. J. Biochem. 1994;220:477–484. doi: 10.1111/j.1432-1033.1994.tb18646.x. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe T., Pfeil-Gardiner O., Kahnt J., Koch J., Shima S., Murphy B.J. Three-megadalton complex of methanogenic electron-bifurcating and CO2-fixing enzymes. Science. 2021;373:1151–1156. doi: 10.1126/science.abg5550. [DOI] [PubMed] [Google Scholar]
- 55.Shlomi T., Cabili M.N., Herrgård M.J., Palsson B.Ø., Ruppin E. Network-based prediction of human tissue-specific metabolism. Nat. Biotechnol. 2008;26:1003–1010. doi: 10.1038/nbt.1487. [DOI] [PubMed] [Google Scholar]
- 56.Zur H., Ruppin E., Shlomi T. iMAT: an integrative metabolic analysis tool. Bioinformatics. 2010;26:3140–3142. doi: 10.1093/bioinformatics/btq602. [DOI] [PubMed] [Google Scholar]
- 57.Bordbar A., Mo M.L., Nakayasu E.S., Schrimpe-Rutledge A.C., Kim Y.M., Metz T.O., Jones M.B., Frank B.C., Smith R.D., Peterson S.N., et al. Model-driven multi-omic data analysis elucidates metabolic immunomodulators of macrophage activation. Mol. Syst. Biol. 2012;8:558. doi: 10.1038/msb.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nilsson A., Nielsen J., Palsson B.O. Metabolic models of protein allocation call for the kinetome. Cell Syst. 2017;5:538–541. doi: 10.1016/j.cels.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Birkel G.W., Ghosh A., Kumar V.S., Weaver D., Ando D., Backman T.W.H., Arkin A.P., Keasling J.D., Martín H.G. The JBEI quantitative metabolic modeling library (jQMM): a python library for modeling microbial metabolism. BMC Bioinf. 2017;18:205. doi: 10.1186/s12859-017-1615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fang X., Lloyd C.J., Palsson B.O. Reconstructing organisms in silico: genome-scale models and their emerging applications. Nat. Rev. Microbiol. 2020;18:731–743. doi: 10.1038/s41579-020-00440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thauer R.K. The Wolfe cycle comes full circle. Proc. Natl. Acad. Sci. USA. 2012;109:15084–15085. doi: 10.1073/pnas.1213193109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith D.R., Doucette-Stamm L.A., Deloughery C., Lee H., Dubois J., Aldredge T., Bashirzadeh R., Blakely D., Cook R., Gilbert K., et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J. Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rinke C., Chuvochina M., Mussig A.J., Chaumeil P.-A., Davín A.A., Waite D.W., Whitman W.B., Parks D.H., Hugenholtz P. A standardized archaeal taxonomy for the Genome Taxonomy Database. Nat. Microbiol. 2021;6:946–959. doi: 10.1038/s41564-021-00918-8. [DOI] [PubMed] [Google Scholar]
- 64.Liesegang H., Kaster A.-K., Wiezer A., Goenrich M., Wollherr A., Seedorf H., Gottschalk G., Thauer R.K. Complete genome sequence of Methanothermobacter marburgensis, a methanoarchaeon model organism. J. Bacteriol. 2010;192:5850–5851. doi: 10.1128/JB.00844-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Center for Biotechnology Information. 1988. https://www.ncbi.nlm.nih.gov/
- 66.Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M., et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deutsch E.W., Csordas A., Sun Z., Jarnuczak A., Perez-Riverol Y., Ternent T., Campbell D.S., Bernal-Llinares M., Okuda S., Kawano S. The ProteomeXchange Consortium in 2017: Supporting the Cultural Change in Proteomics Public Data Deposition. Nucleic Acids Res. 2016;45:gkw936. doi: 10.1093/nar/gkw936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deutsch E.W., Bandeira N., Sharma V., Perez-Riverol Y., Carver J.J., Kundu D.J., García-Seisdedos D., Jarnuczak A.F., Hewapathirana S., Pullman B.S., et al. The ProteomeXchange consortium in 2020: enabling ‘big data’approaches in proteomics. Nucleic Acids Res. 2020;48:D1145–D1152. doi: 10.1093/nar/gkz984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergmann F.T., Adams R., Moodie S., Cooper J., Glont M., Golebiewski M., Hucka M., Laibe C., Miller A.K., Nickerson D.P., et al. COMBINE archive and OMEX format: one file to share all information to reproduce a modeling project. BMC Bioinf. 2014;15:369. doi: 10.1186/s12859-014-0369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glont M., Nguyen T.V.N., Graesslin M., Hälke R., Ali R., Schramm J., Wimalaratne S.M., Kothamachu V.B., Rodriguez N., Swat M.J., et al. BioModels: expanding horizons to include more modelling approaches and formats. Nucleic Acids Res. 2018;46:D1248–D1253. doi: 10.1093/nar/gkx1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malik-Sheriff R.S., Glont M., Nguyen T.V.N., Tiwari K., Roberts M.G., Xavier A., Vu M.T., Men J., Maire M., Kananathan S., et al. BioModels—15 years of sharing computational models in life science. Nucleic Acids Res. 2020;48:D407–D415. doi: 10.1093/nar/gkz1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keating S.M., Waltemath D., König M., Zhang F., Dräger A., Chaouiya C., Bergmann F.T., Finney A., Gillespie C.S., Helikar T., et al. SBML Level 3: an extensible format for the exchange and reuse of biological models. Mol. Syst. Biol. 2020;16:e9110. doi: 10.15252/msb.20199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lieven C., Beber M.E., Olivier B.G., Bergmann F.T., Ataman M., Babaei P., Bartell J.A., Blank L.M., Chauhan S., Correia K., et al. MEMOTE for standardized genome-scale metabolic model testing. Nat. Biotechnol. 2020;38:272–276. doi: 10.1038/s41587-020-0446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.König M. fbc_curation: Reproducibility of constraint-based models. 2022. https://fbc-curation.readthedocs.io/en/latest/index.html
- 76.King Z.A., Dräger A., Ebrahim A., Sonnenschein N., Lewis N.E., Palsson B.O. Escher: a web application for building, sharing, and embedding data-rich visualizations of biological pathways. PLoS Comput. Biol. 2015;11:e1004321. doi: 10.1371/journal.pcbi.1004321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balch W.E., Fox G.E., Magrum L.J., Woese C.R., Wolfe R.S. Methanogens: reevaluation of a unique biological group. Microbiol. Rev. 1979;43:260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen J.N. Approach to steady state in completely mixed flow reactors. J. Environ. Eng. (New. York). 2001;127:13–18. [Google Scholar]
- 79.Cold Spring Harbor Protocols CTAB extraction buffer. Cold Spring Harbor. 2009 http://cshprotocols.cshlp.org/content/2009/10/pdb.rec11984.full [Google Scholar]
- 80.Kanehisa M., Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seaver S.M.D., Liu F., Zhang Q., Jeffryes J., Faria J.P., Edirisinghe J.N., Mundy M., Chia N., Noor E., Beber M.E., et al. The ModelSEED Biochemistry Database for the integration of metabolic annotations and the reconstruction, comparison and analysis of metabolic models for plants, fungi and microbes. Nucleic Acids Res. 2021;49:D575–D588. doi: 10.1093/nar/gkaa746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.UniProt Consortium UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47:D506–D515. doi: 10.1093/nar/gky1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeske L., Placzek S., Schomburg I., Chang A., Schomburg D. BRENDA in 2019: a European ELIXIR core data resource. Nucleic Acids Res. 2019;47:D542–D549. doi: 10.1093/nar/gky1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karp P.D., Billington R., Caspi R., Fulcher C.A., Latendresse M., Kothari A., Keseler I.M., Krummenacker M., Midford P.E., Ong Q., et al. The BioCyc collection of microbial genomes and metabolic pathways. Brief. Bioinform. 2019;20:1085–1093. doi: 10.1093/bib/bbx085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caspi R., Billington R., Keseler I.M., Kothari A., Krummenacker M., Midford P.E., Ong W.K., Paley S., Subhraveti P., Karp P.D. The MetaCyc database of metabolic pathways and enzymes-a 2019 update. Nucleic Acids Res. 2020;48:D445–D453. doi: 10.1093/nar/gkz862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Norsigian C.J., Pusarla N., McConn J.L., Yurkovich J.T., Dräger A., Palsson B.O., King Z. BiGG Models 2020: multi-strain genome-scale models and expansion across the phylogenetic tree. Nucleic Acids Res. 2020;48:D402–D406. doi: 10.1093/nar/gkz1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sayers E.W., Beck J., Bolton E.E., Bourexis D., Brister J.R., Canese K., Comeau D.C., Funk K., Kim S., Klimke W., et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2021;49:D10–D17. doi: 10.1093/nar/gkaa892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Satish Kumar V., Ferry J.G., Maranas C.D. Metabolic reconstruction of the archaeon methanogen Methanosarcina Acetivorans. BMC Syst. Biol. 2011;5:28. doi: 10.1186/1752-0509-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benedict M.N., Gonnerman M.C., Metcalf W.W., Price N.D. Genome-scale metabolic reconstruction and hypothesis testing in the methanogenic archaeon Methanosarcina acetivorans C2A. J. Bacteriol. 2012;194:855–865. doi: 10.1128/JB.06040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nazem-Bokaee H., Gopalakrishnan S., Ferry J.G., Wood T.K., Maranas C.D. Assessing methanotrophy and carbon fixation for biofuel production by Methanosarcina acetivorans. Microb. Cell Fact. 2016;15:10. doi: 10.1186/s12934-015-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peterson J.R., Thor S., Kohler L., Kohler P.R.A., Metcalf W.W., Luthey-Schulten Z. Genome-wide gene expression and RNA half-life measurements allow predictions of regulation and metabolic behavior in Methanosarcina acetivorans. BMC Genom. 2016;17:924. doi: 10.1186/s12864-016-3219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feist A.M., Scholten J.C.M., Palsson B.Ø., Brockman F.J., Ideker T. Modeling methanogenesis with a genome-scale metabolic reconstruction of Methanosarcina barkeri. Mol. Syst. Biol. 2006;2:2006.0004. doi: 10.1038/msb4100046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonnerman M.C., Benedict M.N., Feist A.M., Metcalf W.W., Price N.D. Genomically and biochemically accurate metabolic reconstruction of Methanosarcina barkeri Fusaro, iMG746. Biotechnol. J. 2013;8:1070–1079. doi: 10.1002/biot.201200266. [DOI] [PubMed] [Google Scholar]
- 94.Hamilton J.J., Calixto Contreras M., Reed J.L. Thermodynamics and H2 transfer in a methanogenic, syntrophic community. PLoS Comput. Biol. 2015;11:e1004364. doi: 10.1371/journal.pcbi.1004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goyal N., Widiastuti H., Karimi I.A., Zhou Z. A genome-scale metabolic model of Methanococcus maripaludis S2 for CO2 capture and conversion to methane. Mol. Biosyst. 2014;10:1043–1054. doi: 10.1039/c3mb70421a. [DOI] [PubMed] [Google Scholar]
- 96.Richards M.A., Lie T.J., Zhang J., Ragsdale S.W., Leigh J.A., Price N.D. Exploring hydrogenotrophic methanogenesis: a genome scale metabolic reconstruction of Methanococcus maripaludis. J. Bacteriol. 2016;198:3379–3390. doi: 10.1128/JB.00571-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shoaie S., Karlsson F., Mardinoglu A., Nookaew I., Bordel S., Nielsen J. Understanding the interactions between bacteria in the human gut through metabolic modeling. Sci. Rep. 2013;3:2532. doi: 10.1038/srep02532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Selkov E., Maltsev N., Olsen G.J., Overbeek R., Whitman W.B. A reconstruction of the metabolism of Methanococcus jannaschii from sequence data. Gene. 1997;197:GC11–GC26. doi: 10.1016/s0378-1119(97)00307-7. [DOI] [PubMed] [Google Scholar]
- 99.Hucka M., Bergmann F.T., Dräger A., Hoops S., Keating S.M., Le Novère N., Myers C.J., Olivier B.G., Sahle S., Schaff J.C., et al. The systems biology markup language (SBML): Language specification for level 3 version 1 core. J. Integr. Bioinform. 2018;15:20170081. doi: 10.1515/jib-2017-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Olivier B.G., Bergmann F.T. SBML level 3 package: flux balance constraints version 2. J. Integr. Bioinform. 2018;15:20170082. doi: 10.1515/jib-2017-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hucka M., Smith L.P. SBML level 3 package: groups, version 1 release 1. J. Integr. Bioinform. 2016;13:290. doi: 10.2390/biecoll-jib-2016-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Carey M.A., Dräger A., Beber M.E., Papin J.A., Yurkovich J.T. Community standards to facilitate development and address challenges in metabolic modeling. Mol. Syst. Biol. 2020;16:e9235. doi: 10.15252/msb.20199235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Juty N., Le Novère N., Laibe C. Identifiers. org and MIRIAM Registry: community resources to provide persistent identification. Nucleic Acids Res. 2012;40:D580–D586. doi: 10.1093/nar/gkr1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mahamkali V., McCubbin T., Beber M.E., Noor E., Marcellin E., Nielsen L.K. multiTFA: a Python package for multi-variate thermodynamics-based flux analysis. Bioinformatics. 2021;37:3064–3066. doi: 10.1093/bioinformatics/btab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henry C.S., DeJongh M., Best A.A., Frybarger P.M., Linsay B., Stevens R.L. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat. Biotechnol. 2010;28:977–982. doi: 10.1038/nbt.1672. [DOI] [PubMed] [Google Scholar]
- 106.Marcellin E., Behrendorff J.B., Nagaraju S., DeTissera S., Segovia S., Palfreyman R.W., Daniell J., Licona-Cassani C., Quek L.-E., Speight R., et al. Low carbon fuels and commodity chemicals from waste gases–systematic approach to understand energy metabolism in a model acetogen. Green Chem. 2016;18:3020–3028. [Google Scholar]
- 107.Ebrahim A., Lerman J.A., Palsson B.O., Hyduke D.R. COBRApy: constraints-based reconstruction and analysis for python. BMC Syst. Biol. 2013;7:74. doi: 10.1186/1752-0509-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duboc P., Schill N., Menoud L., Van Gulik W., Von Stockar U. Measurements of sulfur, phosphorus and other ions in microbial biomass: influence on correct determination of elemental composition and degree of reduction. J. Biotechnol. 1995;43:145–158. doi: 10.1016/0168-1656(95)00135-0. [DOI] [PubMed] [Google Scholar]
- 109.Valgepea K., Loi K.Q., Behrendorff J.B., Lemgruber R.d.S.P., Plan M., Hodson M.P., Köpke M., Nielsen L.K., Marcellin E. Arginine deiminase pathway provides ATP and boosts growth of the gas-fermenting acetogen Clostridium autoethanogenum. Metab. Eng. 2017;41:202–211. doi: 10.1016/j.ymben.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 110.Fagbadebo F.O., Kaiser P.D., Zittlau K., Bartlick N., Wagner T.R., Froehlich T., Jarjour G., Nueske S., Scholz A., Traenkle B., et al. A Nanobody-Based Toolset to Monitor and Modify the Mitochondrial GTPase Miro1. Front. Mol. Biosci. 2022;9:835302. doi: 10.3389/fmolb.2022.835302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Broadbent J.A., Broszczak D.A., Tennakoon I.U.K., Huygens F. Pan-proteomics, a concept for unifying quantitative proteome measurements when comparing closely-related bacterial strains. Expert Rev. Proteomics. 2016;13:355–365. doi: 10.1586/14789450.2016.1155986. [DOI] [PubMed] [Google Scholar]
- 112.Maillet N. Rapid Peptides Generator: fast and efficient in silico protein digestion. NAR Genom. Bioinform. 2020;2:lqz004. doi: 10.1093/nargab/lqz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Heirendt L., Arreckx S., Pfau T., Mendoza S.N., Richelle A., Heinken A., Haraldsdóttir H.S., Wachowiak J., Keating S.M., Vlasov V., et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v. 3.0. Nat. Protoc. 2019;14:639–702. doi: 10.1038/s41596-018-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gurobi Optimization LLC Gurobi Optimizer Reference Manual. 2020. https://www.gurobi.com
- 115.Koren S., Walenz B.P., Berlin K., Miller J.R., Bergman N.H., Phillippy A.M. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., McCarthy S.A., Davies R.M., Li H. Twelve years of SAMtools and BCFtools. GigaScience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuznetsov A., Bollin C.J. NCBI genome workbench: desktop software for comparative genomics, visualization, and GenBank data submission. Methods Mol. Biol. 2021;2231:261–295. doi: 10.1007/978-1-0716-1036-7_16. [DOI] [PubMed] [Google Scholar]
- 119.Li T., Zhang X., Luo F., Wu F.-X., Wang J. MultiMotifMaker: a multi-thread tool for identifying DNA methylation motifs from Pacbio reads. IEEE/ACM Trans. Comput. Biol. Bioinform. 2020;17:220–225. doi: 10.1109/TCBB.2018.2861399. [DOI] [PubMed] [Google Scholar]
- 120.Cock P.J.A., Antao T., Chang J.T., Chapman B.A., Cox C.J., Dalke A., Friedberg I., Hamelryck T., Kauff F., Wilczynski B., de Hoon M.J.L. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: architecture and applications. BMC Bioinf. 2009;10:421–429. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 124.Madden T. In: The NCBI Handbook. McEntyre J., Ostell J., editors. National Center for Biotechnology Information (US); 2002. The BLAST Sequence Analysis Tool. [Google Scholar]
- 125.Leimbach A. Bac-Genomics-Scripts: Bovine E. coli Mastitis Comparative Genomics Edition. 2016. https://github.com/aleimba/bac-genomics-scripts/blob/master/README.md#citation
- 126.Galperin M.Y., Wolf Y.I., Makarova K.S., Vera Alvarez R., Landsman D., Koonin E.V. COG database update: focus on microbial diversity, model organisms, and widespread pathogens. Nucleic Acids Res. 2021;49:D274–D281. doi: 10.1093/nar/gkaa1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mojica Prieto F.J., Millero F.J. The values of pK1+ pK2 for the dissociation of carbonic acid in seawater. Geochem. Cosmochim. Acta. 2002;66:2529–2540. [Google Scholar]
- 128.Wang N.S., Stephanopoulos G. Application of macroscopic balances to the identification of gross measurement errors. Biotechnol. Bioeng. 1983;25:2177–2208. doi: 10.1002/bit.260250906. [DOI] [PubMed] [Google Scholar]
- 129.Michael L.S., Kargi F. Prentice-Hall International; 2002. Bioprocess Engineering: Basic Concepts. [Google Scholar]
- 130.Bryant C. ResearchPy. 2018. https://github.com/researchpy/researchpy
- 131.Bushnell B. Lawrence Berkeley National Lab; 2014. BBMap: A Fast, Accurate, Splice-Aware Aligner. [Google Scholar]
- 132.Gordon A., Hannon G. Fastx-toolkit. FASTQ/A Short-Reads Pre-processing Tools. 2010. 2017. http://hannonlab.cshl.edu/fastx_toolkit
- 133.Li H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv. 2013 doi: 10.48550/arXiv.1303.3997. Preprint at. [DOI] [Google Scholar]
- 134.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 135.Patro R., Duggal G., Love M.I., Irizarry R.A., Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods. 2017;14:417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Waardenberg A.J., Field M.A. consensusDE: an R package for assessing consensus of multiple RNA-seq algorithms with RUV correction. PeerJ. 2019;7:e8206. doi: 10.7717/peerj.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mölder F., Jablonski K.P., Letcher B., Hall M.B., Tomkins-Tinch C.H., Sochat V., Forster J., Lee S., Twardziok S.O., Kanitz A. Sustainable Data Analysis with Snakemake. F1000Research. 2021;10 doi: 10.12688/f1000research.29032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 140.Wagner T., Watanabe T., Shima S. In: Biogenesis of Hydrocarbons. Stams A.J., Sousa D., editors. Springer Cham; 2019. Hydrogenotrophic Methanogenesis; pp. 79–107. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Supplemental tables can be found in Data_S1.xlsx. The genome sequences, annotations, and methylation patterns were deposited to NCBI65 and can be found under Bioproject ID PRJNA674001 (Data S1 - Table S1). The transcriptomic data (gene expression data) discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus66 and are accessible through GEO Series accession number GSE218145 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE218145). The mass spectrometry proteomic DDA data were deposited to the ProteomeXchange Consortium via the PRIDE partner repository67,68,69 with the dataset identifier PXD039574. The modeling-related files (Data S2) were deposited in an Open Modeling EXchange format (OMEX)70 to BioModels71,72 and assigned the identifiers MODEL2211290001 (M. thermautotrophicus ΔH), MODEL2211290002 (M. thermautotrophicus Z-245), and MODEL2211290003 (M. marburgensis Marburg). The pan-model is provided as an Excel® model (.xlsx, densely commented). The GEMs for each strain are provided in the following formats: .xml (SBML73), .json, and .mat. Additional files include: the benchmarking reports (.html) from MEMOTE,74 the FROG analysis files (.omex) from fbc_curation,75 and the map files for visualization (.json) with Escher.76 Further, the data used to constrain the models are provided as supplemental data. This includes: the gas fermentation data (as Excel® files, Data S3 and S6) and the individual strain and differential expression analyses data for both the transcriptomics and proteomics (as an Excel® file, Data S4). Data S1 and S3–S6 are available on GitHub at https://github.com/isacasini/Casini_2023_GEM.
-
•
Additional original code (scripts and programs) are found in Data S7. Data S7 is available on GitHub at https://github.com/isacasini/Casini_2023_GEM.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.