Abstract
Background
The high levels of C reactive protein (CRP) to albumin ratio (CAR) is thought to increase the risk of poor outcomes for cancer and cardiovascular disease (CVD). However, the association between CAR and CVD in the Chinese community population has not been investigated.
Objective
The aim of this study was to investigate the association between CAR and CVD in the Chinese community population.
Methods
A total of 62 067 participants without a history of CVD or cancer were included in this study. Kaplan-Meier survival curves were used to calculate the cumulative incidence of endpoint events in CAR quartile groups, and the results were tested by log-rank test. Fine-Gray model was used to analyse the competing risk of death. C-index, Net Reclassification Index (NRI) and Integrated Discrimination Improvement Index (IDI) of different indicators were calculated to distinguish the predictive performance of different indicators.
Results
During an average follow-up period of 10.3±2.1 years, 4025 participants developed CVD. In multivariable Cox regression analysis, compared with Q1 group, model 3 showed that the hazard ratio (HR) (95% confidence interval (95%CI)) of CVD in Q4 group was 1.26 (1.15 to 1.38) (p<0.01), and the HR (95% CI) per 1 SD increase was 1.06 (1.03 to 1.08) (p<0.01). The C-index, continuous NRI and IDI for predicting 10-year CVD were 73.48%, 0.1366 (0.1049 to 0.1684) (p<0.01) and 0.0002 (0.0001 to 0.0004) (p<0.01), respectively, which were higher than those of hs-CRP (C-index:0.7344, NRI:0.0711, IDI: 0.0001) and albumin (C-index:0.7339, NRI: −0.0090, IDI: 0.0000).
Conclusion
High levels of CAR can increase the risk of CVD and the predictive performance of CAR for CVD is better than that of hs-CRP or albumin alone.
Keywords: CARDIOVASCULAR DISEASES, COHORT STUDIES, EPIDEMIOLOGY
WHAT IS ALREADY KNOWN ON THIS TOPIC
Previous studies have found that C reactive protein (CRP) to albumin ratio (CAR) is associated with adverse cardiovascular events in patients with ST-segment elevation myocardial infarction and acute coronary syndrome, but the relationship between CAR and cardiovascular disease (CVD) has not been investigated in the Chinese community population.
WHAT THIS STUDY ADDS
High levels of CAR can increase the risk of CVD, and this risk-increasing effect is higher in young and middle-aged men populations and also shows site dependence, the risk of CVD is higher than that of cerebrovascular disease.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings provide evidence for exploring the mechanism of CAR and CVD. When screening high-risk populations for CVD, we should pay special attention to those with a simultaneous increase in CRP and a decrease in albumin.
Foreword
C reactive protein (CRP) and albumin are both synthesised by the liver and are markers of inflammation and biological reserve, respectively. Both CRP and albumin are independent predictors of cardiovascular disease (CVD).1–6 High-sensitivity CRP to albumin ratio (CAR), a new marker based on inflammation and nutritional status, has been identified as a prognostic marker for infections, rheumatic diseases, malignant tumours and critical diseases.7–16 CAR is a risk stratification index for patients with severe aortic stenosis undergoing transcatheter aortic valve replacement,17 and an independent predictor of death for ST-segment elevation myocardial infarction (STEMI) patients.18 It is more valuable than CRP or albumin in predicting adverse cardiovascular events and recanalisation after percutaneous coronary intervention,19 and predicting new-onset atrial fibrillation after coronary artery bypass surgery.20 However, the existing research on CAR mainly focuses on critical patients, tumour patients, stent and postoperative patients, and there is still a lack of research on the association between CAR and CVD in the Chinese community population. Therefore, the aim of this study was to investigate the association between CAR and CVD in the Chinese community population.
DATA COLLECTION AND METHODS
Study population
The Kailuan study (registration number: ChiCTR-TNC-11001489) is a prospective cohort study based on the Kailuan community in Tangshan City, which is a large and littoral modern city located in the central section of the circulating Bohai Sea Gulf region. The Kailuan community is a functional and comprehensive community owned and managed by Kailuan Group. The study was conducted from 2006 to 2007 at Kailuan General Hospital and its affiliated 11 hospitals, where the first physical examination was performed on employees and retirees of Kailuan Group company. Thereafter, physical examination was performed every 2 years for a total of 7 times until 31 December 2019, a total of 171 086 participants were included. We obtained the list of endpoint events through the Kailuan Medical Insurance and identified them by searching the medical records. The endpoint events were performed annually and have been followed up until 31 December, 2021. The enrolment time was the time of each health examination, and the follow-up time was the time of the occurrence of the endpoint event or the time of death or the end follow-up. Hs-CRP and albumin were detected at the third physical examination in 2010. Because the aim of this study was to investigate the hs-CRP/albumin ratio, people who participated in the 2010 health examination were included as the study subjects.
Inclusion and exclusion criteria
Inclusion criteria: patients who participated in the third health examination and had complete data on hs-CRP and albumin.
Exclusion criteria: patients with a history of myocardial infarction, stroke and cancer history were excluded; hs-CRP>86.0 mg/L (99.95 percentile) and albumin<18.8 g/L (0.05 percentile) or >75.4 g/L (99.95 percentile) were excluded, we did not exclude those patients because the 0.05 percentile for hs-CRP was 0 mg/L. The flow chart of subject inclusion and exclusion is shown in figure 1.
Figure 1.
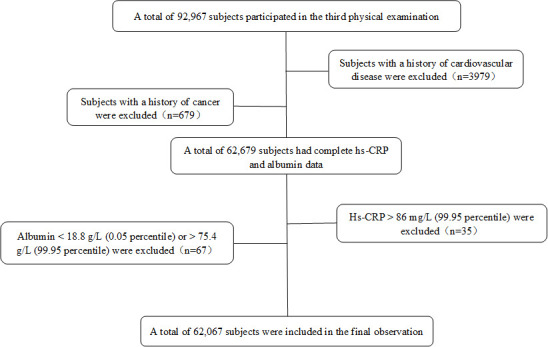
Inclusion and exclusion flow chart. Hs-CRP, high-sensitivity C reactive protein.
Data collection
Information on age, gender, smoking, alcohol use, physical exercise and self-reported medical history (hypertension, diabetes, cancer, etc) was collected through questionnaires by trained professionals. Medication information was obtained from subjects self-reported and outpatient medication information. Fasting blood samples of the subjects were collected for blood routine and blood biochemical measurements. Serum hs-CRP was determined by turbidimetry and serum albumin was determined by bromocresol green method. All tests were conducted at the Central Laboratory of Kailuan Hospital. Details refer to the published articles in our laboratory.21–23
The study used the completion of the 2010 physical examination as the starting point for follow-up. The occurrence of CVD was considered as the endpoint event, with the first occurrence of multiple CVD events being recorded as the occurrence of CVD. The study defined CVD as including acute myocardial infarction and stroke. Stroke was further classified into ischaemic stroke and haemorrhagic stroke, which included subarachnoid haemorrhage and cerebral haemorrhage stroke. The International Statistical Classification of Diseases and Related Health Problems 10th Revision was shown in online supplemental table 1. These events were confirmed by a qualified physician through inpatient medical records.
jech-2023-220760supp001.pdf (688.7KB, pdf)
Calculation formula and grouping of CAR ratio
To make the calculated CAR ratio non-zero, convert hs-CRP from 0 mg/L to 0.01 mg/L. The formula for CAR was hs-CRP divided by albumin. In the absence of accepted CAR cut-off values, the quartile was grouped according to CAR, group 1 (Q1) was less than 0.0136; group 2 (Q2) was between 0.0136 and 0.0267; group 3 (Q3) was between 0.0267 and 0.0622 and group 4 (Q4) was greater than or equal to 0.0622. Using Receiver Operating Characteristic analysis, an optimal cut-off value for the CAR was derived from the point with the maximum Youden index. This cut-off point for CAR was 0.0334, yielding 55.9% sensitivity and 52.7% specificity.
Statistical analysis
The normal measurement data were expressed as mean plus or minus SD ( ±s), and the analysis of variance was used for comparison between groups. Skewed distribution measurement data were expressed as medians (25 percentile and 75 percentile), and intergroup comparison was examined by rank-sum test. Enumeration data were expressed as use cases (%) and intergroup comparisons were performed using the χ2 test.
CAR was calculated and classified into quartiles, with CVD as the endpoint event, and the CAR quartiles as the independent variable, the cumulative incidence of endpoint events in different groups were described using Kaplan-Meier product limit methods and compared by log-rank tests. The multivariate Cox proportional hazard model was used to analyse the risk of endpoint events among different CAR groups. Model 1 adjusted for age (continuous) and gender (male or female); model 2 further adjusted for smoking (yes or no), alcohol intake ≥36 mL/day (yes or no), physical exercise (yes or no), higher education (yes or no), body mass index (BMI) (<28 kg/m2, ≥28 kg/m2), hypertension (yes or no), diabetes (yes or no), low-density lipoprotein cholesterol (continuous), high-density lipoprotein cholesterol (continuous), estimated glomerular filtration rate (<60 mL/min, ≥60 mL/min), alanine aminotransferase (ALT, continuous) on the basis of model 1; model 3 further adjusted for hypertensive drugs (yes or no), hypoglycaemic drugs (yes or no), and lipid-lowering drugs (yes or no) on the basis of model 2; model 4 further adjusted for hs-CRP (continuous) and albumin (continuous) in 2010 based on model 3. Multiplicative interactions between CAR group and age (continuous), gender (male or female), hypertension (yes or no), diabetes (yes or no),and taking cardiovascular medicines (including hypertensive and lipid-lowering drugs) (yes or no) were also included in the Cox model to explore potential interaction in the model 4. An interaction of p<0.1 was considered to be significant.24 If p for interaction <0.1, the Cox model was repeated after stratification by covariates.
To eliminate the effect of medication, acute infection and liver dysfunction on the results, subjects taking hypertensive, lipid-lowering and hypoglycaemic drugs, subjects with hs-CRP>10 mmol/L and positive for hepatitis B surface antigen (HbsAg (+)) were excluded. As death and CVD may compete with each other, the Fine-Gray model was used to control for the risk of death competition, and the differences in CVD risk among different CAR groups were analysed.
To evaluate the predictive performance of CAR for CVD, the C-index, Net Reclassification Index (NRI) and Integrated Discrimination Improvement Index (IDI) of the Prediction Model for Atherosclerotic Cardiovascular Disease Risk in China (China-PAR model), China-PAR model plus hs-CRP, China-PAR model plus albumin and China-PAR model plus CAR were calculated to compare the predictive performance of different indicators for endpoint events.
Statistical analysis were carried out by using SAS V.9.4 (SAS Institute) and p values <0.05 (two-sided test) were regarded as statistically significant.
Results
Basic characteristics
A total of 92 967 subjects were initially included in the third health examination. After excluding 3979 subjects with a history of CVD and 679 subjects with a history of cancer, leaving 66 827 subjects with complete data. At the third health examination, an additional 35 subjects with hs-CRP levels >86.0 mg/L (99.95 percentile) and 67 subjects with albumin levels <18.8 g/L (0.05 percentile) or >75.4 g/L (99.95 percentile) were excluded. Finally, a total of 62 067 subjects were included in the study, of which 46 503 (74.9%) were male. The mean age was 52.4±13.0 years old, mean hs-CRP was 2.4±4.5 mg/L, mean albumin was 46.5±3.1 g/L, and mean CAR was 0.05±0.10 (table 1).
Table 1.
Baseline characteristics of different CAR groups in the total population
| Total (n=62 067) | Q1 (n=15 614) | Q2 (n=15 419) | Q3 (n=15 519) | Q4 (n=15 515) | |
| Male n (%) | 46 503 (74.9) | 11 415 (73.1) | 11 199 (72.6) | 11 858 (76.4) | 12 031 (77.5) |
| Female n (%) | 15 564 (25.1) | 4199 (26.9) | 4220 (27.4) | 3661 (23.6) | 3484 (22.5) |
| Age (years) | 52.4±13.0 | 50.4±13.4 | 53.0±12.9 | 52.9±12.6 | 53.4±13.0 |
| Smoker n (%) | 28 464 (45.9) | 6829 (43.7) | 6746 (43.8) | 7339 (47.3) | 7550 (48.7) |
| Alcohol intake≥36 mL/day, n (%) | 2956 (4.8) | 727 (4.7) | 811 (5.3) | 785 (5.1) | 633 (4.1) |
| Higher education n (%) | 10 607 (17.1) | 3548 (22.7) | 2713 (17.6) | 2338 (15.1) | 2008 (12.9) |
| Physical exercise n (%) | 41 118 (66.2) | 9895 (63.4) | 10 127 (65.7) | 10 471 (67.5) | 10 625 (68.5) |
| Body mass index kg/m2 | 25.0±3.4 | 23.8±3.2 | 24.6±3.2 | 25.5±3.3 | 25.9±3.6 |
| Waist cm | 87.4±10.3 | 84.8±10.2 | 86.7±9.9 | 88.5±10.0 | 89.7±10.4 |
| LDL-c mmol/L | 2.5±0.9 | 2.4±0.8 | 2.6±0.8 | 2.6±0.9 | 2.5±1.0 |
| HDL-c mmol/L | 1.6±0.5 | 1.6±0.5 | 1.7±0.5 | 1.5±0.4 | 1.4±0.4 |
| Total cholesterol mmol/L | 5.0±1.0 | 4.9±1.0 | 4.9±1.0 | 5.0±1.0 | 5.0±1.0 |
| Triglyceride mmol/L | 1.3 (0.9,1.9) | 1.1 (0.8,1.7) | 1.3 (0.9,1.8) | 1.4 (0.9,2.1) | 1.4 (1.0,2.1) |
| Systolic blood pressure mmHg | 130.0±19.5 | 125.8±18.9 | 129.0±19.4 | 131.9±19.2 | 133.1±19.8 |
| Diastolic blood pressure mmHg | 83.8±10.9 | 81.8±10.7 | 83.2±10.8 | 84.9±10.8 | 85.3±11.0 |
| hs-CRP mg/L | 1.2 (0.6, 2.9) | 0.3 (0.1, 0.5) | 0.9 (0.8, 1.0) | 1.9 (1.5, 2.3) | 5.0 (3.7, 7.5) |
| Serum albumin g/L | 46.5±3.1 | 46.6±2.9 | 46.6±3.1 | 46.5±3.2 | 46.3±3.1 |
| Alanine aminotransferase U/L | 21.8±17.0 | 19.4±13.8 | 20.8±14.7 | 23.0±18.6 | 23.9±19.6 |
| Hypertension n (%) | 35 478 (57.2) | 7291 (46.7) | 8527 (55.3) | 9581 (61.7) | 10 079 (65.0) |
| Diabetes n (%) | 6324 (10.2) | 1133 (7.3) | 1429 (9.3) | 1693 (10.9) | 2069 (13.3) |
| eGFR mL/min | 93.4±19.3 | 98.1±18.1 | 95.1±18.4 | 91.4±19.9 | 89.2±19.5 |
| HbsAg (+) n (%) | 14 225 (23.5) | 3829 (25.2) | 3744 (24.9) | 3465 (22.9) | 3187 (21.1) |
| Hypotensive drugs n (%) | 13 640 (22.0) | 2651 (16.7) | 3383 (21.9) | 3614 (23.3) | 4028 (26.0) |
| Lipid-lowing treatment n (%) | 1813 (2.9) | 344 (2.2) | 477 (3.1) | 475 (3.1) | 517 (3.3) |
| Hypoglycaemic drugs n (%) | 3226 (5.2) | 645 (4.1) | 821 (5.3) | 823 (5.3) | 937 (6.0) |
| Family history of CVD n (%) | 7644 (12.3) | 2009 (12.9) | 2124 (13.8) | 1874 (12.1) | 1637 (10.6) |
Measurement data with normal distribution were presented as ( ±s), count data as %, and skewed distribution data as median (25th, 75th).
All p values were significant at a level <0.01.
CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbsAg (+), positive for hepatitis B surface antigen; HDL-c, high-density lipoprotein cholesterol; Hs-CRP, high-sensitivity C reactive protein; LDL-c, low-density lipoprotein cholesterol.
Comparison of baseline data in different groups
The study subjects were divided into four groups according to their CAR levels, subjects in group Q1, Q2, Q3 and Q4 were 15 614, 15 419, 15 519 and 15 515, respectively. Compared with Q1, the Q2, Q3 and Q4 had increased proportions of individuals who smoked, engaged in physical exercise, had hypertension, diabetes and were taking hypertensive drugs. Additionally, the Q2, Q3 and Q4 had increased BMI, waist circumference, hs-CRP, ALT, systolic and diastolic blood pressure levels. On the other hand, the proportion of individuals with higher education and HbsAg (+) showed a decreasing trend. The estimated glomerular filtration rate also showed a decreasing trend, and all the differences were statistically significant (p<0.01) (table 1).
CVD incidence
The starting point of the follow-up was the completion of the third health examination and the last follow-up was conducted on 31 December 2021. The mean follow-up time was 10.3±2.1 years. During the follow-up period, there were 4025 cases of CVD events, including 816 cases of acute myocardial infarction and 3212 cases of stroke (including 344 cases of haemorrhagic stroke and 2942 cases of ischaemic stroke). The cumulative incidence of CVD, acute myocardial infarction, stroke, haemorrhagic stroke and ischaemic stroke among different CAR groups was 5.18% vs 6.30% vs 7.00% vs 8.51%, 0.89% vs 1.07% vs 1.48% vs 2.08%, 4.24% vs 5.32% vs 5.63% vs 6.61%, 0.08% vs 0.06% vs 0.07% vs 0.08% and 3.72% vs 4.82% VS 5.07% vs 5.98%, respectively. Kaplan-Meier analysis and log-rank test indicated significant differences in the cumulative incidence of CVD, acute myocardial infarction, stroke, haemorrhagic stroke and ischaemic stroke among different CAR subgroups (online supplemental figures 1 and 2).
Cox proportional hazard model affecting endpoint events
The Cox proportional hazard regression was conducted with CVD (assignment: no=0, yes=1) as the dependent variable and CAR group as the independent variable. Compared with the Q1 group, in model 3, the hazard ratio (HR) (95% confidence interval (95%CI)) of CVD, acute myocardial infarction, stroke, haemorrhagic stroke and ischaemic stroke in the Q4 group was 1.26 (1.15 to 1.38), 1.61 (1.31 to 1.97), 1.18 (1.07 to 1.31), 0.95 (0.72 to 1.25) (p=0.72) and 1.22 (1.09 to 1.36), respectively. With an increase of 1SD for each additional was 1.06 (1.03 to 1.08), 1.11 (1.06 to 1.16), 1.04 (1.01 to 1.07), 1.06 (0.98 to 1.14) (p=0.15) and 1.04 (1.01 to 1.07), respectively. In model 4, after adjusting for hs-CRP and albumin in 2010, showed results generally consistent with those of model 3 (table 2).
Table 2.
Cox proportional hazards model analysis of different CAR groups and incidence of endpoint events in the total population
| CAR groups | Events/total population | Incidence density/103 person-years | Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
Model 4 HR (95% CI) |
| CVD | ||||||
| Q1 | 773/15 614 | 4.79 | Ref | 1Ref | Ref | Ref |
| Q2 | 931/15 419 | 5.82 | 1.08 (0.98 to 1.19) | 1.01 (0.91 to 1.11) | 1.00 (0.91 to 1.10) | 1.00 (0.91 to 1.10) |
| Q3 | 1043/15 519 | 6.51 | 1.26 (1.15 to 1.38) | 1.07 (0.98 to 1.18) | 1.07 (0.98 to 1.18) | 1.07 (0.97 to 1.18) |
| Q4 | 1278/15 515 | 8.12 | 1.56 (1.42 to 1.71) | 1.27 (1.16 to 1.39) | 1.26 (1.15 to 1.38) | 1.24 (1.12 to 1.38) |
| Increase 1 SD | 1.08 (1.06 to 1.11) | 1.06 (1.03 to 1.08) | 1.06 (1.03 to 1.08) | 1.04 (1.00 to 1.07) | ||
| Acute myocardial infarction | ||||||
| Q1 | 143/15 614 | 0.89 | Ref | Ref | Ref | Ref |
| Q2 | 153/15 419 | 0.96 | 0.96 (0.76 to 1.21) | 0.87 (0.69 to 1.10) | 0.87 (0.69 to 1.09) | 0.87 (0.69 to 1.09) |
| Q3 | 215/15 519 | 1.34 | 1.40 (1.14 to 1.73) | 1.17 (0.95 to 1.45) | 1.17 (0.94 to 1.45) | 1.17 (0.94 to 1.45) |
| Q4 | 305/15 515 | 1.94 | 1.98 (1.63 to 2.42) | 1.61 (1.32 to 1.98) | 1.61 (1.31 to 1.97) | 1.63 (1.29 to 2.05) |
| Increase 1 SD | 1.12 (1.08 to 1.17) | 1.11 (1.06 to 1.16) | 1.11 (1.06 to 1.16) | 1.07 (1.01 to 1.14) | ||
| Stroke | ||||||
| Q1 | 630/15 614 | 3.90 | Ref | Ref | Ref | Ref |
| Q2 | 778/15 419 | 4.86 | 1.11 (1.00 to 1,23) | 1.04 (0.93 to 1.15) | 1.03 (0.93 to 1.14) | 1.03 (0.93 to 1.14) |
| Q3 | 830/15 519 | 5.18 | 1.23 (1.11 to 1.37) | 1.06 (0.95 to 1.17) | 1.05 (0.95 to 1.17) | 1.05 (0.95 to 1.17) |
| Q4 | 974/15 515 | 6.19 | 1.45 (1.31 to 1.60) | 1.19 (1.07 to 1.32) | 1.18 (1.07 to 1.31) | 1.15 (1.02 to 1.30) |
| Increase 1 SD | 1.07 (1.04 to 1.10) | 1.04 (1.01 to 1.07) | 1.04 (1.01 to 1.07) | 1.02 (0.98 to 1.06) | ||
| Haemorrhagic stroke | ||||||
| Q1 | 84/15 614 | 0.52 | Ref | Ref | Ref | Ref |
| Q2 | 79/15 419 | 0.49 | 0.85 (0.63 to 1.16) | 0.82 (0.61 to 1.09) | 0.81 (0.61 to 1.07) | 0.81 (0.61 to 1.07) |
| Q3 | 84/15 519 | 0.52 | 0.94 (0.69 to 1.27) | 0.88 (0.67 to 1.17) | 0.88 (0.67 to 1.16) | 0.88 (0.67 to 1.16) |
| Q4 | 97/15 515 | 0.62 | 1.09 (0.81 to 1.46) | 0.96 (0.73 to 1.27) | 0.95 (0.72 to 1.25) | 0.98 (0.71 to 1.36) |
| Increase 1 SD | 1.06 (0.98 to 1.15) | 1.06 (0.98 to 1.14) | 1.06 (0.98 to 1.14) | 1.05 (0.94 to 1.18) | ||
| Ischaemic stroke | ||||||
| Q1 | 565/15 614 | 3.50 | Ref | Ref | Ref | Ref |
| Q2 | 716/15 419 | 4.47 | 1.14 (1.02 to 1.27) | 1.07 (0.95 to 1.20) | 1.06 (0.95 to 1.19) | 1.05 (0.95 to 1.19) |
| Q3 | 766/15 519 | 4.78 | 1.27 (1.14 to 1.41) | 1.08 (0.97 to 1.21) | 1.08 (0.97 to 1.21) | 1.08 (0.97 to 1.21) |
| Q4 | 895/15 515 | 5.68 | 1.49 (1.34 to 1.65) | 1.22 (1.09 to 1.36) | 1.22 (1.09 to 1.36) | 1.19 (1.05 to 1.35) |
| Increase 1 SD | 1.07 (1.04 to 1.10) | 1.04 (1.01 to 1.07) | 1.04 (1.01 to 1.07) | 1.02 (0.98 to 1.06) | ||
Model 1 adjusted for age (continuous) and gender (male or female); Model 2 further adjusted for smoking (yes or no), alcohol intake ≥36 mL/day (yes or no), physical exercise (yes or no), higher education (yes or no), body mass index (<28 kg/m2, ≥28 kg/m2), hypertension (yes or no), diabetes (yes or no), low-density lipoprotein cholesterol (continuous), high-density lipoprotein cholesterol (continuous), estimated glomerular filtration rate (<60 mL/min, ≥60 mL/min), alanine aminotransferase (continuous) on the basis of model 1; model 3 further adjusted for hypertensive drugs (yes or no), hypoglycaemic drugs (yes or no), and lipid-lowering drugs (yes or no) on the basis of model 2; model 4 further adjusted for hs-CRP (continuous) and albumin (continuous) in 2010 based on model 3.
Bold font indicated statistical significance with p<0.05.
CAR, high-sensitivity C reactive protein to albumin ratio; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; hs-CRP, high-sensitivity C reactive protein; Increase 1 SD, increase high-sensitivity C reactive protein to albumin ratio by 1-unit standard deviation.
Stratified analysis
The p values for interaction between CAR group and gender, age, hypertension, diabetes and taking cardiovascular medicines or not were 0.19, <0.01, 0.16, 0.18 and 0.07, respectively. An interaction of p<0.1 was considered to be significant. Although there was no interaction between CAR group and gender, stratified analysis was conducted due to physiological differences among different subjects. For males versus females, the HR (95% CI) of Q4 for CVD were 1.26 (1.14 to 1.39) vs 1.17 (0.90 to 1.53). For age groups <60 years versus ≥60 years, the HR (95% CI) Of Q4 group for CVD were 1.33 (1.12 to 1.50) vs 1.16 (1.00 to 1.33). For subjects who taking cardiovascular medicines versus subjects who taking no cardiovascular medicines, the HR (95% CI) of Q4 group for CVD were 1.19 (1.02 to 1.38) vs 1.28 (1.14 to 1.44) (online supplemental table 2).
Sensitivity analysis
We excluded 15 880 subjects who had taken hypertensive, lipid-lowering and hypoglycaemic drugs. In model 3, the HR (95% CI) of CVD in the Q4 group was 1.34 (1.19 to 1.51) as compared with Q1 group (online supplemental table 3). A total of 2339 subjects with hs-CRP>10 mg/L and 14 225 subjects with HbsAg (+) were also excluded from the Cox model, respectively. These were shown in online supplemental tables 4 and 5. As there were 4738 all-cause mortality in the overall population, we performed a death competition risk analysis, in model 4, the HR (95% CI) of Q3 and Q4 were 1.10 (1.03 to 1.17) and 1.25 (1.16 to 1.34). The results showed that there was no competitive relationship between CVD and the occurrence of all-cause mortality in this study (online supplemental table 6).
C-index, NRI and IDI
The addition of CAR to the China-PAR model in the overall population resulted in a higher predictive performance for the risk of CVD within 10 years compared with the addition of hs-CRP or albumin. The C-index for predicting the occurrence of CVD was 73.48%, which was a 0.09% improvement compared with the China-PAR model alone (C-index=73.39%). The continuous NRI was 0.1366 (95% CI 0.1049 to 0.1684) and the IDI was 0.0002 (95% CI 0.0001 to 0.0004) (table 3 and online supplemental table 7).
Table 3.
C-index, NRI and IDI were used to compare the predictive performance of different indicators for CVD
| Predictor | C-index (95% CI) | Continuous NRI (95% CI) | P value | Absolute IDI (95% CI) | P value |
| China-PAR model | 0.7339 (0.7270 to 0.7406) | Ref | Ref | ||
| China-PAR model+hs CRP | 0.7344 (0.7276 to 0.7411) | 0.0711 (0.0477 to 0.0946) | <0.01 | 0.0001 (0.0000 to 0.0002) | 0.12 |
| China-PAR model+albumin | 0.7339 (0.7271 to 0.7407) | −0.0090 (-0.0261 to 0.0081) | 0.58 | 0.0000 (0.0000 to 0.0000) | 0.32 |
| China-PAR model+CAR | 0.7348 (0.7280 to 0.7416) | 0.1366 (0.1049 to 0.1684) | <0.01 | 0.0002 (0.0001 to 0.0004) | <0.01 |
CAR, high-sensitivity C reactive protein to albumin ratio; China-PAR model, Prediction Model for Atherosclerotic Cardiovascular Disease Risk in China; CVD, cardiovascular disease; hs-CRP, high-sensitivity C reactive protein; IDI, Integrated Discrimination Index; NRI, Net Reclassification Index.
Discussion
Our main findings are that a new marker based on inflammation and nutrition, CAR, can increase the risk of CVD when elevated, and this risk-increasing effect is independent of the traditional risk factors and hs-CRP and albumin alone. Moreover, this increased risk is higher in young-aged and middle-aged men populations. Its risk also shows site dependence, and the risk of CVD is higher than that of cerebrovascular disease. Its predictive performance for CVD was higher than that of hs-CRP and albumin alone.
In our community-based prospective study of 62 067 subjects with an average follow-up of 10.3±2.1 years, we found that a high CAR was associated with an increased risk of CVD, even after adjusting for other risk factors for CVD. Previous studies focused on populations with acute coronary syndrome or STEMI, but we explored the relationship between CAR and CVD in the Chinese community population for the first time. In our study, the Q4 group risk of CVD was 26% higher than Q1 group, and every additional SD increased the risk of CVD by 6%, the C-index of CVD was 0.7348, which was better predictive performance than hs-CRP (0.7344) and albumin (0.7339). We further adjusted for hs-CRP and albumin at baseline, and the results were consistent with those previously reported, indicated that high CAR increased the risk of CVD independent of hs-CRP and albumin. In a study of 539 patients with STEMI who received direct percutaneous coronary intervention,25 CAR had a higher predictive performance for major adverse cardiovascular events (Area Under the Curve of 0.770) compared with CRP (0.761) and albumin (0.658). Another study of 659 patients with acute coronary syndrome found that the risk of major adverse cardiovascular events increased by 27.6% in the high CAR group compared with the low CAR group.26 Finally, a study of 9375 patients with coronary heart disease receiving drug-eluting stent implantation also found that CAR was an independent predictor of the occurrence of adverse cardiovascular events and myocardial infarction.27 Our findings are consistent with previous studies.
We performed stratified analysis by gender, age and taking cardiovascular medicines or not. We found that high CAR could increase the risk of CVD only in men. We stratified the study population into young-aged and middle-aged (<60 years) and elderly (≥60 years). Compared with the elderly, the CVD risk caused by high CAR was significantly increased in the young and middle-aged population. In the stratified analysis by cardiovascular medicines use or not, high CAR was associated with increased CVD risk regardless of whether taking cardiovascular medicines or not, and the CVD risk was higher in people not taking cardiovascular medicines than in those taking cardiovascular medicines. Therefore, we found that young-aged and middle-aged men should be our focus when preventing CVD in people with high CAR. Moreover, cardiovascular medications (including hypertensive drugs and lipid-lowering drugs) could reduce the high CVD risk attributable to high CAR. However, there has been no relevant research on different age and gender in the Chinese community population. Therefore, more research is needed to further explore its clinical significance.
In addition, we found that the increased CVD risk associated with high CAR was site-dependent. Specifically, in the Chinese community population, we observed a 61% increase in the risk of acute myocardial infarction and an 18% increase in the risk of stroke in group Q4 compared with group Q1. This is consistent with previous studies that have shown a site-specific association between CRP and adverse outcomes, as opposed to albumin. For example, a meta-analysis showed that high CRP was associated with a 59% increased risk of non-fatal myocardial infarction, which was higher than the 39% increased risk of stroke.28 Another study on albumin and CVD found that low albumin was associated with a 25% increased risk of myocardial infarction, which was lower than the 37% increased risk of stroke.4 However, since this is the first study to show site-dependence in the onset risk of CAR, further exploration is needed to fully understand its clinical significance.
After excluding individuals taking drugs that may affect CVD risk, we observed that the Chinese community population had a higher risk of CVD (online supplemental table 3), which was reduced after adjusting for drugs (table 2). This indicates that hypertensive, lipid-lowering and hypoglycaemic drugs may lower the CVD risk associated with high CAR, which is consistent with previous studies.29–31 This finding is clinically significant because the usage rates of these drugs in the Chinese population are still low. In China, hypertension and diabetes treatment rates were 34.9% and 34.1% in 2018, hyperlipidaemia treatment rates were 5.5% in 2015.32–34 We also conducted sensitivity analyses by excluding individuals with hs-CRP>10 mg/L and HbsAg (+), and found results consistent with those in the overall population (online supplemental tables 4 and 5). Additionally, we did not find any competitive relationship between CVD and death in our analysis of death competitive risk. These sensitivity analyses further support the reliability of our study’s findings.
The reason why CAR leads to an increased risk of CVD is still unclear, although it may be related to an increase in CRP and a decrease in albumin. Previous studies have reported that both CRP and albumin are independent predictors of CVD.1–6 In a prospective study of 268 803 subjects with a mean follow-up of 4.49 years, the risk of disease increased by 87% in females and 30% in males in group Q4 compared with group Q1.3 Another cohort study involving 100 520 subjects showed that the CVD risk in the low serum albumin group (albumin<35 g/L) was 22% higher than that in the normal group (40–44.9 g/L).4 Previous studies have shown that both elevated CRP and decreased albumin may increase the risk of CVD, and when both elevated CRP and decreased albumin appear at the same time, the risk of CVD is even higher. Therefore, when screening high-risk populations for CVD, we should pay special attention to those with a simultaneous increase in CRP and decrease in albumin.
Our study has several advantages. First, it was a large prospective cohort study with a mean follow-up of 10.3 years. This allowed us to directly assess the long-term effects of CAR on CVD risk. Second, all outcome events were confirmed by qualified physicians through inpatient records, which enhanced the reliability of events. Third, we corrected for identified confounders, including traditional risk factors such as age, gender, blood lipid and blood pressure, as well as non-traditional risk factors such as estimated glomerular filtration rate and ALT. We also adjusted for baseline hs-CRP and albumin, and performed stratification and sensitivity analyses. These steps made our results relatively reliable.
This study also has some limitations. First, we cannot establish a causal relationship between CAR and CVD risk because it is an observational study. Therefore, our findings need to be confirmed in future studies. Additionally, while we corrected for potential CVD risk factors, this study remains observational, and we cannot rule out the possibility of residual or unmeasured confounding factors. Finally, most of the participants in our study were male enrolled workers. As a result, these findings may not be directly applicable to the Chinese general population.
Conclusion
In summary, high levels of CAR can increase the risk of CVD and with a better predictive performance than hs-CRP and albumin alone.
Acknowledgments
We thank all study participants, their relatives, the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group and the Kailuan Group.
Footnotes
Contributors: XuY and XiY: contributed to the study concept and design, analysis and interpretation of data, drafting of the manuscript, and served as the equally contributing first authors of the manuscript. JY and XW: supervised the analysis, interpreted the data and commented on the drafts. SW: contributed to article writing and data review. LC: contributed to the study concept and design, study supervision or coordination, revisions of the manuscript and served as the corresponding authors of the manuscript. XuY, XiY and LC: guarantors of the paper, and they can accept full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. The datasets used for the present analysis may be made available on reasonable request by contacting the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Ethics Committee of Kailuan General Hospital and conducted in accordance with the principles of the Declaration of Helsinki. All participants provided written informed consent.
References
- 1. Kaptoge S, Di Angelantonio E, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med 2012;367:1310–20. 10.1056/NEJMoa1107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yousuf O, Mohanty BD, Martin SS, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol 2013;62:397–408. 10.1016/j.jacc.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 3. Sung K-C, Ryu S, Chang Y, et al. C-reactive protein and risk of cardiovascular and all-cause mortality in 268 803 East Asians. Eur Heart J 2014;35:1809–16. 10.1093/eurheartj/ehu059 [DOI] [PubMed] [Google Scholar]
- 4. Ronit A, Kirkegaard-Klitbo DM, Dohlmann TL, et al. Plasma albumin and incident cardiovascular disease: results from the CGPS and an updated meta-analysis. Arterioscler Thromb Vasc Biol 2020;40:473–82. 10.1161/ATVBAHA.119.313681 [DOI] [PubMed] [Google Scholar]
- 5. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med 2018;52:8–12. 10.1016/j.ejim.2018.04.014 [DOI] [PubMed] [Google Scholar]
- 6. Manolis AA, Manolis TA, Melita H, et al. Low serum albumin: A neglected Predictor in patients with cardiovascular disease. Eur J Intern Med 2022;102:24–39. 10.1016/j.ejim.2022.05.004 [DOI] [PubMed] [Google Scholar]
- 7. Ranzani OT, Zampieri FG, Forte DN, et al. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One 2013;8:e59321. 10.1371/journal.pone.0059321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang P, Kang W, Li Y, et al. C-reactive protein-to-albumin ratio as an early biomarker to identify sepsis in neonates with pneumonia. Mediators Inflamm 2022;2022:4711018. 10.1155/2022/4711018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. He Y, Tang J, Wu B, et al. Correlation between albumin to fibrinogen ratio, C-reactive protein to albumin ratio and Th17 cells in patients with rheumatoid arthritis. Clin Chim Acta 2020;500:149–54. 10.1016/j.cca.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 10. Yang W-M, Zhang W-H, Ying H-Q, et al. Two new inflammatory markers associated with disease activity Score-28 in patients with rheumatoid arthritis: albumin to fibrinogen ratio and C-reactive protein to albumin ratio. Int Immunopharmacol 2018;62:293–8. 10.1016/j.intimp.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 11. Oubaya N, Soubeyran P, Reinald N, et al. Prognostic value of routinely measured inflammatory biomarkers in older cancer patients: pooled analysis of three cohorts. Cancers (Basel) 2021;13:6154. 10.3390/cancers13246154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou J, Wei W, Hou H, et al. Prognostic value of C-reactive protein, Glasgow Prognostic score, and C-reactive protein-to-albumin ratio in colorectal cancer. Front Cell Dev Biol 2021;9:637650. 10.3389/fcell.2021.637650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishizuka M, Nagata H, Takagi K, et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol 2016;23:900–7. 10.1245/s10434-015-4948-7 [DOI] [PubMed] [Google Scholar]
- 14. Park JE, Chung KS, Song JH, et al. The C-reactive protein/albumin ratio as a Predictor of mortality in critically ill patients. J Clin Med 2018;7:333. 10.3390/jcm7100333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oh TK, Song I-A, Lee JH. Clinical usefulness of C-reactive protein to albumin ratio in predicting 30-day mortality in critically ill patients: A retrospective analysis. Sci Rep 2018;8:14977. 10.1038/s41598-018-33361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: A retrospective cohort study: Erratum. Medicine (Baltimore) 2019;98:e16675. 10.1097/MD.0000000000016675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seoudy H, Shamekhi J, Voigtländer L, et al. C-reactive protein to albumin ratio in patients undergoing Transcatheter aortic valve replacement. Mayo Clin Proc 2022;97:931–40. 10.1016/j.mayocp.2021.11.022 [DOI] [PubMed] [Google Scholar]
- 18. Söğüt Ö, Akdemir T, Can MM. Prognostic value of the C-reactive protein to albumin ratio in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Turk J Med Sci 2021;51:1281–8. 10.3906/sag-2003-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cheng L, Meng Z, Wang Q, et al. The usefulness of C-reactive protein to albumin ratio in the prediction of adverse cardiovascular events in coronary chronic total occlusion undergoing percutaneous coronary intervention. Front Cardiovasc Med 2021;8:731261. 10.3389/fcvm.2021.731261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aksoy F, Uysal D, Ibrişim E. Relationship between C-reactive protein/albumin ratio and new-onset atrial fibrillation after coronary artery bypass Grafting. Rev Assoc Med Bras (1992) 2020;66:1070–6. 10.1590/1806-9282.66.8.1070 [DOI] [PubMed] [Google Scholar]
- 21. Wang F, Wu S, Song Y, et al. Waist circumference, body mass index and waist to hip ratio for prediction of the metabolic syndrome in Chinese. Nutr Metab Cardiovasc Dis 2009;19:542–7. 10.1016/j.numecd.2008.11.006 [DOI] [PubMed] [Google Scholar]
- 22. Wu S, Li Y, Jin C, et al. Intra-individual variability of high-sensitivity C-reactive protein in Chinese general population. Int J Cardiol 2012;157:75–9. 10.1016/j.ijcard.2010.12.019 [DOI] [PubMed] [Google Scholar]
- 23. Wu S, Huang Z, Yang X, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes 2012;5:487–93. 10.1161/CIRCOUTCOMES.111.963694 [DOI] [PubMed] [Google Scholar]
- 24. Sessler DI, Pei L, Li K, et al. Aggressive intraoperative warming versus routine thermal management during non-cardiac surgery (PROTECT): a Multicentre, parallel group, superiority trial. Lancet 2022;399:1799–808. 10.1016/S0140-6736(22)00560-8 [DOI] [PubMed] [Google Scholar]
- 25. Acet H, Güzel T, Aslan B, et al. Predictive value of C-reactive protein to albumin ratio in ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention. Angiology 2021;72:244–51. 10.1177/0003319720963697 [DOI] [PubMed] [Google Scholar]
- 26. Wang W, Ren D, Wang C-S, et al. High sensitivity C-reactive protein to Prealbumin ratio measurement as a marker of the prognosis in acute coronary syndrome. Sci Rep 2019;9:11583. 10.1038/s41598-019-48189-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang J, Li C, Zheng Y, et al. The association between high-sensitivity C-reactive protein/albumin ratio and cardiovascular prognosis in patients undergoing percutaneous coronary intervention. Angiology 2022;73:818–26. 10.1177/00033197221110715 [DOI] [PubMed] [Google Scholar]
- 28. Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67. 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 30. Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents: DPP-4 and SGLT-2 inhibitors. Circ Res 2018;122:1439–59. 10.1161/CIRCRESAHA.117.311588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soppert J, Lehrke M, Marx N, et al. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev 2020;159:4–33. 10.1016/j.addr.2020.07.019 [DOI] [PubMed] [Google Scholar]
- 32. Zhang M, Shi Y, Zhou B, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004-18: findings from six rounds of a national survey. BMJ 2023;380:e071952. 10.1136/bmj-2022-071952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chinese Center for Disease Control and Prevention, Chinese Center for Chronic and Non-communicable Diseases Prevention and Control . Chinese chronic disease and risk factor surveillance report, 2018. Beijing, 2021. [Google Scholar]
- 34. Daqing Z. Interpretation of Chinese guidelines for the management of blood lipids. Pract Med Clin Pract 2023;26:385–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jech-2023-220760supp001.pdf (688.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. The datasets used for the present analysis may be made available on reasonable request by contacting the corresponding author.


