Abstract
Objectives
Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A. BKZ treatment has demonstrated superior efficacy versus placebo (PBO) at Week 16 in biologic disease-modifying antirheumatic drug (DMARD)-naïve patients with active psoriatic arthritis (PsA). Here, we report long-term efficacy and safety to Week 52.
Methods
BE OPTIMAL comprised a 16-week, double-blind, PBO-controlled period, then 36 weeks treatment-blind. Patients were randomised 3:2:1 to subcutaneous BKZ 160 mg every 4 weeks, PBO with switch to BKZ at Week 16, or reference arm (adalimumab (ADA) 40 mg every 2 weeks). Efficacy outcomes included the American College of Rheumatology (ACR) response criteria 20/50/70, Psoriasis Area and Severity Index (PASI) 75/90/100 in patients with baseline psoriasis affecting ≥3% body surface area and minimal disease activity (MDA); non-responder imputation.
Results
ACR20/50/70, PASI75/90/100 and MDA responses were sustained with BKZ to Week 52, consistent with results observed at Week 16. Patients who switched to BKZ at Week 16 demonstrated improvements in efficacy with similar results to BKZ-randomised patients by Week 52.
To Week 52, 555/702 (79.1%) patients had ≥1 treatment-emergent adverse event (TEAE) during BKZ treatment; 113/140 (80.7%) on ADA. On BKZ, 46 (6.6%) patients had serious TEAEs. 54 (7.7%) Candida infections occurred during BKZ treatment and 1 (0.7%) during ADA; all cases were localised and non-serious. One death occurred in a BKZ-treated patient, unrelated to treatment.
Conclusions
The efficacy of BKZ in bDMARD-naïve patients with PsA was sustained from Week 16 to Week 52. BKZ was well tolerated with no new safety signals observed.
Trial registration number
Keywords: psoriatic arthritis; arthritis, psoriatic; biological therapy
Video Abstract.
Disclaimer: this video summarises a scientific article published by BMJ Publishing Group Limited (BMJ). The content of this video has not been peer-reviewed and does not constitute medical advice. Any opinions expressed are solely those of the contributors. Viewers should be aware that professionals in the field may have different opinions. BMJ does not endorse any opinions expressed or recommendations discussed. Viewers should not use the content of the video as the basis for any medical treatment. BMJ disclaims all liability and responsibility arising from any reliance placed on the content.
What is already known on this topic
Psoriatic arthritis (PsA) is a chronic, inflammatory disease that is associated with clinical manifestations across joint and skin domains that can negatively impact quality of life.
Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A.
Primary results from two phase III studies of BKZ treatment in patients with PsA who were biologic disease-modifying antirheumatic drug-naïve (bDMARD-naïve; BE OPTIMAL) or who had a previous inadequate response or intolerance to tumour necrosis factor-α inhibitors (BE COMPLETE) demonstrated a similar magnitude of response in both patient populations at Week 16.
PsA is a chronic, long-term disease, therefore it is important to assess the long-term efficacy and safety of new treatment options for PsA.
What this study adds
The 52-week randomised controlled trial BE OPTIMAL assessed the long-term efficacy of BKZ across joint, skin, enthesitis, dactylitis and radiographic outcomes.
Improvements in joint, skin clearance and composite outcomes observed at Week 16 were sustained to Week 52 in patients initially randomised to BKZ, with over half of BKZ-treated patients achieving American College of Rheumatology response criteria ≥50% improvement, Psoriasis Area and Severity Index 100% improvement or minimal disease activity by Week 52.
For patients who switched from placebo to BKZ at Week 16, improvements in efficacy were similar to those initially randomised to BKZ across joint and skin outcomes at Week 52.
The overall safety profile was consistent with the known safety of BKZ, including higher rates of mucocutaneous Candida infections when compared with adalimumab (ADA).
How this study might affect research, practice or policy
Results to Week 52 from BE OPTIMAL suggest that BKZ is an effective long-term treatment option in bDMARD-naïve patients with active PsA.
Data from the ongoing open-label extension, BE VITAL, will assess the safety and efficacy of BKZ treatment to three years.
Introduction
Psoriatic arthritis (PsA) is a chronic, inflammatory disease that develops in up to 30% of patients with psoriasis, and is associated with a diverse range of clinical features that result in a significant patient burden and reduced quality of life.1–5
Interleukins (IL)-17A and IL-17F have been identified as independent pivotal drivers of joint and skin inflammation in PsA.1 2 IL-17A inhibitors, such as secukinumab and ixekizumab, are currently available and are established therapies shown to be effective treatment options for patients with PsA.6 7 However, inhibition of both IL-17A and IL-17F could be a more effective treatment strategy, as in vitro, dual neutralisation of IL-17A and IL-17F is more effective in reducing inflammation than neutralising IL-17A alone.8 Bimekizumab (BKZ) is a humanised monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A.9 The benefits of dual neutralisation were demonstrated clinically in a phase IIIb clinical trial in psoriasis, where skin clearance was significantly higher with BKZ when compared with secukinumab.6 This strategy may also achieve greater disease control and alleviation of symptoms of inflammatory diseases like PsA, in which both IL-17A and IL-17F are implicated as major effector cytokines.8
The efficacy and safety of BKZ was previously reported up to two years in multiple phase III studies in patients with plaque psoriasis,6 10–12 and in patients with PsA to Week 24 and Week 16 in two phase III studies: BE OPTIMAL and BE COMPLETE, respectively.13 14 BE OPTIMAL included patients with active PsA who were biologic disease-modifying antirheumatic drug (bDMARD)-naïve; BE COMPLETE included patients with active PsA who had previous inadequate response or intolerance to tumour necrosis factor-α inhibitors (TNFi-IR). BE OPTIMAL also included an adalimumab (ADA) reference arm. BKZ demonstrated clinically meaningful improvements across joint, skin clearance, composite and patient-reported outcomes in the respective populations to Week 16 in both studies. BKZ also demonstrated inhibition of structural progression to Week 16 in BE OPTIMAL.13 Safety results were similar to those reported in previous studies of BKZ in psoriasis.6 10 11
PsA is a chronic, long-term disease,15 16 therefore treatment options that provide inflammation control and long-term, durable efficacy and tolerability are needed. Treatment should aim to achieve the lowest possible level of disease activity across domains,4 assessed using more stringent disease measures such as American College of Rheumatology response criteria (ACR) ≥50% improvement, Psoriasis Area and Severity Index (PASI) 100% improvement and minimal disease activity (MDA). Data from BE OPTIMAL to Week 24 were reported in the primary manuscript.13 Here, we report the long-term clinical efficacy and safety of subcutaneous BKZ 160 mg every 4 weeks in adult, bDMARD-naïve patients with active PsA to 52 weeks: the final results of the phase III BE OPTIMAL study.
Methods
Study design and participants
Complete methods details are reported in the primary manuscript.13 In brief, BE OPTIMAL (NCT03895203; ClinicalTrials.gov) was a 52-week, phase III, multicentre, randomised, double-blind, placebo (PBO)-controlled, active reference (ADA) study. The study was conducted in Asia, Eastern Europe, Western Europe and North America, and consisted of a 2–5 week screening period, a 16-week PBO-controlled, double-blind treatment period and a 36-week active treatment-blind period. BE OPTIMAL included an active reference (ADA) arm to provide a reference for the benefit-risk profile of BKZ alongside a standard-of-care treatment. This arm was not powered for statistical comparison to BKZ or PBO.
Patients completing Week 52 and meeting eligibility criteria were eligible for enrolment in the ongoing open-label extension (OLE) study BE VITAL (NCT04009499; ClinicalTrials.gov). A safety follow-up was conducted 20 weeks after the last dose of BKZ for patients not entering the OLE, or who discontinued early (online supplemental figure 1).
ard-2023-224431supp001.pdf (1.3MB, pdf)
Patients
Full inclusion and exclusion criteria were reported previously.13 In brief, eligible patients were ≥18 years and had a documented diagnosis of adult-onset PsA meeting the Classification Criteria for Psoriatic Arthritis for ≥6 months prior to screening. Patients had active PsA with a tender joint count (TJC) ≥3 (of 68), swollen joint count (SJC) ≥3 (of 66) and ≥1 active psoriatic lesion and/or a documented psoriasis history. Patients with current or prior exposure to any biologics for the treatment of PsA or psoriasis were excluded.
Randomisation and masking
Patients were randomised 3:2:1 to receive subcutaneous BKZ 160 mg every 4 weeks, subcutaneous PBO every 2 weeks or the reference (subcutaneous ADA 40 mg every 2 weeks). At Week 16, patients initially randomised to PBO were reallocated to receive subcutaneous BKZ 160 mg every 4 weeks through to Week 52 (PBO/BKZ); patients initially randomised to BKZ or ADA continued their dosing to Week 52. Due to the different dosing schedules, BKZ-treated patients received PBO between BKZ doses to match the reference arm dosing schedule (double-dummy) and maintain treatment blinding up to Week 52.
Study procedures
Study visits occurred every 2 weeks. Efficacy was assessed at baseline and Weeks 2, 4, 8, 12, 16, 20, 24, 36 and 52. Safety was assessed at baseline and each study visit thereafter. Structural damage progression in the hands and feet was assessed on plain radiographs, using the van der Heijde modified Total Sharp Score (vdHmTSS), quantifying the extent of bone erosions and joint space narrowing. Hand and feet radiographs were taken at screening, Week 16 and Week 52. These were read centrally and independently by two experienced readers, who were blind to the treatment assignment and time course of the films.
From Week 16 onwards, non-responder patients as per investigator assessment were eligible for rescue therapy with prespecified background medications. Patients requiring rescue therapy continued their assigned treatment. Further details are in the primary manuscript.13
Outcomes
Clinical efficacy outcomes assessed through Week 52 included ACR20/50/70, PASI75/90/100 (in patients with psoriasis affecting ≥3% body surface area (BSA) at baseline), MDA and very low disease activity (VLDA; achieved if 5/7 or 7/7 of the following criteria were fulfilled, respectively: TJC ≤1, SJC ≤1, BSA ≤3%, patients’ pain visual analogue scale (VAS) ≤15, patient’s global assessment of arthritis VAS ≤20, Health Assessment Questionnaire-Disability Index (HAQ-DI) ≤0.5, tender entheseal points ≤1), ACR50+PASI100 (in patients with psoriasis affecting ≥3% BSA at baseline), radiographic endpoints (vdHmTSS, bone erosion, joint space narrowing and proportion of patients with no radiographic inhibition (vdHmTSS change from baseline (CfB) ≤0.5); radiographic set), enthesitis (in patients with enthesitis at baseline using the Leeds Enthesitis Index (LEI)), dactylitis (in patients with dactylitis at baseline using the Leeds Dactylitis Index (LDI)), the modified Nail Psoriasis Severity Index (mNAPSI; in patients with psoriatic nail disease at baseline; one target nail was selected at baseline) and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; in patients with axial involvement (score ≥4) at baseline). Patient-reported outcome measures reported include the HAQ-DI, Short Form 36-item health survey Physical Component Summary (SF-36 PCS), Psoriatic Arthritic Impact of Disease-12 item Questionnaire (PsAID-12), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue and Patient's Assessment of Arthritis Pain (PtAAP).
Safety is reported through Week 52, including incidence of treatment-emergent adverse events (TEAEs), treatment-emergent serious adverse events (SAEs) and TEAEs leading to study withdrawal. Where applicable, the exposure-adjusted incidence rate (EAIR) per 100 patient-years (PY) is reported.
Prespecified safety topics of interest included infections (serious, opportunistic, fungal and tuberculosis (TB)), neutropenia, hypersensitivity, suicidal ideation and behaviour (SIB), major adverse cardiovascular events (MACE), liver function test changes/enzyme elevations, malignancies and inflammatory bowel diseases (IBD). SIB, MACE and IBD events were adjudicated by external adjudication committees. An independent data monitoring committee periodically reviewed the safety data collected during the study.
Statistical analysis
Statistical powering and sample size determination are reported in the BE OPTIMAL primary manuscript.13 The study was powered to show the statistical superiority of BKZ compared with PBO for the primary end point of ACR50 at Week 16. The study was not powered for statistical comparisons of ADA with BKZ or PBO. No statistical comparisons were made between ADA and BKZ or PBO.
Missing data for binary end points were imputed using non-responder imputation (NRI). P values for these end points were determined using logistic regression adjusted for treatment, region and bone erosion at baseline (0 or ≥1). Observed case data are also reported for binary end points. Continuous outcomes are reported using multiple imputation for missing data. Bone erosion stratification for statistical analyses was based on actual erosion at baseline (assessed using centrally read radiographs, for which readings were more precise than the randomisation stratum for bone erosion). All analyses were done with SAS, V.9.3 or higher.
Results
Patient disposition and baseline characteristics
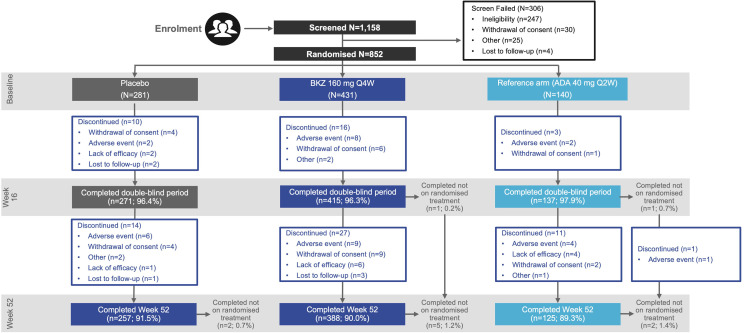
Of the 1,158 patients screened, 852 were randomised. Of those, 770 (90.4%) completed Week 52 (figure 1). Overall, 257/281 (91.5%) PBO/BKZ-treated patients, 388/431 (90.0%) BKZ-treated patients and 125/140 (89.3%) patients in the ADA group completed Week 52. Of the 271 patients who switched to BKZ from PBO at Week 16, 257 (94.8%) completed Week 52. As per the protocol, patients withdrawn from study treatment could return for all remaining study visits; of the patients that remained in the study at Week 52, 9 (1.1%) were not on randomised treatment (2 (0.7%) PBO/BKZ-treated patients; 5 (1.2%) BKZ-treated patients; 2 (1.4%) patients in the ADA group).
Figure 1.
Patient disposition. The percentage of patients completing or not completing treatment periods is calculated with the initial number of patients who were randomised to that group. Completed not on randomised treatment refers to patients that stopped treatment but continued in the study. ADA, adalimumab; BKZ, bimekizumab; Q2W, every 2 weeks; Q4W, every 4 weeks.
The most common reasons for discontinuation in the active treatment-blind period were adverse events (PBO/BKZ: 6 (2.2%); BKZ: 9 (2.2%); ADA: 4 (2.9%)), withdrawal of consent (PBO/BKZ: 4 (1.5%); BKZ: 9 (2.2%); ADA: 2 (1.5%)) and lack of efficacy (PBO/BKZ: 1 (0.4%); BKZ: 6 (1.4%); ADA: 4 (2.9%)).
Important protocol deviations were reported for 29 (3.5%) patients from Weeks 16 to 52. No patients were excluded from the pharmacokinetic per-protocol set. Important protocol deviations for the double-blind treatment period can be found in the BE OPTIMAL primary manuscript13; important protocol deviations for the active treatment-blind period are reported in online supplemental table 1.
As previously reported, baseline patient demographics and disease characteristics were comparable between treatment groups (table 1).13
Table 1.
Patient demographics and baseline disease characteristics
| PBO n=281 |
BKZ 160 mg every 4 weeks n=431 |
Reference arm (ADA 40 mg every 2 weeks) n=140 |
|
| Age, years, mean (SD) | 48.7 (11.7) | 48.5 (12.6) | 49.0 (12.8) |
| Male, n (%) | 127 (45.2) | 201 (46.6) | 71 (50.7) |
| BMI, kg/m2, mean (SD) | 29.6 (6.1) | 29.2 (6.8) | 28.4 (5.9) |
| Race, white,* n (%) | 270 (96.1) | 410 (95.1) | 133 (95.0) |
| Time since PsA diagnosis,† years, mean (SD) | 5.6 (6.5) | 6.0 (7.3) | 6.1 (6.8) |
| cDMARDs at baseline, n (%) | 194 (69.0) | 301 (69.8) | 99 (70.7) |
| Use of MTX at baseline, n (%) | 163 (58.0) | 252 (58.5) | 82 (58.6) |
| BSA affected by PSO ≥3%, n (%) | 140 (49.8) | 217 (50.3) | 68 (48.6) |
| PASI,‡ mean (SD) | 7.9 (5.6) | 8.2 (6.8) | 8.6 (7.6) |
| TJC (of 68 joints), mean (SD) | 17.1 (12.5) | 16.8 (11.8) | 17.5 (13.1) |
| SJC (of 66 joints), mean (SD) | 9.5 (7.3) | 9.0 (6.2) | 9.7 (7.1) |
| Presence of enthesitis (LEI),§¶ n (%) | 70 (24.9) | 143 (33.2) | 36 (25.7) |
| Score,¶** mean (SD) | 2.9 (1.5) | 2.5 (1.5) | 2.3 (1.6) |
| Presence of enthesitis (SPARCC),¶†† n (%) | 90 (32.0) | 166 (38.5) | 44 (31.4) |
| Score,¶** mean (SD) | 4.7 (3.8) | 4.6 (3.8) | 4.4 (3.9) |
| Presence of dactylitis,‡‡§§ n (%) | 33 (11.7) | 56 (13.0) | 11 (7.9) |
| Score,§§¶¶ mean (SD) | 47.3 (41.1) | 46.7 (54.3) | 49.7 (31.9) |
| Nail psoriasis,***††† n (%) | 156 (55.5) | 244 (56.6) | 75 (53.6) |
| Score,†††‡‡‡ mean (SD) | 4.0 (2.1) | 4.1 (2.5) | 3.7 (2.3) |
| HAQ-DI score,§§§ mean (SD) | 0.89 (0.61) | 0.82 (0.59) | 0.86 (0.54) |
| hs-CRP ≥6 mg/L, n (%) | 121 (43.1) | 158 (36.7) | 44 (31.4) |
| BASDAI score ≥4,¶¶¶ n (%) | 213 (75.8) | 311 (72.2) | 107 (76.4) |
| PtAAP,§§§ mean (SD) | 56.8 (23.2) | 53.6 (24.3) | 56.7 (23.9) |
| Patient’s global assessment score,§§§ mean (SD) | 58.6 (23.5) | 54.4 (23.4) | 57.1 (21.8) |
| Physician’s global assessment score,¶¶¶ mean (SD) | 57.2 (15.1) | 57.2 (16.3) | 57.3 (17.5) |
| SF-36 PCS score,‡‡‡‡ mean (SD) | 36.9 (9.7) | 38.1 (9.4) | 37.6 (8.8) |
| PsAID-12 score,§§§ mean (SD) | 4.1 (1.9) | 3.9 (1.9) | 4.3 (1.8) |
| FACIT-Fatigue score,§§§ mean (SD) | 36.0 (10.2) | 37.8 (9.6) | 36.4 (8.8) |
| Bone erosion ≥1 and/or hs-CRP ≥6 mg/L, n (%) | 236 (84.0) | 365 (84.7) | 116 (82.9) |
| vdHmTSS score,**** mean (SD) | 12.3 (22.5) | 12.5 (30.0) | 13.8 (26.5) |
| vdHmTSS score in at-risk set,†††† mean (SD) | 14.5 (23.9) | 14.4 (32.0) | 16.5 (28.4) |
The ADA 40 mg every 2 weeks treatment arm served as an active reference. The study was not powered for statistical comparisons of ADA with BKZ or PBO.
*Race was reported by the patient. Missing for patients enrolled in France (data missing for one patient receiving BKZ and two patients receiving ADA). PBO: 270; BKZ: 410; ADA: 133.
†Data missing for two patients receiving PBO, eight patients receiving BKZ and one patient receiving ADA.
‡In patients with psoriasis affecting ≥3% BSA at baseline; PBO/BKZ: n=140; BKZ: n=217; ADA: n=68.
§LEI >0.
¶Data missing for six patients receiving BKZ and one patient receiving ADA.
**In patients with enthesitis at baseline.
††SPARCC >0.
‡‡LDI >0.
§§Data missing for one patient receiving PBO, seven patients receiving BKZ and one patient receiving ADA.
¶¶In patients with dactylitis at baseline.
***mNAPSI >0.
†††Data missing for seven patients receiving BKZ.
‡‡‡In patients with nail psoriasis at baseline.
§§§Data missing for one patient receiving BKZ.
¶¶¶Data missing for one patient receiving PBO, five patients receiving BKZ and one patient receiving ADA.
****Radiographic set (patients with valid radiographs of hands and feet at baseline, as assessed by ≥2 reviewers); PBO: n=269; BKZ: n=420; ADA: n=135.
††††At‑risk subgroup defined as patients with elevated hs‑CRP and/or with at least one bone erosion at baseline; PBO: n=227; BKZ: n=361; ADA: n=112.
‡‡‡‡Data missing for one patient receiving BKZ and one patient receiving ADA.
ADA, adalimumab; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BKZ, bimekizumab; BMI, body mass index; BSA, body surface area; cDMARD, conventional disease‑modifying antirheumatic drug; FACIT‑Fatigue, Functional Assessment of Chronic Illness Therapy‑Fatigue; HAQ‑DI, Health Assessment Questionnaire‑Disability Index; hs-CRP, high-sensitivity C-reactive protein; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; mNAPSI, modified Nail Psoriasis Severity Index; MTX, methotrexate; PASI, Psoriasis Area and Severity Index; PBO, placebo; PCS, Physical Component Summary; PsAID‑12, Psoriatic Arthritic Impact of Disease‑12 questionnaire; PSO, psoriasis; PtAAP, Patient’s Assessment of Arthritis Pain; SF‑36, Short‑Form 36‑item Health Survey; SJC, swollen joint count; SPARCC, Spondyloarthritis Research Consortium of Canada; TJC, tender joint count; vdHmTSS, van der Heijde modified Total Sharp Score.
Efficacy
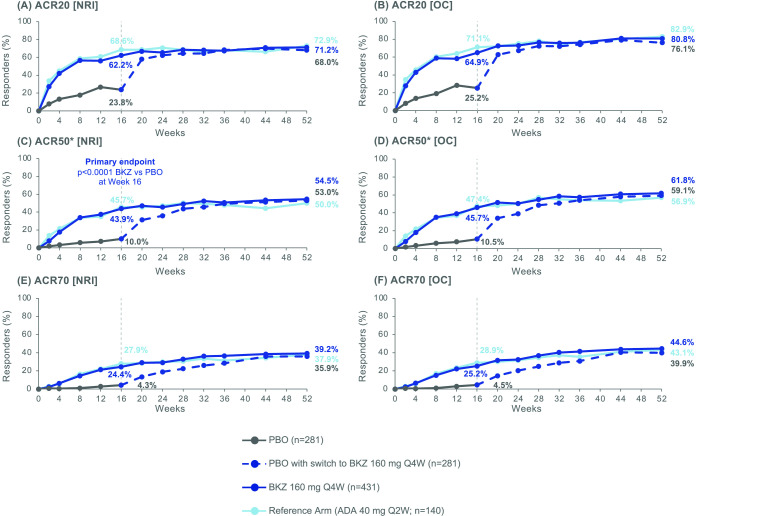
The percentage of patients achieving ACR50 was sustained from Week 16 to Week 52 in patients receiving BKZ. At Week 16, 28/281 (10.0%), 189/431 (43.9%) and 64/140 (45.7%) patients treated with PBO, BKZ and ADA, respectively, achieved ACR50 (NRI; table 2; figure 2C). At Week 52, approximately half of patients continuing active treatment achieved ACR50 (BKZ: 235 (54.5%); ADA: 70 (50.0%)). In patients who switched from PBO to BKZ at Week 16, a similar proportion of patients achieved ACR50 at Week 52 (149 (53.0%); figure 2C) compared to patients initially randomised to BKZ.
Table 2.
Efficacy end points at Week 16 and Week 52
| End point | PBO (Weeks 0–16) n=281 |
BKZ 160 mg every 4 weeks (Weeks 16–52) n=281 |
BKZ 160 mg every 4 weeks (Weeks 0–52) n=431 |
Reference arm (ADA 40 mg every 2 weeks; Weeks 0–52) n=140 |
||
| Week 16 | Week 52 | Week 16 | Week 52 | Week 16 | Week 52 | |
| ACR20 response, n (%) | 67 (23.8) | 191 (68.0) | 268 (62.2) | 307 (71.2) | 96 (68.6) | 102 (72.9) |
| ACR50 response,* n (%) | 28 (10.0) | 149 (53.0) | 189 (43.9) | 235 (54.5) | 64 (45.7) | 70 (50.0) |
| ACR70 response, n (%) | 12 (4.3) | 101 (35.9) | 105 (24.4) | 169 (39.2) | 39 (27.9) | 53 (37.9) |
| PASI75 response,† n/N (%) | 18/140 (12.9) | 119/140 (85.0) | 168/217 (77.4) | 177/217 (81.6) | 45/68 (66.2) | 45/68 (66.2) |
| PASI90 response,† n/N (%) | 4/140 (2.9) | 106/140 (75.7) | 133/217 (61.3) | 155/217 (71.4) | 28/68 (41.2) | 41/68 (60.3) |
| PASI100 response,† n/N (%) | 3/140 (2.1) | 91/140 (65.0) | 103/217 (47.5) | 132/217 (60.8) | 14/68 (20.6) | 33/68 (48.5) |
| MDA response, n (%) | 37 (13.2) | 151 (53.7) | 194 (45.0) | 237 (55.0) | 63 (45.0) | 74 (52.9) |
| VLDA response, n (%) | 3 (1.1) | 62 (22.1) | 63 (14.6) | 125 (29.0) | 22 (15.7) | 39 (27.9) |
| ACR50+PASI100 response,† n/N (%) | 0/140 (0) | 65/140 (46.4) | 60/217 (27.6) | 102/217 (47.0) | 11/68 (16.2) | 24/68 (35.3) |
| PASDAS category,‡ n (%) | ||||||
| REM | 6 (2.1) | 64 (22.8) | 85 (19.7) | 109 (25.3) | 27 (19.3) | 37 (26.4) |
| REM+LDA | 44 (15.7) | 140 (49.8) | 212 (49.2) | 205 (47.6) | 66 (47.1) | 67 (47.9) |
| DAPSA state,§ n (%) | ||||||
| REM | 4 (1.4) | 46 (16.4) | 47 (10.9) | 100 (23.2) | 27 (19.3) | 41 (29.3) |
| REM+LDA | 39 (13.9) | 155 (55.2) | 205 (47.6) | 246 (57.1) | 72 (51.4) | 74 (52.9) |
| Change from baseline in vdHmTSS,¶ mean (SE) | 0.25 (0.07) | 0.17 (0.11) | 0.03 (0.04) | 0.10 (0.07) | −0.09 (0.08) | −0.12 (0.12) |
| Change from baseline in vdHmTSS (at-risk),** mean (SE) | 0.29 (0.09) | 0.19 (0.13) | 0.03 (0.05) | 0.10 (0.08) | −0.14 (0.10) | −0.17 (0.13) |
| Change from baseline in bone erosion subscore,¶ mean (SE) | 0.16 (0.05) | 0.06 (0.08) | 0.02 (0.03) | 0.06 (0.05) | −0.05 (0.06) | −0.16 (0.09) |
| Change from baseline in bone erosion subscore (at-risk),** mean (SE) | 0.18 (0.06) | 0.06 (0.10) | 0.02 (0.04) | 0.06 (0.06) | −0.07 (0.07) | −0.21 (0.10) |
| Change from baseline in joint space narrowing subscore,¶ mean (SE) | 0.06 (0.03) | 0.06 (0.04) | 0.01 (0.02) | 0.04 (0.03) | −0.03 (0.05) | 0.04 (0.05) |
| Change from baseline in joint space narrowing subscore (at-risk),** mean (SE) | 0.07 (0.03) | 0.08 (0.05) | 0.02 (0.02) | 0.05 (0.03) | −0.06 (0.06) | 0.03 (0.06) |
| Complete resolution of enthesitis (LEI),†† n/N (%) | 29/70 (41.4) | 44/70 (62.9) | 72/143 (50.3) | 87/143 (60.8) | 18/36 (50.0) | 21/36 (58.3) |
| Complete resolution of enthesitis (SPARCC),‡‡ n/N (%) | 32/90 (35.6) | 55/90 (61.1) | 83/166 (50.0) | 94/166 (56.6) | 23/44 (52.3) | 25/44 (56.8) |
| Complete resolution of dactylitis,§§ n/N (%) | 18/33 (54.5) | 29/33 (87.9) | 44/56 (78.6) | 45/56 (80.4) | 9/11 (81.8) | 8/11 (72.7) |
| mNAPSI resolution,¶¶ n/N (%) | 29/156 (18.6) | 111/156 (71.2) | 82/244 (33.6) | 160/244 (65.6) | 28/75 (37.3) | 45/75 (60.0) |
| BASDAI change from baseline,*** mean (SE) | −1.1 (0.1) | −3.0 (0.2) | −2.6 (0.1) | −3.2 (0.1) | −2.7 (0.2) | −3.1 (0.2) |
| HAQ-DI MCID response,††† n/N (%) | 71/221 (32.1) | 125/221 (56.6) | 161/318 (50.6) | 182/318 (57.2) | 63/115 (54.8) | 68/115 (59.1) |
| HAQ-DI score change from baseline, mean (SE) | −0.09 (0.03) | −0.38 (0.03) | −0.26 (0.02) | −0.34 (0.02) | −0.33 (0.04) | −0.41 (0.05) |
| FACIT-Fatigue score change from baseline, mean (SE) | 1.5 (0.5) | 5.5 (0.5) | 3.9 (0.4) | 5.3 (0.4) | 5.0 (0.7) | 5.5 (0.8) |
| PsAID-12 total score change from baseline, mean (SE) | −0.5 (0.1) | −2.2 (0.1) | −1.8 (0.1) | −2.3 (0.1) | −2.1 (0.2) | −2.5 (0.2) |
| SF-36 PCS score change from baseline, mean (SE) | 2.3 (0.5) | 8.4 (0.6) | 6.3 (0.4) | 8.1 (0.5) | 6.8 (0.8) | 9.0 (0.9) |
| PtAAP score change from baseline, mean (SE) | −6.2 (1.5) | −31.8 (1.8) | −23.6 (1.3) | −30.4 (1.4) | −25.7 (2.5) | −32.7 (2.5) |
Randomised set. Previously reported data through Week 16 included for reference.13
*ACR50 at Week 16 was the primary end point.
†In patients with psoriasis affecting ≥3% BSA at baseline.
‡Patients achieved REM if PASDAS score was ≤1.9, and REM+LDA if PASDAS score was <3.2.
§Patients achieved REM if DAPSA score was ≤4, and REM+LDA if DAPSA score was ≤14.
¶Radiographic set (patients with valid radiographs of hands and feet at baseline, as assessed by ≥2 reviewers); PBO/BKZ: n=269; BKZ: n=420; ADA: n=135.
**At-risk subgroup defined as patients with elevated hs-CRP and/or with at least one bone erosion at baseline; PBO/BKZ: n=227; BKZ: n=361; ADA: n=112.
††Patients with enthesitis at baseline (LEI >0). Patients achieved complete resolution when LEI=0.
‡‡Patients with enthesitis at baseline (SPARCC >0). Patients achieved complete resolution when SPARCC=0.
§§Patients with dactylitis at baseline (LDI >0). Patients achieved complete resolution when LDI=0.
¶¶Patients with psoriatic nail disease at baseline (mNAPSI score >0). Patients achieved resolution when mNAPSI=0.
***Patients with axial involvement at baseline (BASDAI ≥4); PBO/BKZ: n=213; BKZ: n=311; ADA: n=107.
†††Patients who had a HAQ-DI decrease from baseline of ≥0.35 in patients with HAQ-DI ≥0.35 at baseline.
ACR20/50/70, American College of Rheumatology response criteria ≥20/50/70% improvement; ADA, adalimumab; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BKZ, bimekizumab; BSA, body surface area; DAPSA, Disease Activity Index for Psoriatic Arthritis; FACIT‑Fatigue, Functional Assessment of Chronic Illness Therapy‑Fatigue; HAQ‑DI, Health Assessment Questionnaire‑Disability Index; hs-CRP, high-sensitivity C reactive protein; LDA, low disease activity; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MCID, minimum clinically important difference; MDA, minimal disease activity; mNAPSI, modified Nail Psoriasis Severity Index; PASDAS, Psoriatic Arthritis Disease Activity Score; PASI75/90/100, Psoriasis Area and Severity Index ≥75%/90%/100% improvement; PBO, placebo; PCS, Physical Component Summary; PsAID‑12, Psoriatic Arthritis Impact of Disease 12‑item questionnaire; PtAAP, Patients’ Assessment of Arthritis Pain; REM, remission; SF-36, Short-Form 36-item Health Survey; SPARCC, Spondyloarthritis Research Consortium of Canada; vdHmTSS, van der Heijde modified Total Sharp Score; VLDA, very low disease activity.
Figure 2.
ACR responders to Week 52. Randomised set. P value was calculated using a logistic regression model with treatment, bone erosion at baseline and region as stratification factors. The study was not powered for statistical comparisons of ADA with BKZ or PBO. All data points can be found in table format in online supplemental table 5. *ACR50 at Week 16 was the primary end point. ACR20/50/70, American College of Rheumatology response criteria ≥20%/50%/70% improvement; ADA, adalimumab; BKZ, bimekizumab; NRI, non-responder imputation; OC, observed case; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks.
ACR20 and ACR70 responses were also sustained to Week 52 in BKZ-treated patients (ACR20: 191 (68.0%) PBO/BKZ, 307 (71.2%) BKZ, 102 (72.9%) ADA; ACR70: 101 (35.9%) PBO/BKZ, 169 (39.2%) BKZ, 53 (37.9%) ADA; NRI; figure 2A,E). Observed case data are also shown in figure 2B,D,F. A consistent trend was seen across the individual components of the ACR response criteria with BKZ treatment (online supplemental table 2).
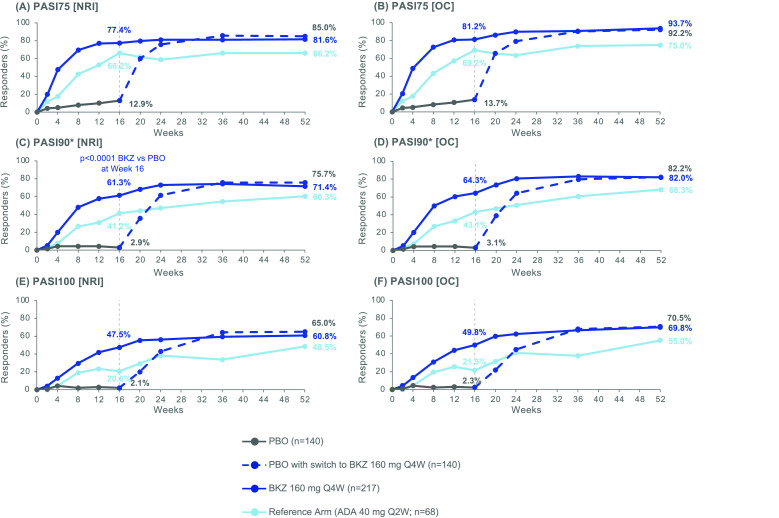
Improvements in skin, including complete clearance, were sustained from Week 16 to Week 52 in patients with ≥3% BSA affected by psoriasis at baseline. At Week 16, 4/140 (2.9%), 133/217 (61.3%) and 28/68 (41.2%) patients treated with PBO, BKZ and ADA, respectively, achieved PASI90. At Week 52, 155 (71.4%) BKZ-treated patients and 41 (60.3%) ADA-treated patients achieved PASI90 at Week 52 (NRI; figure 3C; table 2). Compared to patients initially randomised to BKZ, a similar proportion of patients who switched from PBO to BKZ at Week 16 achieved PASI90 response at Week 52 (106 (75.7%)). At Week 52, 91 (65.0%) PBO/BKZ-treated patients and 132 (60.8%) BKZ-treated patients achieved complete skin clearance (PASI100; NRI; figure 3E). In total, 33 (48.5%) ADA-treated patients achieved PASI100. PASI75 responder rates are shown in figure 3A (NRI). Observed case data are also shown in figure 3B,D,F.
Figure 3.
PASI responders to Week 52. Randomised set, in patients with psoriasis affecting ≥3% BSA at baseline. P value was calculated using a logistic regression model with treatment, bone erosion at baseline and region as stratification factors. The study was not powered for statistical comparisons of ADA with BKZ or PBO. All data points can be found in table format in online supplemental table 6. *PASI90 was a ranked secondary end point. ADA, adalimumab; BKZ, bimekizumab; BSA, body surface area; NRI, non-responder imputation; OC, observed case; PASI75/90/100, Psoriasis Area and Severity Index ≥75%/90%/100% improvement; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks.
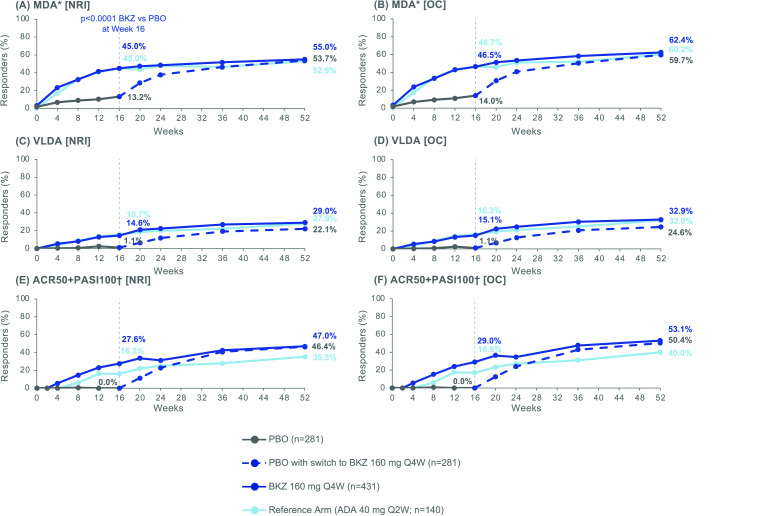
The percentage of patients achieving MDA improved from Week 16 to Week 52 in patients initially randomised to BKZ at baseline. At Week 16, 37 (13.2%), 194 (45.0%) and 63 (45.0%) patients treated with PBO, BKZ and ADA, respectively, achieved MDA (NRI; table 2). At Week 52, 237 (55.0%) BKZ-treated patients and 74 (52.9%) ADA-treated patients achieved MDA. Compared to patients initially randomised to BKZ, a similar proportion of patients who switched from PBO to BKZ at Week 16 achieved MDA response at Week 52 (151 (53.7%); NRI; figure 4A).
Figure 4.
Additional efficacy end points. Randomised set. P value was calculated using a logistic regression model with treatment, bone erosion at baseline and region as stratification factors. The study was not powered for statistical comparisons of ADA with BKZ or PBO. All data points can be found in table format in online supplemental table 7. *MDA was a ranked secondary end point; †In patients with psoriasis affecting ≥3% BSA at baseline; PBO/BKZ: n=140; BKZ: n=217; ADA: n=68. ACR50, American College of Rheumatology response criteria ≥50% improvement; ADA, adalimumab; BKZ, bimekizumab; BSA, body surface area; MDA, minimal disease activity; NRI, non-responder imputation; OC, observed case; PASI100, Psoriasis Area and Severity Index 100% improvement; PBO, placebo; Q2W, every 2 weeks; Q4W, every 4 weeks; VLDA, very low disease activity.
Responder rates were sustained from Week 16 to Week 52 for VLDA (Week 16: 3 (1.1%) PBO, 63 (14.6%) BKZ, 22 (15.7%) ADA; Week 52: 62 (22.1%) PBO/BKZ, 125 (29.0%) BKZ, 39 (27.9%) ADA; NRI; figure 4C) and ACR50+PASI100 (Week 16: 0/140 PBO, 60/217 (27.6%) BKZ and 11/68 (16.2%) ADA; Week 52: 65 (46.4%) PBO/BKZ, 102 (47.0%) BKZ, 24 (35.3%) ADA; NRI; figure 4E). Observed case data are also shown in figure 4B,D,F.
Improvements in enthesitis and dactylitis in patients with enthesitis or dactylitis at baseline were sustained from Week 16 to Week 52 (complete resolution of enthesitis at Week 52 (LEI): 44/70 (62.9%) PBO/BKZ, 87/143 (60.8%) BKZ, 21/36 (58.3%) ADA; complete resolution of dactylitis at Week 52 (LDI): 29/33 (87.9%) PBO/BKZ, 45/56 (80.4%) BKZ, 8/11 (72.7%) ADA; table 2). The percentage of patients achieving complete resolution of nail psoriasis, as measured by mNAPSI, improved from Week 16 to Week 52 (complete resolution of nail psoriasis at Week 52: 111/156 (71.2%) PBO/BKZ, 160/244 (65.6%) BKZ, 45/75 (60.0%) ADA).
Radiographic progression in the radiographic set was minimal to Week 16 (mean CfB (SE) in vdHmTSS: 0.25 (0.07) PBO, 0.03 (0.04) BKZ and −0.09 (0.08) ADA) and to Week 52 (0.17 (0.11) PBO/BKZ, 0.10 (0.07) BKZ and −0.12 (0.12) ADA). The majority of patients experienced no radiographic progression to Week 16 (222/250 (88.8%) PBO, 356/390 (91.3%) BKZ and 109/116 (94.0%) ADA) or to Week 52 (207/237 (87.3%) PBO/BKZ, 326/365 (89.3%) BKZ, 111/118 (94.1%) ADA; observed case data; online supplemental figure 2A). Cumulative probability plots of radiographic inhibition for Weeks 0–16 and Weeks 0–52 can be found in online supplemental figure 2B. Bone erosion and joint space narrowing subscores are reported in table 2.
Improvements in patient-reported physical functioning with BKZ were sustained from Week 16 to Week 52 (table 2); mean (SE) HAQ-DI CfB was −0.38 (0.03) for PBO/BKZ, −0.34 (0.02) for BKZ and −0.41 (0.05) for ADA at Week 52. Improvements in pain and fatigue with BKZ treatment were also sustained from Week 16 to Week 52 (mean (SE) PtAAP CfB: −31.8 (1.8) PBO/BKZ, −30.4 (1.4) BKZ, −32.7 (2.5) ADA; mean (SE) FACIT-Fatigue CfB: 5.5 (0.5) PBO/BKZ, 5.3 (0.4) BKZ, 5.5 (0.8) ADA; table 2). Similar results showing sustained improvement from Week 16 to Week 52 were observed across other health-related quality of life (HRQoL) outcomes such as BASDAI, PsAID-12 and SF-36 PCS (table 2).
Safety
Up to Week 52, 555/702 (79.1%; EAIR/100 PY: 222.5) patients reported ≥1 TEAE while receiving BKZ treatment, including patients who switched from PBO to BKZ at Week 16; 113/140 (80.7%; EAIR/100 PY: 209.4) ADA-treated patients reported ≥1 TEAE to Week 52. Up to Week 52, 46 (6.6%; EAIR/100 PY: 7.9) patients receiving BKZ treatment and 10 (7.1%; EAIR/100 PY: 7.5) patients receiving ADA treatment reported an SAE. In total, 21 (3.0%; EAIR/100 PY: 3.5) patients receiving BKZ and 7 (5.0%; EAIR/100 PY: 5.2) patients receiving ADA discontinued the study. One death occurred, in a BKZ-treated patient, unrelated to treatment (motorcycle accident; table 3). Week 16–52 safety data are reported in online supplemental table 4.
Table 3.
Safety data to Week 52
| Up to Week 16, n (%) | Up to Week 52, n (%) (EAIR/100 PY) | ||||
| PBO n=281 (PYAR: 87.3) |
BKZ 160 mg every 4 weeks n=431 (PYAR: 134.2) |
Reference arm (ADA 40 mg every 2 weeks) n=140 (PYAR: 43.4) |
BKZ 160 mg every 4 weeks Total n=702* (PYAR: 603.4) |
Reference arm (ADA 40 mg every 2 weeks) n=140 (PYAR: 136.8) |
|
| Any TEAE | 139 (49.5) | 257 (59.6) | 83 (59.3) | 555 (79.1) (222.5) | 113 (80.7) (209.4) |
| Severe TEAEs | 0 | 4 (0.9) | 3 (2.1) | 23 (3.3) | 9 (6.4) |
| Study discontinuation due to TEAEs | 3 (1.1) | 8 (1.9) | 3 (2.1) | 21 (3.0) (3.5) | 7 (5.0) (5.2) |
| Drug-related TEAEs | 35 (12.5) | 100 (23.2) | 34 (24.3) | 224 (31.9) | 54 (38.6) |
| Serious TEAEs | 3 (1.1) | 8 (1.9) | 2 (1.4) | 46 (6.6) (7.9) | 10 (7.1) (7.5) |
| Deaths | 0 | 0 | 0 | 1 (0.1)† | 0 |
| Most frequent adverse events‡ | |||||
| Nasopharyngitis | 13 (4.6) | 40 (9.3) | 7 (5.0) | 84 (12.0) (15.2) | 12 (8.6) (9.4) |
| Upper respiratory tract infection | 18 (6.4) | 22 (5.1) | 3 (2.1) | 50 (7.1) (8.7) | 8 (5.7) (6.1) |
| Urinary tract infection | 4 (1.4) | 9 (2.1) | 3 (2.1) | 43 (6.1) (7.3) | 5 (3.6) (3.7) |
| Headache | 7 (2.5) | 19 (4.4) | 2 (1.4) | 41 (5.8) (7.1) | 6 (4.3) (4.5) |
| Oral candidiasis§ | 0 | 9 (2.1) | 0 | 38 (5.4) (6.5) | 1 (0.7) (0.7) |
| Diarrhoea | 7 (2.5) | 16 (3.7) | 5 (3.6) | 36 (5.1) (6.2) | 7 (5.0) (5.3) |
| Hypertension | 11 (3.9) | 12 (2.8) | 4 (2.9) | 29 (4.1) (4.9) | 9 (6.4) (6.8) |
| ALT elevation | 2 (0.7) | 3 (0.7) | 7 (5.0) | 16 (2.3) (2.7) | 11 (7.9) (8.5) |
| AST elevation | 2 (0.7) | 1 (0.2) | 4 (2.9) | 14 (2.0) (2.3) | 7 (5.0) (5.3) |
| Injection site erythema | 0 | 1 (0.2) | 4 (2.9) | 6 (0.9) (1.0) | 7 (5.0) (5.3) |
| Uveitis | 0 | 0 | 0 | 0 | 0 |
| Adjudicated MACE | 0 | 0 | 0 | 4 (0.6) (0.7)¶ | 0 |
| Neutropenia | 1 (0.4) | 5 (1.2) | 1 (0.7) | 11 (1.6) (1.8) | 2 (1.4) (1.5) |
| Infections | |||||
| Serious | 0 | 1 (0.2) | 1 (0.7) | 6 (0.9) (1.0)** | 2 (1.4) (1.5)** |
| Opportunistic | 0 | 0 | 1 (0.7)†† | 9 (1.3) (1.5) | 1 (0.7) (0.7) |
| Active TB | 0 | 0 | 0 | 0 | 0 |
| Hypersensitivity | 6 (2.1) | 18 (4.2) | 3 (2.1) | 59 (8.4) (10.3) | 7 (5.0) (5.3) |
| Injection site reactions | 3 (1.1) | 5 (1.2) | 7 (5.0) | 15 (2.1) (2.5) | 13 (9.3) (10.2) |
| Adjudicated SIB | 0 | 0 | 0 | 0 | 0 |
| Liver function test changes/enzyme elevations | |||||
| ALT >3×ULN | 0‡‡ | 5 (1.2) | 2 (1.4)§§ | 15 (2.1)§§ | 7 (5.0)§§ |
| AST or ALT >3×ULN | 0‡‡ | 5 (1.2) | 3 (2.2)§§ | 24 (3.4)§§ | 9 (6.5)§§ |
| Adjudicated IBD | 0 | 0 | 0 | 2 (0.3) (0.3)¶¶ | 0 |
| Malignancies excluding non-melanoma skin cancer | |||||
| Breast cancer stage 1 | 1 (0.4) | 0 | 0 | 0 | 0 |
| Colon cancer | 0 | 0 | 0 | 1 (0.1) (0.2) | 0 |
| Chronic lymphocytic leukaemia stage 0 | 0 | 0 | 0 | 1 (0.1) (0.2) | 0 |
| Papillary thyroid cancer | 0 | 0 | 0 | 1 (0.1) (0.2) | 0 |
| Non-melanoma skin cancer | |||||
| Squamous cell carcinoma | 0 | 0 | 0 | 1 (0.1) (0.2) | 0 |
| Basal cell carcinoma | 0 | 1 (0.2) | 0 | 3 (0.4) (0.5) | 0 |
Safety set (Week 16) and active medication set for the overall study period (Week 52). TEAEs are reported for all study groups for Weeks 0–16 and Weeks 0–52. For Weeks 0–52, only TEAEs occurring while receiving BKZ are reported; for patients initially randomised to PBO, only events after switching to BKZ at Week 16 are included.
*Includes patients who switched from PBO to BKZ (events after switch only).
†Motorcycle accident; unrelated to treatment.
‡Most frequent adverse events are those occurring in ≥5% of patients in any study arm.
§All infections were mild or moderate and none were serious, one BKZ-treated patient discontinued.
¶One case each of myocardial infarction, cerebrovascular accident, ischaemic stroke and thrombotic cerebral infarction. The case of ischaemic stroke was deemed by the investigator to be related to study medication.
**Six serious infections were reported on the BKZ treatment arm: one cellulitis, one gangrene, one pneumonia, one upper respiratory tract infection, one cystitis and one urinary tract infection. Two ADA-treated patients reported serious infections: one otitis media and one reported both herpes zoster and atypical pneumonia.
††One opportunistic infection event of herpes zoster was reported for the ADA treatment arm during the double-blind period.
‡‡Data missing for two patients.
§§Data missing for one patient.
¶¶Both ulcerative colitis; one in a patient with a prior history of IBD and the other de novo.
ADA, adalimumab; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BKZ, bimekizumab; EAIR, exposure‑adjusted incident rate; IBD, inflammatory bowel disease; MACE, major adverse cardiovascular event; PBO, placebo; PY, patient‑years; PYAR, patient‑years at risk; SIB, suicidal ideation and behaviour; TB, tuberculosis; TEAE, treatment‑emergent adverse event; ULN, upper limit of normal.
The three most common TEAEs to Week 52 in patients receiving BKZ were nasopharyngitis (84 (12.0%); EAIR/100 PY: 15.2), upper respiratory tract infection (50 (7.1%); EAIR/100 PY: 8.7) and urinary tract infection (43 (6.1%); EAIR/100 PY: 7.3; table 3). In patients treated with ADA, the three most common TEAEs were nasopharyngitis (12 (8.6%); EAIR/100 PY: 9.4), ALT elevation (11 (7.9%); EAIR/100 PY: 8.5) and hypertension (9 (6.4%); EAIR/100 PY: 6.8).
In patients receiving BKZ to Week 52, six (0.9%; EAIR/100 PY: 1.0) patients had serious infections: one case each of cellulitis, gangrene of the oral cavity base, pneumonia, upper respiratory tract infection, cystitis and urinary tract infection. Overall, two (1.4%; EAIR/100 PY: 1.5) ADA-treated patients had serious infections, one patient with a case of otitis media and one patient with a case each of herpes zoster and atypical pneumonia. There were no cases of active TB.
Fungal infections were reported by the investigators for 82 (11.7%; EAIR/100 PY: 14.6; online supplemental table 3) patients receiving BKZ and two (1.4%; EAIR/100 PY: 1.5) ADA-treated patients. Of patients receiving BKZ, 54 (7.7%; EAIR/100 PY: 9.3) had a Candida infection, the majority of which were oral candidiasis (38 patients (5.4%); EAIR/100 PY: 6.5). Of ADA-treated patients, one (0.7%; EAIR/100 PY: 0.7) had a Candida infection (oral candidiasis). There were no serious or systemic Candida infections in either treatment arm. One Candida infection (oral candidiasis; 0.1% of BKZ-treated patients; EAIR/100 PY: 0.2) that resulted in study discontinuation occurred in a patient receiving BKZ. Cases of Candida were managed using standard antifungal treatment, with the majority of patients receiving nystatin or fluconazole. In BKZ-treated patients, 12 (1.7%) patients had recurrent candidiasis, as reported by the investigators.
To Week 52, nine (1.3%; EAIR/100 PY: 1.5) patients receiving BKZ and one (0.7%; EAIR/100 PY: 0.7) receiving ADA had an infection defined as opportunistic (definition provided in online supplemental appendix 1). No serious or systemic opportunistic infections were reported in patients treated with BKZ. In BKZ-treated patients, all opportunistic infections were localised, mucocutaneous and fungal in nature; four oesophageal candidiasis, two oropharyngeal candidiasis, one fungal oesophagitis, one fungal laryngitis and one upper respiratory tract fungal infection were reported (online supplemental table 3). The ADA-treated patient had a serious case of herpes zoster.
Four (0.6%; EAIR/100 PY: 0.7; table 3) patients had an event adjudicated as MACE up to Week 52 while receiving BKZ, three cases of stroke (two of which were thrombotic) and one case of myocardial infarction. Each of these patients had a history of cardiovascular events. No events adjudicated as MACE were reported in the reference treatment arm.
Of patients receiving BKZ, there were four cases of non-melanoma skin cancer (three basal cell carcinoma and one squamous cell carcinoma) and one case each of colon cancer, chronic lymphocytic leukaemia (stage 0) and papillary thyroid cancer (table 3). No malignancies were reported in the ADA group.
In total, 15 (2.1%; EAIR/100 PY: 2.5) patients receiving BKZ and 13 (9.3%; EAIR/100 PY: 10.2) ADA-treated patients had injection site reactions (table 3). There were two (0.3%; EAIR/100 PY: 0.3) cases of IBD in patients receiving BKZ; no cases of IBD were reported in patients receiving ADA. There were no cases of adjudicated SIB in any treatment arm.
Discussion
BE OPTIMAL demonstrated long-term efficacy of BKZ up to Week 52 in patients with active PsA who were bDMARD-naïve across a range of PsA domains, including joints, skin, enthesitis, dactylitis and nails, as well as the inhibition of structural progression. Improvements in efficacy as assessed by stringent outcome measures were sustained from Week 16 to Week 52 with BKZ treatment, with a low rate of patient drop-out. Furthermore, safety was as expected with no new safety signals observed.
PsA is a chronic, long-term disease, and treatment goals include achievement of more stringent efficacy outcomes that are sustained over time. Improvements seen with BKZ in the BE OPTIMAL study indicate a long-term, sustained depth of response. Improvements in ACR50 and PASI100 observed at Week 16 continued to improve to Week 52 in patients initially randomised to BKZ. Patients who switched from PBO to BKZ at Week 16 had similar ACR50 and PASI100 responder rates at Week 52 to those initially randomised to BKZ. ACR50 responder rates in all treatment groups were 50% or greater, and PASI100 responder rates for BKZ-treated patients were greater than 60% at Week 52.
A similar trend of improvements sustained from Week 16 to Week 52 was observed for the composite end points MDA, VLDA and ACR50+PASI100, demonstrating the efficacy of BKZ treatment across multiple domains of PsA.4 This is particularly important as international guidelines emphasise the need to reduce disease activity across all active domains of the disease, as this is associated with better outcomes.4 A positive trend in the individual components of ACR indicate that no single component drove the overall response. For patients who switched from PBO to BKZ at Week 16, improvements in composite outcomes were observed to Week 52.
Prevention of structural joint damage is a key goal of treatment.4 Persistent inflammation in patients with PsA can lead to structural damage,17 but the majority of patients treated with BKZ experienced minimal changes in radiographic progression to Week 52. This is especially notable, as BE OPTIMAL did not include joint erosion or elevated C-reactive protein as inclusion criteria. Thus, it was more difficult to demonstrate inhibition of radiographic progression compared with other studies on biologics.
Improvements in efficacy were accompanied by improvements in physical function (HAQ-DI), HRQoL (SF-36 PCS, PsAID-12) and patient-reported pain and fatigue (PtAAP, FACIT-Fatigue), which have been identified by patients as important to address in managing their disease.18
BKZ was well tolerated and the safety profile was similar to previous studies in PsA and consistent with the known safety profile of BKZ.13 14 19 Numerical differences were observed between the safety profile of BKZ and the ADA reference arm. In line with the mechanism of action of BKZ and the contribution of IL-17 in host protection against mucosal fungal infection,20 increased incidence of fungal infections was observed in BKZ-treated patients compared with ADA-treated patients. In the BKZ group, all fungal infections were mucocutaneous and localised. All cases of Candida were mild or moderate in severity and the majority were assessed as treatment related by the investigator. There were no cases of systemic candidiasis and the vast majority of cases did not lead to patients discontinuing treatment. The rate of Candida infections observed with BKZ treatment to Week 52 in BE OPTIMAL was consistent with those reported to Week 52 in the BE COMPLETE sister study and its OLE, BE VITAL, and the phase IIb trial of BKZ in PsA to three years.19 21 Rates were also similar to those reported in axial spondyloarthritis,22 and lower than those reported in psoriasis.23
In BKZ-treated patients, there were four cases of MACE and three malignancies excluding non-melanoma skin cancers. None were observed in the reference arm. Numerically higher rates of injection site reactions and liver enzyme elevations were observed in the ADA treatment group than the BKZ treatment group.
A limitation of this study is the small size of the subgroups of patients with enthesitis or dactylitis at baseline. In the primary analysis described previously, these subgroups were pooled with the BE COMPLETE study as part of the statistical hierarchy, to ensure adequate power for statistical analysis.13 In this manuscript, enthesitis and dactylitis subgroups were reported for BE OPTIMAL only. Despite this, a numerical difference was observed between BKZ and PBO at Week 16, and PBO/BKZ patients demonstrated similar resolution of both enthesitis and dactylitis at Week 52. Another limitation is that the study was not powered for statistical comparisons of ADA with BKZ, therefore no such comparisons between treatments can be made.
BKZ has demonstrated efficacy and tolerability to 52 weeks in bDMARD-naïve patients with active PsA. Data are reported to Week 52, which was the duration of the BE OPTIMAL study. However, PsA is a chronic, lifelong disease, therefore longer-term data are needed to further assess the long-term safety and efficacy profile of BKZ; longer treatment exposure is still required. Data from the BE VITAL OLE will assess BKZ treatment in patients with active PsA over three years to further assess long-term efficacy and tolerability. The long-term safety profile of BKZ, particularly the increased risk of fungal infections, will be important to further characterise and consider in context with the mechanistic benefits of dual inhibition of IL-17A and IL-17F in achieving complete skin clearance and will be particularly relevant for treatment decisions in clinical practice.13 14 24 In addition to the OLE, further observational research on outcomes in real-world datasets would aid extrapolation of these results to routine clinical populations.
In conclusion, dual inhibition of IL-17A and IL-17F with BKZ in bDMARD-naïve patients with active PsA resulted in sustained long-term, clinically meaningful improvements across the PsA disease domains, determined using stringent efficacy measures. Efficacy on BKZ treatment was sustained from Week 16 to Week 52 in patients initially randomised to BKZ; patients who switched from PBO to BKZ at Week 16 achieved similar levels of efficacy at Week 52 to patients initially randomised to BKZ. BKZ was well tolerated and the safety profile was consistent with prior studies. Long-term safety and efficacy data will be assessed in the ongoing OLE study, BE VITAL.
Acknowledgments
The authors thank the patients, the investigators and their teams who took part in this study. A portion of this work has been previously presented at ACR 2022 (abstract number: L02). The authors also acknowledge Heather Edens, PhD, UCB Pharma, Smyrna, GA, USA, for publication coordination and editorial assistance, Nadine Goldammer for her work as Clinical Programme Delivery Lead for the BKZ PsA programme, Deepak Assudani for his work in the BKZ PsA programme and Laura Mawdsley, MSc, and Sona Popat, BA, Costello Medical, Cambridge, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Footnotes
Handling editor: Josef S Smolen
Twitter: @LGossec
Correction notice: This article has been corrected since it published Online First. The results section of the abstract has been updated for clarity.
Contributors: Substantial contributions to study conception and design: CTR, LCC, IBM, PJM, JFM, YT, AA, LG, ABG, RBW, BI, RB, VS, JC, RBML; substantial contributions to analysis and interpretation of the data: CTR, LCC, IBM, PJM, JFM, YT, AA, LG, ABG, RBW, BI, RB, VS, JC, RBML; drafting the article or revising it critically for important intellectual content: CTR, LCC, IBM, PJM, JFM, YT, AA, LG, ABG, RBW, BI, RB, VS, JC, RBML; final approval of the version of the article to be published: CTR, LCC, IBM, PJM, JFM, YT, AA, LG, ABG, RBW, BI, RB, VS, JC, RBML; manuscript guarantor: BI.
Funding: This study was sponsored by UCB Pharma. Support for third-party writing assistance for this article, provided by Laura Mawdsley, MSc, Costello Medical, Cambridge, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). RBW is supported by the NIHR Manchester Biomedical Research Centre (NIHR203308).
Competing interests: CTR: research for AbbVie; consultant for Amgen, AbbVie, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB Pharma. LCC: has received grants/research support from AbbVie, Amgen, Celgene, Eli Lilly, Gilead, Janssen, Novartis, Pfizer and UCB Pharma; worked as a paid consultant for AbbVie, Amgen, BMS, Boehringer Ingelheim, Celgene, Domain, Eli Lilly, Galapagos, Gilead, Janssen, Moonlake, Novartis, Pfizer and UCB Pharma; and has been paid as a speaker for AbbVie, Amgen, Biogen, Celgene, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Medac, Novartis, Pfizer and UCB Pharma. IBM: consulting fees and honoraria from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Cabaletta, Causeway Therapeutics, Celgene, Eli Lilly, Evelo, Janssen, Moonlake, Novartis and UCB Pharma; research support from BMS, Boehringer Ingelheim, Celgene, Janssen, Novartis and UCB Pharma; Associate Editor at Annals of the Rheumatic Diseases. PJM: research grants from AbbVie, Amgen, BMS, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sun Pharma and UCB Pharma; consultancy fees from AbbVie, Acelyrin, Aclaris, Amgen, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Moonlake Pharma, Novartis, Pfizer, Sun Pharma and UCB Pharma; speakers’ bureau from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer and UCB Pharma. JFM: consultant and/or investigator for AbbVie, Amgen, Biogen, BMS, Dermavant, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma and UCB Pharma. YT: speaking fees and/or honoraria from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Chugai, Eisai, Eli Lilly, Gilead, GSK, Pfizer, Taiho and Taisho; received grants from Chugai, Eisai, Mitsubishi-Tanabe and Taisho; Editorial Board Member at Annals of the Rheumatic Diseases. AA: honoraria and/or research grants from AbbVie, Amgen, BMS, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Novartis, Pfizer, Sun Pharma, Taiho Pharma, Torii Pharmaceutical and UCB Pharma. LG: research grants from Sandoz and UCB Pharma; consulting fees from AbbVie, Amgen, BMS, Celltrion, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Sandoz and UCB Pharma; Editorial Board Member at Annals of the Rheumatic Diseases. ABG: received honoraria as an advisory board member and consultant for Amgen, AnaptypsBio, Avotres Therapeutics, BMS, Boehringer Ingelheim, Dice Therapeutics, Eli Lilly, Janssen, Novartis, Sanofi, UCB Pharma and Xbiotech; has received research/educational grants from AnaptypsBio, BMS, Moonlake Immunotherapeutics, Novartis and UCB Pharma (all paid to Mount Sinai School of Medicine). RBW: consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi and UCB Pharma; research grants to his institution from AbbVie, Almirall, Janssen, LEO Pharma, Novartis and UCB Pharma; honoraria from Astellas, DiCE, GSK and Union. BI: employee of UCB Pharma and shareholder of AbbVie, GSK and UCB Pharma. RB, VS, JC are all employees and shareholders of UCB Pharma. RL: consultancy fees from AbbVie, AstraZeneca, BMS, Eli Lilly, Novartis, Pfizer and UCB Pharma. Research grants from AbbVie, Novartis, Pfizer and UCB Pharma. Owner of Rheumatology Consultancy BV, an AMS company under Dutch law; Editorial Board Member at Annals of the Rheumatic Diseases.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data from this manuscript may be requested by qualified researchers six months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password-protected portal.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation guidance for Good Clinical Practice. Ethical approval was obtained from the relevant institutional review boards at participating sites, and all patients provided written informed consent in accordance with local requirements.
References
- 1. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:2095–6. 10.1056/NEJMc1704342 [DOI] [PubMed] [Google Scholar]
- 2. Veale DJ, Fearon U. The pathogenesis of Psoriatic arthritis. Lancet 2018;391:2273–84. 10.1016/S0140-6736(18)30830-4 [DOI] [PubMed] [Google Scholar]
- 3. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of Psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coates LC, Soriano ER, Corp N, et al. Group for research and assessment of psoriasis and Psoriatic arthritis (GRAPPA): updated treatment recommendations for Psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18:465–79.:734. 10.1038/s41584-022-00861-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and Psoriatic arthritis (MAPP) survey. Rheumatol Ther 2016;3:91–102. 10.1007/s40744-016-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus Secukinumab in plaque psoriasis. N Engl J Med 2021;385:142–52. 10.1056/NEJMoa2102383 [DOI] [PubMed] [Google Scholar]
- 7. O’Rielly DD, Rahman P. A review of Ixekizumab in the treatment of Psoriatic arthritis. Expert Rev Clin Immunol 2018;14:993–1002. 10.1080/1744666X.2018.1540931 [DOI] [PubMed] [Google Scholar]
- 8. Burns LA, Maroof A, Marshall D, et al. Presence, function, and regulation of IL-17F-expressing human Cd4+ T cells. Eur J Immunol 2020;50:568–80. 10.1002/eji.201948138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel Humanized Igg1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol 2020;11:1894. 10.3389/fimmu.2020.01894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus Ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, Multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet 2021;397:487–98. 10.1016/S0140-6736(21)00125-2 [DOI] [PubMed] [Google Scholar]
- 11. Warren RB, Cioffi C, Peterson L. Bimekizumab versus Adalimumab in plaque psoriasis. reply. N Engl J Med 2021;385:1150. 10.1056/NEJMc2113092 [DOI] [PubMed] [Google Scholar]
- 12. Thaçi D, Vender R, de Rie MA, et al. Safety and efficacy of Bimekizumab through 2 years in patients with moderate-to-severe plaque psoriasis: longer-term results from the BE SURE randomized controlled trial and the open-label extension from the BE BRIGHT trial. Br J Dermatol 2023;188:22–31. 10.1093/bjd/ljac021 [DOI] [PubMed] [Google Scholar]
- 13. McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with Psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled. Lancet 2023;401:25–37. 10.1016/S0140-6736(22)02302-9 [DOI] [PubMed] [Google Scholar]
- 14. Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active Psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-Α inhibitors: a randomised, double-blind. Lancet 2023;401:38–48. 10.1016/S0140-6736(22)02303-0 [DOI] [PubMed] [Google Scholar]
- 15. Coates LC, Helliwell PS. Psoriatic arthritis: state of the art review. Clin Med (Lond) 2017;17:65–70. 10.7861/clinmedicine.17-1-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gupta S, Syrimi Z, Hughes DM, et al. Comorbidities in Psoriatic arthritis: a systematic review and meta-analysis. Rheumatol Int 2021;41:275–84. 10.1007/s00296-020-04775-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van der Heijde D, Gladman DD, Kavanaugh A, et al. Assessing structural damage progression in Psoriatic arthritis and its role as an outcome in research. Arthritis Res Ther 2020;22:18. 10.1186/s13075-020-2103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogdie A, Michaud K, Nowak M, et al. Patient’s experience of Psoriatic arthritis: a conceptual model based on qualitative interviews. RMD Open 2020;6:e001321. 10.1136/rmdopen-2020-001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coates LC, McInnes IB, Merola JF, et al. Safety and efficacy of Bimekizumab in patients with active Psoriatic arthritis: three-year results from a phase IIb randomized controlled trial and its open-label extension study. Arthritis Rheumatol 2022;74:1959–70. 10.1002/art.42280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian Y, Kang Z, Liu C, et al. IL-17 signaling in host defense and inflammatory diseases. Cell Mol Immunol 2010;7:328–33. 10.1038/cmi.2010.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coates L, Landewé RBM, Mcinnes I, et al. Sustained efficacy and safety of Bimekizumab in patients with active Psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: results from the phase 3 BE COMPLETE study and its open-label extension up to 1 year. Ann Rheum Dis 2023;82:346–7. 10.1136/annrheumdis-2023-eular.1306 [DOI] [Google Scholar]
- 22. Baraliakos X, Deodhar A, Van der Heijde D, et al. Bimekizumab maintained improvements in efficacy endpoints and had a consistent safety profile through 52 weeks in patients with non-radiographic and radiographic axial Spondyloarthritis: results from two parallel phase 3 studies. Ann Rheum Dis 2023;82:873. 10.1136/annrheumdis-2023-eular.1562 36931692 [DOI] [Google Scholar]
- 23. Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol 2022;158:735–44. 10.1001/jamadermatol.2022.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet 2021;397:475–86. 10.1016/S0140-6736(21)00126-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2023-224431supp001.pdf (1.3MB, pdf)
Data Availability Statement
Data are available on reasonable request. Data from this manuscript may be requested by qualified researchers six months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password-protected portal.