Abstract
IL-3 has been reported to be involved in various inflammatory disorders, but its role in inflammatory bowel disease (IBD) has not been addressed so far. Here, we determined IL-3 expression in samples from patients with IBD and studied the impact of Il3 or Il3r deficiency on T cell-dependent experimental colitis. We explored the mechanical, cytoskeletal and migratory properties of Il3r −/− and Il3r +/+ T cells using real-time deformability cytometry, atomic force microscopy, scanning electron microscopy, fluorescence recovery after photobleaching and in vitro and in vivo cell trafficking assays. We observed that, in patients with IBD, the levels of IL-3 in the inflamed mucosa were increased. In vivo, experimental chronic colitis on T cell transfer was exacerbated in the absence of Il-3 or Il-3r signalling. This was attributable to Il-3r signalling-induced changes in kinase phosphorylation and actin cytoskeleton structure, resulting in increased mechanical deformability and enhanced egress of Tregs from the inflamed colon mucosa. Similarly, IL-3 controlled mechanobiology in human Tregs and was associated with increased mucosal Treg abundance in patients with IBD. Collectively, our data reveal that IL-3 signaling exerts an important regulatory role at the interface of biophysical and migratory T cell features in intestinal inflammation and suggest that this might be an interesting target for future intervention.
Keywords: INFLAMMATORY BOWEL DISEASE, INTESTINAL T CELLS, CYTOKINES
WHAT IS ALREADY KNOWN ON THIS TOPIC
Interleukin (IL)-3 has been suggested to be involved in the pathogenesis of several immune-mediated inflammatory diseases, but its role in chronic intestinal inflammation is unclear.
WHAT THIS STUDY ADDS
IL-3 receptor signalling counteracts experimental colitis.
Mechanistically, IL-3 receptor signalling controls the mechanobiology of regulatory T cells (Tregs) and impedes mucosal Treg egress to restore the balance of pro-inflammatory and anti-inflammatory T cells. Thus, for the first time, we identify a factor controlling intestinal mucosal tissue egress of Tregs.
Our data support a fundamentally new concept of immune cell motility as a consequence of the cellular biophysical features
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
IL-3 emerges as a potential future therapeutic strategy in IBD.
Approaches locally modulating the mechanical properties of immune cells in the inflamed mucosa seem highly promising to mitigate intestinal inflammation.
Introduction
The pathogenesis of inflammatory bowel diseases (IBD) such as Crohn’s disease (CD) and ulcerative colitis (UC) is still incompletely understood. According to current concepts, a multifactorial interplay of genetic susceptibility, barrier breakdown and environmental factors associated with intestinal dysbiosis triggers inadequate and pro-inflammatory immune responses in the gut resulting in tissue destruction and symptomatic disease.1–3
Dissecting the mechanisms of pathologic immune cell communication via cytokines in experimental colitis and human IBD has previously led to important insights into these pathogenetic events and has facilitated the development of current backbones in the therapy of IBD such as anti-tumour necrosis factor-α antibodies or anti-interleukin (IL)-12/23 antibodies.4 In particular, signalling of cytokines regulating T cell function and diversity such as IL-12, IL-23 or transforming growth factor (TGF)-β as well as T cell effector cytokines including IL-9, IL-17 or IL-10 via their respective receptors have been implicated in chronic colitis.5 Of note, similar roles of many of these cytokines have been described in related immune-mediated inflammatory diseases.6 However, despite having been described back in the 1980s7 8 and reported to be involved in pathologies such as experimental inflammatory arthritis,9 10 the role of IL-3 signalling via the IL-3 receptor (IL-3R) in the development and perpetuation of chronic intestinal inflammation remains largely unexplored.
So far, IL-3 is mainly known as a hematopoietic growth factor, which is expressed by activated T cells.11 IL-3R is constitutively expressed on basophils, myeloid precursors, plasmacytoid dendritic cells as well as neoplastic mast and B cells, but the expression on regulatory T cells (Tregs) in mice9 and on proliferating12 and activated T helper (Th) cells with a Th2 phenotype in the human blood13 has also been described.
Driven by the successful implementation and use of the anti-α4β7 integrin antibody vedolizumab,14 15 another aspect that has gained substantial attraction in the field of IBD in recent years is immune cell trafficking. Immune cell trafficking comprises all processes controlling immune cell locomotion such as tissue homing, retention or egress.16 Although previous research led to a detailed characterisation of the interaction of surface molecules like integrins, cell adhesion molecules or selectins in cell trafficking,17 cell-intrinsic processes regulating trafficking properties have only poorly been investigated. Particularly, hampered by missing appropriate techniques until recently, cell mechanical properties such as cell elasticity have only begun to be linked with biological processes like immune cell trafficking.
In this study, we aimed to explore the role of IL-3 signalling via IL-3R in experimental colitis as well as human IBD. We show that IL-3 expression is increased in colitis in mice and humans and that Il-3r signalling in T cells alleviates chronic colitis by instructing specific mechanical properties modulating the tissue egress capacity of Tregs. Our findings suggest novel approaches for future therapy of IBD by locally modulating mechano-migratory characteristics of immune cells.
Methods
Methods are available as online supplemental file.
gutjnl-2023-329818supp002.pdf (175.5KB, pdf)
Results
IL-3 expression is increased in IBD
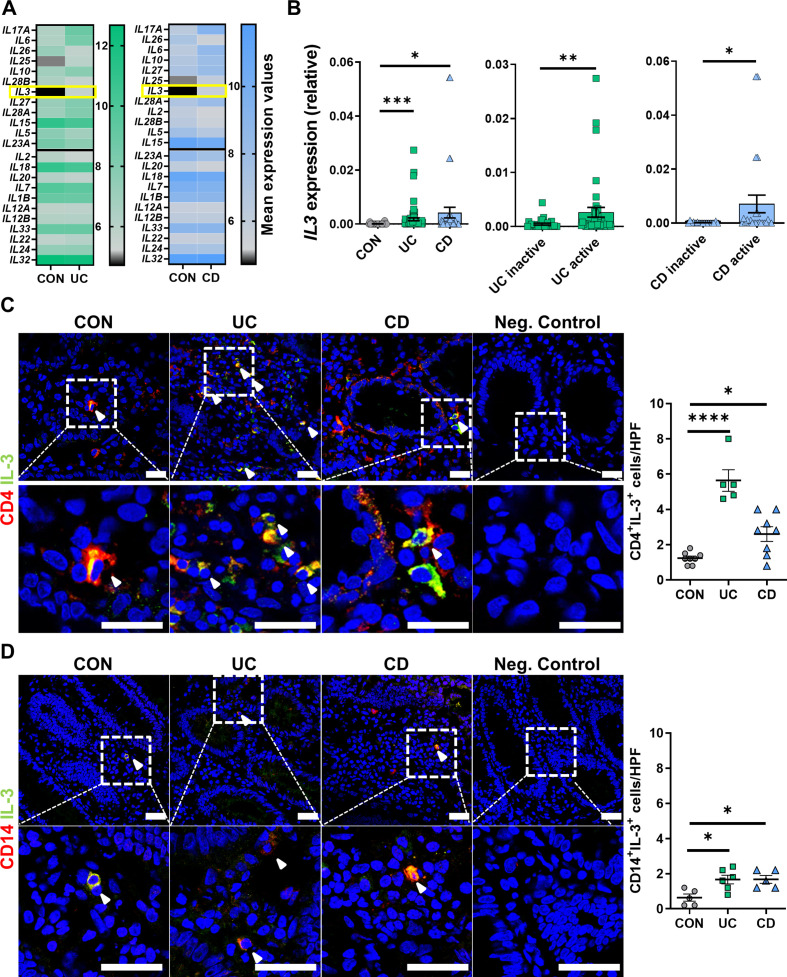
To explore the potential involvement of so far unaddressed cytokines in the pathogenesis of IBD, we re-analysed a previously published microarray data set18 including biopsies from a cohort of patients with UC, CD and non-IBD controls. Consistent with previous reports,19–21 the expression of cytokines like IL-17A, IL-6 or IL-26 was increased in the gut of patients with IBD (figure 1A). However, although an implication in IBD has not yet been described, we also found a significant upregulation of IL-3 both in CD and UC compared with control patients, where mucosal IL-3 expression was low. Thus, we further analysed the expression of IL-3 in colon tissue obtained from biopsies from a local patient cohort (online supplemental table 1). We detected substantially higher IL3 messenger RNA (mRNA) expression in patients with UC or CD compared with non-IBD control donors. Moreover, IL3 mRNA expression in patients with active disease was significantly higher than in patients with inactive disease (figure 1B). To validate this finding on protein level and to determine the source of IL-3 in the gut, we stained colonic specimens of patients with active IBD and non-IBD controls for CD4, CD14 or CD19 and IL-3. We found a marked increase in the number of CD4+IL-3+ T cells in samples from patients with CD and UC (figure 1C), whereas only a few CD14+ macrophages and CD19+ B cells co-expressed IL-3 (figure 1D, online supplemental figure 1A). IL-3 was also detectable in the serum of patients with IBD and control donors, but without relevant differences (online supplemental figure 1B, online supplemental table 2). Thus, these data suggested that CD4+ T cells are a major source of IL-3 in the inflamed colon and indicated a potential involvement of IL-3 in the local signalling in the intestine in IBD.
Figure 1.
IL-3 is upregulated in inflamed tissue of patients with IBD. (A) Microarray analysis of the publicly available data set GSE97012: mean values of selected cytokines plotted for patients with non-IBD (CON, n=27) versus UC (left, n=22) or patients with CD (right, n=19). Genes are ordered from low to high p values (top to bottom) with the black line separating genes with and without significantly different expression (above/below). (B) IL3 mRNA expression in colon tissue from patients with colonic CD (n=41), UC (n=68) and CON (n=21) donors as determined by qPCR. Comparison of levels between the entities (left) and comparison of levels between inactive and active disease (right); Kruskal-Wallis test with Dunn’s multiple comparisons post hoc test. (C,D) Immunofluorescence of cryosections from patients with CD (n=5–8), UC (n=5–6) and CON (n=5–8) for IL-3 (green) and CD4 (C, red) or CD14 (D, red); counterstaining with Hoechst (blue). Left: representative images, white arrowheads highlight double positive cells, scale bars – 25 µm; right: quantification of double positive cells per high power field (HPF); one-way analysis of variance with Tukey’s multiple comparisons post hoc test. CD, Crohn’s disease; CON, non-IBD control IL, interleukin; mRNA, messenger RNA; qPCR, quantitative PCR; UC, ulcerative colitis.
gutjnl-2023-329818supp001.pdf (2MB, pdf)
Il-3 is selectively upregulated in the colon of mice with chronic colitis and counteracts inflammation
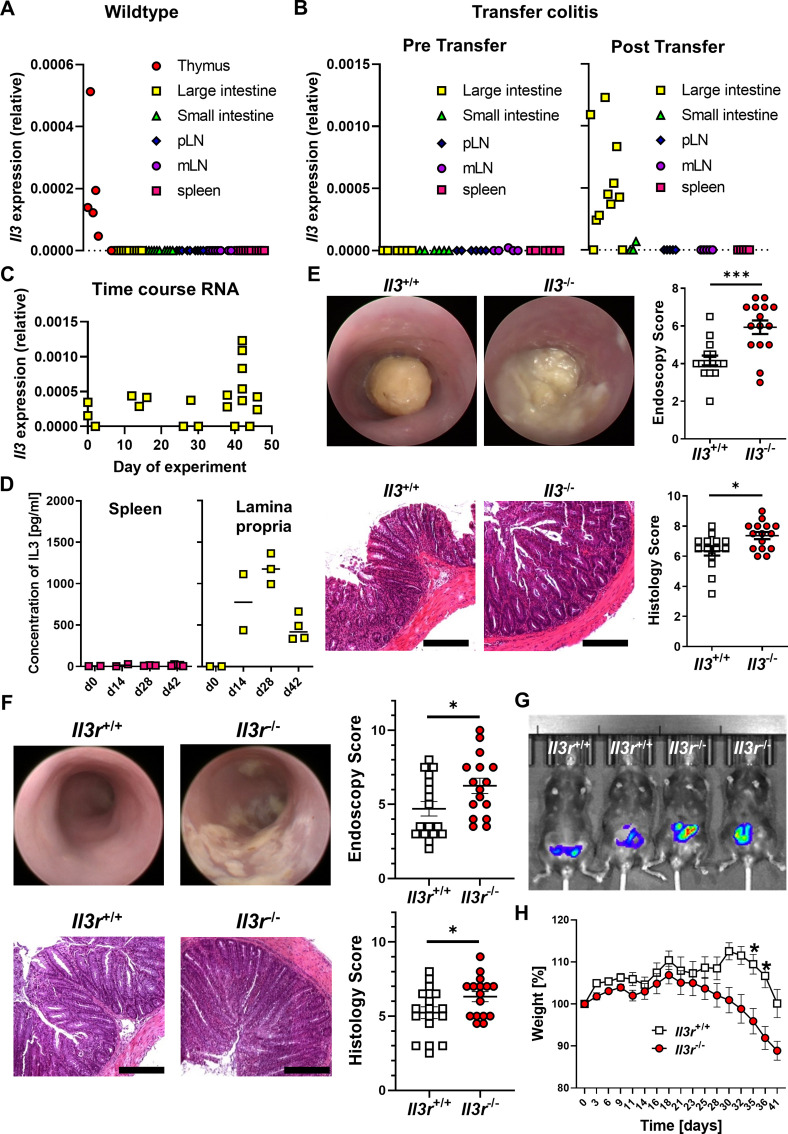
To address the functional role of Il-3, we explored its impact on experimental T cell transfer colitis. We did not detect Il3 mRNA expression in intestinal tissues or lymphoid organs of unchallenged Rag1 −/− mice and only in the thymus, but not in the spleen of wild-type mice. However, Il3 was expressed in colonic lamina propria mononuclear cells (LPMCs) of Rag1 −/− mice with colitis after the transfer of naïve CD4+ T cells from the spleen of wild type donor mice (figure 2A,B). Moreover, Il3 mRNA expression by LPMCs increased over the course of transfer colitis (figure 2C). On protein level, we were also able to detect substantial secretion of Il-3 by re-stimulated LPMCs, but not splenocytes from Rag1 −/− mice with transfer colitis (figure 2D).
Figure 2.
Il-3 alleviates T cell transfer colitis. (A) Il3 mRNA expression in tissue from various organs of unchallenged C57BL6/J mice as determined by qPCR. n=6–10 per group. (B) Il3 mRNA expression in tissue from various organs of unchallenged Rag1 −/− mice (pre transfer, left, n=5–6) and Rag1 −/− mice with established T cell transfer colitis (post transfer, right, n=3–11) as determined by qPCR. (C) Time course of Il3 mRNA expression in lamina propria mononuclear cells (LPMCs) of Rag1 −/− mice with T cell transfer colitis; n=3–11 per time point. (D) Concentration of Il-3 in supernatants of splenocytes and LPMCs re-stimulated with anti-CD3/28 antibodies at different time points of T cell transfer colitis as determined by ELISA; n=2–5 per time point. (E) Colitis in Rag1 −/− mice after transfer of naïve CD4+ T cells from Il3 +/+ and Il3 −/− mice. Upper panels: mini-endoscopy. Lower panels: histology of colon tissue. Left: representative images (scale bars – 12.5 µm), right: quantitative endoscopic and histological scores of disease severity. n=14–15 per group, unpaired t-test. (F–H) Colitis in Rag1 −/− mice after transfer of naïve CD4+ T cells from Il3r +/+ and Il3r −/− mice. (F) Mini-endoscopy (top) and histology of colon tissue (bottom). Left: representative images (scale bars – 12.5 µm), right: quantitative endoscopic and histological scores of disease severity. n=16–17 per group, Mann-Whitney (endoscopy) and unpaired t-test (histology). (G) Representative in vivo IVIS luminescence imaging of reactive oxygen species after i.p. injection of L-012. (H) Weight course. Normalisation to weight on the day adoptive T cell transfer was performed. n=16–17 per group, mixed-effects analysis with Sidak post hoc test. IL, interleukin; i.p., intraperitoneal; mLN, mesenteric lymph node; mRNA, messenger RNA; MPO, myeloperoxidase; pLN, peripheral lymph node; qPCR, quantitative PCR.
Having shown that Il-3 is specifically expressed in the colon during transfer colitis, we subsequently compared the phenotype of transfer colitis in Rag1 −/− mice after transfer of naïve CD4+ T cells from Il-3-deficient (Il3 −/−) and wild-type (Il3 +/+) mice. On colonoscopy, mice having received Il3 −/− T cells displayed increased signs of inflammation such as fibrin exudates, intestinal wall thickening and soft stool. Consistently, histological inflammation scores were higher in these mice (figure 2E) and in vivo imaging demonstrated the increased presence of reactive oxygen species (ROS; online supplemental figure 2A). This was accompanied by increased numbers of CD4+ and myeloperoxidase+ (MPO+) cells in the colon of mice that had received Il3 −/− T cells (online supplemental figure 2B).
We further sought to confirm this phenotype in another T cell-dependent colitis model. Accordingly, we performed oxazolone colitis with Il3 +/+ and Il3 −/− mice and similarly observed increased signs of inflammation in Il3 −/− mice on endoscopy, histology and in vivo imaging of ROS (online supplemental figure 3A,B).
To address whether, consistently, the exposure to Il-3 might mitigate colitis, we chose an overexpression approach based on minicircle vectors containing Il-3 complementary DNA. This led to efficient production of Il-3 (online supplemental figure 3C) and on subsequent induction of oxazolone colitis, mice with Il-3 vector treatment lost less weight (online supplemental figure 3D) and showed lower endoscopic and histological disease activity than mice having been treated with a mock vector (online supplemental figure 3E).
Collectively, these data suggested that Il-3 alleviates experimental colitis
Il-3 receptor signalling in T cells reduces intestinal inflammation
Since T cell transfer colitis is crucially driven by T cells, we wondered whether signalling of Il-3 via Il-3r on intestinal T cells might be responsible for the above effects. Thus, we generated a new mouse line specifically lacking the Il-3 receptor (Il3r −/−; online supplemental figure 4A–C) and isolated naïve CD4+ T cells from these mice for transfer colitis in Rag1 −/− mice. Consistent with the phenotype driven by Il3 −/− T cells, mice that received Il3r −/− CD4+ T cells suffered from severer T cell transfer colitis than mice that received Il3r +/+ CD4+ T cells as demonstrated by endoscopy, histology, in vivo imaging of ROS and weight course (figure 2F–H). Thus, we concluded that Il-3r signalling in T cells seems to be important for Il-3-driven effects in experimental colitis.
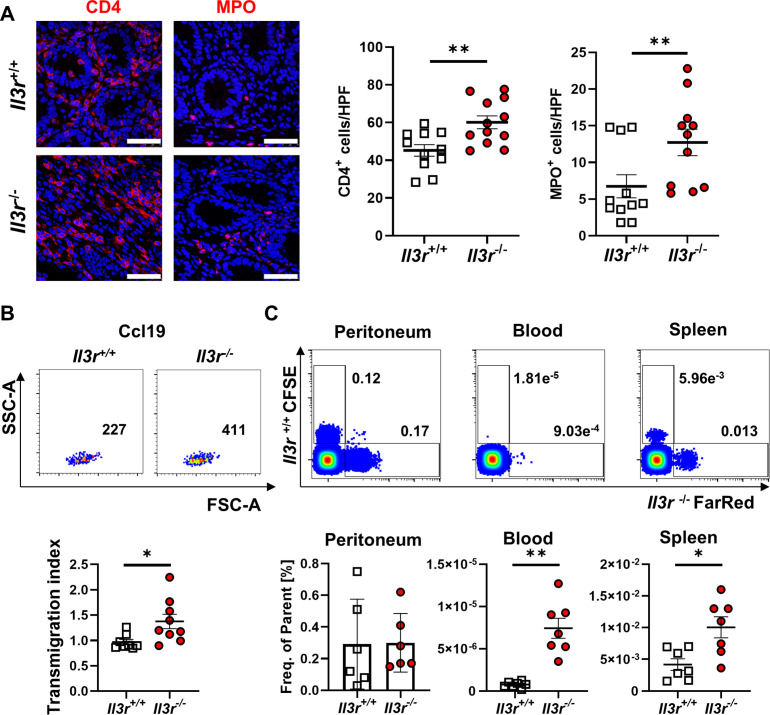
Il3r −/−-driven transfer colitis was marked by increased accumulation of CD4+ and MPO+ cells in the inflamed colon (figure 3A). However, the number of apoptotic CD4+ cells in the large intestine as determined by terminal desoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining was comparable between mice with Il3r −/− and Il3r +/+ CD4+ T cells (online supplemental figure 4D), the in vitro proliferation of Il3r −/− and Il3r +/+ T cells was similar (online supplemental figure 4E) and T cell differentiation as determined by the expression of key T cell transcription factors such as Tbet, Rorgt or Gata3 did not differ (online supplemental figure 4F). We therefore hypothesised that, while the local T cell turnover did not seem to be affected by Il3r deficiency, this might be due to altered T cell influx or efflux during trafficking.
Figure 3.
Il3r alters T cell recirculation. (A) Immunofluorescence staining for CD4 (left, red) and MPO (right, red) in colon tissue of Rag1 −/− mice after transfer of naïve CD4+ T cells from Il3r +/+ and Il3r −/− mice counterstained with Hoechst (blue). Left panels: representative images, white arrows highlight CD4+ or MPO+ cells. Right panels: quantification of CD4+ and MPO+ cells per high power field (HPF). n=11–12 per group, unpaired t-test (CD4) and Mann-Whitney (MPO); scale bars – 50 µm. (B) Migration of Il3r −/− or Il3r +/+ thymus T cells over porous membranes towards rm Ccl19. Upper panels: representative flow cytometry. Lower panel: quantification of Ccl19-specific transmigration; n=8–9 per group, Mann-Whitney test. (C) Recirculation of FarRed-stained Il3r −/− and CFSE-stained Il3r +/+ thymus T cells after i.p. injection. Upper panels: representative flow cytometry of peritoneal, blood and splenic cells. Lower panel: quantification; n=6–7 per group, paired t-test. i.p., intraperitoneal; MPO, myeloperoxidase.
Il3r deficiency increases T cell recirculation
To address this hypothesis, we systematically investigated key steps of intestinal T cell trafficking. Using the spleen as a major source of circulating lymphocytes potentially infiltrating the gut in analogy to transfer colitis, we did not detect any differences in the expression of integrins relevant for homing to the gut such as α4, β7 or β1 (online supplemental figure 5A). We further ruled out differential conformational regulation of α4 integrin dependent on Il-3r signalling (online supplemental figure 5B). Consistently, we observed no differences in the dynamic adhesion of Il3r −/− and Il3r +/+ CD4+ T cells to endothelial cell adhesion molecules such as mucosal addressin cell adhesion molecule 1 (MAdCAM-1) and vascular cell adhesion molecule 1 (VCAM-1) (online supplemental figure 5C). We also employed an established in vivo model of T cell homing to the inflamed gut22 to study the infiltration of Il3r-proficient and Il3r-deficient CD4+ T cells to the inflamed colon and found no differences (online supplemental figure 5D).
Since these data made it unlikely that T cell recruitment into the inflamed tissue differs between Il3r −/− and Il3r +/+ T cells, we considered T cell recirculation from the intestine as an alternative trafficking step controlling T cell abundance in the gut. Due to the large T cell numbers required for the assays and to mimic the local exposure to Il-3, we used T cells from the thymus (enriched for CD4+ T cells to >95%; online supplemental figure 5E), since Il-3 is expressed there similar to the inflamed colon (figure 2A,B). Indeed, the migration of Il3r −/− T cells over porous membranes towards the mesenteric lymph node (mLN) chemokine Ccl19 was increased compared with Il3r +/+ cells, although the expression of Ccr7 as the respective receptor was similar (figure 3B, online supplemental figure 5F).
Seeking to test whether the motility of these cells is also altered in vivo, we adoptively co-transferred differentially labelled Il3r −/− and Il3r +/+ T cells to the peritoneum of Rag1 −/− mice and competitively measured their appearance in the spleen 72 hours later. Consistently, these analyses demonstrated that more Il3r −/− than Il3r +/+ T cells migrated there (figure 3C) despite similar expression of various genes coding for potentially involved trafficking molecules as determined by RNA sequencing and flow cytometry (online supplemental figure 6). In synopsis, these data were consistent with a model, in which the recirculation of Il3r-deficient T cells is increased.
Il3r deficiency instructs altered cytoskeleton architecture and dynamics
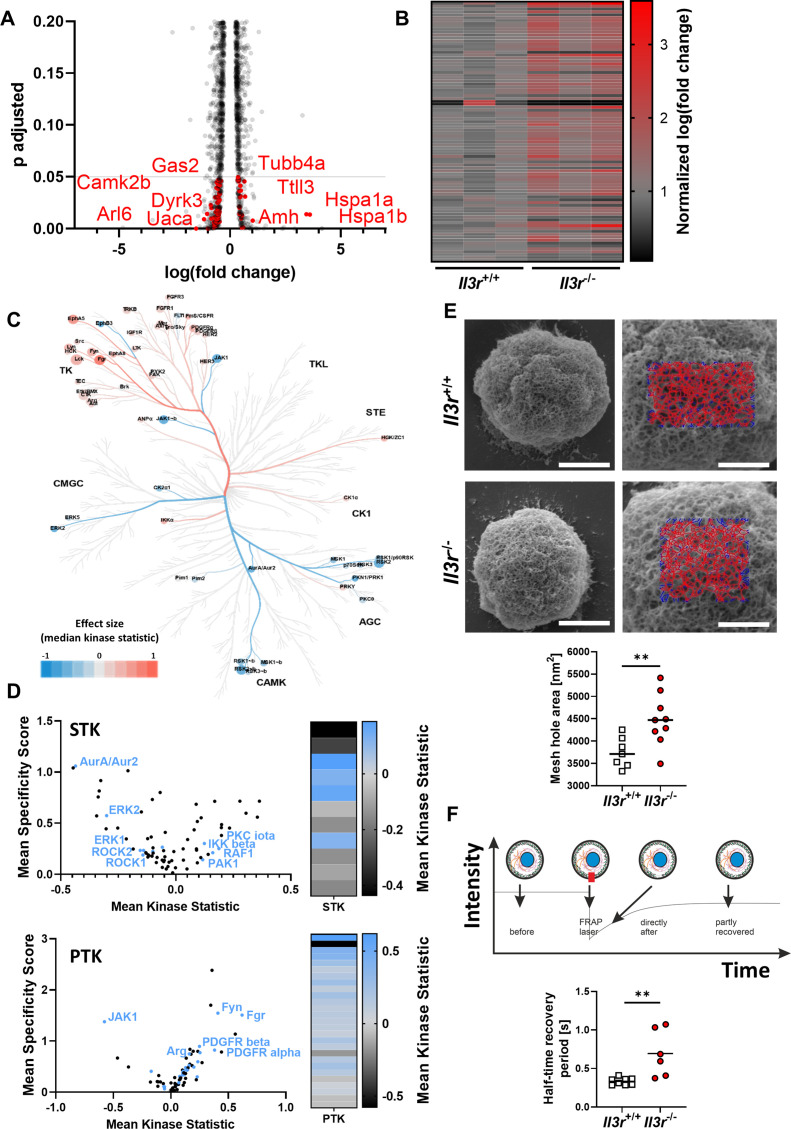
Having shown that, we sought to identify the mechanism for increased Ccl19-directed motility and increased recirculation of Il3r-deficient compared with Il3r-proficient T cells. To this end, we profiled unchallenged Il3r −/− and Il3r +/+ T cell from the thymus by bulk RNA sequencing,23 which revealed 624 differentially expressed genes. Pathway enrichment analysis with the Database for Annotation, Visualization and Integrated Discovery (DAVID) demonstrated a substantial number of differentially expressed genes linked to the cytoskeleton (figure 4A,B), whereas the genes of key surface molecules involved in T cell trafficking including Ccr7 were similarly expressed (online supplemental figure 6). This finding was consistent with the idea that cell-intrinsic processes rather than the interaction of surface molecules drive differential trafficking of Il3r −/− and Il3r +/+ T cells.
Figure 4.
Il3r-deficiency alters structure and dynamics of the cytoskeleton. (A,B) Differentially expressed genes in RNA sequencing of Il3r −/− or Il3r +/+ thymus T cells (n=3 per group). (A) Volcano plot of the top 4245 regulated genes, grey line indicates significance threshold. Significantly differentially expressed cytoskeleton-related genes (as identified by Database for Annotation, Visualization and Integrated Discovery (DAVID) annotation analysis, n=90) highlighted in red, selected gene names indicated. (B) Heatmap of normalised log(fold change) of 90 cytoskeleton-related genes as identified by DAVID annotation analysis. (C) Coral Kinome Tree plotted with data from a Pamgene kinome analysis of Il3r −/− vs Il3r +/+ thymus T cells. Kinases with lower activity in Il3r −/− are highlighted in blue, kinases with higher activity in Il3r −/− are highlighted red. (D) Left panels: volcano plots showing all differently activated serin/threonine kinases (STK) and protein tyrosine kinases (PTK) in Il3r −/− versus Il3r +/+ thymus T cells. Cytoskeleton-associated kinases are highlighted in blue. Right panels: heatmaps of mean kinase statistics for cytoskeleton-associated STK and PTK. (E) Scanning electron microscopy (SEM) of the actin cortex in Il3r −/− or Il3r +/+ thymus T cells. Upper panels: representative SEM pictures (left, scale bars – 2 µm) and magnifications with representative FiNTA software-based quantification grids (right, scale bar – 1 µm). Lower panel: quantification of the mean mesh hole area; n=7–9 per group, unpaired t-test. (F) Fluorescence recovery after photobleaching (FRAP) of the actin cortex in Il3r −/− or Il3r +/+ thymus T cells. Schematic representation of FRAP experiment (upper panel) and quantification of the half-time recovery period (lower panel); n=6–8 per group, unpaired t-test.
To further investigate this idea, we performed a kinome assay assessing the activity of various kinases in unchallenged Il3r −/− and Il3r +/+ T cells from the thymus. Overall, it showed differential activation for numerous members of the protein tyrosine kinases (PTK) and serin/threonine kinase family (STK) for Il3r −/− compared with Il3r +/+ cells (figure 4C). Again, pathway annotation analysis with DAVID revealed an enrichment in kinases associated with cytoskeleton regulation such as Rho kinase 1 and 2 (figure 4D).
We therefore aimed to study the Il-3r-dependent regulation and function of the cytoskeleton in more detail. Since small GTPases are known as key regulators of the cytoskeleton,24 we determined their expression in Il3r +/+ and Il3r −/− T cells by immunofluorescence, but detected no differences (online supplemental figure 7A). However, well in line with a previous study,25 pull-down assays showed increased functional activity of Rac1, a Rho GTPase with particular relevance in T cells,26 in Il3r −/− compared with Il3r +/+ T cells (online supplemental figure 7B), suggesting that Rac1 function rather than Rac1 expression is a target of Il-3r signalling. To investigate how this reflects in the actual cytoskeleton architecture, we employed scanning electron microscopy and studied the actin cortex of Il3r −/− and Il3r +/+ T cells. It turned out that the mesh-hole area of the actin cortex of Il3r −/− T cells is substantially higher than that of Il3r +/+ T cells (figure 4E). In a further series of experiments, we additionally used fluorescence recovery after photobleaching (FRAP) to explore whether Il-3r signalling also affects the dynamics of the actin cortex. Actually, the half-time recovery period for the actin cortex of Il3r −/− T cells was clearly longer than for Il3r +/+ T cells (figure 4F). Collectively, these data indicated that Il3r deficiency results in altered cytoskeleton regulation including disturbed architecture and dynamics of the actin cortex.
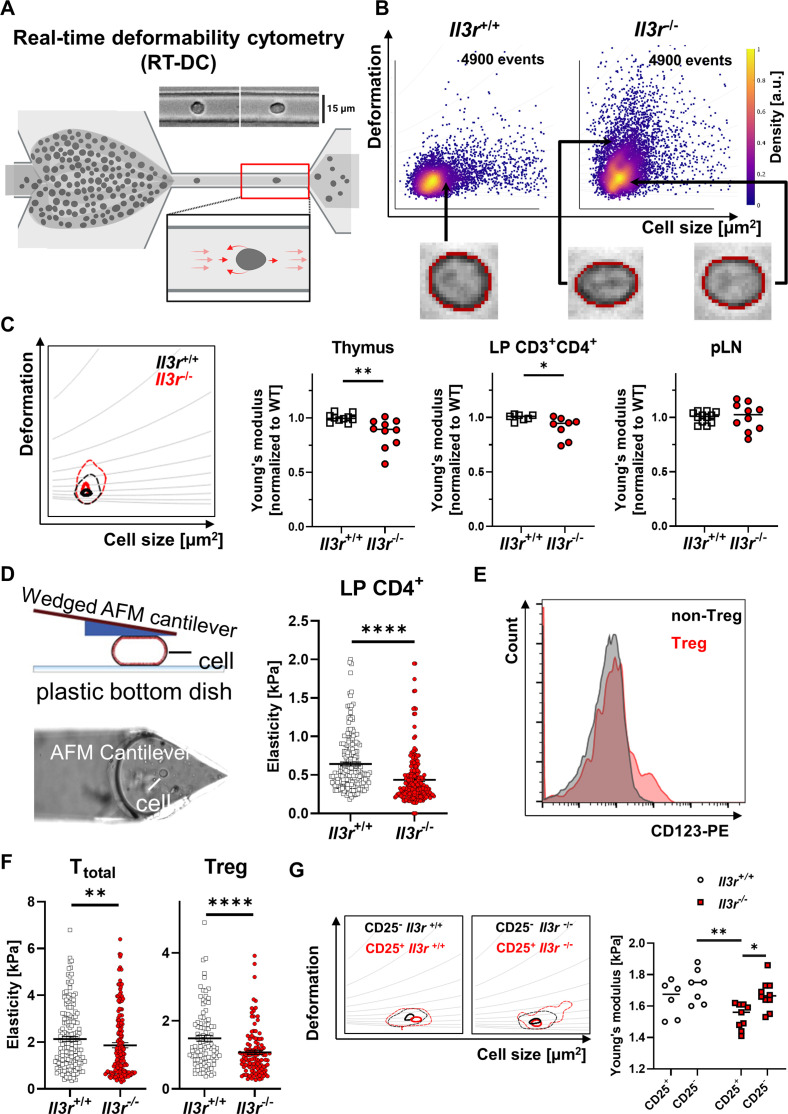
Il3r-deficient T cells exhibit increased deformability, which is particularly pronounced in Tregs
We then wondered how these findings might affect the postulated difference in trafficking of Il3r −/− and Il3r +/+ T cells. Since cell deformation is an important part of T cell egress from tissues as cells cross the basement membrane and undergo reverse transendothelial migration,27 we explored whether cell mechanical properties might differ. To this end, we used real-time deformability cytometry (RT-DC), a microfluidic technique suitable for high throughput assessment of cell elasticity28 (figure 5A). While we observed a very homogenous phenotype of Il3r +/+ lymphocytes from thymus, transfer colitis colon and peripheral lymph nodes (pLN) with only low deformation, a subset of Il3r −/− lymphocytes from the thymus and the inflamed colon (where Il-3 is both expressed), but not pLNs (where Il-3 is not expressed, figure 2A,B) displayed high deformability (figure 5B,C). To corroborate this finding, we employed atomic force microscopy (AFM) using a wedged cantilever measuring the forces necessary to squeeze lamina propria CD4+ T cells from mice with established transfer colitis. Again, we found a higher deformability of Il3r −/− compared with Il3r +/+ T cells (i.e. less force was required for deformation; figure 5D).
Figure 5.
Increased deformability of Il3r −/− lymphocytes. (A) Schematic representation of RT-DC measurement. Cells from a reservoir are pumped through a narrow constriction in a polydimethylsiloxane (PDMS)-based microfluidic chip (15 µm capillary) and deformation ensuing due to hydrodynamic stresses is analysed; figure drawn with licensed BioRender software. (B) Representative RT-DC scatter plot of deformation versus cell size (cross-sectional area) of thymus T cells from Il3r −/− and Il3r +/+ mice highlighting representative cells of the populations. (C) Representative kernel density estimate (KDE) plot (with grey isoelasticity lines) of thymus T cells from Il3r −/− and Il3r +/+ mice (left) and quantification of the calculated Young’s modulus of lymphocytes from thymus and pLN as well as pregated CD3+CD4+ lamina propria T cells from mice with transfer colitis. Quantification normalised to the mean of the Il3r +/+ cells in each independent experiment; n=7–12 per group, Mann-Whitney test; effect size: Cohen’s d=1.468 (Thymus), d=1.363 (CD3+CD4+ LP), d=0.065 (pLN). (D) Schematic representation of atomic force microscopy (AFM) cantilever and cell deformation (left). Quantification of the elasticity of CD4+ lamina propria lymphocytes from mice with established transfer colitis with Il3r −/− and Il3r +/+ T cells by AFM; n=212 each, Mann-Whitney test. (E) Histograms of Cd123 expression on CD3+CD4+ Treg (CD25+CD127−) or non-Treg (CD25−) Il3r +/+ thymocytes as determined by flow cytometry. Data are representative for six mice from four independent experiments. (F) Quantification of the calculated Young’s modulus of total CD4+ and regulatory CD4+ T cells (right) from Il3r −/− and Il3r +/+ mice by AFM; n=100–172 per group, Mann-Whitney test. (G) Representative RT-DC KDE plot (upper panel) and quantification of the calculated mean Young’s modulus (lower panel) of splenic CD3+CD4+ CD25+ and CD25− lymphocytes from Il3r −/− and Il3r +/+ mice; n=6–10 per group, two-way analysis of variance with Tukey’s multiple comparison test; effect size: Cohen’s f=0.071. LP, lamina propria; pLN, peripheral lymph node; Treg, regulatory T cell; WT, wild-type.
In a next step, we wondered whether these alterations in cell mechanical properties might be differentially pronounced in pro-inflammatory and anti-inflammatory T cells and might thereby drive the phenotype observed in experimental colitis. Indeed, confirming previous data,9 we found increased expression of Il-3r on Tregs compared with non-Tregs (figure 5E). Consistently, the increase in deformability of Il3r −/− T cells was clearly more pronounced in isolated Tregs as determined by AFM (figure 5F) as well as in CD25+ compared with CD25− lamina propria T cells as determined by RT-DC coupled with fluorescence (figure 5G). Taken together, our findings indicated that defective Il-3r signalling in T cells and particularly in Tregs modulates cell mechanical properties and increases their deformability, which might explain altered trafficking features.
Altered tissue egress of Il3r −/− T cell to mLNs in experimental colitis disturbs Treg proportions in the inflamed gut
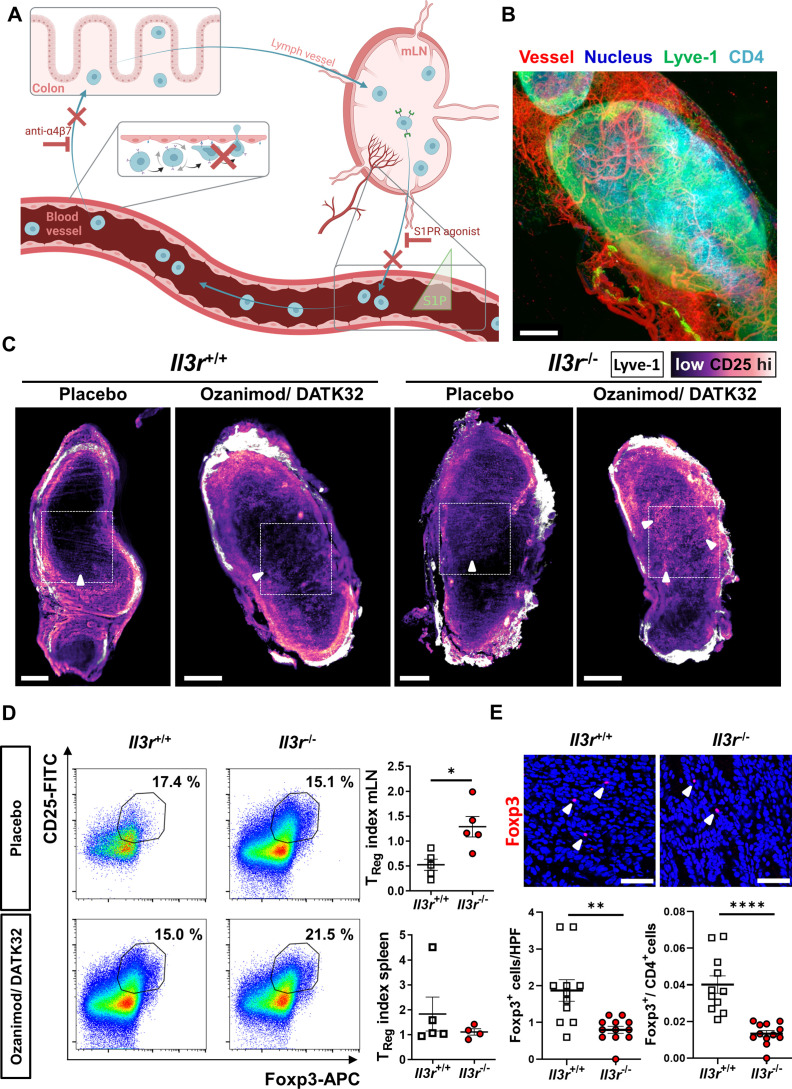
Although demonstrating altered mechano-migratory properties of Il3r −/− T cells, our findings did so far not answer the question, why experimental T cell transfer colitis is aggravated after transfer of Il3r −/− cells. In view of the pronounced mechanical phenotype of Il3r −/− Tregs, we hypothesised that their higher deformability might predispose them for increased egress from the colon to mLNs compared with other T cells, which might lead to an enhanced dysbalance of pro-inflammatory and anti-inflammatory T cells in the gut.
To explore this question, we used Rag1 −/− mice with established Il3r +/+ or Il3r −/− T cell transfer colitis and performed recirculation assays by injecting anti-α4β7 integrin antibodies (DATK32) to block T cell gut homing and the sphingosine-1-phosphate receptor (S1PR) agonist ozanimod to block T cell recirculation from mLNs. In this situation, changes occurring in the T cell composition of mLNs should largely be attributable to T cell egress from the gut, since upstream and downstream pathways are unavailable (figure 6A). Consistently, whole organ imaging of mLNs from mice with transfer colitis using lightsheet fluorescence microscopy demonstrated Lyve-1-expressing afferent lymph vessels and infiltrating CD4+ T cells (figure 6B). In further experiments, we stained mLNs for Lyve-1 and CD25. Analysis by lightsheet microscopy suggested increased infiltration of Il3r −/−, but not Il3r +/+ CD25+ Tregs to mLNs 16 hours after combined DATK32 and ozanimod treatment compared with control mice treated with placebo (figure 6C). To quantify these changes in T cell composition, we determined the fraction of CD25+Foxp3+ Tregs in mLNs and spleens of mice with transfer colitis after treatment with DATK32/ozanimod or with placebo. Interestingly, in mice with Il3r −/− T cells, the Treg fraction in mLNs, but not in spleens, increased after DATK32/ozanimod treatment compared with placebo, while it rather decreased in mice with Il3r +/+ T cells. Consistently, when we compared the ratio of Tregs in DATK32/ozanimod-treated and placebo-treated mice between Il3r +/+-dependent and Il3r −/−-dependent transfer colitis, we detected a significant difference in mLNs, but not in spleen (figure 6D).
Figure 6.
Enhanced recirculation of Il3r −/− regulatory T lymphocytes from the inflamed colon. (A) Schematic representation of recirculation studies in T cell transfer colitis in vivo (cf. main text for details); figure drawn with licensed BioRender software. (B) Representative lightsheet microscopy of Il3r +/+ mLN after staining with vesseldye-CF770 (red) and with antibodies against Lyve-1-eFluor570 (green), CD4-AF647 (light blue) and counterstaining with Hoechst (dark blue); three-dimensional reconstruction with Imaris software V.9.9; scale bar – 200 µm. (C) Representative virtual sections of mLNs from Rag1 −/− mice with transfer colitis induced by Il3r −/− or Il3r +/+ T cells and treated with ozanimod/DATK32 or placebo control analysed by lightsheet microscopy. Whole-organ staining with antibodies against Lyve-1-eFluor570 (white) and CD25-AF488 (scale from black to light magenta); scale bars – 400 µm, dashed square highlights the mLN centre, white arrowheads highlight representative CD25 signal. (D) Representative flow cytometry of mLNs (left, gated on CD3+CD4+) and quantification of the CD25+Foxp3+ regulatory T cell (Treg) index (right, Treg fraction in ozanimod/DATK32 per fraction in placebo) in mLNs (upper panel) and spleens (lower panel). n=4–5 per group, Mann-Whitney test. (E) Immunofluorescence staining for Foxp3 in colon tissue of Rag1 −/− mice with transfer colitis induced by Il3r −/− or Il3r +/+ T cells: Representative confocal microscopy (upper panels) and quantification of Foxp3+ cells as well as the ratio of Foxp3+ to CD4+ cells (stained on sequential sections, lower panels). n=11–12 per group, Mann-Whitney (Foxp3) and unpaired t-test (ratio); scale bars – 50 µm, white arrowheads highlight Foxp3+ cells. HPF, high power field; mLN, mesenteric lymph node; S1P, sphingosine-1-phosphate; Treg, regulatory T cell.
These data suggested that the egress of Il3r −/− Tregs from the inflamed colon to draining mLNs surpasses that of Il3r −/− non-Tregs. To evaluate the consequences of this observation for T cell composition in the inflamed colon, we stained Foxp3 on colon cryosections from mice with transfer colitis (without additional treatment, as shown in figure 2F–H). We observed a clear reduction of Foxp3+ Tregs in the colon of Rag1 −/− mice with Il3r −/− compared with Il3r +/+ T cells resulting in a decreased ratio of Tregs to total CD4+ T cells (figure 6E). Importantly, Treg differentiation and effector cell suppression per se were not altered by Il3r deficiency and addition of Il-3 (online supplemental figure 8A,B).
Thus, together, these findings suggested that aggravated inflammation in Il3r −/−-dependent T cell transfer colitis can be explained as a result of increased Treg egress to mLNs leading to reduced anti-inflammatory activity in the gut with consecutively enhanced effector T cell activity.
Il3r-dependent mechanical and migratory properties are recapitulated in human T cells and IL-3 expression in the intestine is associated to Treg abundance
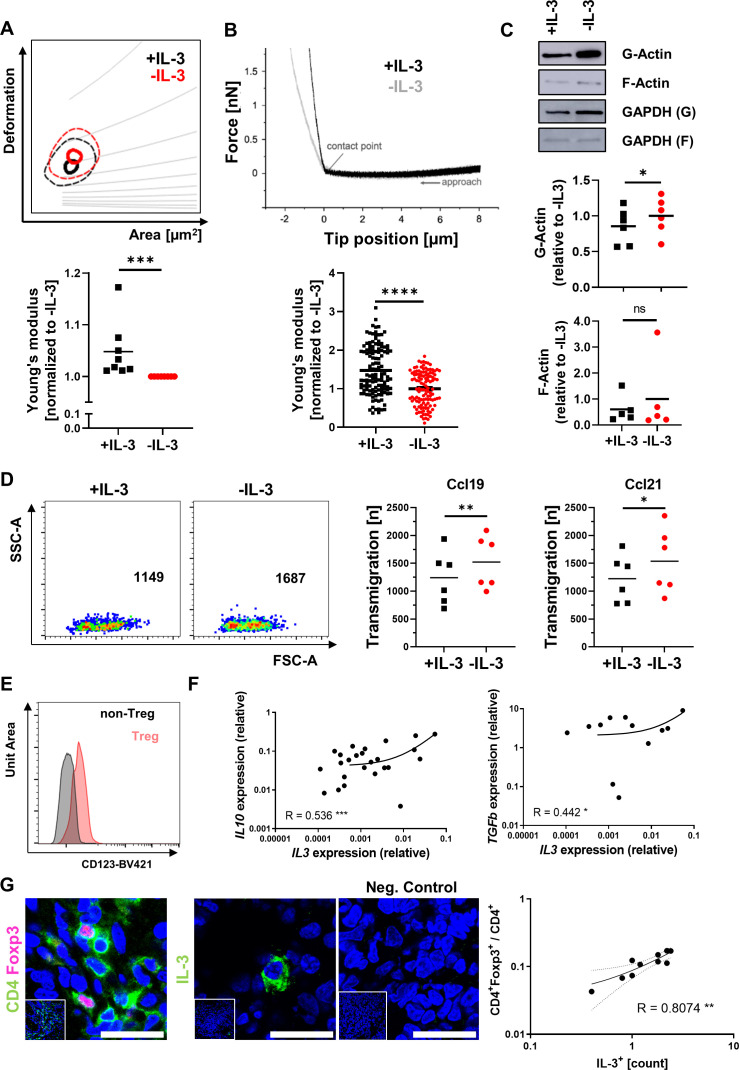
We next sought to answer the question whether similar mechanisms apply for human T cells. To this end, we isolated CD4+ T cells from the peripheral blood, stimulated them with anti-CD3/CD28 antibodies to induce IL-3R expression (online supplemental figure 9A), and treated them with or without IL-3.
In a first series of experiments, we confirmed that IL-3 does not impact on the expression of gut homing markers or the dynamic adhesion to MAdCAM-1 and VCAM-1 similar to the situation in mice (online supplemental figure 9B,C). We then characterised their mechanical phenotype by RT-DC. Consistent with the findings in mice, IL-3-exposed T cells exhibited lower deformability, whereas IL-6 or interferon-γ did not induce similar changes (figure 7A, online supplemental figure 9D). AFM corroborated decreased deformability of IL-3-exposed cells (figure 7B). Although phalloidin staining revealed similar expression of total actin in T cells treated with or without IL-3 (online supplemental figure 9E), separation of globular (G-) and filamentous (F-) actin from these cells and subsequent western blot demonstrated increased G-actin in IL-3-unexposed CD4+ T cells (figure 7C). This was fitting to the concept that IL-3R signalling also triggers cytoskeletal alterations in human T cells as it does in mice.
Figure 7.
IL-3 stiffens human CD4+ T cells and correlates to Tregs in the inflamed gut. (A) Representative RT-DC kernel density estimate plot (with grey isoelasticity lines) of CD4+ peripheral blood T cells stimulated with anti-CD3/CD28 antibodies and treated with or without rh IL-3 (upper panel) and quantification of the calculated mean Young’s modulus normalised to values of untreated samples (lower panel); n=8 per group, Wilcoxon signed-rank test; effect size: Cohen’s d=1.323. (B) Representative atomic force microscopy force curve (upper panel) and quantification of the calculated Young’s modulus relative to the mean of untreated cells (lower panels) of CD4+ peripheral blood T cells stimulated with anti-CD3/CD28 antibodies and treated with or without rh IL-3; n=59–62 per group, paired Mann-Whitney test. (C) Representative western blot analysis (upper panels) and quantification (lower panels) of G-Actin and F-Actin (as well as GAPDH control) in CD4+ T cells stimulated with anti-CD3/CD28 antibodies and treated with or without rh IL-3; n=5–6 per group, Wilcoxon matched pairs signed-rank test. (D) Migration of CD4+ peripheral blood T cells over porous membranes towards rh CCL19 or rh CCL21. Left panels: Representative flow cytometry. Right panels: Quantification of transmigration; n=6 per group, paired t-test. (E) Flow cytometry of lamina propria mononuclear cells isolated from biopsies of patients with IBD. Representative histogram of CD123 staining on CD4+CD25+Foxp3+ Treg cells and CD4+CD25−Foxp3− non-Treg cells (left). Data are representative for five independent experiments. (F) Correlation of IL3 mRNA with IL10 and TGFB1 mRNA expression as determined by qPCR in colon tissue from patients with IBD (n=21–45). Spearman’s R, significance levels and a regression line are indicated. (G) Immunofluorescence of cryosections from patients with IBD (CD or UC, each n=5) for CD4 (green) and Foxp3 (magenta) or IL-3 (green); counterstaining with Hoechst (blue). Left panels: representative images, scale bars – 25 µm; right panel: correlation of IL-3+ cells and CD4+Foxp3+ per total CD4+ cells; Spearman’s R, significance levels and a regression line are indicated. CD, Crohn’s disease; F-Actin; filamentous actin; G-Actin, globular actin; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IBD, inflammatory bowel disease; IL, interleukin; mRNA, messenger RNA; qPCR, quantitative PCR; rh, recombinant human; RT-DC, real-time deformability cytometry; TGF, transforming growth factor; Tregs, regulatory T cells; UC, ulcerative colitis.
We further aimed to investigate the impact of IL-3R signalling on human T cells on a functional level. Thus, we investigated their migration over porous membranes towards the mLN chemokines CCL19 and CCL21 on previous incubation with or without IL-3. Indeed, fewer IL-3-exposed T cells migrated through those membranes than unexposed T cells (figure 7D), suggesting that lower deformability results in reduced motility similar to the situation in mice.
Finally, we aimed at elucidating whether these mechanisms might also be relevant for human IBD. To this end, we stained LPMCs from colon biopsies of patients with IBD for flow cytometry. Importantly, we could show increased expression of IL-3R (CD123) on Tregs compared with conventional T cells in the lamina propria (figure 7E). This suggested that IL-3 released in the inflamed human gut can locally determine the mechanobiology and motility of IL-3R-expressing Tregs.
Indeed, the expression of IL3 mRNA in the colon of patients with IBD was significantly correlated with Treg cytokines such as IL10 or TGFB,29 but not with pro-inflammatory mediators such as IL13 or IL18 (figure 7F, online supplemental figure 10). Similarly, when we stained colon samples from patients with IBD for CD4+Foxp3+ Tregs and for IL-3+ cells, we observed a strong correlation (figure 7G), which was consistent with the idea that IL-3 promotes mucosal residence of Tregs also in the human gut.
Discussion
Various cytokines have been implicated in the pathogenesis of IBD,30 but the role of IL-3 remained to be explored until now. In this study, we identify an important function of IL-3R signalling in CD4+ T cells in experimental colitis and human IBD.
We observed increased expression of IL-3 in the inflamed mucosa of patients with CD and UC, whereas IL-3 was barely expressed in patients without IBD. Moreover, IL-3 was strongly correlated to Treg cytokines and abundance. Thus, our data are the first to provide evidence for a key role of IL-3 in the pathogenesis of IBD.
Moreover, our findings uncovered a crucial role of IL-3 in driving Treg mechanobiology and tissue egress. To our knowledge, these observations identify the first factor controlling mucosal tissue egress of Tregs and suggest new avenues to specifically interfere with T cell trafficking in order to treat chronic intestinal inflammation.
We observed an increase of Il-3 expression during experimental T cell transfer colitis suggesting that IL-3 and associated IL-3R signalling not only impacts on human disease but also on experimental models of IBD. Compatibly, Il-3 had previously been linked with experimental models of several other immune-mediated inflammatory diseases such as rheumatoid arthritis, lupus erythematodes, asthma or multiple sclerosis,9 10 31 32 whereas insights into chronic intestinal inflammation apart from an established role on basophil expansion33 had been lacking so far. Taking advantage of two T cell-dependent models covering different aspects of intestinal inflammation,34 35 our further experiments revealed that Il-3 and Il-3r signalling counteract intestinal inflammation. Regarding the marked upregulation of IL-3 expression particularly in active IBD, this might appear counterintuitive on first view. However, one has to consider that active inflammation also triggers regulatory pathways, which limit tissue destruction and may lead to resolution of inflammation.36 Consistently, increased expression of the key Treg cytokine IL-10 and an increased presence of functional Tregs in active IBD have earlier been demonstrated29 37 and IL-3 was strongly correlated with Treg cytokines and abundance. However, in the inflammatory network present in the mucosa in IBD, such compensatory attempts fall short of controlling disease due to an even more pronounced expansion of pro-inflammatory signalling and cells.5 37 Thus, we interpret our findings in human IBD as the abortive effort of the intestinal immune system to mount anti-inflammatory strategies to resolve inflammation. This idea is also promoted by the virtual absence of IL-3 expression in the uninflamed gut suggesting that IL-3 does not control homeostasis, but is released as a counter-regulatory signal in inflammation.
Of note, the role of IL-3 seems to differ between different organs and contexts. Whereas detrimental effects have been suggested in diseases such as chronic inflammation of the central nervous system,31 other authors report a regulatory role, for example, in asthma.9 38 39 Importantly, many of the latter reports associate the regulatory function with Tregs. Thus, our data further underscore that the role of IL-3 in various pathologies is pleiotropic, but match well with other settings, where IL-3 restrains inflammation.
On a mechanistic level, our studies implicate IL-3 in the regulation of T cell trafficking, particularly in the prevention of Treg egress from the colon lamina propria. This observation is noteworthy for two reasons: (1) T cell egress from the intestine has barely been studied so far and (2) our data suggest that cell-intrinsic processes rather than the interaction of surface molecules orchestrate this effect.
In the light of an anti-α4β7 integrin antibody (vedolizumab) and an S1PR agonist (ozanimod) being available for the treatment of IBD14 15 40 and other anti-trafficking agents in the pipeline,41 42 T cell trafficking has gained increasing attraction in the field. However, while previous research extensively studied T cell homing and retention for their role in regulating the in situ T cell pool in the intestine,22 43 44 T cell egress from that pool has largely been neglected. While a role of Ccr7 and sphingosine-1-phosphate (S1P) have been demonstrated as in other organs,45 46 dedicated studies on local factors calibrating intestinal T cell egress in IBD are missing. This even capitalises in the so far unanswered question, whether clinically available and investigated S1PR agonists might also impact on T cell recirculation from the mucosa in addition to recirculation from secondary lymphoid organs. Thus, our data provide important new insights into a so far understudied aspect of T cell trafficking. Of note, in line with our data suggesting that deficient Il3r signalling induces a preferential increase of Treg egress, a previous study has demonstrated that Treg recirculation between the colon and mLNs is vital in experimental colitis.45
Moreover, despite recent major breakthroughs in the technical prerequisites and equipment to assess cell mechanical properties,47 the functional role of cell mechanics for different cellular functions such as cell migration and motility has so far largely been studied in cancer,48 but not sufficiently in T cells and in chronic inflammation. In fact, to our best knowledge, our study is the first to show the impact of a specific cytokine on the mechanical phenotype of T cells. Mechanistically, this seems to be driven by Il3r-dependent signalling altering the architecture and dynamics of the cytoskeleton. This is well in line with concepts previously demonstrated in other cell types.49
While we acknowledge as a potential limitation that some of these characterisations were performed with non-intestinal T cells and under unchallenged conditions, our data on the biomechanics of lamina propria T cells and recirculation during T cell transfer colitis strongly support that this concept is relevant in intestinal inflammation. Essentially, by linking cytokine signalling-dependent T cell mechanical properties with their trafficking features and a resulting effect on chronic intestinal inflammation, we underscore that the regulation of T cell trafficking is not limited to the sole interaction of surface molecules, but importantly involves intracellular events. Thereby, we also provide a completely novel and orthogonal concept explaining aspects of the pathogenesis of chronic intestinal inflammation.
Importantly, studies with human cells and tissue recapitulated key aspects of the mechano-migratory phenotype imprinted on T cells by IL-3R signalling in mice. Thus, they suggest that a similar concept might apply in IBD. This might open avenues for novel future approaches in patients with CD and UC. In particular, since it has previously been shown that Tregs are a useful strategy for IBD therapy,50 51 it fuels the idea that locally modulating mechanical Treg properties with IL-3 might be a suitable and organ-selective treatment approach, for example, by application via enema or by targeted delivery through engineered commensal bacteria as previously suggested for IL-10.52
Taken together, we demonstrate a beneficial role of IL-3 in chronic intestinal inflammation that is driven by alterations in the cytoskeleton promoting mucosal residence of Tregs. This suggests novel treatment approaches for IBD and stimulates necessary further studies in this direction
Acknowledgments
The research of TMM, IA, SF, BW, RL-P, IP, RA, MFN and SZ was supported by the Interdisciplinary Center for Clinical Research (IZKF) and the ELAN programme of the University Erlangen-Nuremberg, the Fritz-Bender-Stiftung, the Else Kröner-Fresenius-Stiftung, the DFG Collaborative Research Centers 643, 796, 1181 and TRR241, the Kenneth Rainin Foundation and the Litwin IBD Pioneers programme of the Crohn’s and Colitis Foundation of America (CCFA); FL was supported by SFB 1027. The authors thank J Marcks, D Dziony, J Schuster, S Hofmann, L-S Becker, M Döbrönti, S Abuhattum, C Büttner, A Werlein, L Sologub, H Knott and A-L. Vögele for excellent support and/or technical assistance.
Footnotes
Contributors: KAU, JD, CB, GR, SU, LLS, L-JL, MS, LK, OBB, TMM, N-MS and SZ performed the experiments. KAU, SU, MK, EB, IA, SF, IP, BW, SW, RL-P, RA, FL, JG, MFN and SZ provided clinical samples, protocols, reagents or designed experiments. ABE performed and MD analysed RNA sequencing. KAU, JD, CB, GR, SU, LLS, LK, TMM, FL, JG, MFN and SZ analysed and interpreted the data. SZ and KAU drafted the manuscript with the help of MFN. All authors critically revised the manuscript for important intellectual content. Guarantors: MFN, SZ.
Funding: This work was funded by grants of the German Research Foundation (ZU 377/4-1; TRR 241 – 375876048; KFO 5024 – GB.com), the German Crohn’s and Colitis Foundation (DCCV), the Interdisciplinary Center for Clinical Research (IZKF, J63), the Fritz Thyssen Stiftung (10.21.2.018MN) and the Else Kröner-Fresenius-Stiftung (2021_EKCS.23).
Competing interests: MFN has served as an advisor for Pentax, Giuliani, MSD, AbbVie, Janssen, Takeda and Boehringer. SZ received honoraria from Takeda, Roche, Galapagos, Ferring, Falk, Lilly and Janssen. MFN and SZ received research support from Takeda, Shire (a part of Takeda) and Roche. JG and MK are co-founders of Rivercyte GmbH, a company that develops biomedical applications for real-time deformability cytometry. The other authors declare no conflicts of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. RNA sequencing data are deposited on Zenodo (https://doi.org/10.5281/zenodo.8068464). All other data that support the findings of this study are either included in the article, uploaded as supplementary information or available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by ethics committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg (249_13 B; 135_20B, 426_20B). Participants gave informed consent to participate in the study before taking part.
References
- 1. Ananthakrishnan AN, Bernstein CN, Iliopoulos D, et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol 2018;15:39–49. 10.1038/nrgastro.2017.136 [DOI] [PubMed] [Google Scholar]
- 2. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers 2020;6:74. 10.1038/s41572-020-0205-x [DOI] [PubMed] [Google Scholar]
- 3. Tschurtschenthaler M, Adolph TE, Ashcroft JW, et al. Defective ATG16L1-mediated removal of Ire1Α drives Crohn's disease-like Ileitis. J Exp Med 2017;214:401–22. 10.1084/jem.20160791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease. Medical Treatment J Crohns Colitis 2020;14:4–22. 10.1093/ecco-jcc/jjz180 [DOI] [PubMed] [Google Scholar]
- 5. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol 2014;14:329–42. 10.1038/nri3661 [DOI] [PubMed] [Google Scholar]
- 6. Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med 2021;385:628–39. 10.1056/NEJMra1909094 [DOI] [PubMed] [Google Scholar]
- 7. Ihle JN, Pepersack L, Rebar L. Regulation of T cell differentiation: in vitro induction of 20 alpha-hydroxysteroid dehydrogenase in splenic lymphocytes from Athymic mice by a unique lymphokine. J Immunol 1981;126:2184–9. 10.4049/jimmunol.126.6.2184 [DOI] [PubMed] [Google Scholar]
- 8. Fung MC, Hapel AJ, Ymer S, et al. Molecular cloning of cDNA for murine Interleukin-3. Nature 1984;307:233–7. 10.1038/307233a0 [DOI] [PubMed] [Google Scholar]
- 9. Srivastava RK, Tomar GB, Barhanpurkar AP, et al. IL-3 attenuates collagen-induced arthritis by Modulating the development of Foxp3+ regulatory T cells. J Immunol 2011;186:2262–72. 10.4049/jimmunol.1002691 [DOI] [PubMed] [Google Scholar]
- 10. Yogesha SD, Khapli SM, Srivastava RK, et al. IL-3 inhibits TNF-alpha-induced bone resorption and prevents inflammatory arthritis. J Immunol 2009;182:361–70. 10.4049/jimmunol.182.1.361 [DOI] [PubMed] [Google Scholar]
- 11. Dougan M, Dranoff G, Dougan SK. GM-CSF, IL-3, and IL-5 family of Cytokines: regulators of inflammation. Immunity 2019;50:796–811. 10.1016/j.immuni.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renner K, Metz S, Metzger A-M, et al. Expression of IL-3 receptors and impact of IL-3 on human T and B cells. Cell Immunol 2018;334:49–60. 10.1016/j.cellimm.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 13. Kumar A, Rani L, Mhaske ST, et al. IL-3 receptor expression on activated human th cells is regulated by IL-4, and IL-3 Synergizes with IL-4 to enhance Th2 cell differentiation. J Immunol 2020;204:819–31. 10.4049/jimmunol.1801629 [DOI] [PubMed] [Google Scholar]
- 14. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. 10.1056/NEJMoa1215734 [DOI] [PubMed] [Google Scholar]
- 15. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. 10.1056/NEJMoa1215739 [DOI] [PubMed] [Google Scholar]
- 16. Zundler S, Becker E, Schulze LL, et al. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut 2019;68:1688–700. 10.1136/gutjnl-2018-317977 [DOI] [PubMed] [Google Scholar]
- 17. Habtezion A, Nguyen LP, Hadeiba H, et al. Leukocyte trafficking to the small intestine and colon. Gastroenterology 2016;150:340–54. 10.1053/j.gastro.2015.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang MS, Bowcutt R, Leung JM, et al. Integrated analysis of biopsies from inflammatory bowel disease patients identifies SAA1 as a link between mucosal microbes with TH17 and TH22 cells. Inflamm Bowel Dis 2017;23:1544–54. 10.1097/MIB.0000000000001208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol 2019;16:185–96. 10.1038/s41575-018-0084-8 [DOI] [PubMed] [Google Scholar]
- 20. Corridoni D, Antanaviciute A, Gupta T, et al. Single-cell Atlas of colonic CD8+ T cells in ulcerative colitis. Nat Med 2020;26:1480–90. 10.1038/s41591-020-1003-4 [DOI] [PubMed] [Google Scholar]
- 21. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity 2019;50:992–1006. 10.1016/j.immuni.2019.03.017 [DOI] [PubMed] [Google Scholar]
- 22. Zundler S, Schillinger D, Fischer A, et al. Blockade of ΑEβ7 integrin suppresses accumulation of CD8+ and Th9 lymphocytes from patients with IBD in the inflamed gut in vivo. Gut 2017;66:1936–48. 10.1136/gutjnl-2016-312439 [DOI] [PubMed] [Google Scholar]
- 23. Ullrich K, Dedden M, Müller TM, et al. IL-3 receptor signaling suppresses chronic intestinal inflammation by controlling Mechanobiology and tissue egress of regulatory T cells. 22 June 2023. 10.5281/zenodo.8068464 [DOI] [PMC free article] [PubMed]
- 24. Müller PM, Rademacher J, Bagshaw RD, et al. Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat Cell Biol 2020;22:498–511. 10.1038/s41556-020-0488-x [DOI] [PubMed] [Google Scholar]
- 25. Romanova LY, Alexandrov IA, Blagosklonny MV, et al. Regulation of actin cytoskeleton in lymphocytes: PKC-Delta disrupts IL-3-induced membrane ruffles downstream of Rac1. J Cell Physiol 1999;179:157–69. [DOI] [PubMed] [Google Scholar]
- 26. Tiede I, Fritz G, Strand S, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003;111:1133–45. 10.1172/JCI16432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. James KD, Jenkinson WE, Anderson G. T-cell egress from the thymus: should I stay or should I go? J Leukoc Biol 2018;104:275–84. 10.1002/JLB.1MR1217-496R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Otto O, Rosendahl P, Mietke A, et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat Methods 2015;12:199–202. 10.1038/nmeth.3281 [DOI] [PubMed] [Google Scholar]
- 29. Wittmann Dayagi T, Werner L, Pinsker M, et al. Mucosal IL-10 and IL-10 receptor expression patterns in paediatric patients with ulcerative colitis. Int J Exp Pathol 2021;102:4–10. 10.1111/iep.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marafini I, Sedda S, Dinallo V, et al. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther 2019;19:1207–17. 10.1080/14712598.2019.1652267 [DOI] [PubMed] [Google Scholar]
- 31. Renner K, Hellerbrand S, Hermann F, et al. IL-3 promotes the development of experimental autoimmune encephalitis. JCI Insight 2016;1:e87157. 10.1172/jci.insight.87157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rignault-Bricard R, Machavoine F, Mecheri S, et al. IL-3-producing Basophils are required to exacerbate airway hyperresponsiveness in a murine inflammatory model. Allergy 2018;73:2342–51. 10.1111/all.13480 [DOI] [PubMed] [Google Scholar]
- 33. Gomez MR, Talke Y, Hofmann C, et al. Basophils control T-cell responses and limit disease activity in experimental murine colitis. Mucosal Immunol 2014;7:188–99. 10.1038/mi.2013.38 [DOI] [PubMed] [Google Scholar]
- 34. Wirtz S, Popp V, Kindermann M, et al. Chemically induced Mouse models of acute and chronic intestinal inflammation. Nat Protoc 2017;12:1295–309. 10.1038/nprot.2017.044 [DOI] [PubMed] [Google Scholar]
- 35. Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015;1:154–70. 10.1016/j.jcmgh.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen Z, Bozec A, Ramming A, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol 2019;15:9–17. 10.1038/s41584-018-0109-2 [DOI] [PubMed] [Google Scholar]
- 37. Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology 2005;128:1868–78. 10.1053/j.gastro.2005.03.043 [DOI] [PubMed] [Google Scholar]
- 38. Drube S, Müller S, Weber F, et al. IL-3 is essential for ICOS-L stabilization on mast cells, and sustains the IL-33-induced RORγt+ TReg generation via enhanced IL-6 induction. Immunology 2021;163:86–97. 10.1111/imm.13305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krammer S, Yang Z, Zimmermann T, et al. An Immunoregulatory role of Interleukin-3 in allergic asthma. Front Immunol 2022;13:821658. 10.3389/fimmu.2022.821658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2021;385:1280–91. 10.1056/NEJMoa2033617 [DOI] [PubMed] [Google Scholar]
- 41. Matsuoka K, Watanabe M, Ohmori T, et al. Ajm300 (Carotegrast methyl), an oral antagonist of Α4-integrin, as induction therapy for patients with moderately active ulcerative colitis: a Multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol Hepatol 2022;7:648–57. 10.1016/S2468-1253(22)00022-X [DOI] [PubMed] [Google Scholar]
- 42. Sandborn WJ, Peyrin-Biroulet L, Zhang J, et al. Efficacy and safety of Etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology 2020;158:550–61. 10.1053/j.gastro.2019.10.035 [DOI] [PubMed] [Google Scholar]
- 43. Dai B, Hackney JA, Ichikawa R, et al. Dual targeting of lymphocyte homing and retention through Α4Β7 and ΑEβ7 inhibition in inflammatory bowel disease. Cell Rep Med 2021;2:100381. 10.1016/j.xcrm.2021.100381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Belarif L, Danger R, Kermarrec L, et al. IL-7 receptor influences anti-TNF responsiveness and T cell gut homing in inflammatory bowel disease. J Clin Invest 2019;129:1910–25.:121668. 10.1172/JCI121668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakanishi Y, Ikebuchi R, Chtanova T, et al. Regulatory T cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol 2018;11:437–48. 10.1038/mi.2017.64 [DOI] [PubMed] [Google Scholar]
- 46. McNamee EN, Masterson JC, Veny M, et al. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn'S-like murine Ileitis. J Leukoc Biol 2015;97:1011–22. 10.1189/jlb.3HI0614-303R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Urbanska M, Muñoz HE, Shaw Bagnall J, et al. A comparison of microfluidic methods for high-throughput cell deformability measurements. Nat Methods 2020;17:587–93. 10.1038/s41592-020-0818-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mierke CT. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep Prog Phys 2019;82:064602. 10.1088/1361-6633/ab1628 [DOI] [PubMed] [Google Scholar]
- 49. Lautenschläger F, Paschke S, Schinkinger S, et al. The regulatory role of cell mechanics for migration of differentiating myeloid cells. Proc Natl Acad Sci U S A 2009;106:15696–701. 10.1073/pnas.0811261106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Desreumaux P, Foussat A, Allez M, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology 2012;143:1207–17. 10.1053/j.gastro.2012.07.116 [DOI] [PubMed] [Google Scholar]
- 51. Voskens C, Stoica D, Rosenberg M, et al. Autologous regulatory T-cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut 2023;72:49–53. 10.1136/gutjnl-2022-327075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Braat H, Rottiers P, Hommes DW, et al. A phase i trial with transgenic bacteria expressing Interleukin-10 in Crohn's disease. Clin Gastroenterol Hepatol 2006;4:754–9. 10.1016/j.cgh.2006.03.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2023-329818supp002.pdf (175.5KB, pdf)
gutjnl-2023-329818supp001.pdf (2MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. RNA sequencing data are deposited on Zenodo (https://doi.org/10.5281/zenodo.8068464). All other data that support the findings of this study are either included in the article, uploaded as supplementary information or available from the corresponding author upon reasonable request.