Abstract
Background
Our study investigated the rate of breakthrough SARS-CoV-2 infection and clinical outcomes in a cohort of multiple sclerosis (MS) patients who were treated with the anti-CD20 monoclonal antibody (Ab), ocrelizumab, before first, second and third BNT162b2 mRNA vaccinations. To correlate clinical outcomes with the humoral and cellular response.
Methods
The study was a prospective non-randomised controlled multicentre trial observational study. Participants with a diagnosis of MS who were treated for at least 12 months with ocrelizumab prior to the first BNT162b2 mRNA vaccination were prospectively followed up from January 2021 to June 2022.
Results
Out of 54 participants, 32 (59.3%) developed a positive SARS-CoV-2 PCR test in the study period. Mild infection was observed in all infected participants. After the third vaccination, the non-infected participants had higher mean Ab levels compared to the infected participants (54.3 binding antibody unit (BAU)/mL vs 26.5 BAU/mL, p=0.030). The difference in reactivity between spike-specific CD4+ and CD8+ T lymphocytes in the two groups was not significant.
Conclusion and relevance
The study results demonstrate rates of 59% in breakthrough infections after the third SARS-CoV-2 mRNA vaccination in ocrelizumab-treated patients with MS, without resulting in critical disease courses. These findings suggest the need for continuous development of prophylactic treatments when proved important in the protection of severe breakthrough infection.
Keywords: MULTIPLE SCLEROSIS, IMMUNOLOGY
Background
Treatment with anti-CD20 (a-CD20) monoclonal antibodies (Ab) is widely used for autoimmune diseases, including multiple sclerosis (MS). While MS alone does not affect humoral and cellular immunity, a-CD20 treatment leads to B-cell depletion, thereby impairing humoral immunity. More severe COVID-19 outcomes were reported in 2020 and 2021 in patients with MS treated with a-CD20 therapy.1 After SARS-CoV-2 mRNA vaccination, numerous studies have demonstrated absent or decreased humoral response in a-CD20-treated patients, while cellular responses are generally preserved.2–5 The dominating subtypes of SARS-CoV-2 shifted from Delta to Omicron (BA.1–5), and by the end of December 2021, >90% of positive PCR tests sequenced in Denmark and the USA identified the Omicron variant.6 7 Increased risk of breakthrough COVID-19 has been reported for a-CD20-treated patients despite vaccination.8 Early booster administration2 and treatment with anti-spike monoclonal Abs for vulnerable patients have been common practice worldwide. Additionally, in the USA, the administration of Evusheld has been frequently used as prophylaxis in B-cell depleted patients.9 The relationship between clinical outcomes after infection with SARS-CoV-2 in a-CD20-treated patients and levels of humoral and cellular reactivity after vaccination is unknown. The current study evaluates clinical outcomes and their correlation with humoral and cellular immunity in a cohort of patients with MS prospectively followed up through their three mRNA SARS-CoV-2 vaccinations.2 3
Methods
This study followes 54 participants with MS in Denmark and the USA treated with ocrelizumab before the first, second, and third BNT162b2 mRNA vaccine doses. Levels of receptor binding domain (RBD) Abs and spike-reactive T cells before and after first, second and third vaccinations were measured as previously published.2 3 Spike-specific CD4+ and CD8+ T-cell responses were measured by activation-induced marker (AIM) expression as previously described.4 At the time of vaccination, only one participant had a prior clinical history of SARS-CoV-2 infection.
Participants received the third vaccination between August 8th and October 2021, and were followed for COVID-19 infection until 26 May 2022. SARS-CoV-2 PCR test and virus characterisation results, including the most frequent viral variants, were extracted from the Danish National Microbiology Database, MiBa (Statens Serum Institut, Copenhagen, Denmark). Results from the USA were obtained by reviewing the electronic medical records of all American participants. Clinical outcomes and demographics were scored with the WHO progression scale based on patient records and interviews.10
Anonymised data will be shared on request from any qualified investigator under approval from the Danish Data Protection Agency.
All data were analysed for normal distribution with D’Agostino-Pearson normality test and with a visual inspection (online supplemental table 1). Analysis of Abs levels and cellular response between infected and uninfected participants was done with the Mann-Whitney test. The level of significance was defined as p<0.05.
jnnp-2022-330757supp001.pdf (310.8KB, pdf)
Results
Participants’ demographic and clinical characteristics are summarised in table 1A. Out of 54 participants, 32 (59.3%) developed a positive SARS-CoV-2 PCR test in the study period. The first recorded infection was on 17 December 2021, and the last infection recorded was on 20 April 2022, indicating that all infections occurred after the third mRNA vaccination (Ultimo October 2021). Out of the infected participants, 9.4% tested positive during December 2022, 40.6% in January 2022, 31.3% in February 2022, 15.6% in March 2022 and 3.1% in April 2022. When comparing the two geographical locations, altogether during the study period, 73.2% of the Danish cohort and 15.4% of the US cohort had a positive PCR test.
Table 1.
Demographic characteristics, treatment and clinical outcomes in the positive PCR cohort
| A | SARS-CoV-2 positive | SARS-CoV-2 negative |
| N (% of total) | 32 (59.3) | 22 (40.7) |
| DK | 30 (73.2) | 11 (26.8) |
| US | 2 (15.4) | 11 (84.6) |
| Female, n (%) | 25 (83.3) | 17 (70.8) |
| DK | 23 (82.1) | 8 (72.7) |
| US | 2 (100.0) | 9 (81.8) |
| Age, median (range) | 48 (25–64) | 47 (26–67) |
| DK | 48 (25–64) | 47 (28–67) |
| US | 46 (40-52) | 47 (26–54) |
| Duration of ocrelizumab treatment (years), median (range) | 2.2 (1.0–5.7) | 2.0 (1.6–7.0) |
| DK | 2.8 (1.0–5.7) | 2.5 (1.7–3.8) |
| US | 4.1 (3.6–4.5) | 4.4 (1.6–7.0) |
| Latest Expanded Disability Status Scale (EDSS), median (range) | 2.75 (0.0–6.0) | 2.0 (0.0–6.0) |
| DK | 2.25 (0.0–5.0) | 1.5 (1.5–6.0) |
| US | 5.75 (5.5–6.0) | 1.5 (0.0–3.5) |
| B | SARS-CoV-2 positive | DK cohort | US cohort |
| Remdesivir taken, n | 4 | 4 | 0 |
| Sotrovimab taken, n | 13 | 12 | 1 |
| Evusheld prophylaxis, n | 1 | 0 | 7 (6 uninfected) |
| WHO score, median (range) | 2 (1–4) | 2 (1–4) | 2 (2) |
| Days from last OCR treatment until COVID-19, median (range) | 79.0 (14.0–393.0) | 81.5 (15.0–393.0) | 39.5 (14.0–65.0) |
| Days from third vaccine to COVID-19, median (range) | 114.0 (47.0–188.0) | 114.0 (47.0–188.0) | 106.5 (85.0–128.0) |
DK, Danish cohort; OCR, ocrelizumab.
In Denmark, Omicron BA.1 was the dominant variant from December 2021 to January 2022, whereas in the USA, the BA.1 variant was dominant December 2021 until the end of March 2022. Thereafter, Omicron BA.2 was the dominant variant from February to May 2022 in both locations (end of the study period). Routine variant determination for all SARS-CoV-2 subtypes was stopped on 21 December 2021. Therefore, based on the assumption that the patients were infected on average with the most prevalent SARS-CoV-2 subtype, we found the following distribution: BA.1: 25.0% (n=8) and BA.2:75.0% (n=24).
Of the 32 participants with positive PCR, 17 (53.1%) received treatment after infection. For the infected Danish participants, sotrovimab (anti-spike SARS-CoV-2 monoclonal Ab) was administrated to 13 participants and 4 participants also received remdesivir. Seven of the US participants received Evusheld as prophylaxis between April and June 2022, one of whom tested positive for SARS-CoV-2. In general, the participants had mild disease courses with WHO scores of 1–4 (table 1B). One patient was hospitalized (= WHO score of 4) but discharged after one day without need for treatment or oxygen.
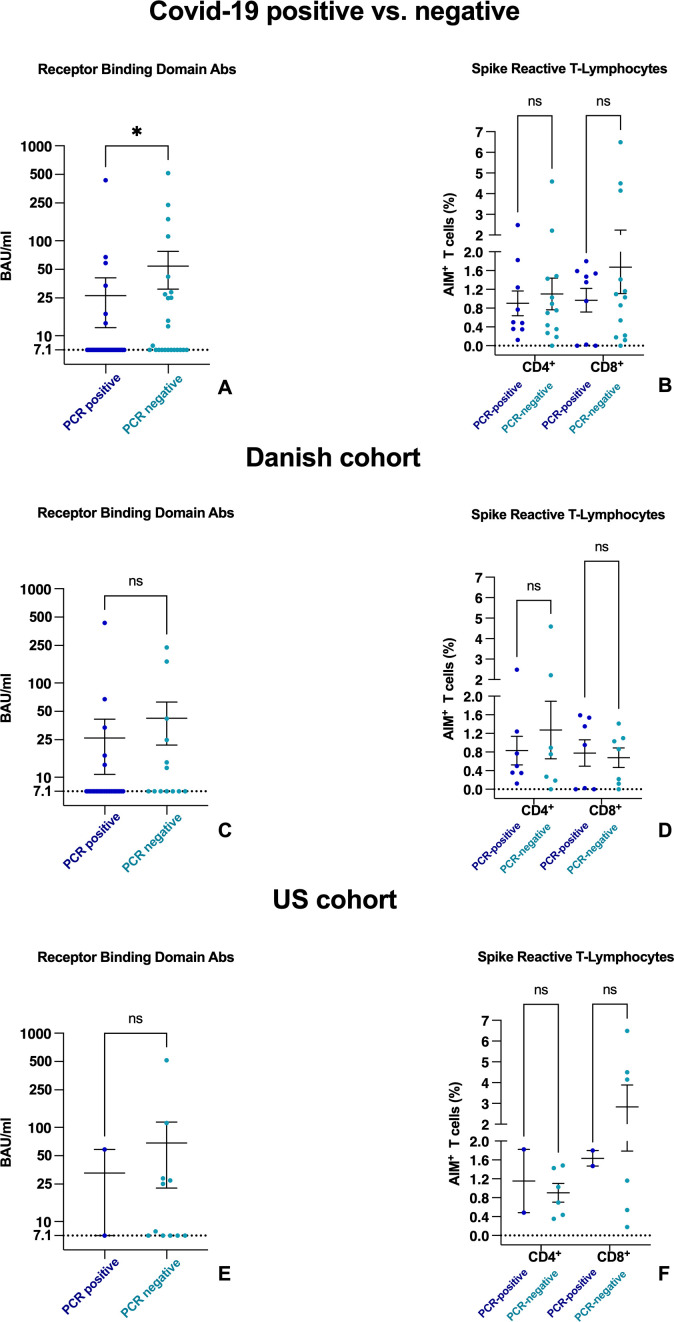
When comparing the humoral and cellular immunity between the infected and non-infected participants, in both the Danish and American cohorts, the non-infected participants had higher mean Ab levels compared with the infected participants (non-infected mean 54.3, infected mean 26.5 binding Ab unit/mL; p=0.030) after the third vaccination (figure 1A). No significant difference was found between the Danish and American cohorts (figure 1C,E). However, the frequencies of spike-specific CD4+ and CD8+ T-lymphocytes did not differ between the infected and non-infected groups (figure 1B, D and F).
Figure 1.
Dot plot that compares levels of RBD Abs (A,C,E) and spike-reactive T lymphocytes (B,D,F) in patients with a negative and positive SARS-CoV-2 PCR after first, second and third mRNA vaccinations. Positive and negative patients are shown in the full cohort (A,B), in the Danish cohort (C,D) and in the US cohort (E,F). Levels and means of RBD Abs are shown with SEM given in BAU per millilitre logarithmically scaled. Frequencies of spike-reactive CD4+ and CD8+ cells are demonstrated with means of AIMs of positive T cells and SEM. The y-axis is split in two segments from 0 to 2×109 cells/L and 2–7×109 cells/L. Ab, antibody; AIM, activation-induced marker; BAU, binding antibody unit; ns, not significant; RBD, receptor binding domain.
Discussion
The current study describes the clinical outcomes and immune correlations in a cohort of participants with MS and breakthrough SARS-CoV-2 infection while in treatment with ocrelizumab before and after SARS-CoV-2 mRNA vaccination. Of the study cohort, 59.3% experienced breakthrough infection, exceeding the 8.3% reported from the background population in a recent Danish study with a shorter observation period.11 Severe SARS-CoV-2 disease has also been associated with higher MS-related disability.1 Consequently, patients with MS and high disease burden and/or other risk factors are recommended to avoid the risk of exposure to COVID-19 infection more than the general population. Overall, the current findings suggest that vaccine-conferred protection against Omicron variants is lower in a-CD20-treated patients with MS than has been reported in healthy individuals.
Evaluating specific immune responses to vaccinations, we found that the participants who were not infected had significantly higher levels of RBD Abs before infection than the participants who became infected (figure 1). Overall, the Abs levels were low in both the infected and non-infected groups compared to those described in healthy control groups.12–14 This supports prior findings that the spike-specific Abs capable of SARS-CoV-2 neutralisation are vital for protection against infection. In contrast, no differences were observed between spike-specific CD4+ and CD8+ T-cell response in infected and uninfected participants. Despite lower RBD Abs levels, in general, our participants demonstrated a mild disease course of SARS-CoV-2 infection similar to the disease course observed in the background population11 and vaccinated MS-cohorts.8 15
This study has limitations. The current study was designed neither to examine the severity of the disease course following SARS-CoV-2 infection nor to adjust for potentially mediating factors including behavioural factors (such as isolation, distancing, mask use and hygiene) and comorbidities. Denmark and the USA had different strategies, for example, lockdown strategies resulting in geographical variations in patterns of serological prevalence, and accessibility to Evusheld prophylaxis. Patients undergoing B-cell depleting therapy were prioritised and vaccinated months earlier than the healthy background population since B-cell therapy was considered a risk factor for severe COVID-19. Therefore, including a matched healthy control group was not possible. Likewise, delaying the vaccination of our participants was considered unethical in the effort to recruit a matched control group. This study did not include other MS-specific DMTs since B-cell depleting therapy was the only DMT considered a risk factor of severe COVID-19 at the time of study initiation. We therefore prioritised patients treated with ocrelizumab compared with other DMTs.
To summarise, this study demonstrates a breakthrough infection rate of 59% in a Danish and American MS cohorts after the participants received the third SARS-CoV-2 mRNA vaccine while being treated with anti-CD2 therapy. In addition, the geographical difference regarding prophylaxis is evident when comparing different treatment strategies and infection rates. Our results suggest that vaccination of B-cell depleted patients causes only low protection against infection. However, the infected participants all experienced mild disease courses without any need for hospitalisation. The findings call for the continuing deployment of more effective prophylactic options and altered vaccination strategies to enhance protection against breakthrough COVID-19 infection.
Acknowledgments
We acknowledge the great help received from patients participating in this trial and our team of technicians and study coordinators represented by Gunhild Brixen Nielsen, Sarah Andersen, and Pia Hannesbo. We acknowledge grants given by the Lundbeck Neurological Scholarship delegated by the Danish Neurological Society and by Roche.
Footnotes
Contributors: FN, HMB, TS, JJS, ISJ, K-EB, and RB all contributed to study design. FN, HMB, TS, JJS, WR, MVMH, KØ all contributed to patient recruitment and patient data. FN, TS, JS, ACN, CN, KM, and WR all contributed to sample processing. FN, HMB, TS, JJS, ACN, CN, ISJ, K-EB, MVMH, KØ, SZ, and RB all contributed to data analysis/interpretation.
Funding: This study was funded by Roche.
Competing interests: HMB, ACN, K-EB, ISJ, CN, DKH, MVMH, KM and WR have nothing to disclose. RB received research support from Biogen, Roche Genentech and Novartis; personal consulting fees from Alexion, Biogen, EMD Serono, Novartis, Roche Genentech and Sanofi Genentech; and funding from Harry Weaver Award from the National Multiple Sclerosis Society and the National Institutes of Health. SZ received consulting honoraria from Alexion, Biogen-Idec, EMD-Serono, Genzyme, Novartis, Roche/Genentech and Teva Pharmaceuticals, Inc, and served on data safety monitoring boards for Lilly, BioMS, Teva and Therapeutics. JJS received research support from Novartis. TS received travel grants from Biogen, Merck, Novartis and Roche, and research grants from Biogen, and served on advisory boards for Biogen, Merck and Novartis. FN served on advisory boards for Sanofi and received travel grants from Sanofi and Merck.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by the Danish National Committee on Health Research Ethics (protocol number S-20200068C).The study, conducted by University of California, San Francisco (UCSF), was approved by the institutional review board of UCSF Committee on Human Research (protocol # 21–33,240) and the Danish Data Protection Agency (journal no. 20/19,878). The participants gave informed consent to participate in the study before taking part. Both written and oral consent was received from all participants before inclusion. The Danish study adhered to national laws for good clinical practice.
References
- 1. Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-cov-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol 2021;78:699–708. 10.1001/jamaneurol.2021.0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bajwa HM, Novak F, Nilsson AC, et al. Persistently reduced humoral and sustained cellular immune response from first to third SARS-cov-2 mrna vaccination in anti-CD20-treated multiple sclerosis patients. Mult Scler Relat Disord 2022;60:103729. 10.1016/j.msard.2022.103729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Novak F, Nilsson AC, Nielsen C, et al. Humoral immune response following SARS-cov-2 mrna vaccination concomitant to anti-CD20 therapy in multiple sclerosis. Mult Scler Relat Disord 2021;56:103251. 10.1016/j.msard.2021.103251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabatino JJ Jr, Mittl K, Rowles WM, et al. Multiple sclerosis therapies differentially affect SARS-cov-2 vaccine-induced antibody and T cell immunity and function. JCI Insight 2022;7:e156978. 10.1172/jci.insight.156978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-cov-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021;27:1990–2001. 10.1038/s41591-021-01507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institut SS . Covid-19. Rapport om omikronvarianten. 2022.
- 7. Center for disease control and prevention - COVID data tracker.
- 8. Sormani MP, Schiavetti I, Inglese M, et al. Breakthrough SARS-cov-2 infections after COVID-19 mrna vaccination in MS patients on disease modifying therapies during the delta and the omicron waves in italy. EBioMedicine 2022;80:104042. 10.1016/j.ebiom.2022.104042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of covid-19. N Engl J Med 2022;386:2188–200. 10.1056/NEJMoa2116620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection . A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020;20:e192–7. 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stærke NB, Reekie J, Nielsen H, et al. Levels of SARS-cov-2 antibodies among fully vaccinated individuals with delta or omicron variant breakthrough infections. Nat Commun 2022;13:4466. 10.1038/s41467-022-32254-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canaday DH, Oyebanji OA, White E, et al. Significantly elevated antibody levels and neutralization titers in nursing home residents after SARS-cov-2 bnt162b2 mRNA booster vaccination. MedRxiv 2021:2021.12.07.21267179. 10.1101/2021.12.07.21267179 [DOI] [Google Scholar]
- 13. Falsey AR, Frenck RW, Walsh EE, et al. SARS-cov-2 neutralization with bnt162b2 vaccine dose 3. N Engl J Med 2021;385:1627–9. 10.1056/NEJMc2113468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goel RR, Painter MM, Apostolidis SA, et al. MRNA vaccines induce durable immune memory to SARS-cov-2 and variants of concern. Science 2021;374:abm0829. 10.1126/science.abm0829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jakimovski D, Zakalik K, Awan S, et al. COVID-19 vaccination in multiple sclerosis and inflammatory diseases: effects from disease-modifying therapy, long-term seroprevalence and breakthrough infections. Vaccines (Basel) 2022;10:695. 10.3390/vaccines10050695 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2022-330757supp001.pdf (310.8KB, pdf)