Summary
Background
Few studies have examined effectiveness of COVID-19 vaccines against COVID-19 and all-cause mortality across different pandemic periods in 2022.
Methods
We used linked whole-of-population data from the 2021 Australian Census, Australian Immunisation Register, death registrations and other national datasets including migration data. Among 3.8 million adults aged 65+ years and >170,000 aged care residents, we used survival analysis to estimate vaccine effectiveness (VE) against COVID-19 specific mortality and all-cause mortality, by vaccine dose and time since receipt, adjusted for age, sex and other factors. We also estimated absolute COVID-19 mortality rates.
Findings
From January–May 2022 (Omicron BA.1/2), 3250 COVID-19 deaths occurred; from June–November (Omicron BA.4/5) 3185 COVID-19 deaths occurred. During January–May, VE of a 3rd COVID-19 vaccine dose within 3 months was 93% (95% CI 93–94%) whilst VE of a 2nd dose >6 months since receipt was 34% (26–42%). During June–November, VE of a 4th COVID-19 vaccine dose within 3 months was 84% (82–86%) whilst VE of a 3rd dose >6 months since receipt was 56% (50–62%). VE estimates for aged care residents were similar, but absolute risk reductions were substantially greater. During June–November 2022, for all-cause mortality, VE of a 4th dose within 3 months was 58% (56–59%) whilst VE of a 3rd dose >6 months since receipt was 19% (16–22%).
Interpretations
COVID-19 vaccination is highly effective against COVID-19 mortality among older adults although effectiveness wanes with time since the last dose. Our findings emphasise the importance of continuing to administer booster doses, particularly to those at highest risk.
Funding
This study was funded by the Health Economics Research Division in the Australian Government Department of Health and Aged Care.
Keywords: COVID-19, Vaccine effectiveness, Mortality, Waning effectiveness
Research in context.
Evidence before this study
Many studies have estimated COVID-19 vaccine effectiveness against COVID-19 specific disease outcomes including symptomatic infection and severe disease, often measured using hospitalisations and deaths with a related SARS-CoV-2 diagnosis. Defining severe COVID-19 has become more difficult in the Omicron variant era as infection is common and misclassification of attribution can lead to uncertainty and variations in vaccine effectiveness estimates. Few studies have examined COVID-19 vaccine effectiveness against COVID-19 mortality based on death certifications and assessed the effectiveness of boosters against COVID-19 mortality more than six months after receipt. There are also limited studies focussed on high-risk populations such as residents in aged care.
Added value of this study
In this whole of Australian population analysis, we followed 3.8 million people aged 65+ years for COVID-19 specific and all-cause mortality based on death certifications throughout 2022, a period during which Australia transitioned from a country with almost no population-based natural immunity to a setting where most people experienced SARS-CoV-2 Omicron infection. We used a robust method to ascertain COVID-19 death and had minimal loss to follow-up. We showed that COVID-19 vaccine effectiveness against COVID-19 mortality is high after initial vaccine receipt but wanes over time; however, protection more than 6 months after booster receipt remains over 50%. The estimate of vaccine effectiveness was also observed to be affected by increasing population hybrid immunity whereby the relative contribution of protection from a vaccine dose reduces. Estimates of vaccine effectiveness were similar among residents of aged care facilities as that in the general population, but the substantially higher rates of COVID-19 mortality in aged care mean the absolute benefit of vaccination is much greater. COVID-19 vaccination was also found to be effective against all-cause mortality, likely from both direct effects and the impact on other outcomes, such as cardiovascular diseases, known to occur following COVID-19.
Implications of all the available evidence
COVID-19 vaccination is highly effective in preventing COVID-19 death and also reduces all-cause mortality. The effectiveness of boosters against mortality wanes with time but a booster still provides substantial residual protection six months after receipt. Increasing population hybrid immunity is likely to reduce observed vaccine effectiveness as the pandemic progresses but COVID-19 boosters continue to provide significant benefits in mortality reduction, particularly in high-risk populations such as those aged 65+ years and those resident in aged care facilities.
Introduction
Since the introduction of COVID-19 vaccines, numerous studies, particularly on mRNA and adenoviral vector vaccines, have estimated their effectiveness against SARS-CoV-2 infection, and serious outcomes such as hospitalisation or death.1,2 Recent studies have focussed on Omicron, the current dominant viral variant,3 and shown collectively how effectiveness against infection wanes a few months after administration. Protection against severe outcomes is more robust, but there is uncertainty regarding waning effectiveness of booster vaccination against severe disease, in particular death, six months or more after vaccine receipt.4, 5, 6
In Australia, only small incursions of SARS-CoV-2 occurred until late 20217 when international and internal border restrictions were progressively removed. Up until then, immunity in the adult population was highly reliant on vaccination. The Australian COVID-19 vaccine program commenced in February 2021 with prioritisation based on age and for some high risk groups.8 The Pfizer BNT162b2 mRNA and AstraZeneca ChAdOx1 vaccines were available in a two dose homologous primary course with BNT162b2 used in aged care facilities and among health care workers and preferencing of BNT162b2 for adults aged <50 years. As a result, most community-dwelling adults aged 65+ years received the AstraZeneca ChAdOx1 for the primary course.
During 2022, Australia experienced three SARS-CoV-2 infection waves; one from December 2021–May 2022 dominated by Omicron BA.1/BA.2 variants; a second from June to approximately November 2022, dominated mostly by Omicron BA.4/BA.5 and a third from December 2022 dominated by Omicron sub- and recombinant variants.9 In addition to a primary course of COVID-19 vaccination for which Australian adults had very high uptake (>94%),10 all adults were recommended to receive a first booster dose in late 2021. From late March 2022 all adults aged 65+ years and some younger individuals with comorbidities were recommended to receive a ‘winter’ or second booster, which was for most, a fourth dose.11 Almost all boosters administered in Australia have been mRNA vaccines with the bivalent formulations only registered for use from October 2022.
Few studies internationally have analysed COVID-19 vaccine effectiveness against COVID-19 death and all-cause mortality using death certification1 and documented how this has changed with growing population hybrid immunity. We sought to describe this in the Australian population.
Methods
Study population and data sources
The Multi-Agency Data Integration Project (MADIP) is a data asset managed by the Australian Bureau of Statistics. It uses a unique person-linkage spine to securely link together individual-level data on population demographics (from the Census), taxation records, and a range of health datasets including data on services and prescriptions subsidised under Australia’s universal health insurance program (Medicare).
We used the following datasets linked in MADIP: Australian 2021 Census, Australian Immunisation Register (AIR), Medicare Benefits Schedule (MBS), Pharmaceutical Benefits Scheme (PBS), overseas migration, national residential aged care data and death registrations. Australia conducts a whole of population census every 5 years with the most recent in August 2021. Census data include age, sex, income, area of residence; over 96% of Australian dwellings were estimated to have completed this Census.12 The Australian Immunisation Register (AIR)13 records all vaccines administered in Australia; notification of vaccines provided under national programs is mandatory. The AIR records the brand, dose and date of vaccination. A small proportion (<1%) of individuals opt out of immunisation record linkage. The MBS records all health services subsidised by Medicare, Australia’s universal health insurance scheme including general practitioner (GP) visits.14 The PBS records all prescription medicines dispensed under this scheme and includes oral antivirals prescribed for treatment of COVID-19; PBS data include the pharmaceutical item, formulation, dose, and date of dispensing. Overseas migration data include monthly data on whether an individual is absent from Australia. Aged care data include information on residence in an aged care facility and date of admission. Death registrations contain a record of all deaths registered in Australia, and include the WHO International Classification of Diseases version 10 (ICD-10) coded data on the underlying cause of death, contributing causes and date of death. The use of a centrally maintained data spine, and national data collections and mandated reporting to registers in a setting with universal healthcare supports high data quality and completeness. Linkage rates to the spine for Census was 93%, for the Immunisation Register and death registrations 98% and for aged care 99%.
For the primary analyses, the study population included all individuals in the Australian 2021 Census. Exclusions (outlined in Supplementary Fig. S1) included those with significant missing data, age < 65 years, as there were relatively few deaths in those ages, and migration or death prior to follow-up commencement (baseline). In 2022 individuals who received a fifth vaccine dose would have done so as part of a three-dose primary/two dose booster schedule recommended for a small number of individuals with significant immunocompromising conditions; as such we also excluded people who received a fifth vaccine dose prior to baseline. We conducted a secondary analysis among residents in aged care facilities aged 65+ years, ascertained based on a record of admission as a permanent resident in a facility prior to baseline; this population was not derived from the Census 21 population (see Supplementary Fig. S2 for exclusions).
Study definitions
The main study outcome was a death registration where COVID-19 was recorded as the underlying cause of death (ICD-10 code, U07.1 and U07.2).15 The secondary outcome was all-cause mortality. We obtained COVID-19 vaccination status from the AIR. For the main analyses, model covariates included age, sex and jurisdiction of residence from the MADIP spine, household equivalised income from Census data, number of comorbidities (based on a validated measure using individual-level PBS dispensing records over the previous six months prior to study entry16), number of GP consultations in the year prior to study entry as a measure of health service access from MBS data, and receipt of an influenza vaccine in 2021 from the AIR. These factors were prespecified in our analysis plan based on factors known to influence the uptake of COVID-19 vaccine and potentially mortality risk. For analyses of permanent residents in aged care facilities, we included all model covariates above except household equivalised income.
Analysis
We used survival analysis with a cause-specific competing risk approach for the main study outcome where COVID-19 death was the event of interest and any other death the competing event. We used a Cox model for all-cause mortality. We followed individuals for two periods: 1 January to 31 May 2022 (BA.1/2 wave) and 1 June to 30 November 2022 (BA.4/5 wave).9 We followed study participants from the start of each period until receipt of a fifth dose, death or the end of the study period. Vaccination status was a time-varying covariate based on the date of vaccination with classification including the number of doses received and time since receipt in fixed intervals determined a priori based on commonly used intervals in other studies4 (8–90, 91–180, >180 days; the 0–7-day interval was included in analyses but not shown due to small numbers). We adjusted models for the covariates listed previously and all individuals were included in analyses except the very small number with unclassified or unknown gender or state of residence (<0.04%). We estimated adjusted hazard ratios and 95% confidence intervals (aHR) using the unvaccinated population as the reference group and calculated vaccine effectiveness (VE) using the formula (1-aHR)∗100%. Unadjusted Quasi-Poisson regression was used to compute the crude rates of COVID-19 death with their 95% confidence intervals. We used SAS version 9.4 and R studio version 2022.07.2 for data management and statistical analysis. Additional details on analytical methods are described in the Supplementary methods section.
Supplementary analyses focussed on the period June–November 2022. We estimated relative VE of four compared to three vaccine doses as a pre-specified comparison. We restricted analyses to individuals who received an influenza vaccination in 2021 to reduce healthy vaccinee bias, and also to individuals who had at least one GP visit in the last year to reduce potential bias from health service access. We adjusted analyses for receipt of an oral antiviral medicine using PBS data where antiviral use was a time-varying covariate. We also examined alternate study outcomes including deaths where COVID-19 was classified as the underlying cause of death or a contributing cause of death, and deaths where the underlying cause was ischaemic heart disease (ICD-10 I20–I25), cerebrovascular disease (I60–I69), respiratory disorder (ICD-10 codes beginning with “J”), dementia (F01, F03, G30) or cancer (C00–C97) and COVID-19 (U07.1, U07.2) was not a contributing cause of death. Finally, we estimated COVID-19 mortality rates by vaccine status by using the rate in the unvaccinated population and multiplying this by the adjusted hazard ratios to derive adjusted rates. From this we calculated absolute risk reductions by subtracting the adjusted rate in one vaccinated group from another.
Analyses were conducted under the Australian Government’s evaluation of the COVID-19 vaccine program and no ethics approvals were required. No individual identifying details were available in the databases and all results where counts or rates are presented were perturbed or suppressed according to the Australian Bureau of Statistics methods to prevent disclosure of small numbers and potential re-identification.17
Role of the funding source
The funders did not have any role in study design, data analysis, interpretation, writing of the report. The funders did facilitate access to the data that were collected by various Australian government entities.
Results
Characteristics of study population
Analyses for the Australian population aged 65+ years included 3.867 million and 3.877 million individuals for follow-up periods commencing on 1 January and 1 June 2022 respectively. Characteristics of the population according to the number of vaccine doses received at the start of follow-up are shown in Table 1, Table 2. At 1 January 2022, 70.9% had received two COVID-19 vaccine doses and 23.1% had received more than two doses; only 5.0% of the population were unvaccinated. By 1 June 2022, 53.6% had received three COVID-19 vaccine doses, 33.5% had received four doses and 4.3% remained unvaccinated. The majority (∼85%) of the vaccinated population had received the AstraZeneca COVID-19 ChAdOx1 vaccine for their primary course and most (>98%) received an mRNA COVID-19 vaccine for boosters (see Table 1 and Supplementary Table S1).
Table 1.
Characteristics of Australian population aged 65+ overall and according to vaccine doses received at 1 January 2022.
| Mean age (SD) | Unvaccinated (N = 195,138) |
1 dose (N = 36,654) |
2 doses (N = 2,740,926) |
3 doses (N = 893,431) |
4 doses (N = 877) |
Total (N = 3,867,026) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 74.5 (7.9) |
75.9 (8.5) |
74.2 (7.3) |
75.8 (7.3) |
76.4 (7.5) |
74.6 (7.4) |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Age group (years) | ||||||||||||
| 65–79 | 147,784 | 75.7% | 25,141 | 68.6% | 2,120,660 | 77.4% | 645,879 | 72.3% | 618 | 70.5% | 2,940,082 | 76.0% |
| 80+ | 47,354 | 24.3% | 11,513 | 31.4% | 620,266 | 22.6% | 247,552 | 27.7% | 259 | 29.5% | 926,944 | 24.0% |
| Sexa | ||||||||||||
| Men | 85,108 | 43.6% | 15,630 | 42.6% | 1,276,932 | 46.6% | 422,532 | 47.3% | 479 | 54.6% | 1,800,681 | 46.6% |
| Women | 109,914 | 56.3% | 21,005 | 57.3% | 1,462,692 | 53.4% | 470,591 | 52.7% | 397 | 45.3% | 2,064,599 | 53.4% |
| Weekly household income (AUD) | ||||||||||||
| 0–499 | 63,624 | 32.6% | 11,174 | 30.5% | 762,630 | 27.8% | 185,864 | 20.8% | 172 | 19.6% | 1,023,464 | 26.5% |
| 500–999 | 64,793 | 33.2% | 11,553 | 31.5% | 958,656 | 35.0% | 292,699 | 32.8% | 258 | 29.4% | 1,327,959 | 34.3% |
| 1000–1499 | 20,473 | 10.5% | 3640 | 9.9% | 363,170 | 13.2% | 123,761 | 13.9% | 129 | 14.7% | 511,173 | 13.2% |
| 1500–1999 | 7916 | 4.1% | 1487 | 4.1% | 164,376 | 6.0% | 62,957 | 7.0% | 55 | 6.3% | 236,791 | 6.1% |
| 2000+ | 6452 | 3.3% | 1203 | 3.3% | 157,608 | 5.8% | 83,639 | 9.4% | 100 | 11.4% | 249,002 | 6.4% |
| Other (incl not stated) | 31,885 | 16.3% | 7601 | 20.7% | 334,491 | 12.2% | 144,515 | 16.2% | 163 | 18.6% | 518,655 | 13.4% |
| State of residencea | ||||||||||||
| New South Wales | 53,961 | 27.7% | 6115 | 16.7% | 889,576 | 32.5% | 303,876 | 34.0% | 362 | 41.3% | 1,253,890 | 32.4% |
| Victoria | 40,229 | 20.6% | 5756 | 15.7% | 703,405 | 25.7% | 221,595 | 24.8% | 231 | 26.3% | 971,216 | 25.1% |
| Queensland | 49,776 | 25.5% | 9742 | 26.6% | 518,287 | 18.9% | 189,368 | 21.2% | 135 | 15.4% | 767,308 | 19.8% |
| South Australia | 17,717 | 9.1% | 5032 | 13.7% | 228,814 | 8.3% | 66,160 | 7.4% | 70 | 8.0% | 317,793 | 8.2% |
| Western Australia | 25,966 | 13.3% | 8107 | 22.1% | 281,699 | 10.3% | 64,458 | 7.2% | 53 | 6.0% | 380,283 | 9.8% |
| Tasmania | 4743 | 2.4% | 1457 | 4.0% | 71,216 | 2.6% | 24,848 | 2.8% | 17 | 1.9% | 102,281 | 2.6% |
| Australian Capital Territory | 1546 | 0.8% | 250 | 0.7% | 36,973 | 1.3% | 18,651 | 2.1% | 13 | 1.5% | 57,433 | 1.5% |
| Northern Territory | 1177 | 0.6% | 198 | 0.4% | 10,631 | 0.4% | 4412 | 0.5% | 5 | 0.6% | 16,423 | 0.4% |
| No. of comorbiditiesb | ||||||||||||
| 0 | 50,066 | 25.7% | 4488 | 12.2% | 249,397 | 9.1% | 50,237 | 5.6% | 55 | 6.3% | 354,243 | 9.2% |
| 1 | 28,265 | 14.5% | 3810 | 10.4% | 314,684 | 11.5% | 81,884 | 9.2% | 77 | 8.8% | 428,720 | 11.1% |
| 2 | 26,653 | 13.7% | 4541 | 12.4% | 403,206 | 14.7% | 118,049 | 13.2% | 98 | 11.2% | 552,547 | 14.3% |
| 3+ | 90,154 | 46.2% | 23,816 | 65.0% | 17,73,640 | 64.7% | 643,266 | 72.0% | 655 | 74.7% | 2,531,531 | 65.5% |
| No. of GP visits in year prior | ||||||||||||
| 0 | 29,217 | 15.0% | 2422 | 6.6% | 94,832 | 3.5% | 24,130 | 2.7% | 34 | 3.9% | 150,635 | 3.9% |
| 1–2 | 23,826 | 12.2% | 3079 | 8.4% | 184,787 | 6.7% | 38,973 | 4.4% | 45 | 5.1% | 250,710 | 6.5% |
| 3–6 | 50,087 | 25.7% | 8132 | 22.2% | 725,127 | 26.5% | 193,236 | 21.6% | 149 | 17.0% | 976,731 | 25.3% |
| 7–12 | 46,365 | 23.8% | 9780 | 26.7% | 906,595 | 33.1% | 301,453 | 33.7% | 299 | 34.1% | 1,264,492 | 32.7% |
| 13+ | 45,645 | 23.4% | 13,246 | 36.1% | 829,587 | 30.3% | 335,636 | 37.6% | 359 | 40.9% | 1,224,473 | 31.7% |
| Influenza vaccine in 2021 | 35,139 | 18.0% | 14,918 | 40.7% | 1,972,934 | 72.0% | 762,768 | 85.4% | 739 | 84.3% | 2,786,498 | 72.1% |
| In residential aged care facility | 7404 | 3.8% | 3429 | 9.4% | 52,012 | 1.9% | 64,961 | 7.3% | 77 | 8.8% | 127,883 | 3.3% |
| COVID-19 vaccine primary doses branda | ||||||||||||
| Pfizer (BNT162b2) | 16,657 | 45.4% | 340,014 | 12.4% | 128,655 | 14.4% | 192 | 21.9% | 485,518 | 12.6% | ||
| Astra Zeneca (ChAdOx1) | 15,689 | 42.8% | 2,353,786 | 85.9% | 764,560 | 85.6% | 682 | 77.8% | 3,134,717 | 81.1% | ||
| Moderna (mRNA-1273) | 4236 | 11.6% | 45,478 | 1.7% | 43 | 0.0% | 0 | 0.0% | 49,757 | 1.3% | ||
| Mean number of weeks since receipt of most recent dose (SD) | 9.1 (9.2) | 16.5 (6.1) | 2.2 (2.3) | 3.3 (4.1) | ||||||||
All numbers were perturbed to maintain confidentiality. See methods for details.
For COVID-19 vaccine primary doses the % is of all those receiving that dose but for the overall population it includes the unvaccinated.
See methods for how co-morbid conditions were determined.
Table 2.
Characteristics of Australian population aged 65+ years overall and according to vaccine doses received at 1 June 2022.
| Mean age (SD) | Unvaccinated (N = 167,421) |
1 dose (N = 10,640) |
2 doses (N = 321,787) |
3 doses (N = 2,078,884) |
4 doses (N = 1,299,002) |
Total (N = 3,877,734) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 74.3 (7.7) |
76.0 (8.6) |
73.4 (7.4) |
74.3 (7.5) |
75.6 (7.1) |
74.6 (7.4) |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Age group (years) | ||||||||||||
| 65–79 | 129,520 | 77.4% | 7268 | 68.3% | 257,740 | 80.1% | 1,593,966 | 76.7% | 948,715 | 73.0% | 2,937,209 | 75.7% |
| 80+ | 37,901 | 22.6% | 3372 | 31.7% | 64,047 | 19.9% | 484,918 | 23.3% | 350,287 | 27.0% | 940,525 | 24.3% |
| Sexa | ||||||||||||
| Men | 73,245 | 43.7% | 4377 | 41.1% | 146,828 | 45.6% | 970,170 | 46.7% | 606,928 | 46.7% | 1,801,548 | 46.5% |
| Women | 94,074 | 56.2% | 6245 | 58.7% | 174,693 | 54.3% | 1,107,757 | 53.3% | 691,639 | 53.2% | 2,074,408 | 53.5% |
| Weekly household income (AUD) | ||||||||||||
| 0–499 | 54,785 | 32.7% | 3210 | 30.2% | 95,855 | 29.8% | 562,366 | 27.1% | 304,067 | 23.4% | 1,020,283 | 26.3% |
| 500–999 | 56,087 | 33.5% | 3286 | 30.9% | 106,556 | 33.1% | 703,189 | 33.8% | 463,149 | 35.7% | 1,332,267 | 34.4% |
| 1000–1499 | 18,016 | 10.8% | 1054 | 9.9% | 42,657 | 13.3% | 279,754 | 13.5% | 179,761 | 13.8% | 521,242 | 13.4% |
| 1500–1999 | 7050 | 4.2% | 389 | 3.7% | 18,076 | 5.6% | 132,375 | 6.4% | 86,060 | 6.6% | 243,950 | 6.3% |
| 2000+ | 5702 | 3.4% | 353 | 3.3% | 14,384 | 4.5% | 131,048 | 6.3% | 103,847 | 8.0% | 255,334 | 6.6% |
| Other (incl not stated) | 25,786 | 15.4% | 2345 | 22.0% | 44,255 | 13.8% | 270,156 | 13.0% | 162,116 | 12.5% | 504,658 | 13.0% |
| State of residencea | ||||||||||||
| New South Wales | 50,137 | 29.9% | 3136 | 29.5% | 115,231 | 35.8% | 672,629 | 32.4% | 414,264 | 31.9% | 1,255,397 | 32.4% |
| Victoria | 36,572 | 21.8% | 2361 | 22.2% | 85,988 | 26.7% | 518,087 | 24.9% | 328,251 | 25.3% | 971,259 | 25.0% |
| Queensland | 44,521 | 26.6% | 2310 | 21.7% | 65,685 | 20.4% | 408,294 | 19.6% | 251,179 | 19.3% | 771,989 | 19.9% |
| South Australia | 14,759 | 8.8% | 1183 | 11.1% | 22,692 | 7.1% | 171,384 | 8.2% | 109,416 | 8.4% | 319,434 | 8.2% |
| Western Australia | 15,066 | 9.0% | 1096 | 10.3% | 21,054 | 6.5% | 217,865 | 10.5% | 126,902 | 9.8% | 381,983 | 9.9% |
| Tasmania | 3925 | 2.3% | 343 | 3.2% | 6739 | 2.1% | 54,885 | 2.6% | 37,129 | 2.9% | 103,021 | 2.7% |
| Australian Capital Territory | 1426 | 0.9% | 132 | 1.2% | 2948 | 0.9% | 25,620 | 1.2% | 27,384 | 2.1% | 57,510 | 1.5% |
| Northern Territory | 988 | 0.6% | 73 | 0.7% | 1433 | 0.4% | 9890 | 0.5% | 4329 | 0.3% | 16,713 | 0.4% |
| No. of comorbiditiesb | ||||||||||||
| 0 | 48,254 | 28.8% | 1604 | 15.1% | 49,035 | 15.2% | 187,536 | 9.0% | 73,223 | 5.6% | 359,652 | 9.3% |
| 1 | 25,303 | 15.1% | 1174 | 11.0% | 43,333 | 13.5% | 236,106 | 11.4% | 123,202 | 9.5% | 429,118 | 11.1% |
| 2 | 23,046 | 13.8% | 1301 | 12.2% | 47,165 | 14.7% | 305,279 | 14.7% | 179,381 | 13.8% | 556,172 | 14.3% |
| 3+ | 70,820 | 42.3% | 6557 | 61.6% | 182,248 | 56.6% | 1,349,961 | 64.9% | 923,193 | 71.1% | 2,532,779 | 65.3% |
| No. of GP visits in year prior | ||||||||||||
| 0 | 28,559 | 17.1% | 831 | 7.8% | 19,840 | 6.2% | 71,934 | 3.5% | 31,007 | 2.4% | 152,171 | 3.9% |
| 1–2 | 22,600 | 13.5% | 931 | 8.8% | 32,126 | 10.0% | 145,968 | 7.0% | 59,433 | 4.6% | 261,058 | 6.7% |
| 3–6 | 43,702 | 26.1% | 2189 | 20.6% | 85,619 | 26.6% | 553,763 | 26.6% | 309,160 | 23.8% | 994,433 | 25.6% |
| 7–12 | 38,022 | 22.7% | 2653 | 24.9% | 92,502 | 28.7% | 681,663 | 32.8% | 458,323 | 35.3% | 1,273,163 | 32.8% |
| 13+ | 34,541 | 20.6% | 4036 | 37.9% | 91,699 | 28.5% | 625,559 | 30.1% | 441,086 | 34.0% | 1,196,921 | 30.9% |
| Influenza vaccine in 2021 | 24,866 | 14.9% | 3450 | 32.4% | 127,811 | 39.7% | 1,481,097 | 71.2% | 1,144,487 | 88.1% | 2,781,711 | 71.7% |
| In residential aged care facility | 4692 | 2.8% | 1287 | 12.1% | 8320 | 2.6% | 64,989 | 3.1% | 48,657 | 3.7% | 127,945 | 3.3% |
| COVID-19 vaccine primary doses branda | ||||||||||||
| Pfizer (BNT162b2) | 5229 | 49.1% | 113,630 | 35.3% | 289,125 | 13.9% | 93,218 | 7.2% | 501,202 | 12.9% | ||
| Astra Zeneca (ChAdOx1) | 3698 | 34.8% | 177,339 | 55.1% | 1,759,937 | 84.7% | 1,205,137 | 92.8% | 3,146,111 | 81.1% | ||
| Moderna (mRNA-1273) | 956 | 9.0% | 24,261 | 7.5% | 28,436 | 1.4% | 525 | 1.4% | 54,178 | 1.4% | ||
| Novavax (Nuvaxovid) | 732 | 6.9% | 6020 | 1.9% | 36 | 0.0% | 0 | 0.0% | 6788 | 0.2% | ||
| Mean number of weeks since receipt of most recent dose (SD) | 26.5 (14.1) | 29.7 (8.1) | 16.9 (4.4) | 3.0 (2.5) | ||||||||
All numbers were perturbed to maintain confidentiality. See methods for details.
For COVID-19 vaccine primary doses the % is of all those receiving that dose but for the overall population it includes the unvaccinated.
See methods for how co-morbid conditions were determined.
The unvaccinated population, while having a similar age distribution as the vaccinated population, were more likely to be women, have lower incomes, fewer co-morbidities, fewer GP visits, and less likely to have had an influenza vaccine in 2021. By 1 June 2022 there was a gradient of increasing age, household income, number of comorbid conditions, GP visits and likelihood of receipt of influenza vaccine with increasing numbers of vaccine doses received (Table 2). Differences in the likelihood of remaining unvaccinated changed over time by state of residence; at 1 January 2022 two of eight states (Queensland, Western Australia) were over-represented among the unvaccinated population and by 1 June 2022 only Queensland residents were over-represented.
There were 177,630 and 174,780 individuals classified as permanent residents in aged care facilities on 1 January 2022 and 1 June 2022, respectively. Similar to our primary analyses, unvaccinated residents were less likely to have comorbidities, have fewer GP visits and were less likely to have had influenza vaccine in 2021 (Table 3, Table 4). In line with government policy, the Pfizer BNT162b2 vaccine was mostly used for the primary vaccine course in this population.
Table 3.
Characteristics of Australians resident in aged care, aged 65+ years overall and according to vaccine doses received at 1 January 2022.
| Mean age (SD) | Unvaccinated (N = 10,927) |
1 dose (N = 5004) |
2 doses (N = 72,740) |
3 doses (N = 88,857) |
4 doses (N = 102) |
Total (N = 177,630) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 85.3 (8.5) |
84.9 (8.2) |
85.2 (7.8) |
86.1 (7.9) |
85.0 (8.1) |
85.7 (7.9) |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Age group (years) | ||||||||||||
| 65–79 | 2749 | 25.2% | 1310 | 26.2% | 17,042 | 23.4% | 18,224 | 20.5% | 28 | 27.5% | 39,353 | 22.2% |
| 80+ | 8178 | 74.8% | 3694 | 73.8% | 55,698 | 76.6% | 70,633 | 79.5% | 74 | 72.5% | 138,277 | 77.8% |
| Sexa | ||||||||||||
| Men | 3635 | 33.3% | 1840 | 36.8% | 25,709 | 35.3% | 28,347 | 31.9% | 51 | 50.0% | 59,582 | 33.5% |
| Women | 7285 | 66.7% | 3160 | 63.1% | 47,002 | 64.6% | 60,465 | 68.0% | 54 | 52.9% | 117,966 | 66.4% |
| State of residence | ||||||||||||
| New South Wales | 2758 | 25.2% | 1372 | 27.4% | 22,367 | 30.7% | 32,013 | 36.0% | 46 | 45.1% | 58,556 | 33.0% |
| Victoria | 2046 | 18.7% | 957 | 19.1% | 17,093 | 23.5% | 25,238 | 28.4% | 25 | 24.5% | 45,359 | 25.5% |
| Queensland | 3025 | 27.7% | 1276 | 25.5% | 15,201 | 20.9% | 15,517 | 17.5% | 10 | 9.8% | 35,029 | 19.7% |
| Others | 3102 | 28.4% | 1401 | 28.0% | 18,081 | 24.9% | 16,088 | 18.1% | 26 | 25.5% | 38,698 | 21.8% |
| No. of comorbiditiesb | ||||||||||||
| 0 | 3222 | 29.5% | 1051 | 21.0% | 12,584 | 17.3% | 15,800 | 17.8% | 21 | 20.6% | 32,678 | 18.4% |
| 1 | 2881 | 26.4% | 1257 | 25.1% | 17,852 | 24.5% | 22,742 | 25.6% | 22 | 21.6% | 44,754 | 25.2% |
| 2 | 2497 | 22.9% | 1206 | 24.1% | 19,179 | 26.4% | 23,819 | 26.8% | 33 | 32.4% | 46,734 | 26.3% |
| 3+ | 2325 | 21.3% | 1492 | 29.8% | 23,129 | 31.8% | 26,495 | 29.8% | 33 | 32.4% | 53,474 | 30.1% |
| No. of GP visits in year prior | ||||||||||||
| 0–10 | 2561 | 23.4% | 1004 | 20.1% | 12,320 | 16.9% | 16,745 | 18.8% | 14 | 13.7% | 32,644 | 18.4% |
| 11–20 | 3035 | 27.8% | 1375 | 27.5% | 20,587 | 28.3% | 22,634 | 25.5% | 30 | 29.4% | 47,661 | 26.8% |
| 21–30 | 2376 | 21.7% | 1153 | 23.0% | 17,337 | 23.8% | 20,299 | 22.8% | 18 | 17.6% | 41,183 | 23.2% |
| 31–40 | 1397 | 12.8% | 683 | 13.6% | 10,778 | 14.8% | 12,763 | 14.4% | 14 | 13.7% | 25,635 | 14.4% |
| 41–50 | 836 | 7.7% | 446 | 8.9% | 6130 | 8.4% | 8181 | 9.2% | 13 | 12.7% | 15,606 | 8.8% |
| 51–70 | 576 | 5.3% | 281 | 5.6% | 4425 | 6.1% | 6530 | 7.3% | 6 | 5.9% | 11,818 | 6.7% |
| 71+ | 143 | 1.3% | 65 | 1.3% | 1163 | 1.6% | 1701 | 1.9% | 3 | 2.9% | 3075 | 1.7% |
| Influenza vaccine in 2021 | 3978 | 36.4% | 2559 | 51.1% | 45,796 | 63.0% | 54,650 | 61.50% | 54 | 52.9% | 107,037 | 60.3% |
| COVID-19 vaccine primary doses branda | ||||||||||||
| Pfizer (BNT162b2) | 3486 | 69.7% | 49,490 | 68.0% | 84,222 | 94.8% | 93 | 91.2% | 137,291 | 77.3% | ||
| Astra Zeneca (ChAdOx1) | 1412 | 28.2% | 22,924 | 31.5% | 4627 | 5.2% | 11 | 10.8% | 28,974 | 16.3% | ||
| Moderna (mRNA-1273) | 90 | 1.8% | 260 | 0.5% | 0 | 0.0% | 0 | 0.0% | 350 | 0.2% | ||
| Mean number of weeks since receipt of most recent dose (SD) | 16.7 (13.4) | 23.7 (10.4) | 4.2 (2.0) | 4.4 (3.6) | ||||||||
For COVID-19 vaccine primary doses the % is of all those receiving that dose but for the overall population it includes the unvaccinated.
See methods for how co-morbid conditions were determined. All numbers were perturbed to maintain confidentiality. See methods for details.
Table 4.
Characteristics of Australians resident in aged care, aged 65+ years overall and according to vaccine doses received at 1 June 2022.
| Mean age (SD) | Unvaccinated (N = 6905) |
1 dose (N = 1882) |
2 doses (N = 11,832) |
3 doses (N = 89,016) |
4 doses (N = 65,145) |
Total (N = 174,780) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 85.3 (8.5) |
84.4 (8.3) |
84.6 (8.1) |
85.5 (7.8) |
86.1 (7.8) |
85.7 (7.9) |
|||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Age group (years) | ||||||||||||
| 65–79 | 1717 | 24.9% | 549 | 29.2% | 3155 | 26.7% | 19,947 | 22.4% | 13,401 | 20.6% | 38,769 | 22.2% |
| 80+ | 5188 | 75.1% | 1333 | 70.8% | 8677 | 73.3% | 69,069 | 77.6% | 51,744 | 79.4% | 136,011 | 77.8% |
| Sexa | ||||||||||||
| Men | 2203 | 31.9% | 677 | 36.0% | 4188 | 35.4% | 30,312 | 34.1% | 20,997 | 32.2% | 58,377 | 33.4% |
| Women | 4693 | 68.0% | 1205 | 64.0% | 7632 | 64.5% | 58,669 | 65.9% | 44,121 | 67.7% | 116,320 | 66.6% |
| State of residence | ||||||||||||
| New South Wales | 1942 | 28.1% | 550 | 29.2% | 4009 | 33.9% | 30,075 | 33.8% | 20,598 | 31.6% | 57,174 | 32.7% |
| Victoria | 1516 | 22.0% | 337 | 17.9% | 2347 | 19.8% | 19,106 | 21.5% | 21,309 | 32.7% | 44,615 | 25.5% |
| Queensland | 1898 | 27.5% | 416 | 22.1% | 2615 | 22.1% | 18,907 | 21.2% | 11,058 | 17.0% | 34,894 | 20.0% |
| Others | 1545 | 22.4% | 581 | 30.9% | 2856 | 24.1% | 20,926 | 23.5% | 12,184 | 18.7% | 38,092 | 21.8% |
| No. of comorbiditiesb | ||||||||||||
| 0 | 2304 | 33.4% | 473 | 25.1% | 2583 | 21.8% | 16,459 | 18.5% | 11,658 | 17.9% | 33,477 | 19.2% |
| 1 | 1856 | 26.9% | 520 | 27.6% | 3091 | 26.1% | 22,535 | 25.3% | 16,578 | 25.4% | 44,580 | 25.5% |
| 2 | 1504 | 21.8% | 450 | 23.9% | 2959 | 25.0% | 23,636 | 26.6% | 17,438 | 26.8% | 45,987 | 26.3% |
| 3+ | 1235 | 17.9% | 436 | 23.2% | 3195 | 27.0% | 26,388 | 29.6% | 19,471 | 29.9% | 50,725 | 29.0% |
| No. of GP visits in year prior | ||||||||||||
| 0–10 | 1771 | 25.6% | 430 | 22.8% | 2356 | 19.9% | 16,660 | 18.7% | 12,280 | 18.9% | 33,497 | 19.2% |
| 11–20 | 1908 | 27.6% | 540 | 28.7% | 3403 | 28.8% | 24,801 | 27.9% | 17,479 | 26.8% | 48,131 | 27.5% |
| 21–30 | 1434 | 20.8% | 423 | 22.5% | 2680 | 22.7% | 20,777 | 23.3% | 14,838 | 22.8% | 40,152 | 23.0% |
| 31–40 | 855 | 12.4% | 235 | 12.5% | 1603 | 13.5% | 12,725 | 14.3% | 9280 | 14.2% | 24,698 | 14.1% |
| 41–50 | 498 | 7.2% | 136 | 7.2% | 918 | 7.8% | 7434 | 8.4% | 5691 | 8.7% | 14,677 | 8.4% |
| 51–70 | 357 | 5.2% | 101 | 5.4% | 698 | 5.9% | 5251 | 5.9% | 4420 | 6.8% | 10,827 | 6.2% |
| 71+ | 83 | 1.2% | 19 | 1.0% | 164 | 1.4% | 1368 | 1.5% | 1160 | 1.8% | 2794 | 1.6% |
| Influenza vaccine in 2021 | 2148 | 31.1% | 827 | 43.9% | 6158 | 52.0% | 56,958 | 64.0% | 42,394 | 65.1% | 108,485 | 62.1% |
| COVID-19 vaccine primary doses branda | ||||||||||||
| Pfizer (BNT162b2) | 1497 | 79.5% | 8772 | 74.1% | 63,061 | 70.8% | 51,970 | 79.8% | 125,300 | 71.7% | ||
| Astra Zeneca (ChAdOx1) | 305 | 16.2% | 2665 | 22.5% | 25,505 | 28.7% | 13,147 | 20.2% | 41,622 | 23.8% | ||
| Moderna (mRNA-1273) | 66 | 3.5% | 318 | 2.7% | 391 | 0.4% | 22 | 0.0% | 797 | 0.5% | ||
| Mean number of weeks since receipt of most recent dose (SD) | 24.8 (17.7) | 29.8 (17.1) | 19.1 (6.6) | 2.8 (2.5) | ||||||||
All numbers were perturbed to maintain confidentiality. See methods for details.
For COVID-19 vaccine primary doses the % is of all those receiving that dose but for the overall population it includes the unvaccinated.
See methods for how co-morbid conditions were determined.
Effectiveness of COVID-19 vaccines
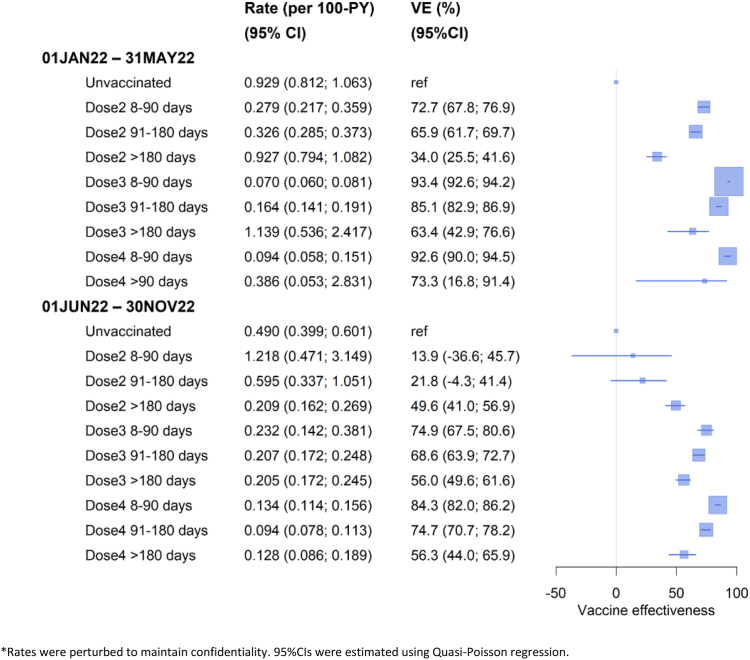
There were 3235 COVID-19 specific deaths over 1.578 million person-years during the January–May 2022 and 3185 COVID-19 deaths over 1.906 million person-years during June–November 2022. Fig. 1 shows the rates of COVID-19 mortality and vaccine effectiveness (VE) according to dose and time since receipt in each period. During January–May 2022, effectiveness of two COVID-19 vaccine doses in preventing COVID-19 death within three months of receipt (defined as ‘recent’) was 73% (95% CI 68–77%); this compared to 93% (95% CI 93–94%) for recent receipt of three doses or 93% (95% CI 90–95%) for four doses. VE waned with time following receipt; and waning was greater for those who only had two compared to three vaccine doses (at >6 months since receipt 34% versus 63%).
Fig. 1.
Rates∗ and vaccine effectiveness (VE) of COVID-19 specific mortality during 1 Jan–31 May 2022 and 1 Jun–30 Nov 2022 by vaccination status in the Australian population aged 65+ years. ∗Rates were perturbed to maintain confidentiality. 95% CIs were estimated using Quasi-Poisson regression.
During June–November 2022 (Fig. 1), VE against COVID-19 death with three vaccine doses within 3 months of receipt was 75% (95% CI 68–81%), whilst effectiveness of four doses within 3 months of receipt was 84% (95% CI 82–86%). The effectiveness of a third and fourth COVID-19 vaccine dose given more than 6 months earlier, whilst lower than recent vaccination, remained above 50%. During this latter wave the relative effectiveness of a recent fourth dose compared to a third dose given more than 6 months earlier was 64% (95% CI 59–68%).
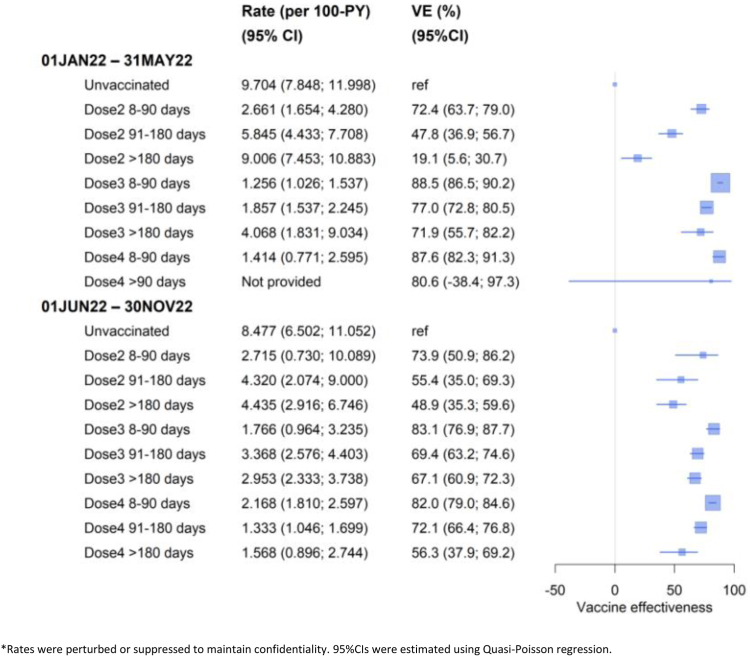
For people resident in aged care, VE against COVID-19 mortality was similar compared to the general population aged 65+ years; protection was higher after recent booster receipt, and then waned (Fig. 2). However the absolute rates of COVID-19 mortality were over ten times that in the general population. During June–November 2022, among the unvaccinated, the COVID-19 mortality rate was 8.5/100 person-years in aged care residents versus 0.49/100 person-years in the population aged 65+ years. The difference in adjusted rates between those who received three doses >6 months ago compared to a recent fourth dose was 0.14/100 person-years in the general population and 1.27/100 person-years in aged care residents. The rate differences in these two groups illustrates the greater impact of booster vaccination in aged care residents, despite similar VE in that group compared to the general population aged 65+ years.
Fig. 2.
Rates∗ and vaccine effectiveness (VE) against of COVID-19 specific mortality during 1 Jan–31 May 2022 and 1 Jun–30 Nov 2022 by vaccination status in Australians resident in aged care aged 65+ years. ∗Rates were perturbed or suppressed to maintain confidentiality. 95% CIs were estimated using Quasi-Poisson regression.
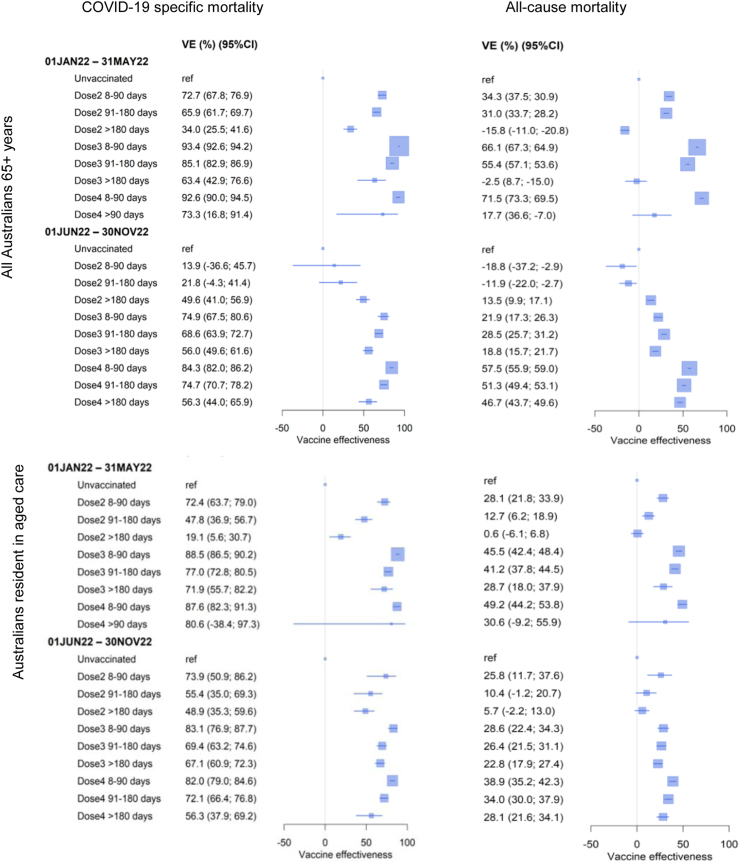
Fig. 3 shows VE against all-cause mortality compared to COVID-19 specific mortality in the general population and in aged care residents. There were 49,195 and 44,689 deaths respectively during each period. We observed similar VE patterns with vaccine dose and time since receipt over the two waves for both outcomes. However, the estimated VE was substantially greater for the specific outcome (COVID-19 mortality) compared to the non-specific outcome (all-cause mortality). For example, during June–November 2022, VE of a recent fourth dose was 84% (95% CI 82–86%) for COVID-19 mortality compared to 58% (95% CI 56–59%) against all-cause mortality.
Fig. 3.
Vaccine effectiveness (VE) against COVID-19 specific mortality and all-cause mortality during 1 Jan–31 May 2022 and 1 Jun–30 Nov 2022 by vaccination status in Australians aged 65+ years overall and those resident in aged care.
Analyses examining other causes of death during June–November 2022 are shown in Supplementary Fig. S3. VE against COVID-19 mortality as both the underlying or contributing cause, which included 4260 deaths (an additional 1074 deaths to the main study outcome) was similar to analyses of COVID-19 as the underlying cause of death. For other pre-specified causes of death, VE varied. Against cerebrovascular, ischaemic heart disease, respiratory and dementia deaths, there was less waning of effectiveness observed with the fourth vaccine dose; the VE patterns for death due to cancer were more similar to that for COVID-19 mortality, although again of a substantially lower magnitude.
Findings from additional analyses focused on the June–November period and including only the 2.781 million individuals who had received an influenza vaccine in 2021 or alternatively, the 3.725 million who had seen a GP at least once in the previous year, were similar to the main analyses (Supplementary Fig. S4). There were 267,897 individuals who received COVID-19 antivirals with the majority (77.4%) receiving molnupiravir. Of those dispensed an antiviral by 30 November 2022, 75% had at least four COVID-19 vaccine doses, 20% had received three vaccine doses, 1.5% were unvaccinated. VE analyses adjusted for antiviral receipt provided slightly higher estimates than the main analyses (Supplementary Fig. S4).
Discussion
While many studies have described the effectiveness of COVID-19 vaccines against SARS-CoV-2 infection and hospitalisation as a proxy for severe disease, few have had sufficient statistical power to examine the outcome of COVID-19 death.6 In VE studies of COVID-19 death, ascertainment has usually been based on a death temporally associated with a SARS-CoV-2 diagnosis.18, 19, 20 This can be imprecise and may lead to underestimation of effects.21 A key strength of this study, which included almost the entire Australian population aged 65+ years, was the use of death registrations where cause of death is based on death certification by a medical practitioner. While it is difficult to directly compare VE estimates across countries and populations due to differences in the types of vaccines used and underlying rates of infection, our estimates of the effectiveness of COVID-19 vaccines against COVID-19 mortality during two waves of Omicron SARS-CoV-2 infection in Australia are mostly consistent with the international literature on severe disease, with high effectiveness in the 3 month period after vaccine administration.1 However, uniquely we show that during each new Omicron variant wave in Australia, effectiveness of a COVID-19 vaccine booster against COVID-19 death in older adults wanes significantly with time since receipt, although VE in those who received a booster more than 6 months earlier remained >50%.
During 2022, Australia transitioned from a country with very little SARS-CoV-2 infection-induced immunity to a population where the majority had experienced infection (anti-nucleocapsid antibody seroprevalence estimates rose from 6.4% in February 2022 to 51.4% in November 2022 among adults aged 70+ years).22 Infection is known to provide significant protection against subsequent disease23 and likely influenced the temporal differences in the estimated VE observed between infection waves. During the first Omicron wave (BA.1/2), where there was little infection-induced immunity, recent receipt of two vaccine doses provided strong protection against COVID-19 death (VE 73%), but this effectiveness waned substantially by 6 months after receipt (VE 34%), and was markedly reduced compared with individuals who received a recent third or fourth dose (both VE 93%). However, during the infection wave from 1 June 2022, effectiveness of a recent third or fourth dose (VE 75% and 84% respectively) while still high, was lower than that observed in the earlier wave. Rather than signifying an overall reduction in protection, this is potentially due to increasing ‘hybrid immunity’ across all sectors of the population resulting in a reduction in the relative ‘immunity gap’ between those who were unvaccinated or had few vaccine doses and those with many vaccine doses. Consistent with this effect, we also observed a lower COVID-19 death rate in the unvaccinated population in the second compared to the first Omicron wave (0.49 versus 0.93 per 100 person-years, respectively).
During June–November 2022, despite growing hybrid immunity in the population, waning VE was also evident regardless of the number of vaccine doses received. There was a 20–35% absolute VE reduction comparing receipt of a booster (either the third or fourth dose) within 3 months to a booster more than 6 months earlier. These findings suggest that even with high population hybrid immunity, in the Australian population aged 65+ years substantial reductions in mortality can be gained by providing a booster to older adults from 6 months after the last dose. The absolute risk reductions demonstrate that boosters are particularly important among adults resident in aged care who have significantly higher COVID-19 mortality rates. Given similar VE for third and fourth doses in relation to time since receipt, recency of a booster appears most important in preventing death, rather than the total number of boosters received.
As COVID-19 deaths only represented a small proportion of all deaths (6.6% in January–May; 6.0% in June–November) the similar pattern of VE estimates for all-cause mortality and COVID-19 mortality is unlikely to be explained entirely by the contribution of acute COVID-19 mortality alone. COVID-19 vaccines also appeared effective against other specific causes of death (Supplementary Fig. S4), although the magnitude of the effect size was less, and patterns of waning effectiveness were inconsistent. Possible explanations for these observations of VE against such non-specific outcomes include that COVID-19 may increase the risk of death from other causes, such as cardiovascular and respiratory disease and thus vaccination provides some protection against these outcomes,24 or uncontrolled confounding by behavioural aspects associated with vaccine uptake for example, the healthy vaccinee effect, whereby those who are more likely to get multiple vaccine doses, or to be vaccinated earlier are healthier and less likely to die from any cause. Although we adjusted for characteristics such as household income, influenza vaccination, and GP consults, other unmeasured behavioural differences, or characteristics such as Indigenous status, remain a plausible cause of residual confounding. It is also notable that VE for all-cause mortality was negative for some of the dose 2 intervals examined. These estimates were for small groups with relatively short follow-up and are statistically less robust. They were also unusual in that they represented people who either completed their primary vaccine course over a year later than when they would have first been eligible (June to November period) or had their primary vaccine course early in the program but no booster (January to May period). We hypothesise that they are predominantly individuals who may have serious health conditions meaning they were unable to have primary vaccination until very late, or unable to be subsequently vaccinated, and this may explain their increased risk of death.
Incomplete adjustment for serious comorbidities could also bias our findings. In Australia, and other countries, COVID-19 vaccines were recommended for both older adults and those with specific comorbidities8,11 due to higher risk of severe disease.25 While we adjusted for comorbidities using a score based on medicine dispensing data, this may not capture the breadth and severity of comorbidities. Also, severely immunocompromised people were recommended to have three vaccine doses for their primary course26 so they received a fourth dose (first booster) when most of the population was recommended to have a third dose, and a fifth dose when others were recommended to receive a fourth. These factors may counterbalance the healthy vaccinee effect as sicker individuals may be more likely to get boosters and to have these earlier.
Our dataset did not include comprehensive data on COVID-19 infections or hospitalisations so we were unable to evaluate the impact of COVID-19 vaccination on these outcomes or account for previous infections. However in Australia during 2022 there was an increasing reliance on rapid antigen testing and the completeness of reporting positive results has declined over time.9 Furthermore, positive tests for COVID-19 were increasingly found incidentally in patients hospitalised for other reasons. The focus on deaths limited our ability to measure the overall health impacts of vaccines, but the data presented here represent a very important measure of their effects.
Other factors not accounted for include past infections which are known to affect subsequent risk of severe disease27 and vaccine type. An earlier analysis in Australia that included infection and vaccine uptake data, showed that those with SARS-CoV-2 infection either delay their next due dose or are more likely than the uninfected to never receive it at all28 suggesting differential levels of hybrid immunity according to vaccine status. While some reports suggest differences in effectiveness of vaccine brands against mortality,1 the majority of the population in this age group residing outside of aged care facilities received the adenoviral vector vaccine, ChAdOx1 for their primary course. Moreover, almost all boosters administered in Australia have been mRNA vaccines (BNT162b2 or mRNA-1273)29; bivalent COVID-19 boosters containing equal quantities of ancestral and the BA.1 subvariant were only available for use in Australia from mid-October 2022. Therefore, we were unable to make comparisons by vaccine type in this study. Further, VE was estimated in relation to an unvaccinated group. Individuals classified as unvaccinated may include those who opted out of linking their vaccination record to other administrative data sources. This too could have resulted in underestimated VE. Finally we lacked genotyping data on the COVID-19 deaths so VE is based on assumptions regarding the predominant SARS-CoV-2 Omicron subvariant present at the time from surveillance data.9
In summary, this study quantifies the effectiveness of COVID-19 vaccines against mortality in the Australian population aged 65+ years during 2022 when the SARS-CoV-2 Omicron variant dominated. We found that both the primary course and subsequent boosters provide significant protection against death from COVID-19. However, effectiveness against COVID-19 mortality was observed to wane with time since receipt, and by >6 months since booster receipt, VE was estimated at just over 50%, suggesting that boosting after 6 months is beneficial. The high mortality rates in aged care residents also suggest that the absolute benefits of COVID-19 boosters will be greater in this population.
Contributors
BL, SP, and KM conceived the study and acquired funding; BL, SS, HG wrote the analysis plan. SS conducted the analyses; BL, SS, and HG accessed and verified the underlying data. BL and SS drafted the manuscript. All authors contributed equally to refining the methodology and analysis plan, data interpretation and reviewing and editing the manuscript.
Data sharing statement
The study investigators do not own the data. Requests for sharing of de-identified data should be directed to the Australian Government Department of Health and Aged Care.
Declaration of interests
BL declares funding from the Department of Health and Aged Care for this work, and the NHMRC, and payment for time as a member of the Australian Technical Advisory Group on Immunisation (ATAGI); HG declares funding from the NHMRC and payment from Seqirus for Q fever advisory board meeting; RK declares funding from the Department of Health and Aged Care for this work; SP declares funding from the NHMRC; NP declares funding from NHMRC and payment for time as a Member of Australian Government Drug Utilisation Sub Committee; CV declares funding from Australian Government Department of Health and Aged Care, NHMRC, World Cancer Research Fund, Australian Research Data Commons and Australian Research Council, participation at QIMR Berghofer Medical Research Institute, Deputy Chair, NSW Population & Health Services Research Ethics Committee, AIHW Refugee and Humanitarian Entrant Health Expert Advisory Group, Australian Research Council Medical Research Advisory Group, Steering Committee Member, Brain Cancer Biobanking Australia Commonwealth Therapeutic Goods Administration Expert Advisory Panel for Breast Implant Associated Anaplastic Large Cell Lymphoma, Australian Institute of Health and Welfare Cancer Monitoring Advisory Group, National Breast Cancer Foundation Access Committee; JW declares funding from the Department of Health and Aged Care; KM declares funding from the Australian and state government departments of health, NHMRC Australia, Gavi, WHO and Welcome Trust, payment as an expert witness on COVID-19 for state governments and support for attending meetings and/or travel only in context of grants above. All other authors have no declarations of interest.
Acknowledgements
We acknowledge the assistance of Allison Clarke, Clement Schlegel, Caroline Roga, Joe Lu, Michael Agnew, Greg Hood, Brandon Hao, from the Health Economics Research Division in the Australian Government Department of Health and Aged Care with use of the MADIP linked to AIR data collection. We acknowledge the assistance of Jiahui Qian and Shayal Prasad from the National Centre for Immunisation Research and Surveillance for preparation of figures and the report presentation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100928.
Appendix A. Supplementary data
References
- 1.International Vaccine Access Center COVID-19 data - vaccine studies. https://view-hub.org/covid-19/effectiveness-studies
- 2.UK Health Security Agency COVID-19 vaccine surveillance report 1 December 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1134074/vaccine-surveillance-report-week-48-2022.pdf
- 3.Viana R., Moyo S., Amoako D.G., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–686. doi: 10.1038/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feikin D.R., Higdon M.M., Abu-Raddad L.J., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944. doi: 10.1016/s0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higdon M.M., Baidya A., Walter K.K., et al. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect Dis. 2022;22(8):1114–1116. doi: 10.1016/s1473-3099(22)00409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu N., Joyal-Desmarais K., Ribeiro P.A.B., et al. Long-term effectiveness of COVID-19 vaccines against infections, hospitalisations, and mortality in adults: findings from a rapid living systematic evidence synthesis and meta-analysis up to December, 2022. Lancet Respir Med. 2023;11(5):439–452. doi: 10.1016/S2213-2600(23)00015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Health Communicable diseases intelligence - COVID-19 Australia: epidemiology report 57. https://www1.health.gov.au/internet/main/publishing.nsf/Content/C50CAE02452A48A7CA2587320081F7BF/%24File/covid_19_australia_epidemiology_report_57_reporting_period_ending_16_january_2022.pdf [DOI] [PubMed]
- 8.Department of Health and Aged Care ATAGI – preliminary advice on general principles to guide the prioritisation of target populations in a COVID-19 vaccination program in Australia. 2020. https://www.health.gov.au/sites/default/files/documents/2020/11/atagi-preliminary-advice-on-general-principles-to-guide-the-prioritisation-of-target-populations-in-a-covid-19-vaccination-program-in-australia_0.pdf
- 9.Department of Health and Aged Care Communicable diseases intelligence - COVID-19 Australia: epidemiology report 70. https://www1.health.gov.au/internet/main/publishing.nsf/Content/C50CAE02452A48A7CA2587320081F7BF/%24File/covid_19_australia_epidemiology_report_70_reporting_period_ending_15_january_2023.pdf [DOI] [PubMed]
- 10.Department of Health COVID-19 vaccine rollout. 2021. https://www.health.gov.au/sites/default/files/documents/2021/12/covid-19-vaccine-rollout-update-23-december-2021.pdf
- 11.Department of Health and Aged Care Expanded ATAGI recommendations on winter COVID-19 booster doses for people at increased risk of severe COVID-19. 2022. https://www.health.gov.au/news/expanded-atagi-recommendations-on-winter-covid-19-booster-doses-for-people-at-increased-risk-of-severe-covid-19
- 12.Australian Bureau of Statistics Participation in the 2021 census - information on how the 2021 census was conducted. https://www.abs.gov.au/census/about-census/2021-census-overview/participation-2021-census
- 13.Services Australia Australian Immunisation Register - the national register where your vaccinations are recorded. https://www.servicesaustralia.gov.au/australian-immunisation-register
- 14.Department of Health and Aged Care MBS Online. 2022. http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home
- 15.Australian Bureau of Statistics COVID-19 mortality in Australia: deaths registered until 31 January 2023. 24 February 2023. https://www.abs.gov.au/articles/covid-19-mortality-australia-deaths-registered-until-31-january-2023
- 16.Pratt N.L., Kerr M., Barratt J.D., et al. The validity of the Rx-risk comorbidity index using medicines mapped to the anatomical therapeutic chemical (ATC) classification system. BMJ Open. 2018;8(4) doi: 10.1136/bmjopen-2017-021122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meindl B., Enderle T. cellKey -consistent perturbation of statistical tables. Conference of European Statisticians. 2019. https://unece.org/fileadmin/DAM/stats/documents/ece/ces/ge.46/2019/mtg1/SDC2019_S7_Austria_and_Germany_cellKey_Meindl_AD.pdf
- 18.Lin D.-Y., Gu Y., Wheeler B., et al. Effectiveness of covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386(10):933–941. doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma A., Oda G., Holodniy M. Effectiveness of messenger RNA–based vaccines during the emergence of the severe acute respiratory syndrome coronavirus 2 omicron variant. Clin Infect Dis. 2022;75(12):2186–2192. doi: 10.1093/cid/ciac325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan V.K.C., Wan E.Y.F., Ye X., et al. Effectiveness of BNT162b2 and CoronaVac vaccinations against mortality and severe complications after SARS-CoV-2 Omicron BA.2 infection: a case-control study. Emerg Microbes Infect. 2022;11(1):2304–2314. doi: 10.1080/22221751.2022.2114854. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feikin D.R., Abu-Raddad L.J., Andrews N., et al. Assessing vaccine effectiveness against severe COVID-19 disease caused by omicron variant. Report from a meeting of the World Health Organization. Vaccine. 2022;40(26):3516–3527. doi: 10.1016/j.vaccine.2022.04.069. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.University of New South Wales - The Kirby Institute Seroprevalence of SARS-CoV-2-specific antibodies among Australian blood donors: round 4 update Serosurveillance for SARS-CoV-2 infection to inform public health responses. 2023. https://kirby.unsw.edu.au/sites/default/files/COVID19-Blood-Donor-Report-Round4-Nov-Dec-2022.pdf
- 23.Stein C., Nassereldine H., Sorensen R.J.D., et al. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. Lancet. 2023;401(10379):833–842. doi: 10.1016/S0140-6736(22)02465-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu B., Spokes P., He W., Kaldor J. High risk groups for severe COVID-19 in a whole of population cohort in Australia. BMC Infect Dis. 2021;21(1):685. doi: 10.1186/s12879-021-06378-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Department of Health and Aged Care Considerations for special populations. https://www.health.gov.au/our-work/covid-19-vaccines/advice-for-providers/clinical-guidance/clinical-recommendations#considerations-for-special-populations
- 27.Bobrovitz N., Ware H., Ma X., et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. 2023 doi: 10.1016/s1473-3099(22)00801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gidding H., Stepien S., Qian J., Macartney K., Liu B. COVID-19 vaccine uptake by infection status in New South Wales, Australia. Emerg Infect Dis. 2023;29(5):1070–1073. doi: 10.3201/eid2905.230047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu B., Gidding H., Stepien S., Cretikos M., Macartney K. Relative effectiveness of COVID-19 vaccination with 3 compared to 2 doses against SARS-CoV-2 B.1.1.529 (Omicron) among an Australian population with low prior rates of SARS-CoV-2 infection. Vaccine. 2022;40(43):6288–6294. doi: 10.1016/j.vaccine.2022.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.