Highlights
-
•
In non-DP patients, TGF-β and IL-6 first decrease, then they successively increase.
-
•
In non-DP patients, IFN-γ increases, while IL-8 and IL-10 decrease at the end of follow up.
-
•
In DP patients IL-6 increases when patients progressed.
-
•
In patients with pseudoprogression IL-6 and IL-10 increase.
-
•
Baseline TGF-β, IFN-γ, IL-6, and IL-10 are increased in patients with irAEs.
Keywords: Metastatic melanoma patients, BRAF wild type, Pembrolizumab, Cytokines, Monitoring, Outcome predictors
Abstract
Background
The biomarkers of immune checkpoint inhibitors (ICIs) efficacy and safety are still urgently needed. As cytokines are easily detected and monitored in circulation, they could be used as potential predictors of response and immune-related adverse events (irAEs) for ICIs therapy.
Methods
The levels of TGF-β, IFN-γ, IL-6, IL-8, IL-10 were measured in sera and plasma by ELISA method of 30 healthy controls (HC) and 32 BRAF wild type (wt) MM patients before and after every 12 weeks of Pembrolizumab, PD-1 inhibitor, until one year or disease progression (DP).
Results
Higher pretherapy levels of circulating TGF-β, IFN-γ, IL-6, and IL-10 were shown in MM patients compared to HC. In patients with disease control, TGF-β and IL-6 first decreased during the therapy, while then they started to successively increase reaching the initial values by the end of the follow up. Furthermore, in this group of patients IFN-γ increased, while IL-8 and IL-10 decreased at final points of the follow up. In patients with DP IL-6 increased at the time of progression, while IL-8 decreased when the best response was achieved. In patients with pseudoprogression IL-6 and IL-10 significantly increased compared to the pretreatment values. Melanoma patients with irAEs had increased baseline values of TGF-β, IFN-γ, IL-6, and IL-10 compared to HC. However, no significant changes in cytokines levels were found in these patients during therapy.
Conclusions
Inflammatory cytokines monitoring in circulation of BRAFwt MM patients could help in the selection of patients who will have the benefit from Pembrolizumab therapy.
Graphical abstract
Introduction
Melanoma is a highly aggressive skin tumor that originates from the pigment cells, melanocytes. This cancer is characterized by invasive growth and early lymphogenic and hematogenic metastasis, with a 5-year survival rate of 32 % in metastatic melanoma patients [1]. The clinical application of immune checkpoint inhibitors (ICIs), monoclonal antibodies that block inhibitory molecules, primarily CTLA-4 (Cytotoxic T-lymphocyte Antigen-4), PD-1 (Programmed Cell Death-1), PD-L1 (Programmed Cell Death Ligand-1), has dramatically improved the outcome of patients with metastatic melanoma by increasing the antitumor immune response of these patients [2]. It has been shown that the drugs that block the PD-1 pathway (Pembrolizumab and Nivolumab) provide higher response rates with a less toxicity than the blocker of CTLA-4 molecule, Ipilimumab [2,3]. However, despite the promising results of anti-PD-1 antibodies in melanoma, clinical outcomes remain highly variable and only a subset of patients shows long-term response [4]. Moreover, ICIs therapies can result in severe and potentially life-threating side effects termed immune-related adverse events (irAEs) [5]. Therefore, predictive biomarkers that can aid in more precise delivery of immunotherapy to melanoma patients are urgently needed. However, few biomarkers have been established that can predict treatment success and positive clinical outcomes for patients [6]. As mutations in tumor cells are thought to be very important for the generation of neoantigens that initiate anti-tumor immune response, tumor mutational burden (TMB) may have a significant correlation with response rate and prognosis in these patients [7]. Moreover, transmembrane protein PD-L1, expressed on many tumor cells, plays a major role in suppressing the immune system via engagement of PD-1 expressed on activated T cells and natural killer (NK) cells [8]. However, none of the investigated parameters has demonstrated a clinically-useful role in distinguishing between responder and non-responder patients. The clinical use of these markers is still limited due to the discrepancy of results and the requirement of tissue samples that have to be obtained by the invasive tumor biopsies. Therefore, the identification of prognostic and predictive biomarkers in peripheral blood may represent an alternative solution to avoid these problems [9]. Assessment of cytokines in plasma and sera may greatly increase our understanding of a patient’ immune response to the tumor before and during the treatment [10].
Cytokines are small soluble proteins that are secreted by numerous cells and have various biological activities in physiological and pathological conditions. In complex microenvironment of tumor, they are involved in the regulation of development and progression of tumor cells, as well as they have the role in the modulation of host immune response. Therefore, cytokines represent a bridge between immune and tumor cells [11]. IFN-γ, multipotent cytokine mainly produced by activated helper 1 T (Th1) cells and NK cells, increases tumor immunogenicity, inhibits the proliferation of tumor cells and promotes Th1, NK cells and cytotoxic T lymphocytes (CTLs) anti-tumor immune response. Contrary to IFN-γ, TGF-β, IL-6, IL-8 and IL-10 produced by various immune and tumor cells directly or indirectly, by recruitment and activation of the immunosuppressive regulatory T (Treg) cells, myeloid derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), inhibit anti-tumor immune response [12].
Although the role of cytokines in the regulation of the immune response is well known and many studies have evaluated the baseline levels and changes of cytokines in patients with various tumors during ICI treatment [10,13,14], the implication of cytokines as potential biomarkers of response to this therapy is still controversial. In this study, we explored the biomarkers associated with clinical benefits such as tumor response, and onset of irAEs. The aim of this study is to investigate if serum or plasma TGF-β, IFN-γ, IL-6, IL-8, IL-10 can play a predictive role in BRAF wild type (wt) metastatic melanoma (MM) patients treated with Pembrolizumab and to assess any potential correlations between their levels and the treatment response and clinical safety.
Patients and methods
Patients’ characteristics, treatment details and response to therapy
Thirty-two BRAFwt MM patients (stage IV according to 8th modified AJCC/UICC staging system) [15] of the Institute of Oncology and Radiology of Serbia and 30 healthy controls (HC) were involved in the study. The study was approved by the Ethical Committee of Institute of Oncology and Radiology of Serbia and all patients and HC signed the informed consent before inclusion in the study. The patients were treated only with anti-PD-1 therapy, Pembrolizumab, until disease progression or intolerable toxicity. The patients and HC were age and sex matched with no evidence of infection, autoimmune or any other disease. In patients, tumor assessment was performed at baseline and every 12 weeks of therapy and clinical response was classified according to immune-response evaluation criteria in solid tumors (iRECIST) [16]. Clinicopathological data and treatment outcomes were collected retrospectively from the patients’ database of the Institute and updated on August 15, 2022. Therefore, patients were divided into two groups: 14 patients were with disease control, i.e. without disease progression (Non-DP) (patients who achieved complete response (CR), partial response (PR), or stable disease (SD)) and 18 patients achieved disease progression (DP). Furthermore, iRECIST criteria define pseudoprogression of disease (PSPD) that represents initially growth of tumor typically within 12 weeks of immunotherapy followed by a subsequent response to immunotherapy. In this study 8 patients were observed as PSPD after 12 weeks of the therapy. However, only one patient with PSPD had disease control (PR). Peripheral blood samples obtained from patients were collected prior to the first administration of therapy and every 12 weeks of therapy until one year or until DP, between December 2017 and April 2020. The characteristics of MM patients and HC are listed in Table 1.
Table 1.
The characteristics of patients and healthy controls.
| Characteristics | MM | HC |
|---|---|---|
| Gender | ||
| Male | 14 (43.75 %) | 16 (53.33 %) |
| Female | 18 (56.25 %) | 14 (46.67 %) |
| Age (years) | ||
| Mean (SD) | 59.34 (9.18) | 60.53 (10.89) |
| Median (Range) | 59 (39–84) | 58 (40–80) |
| Leukocytes (x109/L) | ||
| Mean (SD) | 8.69 (4.21) | |
| Median (Range) | 7.65 (3.6–20.7) | |
| Lymphocytes (x109/L) | ||
| Mean (SD) | 1.53 (0.64) | |
| Median (Range) | 1.60 (0.5–2.8) | |
| Neutrophils (x109/L) | ||
| Mean (SD) | 6.00 (3.60) | |
| Median (Range) | 5.00 (2.4–18.4) | |
| Performance status | ||
| 0 | 11 (34.37 %) | |
| 1 | 10 (31.25 %) | |
| 2 | 7 (21.88 %) | |
| 3 | 4 (12.50 %) | |
| 4 | 0 (0 %) | |
| Metastasis stage | ||
| M1a | 2 (6.25 %) | |
| M1b | 10 (31.25 %) | |
| M1c | 12 (37.5 %) | |
| M1d | 8 (25 %) | |
| Immune-related adverse events | ||
| Hepatotoxicity | 1 (3.12 %) | |
| Eosinophilia | 1 (3.12 %) | |
| Hypothyroidism | 1 (3.12 %) | |
| Hyperbilirubinemia | 1 (3.12 %) | |
| Neutropenia | 1 (3.12 %) | |
| Baseline LDH | ||
| Normal | 20 (62.50 %) | |
| Elevated | 12 (37.50 %) | |
| AST (U/L) | ||
| Mean (SD) | 25.62 (17.85) | |
| Median (Range) | 19.50 (10–88) | |
| ALT (U/L) | ||
| Mean (SD) | 24.78 (21.56) | |
| Median (Range) | 17.5 (9–108) | |
| γ-GT (U/L) | ||
| Mean (SD) | 48.37 (48.12) | |
| Median (Range) | 33.50 (10–203) | |
| Pembrolizumab cycles | ||
| Mean (SD) | 29.28 (24.19) | |
| Median (Range) | 26.5 (1–68) | |
| Therapy responses | ||
| Non-DP | 14 (43.75 %) | |
| CR | 6 (42.86 %) | |
| PR | 5 (35.71 %) | |
| SD | 3 (21.43 %) | |
| DP | 18 (56.25 %) | |
| Total | 32 (100 %) | 30 (100 %) |
Measurement of cytokines by ELISA
Blood samples of MM patients and HC were collected by venipuncture. The sera and plasma samples were isolated and frozen immediately at -80 °C until analysis. The concentrations of TGF-β, IFN-γ, IL-8 and IL-10 in sera and IL-6 in plasma of the investigated patients and HC were determined by commercial uncoated ELISA kits, according to manufacturer’ instructions (Invitrogen) as previously described [17]. The quantification of IL-6 was carried in plasma samples, due to the low and often undetectable level that is below the range of standard curve in sera.
Statistical analysis
Significance of difference for cytokine levels in sera and plasma of MM patients compared to HC was done by nonparametric Mann-Whitney exact test. The dynamics of changes in the cytokine levels during the Pembrolizumab therapy were analyzed by Wilcoxon matched-pairs signed rank test. For both tests the p values bellow or equal 0.05 were considered significant.
Results
In this study, peripheral blood of 32 MM patients was collected prior to anti-PD therapy and at time points of clinical evaluation that was performed after every 4 cycles of therapy. Namely, in the sera and plasma of MM patients inflammatory cytokines TGF-β, IFN-γ, IL-6, IL-8, and IL-10 were measured before therapy and after the 4th, 8th, 12th, and 16th cycle of therapy, or until the disease progression. A cycle was defined as 3 weeks of treatment.
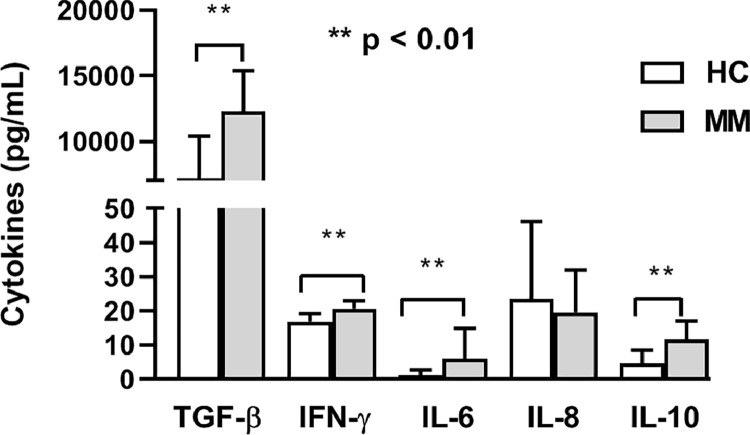
Pretherapy values of the investigated inflammatory cytokines in MM patients were compared to age-matched healthy controls. The obtained data showed significantly higher baseline levels of TGF-β, IFN-γ, IL-6, and IL-10 in MM patients (p < 0.01, Mann–Whitney exact test) but similar level of IL-8 (p > 0.05, Mann–Whitney exact test) (Fig. 1).
Fig. 1.
Pretherapy cytokines levels. Metastatic melanoma patients (MM) have significantly higher levels of TGF-β, IFN-γ, IL-6, and IL-10 compared to healthy controls (HC) (Mann-Whitney test). The results are presented as mean value with standard deviation.
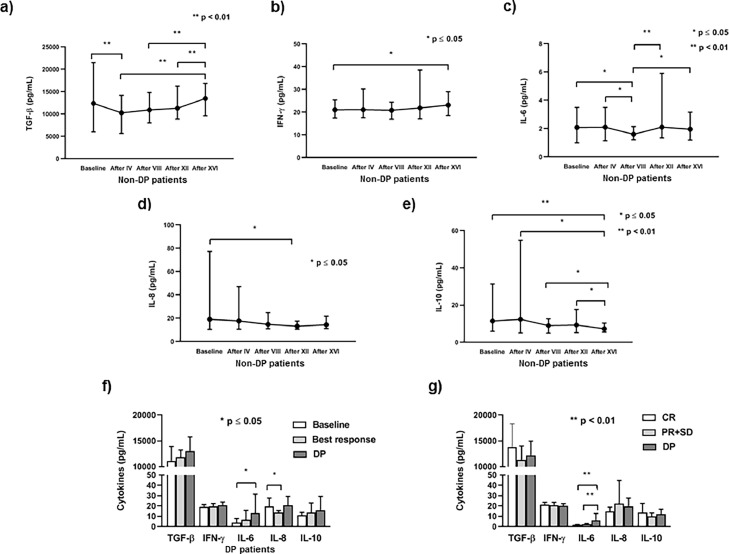
The dynamics of cytokine fluctuation in peripheral circulation during anti-PD-1 immunotherapy was evaluated by Wilcoxon matched-pairs signed rank test at time points of clinical evaluation. In this sense, in patients without disease progression (non-DP) the level of TGF-β significantly decreased after the 4th cycle of therapy compared to the baseline value, while after the 8th cycle it started to successively increase reaching the level similar to the initial value by the end of the follow up (Fig. 2a). The level of IFN-γ significantly increased at the end of the follow up compared to the pretherapy level (Fig. 2b). Similarly to TGF-β, the level of circulating IL-6 fluctuated, showing the significant decrease after the 8th cycle and significant augmentation through the 12th cycle till the end of the follow up when the concentration similar to the baseline was reached (Fig. 2c). However, the level of IL-8 significantly decreased after the 12th cycle of anti-PD-1 therapy compared to the baseline value (Fig. 2d), while circulating IL-10 continuously decreased after the 4th cycle until the end of the follow up (Fig. 2e).
Fig. 2.
Cytokines levels in metastatic melanoma (MM) patients without (non-DP) and with disease progression (DP). The dynamics of changes in a) TGF-β, b) IFN-γ, c) IL-6, d) IL-8 and e) IL-10 from baseline (pretherapy) value and after IV, VIII, XII and XVI cycles of anti-PD-1 therapy (Wilcoxon matched-pairs signed rank test) in non-DP MM patients. Results are presented as mean value with range (minimum and maximum); f) In MM patients with DP the level of circulating IL-6 increases at the time of progression, while IL-8 decreases when the best response was achieved compared to the baseline level (Wilcoxon matched-pairs signed rank test). The results are presented as mean value with standard deviation; g) Patients with DP have significantly higher baseline level of IL-6 compared to patients with disease control (complete response (CR), partial response (PR), and stable disease (SD)) (Mann-Whitney test). The results are presented as mean value with standard deviation.
In patients who achieved progression of the disease (DP) pretherapy levels of circulating cytokines were compared with the level detected at the time of best response and DP of these patients. Our results show that the level of IL-6 significantly increased at DP, while the level of IL-8 significantly decreased at best response, while the level of circulating TGF-β, IFN-γ and IL-10 remained similar at the best response and DP compared to the baseline values (p ≤ 0.05, Wilcoxon matched-pairs signed rank test) (Fig. 2f). Furthermore, analyzing baseline cytokines levels we have shown that patients with DP had significantly higher level of IL-6 compared to patients with disease control (CR, PR, SD) (p < 0.01, Mann–Whitney exact test). The other analyzed cytokines had similar baseline levels in investigated groups of MM patients (p > 0.05, Mann–Whitney exact test) (Fig. 2g).
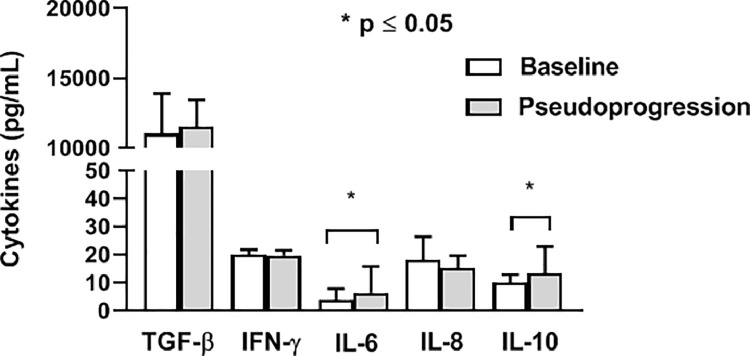
In patients with pseudoprogression of disease after 12 weeks of the therapy the level of circulating IL-6 and IL-10 significantly increased compared to the baseline values (p ≤ 0.05, Wilcoxon matched-pairs signed rank test) (Fig. 3).
Fig. 3.
Cytokines levels during pseudoprogression. The levels of circulating IL-6 and IL-10 significantly increase during pseudoprogression, after 12 weeks of the therapy, compared to the baseline values (Wilcoxon matched-pairs signed rank test). The results are presented as mean value with standard deviation.
Analyzing the peripheral blood leukocytes in investigated BRAFwt MM patients, we have shown that there were not any changes in their counts during Pembrolizumab therapy in MM patients without progression (non-DP), as well as in MM patients with DP (p > 0.05, Wilcoxon matched-pairs signed rank test) (Table 2).
Table 2.
Leukocyte count in metastatic melanoma patients during therapy.
| Leukocytes (x 109/L) | Baseline | After IV | After VIII | After XII | After XVI |
|---|---|---|---|---|---|
| Non-DP | 8.32 ± 4.12* | 7.75 ± 4.60 | 7.78 ± 2.99 | 7.66 ± 3.22 | 7.45 ± 3.21 |
| DP | 8.97 ± 4.74 | 8.72 ± 3.35 | 9.09 ± 4.45 | 8.93 ± 3.74 | 9.02 ± 5.09 |
Results are presented as mean ± standard deviation.
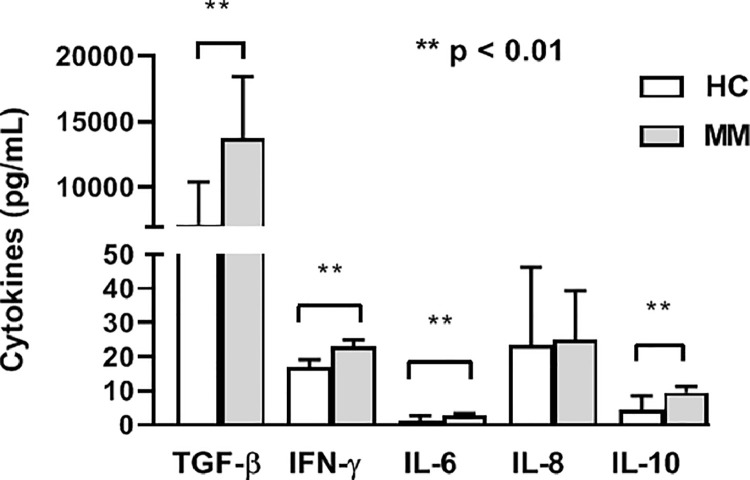
Immune-related adverse events (irAEs) for investigated MM patients treated with Pembrolizumab are shown in Table 1. Also, we have shown that these patients had significantly higher baseline levels of TGF-β, IFN-γ, IL-6, and IL-10 compared to HC (p < 0.01, Mann–Whitney exact test) (Fig. 4). However, during the therapy no significant changes of the investigated cytokines levels were noted in patients with appearance of irAEs (p > 0.05, Wilcoxon matched-pairs signed rank test) (data not shown).
Fig. 4.
Cytokines levels in metastatic melanoma (MM) patients with immune-related adverse events. MM patients with immune-related adverse events have significantly higher baseline levels of TGF-β, IFN-γ, IL-6, and IL-10 compared to HC (Mann–Whitney exact test). The results are presented as mean value with standard deviation.
Discussion
The microenvironment of melanoma is a complex network that consists of various types of cells, cytokines and enzymes. The cytokines, low weight molecules, mediate immune cell-tumor cell communication [18]. In this study BRAFwt MM patients had statistically higher baseline levels of TGF-β, IFN-γ, IL-6, and IL-10 compared to HC that is in accord with various data in literature [10,[19], [20], [21]]. These cytokines are important components of chronic inflammatory milieu in melanoma and they by various mechanisms directly or indirectly activate or inhibit immune cells [10,18].
Changes in cytokines levels during ICIs therapy regulate tumor microenvironment (TME), change the proliferation, differentiation and activation of immune cells, and influence the metastasis of cancer cells. Therefore, in this study we also analyzed the concentrations of investigated cytokines during Pembrolizumab therapy.
In MM patients without disease progression the level of TGF-β significantly decreased after the 4th cycle of therapy compared to the baseline value, while after that it started to successively increase reaching the level similar to the initial value by the end of the follow up. Contrary, in patients who achieved progression of the disease level of circulating TGF-β remained similar at the best response and DP compared to their baseline values. Therefore, early decrease of TGF-β during Pembrolizumab therapy could be the potential biomarker of therapy response in MM patients. TGF-β is pleiotropic cytokine produced by various cancer cells including melanoma, as well as immunosuppressive cells, such Treg and MDSCs. It is known for late-stage of melanoma that TGF-β induces tumor progression by enhancing metastasis, treatment resistance, and immune escape. The immunosuppressive effects of TGF-β on the TME are multiple. TGF-β suppresses the cytotoxicity of T cells and NK cells, induces Treg cells differentiation, recruits MDSCs, and inhibits the maturation and functions of dendritic cells (DCs) [22]. Therapeutic application of TGF-β/TGF-βR blockators in combination with ICIs is currently being tested in patients with metastatic melanoma [23].
The role of IFN-γ in the prediction of the efficacy of ICI immunotherapy is intensively evaluated. In this study in patients without progression, IFN-γ significantly increased at the end of the follow up compared to its pretherapy level that is in accord with numerous results in the studies of MM and non-small cell lung cancer (NSCLC) patients [24], [25], [26]. In patients with DP there was no difference in IFN-γ level during Pembrolizumab therapy. Therefore, in patients with disease control anti-PD-1 therapy increases the production of IFN-γ which then increases tumor immunogenicity, inhibits tumor cell proliferation, and enhances the cytotoxic function of NK cells and CTLs. Additionally, this cytokine induces the secretion of various chemokines such as CXCL9 and CXCL10 that recruit tumor-activated T cells [27]. Moreover, IFN-γ released by T and NK cells stimulates DCs to produce IL-12, which in turn promotes the production of IFN-γ by stimulated lymphocytes and a positive feedback loop occurs [28]. Therefore increased IFN-γ may represent a positive biomarker for ICI therapy response.
Interleukin-6 is a proinflammatory cytokine that is involved in cell growth, immune regulation and inflammation. In melanoma microenvironment this cytokine is chronically elevated and it has the role in carcinogenesis and tumor metastasis, as well as in the inhibition of anti-tumor immune response [29]. High baseline IL-6 could be a biomarker of tumor progression and poor prognosis in MM patients receiving ICIs [14,30,31]. Therefore, in this study we showed high pretherapy IL-6 values in patients with disease progression compared to patients with disease control i.e. CR, PR, and SD. It is known that IL-6 interferes with the development of DCs or it induces the differentiation and activation of immunosuppressive MDSCs [29]. In this study in non-DP patients plasma IL-6 showed the significant decrease after the 8th cycle of Pembrolizumab and significant augmentation through the 12th cycle till the end of the follow up when the concentration similar to the baseline was reached as we have shown for TGF-β. Contrary, in patients who achieved progression the level of IL-6 significantly increased at the time of progression. Tsukamoto et al. [20] have shown in patients with melanoma, treated with anti-PD-1 inhibitor, Nivolumab, increased systemic level of IL-6, which was associated with poor clinical response. Therefore, continual increase in IL-6 level during ICI therapy could be a negative biomarker of prognosis in melanoma patients.
Furthermore, we analyzed the level of IL-8 during Pembrolizumab therapy. This proinflammatory chemokine (CXCL8), produced by various cell types, has the role in the recruitment of leukocytes in healthy or injured tissue. It also has the important role in the process of tumor progression. Tumor-derived IL-8 promotes the trafficking of neutrophils and MDSCs into the tumor microenvironment and has the ability to dampen anti-tumor immune response [32]. In this study, the level of IL-8 in patients without progression significantly decreased after the 12th cycle of Pembrolizumab (after 9 months) compared to the baseline value. Contrary to non-DP patients, in DP patients the level of circulating IL-8 remained similar at the time of progression compared to the baseline values. Agulló-Ortuño et al. [33] have shown that the increased level of IL-8 (2 months after immunotherapy) was associated with poor overall survival (OS) in NSCLC patients, while Boutsikou et al. [25] have reported that increased level of IL-8 (3 months after immunotherapy) was correlated with prolonged OS in 26 NSCLC patients treated with anti-PD-1 in first or second line. Furthermore, in the patients who achieved progression we have shown that the level of IL-8 was significantly decreased at best response, compared to the baseline values, but increased upon progression that is in accord with results of Sanmamed et al. [34].
The role of IL-10 in tumor pathogenesis is controversial, with some findings showing that IL-10 promotes tumor development, while the other results show that this cytokine inhibits tumor growth and metastasis [35]. In immunosuppressive TME IL-10, produced by cancer cells, MDSCs, TAMs, Treg cells, enhances tumor cell survival, proliferation and metastasis by the inhibition of NK and CTL cells. Furthermore, together with IL-1, IL-6, TNF, prostaglandin E2 IL-10 promotes myelopoiesis, skews differentiation of monocyte progenitors into mMDSCs. Also, IL-10 induces the expression of Foxp3, TGF-β receptor 2 and TGF-β on activated Treg cells, and thus stabilizes the phenotype and function of these cells [36,37]. Contrary to patients with progression, in the patients without DP in this study, serum IL-10 continuously decreased after the 4th cycle of therapy until the end of the follow up. Increased IL-10 in serum of various cancer patients often correlates with a poor prognosis [19]. It has been shown that PD-1 or IL-10 blockade as monotherapy is less efficient compared to therapeutical application of their combination that delays tumor growth by decrease of MDSCs infiltration [38].
In this study we show for the first time increased values of IL-6 and IL-10 in MM patients with pseudoprogression observed after 4 cycles of Pembrolizumab therapy (12 weeks). Monitoring the changes in cytokines levels during ICI therapy may also be useful in identifying pseudoprogression [39]. During this process, increase in the infiltration of immune cells in tumor lesion impacts on the increase of the volume of tumor mass. Current imaging techniques cannot differentiate the composition of cells inside the growing tumor lesion. However, measurement of circulating cytokines might reflect more accurately changes in the tumor compartment. Sanmamed et al. [34] show that serum IL-8 levels significantly decreased compare to baseline values during the increase in tumor size shown by imaging evaluation. Our results of increased circulating IL-6 and IL-10 in PSPD generate the hypothesis that changes in these cytokines levels might become a useful tool to diagnose and follow pseudoprogression. However, only one patient with PSPD in this study had disease control. The relationships between cytokines levels in circulation and anti‐PD‐1 treatment outcomes are not well established. Larger series of patients experiencing pseudoprogression need to be studied to confirm these findings.
Furthermore, in this study, we analyzed the peripheral blood leukocytes in BRAFwt MM patients and have shown that there were not any changes in their counts during Pembrolizumab therapy in MM patients without progression, as well as in patients with DP. Therefore, the number of leukocytes in this study does not have any association with changes in cytokine values. Analysis of changes in various leukocyte subsets during Pembrolizumab therapy in BRAFwt MM patients should be addressed in the future studies.
Immune-related adverse events are consequence of over-activated immune system and various cytokines are extensively studied to predict them. The cytokine profile of irAEs varies and in several malignancies including melanoma increased baseline IL-17 was found in pneumonitis and colitis, elevated baseline IL-1β, IL-2, and granulocyte-macrophage colony-stimulating factor predicted thyroid irAE, while dermatitis was associated with high IL-6 and IL-10 [40,41]. In this study five patients developed hepatotoxicity, hypothyroidism, hyperbilirubinemia, eosinophilia or neutropenia. However, all of these patients were with disease control (non-DP). We have shown for the first time that these patients had increased pretherapy values of TGF-β, IFN-γ, IL-6 and IL-10 compared to values of these cytokines in healthy controls. Therefore, these cytokines could associate with irAE risk in BRAFwt MM patients receiving Pembrolizumab. However, we have also shown no association between the appearance of irAEs and cytokine levels during Pembrolizumab therapy. More research with larger group of patients is needed to determine the impact of cytokines on the development of irAEs.
Conclusions
In this prospective and longitudinal study we investigated potential biomarkers associated with clinical benefits such as tumor response, and onset of irAEs by monitoring the dynamics of cytokine fluctuation in peripheral circulation during the course of anti-PD-1 immunotherapy in BRAFwt MM patients. Our results show that in patients with disease control TGF-β and IL-6 first significantly decreased following the onset of therapy, while, after that, they started to successively increase reaching the level similar to the initial value by the end of the follow up. Furthermore, in this group of patients the level of IFN-γ increased while the levels of IL-8 and IL-10 decreased at final points of the clinical follow up. In patients with progression the level of circulating IL-6 increased at the time of progression compared to the baseline level, while IL-8 decreased when the best response was achieved. Also, in patients with pseudoprogression of disease the level of circulating IL-6 and IL-10 significantly increased compared to the baseline values. Furthermore, MM patients with irAEs had elevated pretherapy values of TGF-β, IFN-γ, IL-6, and IL-10 compared to HC, although no significant changes in the investigated cytokines levels were found in these patients during therapy. Changes in circulating cytokine level found in this study could be used to monitor and predict clinical benefit from Pembrolizumab therapy in BRAFwt MM patients. Dynamic of changes in these potential biomarkers might be a promising tool to characterize long-term responders, and also may predict a patient's risk for irAEs development.
Funding
This work was supported by the Ministry of Science, Technological Development and Innovation (Serbia) (Grant Nos. 451–03–47/2023–01/200043 and 451–03-47/2023–01/200111).
CRediT authorship contribution statement
Katarina Mirjačić Martinović: Investigation, Conceptualization, Methodology, Writing – original draft, Supervision. Ana Vuletić: Investigation, Conceptualization, Methodology, Writing – original draft, Validation. Nevena Tišma Miletić: Investigation, Methodology, Writing – original draft. Irina Besu Žižak: Investigation, Methodology, Writing – original draft. Jelena Milovanović: Investigation, Methodology, Writing – original draft. Suzana Matković: Conceptualization, Data curation, Writing – review & editing. Vladimir Jurišić: Investigation, Conceptualization, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to thank Mrs. Jasna Popović Basić for excellent technical work.
References
- 1.Schadendorf D., van Akkooi A.C.J., Berking C., Griewank K.G., Gutzmer R., Hauschild A., Stang A., Roesch A., Ugurel S. Melanoma. Lancet. 2018;392(10151):971–984. doi: 10.1016/S0140-6736(18)31559-9. Sep 15Erratum in: Lancet. 2019 Feb 23;393(10173):746. [DOI] [PubMed] [Google Scholar]
- 2.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P., Allison J.P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darvin P., Toor S.M., Sasidharan Nair V., Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 2018;50(12):1–11. doi: 10.1038/s12276-018-0191-1. Dec 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy L.B., Salama A.K.S. A review of immune-mediated adverse events in Melanoma. Oncol. Ther. 2019;7(2):101–120. doi: 10.1007/s40487-019-0096-8. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyhan A.A., Carini C. Insights and strategies of Melanoma immunotherapy: predictive biomarkers of response and resistance and strategies to improve response rates. Int. J. Mol. Sci. 2022;24(1):41. doi: 10.3390/ijms24010041. Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeckel C., Bakhl K., Georgakopoulos-Soares I., Zaravinos A. The efficacy of tumor mutation burden as a biomarker of response to immune checkpoint inhibitors. Int. J. Mol. Sci. 2023;24(7):6710. doi: 10.3390/ijms24076710. Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paver E.C., Cooper W.A., Colebatch A.J., Ferguson P.M., Hill S.K., Lum T., Shin J.S., O'Toole S., Anderson L., Scolyer R.A., Gupta R. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology. 2021;53(2):141–156. doi: 10.1016/j.pathol.2020.10.007. (Phila)Feb. [DOI] [PubMed] [Google Scholar]
- 9.Buder-Bakhaya K., Hassel J.C. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment-a review from the melanoma perspective and beyond. Front. Immunol. 2018;9:1474. doi: 10.3389/fimmu.2018.01474. Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M., Zhai X., Li J., Guan J., Xu S., Li Y., Zhu H. The role of cytokines in predicting the response and adverse events related to immune checkpoint inhibitors. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.670391. Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4(1):11–22. doi: 10.1038/nrc1252. Jan. [DOI] [PubMed] [Google Scholar]
- 12.Kartikasari A.E.R., Huertas C.S., Mitchell A., Plebanski M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.692142. Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim J.U., Yoon H.K. Potential predictive value of change in inflammatory cytokines levels subsequent to initiation of immune checkpoint inhibitor in patients with advanced non-small cell lung cancer. Cytokine. 2021;138 doi: 10.1016/j.cyto.2020.155363. Feb. [DOI] [PubMed] [Google Scholar]
- 14.Mirjačić Martinović K., Vuletić A., Tišma Miletić N., Matković S., Gavrilović D., Ninković A., Jurišić V., Babović N. Circulating IL-6 is associated with disease progression in BRAFwt metastatic melanoma patients receiving anti-PD-1 therapy. J. Clin. Pathol. 2023 doi: 10.1136/jcp-2022-208615. Feb 8. [DOI] [PubMed] [Google Scholar]
- 15.Keohane S.G., Proby C.M., Newlands C., Motley R.J., Nasr I. Mohd Mustapa MF; British association of dermatologists (Squamous and Basal cell carcinoma guideline development groups); Slater DN; Royal College of Pathologists (Skin Cancer Lead). the new 8th edition of TNM staging and its implications for skin cancer: a review by the British Association of Dermatologists and the Royal College of Pathologists, U.K. Br. J. Dermatol. 2018;179(4):824–828. doi: 10.1111/bjd.16892. Oct. [DOI] [PubMed] [Google Scholar]
- 16.Seymour L., Bogaerts J., Perrone A., Ford R., Schwartz L.H., Mandrekar S., Lin N.U., Litière S., Dancey J., Chen A., Hodi F.S., Therasse P., Hoekstra O.S., Shankar L.K., Wolchok J.D., Ballinger M., Caramella C., de Vries E.G.E., RECIST working group iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. MarEpub 2017 Mar 2. Erratum in: Lancet Oncol. 2019 May;20(5):e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanojković T.P., Matić I.Z., Petrović N., Stanković V., Kopčalić K., Besu I., Đorđić Crnogorac M., Mališić E., Mirjačić-Martinović K., Vuletić A., Bukumirić Z., Žižak Ž., Veldwijk M., Herskind C., Nikitović M. Evaluation of cytokine expression and circulating immune cell subsets as potential parameters of acute radiation toxicity in prostate cancer patients. Sci. Rep. 2020;10(1):19002. doi: 10.1038/s41598-020-75812-0. Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharouf N., Flanagan T.W., Hassan S.Y., Shalaby H., Khabaz M., Hassan S.L., Megahed M., Haikel Y., Santourlidis S., Hassan M. Tumor microenvironment as a therapeutic target in melanoma treatment. Cancers. 2023;15(12):3147. doi: 10.3390/cancers15123147. (Basel)Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S., Wu D., Wu P., Wang Z., Huang J. Serum IL-10 Predicts worse outcome in cancer patients: a meta-analysis. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139598. Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsukamoto H., Fujieda K., Miyashita A., Fukushima S., Ikeda T., Kubo Y., Senju S., Ihn H., Nishimura Y., Oshiumi H. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–5022. doi: 10.1158/0008-5472.CAN-18-0118. Sep 1. [DOI] [PubMed] [Google Scholar]
- 21.Mirjačić Martinović K, Vuletić A, Mališić E, Srdić-Rajić T, Tišma Miletić N, Babović N, Jurišić V. Increased circulating TGF-β1 is associated with impairment in NK cell effector functions in metastatic melanoma patients. Growth Factors. 2022;40(5–6):231–239. doi: 10.1080/08977194.2022.2124915. Nov. [DOI] [PubMed] [Google Scholar]
- 22.Derynck R., Turley S.J., Akhurst R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021;18(1):9–34. doi: 10.1038/s41571-020-0403-1. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson C., Oronsky B., Reid T. AdAPT-001, an oncolytic adenovirus armed with a TGF-β trap, overcomes in vivo resistance to PD-L1-immunotherapy. Am J Cancer Res. 2022;12(7):3141–3147. Jul 15. [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki N., Kiyohara Y., Uhara H., Iizuka H., Uehara J., Otsuka F., Fujisawa Y., Takenouchi T., Isei T., Iwatsuki K., Uchi H., Ihn H., Minami H., Tahara H. Cytokine biomarkers to predict antitumor responses to nivolumab suggested in a phase 2 study for advanced melanoma. Cancer Sci. 2017;108(5):1022–1031. doi: 10.1111/cas.13226. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutsikou E., Domvri K., Hardavella G., Tsiouda D., Zarogoulidis K., Kontakiotis T. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther. Adv. Med. Oncol. 2018;10 doi: 10.1177/1758835918768238. Apr 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirashima T., Kanai T., Suzuki H., Yoshida H., Matsushita A., Kawasumi H., Samejima Y., Noda Y., Nasu S., Tanaka A., Morishita N., Hashimoto S., Kawahara K., Tamura Y., Okamoto N., Tanaka T. The levels of interferon-gamma release as a biomarker for non-small-cell lung cancer patients receiving immune checkpoint inhibitors. Anticancer Res. 2019;39(11):6231–6240. doi: 10.21873/anticanres.13832. Nov. [DOI] [PubMed] [Google Scholar]
- 27.Dulos J., Carven G.J., van Boxtel S.J., Evers S., Driessen-Engels L.J., Hobo W., Gorecka M.A., de Haan A.F., Mulders P., Punt C.J., Jacobs J.F., Schalken J.A., Oosterwijk E., van Eenennaam H., Boots A.M. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J. Immunother. 2012;35(2):169–178. doi: 10.1097/CJI.0b013e318247a4e7. Feb-Mar. [DOI] [PubMed] [Google Scholar]
- 28.Garris C.S., Arlauckas S.P., Kohler R.H., Trefny M.P., Garren S., Piot C., Engblom C., Pfirschke C., Siwicki M., Gungabeesoon J., Freeman G.J., Warren S.E., Ong S., Browning E., Twitty C.G., Pierce R.H., Le M.H., Algazi A.P., Daud A.I., Pai S.I., Zippelius A., Weissleder R., Pittet M.J. Successful Anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49(6):1148–1161. doi: 10.1016/j.immuni.2018.09.024. Dec 18.e7Epub 2018 Dec 11. Erratum in: Immunity. 2022 Sep 13;55(9):1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fisher D.T., Appenheimer M.M., Evans S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014;26(1):38–47. doi: 10.1016/j.smim.2014.01.008. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laino A.S., Woods D., Vassallo M., Qian X., Tang H., Wind-Rotolo M., Weber J. Serum interleukin-6 and C-reactive protein are associated with survival in melanoma patients receiving immune checkpoint inhibition. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2020-000842. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi N., Lee K.A., Bermudez M.V., Visconti A., Thomas A.M., Bolte L.A., Björk J.R., de Ruijter L.K., Newton-Bishop J., Harland M., Shaw H.M., Harries M., Sacco J., Board R., Lorigan P., de Vries E.G.E., Segata N., Taams L., Papa S., Spector T.D., Nathan P., Weersma R.K., Hospers G.A.P., Fehrmann R.S.N., Bataille V., Falchi M. Circulating inflammatory proteins associate with response to immune checkpoint inhibition therapy in patients with advanced melanoma. eBioMedicine. 2022;83 doi: 10.1016/j.ebiom.2022.104235. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David J.M., Dominguez C., Hamilton D.H., Palena C. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines. 2016;4(3):22. doi: 10.3390/vaccines4030022. (Basel)Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agulló-Ortuño M.T., Gómez-Martín Ó., Ponce S., Iglesias L., Ojeda L., Ferrer I., García-Ruiz I., Paz-Ares L., Pardo-Marqués V. Blood predictive biomarkers for patients with non-small-cell lung cancer associated with clinical response to Nivolumab. Clin. Lung Cancer. 2020;21(1):75–85. doi: 10.1016/j.cllc.2019.08.006. Jan. [DOI] [PubMed] [Google Scholar]
- 34.Sanmamed M.F., Perez-Gracia J.L., Schalper K.A., Fusco J.P., Gonzalez A., Rodriguez-Ruiz M.E., Oñate C., Perez G., Alfaro C., Martín-Algarra S., Andueza M.P., Gurpide A., Morgado M., Wang J., Bacchiocchi A., Halaban R., Kluger H., Chen L., Sznol M., Melero I. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. 2017;28(8):1988–1995. doi: 10.1093/annonc/mdx190. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlini V., Noonan D.M., Abdalalem E., Goletti D., Sansone C., Calabrone L., Albini A. The multifaceted nature of IL-10: regulation, role in immunological homeostasis and its relevance to cancer, COVID-19 and post-COVID conditions. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1161067. Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dennis K.L., Blatner N.R., Gounari F., Khazaie K. Current status of interleukin-10 and regulatory T-cells in cancer. Curr. Opin. Oncol. 2013;25(6):637–645. doi: 10.1097/CCO.0000000000000006. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirlekar B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: its implications in cancer immunotherapy. SAGE Open Med. 2022;10:1–15. doi: 10.1177/20503121211069012. 20503121211069012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamichhane P., Karyampudi L., Shreeder B., Krempski J., Bahr D., Daum J., Kalli K.R., Goode E.L., Block M.S., Cannon M.J., et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res. 2017;77:6667–6678. doi: 10.1158/0008-5472.CAN-17-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y., Liu X., Du J., Zhang D., Liu J., Chen M., Zhao J., Zhong W., Xu Y., Wang M. Circulating cytokines associated with clinical outcomes in advanced non-small cell lung cancer patients who received chemoimmunotherapy. Thorac. Cancer. 2022;13(2):219–227. doi: 10.1111/1759-7714.14248. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyan K., Baginska J., Brainard M., Giobbie-Hurder A., Severgnini M., Manos M., Haq R., Buchbinder E.I., Ott P.A., Hodi F.S., Rahma O.E. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021;70(8):2209–2221. doi: 10.1007/s00262-021-02855-1. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chennamadhavuni A., Abushahin L., Jin N., Presley C.J., Manne A. Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.779691. Apr 26. [DOI] [PMC free article] [PubMed] [Google Scholar]