Abstract
Myosin-7a is an actin-based motor protein essential for vision and hearing. Mutations of myosin-7a cause type 1 Usher syndrome, the most common and severe form of deafblindness in humans. The molecular mechanisms that govern its mechanochemistry remain poorly understood, primarily because of the difficulty of purifying stable intact protein. Here, we recombinantly produce the complete human myosin-7a holoenzyme in insect cells and characterize its biochemical and motile properties. Unlike the Drosophila ortholog that primarily associates with calmodulin (CaM), we found that human myosin-7a utilizes a unique combination of light chains including regulatory light chain, CaM, and CaM-like protein 4. Our results further reveal that CaM-like protein 4 does not function as a Ca2+ sensor but plays a crucial role in maintaining the lever arm’s structural–functional integrity. Using our recombinant protein system, we purified two myosin-7a splicing isoforms that have been shown to be differentially expressed along the cochlear tonotopic axis. We show that they possess distinct mechanoenzymatic properties despite differing by only 11 amino acids at their N termini. Using single-molecule in vitro motility assays, we demonstrate that human myosin-7a exists as an autoinhibited monomer and can move processively along actin when artificially dimerized or bound to cargo adaptor proteins. These results suggest that myosin-7a can serve multiple roles in sensory systems such as acting as a transporter or an anchor/force sensor. Furthermore, our research highlights that human myosin-7a has evolved unique regulatory elements that enable precise tuning of its mechanical properties suitable for mammalian auditory functions.
Keywords: myosin, myosin regulation, calcium, ATPase, in vitro motility
The myosin superfamily plays a fundamental role in many forms of cellular motility and cytoskeletal organization (1). Myosin-7a is expressed in actin-rich membrane protrusions of the inner ear and retina. More specifically, it is found in the hair bundles and synapses of the inner ear hair cells, in photoreceptors, and in the retinal pigment epithelium. In mammals, defects in myosin-7a lead to deafness and retinal degeneration known as the type 1B Usher syndrome (2, 3, 4).
The full-length myosin-7a consists of a motor domain, followed by a neck region containing 5 IQ motifs, which serves as binding sites for calmodulin (CaM) family member light chains. Its tail contains two MyTH4-FERM (myosin tail homology; band 4.1/ezrin/radexin/moesin homology) domains separated by an SH3 domain (5).
Unconventional myosins are known to bind CaM, CaM-like proteins, regulatory light chains (RLCs), or essential light chains (ELCs) (6). To date, myosin-7a has been coexpressed with CaM only (7). Previous research has demonstrated the potential of the human myosin-7a IQ motifs to bind RLC and ELC, but no in-depth analysis has been carried out to describe the precise light chain composition (8). The possibility of myosin-7a binding to other light chains was raised by the recent identification of CaM-like protein 4 (CALML4) as the endogenous light chain for myosin-7b (9, 10).
Recent studies reveal that the cochlea expresses two myosin-7a isoforms, differing by a short N-terminal extension (11). The canonical long isoform, with an 11 amino acid extension, is predominantly expressed in the inner hair cells, whereas in outer hair cells (OHCs), its expression varies in a tonotopic manner. Its level increases from the base to the apex of the cochlea. The expression pattern of the short isoform, which lacks the 11 amino acid N-terminal extension, is expected to inversely correlate to the canonical myosin-7a (3, 11).
Several studies have demonstrated that the short putative coiled-coil sequence found in the tail of myosin-7a is insufficient to form a dimer. Instead, it is predicted to form a stable single alpha-helix known as the SAH domain (8, 12). Dimerization can potentially be mediated by binding partners. One such binding partner, MyRIP (myosin and Rab interacting protein, also known as exophilin-8/Slac-2c), has been shown to promote movement of myosin-7a in the cellular environment. MyRIP functions as a linker between Rab27a and myosin-7a, and the resulting tripartite complex is hypothesized to be involved in melanosome transport in the retinal pigment epithelium (13, 14).
In this study, we show that a combination of intramolecular and intermolecular regulatory mechanisms are employed to tune the conformation, activity, and oligomeric state of human myosin-7a. Alternative splicing in the motor domain creates variants with distinct kinetic properties, allowing the mechanical output to be controlled by varying the proportion of fast and slow isoforms. Myosin-7a remains monomeric and therefore unprocessive in isolation. In contrast, it makes long duration processive runs on actin in the presence of binding partners such as MyRIP.
Results
Production of full-length human myosin-7a holoenzyme
In our efforts to recombinantly produce a human myosin-7a from baculovirus-infected Sf9 cells, we first attempted to coexpress the full-length myosin-7a (isoform 1, NM_000260.4) with CaM. This approach did not produce soluble intact protein, and instead, we observed the presence of many proteolytic fragments following chromatography over a FLAG-affinity column. Previous studies showed that truncated myosin-7a IQ motifs can bind to RLC and ELC (8). In addition, a small EF-hand protein CALML4 has recently been discovered as a critical light chain for myosin-7b in mammalian gut brush border, and it can interact with myosin-7a in pull-down assays (9, 15). Intriguingly, CALML4 is also identified as a candidate gene for Usher syndrome (USH1H) (16). In view of these observations, we hypothesized that multiple light chain components may be required for producing intact human myosin-7a protein.
To determine the exact light chain composition of the holoenzyme, we coexpressed full-length myosin-7a heavy chain with baculoviruses encoding RLC, ELC, CaM, and CALML4. We selected the short isoform of CALML4 for our studies as it was shown to be the dominant variant in the tissues (gut epithelium) analyzed to date (9). Furthermore, we included a baculovirus expressing both human UNC45B and human HSP90AA1 (Fig. S1A), as previous studies have shown that these chaperones can facilitate the folding of myosin-15, a close family member of myosin-7a found in hair cell stereocilia (17, 18). Using these methods, we were able to observe protein bands consistent with the size of full-length myosin-7a in elution fractions from anti-FLAG resin. Mass spectrometric analyses identified that RLC, CaM, and CALML4 were copurified with myosin-7a but not ELC.
To further optimize the production, we adopted the MultiBac system (19) to coexpress myosin-7a heavy chain, RLC, CaM, and CALML4 via a single baculovirus backbone along with the chaperone-containing baculovirus. This approach ultimately resulted in high-yield and purified myosin-7a holoenzyme, with the heavy chain and three types of light chains clearly visible on the gel (Fig. 1, A and B). CaM and CALML4 have very similar molecular weights but were resolved on 16% SDS gels. The presence of CALML4 was further confirmed by immunoblotting (Fig. S1B).
Figure 1.
Purified full-length human myosin-7a requires multiple light chains and adopts the autoinhibited conformation at physiological conditions.A, domain organizations of human myosin-7a. (Schematics are in scale with the length of their primary sequences). B, SDS-PAGE analysis of purified human myosin-7a proteins. Lane 1: molecular mass marker (Mark12; Invitrogen); lane 2, FLAG-affinity purified myosin-7a-S1 consisting of an 86 kDa heavy chain and 20 kDa RLC light chain; lane 3, FLAG-affinity purified myosin-7a-FL consisting of a 255 kDa heavy chain, 20 kDa RLC, and 17 kDa calmodulin and CALML4 light chains. C and D, single-molecule mass photometry assays. Histograms of the molecular weights of individual particles with a bin width of 5 kDa for the particular protein (C: myosin-7a motor + 1IQ, D: myosin-7a-FL). Lines are the Gaussian fit to the data yielding the molecular weight of the samples. E and F, class averages from negative-stain electron microscopy images of myosin-7a in (E) in 150 mM salt buffer and (F) in 500 mM salt buffer in the presence of ATP. Myosin-7a in low salt conditions exhibits a compact autoinhibited conformation, whereas in high salt conditions, the myosin exhibits a more extended conformation. Scale bars represent 10 nm. G, actin activated ATPase activity of subfragment 1 (pink triangle) (vmax = 1.5 ± 0.1 s−1, KATPase = 11 ± 2.6 μM) and full-length myosin-7a in the absence (blue circle) (vmax = 0.2 ± 0.11 s−1, KATPase = 54 ± 4.5 μM) and in the presence of calcium (black square) (vmax = 0.98 ± 0.27 s−1, KATPase = 27 ± 7 μM). Values are mean ± SD from four independent experiments. CALML4, calmodulin-like protein 4; RLC, regulatory light chain.
Coexpression of myosin-7a subfragment-1 (M7a-S1) containing only the motor domain and the first IQ motif with multiple light chain candidates revealed that only RLC copurified with the heavy chain. This observation suggests that the first IQ motif preferentially binds to RLC (Fig. 1B).
Human myosin-7a is monomeric and adopts an evolutionarily conserved autoinhibitory conformation
To gain insight into the biophysical properties of human myosin-7a, we first utilized mass photometry to determine its oligomeric states. Mass photometry analysis of M7a-S1 showed a single peak at the mass of 103 kDa, consistent with the molecular weight of one M7a-S1 (∼89 kDa) plus one light chain (RLC, ∼20 kDa) (Fig. 1C). The mass photometry profile of full-length myosin-7a resulted in a major mass peak at 325 kDa, which matches the molecular weight of one full-length myosin-7a heavy chain (∼255 kDa) plus four light chains (RLC: ∼20 kDa; CaM and CALML4: ∼17 kDa). It should be noted, however, that the mass photometry is unlikely to resolve multiple populations differing by the mass of one or two light chains. Hence, in scenarios where the myosin contains a mixture of light chain occupancies (e.g., four and five light chains), we are not able to resolve the exact proportion of each. Nevertheless, the peak of the distribution corresponds best to a lever with four light chains bound. The total mass also indicates that the full-length myosin is a monomer. The smaller peaks observed in the ∼50 to 150 kDa range were attributed to proteolytic fragments, which were also detected in SDS gels (Fig. 1B). No distinct peak was observed at a position that would correspond to dimer (or other multimers) (Fig. 1D).
Using negative-stain electron microscopy, we observed a bent conformation of human myosin-7a at low ionic strength, in which the tail region folds back to contact the motor domain (Fig. 1E). The size of the particles was consistent with a monomeric myosin as predicted by the mass photometry. The contour from the probable motor domain-lever junction to the bend point measures 20.7 ± 1.9 nm. This is consistent with the expected length of the lever with 5IQ motifs (∼19 nm) (20). Since the SAH domain is expected to add an additional ∼9 nm to the lever arm, we conclude that the SAH domain does not contribute to the length of the structure leading up to the bend but rather forms part of the return leg, along with the rest of the tail domain motifs. The results from mass photometry suggest weak occupancy at one of the IQ domains (Fig. 1D). One possibility is that lack of the light chain at the fifth IQ can contribute to the bending of the tail (Fig. S2). Indeed, it is known that the fifth IQ-SAH region can undergo changes in CaM binding depending on Ca2+ concentration (20). However, previous studies have relied on only partial lever fragments in which the context of other light chains is lost. Higher resolution structures of the intact molecule would be needed to further determine this.
The autoinhibition of Drosophila myosin-7a was shown to be regulated via electrostatic interactions facilitated by charged amino acids within the second FERM domain (21). Increased salt concentration also led to an open conformation of human myosin-7a, suggesting an evolutionarily conserved autoinhibitory mechanism (Fig. 1F). The interaction of the tail with the motor domain results in suppression of the enzymatic function. Actin-activated steady-state activity was measured by a NADH-coupled assay. The maximum extrapolated ATPase rate (vmax) of the full-length protein was 0.2 ± 0.11 s−1 with an apparent actin affinity (KATPase) of 54 ± 4.5 μM. In contrast, S1 that lacks the tail domain exhibited a ∼7.5-fold higher maximum ATPase rate at 1.5 ± 0.1 s−1 and ∼5-fold higher actin affinity at 11 ± 2.6 μM (Fig. 1G). The autoinhibition can be alleviated by calcium ions, and following the addition of calcium, the maximum extrapolated ATPase rate of myosin-7a-FL increased to 0.98 ± 0.27 s−1 (Fig. 1G, Table 1).
Table 1.
Steady-state ATPase activity of myosin-7a constructs
| Parameter | FL | FL (+Ca2+) | S1 |
|---|---|---|---|
| vmax | 0.2 ± 0.11 s−1 | 0.98 ± 0.27 s−1 | 1.5 ± 0.1 s−1 |
| KATPase | 54 ± 4.5 μM | 27 ± 7 μM | 11 ± 2.6 μM |
Abbreviation: FL, full length.
CALML4 and CaM display distinct Ca2+-regulation properties
Calcium influences many CaM-like and other EF-hand containing proteins via their calcium-binding sites (22). CaM and CALML4 are 44% identical, allowing their sequences to be aligned and any putative calcium-binding sites examined. When the amino acid sequence of CALML4 is compared with that of CaM, it is evident that the crucial amino acids required for Ca2+ chelation are absent in each of the EF-hands of CALML4 (Fig. 2A). The presence of some conserved amino acids in the fourth EF-hand suggests it might retain the ability to bind calcium. We therefore performed an electrophoretic mobility shift assay using purified CaM and CALML4 to assess CALML4’s calcium-binding capability. CaM exhibited altered electrophoretic mobility upon incubation with Ca2+ because of calcium-dependent conformational changes that are known to be detectable in SDS-PAGE as increased mobility (23). In contrast, under the same conditions, the mobility of the CALML4 samples remained unchanged following addition of calcium (Fig. 2B). Consistently, microscale thermophoresis (MST) studies show that while CaM binds to Ca2+ in the tested concentration range, CALML4 does not bind with calcium under the same conditions (Fig. 2C) (Fig. S3). These findings collectively suggest that CALML4 is an EF-hand protein that has lost the ability to bind to Ca2+.
Figure 2.
The molecular details of light chain binding to myosin-7a reveala Ca2+-dependent regulatory mechanism.A, amino acid comparison of human calmodulin and CALML4. Amino acids critical for calcium binding are highlighted. B, electrophoretic mobility shift assay of purified human calmodulin and CALML4. Samples were incubated in the presence of CaCl2 or EGTA and then run on 16% Tris–glycine gel. C, normalized fluorescence plotted against calcium concentration of CALML4 (light blue, circles) and calmodulin (dark blue, squares). D, SDS-PAGE analysis of purified myosin-7a 5IQ S1 constructs coexpressed with indicated light chains. Red arrow: endogenous Sf9 calmodulin. E, interferometric single-molecule mass photometry assay demonstrating the effect of calcium on full-length myosin-7a proteins. The samples were incubated in the presence of CaCl2 (black) or EGTA (blue). Histograms show the molecular weights of individual particles with a bin width of 5 kDa. Lines are the Gaussian fit to the data yielding the molecular weight of the samples. F, in vitro motility actin gliding assay of full-length long N-terminal myosin-7a in the presence (black square) (v = 6.7 ± 3.7 nm/s) and the absence (blue circle) (v = 22.1 ± 2.3 nm/s) of calcium. G, graphical representation of myosin-7a during mechanoelectrical transduction. The increase in intracellular calcium level initiates light chain dissociation from the heavy chain, which results in releasing the tension as the rigidity of the neck region changes. CALML4, calmodulin-like protein 4.
Interestingly, despite its inability to respond to Ca2+ signals, CALML4 appears to be crucial for maintaining the structural–functional integrity of human myosin-7a. In our initial purification attempts, we found that the yield and integrity of full-length myosin-7a was significantly improved when coexpressed with CALML4. Furthermore, we discovered that CaM copurified with our 5IQ S1 constructs only when CALML4 was present, implying that CALML4 may stabilize the interaction of the light chain–binding region with CaM (Fig. 2D). This hypothesis is supported by the recruitment of endogenous Sf9 CaM by CALML4 as detected on SDS-PAGE after purification when no CaM was coexpressed (Fig. 2D, arrow).
Light chain binding of myosin-7a is calcium dependent
Using mass photometry, we were also able to visualize effects of calcium on the subunit composition of the purified holoenzyme. In EGTA conditions, the full-length myosin-7a construct exhibited a major mass of 325 ± 21 kDa, corresponding to one heavy chain and four light chains (Fig. 1D). Upon the addition of calcium, we observed a shift toward a smaller mass peak at 293 ± 25 kDa, which corresponds to one heavy chain plus an extra 38 kDa. Given that RLC and CALML4 are inert to calcium, we attribute this 38 kDa mass to the combined presence of one RLC and one CALM4 (Fig. 2E). The mass photometer does not detect proteins less than 25 kDa, and thus, any dissociated free light chains in this experiment are not detected.
We carried out in vitro actin gliding assays to examine the impact of this calcium-induced light chain detachment on the myosin's mechanical activity. The full-length myosin-7a is in an “open” conformation when attached to the surface as the autoinhibition is disrupted, hence it is able to glide actin. Surface-bound myosin moved the fluorescently labeled actin at 22.1 ± 2.3 nm/s. However, in the presence of calcium, the gliding velocity decreased to 6.7 ± 3.7 nm/s (Fig. 2F), suggesting that the myosin’s motility is compromised as a result of CaM dissociation from the holoenzyme even though free CaM was present in the assay.
The short N-terminal extension of myosin-7a influences the motor activity
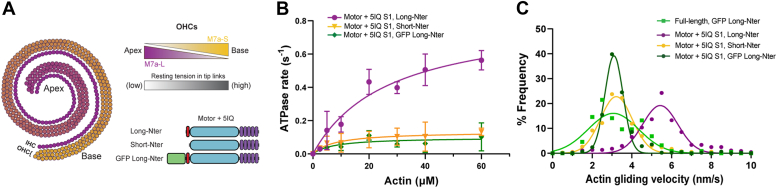
Two isoforms of myosin-7a, termed long and short, are expressed in mammalian cochlea. They are generated via alternative splicing with varying translation start sites and only differ by 11 amino acids at the N terminus preceding the motor domain (Fig. 3A). Myosin-7a is believed to directly control the tip link resting tension and thereby regulate the open probability of the mechanoelectrical channel (11). Intriguingly, recent studies on hair bundle mechanics have revealed a tonotopic variation in the tip-link tension of OHCs, wherein the tension gradually increases toward the base (24) (Fig. 3A). These observations collectively suggest that the two myosin-7a isoforms may have distinct mechanochemistry that contributes to the tonotopic gradients of the tip link tension.
Figure 3.
The short N-terminal extension of myosin-7a regulates the myosin enzymatic activity.A, graphical illustration of the expression pattern of the two different myosin-7a isoforms in the outer hair cells of the cochlea (11). As the expression of the short isoform increases, so does the tension within the hair cells stereocilia (24). Domain organizations of myosin-7a-5IQ S1 constructs used in the experiments described in B and C. B, steady-state ATPase assay of myosin-7a-5IQ S1 constructs yielding the kinetic parameters: long N-terminal (purple) isoform vmax = 0.83 ± 0.14 s−1, KATPase = 27 ± 5 μM; short N-terminal (yellow) isoform vmax = 0.13 ± 0.06 s−1, KATPase = 8 ± 1.1 μM; GFP-tagged long N-terminal (green) isoform vmax = 0.1 ± 0.07 s−1, KATPase = 8 ± 5.5 μM. Values plotted are mean ± SD from four independent experiments. C, in vitro motility actin gliding assay of different motor + 5IQ S1 myosin-7a yielding the kinetic parameters: long N-terminal motor + 5IQ S1 (purple) isoform v = 5.4 ± 0.9 nm/s; short N-terminal motor + 5IQ S1 (yellow) isoform v = 3 ± 0.9 nm/s; GFP-tagged long N-terminal motor + 5IQ S1 (green) isoform v = 3.1 ± 0.5 nm/s; GFP-tagged long N-terminal full-length (light green) isoform v = 3.1 ± 1.3 nm/s.
To explore this hypothesis further, we purified the two isoforms and examined the role of the N-terminal extension in the function of myosin-7a. In addition, the common use of an N-terminal GFP tag on myosin-7a (8, 25, 26, 27) raises questions about its potential impact on the regulation by the N-terminal extension. We therefore also engineered a long N-terminal construct that includes a GFP moiety before the N terminus (Fig. 3A). In addition to the full-length proteins, we engineered constructs containing the motor domain and the 5IQ motifs (M7a-5IQ S1) but no tail to avoid the complication of tail-dependent autoinhibition (Fig. 3A).
Steady-state ATPase assays reveal that the long N-terminal 5IQ S1 isoform has a maximal actin-activated ATPase activity of approximately 0.83 ± 0.14 s−1, which is comparable to the activity of the mammalian myosin-7a motor, as measured both in our studies (Fig. 1G, Table 2) and in previous work conducted by other groups (7, 28). In contrast, we observed a significant reduction in ATPase activity (∼0.1 s−1) in the absence of the N-terminal extension (0.13 ± 0.06 s−1) (Fig. 3B, Table 2). In vitro actin gliding assays show that the long N-terminal isoform moves actin filaments at a speed of approximately 22 and 5 nm/s for the full-length and 5IQ S1 constructs, respectively (Figs. 2E and 3C). We reason that the faster actin movement produced by the full-length molecule is in part because of the presence of the SAH domain, which results in a longer lever arm, and therefore longer step-size, compared with the 5IQ S1 constructs (20). The slower motility rates for the 5IQ S1 fragment likely are due in part to its attachment to the nitrocellulose surface, which may compromise the length of its power stroke. It has been well known that full-length myosin and longer myosin-2 fragments such as HMM move actin filaments faster than S1 (29). For the 5IQ S1 fragment, the lack of SAH domain added to the fact that some of the lever is bound to the surface, resulting in compromised motility rates. The short N-terminal isoform exhibited slower gliding speed (∼3 nm/s) (Fig. 3C).
Table 2.
Steady-state ATPase activity and actin gliding velocity of human myosin-7a-5IQ S1 constructs
| Parameter | Long N-terminal | Short N-terminal | GFP-Long N-terminal |
|---|---|---|---|
| vmax | 0.83 ± 0.14 s−1 | 0.13 ± 0.06 s−1 | 0.1 ± 0.07 s−1 |
| Km | 27 ± 5 μM | 8 ± 1.1 μM | 8 ± 5.8 μM |
| Actin gliding velocity | 5.4 ± 0.9 nm/s | 3 ± 0.9 nm/s | 3.1 ± 0.5 nm/s |
Interestingly, the GFP-tagged long 5IQ S1 isoform also had a significant reduction in ATPase activity (0.1 ± 0.07 s−1) (Table 2) and exhibited gliding speed (∼3 nm/s) similar to the short 5IQ S1 isoform. The N-terminal GFP fusion of the full-length myosin-7a caused a similar reduction in gliding speed compared with that of the untagged version (Fig. 3C).
Human myosin-7a moves processively along actin upon dimerization
Drosophila myosin-7a has been shown to dimerize in a cargo-dependent manner and move processively along actin filaments (30). To investigate whether this mechanism is conserved in the human homolog, we engineered a construct to induce artificial dimerization by adding a leucine zipper motif to the myosin-7a-5IQ + SAH sequence (Fig. 4A). This construct also contains an N-terminal GFP tag for single-molecule imaging. A major mass species of approximately 406 kDa was detected by mass photometry, matching the sum of two heavy chains (141 kDa each) plus six to eight light chains (Fig. 4B). Using the single-molecule in vitro motility assay, where actin filaments bound to the coverslip surface serve as tracks for the motors, we were able to observe the processive movements of individual fluorescently labeled myosin molecules (Fig. 4C). In the presence of 150 mM NaCl and ATP, artificially dimerized myosin-7a moved slowly (4.3 ± 1.5 nm/s) along the surface-bound actin filaments (Fig. 4, D and E), with a characteristic run length of 378 nm (Fig. 4F) and duration time of 137 s (Fig. 4G).
Figure 4.
Human myosin-7a is a processive motor when dimerized.A, domain organization of human GFP-myosin-7a-S1-SAH-Zipper with an N-terminal GFP moiety. B, single-molecule mass photometry assay of GFP-myosin-7a-S1-SAH-Zipper proteins. Histograms show the molecular weights of individual particles with a bin width of 5 kDa. Lines are the Gaussian fit to the data yielding the molecular weight of the samples. C, a single time frame of GFP-myosin-7a-S1SAH-Zipper on immobilized actin filaments (labeled with rhodamine phalloidin, red). Scale bar represents 5 μm. D, kymograph analysis showing the processive movements (diagonal lines) of GFP-Myosin-7a-S1SAH-Zipper. E–G, frequency distribution histograms of the velocity (4.3 ± 1.5 nm/s) (E), characteristic run length (378 nm) (F), and characteristic run duration (137 s) (G), respectively, for the GFP-myosin-7a-S1SAH-Zipper. The solid line shows the best fit to a Gaussian or a single exponential equation as appropriate. H, a single time frame of myosin-7a-FL and mCherry-MyRIP (red) on immobilized actin filaments (labeled with Alexa-Flour 647, blue). Myosin-7a is visualized by GFP-RLC. Scale bar represents 5 μm. I, kymograph analysis reveals diagonal lines of GFP-RLC and mCherry-MyRIP are overlaid. J–L, frequency distribution histograms of the velocity (7.8 ± 4.2 nm/s) (J), characteristic run length (552 nm) (K), and characteristic run duration (113 s) (L), respectively, for the myosin-7a-FL. The solid line shows the best fit to a Gaussian or a single exponential equation. MyRIP, myosin and Rab interacting protein; RLC, regulatory light chain; SAH, SAH, stable single alpha-helix.
We next tested binding protein–mediated processivity in human myosin-7a. MyRIP, a known myosin-7a binding partner, has been shown to induce processivity of myosin-7a when the two proteins are cotransfected in cells (25). We expressed and purified mCherry-tagged MyRIP in Sf9 cells (Fig. S4). To characterize the motility of the motor–adaptor complex, we performed single-molecule in vitro assays using equimolar concentration of purified mCherry-MyRIP preincubated with full-length myosin-7a that had no tag at the N terminus. A GFP tag was fused to RLC to visualize myosin-7a under total internal reflection fluorescence (TIRF) microscopy. We observed processive movement of myosin-7a–MyRIP complex (Fig. 4H). Simultaneous two-color imaging reveals that MyRIP and myosin-7a comigrate (Fig. 4I). The complex moves slowly on actin filaments (7.8 ± 4.2 nm/s) (Fig. 4J), with a characteristic run length of 552 nm (Fig. 4K) and average time of attachment of 113 s (Fig. 4L).
Discussion
The regulation of myosin-7a has been a focus of research for several years because of its importance in human sensory functions (2, 31). Despite this, many questions remain unanswered because of the challenges in obtaining a high-quality holoenzyme. The present study demonstrates a method for purifying intact human myosin-7a. We show that mammalian myosin-7a has evolved multiple unique regulatory elements, enabling a stringent control of its mechanical function. We propose that this adaptation allows myosin-7a to fulfill its varied roles in cochlear hair cells, such as cargo transport and dynamic gating of mechanoelectrical channels.
The light chain composition and calcium regulation of human myosin-7a
Using the baculovirus/Sf9 system, we demonstrate that human myosin-7a copurifies with RLC, CaM, and CALML4. Although copurification does not directly confirm their roles as native light chains, it is worth noting that RLC, CaM, and CALML4 have all been identified in hair cell stereocilia according to previous mass spectrometry (MS) analyses (32, 33). Our results further demonstrate that the first IQ motif of myosin-7a preferentially binds to RLC. This finding is reminiscent of a previous report on myosin-15, another stereociliar MyTH4-FERM myosin, which was also shown to bind RLC at its first IQ motif (17). Earlier studies using purified IQ segments showed that the third IQ motif of myosin-7a does not bind to either CaM or RLC (8). Recently, Choi et al. (http://rave.ohiolink.edu/etdc/view?acc_num=toledo1532947508319487) used pull-down experiments to show that CALML4 only associated with the neck region when the third IQ motif was present. Our mass photometry results suggest that in the presence of calcium, while CaM dissociates, myosin-7a maintains its stable association with one RLC and one CALML4. Taken together, we propose a model for the spatial configuration of the light chains along the lever arm: RLC and CALML4 binds to the first and third IQ motifs of myosin-7a, respectively, independent of environmental Ca2+ concentrations. The remaining IQs motifs, on the other hand, are able to dynamically interact with CaM in a calcium-dependent manner, potentially serving as regulatory elements that modulate the mechanoactivity of myosin-7a in response to Ca2+ signaling (Fig. 2G). The third IQ of myosin-7a across a wide range of species (Fig. S5) is somewhat divergent from the classical IQxxxRGxxxR with the glycine and second arginine being absent. How this relates to the specificity of light chain binding is currently unclear, but it should be noted that this divergence likely occurred prior to the emergence of CALML4 in evolution (Fig. S5).
Previous structural studies on the CaM and IQ5-SAH complex show that increased Ca2+ concentration causes CaM to orientate toward the N terminus, resulting in a lever arm that is less rigid, as evidenced by increased structural flexibility of the IQ5-SAH segment (20). It was also shown that the Ca2+-bound CaM does not dissociate but instead partially binds to the IQ4 region. The authors propose that the retained Ca2+-bound CaM may play a role in maintaining the lever arm to be partially structured, thereby enabling a quicker restoration of motor function when Ca2+ concentrations return to resting levels (20). In line with this, our in vitro motility assays demonstrate that elevated Ca2+ concentration hampers the motor’s ability to move actin filaments, indicating a compromised mechanical function. Intriguingly, we found that the reduced motor function is accompanied by dissociation of CaM. We reason that this observed difference may be due to the fact that our protein contains an intact neck region, which allows detection of light chain dissociation potentially triggered by the translocation. We further propose that the Ca2+-insensitive CALML4 may serve to maintain the lever arm’s structural integrity when cellular Ca2+ concentration rises. This hypothesis is supported by the observation that in the copurification experiments, the association of CaM with myosin-7a heavy chain is dependent on the presence of CALML4.
Regulation of the mechanoenzymatic activity by the N-terminal extension
Recent studies reveal that mammalian cochlea expresses two myosin-7a splice isoforms, produced by alternative translation starting sites. Analysis of isoform-specific transgenic mouse models suggests that the two isoforms are expressed in opposing gradients along the tonotopic axis in OHCs, with the short variant being dominant at the cochlear base (11). Using purified proteins, we demonstrate that the short isoform possesses significantly slower mechanoenzymatic activity compared with the long isoform, characterized by an approximately eightfold decrease in both steady-state ATPase activity and actin gliding motility. These findings align with recent mechanical studies on hair cells, showing that the resting tension of single tip links also follows the tonotopic gradient, progressively increasing toward the base (24). We speculate that the slow ATP turnover of the short isoform may allow this variant to generate higher tension on the tip links at the base at a lower energy cost. To test this hypothesis, further kinetic studies will be needed to determine the parameters of kinetic intermediates within the actomyosin ATPase cycle of these isoforms. Furthermore, considering that their expression patterns correlate with the gradient of the tip link tension, we propose that OHCs may regulate their mechanosensitivity by adjusting the expression levels of the two myosin-7a isoforms. Examining the effect of mechanical load in optical trapping experiments could test this hypothesis.
N-terminal alternative splicing has been observed in multiple myosin classes as a mechanism to establish kinetic and functional diversity (34, 35, 36). Furthermore, it has been demonstrated that N-terminal extension prior to the motor domain can influence myosin motor activity (34, 37). One well-studied example is myosin-1b. Its N-terminal extension was shown to position near the converter-lever arm junction and can impact the myosin’s force sensitivity and mechanical output (37, 38, 39). The structural basis for the regulation of the N-terminal extension in human myosin-7a is currently unknown. However, our findings suggest that this N-terminal segment plays a crucial role in accelerating the mechanoenzymatic activity of myosin-7a. When a GFP moiety is added prior to the N-terminal extension, the long isoform displayed kinetic and motile behavior similar to that of the short isoform, suggesting a potential disruption of the normal configuration and function of the N-terminal extension. These findings also suggest that caution should be exercised in the use of N-terminal-tagged GFP-myosin-7a in cellular studies. It is worth noting that this N-terminal extension splicing mechanism appears to be specific to mammals and does not exist in the Drosophila ortholog. Instead, Drosophila myosin-7a incorporates an SH3 domain prior to the motor domain (40), and the presence of an N-terminal tag does not seem to affect its mechanoenzymatic activity (30).
The processive motility of human myosin-7a
Myosin-7a is proposed to participate in cargo transport within hair cell stereocilia, in addition to maintaining the resting tension of the tip link (3, 41). We show that purified intact human myosin-7a is monomeric, adopting an evolutionarily conserved autoinhibited conformation (21). This indicates that for myosin-7a to move processively, dimerization or oligomerization is required. By adding a leucine zipper motif to the end of the lever arm, we demonstrate that dimerized myosin-7a can move processively along actin filaments. It is interesting to note that this construct contains an N-terminal GFP tag, which we show can alter the motor’s enzymatic activity into short-isoform like. The observed processive motility indicates that the short isoform may also be able to transport cargoes, albeit slowly. This notion is supported by the observation that in the long-isoform specific knockout mice, the hair bundle morphology developed normally, and the putative myosin-7a cargo proteins remained present in the stereocilia (11).
We further tested the mechanism of binding protein–mediated myosin-7a processivity. We examined MyRIP, a known binding partner for myosin-7a and Rab27a, and that has been shown to activate the myosin in vivo (25). We show that MyRIP can bind to human myosin-7a as purified components, and the resulting motor–adaptor complex comigrates along actin filaments. While the exact mechanism of MyRIP-induced processivity is not the main focus of this study, our previous work has extensively characterized its Drosophila homolog M7BP and provided detailed mechanistic insights into its role in mediating myosin-7a processivity (Table S1) (30). Together, our results support the notion that human myosin-7a can move processively along actin upon dimerization and potentially serve as a cargo transporter in living cells.
Functional mechanisms of myosin-7a in mammalian cochlea
In summary, this work uncovers several regulatory mechanisms that appear to be unique for mammalian myosin-7a and its roles in the Organ of Corti. Our findings collectively support a model in which myosin-7a localizes to the tip ends of stereocilia through its processive motility and regulates the tip link’s tension and hair cell mechanosensitivity. We propose that multiple myosin-7a splicing isoforms, each with distinct mechanoenzymatic properties, are employed to establish characteristic tip-link tension within the cochlear tonotopic map. The successful production of intact human myosin-7a protein provides a valuable tool for future investigations into the molecular details of human vision and hearing loss caused by myosin-7a defects.
Experimental procedures
Cloning and optimization of myosin-7a expression
Full-length human myosin-7a heavy chain (NM_000260.4) with a C-terminal FLAG purification tag, RLC (NM_001144944.1), CaM (NM_001329922.1), and CALML4 (NM_033429.3) sequences were cloned separately into pACEBac1 (Geneva Biotech) vectors. InFusion (TakaraBio) was used to ligate the PCR product and the EcoRI digested vector backbone. The separate gene cassettes were digested with BstXI and I-CeuI and then assembled with a BstXI digested vector using T4 DNA ligase. Sf9 insect cells were coinfected with MultiBac baculovirus containing all four subunits of the myosin-7a holoenzyme and baculovirus expressing both human UNC45B and human HSP90AA1.
Purification of myosin-7a
Myosin-7a-FLAG recombinant protein was expressed in Sf9 insect cells, which were previously infected with the recombinant baculoviruses. The cells were centrifuged after 72 h expression, and the pellet was frozen in liquid nitrogen and stored at −80 °C until it was used. Every process of the purification was performed at 4 °C. The frozen pellet was thawed on ice, washed, and homogenized with buffer containing 10 mM MOPS, 500 mM NaCl, 10 mM MgCl2, 1 mM EGTA, 0.3 mM NaN3, 1 mM DTT, 0.1 mM PMSF, 4 μg/ml leupeptin, 5 mM ATP, Pierce protease inhibitor tablet (ThermoFisher) (pH 7.4). After sonication (with Misonix Xl2020 Ultra Sonic Processor, for 5 min, 5 s impulses with 3 s breaks, 60% amplitude), the cellular debris was removed by ultracentrifugation (48,000g, 30 min). Monoclonal anti-FLAG M2 resin (2 ml) (Sigma) was added to the supernatant and incubated for 1 h at 4 °C while shaking. After 1 h of gentle shaking, the resin was loaded into a column. The resin was then washed with 500 mM NaCl, 10 mM MOPS, 0.1 mM EGTA, 3 mM NaN3, 0.1 mM DTT, 0.1 mM PMSF, 10 mM MgCl2, 5 mM ATP, 4 μg/ml leupeptin (pH 7.4); followed by a washing with 10 mM MOPS, 0.1 mM EGTA, 3 mM NaN3, 0.1 mM PMSF, 4 μg/ml leupeptin (pH 7.4). Myosin-7a was eluted with a FLAG protein containing buffer (500 mM NaCl, 2.5 mM MOPS, 25 μM EGTA, 0.75 mM NaN3, 5 mg FLAG peptide [pH 7.4]). About 300 μl fractions was collected, then pooled, and dialyzed against a buffer containing 500 mM NaCl, 2 mM MgCl2, 0.1 mM EGTA, 10 mM MOPS, and 1 mM DTT (pH 7.4).
Purification of other proteins
MyRIP (NM_001284423.2) with a C-terminal FLAG purification was expressed in Sf9 insect cells. The protein purification was carried out as described previously. CaM was purified according to previously published protocols (36).
CALML4-His was cloned into pET15b (Addgene, Plasmid #26092) plasmid and then expressed in KRX (Promega) cells. The cells were inoculated into an overnight starter culture and then diluted into 1 liter of LB (KD Medical). The expression was induced with the addition of 1 mM IPTG. The cells were harvested after 10 h expression, and the pellet was frozen in liquid nitrogen and stored at −80 °C until it was used. The frozen pellet was thawed on ice, washed, and homogenized with buffer (2× PBS). After sonication (5 min, 5 s impulses with 3 s breaks, 60% amplitude), the cellular debris was removed by ultracentrifugation (48,000g, 1 h). NEBExpress Ni Resin was added to the supernatant and incubated for 1 h at 4 °C while shaking. After 1 h, the resin was loaded into a column and allowed it to sediment. It was washed with a buffer (2× PBS, 5 mM imidazole). CALML4-His was eluted with 500 mM imidazole (in 2× PBS). Tobacco etch virus protease (Sigma) was added (1:100 dilution), and the sample was dialyzed overnight. After overnight dialysis, the sample was loaded into NEBExpress Ni Resin containing column, allowed it to sediment, then collected the flow through, and dialyzed against a buffer containing 500 mM NaCl, 2 mM MgCl2, 0.1 mM EGTA, 10 mM MOPS, and 1 mM DTT (pH 7.4).
GFP-RLC was cloned into pFastBac1 plasmid and expressed in Sf9 insect cells. The cells were centrifuged after 72 h expression, and the pellet was frozen in liquid nitrogen and stored at −80 °C until it was used. Every process of the purification was performed at 4 °C. The frozen pellet was thawed on ice, washed, and homogenized with buffer containing 2 M urea, 25 mM Tris–HCl, 0.1 mM PMSF, 1 mM DTT, and 0.001% NaN3 (pH 7.5). In the rest of this paragraph, we refer to this buffer. The cellular debris was removed by ultracentrifugation (48,000g, 1 h). The supernatant was applied to Q-Sepharose column, washed with buffer containing 200 mM NaCl, and eluted with buffer containing 300 mM NaCl. The eluant was diluted fourfold into buffer to reduce the ionic strength and applied to a DEAE–Sepharose column (Sigma). After washing with buffer, protein was eluted using buffer containing a salt gradient from 0 to 300 mM NaCl. The eluant was precipitated using ammonium sulfate in 5% increments, and precipitates were resuspended in PBS, followed by dialysis against PBS (+5 mM DTT). The fractions were assessed by SDS-PAGE, and the purest was applied to a Superdex 200 Increase 10/300 GL column (Sigma). Flow through was collected, then SDS-PAGE was used to assess the purest fractions, and these were drop frozen in liquid nitrogen.
Actin gliding motility assay
Flow chambers were prepared using No. 1.5 glass coverslips and glass slides. The coverslips were covered with 1% nitrocellulose solution in amyl acetate and then fixed on a cleaned slide with double-sided tape. Full-length myosin-7a was bound to the coverslip in low salt conditions (∼0.2 mg/ml myosin in 150 mM NaCl, 20 mM MOPS, 5 mM MgCl2, 0.1 mM EGTA, pH 7.4). After thorough washing steps, rhodamine–phalloidin-labeled actin (20 nM) was flushed in the chamber. The actin motility was observed in the final assay buffer (150 mM NaCl, 20 mM MOPS, 5 mM MgCl2, 0.1 mM EGTA, 50 mM DTT, 5 mM ATP, 2.5 mg/ml glucose, 100 μg/ml glucose oxidase, 2 μM GFP-RLC, 2 μM CaM, 2 μM CALML4, and 100 μM CaCl2). Movies were collected on an inverted Nikon Eclipse Ti-E microscope with an H-TIRF module attachment, a CFI60 Apochromat TIRF 100× Oil Immersion Objective Lens (numerical aperture = 1.49, working distance = 0.12 mm), and an EMCCD camera (Andor iXon Ultra 888 EMCCD, 1024 × 1024 array, 13 μm pixel). The excitation light source was a Nikon LU-N4 Laser Unit equipped with four lasers (405, 488, 561, and 640 nm). Where required, movies were drift corrected using the ImageJ/FIJI plugin image stabilizer. Actin filament centroid positions were determined using a FIJI macro based on the ridge detector plugin. Detected centroid positions were converted to a new image with a circle representing each centroid, and the movement of these circles was then analyzed using the TrackMate plugin for ImageJ. TrackMate settings were LoG detector (estimated blob diameter = 1 μm, threshold = not set), initial threshold = not set, view = HyperStack Displayer, filters on spots = not set, tracker = simple LAP tracker (linking maximum distance = 0.2 μm, gap-closing maximum distance = 0.2 μm, gap-closing maximum frame gap = 2), and filters on tracks: number of spots in track (above 5). Raw data were obtained from the analysis tab of the TrackMate plugin and imported into Prism 7 (GraphPad Software, Inc) for analysis. Track mean line speed was used as the output velocity. Gaussian fits of the velocity histograms were used to determine average velocity ().
Single-molecule motility assay
Flow chambers were prepared using No. 1.5 glass coverslips and glass slides. The coverslips were repeatedly washed with 100% ethanol and distilled water and then thoroughly dried with a stream of filtered air and plasma cleaned (by Diener-Zepto Plasma Cleaner) for 3 min in argon (90%/ power). Biotin–PEG mixture (Laysan Bio) was added to the surface of the coverslip. After a 20 min incubation at 70 °C, the coverslips were rinsed thoroughly with distilled water and then fixed on a cleaned slide with double-sided tape. Biotin–phalloidin (ThermoFisher) actin (200 nM) was bound to the surface of the coverslip via NeutrAvidin (Fisher Scientific; 2 mg/ml). Myosin (∼0.01 mg/ml in 150 mM NaCl, 20 mM MOPS, 5 mM MgCl2, 0.1 mM EGTA, [pH 7.4]) was added. The motility was observed in the final assay buffer (150 mM NaCl, 20 mM MOPS, 5 mM MgCl2, 0.1 mM EGTA, 50 mM DTT, 5 mM ATP, 2.5 mg/ml glucose, 100 μg/ml glucose oxidase, 2 μM GFP-RLC, 2 μM CaM, and 2 μM CALML4).
Movies were collected as mentioned before. Where required, movies were drift corrected using the ImageJ/FIJI plugin Image Stabilizer. Processive runs were analyzed using the TrackMate plugin for ImageJ. TrackMate settings were LoG detector (estimated blob diameter = 0.5–0.7 μm, threshold = 40–100), initial threshold = not set, view = HyperStack Displayer, filters on spots = not set, tracker = simple LAP tracker (linking maximum distance = 0.2 μm, gap-closing maximum distance = 0.2 μm, gap-closing maximum frame gap = 2), filters on tracks: duration of track (above 30 s), and track displacement (above 0.4 μm). Raw data were obtained from the analysis tab of the TrackMate plugin and imported into Prism 7 for analysis. Track mean line speed was used as the output velocity. Gaussian fits of the velocity histograms were used to determine average velocity. Characteristic run lengths and run durations were determined via exponential fits of the corresponding histograms ().
Single-molecule mass photometry
Mass photometry is a novel bioanalytical tool that allows us to measure the molecular mass of individual biomolecules (42, 43). Refeyn One MP mass photometer was used to acquire single-molecule landing data. Microscope slides were cleaned consecutively with water, ethanol, and isopropanol and then dried using a clean stream of nitrogen. A silicone gasket was cleaned with water, dried using a clean stream of nitrogen, and then positioned on a cleaned microscope slide. About 20 nM of each sample or mixture was loaded into a single gasket. Data were collected for 1 min. All measurements were carried out at room temperature (∼24 °C). Images were processed using manufacturer supplied software (Refeyn). The conversion between molecular mass and contrast was calibrated with protein standards of known molecular weight by manufacturer supplied software (Refeyn) as it was described earlier (44). For each sample, a histogram of mass distribution was plotted and fitted with a Gaussian fit.
Steady-state ATPase assay
Steady-state ATPase activities were measured in SpectraMax ID3 microplate reader (Molecular Devices, Inc) at 37 °C in buffers containing 10 mM MOPS, 1 mM ATP, 50 mM NaCl, 2 mM MgCl2, 0.1 mM EGTA (pH 7.2). Data were collected with Softmax Pro 7.1 software (Molecular Devices) and plotted by Graphpad Prism 7.0. The buffers also contained an NADH-coupled ATP-regenerating system including 40 units/ml lactate dehydrogenase, 200 units/ml pyruvate kinase, 200 μM NADH, and 1 mM phosphoenolpyruvate. The rate of ATP hydrolysis was measured from the decrease in absorbance at 340 nm caused by the oxidation of NADH and calculated based on Michaelis–Menten kinetics ().
MS
This MS analysis was done by Poochon Scientific. Samples were processed for trypsin/lys-C digestion followed by LC/MS/MS analysis. The MS raw file was analyzed using Proteome Discoverer 2.5 (Thermo Fisher Scientific) against human protein sequences database and host Sf9 protein sequences database.
Electrophoretic mobility shift assay
Ca2+-dependent electrophoretic shift assay was performed using the method described previously (45) with some modifications. Purified CaM (1 μM) and CALML4 (1 μM) were incubated at room temperature for 20 min in the presence of 5 μM CaCl2 or 1 μM EGTA. The samples were then subjected to 16% SDS-PAGE, which was run with a constant current of 30 mA in Tris–glycine buffer for 2 h at room temperature.
Immunoblotting and antibodies
SDS-PAGE was used for protein separation followed by the semidry transferring to nitrocellulose membrane (Bio-Rad). After transferring, the membrane was blocked using LI-COR Blocking Buffer for an hour at room temperature. The primary antibody (anti-CALML4: 1:5000 dilution; ProteinTech, catalog no.: 15894 1 AP; anti-M7a: 1:1000 dilution; Santa Cruz Biotechnology, catalog no.: Sc-74516; and anti-UNC45B: 1:500 dilution, Invitrogen, catalog no.: PA5 114191) was incubated overnight at 4 °C. After thorough washing with PBS, the secondary antibody (antimouse: 1:10,000 dilution, LI-COR, catalog no.: D10901 11; anti-rabbit: 1:10,000 dilution, LI-COR, catalog no.: D11103 01) was incubated at room temperature for an hour. Imaging was done with Azure 600 (Azure Biosystems).
Negative-stain electron microscopy and image processing
Proteins were diluted in buffer containing 150 mM NaCl (500 mM NaCl was stated in the text), 10 mM MOPS, 2 mM MgCl2, and 0.1 mM EGTA. Dilution buffer for myosin also contained 100 μM ATP (pH 7.0). Samples were applied to UV-treated and carbon-coated EM grids and stained immediately using 1% uranyl acetate. Micrographs were recorded on a JEOL 1200EX microscope using an AMT XR-60 CCD camera at a nominal magnification of 60,000×. Reference-free image alignments and K-means classification were conducted using SPIDER software (developed by Wadsworth Center, Albany, NY).
MST
MST measurements were carried out using a Monolith NT.115 instrument. CALML4-His and CaM-His (MilliporeSigma) were labeled with RED-Tris–NTA dye (Nanotemper) according to the manufacturer’s instructions. A solution of 100 nM CALML4 or CaM was combined with 2 μM Ca2+ to perform a binding check. A calcium dilution series (5 mM–150 nM) combined with 100 nM CaM was performed using 16 capillaries. Measurements were carried out in buffer containing 20 mM HEPES (pH 7.4) and 0.02% Tween-20 using Monolith capillaries (Nanotemper). The MST laser power was set to 60%. Analysis of MST traces was completed using MO.Affinity Analysis software (Nanotemper) (46).
Data availability
All data are contained within the article and supporting information.
Supporting information
This article contains supporting information (30, 47).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank the Electron Microscopy and Biophysics Core Facilities of the National Heart, Lung and Blood Institute (NHLBI, National Institutes of Health) for their support and the use of facilities. We thank Fang Zhang for the help with reagent and actin preparation. We thank the Microscopy Imaging Facility and Visual Function and Morphology Core at West Virginia University for help with image analysis.
Author contributions
J. R. S. and R. L. conceptualization; A. H. and R. L. methodology; N. B. software; A. H. and N. B. formal analysis; A. H. investigation; A. H. writing–original draft; N. B., Y. T., A. K., J. R. S., and R. L. writing–review & editing; J. R. S. and R. L. supervision.
Funding and additional information
R. L. is a recipient of a postdoctoral fellowship from NHLBI (Lenfant Biomedical Fellowship) and the tenure-track startup funds from West Virginia University. R.L. and N.B. are supported by National Institute of General Medical Sciences (NIGMS) Visual Sciences Center of Biomedical Reserach Excellence (Vs-CoBRE) (P20GM144230). This work is also supported by the Intramural Research Program of the NHLBI (grant no.: HL004232; to J. R. S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Enrique De La Cruz
Contributor Information
James R. Sellers, Email: sellersj@nhlbi.nih.gov.
Rong Liu, Email: rong.liu@hsc.wvu.edu.
Supporting information
Supporting Figure S1.

Supporting Figure S2.
Supporting Figure S3.
Supporting Figure S4.
Supporting Figure S5.
References
- 1.Berg J.S., Powell B.C., Cheney R.E. A millennial myosin census. Mol. Biol. Cell. 2001;12:780–794. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathur P., Yang J. Usher Syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim. Biophys. Acta. 2015;1852:406–420. doi: 10.1016/j.bbadis.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreland Z.G., Bird J.E. Myosin motors in sensory hair bundle assembly. Curr. Opin. Cell Biol. 2022;79 doi: 10.1016/j.ceb.2022.102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matoo S., Graves M.J., Acharya P., Choi M.S., Storad Z.A., Idris R., et al. Comparative analysis of the MyTH4-FERM myosins reveals insights into the determinants of actin track selection in polarized epithelia. Mol. Biol. Cell. 2021;32:ar30. doi: 10.1091/mbc.E20-07-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z.-Y., Hasson T., Kelley P.M., Schwender B.J., Schwartz M.F., Ramakrishnan M., Kimberling W.J., Mooseker M.S., Corey D.P. Molecular cloning and domain structure of human myosin-VIIa, the gene product defective in Usher syndrome 1B. Genomics. 1996;36:440–448. doi: 10.1006/geno.1996.0489. [DOI] [PubMed] [Google Scholar]
- 6.Heissler S.M., Sellers J.R. Myosin light chains: teaching old dogs new tricks. Bioarchitecture. 2014;4:169–188. doi: 10.1080/19490992.2015.1054092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haithcock J., Billington N., Choi K., Fordham J., Sellers J.R., Stafford W.F., et al. The kinetic mechanism of mouse myosin VIIA. J. Biol. Chem. 2011;286:8819–8828. doi: 10.1074/jbc.M110.163592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai T., Jung H.S., Sato O., Yamada M.D., You D.J., Ikebe R., et al. Structure and regulation of the movement of human myosin VIIA. J. Biol. Chem. 2015;290:17587–17598. doi: 10.1074/jbc.M114.599365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi M.S., Graves M.J., Matoo S., Storad Z.A., El Sheikh Idris R.A., Weck M.L., et al. The small EF-hand protein CALML4 functions as a critical myosin light chain within the intermicrovillar adhesion complex. J. Biol. Chem. 2020;295:9281–9296. doi: 10.1074/jbc.RA120.012820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapustina M., Cheney R.E. A new light chain for myosin-7. J. Biol. Chem. 2020;295:9297–9298. doi: 10.1074/jbc.H120.014595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S., Mecca A., Kim J., Caprara G.A., Wagner E.L., Du T.T., et al. Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex. Nat. Commun. 2020;11:2066. doi: 10.1038/s41467-020-15936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peckham M., Knight P.J. When a predicted coiled coil is really a single α-helix, in myosins and other proteins. Soft Matter. 2009;5:2493–2503. [Google Scholar]
- 13.Klomp A.E., Teofilo K., Legacki E., Williams D.S. Analysis of the linkage of MYRIP and MYO7A to melanosomes by RAB27A in retinal pigment epithelial cells. Cell Motil. Cytoskeleton. 2007;64:474–487. doi: 10.1002/cm.20198. [DOI] [PubMed] [Google Scholar]
- 14.Kuroda T.S., Fukuda M. Functional analysis of Slac2-c/MyRIP as a linker protein between melanosomes and myosin VIIa. J. Biol. Chem. 2005;280:28015–28022. doi: 10.1074/jbc.M501465200. [DOI] [PubMed] [Google Scholar]
- 15.Morgan C.P., Krey J.F., Grati M., Zhao B., Fallen S., Kannan-Sundhari A., et al. PDZD7-MYO7A complex identified in enriched stereocilia membranes. Elife. 2016;5 doi: 10.7554/eLife.18312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed Z.M., Riazuddin S., Khan S.N., Friedman P.L., Riazuddin S., Friedman T.B. USH1H, a novel locus for type I Usher syndrome, maps to chromosome 15q22-23. Clin. Genet. 2009;75:86–91. doi: 10.1111/j.1399-0004.2008.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird J.E., Takagi Y., Billington N., Strub M.P., Sellers J.R., Friedman T.B. Chaperone-enhanced purification of unconventional myosin 15, a molecular motor specialized for stereocilia protein trafficking. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12390–12395. doi: 10.1073/pnas.1409459111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang F., Takagi Y., Shams A., Heissler S.M., Friedman T.B., Sellers J.R., et al. The ATPase mechanism of myosin 15, the molecular motor mutated in DFNB3 human deafness. J. Biol. Chem. 2021;296 doi: 10.1074/jbc.RA120.014903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sari D., Gupta K., Thimiri Govinda Raj D.B., Aubert A., Drncova P., Garzoni F., et al. The MultiBac baculovirus/insect cell expression vector system for producing complex protein Biologics. Adv. Exp. Med. Biol. 2016;896:199–215. doi: 10.1007/978-3-319-27216-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J., Chen Y., Deng Y., Unarta I.C., Lu Q., Huang X., et al. Ca(2+)-Induced rigidity change of the myosin VIIa IQ motif-single alpha helix lever arm extension. Structure. 2017;25:579–591.e574. doi: 10.1016/j.str.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y., Baboolal T.G., Siththanandan V., Chen M., Walker M.L., Knight P.J., et al. A FERM domain autoregulates Drosophila myosin 7a activity. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4189–4194. doi: 10.1073/pnas.0808682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhyner J.A., Koller M., Durussel-Gerber I., Cox J.A., Strehler E.E. Characterization of the human calmodulin-like protein expressed in Escherichia coli. Biochemistry. 1992;31:12826–12832. doi: 10.1021/bi00166a017. [DOI] [PubMed] [Google Scholar]
- 23.Chinpongpanich A., Wutipraditkul N., Thairat S., Buaboocha T. Biophysical characterization of calmodulin and calmodulin-like proteins from rice, Oryza sativa L. Acta Biochim. Biophys. Sin. (Shanghai) 2011;43:867–876. doi: 10.1093/abbs/gmr081. [DOI] [PubMed] [Google Scholar]
- 24.Tobin M., Chaiyasitdhi A., Michel V., Michalski N., Martin P. Stiffness and tension gradients of the hair cell's tip-link complex in the mammalian cochlea. Elife. 2019;8 doi: 10.7554/eLife.43473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai T., Umeki N., Ikebe R., Ikebe M. Cargo binding activates myosin VIIA motor function in cells. Proc. Natl. Acad. Sci. U. S. A. 2011;108:7028–7033. doi: 10.1073/pnas.1009188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senften M., Schwander M., Kazmierczak P., Lillo C., Shin J.B., Hasson T., et al. Physical and functional interaction between protocadherin 15 and myosin VIIa in mechanosensory hair cells. J. Neurosci. 2006;26:2060–2071. doi: 10.1523/JNEUROSCI.4251-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grati M., Kachar B. Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11476–11481. doi: 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heissler S.M., Manstein D.J. Functional characterization of the human myosin-7a motor domain. Cell. Mol. Life Sci. 2012;69:299–311. doi: 10.1007/s00018-011-0749-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toyoshima Y.Y., Kron S.J., McNally E.M., Niebling K.R., Toyoshima C., Spudich J.A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987;328:536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu R., Billington N., Yang Y., Bond C., Hong A., Siththanandan V., et al. A binding protein regulates myosin-7a dimerization and actin bundle assembly. Nat. Commun. 2021;12:563. doi: 10.1038/s41467-020-20864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whatley M., Francis A., Ng Z.Y., Khoh X.E., Atlas M.D., Dilley R.J., et al. Usher syndrome: genetics and molecular links of hearing loss and directions for therapy. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.565216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin J.B., Krey J.F., Hassan A., Metlagel Z., Tauscher A.N., Pagana J.M., et al. Molecular architecture of the chick vestibular hair bundle. Nat. Neurosci. 2013;16:365–374. doi: 10.1038/nn.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahim S., Avenarius M.R., Grati M., Krey J.F., Windsor A.M., Sousa A.D., et al. Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like. Nat. Commun. 2016;7 doi: 10.1038/ncomms10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita-Becker S., Tsiavaliaris G., Ohkura R., Shimada T., Manstein D.J., Sutoh K. Functional characterization of the N-terminal region of myosin-2. J. Biol. Chem. 2006;281:36102–36109. doi: 10.1074/jbc.M605171200. [DOI] [PubMed] [Google Scholar]
- 35.Mansfield G.S., Al-Shirawi D.Y., Ketchum A.S., Newbern C.E., Kiehart D.P. Molecular organization and alternative splicing inzipper, the gene that encodes theDrosophilaNon-muscle myosin II heavy chain. J. Mol. Biol. 1996;255:98–109. doi: 10.1006/jmbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- 36.Guzik-Lendrum S., Heissler S.M., Billington N., Takagi Y., Yang Y., Knight P.J., et al. Mammalian myosin-18A, a highly divergent myosin. J. Biol. Chem. 2013;288:9532–9548. doi: 10.1074/jbc.M112.441238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg M.J., Lin T., Shuman H., Ostap E.M. Mechanochemical tuning of myosin-I by the N-terminal region. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3337–E3344. doi: 10.1073/pnas.1506633112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shuman H., Greenberg M.J., Zwolak A., Lin T., Sindelar C.V., Dominguez R., et al. A vertebrate myosin-I structure reveals unique insights into myosin mechanochemical tuning. Proc. Natl. Acad. Sci. U. S. A. 2014;111:2116–2121. doi: 10.1073/pnas.1321022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mentes A., Huehn A., Liu X., Zwolak A., Dominguez R., Shuman H., et al. High-resolution cryo-EM structures of actin-bound myosin states reveal the mechanism of myosin force sensing. Proc. Natl. Acad. Sci. U. S. A. 2018;115:1292–1297. doi: 10.1073/pnas.1718316115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiehart D.P., Franke J.D., Chee M.K., Montague R.A., Chen T.L., Roote J., et al. Drosophila crinkled, mutations of which disrupt morphogenesis and cause lethality, encodes fly myosin VIIA. Genetics. 2004;168:1337–1352. doi: 10.1534/genetics.104.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houdusse A., Titus M.A. The many roles of myosins in filopodia, microvilli and stereocilia. Curr. Biol. 2021;31:R586–R602. doi: 10.1016/j.cub.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young G., Hundt N., Cole D., Fineberg A., Andrecka J., Tyler A., et al. Quantitative mass imaging of single biological macromolecules. Science. 2018;360:423–427. doi: 10.1126/science.aar5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D., Piszczek G. Measuring the affinity of protein-protein interactions on a single-molecule level by mass photometry. Anal. Biochem. 2020;592 doi: 10.1016/j.ab.2020.113575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu D., Piszczek G. Standard protocol for mass photometry experiments. Eur. Biophys. J. 2021;50:403–409. doi: 10.1007/s00249-021-01513-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgess W.H., Jemiolo D.K., Kretsinger R.H. Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim. Biophys. Acta. 1980;623:257–270. doi: 10.1016/0005-2795(80)90254-8. [DOI] [PubMed] [Google Scholar]
- 46.Jerabek-Willemsen M., André T., Wanner R., Roth H.M., Duhr S., Baaske P., et al. MicroScale Thermophoresis: interaction analysis and beyond. J. Mol. Struct. 2014;1077:101–113. [Google Scholar]
- 47.Sato O., Komatsu S., Sakai T., Tsukasaki Y., Tanaka R., Mizutani T., et al. Human myosin VIIa is a very slow processive motor protein on various cellular actin structures. J. Biol. Chem. 2017;292:10950–10960. doi: 10.1074/jbc.M116.765966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article and supporting information.