Abstract
Chronic obstructive pulmonary disease (COPD) is a significant global cause of morbidity and mortality currently. Long-term exposure of cigarette smoke (CS) inducing persistent inflammation, small airway remodeling and emphysematous lung are the distinguishing features of COPD. Ferroptosis, occurred in lung epithelial cells has recently been reported to be associated with COPD pathogenesis. DNA dioxygenase ten-eleven translocation 2 (TET2) is an important demethylase and its genetic mutation is associated with low forced expiratory volume in 1 s (FEV1) of lung function. However, its role in COPD remains elusive. Here, we found that TET2 regulates CS induced lipid peroxidation through demethylating glutathione peroxidase 4 (GPx4), thus alleviating airway epithelial cell ferroptosis in COPD. TET2 protein levels were mainly reduced in the airway epithelia of COPD patients, mouse models, and CS extract-treated bronchial epithelial cells. The deletion of TET2 triggered ferroptosis and further exaggerated CS-induced airway remodeling, inflammation, and emphysema in vivo. Moreover, we demonstrated that TET2 silencing intensified ferroptosis, while TET2 overexpression inhibited ferroptosis in airway epithelial cell treated with CSE. Mechanically, TET2 protected airway epithelial cells from CS-induced lipid peroxidation and ferroptosis through demethylating the promoter of glutathione peroxidase 4 (GPx4). Finally, co-administration of methylation inhibitor 5′-aza-2′-deoxycytidine (5-AZA) and the antioxidant N-acetyl-cysteine (NAC) have more protective effects on CS-induced COPD than either administration alone. Overall, our study reveals that TET2 is an essential modulator in the lipid peroxidation and ferroptosis of airway epithelial cell, and could act as a potential therapeutic target for CS-induced COPD.
Graphical abstract
Highlights
-
•
TET2 deficiency promotes the development of cigarette smoke induced COPD.
-
•
TET2 regulates cigarette smoke induced ferroptosis of airway epithelial cell by demethylating GPX4 gene promoter.
-
•
Co-administration of low-dose methylation inhibitor and antioxidants might be a potential therapeutic option for COPD treatment.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung condition characterized by irreversible and persistent airflow obstruction, and is the third leading cause of morbidity and mortality worldwide [1]. The major environmental risk factor for COPD is cigarette smoking (CS). Chronic CS exposure activated oxidative pathways, leading to lung cell death, chronic airway inflammation, and emphysema, and finally developing COPD [2,3]. However, the pathogenesis of COPD is still poorly understood. Death and dysfunction of epithelial cells within the airway and alveolar niches are reported to play an important role in COPD development [4,5].
Ferroptosis, an iron-dependent regulated cell death, was first proposed by Dixon et al., in 2012 [6]. The morphology and biochemistry of ferroptosis differ from other forms of cell death. The morphology of ferroptosis is characterized by high mitochondrial membrane density, fewer or absent of mitochondrial cristae, and folded and crumpled mitochondrial membranes. Moreover, its biochemical process includes abnormal iron metabolism, redox imbalance, and lipid peroxidation, which are the leading causes of ferroptosis [7]. Several recent studies show that ferroptosis of airway epithelial cells was involved in the pathogenesis of COPD [[8], [9], [10]]. CS induces GPx4 depletion, which is linked to the development of emphysema [11,12]. However, the mechanisms underlying this process remain unknown.
Interactions between several genetic and environmental factors contribute to the etiology of COPD [13]. Epigenetics unveil the intricate interactions between genetic variations and environmental factors such as cigarette smoke, thus resulting in alterations in gene expression that potentially heighten the risk of developing COPD [14]. Studies have shown that CS exposure causes alterations in DNA methylation patterns, including global hypomethylation and site-specific hypermethylation [15,16]. The initiation and inhibition of DNA methylation require the precise enzymatic activity of multiple proteins, which is crucial for the survival of the organism. A DNMT enzyme family adds methyl groups, whereas the TET enzyme family removes these groups during DNA methylation and demethylation [17]. Our previous studies found that the Bcl-2 promoter is hypermethylated in response to DNMT1-mediated apoptosis in the emphysema model [18]. Understanding how the TET enzymes regulated DNA methylation in the context of COPD is indeed an intriguing area of study.
TET2, a member of the ten-eleven translocation (TET) family, is a DNA demethylase that catalyzes hydromethylation cytosine and subsequently remodels chromatin to alter gene expression [19]. Previous studies have demonstrated TET2 plays a pivotal role in myelopoiesis [[20], [21], [22]], inflammation regulation [19,23], and lung disorders [[24], [25], [26], [27]]. The GWAS study showed that TET2 gradually increases during the development of fetal lung, and it is a novel locus significantly associated with the decline of forced expiratory volume in 1 s (FEV1) [24]. Besides, a recent cohort study of clonal hematopoiesis shows that mutations in TET2 are associated with COPD [28]. Inactivating TET2 in mouse hematopoietic cells exacerbated the development of emphysema and inflammation induced by CS short-term exposure [29]. However, the role of TET2 in lung cells fate and the development of CS long-term exposure induced COPD is still unclear.
This study was aimed to delineate the function of TET2 in the pathogenesis of CS-induced COPD. To address this issue, TET2 levels in the lungs of COPD patients and the CS-exposed murine model were measured. TET2 was deleted in a murine model of COPD to determine that reduced expression of TET2 aggravates COPD development by triggering ferroptosis. Besides, the activity of TET2 in airway epithelial cells downregulated DNA methylation levels of the GPx4 promoter and subsequently inhibited ferroptosis. This study also aimed to resolve the controversy on the application of NAC in treating COPD. This study proved that sufficient GSH provided by NAC needs high-level of GPx4. According to these studies, targeting TET2 is a potential therapeutic option for treating CS-induced ferroptosis.
2. Materials and methods
2.1. Ethics statement
The protocol for animal experiments was approved by the Committee on the Use and Care of Animals of the Second Xiangya Hospital of Central South University. All human studies were approved by the Institutional Review Board of the Second Xiangya Hospital of Central South University and were performed in compliance with the relevant ethical regulations.
2.2. Patients and samples
The Second Xiangya Hospital recruited 39 patients who had operable pulmonary nodules that were not cancerous, and obtained samples of their lung tissue. Their average ages were similar. The informed consent of patients was obtained before the operations, and the project was approved by the Ethics Committee of the Second Xiangya Hospital. The selected patients were divided into three groups based on their smoking history and lung function tests, then grouped into three groups: nonsmokers(n = 13), smokers(n = 13), and smokers with COPD(n = 13). Smokers refer to non-COPD smokers with normal pulmonary function, and smokers with COPD were classified by guidelines of the Global Initiative for COPD Prevention and Treatment (GOLD2018). An FEV1/FVC (forced expiratory volume in the first second/strength vital capacity) ratio of <70% and the clinical diagnosis confirmed COPD patients with the emphysema phenotype. The patients who underwent had no underlying lung disease or severe cardiopulmonary disease. The clinical characteristics of the patients were listed in Supplementary Table S1.
2.3. Tet2-/- mice genotyping
Professor Xu Guoliang in Shanghai Institute of Biological Sciences, Chinese Academy of Sciences donated the breeding pairs of Tet2+/− mice with a C57BL/6J background [30]. For genotyping, DNA was extracted from mice using DNA extraction kits (Shenggong company, Shanghai, China) according to the manufacturer's instructions. The TET2 gene was genotyped by amplifying it using PCR with genomic DNA, and specific primers were created for this purpose. A list of PCR primer sequences is provided in Supplementary Table S2. Cycling of PCR was 30 cycles of 94 (1 min) for denaturation, 51 °C(1 min) for the annealing step, and 72 °C (2 min) for extension with a T100 PCR Thermal Cycler (Bio-rad, Hercules, CA, USA). The DNA fragments of TET2 from WT, Tet2+/− and Tet2-/- mice, respectively. PCR products were separated at 100 V for 35 min using 1% agarose gel electrophoresis and visualized with a ChemiDoc MP system (Bio-Rad, Hercules, CA, USA).
2.4. Animal experiment
Herein, six to eight weeks old male WT and Tet2-/- mice were whole-body exposed to cigarette smoke (CS, China Tobacco Hunan Industrial Co., Ltd.) using a clear plastic box twice daily with 90 min rest in between, five times per week for six months as previously described [11]. The control mice were exposed to room air (RA). All mice were maintained under pathogen-free conditions. To evaluate the effect of NAC or 5′-aza-2′-deoxycytidine (alone or in combination) in the CS-exposed mouse model. 40 of six to eight weeks old male C57BL/6J mice were randomly divided into five groups:RA, CS, NAC + CS, 5-AZA + CS or NAC+5-AZA + CS group. Mice were injected intraperitoneally twice weekly endotoxin-free 0.156 mg/kg 5′-aza-2′-deoxycytidine or dimethyl sulfoxide (Sangon Biotech Co. Ltd) [31] and supplied with 1 g/kg body weight NAC (Zhejiang CONBA Pharmaceutical Co., Ltd.) added to regular drinking water [32]. All CS exposure group received CS exposure for six months and RA group were kept in the same environment without CS exposure.
2.5. Micro-computed tomography (micro CT)
Micro-CT (PerkinElmer, CA, USA). Was performed on mice three days before the conclusion of the study. Ketamine (75 mg/kg) and xylazine (11 mg/kg) were intraperitoneally (i.p.) injected to sedate mice. Then the mice were placed in a plastic cradle and laydown imaged using radiography. The radiography parameters were 80 kVp, 220 mA, and 12-ms exposure per projection.
2.6. Immunohistochemistry (IHC)
Five human or four mice samples from each group were applied for IHC. Specifically, the lung tissue samples were treated with a fixative, dehydrated using alcohol and xylene, embedded in paraffin, and then sliced into sections with a thickness of 4 μm with the help of a paraffin microtome. According to the manufacturer's recommendations, TET2 (Cat#: 21207-1-AP, Proteintech, USA) and diamino-beenzidine (DAB) staining were used in IHC staining (Solarbio Life Science, China). The slices were observed by microscope (NIKON ECLIPSE C1, Japan) and scanned with 3DHISTECH Panoramic 250 digital slide scanner (3DHISTECH, Hungary). Positive expression was observed as yellow or brown staining and then chose five different representative nonoverlapping fields selected from each lung section to calculate the relative protein expression level. The values were obtained and analyzed by the Image Pro Plus software (version 6.0, Media Cybernetics). H-SCOREs ranged from 0 to 300 [33]. A quantitative imaging method was used to evaluate all staining, and both the percentage of immunostaining and staining intensity were determined. Three different fields were selected from a lung section and three sections per animal were evaluated to obtain a mean value.
2.7. Masson trichome and H&E staining
To measure the airway remodeling and emphysema-like alveolar enlargement, Masson trichome and H&E staining were used. Specifically, mouse lungs were perfused, inflated, formalin-fixed, and cut into pieces of 3–4 μm thickness. Slides were deparaffinized serial passage twice in xylene, followed by a graded ethanol series. Slides were stained with Masson trichome (Servicebio, Wuhan, China) to identify collagen. Six small airways were randomly selected in each mouse lung, and collagen deposition (blue color) around the small airways was normalized to the perimeter of the airways. For morphological changes, lung slides were stained using hematoxylin and eosin (H&E, Servicebio, Wuhan, China). Four small airways were randomly selected in each mouse lung and epithelial cell size in the small airways was normalized to the perimeter of the airways. Emphysema-like alveolar enlargements were assessed in the lung parenchyma (excluding airways and blood vessels) using mean linear intercepts (MLIs) and destructive indexes (DIs) as previously described [11,12].
2.8. Bronchoalveolar lavage fluid (BALF)
BALF was obtained from mouse lungs by washing twice with 500 μl salt solution. Total cells were incubated with red cell lysis buffer (on ice, 5 min) and collected after centrifugation (125×g, 4 °C, 10 min). Slides were stained with total cell numbers, and BALF was determined using Trypan blue exclusion staining and a hemocytometer. Differential cell counts were enumerated using light microscopy. Wright-Giemsa Staining (Servicebio, Wuhan, China) was used to identify the types of cells.
2.9. Transmission electron microscopy (TEM)
The morphological changes of mitochondria in cells were observed by TEM (Tecnai G2 Spirit Twin, Czech). After treatment with different types of drugs, cells were collected and fixed in 4% glutaraldehyde overnight at 4 °C, and then postfixed in 1% osmium tetroxide (OsO4) at 4 °C. Afterward, samples were dehydrated with a series of alcohol concentrations and embedded in Epon-Araldite resin. Ultrathin sections were obtained by an ultramicrotome and stained with uranyl acetate and lead citrate. Subsequently, ultra-structures were observed and images were examined using TEM.
2.10. GSH and GSSG assay
Total GSSG and GSH levels were measured by using the GSH and GSSG assay kit (Beyotime, S0053). Following the manufacturer's instruction, lung tissue or cells were collected in a centrifuge tube and immediately add 3 vol of ice-cold 5% metaphosphoric acid, then frozen and thawed twice using liquid nitrogen and 37 °C water. The samples were centrifuged at 12000 rpm for 10 min at 4 °C and the supernatant was used for GSH and GSSG assays. The GSH/GSSG ratio were calculated using the following equation: GSH/GSSG = [Total GSH-(2 × GSSG)]/GSSG.
2.11. Perls’ DAB staining
Dewaxing and hydration the paraffin embedded lung sections and put slides into the fleshly prepared Prussian blue solution (Sino reagent, China) staining for 30 min, and then Slides were stained with DAB solution (Sino reagent, China) for about 5–10 min, degree of color development should be controlled under a microscope. The iron-containing parts of the tissue are brown, the cell nucleus is light blue, and the background is light brown or colorless.
2.12. Flow cytometry
Lipid ROS levels were measured using the BODIPY 581/591C11 probe (Invitrogen, MA, USA) following the manufacturer's instructions. Briefly, BEAS-2b cells were cultured into 6-well plates and incubated for 24 h to allow for adherence. After treatment according to experimental groups, lung cells were harvested with C11-BODIPY (1 μM) dissolved in complete medium at 37 °C for 30 min in the dark. Lung lobes were chopped and digested in Cell Dissociation Solution (Absin reagents, shanghai, china) for 1 h at 37 °C. Tissue was then passed through a 70 μm sieve (BD Bioscience), washed, and resuspended in 1 mL of complete media. Add C11-BODIPY (1 μM) at 37 °C for 30 min in the dark. Finally, cells were rinsed thrice and fluorescence intensity was detected by a flow cytometer (BD, CA, USA).
2.13. Cell culture and treatment
The BEAS-2b cells were bought from the Cell Biology of Central South University in Changsha, China. These cells were cultured in RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco), 100 U/mL penicillin (Gibco), and 100 mg/mL streptomycin (Gibco), and kept in an incubator with 5% CO2 at 37 °C. These cells were subjected to treatment with 5% CSE and gene editing.
2.14. Preparation of CSE
CSE was prepared as previously reported [34]. Briefly, the smoke of a cigarette was bubbled into a flask containing 10 mL of warm (37 °C) PBS using a vacuum pump at a constant speed. Each cigarette was smoked for 5 min. The CSE solution was adjusted to pH 7.4 and then sterilized by filtration through a 0.22-μm pore filter (Pall Corporation, Port Washington, NY, USA). For quality control, the solution was standardized by monitoring the absorbance at 320 nm (A320) and 540 nm (A540). CSE quality was accepted if ΔOD (A320-A540) was between 0.9 and 1.2. The resulting solution was regarded as 100% CSE and, and was diluted with medium for use in experiments within 1 h.
2.15. Endogenous TET2 activation by CRISPR SAM
Endogenous TET2 was activated using the CRISPR-Cas9 Synergistic Activation Mediator (SAM) system (Genechem Technology, Shanghai, China.). Beas-2b cells were infected with the lentiviruses of Lenti dCAS9-VP64 for 72 h, the cells were then treated with puromycin. Next, lentivirus containing sgRNA-TET2 was used to infect these cells followed by G418 selection for 14 days, and then TET2 overexpression stable cell line was obtained.
2.16. TET2 silence
TET2 siRNAs and negative control siRNAs were designed and synthesized by RiboBio (RiboBio Biotechnology, Guangzhou, China). Beas-2b cells were transfected with TET2 siRNA or control siRNA by employing Lipo3000 transfection reagent (Invitrogen, USA).
2.17. Cell viability assay
Cell viability was evaluated using cell counting kit-8 (TOPSCIENCE, China) according to the manufacturer's instructions. Briefly, BEAS-2b cells (3000/well) were seeded in 96-well plates and incubated for 24 h to allow for adherence. Each group of cells was treated with corresponding drugs, inhibitors, and overexpression or silence plasmids, followed by CSE exposure. Next, the culture medium was replaced with 90 μl of fresh medium and 10 μl of CCK-8 solution. After incubation at 37 °C for 2 h, absorbance was measured at 450 nm using a microplate reader (PerkinElmer EnSpire, Singapore).
2.18. Protein extraction
To prepare mouse lung tissues, a tissue grinder was used to homogenize the tissues in a RIPA buffer (CWBIO, China) supplemented with PhosSTOP phosphatase inhibitor (TOPSCIENCE, C0004) and protease inhibitors (TOPSCIENCE, C0001). To obtain lysates from cells, the lysate buffer was added to plates and scratched. The total protein concentrations of both mouse lungs and cell lysates were measured with the help of BCA protein assay kits (Pierce Biotechnology, Thermo Fisher Scientific, Waltham, MA, USA).
2.19. Immunoblotting
Proteins were separated using SDS-PAGE and transferred to PVDF membranes (Cat#IPVH00010, Millipore, Merck, USA). TET2 was purchased from Millipore (Cat#: MABE462). GPx4 (Cat#sc166570) was purchased from Santa cruz, SLC7A11 (Cat#12691) was purchased from CST β-actin (Cat#60008-1-lg) was purchased from Proteintech. Blots were stained with TET2(1:1000), GPx4(1:500), SLC7A11(1:1000), or β-actin (1:5000) antibodies overnight, and were incubated with anti-rabbit or anti-mouse secondary antibody (room temperature) for 1 h. Super-signal West Femto substrates were used to detect proteins, and images were taken with a ChemiDoc MP system (Bio-Rad, Hercules, CA, USA). The densitometry of proteins was measured using Image J (NIH, USA) and normalized to the density of the control protein (β-actin). Values are presented as fold change of experimental groups in comparison to controls.
2.20. RNA extraction and real-time quantitative (q)PCR
RNA was extracted from homogenized mouse lungs and cells using TRIzolTM reagent (Invitrogen, Thermo Fisher Scientific, USA), according to the manufacturer's instructions. Furthermore, concentrations of RNA were measured using a NanoDrop One (Thermo Fisher Scientific, USA). RNA (1000 ng) was reverse transcribed with reverse transcriptase (Bioscript, Bioline, London, UK) using RT-PCR. The primers for quantitative real-time PCR (qPCR) are below, and the products were detected with SYBR reagents using qPCR (Bio-rad, Hercules, CA, USA). Primer for RT-qPCR were listed in Supplementary Table S3.
2.21. Methylation-specific PCR (MSP)
DNA was extracted from cells and then were treated with bisulfite to convert unmethylated cytosine to uracil by the EZ DNA Methylation-Lightning kit (Zymo research). For each bisulfite-treated sample, two sets of primers were used to amplify the methylated DNA (methylated-MSP, M-MSP) and unmethylated DNA (unmethylated-MSP, U-MSP) respectively. Primers and conditions for M-MSP and U-MSP of TET2 were listed in Supplementary Table S4. The PCR products were loaded onto an agarose gel for electrophoresis.
2.22. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM), from mouse samples for triplicate experiments (n = 3–6). Cell culture experiments were conducted at least three times independently. Comparisons between two groups were made using the student's t-test, a non-parametric test of the Mann-Whitney U test, or Fisher's exact test. Multiple comparisons (>2 groups) were made using one-way ANOVA with Bonferroni post-test, Kruskal-Wallis test with Dunn's multiple comparison test or Two-way ANOVA with Turkey's multiple comparison test depending on the characteristics of the data, through GraphPad Prism Software version 9 (San Diego, CA, USA). A P-value <0.05 was considered statistically significant.
3. Results
3.1. TET2 levels are decreased in the lung tissue of COPD patients and mice, as well as airway epithelium treated with CSE
The lung tissues from nonsmokers(n = 13), smokers (n = 13), and COPD patients (n = 13) were first analyzed to investigate the relative expression of TET2 in the lungs of COPD. Immunohistochemical (IHC) analysis showed that, TET2 was mainly expressed in airway epithelial cells and alveolar epithelium of human lungs, and was significantly reduced in the smokers with COPD (Fig. 1A and B). However, there is no significant difference in TET2 expression between nonsmoker and smokers without COPD. Further immunoblotting confirmed that TET2 protein was downregulated severely in the homogenate of lung tissue of COPD patients (Fig. 1C and D). In addition, an experimental COPD murine model was established by whole body exposure to CS for 6 months. Consistent with the reduction of TET2 in COPD patients, downregulated TET2 expression was also observed in lung tissue cells, especially airway epithelial cells in CS- exposed mice by IHC (Fig. 1E and F). Besides, BEAS-2b cells (immortalized human bronchial epithelial cell) stimulated with cigarette smoke extract (CSE) were used in vitro to confirm these findings. Immunoblotting analyses showed that TET2 protein expression decreased in a CSE concentration-dependent manner (Fig. 1G and H). In summary, these data show that TET2 expression reduced in COPD lungs and highlight a potential role in COPD pathogenesis.
Fig. 1.
TET2 protein levels are reduced in the lungs of chronic obstructive pulmonary disease (COPD) patients, mouse models, and cigarette smoke extract (CSE)-treated lung epithelial cells. A-B Immunohistochemical staining of human lung sections from nonsmokers, smokers without COPD, and COPD patients. (Three subjects from each group are shown, which were representative of five subjects from each group) (scale bar: 50 μm). Results are expressed as mean ± SEM. C-D Western blot analysis of TET2 protein levels in normal human lungs or lungs from patients with COPD or smokers analyzed (NS, n = 13; Smoker, n = 13; COPD, n = 13). E-F Mouse lung sections exposed to room air or cigarette smoke for 6 months. (Two subjects from each group are shown, which were representative of four subjects from each group). G-H Human airway epithelial cells (BEAS-2b) were treated with various concentrations of CSE for 24 h and TET2 protein was measured using Western blot analysis. Data are representative of at least three independent experiments. Results are expressed as mean ± SEM. For B, D, F and H, data are presented as mean ± SD of independent experiments. Statistical differences were determined using Kruskal-Wallis test with Dunn's multiple comparison test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
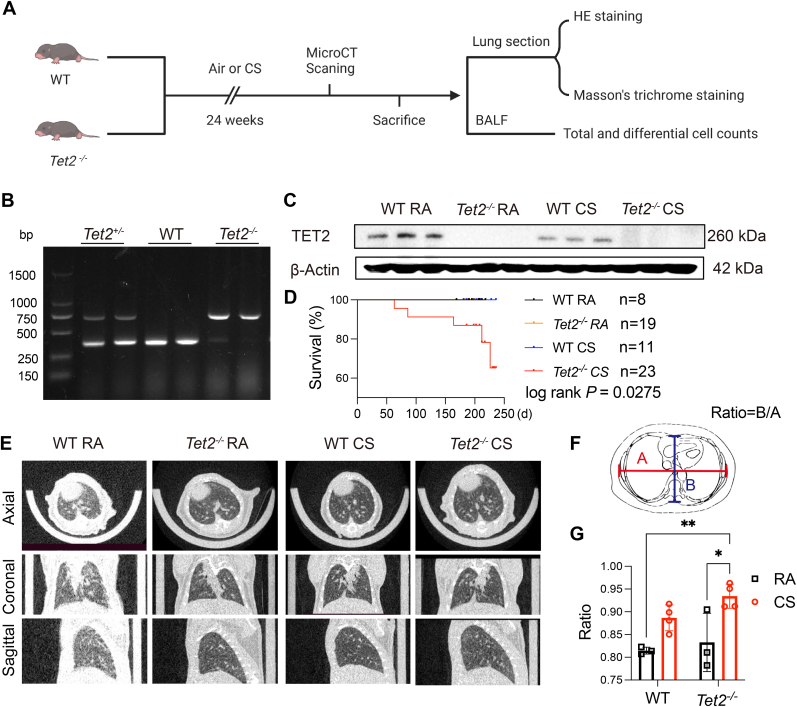
3.2. Tet2-/- mice are more susceptible to CS to develop COPD
To determine whether TET2 reduction is associated with the development of COPD, a Tet2 knockout (Tet2-/-) mice were generated and applied to CS exposure and observed for 6 months (Fig. 2A). The Tet2 knockout was confirmed using genotyping and Western blot, which suggest Tet2-/- mouse is successfully established (Fig. 2B and C). Among all the mice, only Tet2 knockout mice with CS exposure died during the observation period (Fig. 2D). To further evaluate the radiographic morphology of the lung, all the mice were applied to micro-CT scanning. Mice with Tet2 deletion and CS exposure showed lower radiographic density of pulmonary field, comparing to other groups (Fig. 2E). The enlargement change of lung field was quantified by measuring the anteroposterior-to-transverse diameter of the thorax on micro-CT images (Fig. 2F and G), which is considerate as a clinical examination finding in the diagnosis of COPD [35,36]. The chronic inflammation, airway remodeling and emphysematous changes of lungs are typical signs of COPD. After 6 months of CS exposure, the lungs of mice were collected, and the chronic inflammation, airway remodeling and emphysematous changes of lungs were measured. HE staining showed that the distal airspaces of Tet2−/− mice were moderately enlarged than that of WT mice, but less than that of Tet2−/− mice exposed to smoke for 6 months. Moreover, compared with WT mice, the distal airspaces of Tet2−/− mice exposed to CS, which represented higher mean linear intercepts (MLIs) and destructive indexes (DIs) (Fig. 3A–C). CS exposure caused the thickening of epithelial cells in the small airways of WT mice. However, after 6 months of exposure to CS, deletion of Tet2 exacerbated airway epithelial thickening (Fig. 3D and E). Masson's trichrome staining revealed marked collagen accumulation around small airways of Tet2−/− mice after CS exposure, which is the hall marker of airway remodeling. Although collagen accumulation was observed in WT mice exposed to CS, the extent of accumulation was comparatively lower than that observed in Tet2−/− mice under the same conditions (Fig. 3F and G). Inflammatory cells in BALF were counted. Compared with normal air-exposed controls, WT mice had more leukocytes in an CS exposed murine model. Besides, neutrophil count was higher in Tet2 KO with CS exposure, than that in WT mice with CS exposure (Fig. 3H–J). Our in vivo findings suggest that downregulation of TET2 expression promoted cigarette smoke induced emphysema and COPD.
Fig. 2.
Genotyping and basic characteristics of Tet2−/−mice. A Wild type (WT) and Tet2−/−mice were exposed to CS or normal air for 6 months. Histology and inflammation were detected. B Genomic DNA was obtained from the tails of WT and Tet2−/− C57BL/6 mice, and mice were genotyped using a previously described PCR approach and agarose gel running. C Lung proteins were extracted from WT and Tet2−/− mice and TET2 protein was detected using immunoblot analysis. D Proportions of surviving Tet2−/− and WT mice were plotted. E Representative axial (top row), coronal (middle row), and sagittal (bottom row) images of micro-computed x-ray tomography (micro-CT) (n = 3–4 per group). A healthy lung is slightly white, and a diseased lung is increasingly black (decreased density). F-G Ratio of left and right diameters to front and rear diameters. Data were mean ± SEM, Statistical differences were determined using Two-way ANOVA with Turkey's multiple comparison test. *P ≤ 0.05; **P ≤ 0.01.
Fig. 3.
TET2 knockout aggravates cigarette smoke (CS)-induced COPD. WT and Tet2−/− mice were exposed to CS or normal air for 6 months. A-C Mouse lung sections were stained with hematoxylin and eosin (H&E) (scale bar: 100 μm) and emphysema-like alveolar enlargement was quantified based on the measurement of the mean linear intercept and destructive index (n = 6). D-E Mouse lung sections were stained with H&E (scale bar: 50 μm; inserts show the expanded image of the indicated region). Quantification of epithelial thickness in the small airways was normalized to the perimeter of the basement membrane. F-G Collagen deposition around small airways in lung sections was assessed using Masson's trichrome histochemistry (blue color indicates collagen, scale bar: 50 μm; inserts show the expanded image of the indicated region). Quantification of the collagen area around the small airways was normalized to the perimeter of the basement membrane. H-J Differential inflammatory cell counts in the bronchoalveolar lavage fluid. Data were mean ± SEM, Statistical differences were determined using Two-way ANOVA with Turkey's multiple comparison test *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. TET2 KD increases CSE-induced ferroptosis in lung epithelial cells in vivo and in vitro
It is interested to know the cell fate under cigarette smoke stimulation with TET2 knockdown. The cells transfected with TET2 siRNA or control siRNA were treated with CSE or PBS, and then the cell morphology and viability was monitored. Results showed that ablation of TET2 did not contribute to the cell death at the baseline, while increased the CSE-induced cell death according to the morphological and quantitative analysis of cell viability (Fig. 4A and B). Next, we compared the therapeutic efficacy of a number of pharmaceuticals including the ferroptosis inhibitor ferrostatin-1 (Fer-1, 1 μM), liproxstatin-1 (Lip-1, 5 μM), apoptosis inhibitor Z-VAD-FMK (ZVF, 20 μM), necroptosis inhibitor necrostatin-1 (Nec-1, 5 μM) or autophagy inhibitor 3-Methyladenine (3-MA, 2 mM), respectively. We found that the ferroptosis inhibitor Fer-1 and Lip-1 exhibited remarkable protective effects, comparing to those of other inhibitors (Fig. 4B). Though ZVF slightly improved cell viability, there was no statistically significant difference between the groups under CSE treatment and TET2 knockdown with Fer-1 or ZVF treatment. Since the morphologic change of mitochondria is a typical ferroptosis characterization [6], transmission electron microscopy (TEM) was applied to observe mitochondrial morphology both in vivo and in vitro (Fig. 4C and E). Shrunken mitochondria with increased membrane density and reduced mitochondrial cristae were observed in the lung cells of CS exposed mice or CSE treated lung epithelial cells. TET2 KO in mice and KD in lung epithelial cells, combined with cigarette smoke (CS) or cigarette smoke extract (CSE) treatment, resulted in pronounced mitochondrial shrinkage and reduction in cristae structure. Next, we detected the peroxidized lipids within cells indicative of ferroptosis, using lipid peroxidation (LipidRos) -sensitive dye C11-BODIPY staining by flow cytometry (Fig. 4D). Our results clearly demonstrated that TET2 knockdown alone led to an increased accumulation of lipid peroxides compared to control cells. Moreover, there was no significant difference observed between the group treated with cigarette smoke extract (CSE) and the TET2 knockdown group. However, TET2 silencing significantly augmented the accumulation of lipid ROS induced by CSE. Considering the iron dependence of ferroptosis, we investigated whether CS heightens intracellular labile iron in COPD-related experimental mice models. Perls' DAB staining revealed elevated levels of non-heme iron in bronchial epithelial cells from CS-exposed mice compared to those from RA-exposed mice (Fig. 4F–G). Regrettably, TET2 knockout had no effects on labile iron pool (LIP). These findings suggest that low level of TET2 increased cigarette smoke induced ferroptosis of lung epithelial cells.
Fig. 4.
TET2 deletion exhibit a ferroptotic signature in vivo and in vitro.
A BEAS-2b cells were transfected with TET2-targeting siRNA (siTET2) or negative control (NC). Representative phase-contrast microscopy images of cells. B Ferroptosis inhibitor Fer-1 (1 μM) and Lip-1 (5 μM), apoptosis inhibitor Z-VAD-FMK (ZVF, 20 μM), necroptosis inhibitor Necrostatin-1 (Nec-1, 5 μM) or autophagy inhibitor 3-Methyladenine (3-MA, 2 mM) was added to cells 1 h before control or 5% CSE treatment. Cell viability was measured using a CCK8 assay. C Representative transmission electron microscopy (TEM) images of cells after CSE treatment. Enlarged mitochondria (bottom rows). Scale bars: 5 μm (Left Column), 1 μm (Right Column). D Lipid peroxidation as assayed by flow cytometry using the C11-BODIPY probe. Representative histogram data and statistical results are shown. E WT and Tet2−/− mice were exposed to CS or normal air for 6 months. Electron microscope images of mitochondria in mouse lung sections. (Scale bar: 2 μm (Top row), and 500 nm (bottom row)). F-G Ferric iron deposits were stained with Perls/diaminobenzidine (DAB) staining in lung samples. (Scale bar: 50 μm) and quantification of Perls/DAB staining. Data were mean ± SEM, For B and D, Statistical differences were determined using one-way ANOVA with Bonferroni post-test. For G Statistical differences were determined using Two-way ANOVA with Turkey's multiple comparison test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001.
3.4. TET2 KD mainly increases susceptibility to ferroptosis by targeting GPx4 to disrupt the antioxidant system
Elevated labile iron pool (LIP) levels and disrupted oxidation balance are critical factors in the occurrence of ferroptosis. Based on the results of Perls' DAB staining, CS exposure has already provided sufficient LIP and TET2 knockout did not increase the non-heme iron level. Then, we assessed the accumulation of 4-hydroxy-2-nonenal (4-HNE), the major products of phospholipid peroxidation, in the lung of Tet2-/- mice exposed to CS (Fig. 5A and B). The results revealed a distinction between the WT and Tet2-/- groups; however, the observed difference did not reach statistical significance. As expected, CS exposure significantly increased the level of 4-HNE, while Tet2 knockout further exaggerated the accumulation of 4-HNE. Therefore, we suppose that TET2 is involved in the regulation of ferroptosis in COPD due to its influence on the oxidative balance homeostasis. Glutathione (GSH) and GSH-related enzymes constitute an important antioxidant defense system that protects cells from chemotherapy attacks [37]. We further detected the proportion of GSH and GSSG in vivo and in vitro to validate the hypothesis. Interestingly, both GSH and the GSH/GSSG ratio were significantly lower in Tet2-/- mice than in WT mice after CS exposure, but GSH content was not altered in Tet2-/- mice with air exposure (Fig. 5C and D). Additionally, TET2 knockdown and CS exposure resulted in the lowest ratio of GSH/GSSG in airway epithelial cells, which indicated the intensified effects of TET2 KD on CSE induced GSH depletion (Fig. 5E). GSH is converted into GSSG by GPx4 and the cystine transporter SLC7A11 critically restores/maintains intracellular GSH [38]. We observed lower levels of GPx4 in Tet2-/- mice compared to WT mice, regardless of CS exposure. However, there was no significant difference in SLC7A11 between Tet2-/- and WT mice. Notably, SLC7A11 significantly decreased in Tet2-/- mice after CS exposure (Fig. 5F). Therefore, we further observed the expression of GPx4 and SLC7A11 after silencing TET2 in Beas-2B cells. Consistent with the in vivo results, we discovered that TET2 knockdown specifically influenced the expression of GPx4, rather than SLC7A11 (Fig. 5G). We further detected the expression of GPx4 in Beas-2B cells after transfection with TET2 siRNA and subsequent CSE exposure, and found that TET2 knockdown substantially decreased GPx4 expression regardless of CSE stimulation (Fig. 5H). These results indicated that TET2 may influence ferroptosis potentially by regulating the lipid peroxidation through GPx4-GSH antioxidant system.
Fig. 5.
TET2 deletion increased susceptibility to ferroptosis by silencing GPx4 to disrupt the antioxidant system
A-B 4-Hydroxynonenal (4-HNE) staining of lung sections of WT and Tet2-/- mice (scale bars: 20 mm) and quantification of 4-HNE staining. C-D Measurement of GSH level and GSH/GSSG ratio in mouse lung tissues of WT and Tet2-/- mice. E Representative data of the GSH/GSSG ratio in cells treated with 5% CSE for 24 h. F Quantification and representative immunoblot analysis of the protein levels of glutathione peroxidase 4 (GPx4) and solute carrier family 7 member 11 (SLC7A11). β-actin was used as a loading control. G Western blot analysis of the protein levels of GPx4 and SLC7A11 in response to TET2 knockdown. H Quantification and representative immunoblot analysis of GPx4 proteins in cells after treatment with CSE. Data were mean ± SEM, For E and H, Statistical differences were determined using one-way ANOVA with Bonferroni post-test. For B, C, D, and F, Statistical differences were determined using Two-way ANOVA with Turkey's multiple comparison test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001.
3.5. TET2 overexpression reduces lipid peroxidation and ferroptosis in airway epithelial cells induced by CS
To further verify the effects of TET2 on CS induced ferroptosis of lung epithelial cells, stable TET2 overexpressed (TET2-OE) BEAS-2b cell lines were established using the CRISPR SAM system. As expected, overexpression of TET2 significantly mitigated cell death induced by CSE stimulation (Fig. 6A and B). Next, TEM results showed that overexpressed TET2 alleviated CSE induced mitochondria shrinkage (Fig. 6C). Consistent with TEM findings, overexpression of TET2 reduced CSE-induced deposition of lipid ROS (Fig. 6D). In addition, GSH/GSSG ratio increased in the TET2 OE group, which indicated a GSH recovery after TET2 restoration (Fig. 6E). Subsequently, expression of SLC7A11 and GPx4 were checked. TET2 significantly increased the GPx4 expression in two TET2-OE cell lines but had no effect on SLC7A11 expression (Fig. 6F). As expected, GPx4 expression was activated after the restoration of TET2 under CSE treatment (Fig. 6G and H). These results provided further evidence that TET2 alleviates ferroptosis in lung airway epithelial cells by regulating the GSH-GPx4 axis, rather than the SLC7A11 pathway.
Fig. 6.
TET2 overexpression reduces lipid peroxidation and ferroptosis in airway epithelial cells induced by CS
TET2 stable overexpression cell lines were developed using the CRISPR-Cas9 Synergistic Activation Mediator (SAM) system and then were treated with CSE solutions. A-B Cell viability was detected using a CCK8 assay kit and representative phase-contrast microscopy images of cells were obtained. C Representative transmission electron microscopy (TEM) images of cells after CSE treatment. Enlarged mitochondria (bottom rows). Scale bars: 5 μm (top row), 1 μm (bottom row). D Lipid peroxidation was assayed by flow cytometry using the C11-BODIPY probe. E Representative data of the GSH/GSSG ratio in cells treated with 5% CSE for 24 h. F Western blot analysis of GPx4 and SLC7A11 proteins levels in response to TET2 overexpression. G-H Quantification and representative immunoblot analysis of GPx4 proteins in cells after treatment with CSE. Data were mean ± SEM, Statistical differences were determined using one-way ANOVA with Bonferroni post-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001.
3.6. TET2 regulates ferroptosis through demethylation of gpx4 promoter
Previously results indicated that TET2 regulated ferroptosis through the GSH-GPx4 pathway, molecular mechanism of TET2 regulating GPx4 was investigated. The transcription of GPx4 mRNA in TET2 KD and OE cells under CSE treatment was checked by RT-qPCR. Silencing TET2 promoted the reduction of GPx4 mRNA after CSE treatment (Fig. 7A). However, overexpression of full length TET2 attenuated the downregulation of GPx4 mRNA level induced by CSE application (Fig. 7B). We further confirmed that comparing to healthy nonsmokers and smokers, the mRNA level of GPx4 in COPD was lower (Fig. 7C). Reduction of GPx4 mRNA expression was also confirmed in CS-exposed COPD mice. TET2 knockout significantly decreased the transcription of mRNA for GPx4 in both RA and CS exposure mice (Fig. 7D). TET2 is an important DNA demethylase that regulates gene methylation. And, GPx4 expression is regulated by DNA methylation [39,40]. We hypothesized that TET2 regulates GPx4 through its demethylase activity. To clarify this, we applied methylation inhibitor 5-AZA to BEAS-2b cells and found that 5-AZA restored both the transcription of mRNA and expression of GPx4 (Fig. 7E–G). Besides, transfection of the TET2 fragment without methylase residues did not restore the mRNA transcription and protein expression of GPx4 (Fig. 7H and I). The methylation-specific PCR (MSP) showed that TET2-OE upregulated GPx4 promoter methylation after CSE treatment (Fig. 7J). The above results indicated that TET2 regulates GPx4 expression by demethylating the GPx4 gene promoter involving in CSE induced ferroptosis.
Fig. 7.
TET2 deletion induces hypermethylation of the GPx4 promoter. A 5% CSE or phosphate-buffered saline (PBS) was added to BEAS-2b cells after transfection with TET2-targeting siRNA (siTET2) or negative control (NC). GPx4 mRNA levels were detected by real-time quantitative PCR (RT-qPCR) after 24 h. B 5% CSE or PBS was added to TET2 overexpression cell lines and controls. GPx4 mRNA levels were detected by qPCR after 24 h. C qPCR analysis of GPx4 mRNA transcript levels in the lung tissues from nonsmokers (n = 13), non-COPD smokers (n = 13), and COPD patients (n = 13). D qPCR analysis of GPx4 mRNA transcript levels in the lung tissues from WT and Tet2 knockout mice exposed to CS or normal air for 6 months (n = 4). E-G Dimethyl sulfoxide (DMSO) or 5-AZA (5 μM) was added to BEAS-2b cells. GPx4 protein or mRNA levels were detected by WB or qPCR after 24 h. H–I 5% CSE or PBS was added to BEAS-2b cells after transfection with a TET2 isoform or vector. GPx4 protein or mRNA levels were detected by WB or qPCR after 24 h. J TET2 overexpressed BEAS-2b cells were treated with CSE or PBS. GPx4 promoter methylation levels were detected by methylation-specific PCR. Data were mean ± SEM, Statistical differences were determined using one-way ANOVA with Bonferroni post-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001.
3.7. Co-administration of 5-AZA and NAC alleviates CS-induced COPD by inhibiting cell ferroptosis
In chronic obstructive pulmonary disease, NAC is used as an antioxidant to provide GSH and mucus-dissolving agents. Long-term NAC use reduces the recurrence of COPD acute exacerbation [41]. However, NAC alone cannot prevent COPD progression [42]. This research showed that a balanced antioxidant system requires stable GPx4 expression. Therefore, a low-dose DNA methylation inhibitor was applied to investigate whether restored GPx4 expression combined with antioxidants to provide GSH could prevent cell ferroptosis from CS induced-COPD. Mice were treated with low dose of NAC and/or 5-AZA for 6 months with concurrent CS exposure to test the potential therapeutic effect of co-administration of anti-oxidant and methylation inhibitor (Fig. 8A). As expected, NAC protected against COPD. However, the combined application of 5-AZA and NAC further magnified the therapeutic effect of NAC. Firstly, the morphology of mitochondria under TEM was observed. It was noted that co-administration of 5-AZA and NAC alleviated the destruction of mitochondria induced by CS (Fig. 8B). Moreover, the lipid peroxide condition and GSH/GSSG level were detected. The result revealed that the lipid peroxidation caused by CS exposure was mostly reduced under the 5-AZA and NAC co-administration. As expected, 5-AZA and NAC collectively decreased the level of lipid peroxidation (Fig. 8C). However, GSH/GSSG ratio was substantial decreased in CS exposed mice, which indicating a depletion of GSH under CS stimulation. Co-administration of 5-AZA and NAC partially rescued the expression of GSH, resulting in a partial restoration of the GSH/GSSG ratio (Fig. 8D). Regarding the mechanism, 5-AZA was found to enhance GPx4 in control cells, while NAC cannot rescue the expression of GPx4 in mice lung tissue. In addition, 5-AZA and NAC co-administration substantially increased the level of GPx4 protein (Fig. 8E and F).
Fig. 8.
N-acetylcysteine (NAC) combined with a low-dose adjuvant epigenetic therapy suppresses CS induced ferroptosis. A Flowchart of mouse experiments. Mice were exposed to 6 months of cigarette smoke (CS), while controls were exposed to room air. Mice were treated with NAC orally or/and 5-AZA intraperitoneally (i.p) from week 4–6 months of CS exposure and vehicle-treated controls received an equal volume of PBS. B Electron microscope images of mitochondria in mouse lung sections. (Scale bars: 2 μm and 500 nm). C Lipid peroxidation as assayed by flow cytometry using the C11-BODIPY probe. D Representative data of the GSH/GSSG ratio in mouse lung sections. E, F Quantification and representative immunoblot analysis of GPx4. β-actin was used as a loading control. Data were mean ± SEM, Statistical differences were determined using one-way ANOVA with Bonferroni post-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001.
We then assessed the hallmark of experimental COPD. The combination treatment of 5-AZA and NAC exhibited enhanced suppression of emphysema-like alveolar development compared to individual administration of 5-AZA or NAC alone (Fig. 9A–C). Besides, 5-AZA and NAC co-administration significantly attenuated CS induced epithelial accumulation and small airway enlargement (Fig. 9D–G). These findings suggested that co-administration of 5-AZA and NAC rescue emphysema and small airway remodeling in CS-induced COPD through inhibiting cell ferroptosis.
Fig. 9.
Co-administration of N-acetylcysteine (NAC) and 5-AZA are superior in suppressing hallmark features of COPD. A-C Mouse lung sections were stained with hematoxylin and eosin (H&E) (scale bar: 100 μm) and emphysema-like alveolar enlargement was quantified based on the measurement of mean linear intercept and destructive index (n = 6). D, F Mouse lung sections were stained with H&E (scale bar: 50 μm; inserts show the expanded image of the indicated region) and quantification of epithelial thickness in the small airways was normalized to the perimeter of the basement membrane (n = 4). E, G Collagen deposition around small airways in lung sections was assessed using Masson's trichrome histochemistry (blue color indicates collagen, scale bar: 50 μm; inserts show the expanded image of the indicated region)(n = 4). Quantification of the collagen area around the small airways was normalized to the perimeter of the basement membrane. Data were presented as mean ± SEM, Statistical differences were determined using one-way ANOVA with Bonferroni post-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ***P ≤ 0.0001. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Several studies demonstrate that epigenetic modulators involve in COPD development. Herein we demonstrated that TET2 deletion contributes to COPD pathogenesis. TET2 expression was lower in the lung cells of COPD patients and the CS exposed murine model, compared to normal controls. Furthermore, TET2-/- mice model showed that the loss of TET2 significantly impacts experimental COPD development, as TET2 deficiency exaggerated emphysema and small airway remodeling after CS exposure. Additionally, we found that TET2 protects against the CS induced ferroptosis of epithelial cells through demethylating of GPx4 promoter and lipid peroxidation. Notably, N-acetylcysteine (NAC) combined with a low-dose adjuvant epigenetic therapy suppressed experimental COPD morphologic changes.
Small airway remodeling, inflammation, and emphysema are the three main intractable and pathologic features of COPD. Herein, we demonstrated that TET2 was down-regulated in the lung tissue of COPD and airway epithelial cells treated with CSE. Moreover,the absence of TET2 has a notable detrimental effect on experimental COPD, as TET2 deficiency exacerbates both emphysema and airway remodeling following exposure to CS. Besides, by utilizing Tet2-/- and C57BL/6 mice subjected to long-term CS exposure, this study revealed that TET2 deficiency led to mortality in mice and exhibited downregulation in lung and airway epithelial cells in the context of COPD. Although Tet2-/- mice showed significant mortality under CS exposure, which likely introduced a bias that could have influenced the analysis. The inclusion of Tet2 heterozygous mice in the study would be more advantageous and allow for a more nuanced assessment of the function of TET2. Previous studies have demonstrated the pivotal role of TET2 in myelopoiesis [20,21], inflammation regulation [19,22,23], and lung disorders [[24], [25], [26], [27]]. TET2 enhanced intracellular connections between epithelial cells in the early phase of pneumonia caused by P. aeruginosa [25]. Furthermore, TET2 was downregulated in pulmonary arterial hypertension (PAH), and pulmonary arterial hypertension develops spontaneously in homozygous Tet2-/- mice [26]. Also, TET2 participated in cellular oxidative stress caused by arsenic in bronchial epithelial cells [27]. Notably, specific deletion of TET2 in myeloid cells exacerbated inflammation and destruction of lung tissue caused by LPS [23]. Besides, both GWAS and gene-targeted deep sequencing studies showed that TET2 mutation is a risk factor for COPD [24,28]. Recent studies have revealed that ablation of TET2 in hematopoietic myeloid cells aggravated emphysema and inflammation in mouse models induced by CS short-term exposure and CS/poly(I:C) stimulation [29]. The single-cell RNA sequencing analysis revealed that specific deletion of TET2 in hematopoietic myeloid cells did not have a significant impact on the distribution of cell types, but upregulated interferon type I (IFN–I) and IFN-II signaling [29]. However, our data only indicated a difference in the numbers of neutrophils between WT mice and Tet2-/- mice with CS exposure. After all, neutrophilic airway inflammation is a pivotal part of COPD pathophysiology. Our in vivo findings suggest that genetic ablation of TET2 is not sufficient to determine the onset and severity of COPD without CS exposure but makes a dramatic contribution to increase the susceptibility of CS-induced COPD. Our findings further revealed the role of TET2 in the bronchial epithelial cells and COPD development.
The main etiology of COPD is that it is a progressive condition that is CS-induced and causes cell death, including apoptosis, necroptosis and ferroptosis [43]. The COPD lungs showed evidence of apoptosis in T lymphocytes, alveolar epithelial cells, and airway epithelial cells in conjunction with tissue destruction [44]. In CS-exposed animal models and human COPD lungs, necroptosis occurred, and its regulatory protein RIPK1 was overexpressed in lung epithelial cells [45]. A study by Yoshida demonstrated that ferroptosis in human bronchial epithelial cells (HBECs) contributes partially to cell death induced by CSE [8]. Previously we stated that cigarette smoke causes oxidative stress, ferroptosis, and emphysema in the lung tissue of model mice [11]. Dihydroquercetin (DHQ), an anti-oxidant intraperitoneally injected reduces emphysema and prevents HBEC ferroptosis efficiently [11]. This study further demonstrated that ferroptosis mainly occurred in airway epithelial cells is involved in CS long-term exposure induced COPD. Besides, airway epithelial cell ferroptosis might further cause tissue destruction and amplify inflammation, oxidative stress, and collagen deposition. This will induce small airway remodeling and emphysematous morphology change. Interestedly, though the apoptosis inhibitor slightly decreased CSE and TET2 KD induced cell death, the difference did not reach statistical significance. However, ferroptosis inhibitor vigorously alleviated epithelial cell death induced by CSE and TET2 KD, which suggested that TET2 deficiency induced cell death is ferroptosis dependent. The above observations also suggested a potential relationship between apoptosis and ferroptosis, aligning with previous reports [46,47]. Above all, TET2 deficiency promotes CS-induced epithelial cell ferroptosis and is involved in COPD pathogenesis, other potential mechanisms including apoptosis, pyroptosis and necroptosis contributing to COPD development may also exist.
Herein, the mechanism by which TET2 regulated ferroptosis was investigated. As previously reported [48], iron metabolism and the SLC7A11-GSH-GPx4 pathway are the most prominent mechanisms in ferroptosis. Our results demonstrated that TET2 has no effect on iron accumulation, while plays an important role in GSH consumption. However, TET2 knockdown severely worsen the decline of GSH/GSSG ratio, rather than reducing GSH level itself. Moreover, CS-induced GSH depletion and increased lipid dioxide release, and TET2 deficiency exacerbated this phenomenon. Restoring TET2 expression abrogated the GSH depletion and reduced lipid peroxide products. SLC7A11 acts as the “gatekeeper” regulating GSH transportation, whereas GPx4 catalyzed GSH to GSSG to maintain an anti-oxidation balance [[49], [50], [51], [52]]. Our in vitro study demonstrated that overexpression of TET2 decreased the ferroptosis of airway epithelial cells through the GSH-GPx4 system but not SLC7A11. Several studies showed that GPx4 is regulated through DNA methylation [39,40]. GPx4 is hypomethylated upstream and undergoes active epigenetic modifications at H3K4me3 (trimethylation of histone H3 at lysine 4) and H3K27ac (acetylation of histone H3 at lysine 27) of its transcription start site [53]. Besides, high levels of GPx4 in cancer are resulted from epigenetic regulation [54]. Meanwhile, homocysteine enhanced the methylation of GPx4 to promote induce oxidative stress and ferroptosis in the nucleus pulposus, contributing to disc degeneration [40]. This study broadened the paradigm that epigenetic modulator TET2 alleviated ferroptosis of lung epithelial cells induced by CS through demethylating GPx4 promoter and participated in the development of CS induced COPD.
Clinical studies showed that long-term use of NAC reduced the frequency of occurrence of acute exacerbation of COPD [41,55]. However, it has no significant effects on lung function deterioration and disease progression [42]. This suggests that the treatment of COPD with NAC has limitations, probably due to its inability to block the pathogenesis of COPD. This study co-administrated DNA methylation inhibitors and antioxidants in COPD mice models and found a significant protective effect on COPD development, which through inhibiting ferroptosis of lung epithelial cells. The mechanism underlying the inhibition of ferroptosis may be associated with the enhanced rescuing of GSH depletion and GPx4 levels. Additional studies are required to optimize the treatment regimen of the combination of NAC and low-dose adjuvant epigenetic therapy and assess its long-term efficacy and safety in clinical settings in the future. Overall, exploring dual or triple combinations of medications targeting COPD pathogenesis could be a promising therapeutic option for COPD, warranting further investigation.
In conclusion, TET2 deficiency promotes small airway remodeling induced by CS, chronic inflammation, and emphysema in COPD. Furthermore, TET2 deficiency demethylates the GPx4 promoter, which exacerbates GSH depletion induced by CS and airway epithelial cell ferroptosis. A combination of methylation inhibitors and antioxidants is a potential novel therapeutic application for COPD.
5. Conclusion
Our study highlights the critical role of TET2 as a modulator in airway epithelial cell lipid peroxidation and ferroptosis, suggesting its potential as a therapeutic target for CS-induced COPD.
Funding sources
This work was supported by the National Natural Science Foundation of China (No. 81873410, 82070049 and 81400032), Natural Science Foundation of Hunan Province (No. 2022JJ30060), the Fundamental Research Funds for the Central Universities of Central South University (No. 2018zzts046 and 2021zzts0369) and the Hunan Provincial Innovation Foundation for Postgraduate (CX20210371).
Author contributions
ZH Zeng, T Li, and Y Chen conceived and designed the research; ZH Zeng, T Li, XM Liu, YM Ma, LJ Luo and ZQ Zhao performed experiments; ZH Zeng and T Li analyzed the data; ZH Zeng and T Li drafted the manuscript; all the authors reviewed and approved the final version of the manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Acknowledgements
The authors would like to thank all the participants involved in this study. are grateful to professor Xu Guoliang (Shanghai Institute of Biological Sciences, Chinese Academy of Sciences) for providing the TET2 knockout mice.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102916.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Agustí A., Celli B.R., Criner G.J., et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir. J. 2023;(4):61. doi: 10.1183/13993003.00239-2023. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes P.J. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). Jul 1 2017;131(13):1541–1558. doi: 10.1042/cs20160487. [DOI] [PubMed] [Google Scholar]
- 3.Wang C., Zhou J., Wang J., et al. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct Target Ther. Oct 27 2020;5(1):248. doi: 10.1038/s41392-020-00345-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Y.Y., Zhang D.W., Ye J.J., et al. Interleukin-6 neutralizing antibody attenuates the hypersecretion of airway mucus via inducing the nuclear translocation of Nrf2 in chronic obstructive pulmonary disease. Biomed. Pharmacother. 2022;152 doi: 10.1016/j.biopha.2022.113244. Aug. [DOI] [PubMed] [Google Scholar]
- 5.Xu H., Xu F., Lu H., et al. S1PR2 is important for cigarette smoke-induced pyroptosis in human bronchial epithelial cells. Arch. Med. Res. 2023 doi: 10.1016/j.arcmed.2023.03.005. Mar 27. [DOI] [PubMed] [Google Scholar]
- 6.Dixon S.J., Lemberg K.M., Lamprecht M.R., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. May 25 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W., Deng H., Hu S., et al. Role of ferroptosis in lung diseases. J. Inflamm. Res. 2021;14:2079–2090. doi: 10.2147/jir.S307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida M., Minagawa S., Araya J., et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat. Commun. Jul 17 2019;10(1):3145. doi: 10.1038/s41467-019-10991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Fu C., Liu J., et al. Hypermethylation of the Nrf2 promoter induces ferroptosis by inhibiting the nrf2-GPX4 Axis in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2021;16:3347–3362. doi: 10.2147/copd.S340113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia H., Wu Y., Zhao J., et al. N6-Methyladenosine-modified circSAV1 triggers ferroptosis in COPD through recruiting YTHDF1 to facilitate the translation of IREB2. Cell Death Differ. 2023 doi: 10.1038/s41418-023-01138-9. Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Ma Y., Luo L., et al. Dihydroquercetin suppresses cigarette smoke induced ferroptosis in the pathogenesis of chronic obstructive pulmonary disease by activating Nrf2-mediated pathway. Phytomedicine. 2022;96 doi: 10.1016/j.phymed.2021.153894. Feb. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y., Luo L., Zeng Z., et al. MFG-E8 stabilized by deubiquitinase USP14 suppresses cigarette smoke-induced ferroptosis in bronchial epithelial cells. Cell Death Dis. Jan 3 2023;14(1):2. doi: 10.1038/s41419-022-05455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X., Mu X., Deng L., et al. The etiologic origins for chronic obstructive pulmonary disease. Int. J. Chronic Obstr. Pulm. Dis. 2019;14:1139–1158. doi: 10.2147/copd.S203215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Vries M., van der Plaat D.A., Nedeljkovic I., et al. From blood to lung tissue: effect of cigarette smoke on DNA methylation and lung function. Respir. Res. 2018;19(1):212. doi: 10.1186/s12931-018-0904-y. Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson E., Korologou-Linden R., Wootton R.E., et al. Smoking, DNA methylation, and lung function: a mendelian randomization analysis to investigate causal pathways. Am. J. Hum. Genet. 2020;106(3):315–326. doi: 10.1016/j.ajhg.2020.01.015. Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zong D., Liu X., Li J., Ouyang R., Chen P. The role of cigarette smoke-induced epigenetic alterations in inflammation. Epigenet. Chromatin. Nov 11 2019;12(1):65. doi: 10.1186/s13072-019-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohli R.M., Zhang Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature. Oct 24 2013;502(7472):472–479. doi: 10.1038/nature12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng H., Li T., He X., et al. Oxidative stress mediates the apoptosis and epigenetic modification of the Bcl-2 promoter via DNMT1 in a cigarette smoke-induced emphysema model. Respir. Res. Sep 3 2020;21(1):229. doi: 10.1186/s12931-020-01495-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cong B., Zhang Q., Cao X. The function and regulation of TET2 in innate immunity and inflammation. Protein Cell. 2021;12(3):165–173. doi: 10.1007/s13238-020-00796-6. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solary E., Bernard O.A., Tefferi A., Fuks F., Vainchenker W. The Ten-Eleven Translocation-2 (TET2) gene in hematopoiesis and hematopoietic diseases. Leukemia. 2014;28(3):485–496. doi: 10.1038/leu.2013.337. Mar. [DOI] [PubMed] [Google Scholar]
- 21.Moran-Crusio K., Reavie L., Shih A., et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. Jul 12 2011;20(1):11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Q., Zhang Q., Shi Y., et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature. 2018;554(7690):123–127. doi: 10.1038/nature25434. Feb 1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q., Zhao K., Shen Q., et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. Sep 17 2015;525(7569):389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wain L.V., Shrine N., Miller S., et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir. Med. 2015;3(10):769–781. doi: 10.1016/S2213-2600(15)00283-0. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin W., Brands X., Van't Veer C., de Vos A.F., Scicluna B.P., van der Poll T. Bronchial epithelial Tet2 maintains epithelial integrity during acute Pseudomonas aeruginosa pneumonia. Infect. Immun. Dec 15 2020;(1):89. doi: 10.1128/iai.00603-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potus F., Pauciulo M.W., Cook E.K., et al. Novel mutations and decreased expression of the epigenetic regulator TET2 in pulmonary arterial hypertension. Circulation. Jun 16 2020;141(24):1986–2000. doi: 10.1161/circulationaha.119.044320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q., Wang W., Zhang A. TET-mediated DNA demethylation plays an important role in arsenic-induced HBE cells oxidative stress via regulating promoter methylation of OGG1 and GSTP1. Toxicol. Vitro. Apr 2021;72 doi: 10.1016/j.tiv.2020.105075. [DOI] [PubMed] [Google Scholar]
- 28.Buscarlet M., Provost S., Zada Y.F., et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood. Aug 10 2017;130(6):753–762. doi: 10.1182/blood-2017-04-777029. [DOI] [PubMed] [Google Scholar]
- 29.Miller P.G., Qiao D., Rojas-Quintero J., et al. Association of clonal hematopoiesis with chronic obstructive pulmonary disease. Blood. 2022;139(3):357–368. doi: 10.1182/blood.2021013531. Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X., Zhang L., Mao S.Q., et al. Tet and TDG mediate DNA demethylation essential for mesenchymal-to-epithelial transition in somatic cell reprogramming. Cell Stem Cell. Apr 3 2014;14(4):512–522. doi: 10.1016/j.stem.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Dakhlallah D., Batte K., Wang Y., et al. Epigenetic regulation of miR-17∼92 contributes to the pathogenesis of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2013;187(4):397–405. doi: 10.1164/rccm.201205-0888OC. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lao T., Jiang Z., Yun J., et al. Hhip haploinsufficiency sensitizes mice to age-related emphysema. Proc. Natl. Acad. Sci. U. S. A. Aug 9 2016;113(32):E4681–E4687. doi: 10.1073/pnas.1602342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S., Liu D., Luo S., et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat. Commun. Jul 16 2019;10(1):3108. doi: 10.1038/s41467-019-10902-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang N., Chen P., Chen Y., Zeng H., He X., Zhu Y. PRMT6 mediates CSE induced inflammation and apoptosis. Int. Immunopharm. 2015;24(1):95–101. doi: 10.1016/j.intimp.2014.10.029. Jan. [DOI] [PubMed] [Google Scholar]
- 35.Pierce J.A., Ebert R.V. The barrel deformity of the chest, the senile lung and obstructive pulmonary emphysema. Am. J. Med. 1958;25(1):13–22. doi: 10.1016/0002-9343(58)90193-1. Jul. [DOI] [PubMed] [Google Scholar]
- 36.Mattos W.L., Signori L.G., Borges F.K., Bergamin J.A., Machado V. Accuracy of clinical examination findings in the diagnosis of COPD. J. Bras. Pneumol. 2009;35(5):404–408. doi: 10.1590/s1806-37132009000500003. May. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Z., Du S., Du Y., Ren J., Ying G., Yan Z. Glutathione reductase mediates drug resistance in glioblastoma cells by regulating redox homeostasis. J. Neurochem. 2018;144(1):93–104. doi: 10.1111/jnc.14250. Jan. [DOI] [PubMed] [Google Scholar]
- 38.Ye Y., Chen A., Li L., et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. Dec 2022;102(6):1259–1275. doi: 10.1016/j.kint.2022.07.034. [DOI] [PubMed] [Google Scholar]
- 39.Ling H., Li M., Yang C., et al. Glycine increased ferroptosis via SAM-mediated GPX4 promoter methylation in rheumatoid arthritis. Rheumatology. Feb 8 2022 doi: 10.1093/rheumatology/keac069. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X., Huang Z., Xie Z., et al. Homocysteine induces oxidative stress and ferroptosis of nucleus pulposus via enhancing methylation of GPX4. Free Radic. Biol. Med. 2020;160:552–565. doi: 10.1016/j.freeradbiomed.2020.08.029. Nov 20. [DOI] [PubMed] [Google Scholar]
- 41.Zheng J.P., Wen F.Q., Bai C.X., et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir. Med. 2014;2(3):187–194. doi: 10.1016/s2213-2600(13)70286-8. Mar. [DOI] [PubMed] [Google Scholar]
- 42.Decramer M., Rutten-van Mölken M., Dekhuijzen P.N., et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. Apr 30-May 6 2005;365(9470):1552–1560. doi: 10.1016/s0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 43.Sauler M., Bazan I.S., Lee P.J. Cell death in the lung: the apoptosis-necroptosis Axis. Annu. Rev. Physiol. 2019;81:375–402. doi: 10.1146/annurev-physiol-020518-114320. Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung K.F., Adcock I.M. Multifaceted mechanisms in COPD: inflammation, immunity, and tissue repair and destruction. Eur. Respir. J. 2008;31(6):1334–1356. doi: 10.1183/09031936.00018908. Jun. [DOI] [PubMed] [Google Scholar]
- 45.Van Eeckhoutte H.P., Donovan C., Kim R.Y., et al. RIPK1 kinase-dependent inflammation and cell death contribute to the pathogenesis of COPD. Eur. Respir. J. 2023;61(4) doi: 10.1183/13993003.01506-2022. Apr. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Chen X., Liu N., et al. A nuclear long non-coding RNA LINC00618 accelerates ferroptosis in a manner dependent upon apoptosis. Mol. Ther. 2021;29(1):263–274. doi: 10.1016/j.ymthe.2020.09.024. Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye Z., Zhuo Q., Hu Q., et al. FBW7-NRA41-SCD1 axis synchronously regulates apoptosis and ferroptosis in pancreatic cancer cells. Redox Biol. 2021;38 doi: 10.1016/j.redox.2020.101807. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockwell B.R. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. Jul 7 2022;185(14):2401–2421. doi: 10.1016/j.cell.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi: 10.1038/s41422-020-00441-1. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu Y., Cao Y., Cao W., Jia Y., Lu N. The application of ferroptosis in diseases. Pharmacol. Res. 2020;159 doi: 10.1016/j.phrs.2020.104919. Sep. [DOI] [PubMed] [Google Scholar]
- 51.Jiang X., Stockwell B.R., Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen X., Kang R., Kroemer G., Tang D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021;7(6):218. doi: 10.1084/jem.20210518. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., Sui S., Wang L., et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J. Cell. Physiol. 2020;235(4):3425–3437. doi: 10.1002/jcp.29232. Apr. [DOI] [PubMed] [Google Scholar]
- 54.Wu Y., Zhang S., Gong X., et al. The epigenetic regulators and metabolic changes in ferroptosis-associated cancer progression. Mol. Cancer. Feb 27 2020;19(1):39. doi: 10.1186/s12943-020-01157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poole P., Sathananthan K., Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. May 20 2019;5(5) doi: 10.1002/14651858.CD001287.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.