Abstract
Background
Botulinum toxin type A (BTX-A) injection is being widely used off-label for muscular hypertrophy, including deltoid muscle hypertrophy. However, very few studies have evaluated the optimal dosage and its clinical response.
Objective
This study aimed to assess the efficacy and safety of different doses of Prabotulinum toxin A (PBoNT) for treating deltoid muscle hypertrophy.
Methods
Twelve particiapants with bilateral deltoid muscle hypertrophy were enrolled and randomly received either 16 U or 32 U of PBoNT. In each participant, the same dose was administered to both deltoid muscles. Both participants and evaluators were blinded. Deltoid muscle thickness and upper arm circumference were measured on day 0, and weeks 2, 4, and 12 after the PBoNT injection.
Results
Upper arm circumference significantly decreased in both groups; however, deltoid muscle thickness was reduced in the 16 U group only. No major complications were reported in both groups. However, a few minor complications were reported in the 16 U injection group.
Conclusion
Both 16 U and 32 U of PBoNT intramuscular injections are safe and effective in treating deltoid hypertrophy.
Keywords: Botulinum toxins, Deltoid muscle, Hypertrophy
INTRODUCTION
Asian women have recently become more interested in upper arm contouring. Although brachioplasty, liposuction, and other procedures are being tried for upper arm contouring, these techniques are mainly for contouring arm flab1. To date, very few studies have been performed on deltoid contouring.
Botulinum toxin type A (BTX-A) injection into the masseter muscle has been used as an off-label treatment to improve facial contours. For the same aesthetic purpose, BTX-A injections are used to treat gastrocnemius and trapezius hypertrophy2,3,4,5,6.
In a recent pilot study, it was observed that injecting 25 U of BTX-A significantly reduces the thickness of the deltoid muscle after 2 to 12 weeks; however, participant satisfaction and clinical improvement were not significant7. This study was intended to evaluate the effectiveness and safety of different dosages of BTX-A in treating deltoid muscle hypertrophy and upper arm contouring.
MATERIALS AND METHODS
Twelve healthy Korean women (age, 28~40 years) with hypertrophy of both deltoid muscles were enrolled in this double-blinded, randomized multi-center clinical trial after obtaining informed consent. To reduce the effect of fat on deltoid hypertrophy, women with a body mass index (BMI) of less than 25 were selected. The study was approved by the Institutional Review Board of Chung-Ang University Hospital (IRB No. 2020-007-445). We received the patient’s consent form about publishing all photographic materials.
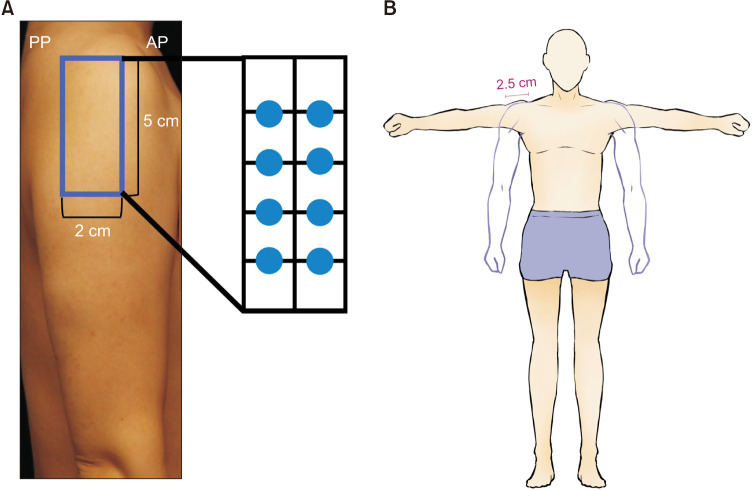
Prabotulinum toxin A (PBoNT) (NABOTA®; Daewoong Pharmaceutical Co. Ltd.) was used in the study. Participants randomly received either 16 U or 32 U of PBoNT via intramuscular injection in the deltoid muscle. PBoNT was injected at 8 points, each 1 cm apart within a 2×5 cm region situated 5 cm below the acromion, where nerves and arteries do not pass (Fig. 1). For 16 U group (2 U per point), PBoNT 100 U was diluted in normal saline 2.5 ml. PBoNT 100 U was diluted in normal saline 5 ml in 32 U group (4 U per point).
Fig. 1. (A) Participants received botulinum toxin type A injections at 8 different points that were 1 cm apart in a 2×5 cm region located 5 cm below the lower edge of the acromion. (B) The circumference of the upper arm was measured using a measuring tape at a point 2.5 cm below the acromion between the anterolateral point (AP) and posterolateral point (PP).
Particiapants were assessed and photographs were taken at baseline and 2, 4, and 12 weeks after the PBoNT injection. The size of the deltoid muscle was calculated by measuring the thickness of the deltoid muscle and upper arm circumference. The thickness of the deltoid muscle was measured twice by ultrasound, once in the anatomical position (relaxed) and the other with arms vertically raised (contracted). The thickness was determined by dividing the ultrasound image screen into 5 equal parts and the average of the four shortest straight distances from the boundary line was calculated. The evaluation of the thickness was conducted by the same investigator to minimize variation and bias. The measurement technique was identical to that used in our pilot study7. The circumference of the upper arm was measured using a measuring tape at a point 2.5 cm below the acromion, between the anterolateral point and the posterolateral point of the deltoid muscle.
Participant satisfaction score was assessed using a 5-point scoring system (1, unsatisfied; 2, slightly unsatisfied; 3, neutral; 4, slightly satisfied; and 5, satisfied). Clinical improvement was assessed by two blinded dermatologists using paired pre- and post-treatment photographs with a 5-point scoring system (1, improved; 2, minimally improved; 3, steady-state; 4, minimally worsened; 5, worsened). All adverse events were recorded throughout the study period.
IBM SPSS version 26.0 for Windows (IBM Corp.) was used for statistical analyses. Wilcoxon’s signed-rank test was used to compare the thickness of the deltoid muscles and the circumference of the upper arm before and after the treatment. Two-way repeated measures analysis of variance (ANOVA) was used to assess differences in deltoid thickness and upper arm circumference in both groups at different time points. The agreement between the assessments of the two blinded dermatologists was evaluated by Cohen’s kappa. The frequency of adverse effects was evaluated using the chi-square test. p-values <0.05 were considered statistically significant.
RESULTS
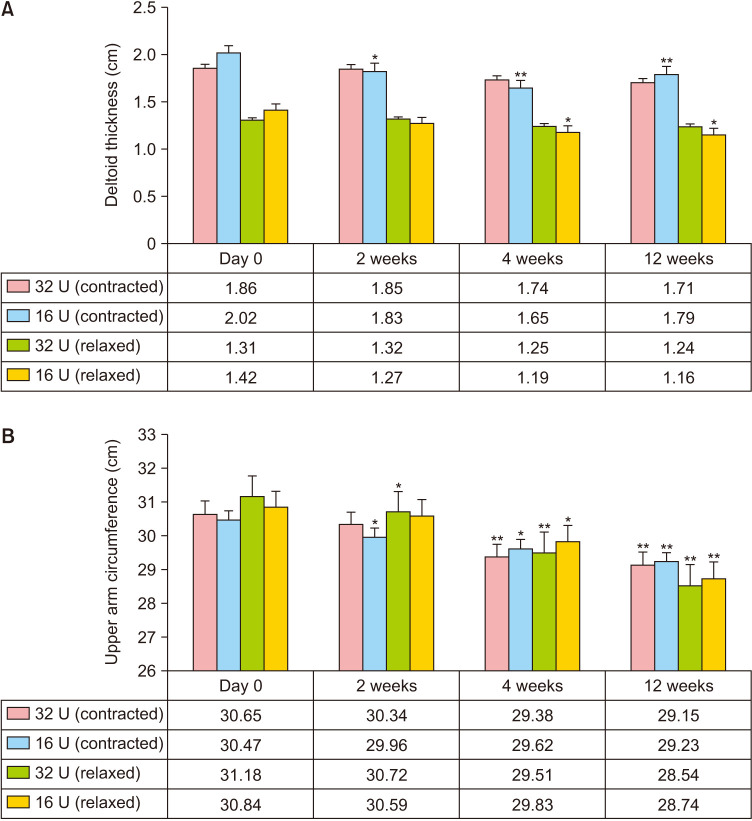
Twelve participants were enrolled and none were lost to follow-up. The average thickness of the deltoid muscles and the circumference of the upper arms at each visit are shown in Fig. 2. At the baseline, there were no significant differences in upper arm circumference and deltoid thickness between the 32 U and 16 U groups. The average thickness of the deltoid muscle showed a decreasing trend following PBoNT injection in both groups; however, the 16 U injection group showed a significant reduction in the upper shoulder at 2, 4, and 12 weeks and lower shoulder at 4 and 12 weeks (Fig. 2A). Upper arm circumference also decreased after PBoNT injection in both groups. In the 32 U injection group, a significant decrease was observed compared to baseline at 4 and 12 weeks in the contracted position and weeks 2, 4, and 12 in the relaxed position. In the 16 U injection group, a significant decrease was observed at 2, 4, and 12 weeks in the contracted position and weeks 2 and 4 in the relaxed position (Fig. 2B). Both the 32 U group and the 16 U group showed a reduction in deltoid muscle thickness and upper arm circumference with time, but there were no significant differences between the two groups. Fig. 3 shows representative photographs of the 16 U injection group.
Fig. 2. Analysis of the deltoid muscle size. (A) Deltoid hypertrophy was significantly improved in the 16 U injection group at weeks 4 and 12 compared to the baseline. (B) The upper arm circumference of participants in both groups was significantly improved at weeks 8 and 12 compared to baseline (*p<0.05, **p<0.01).
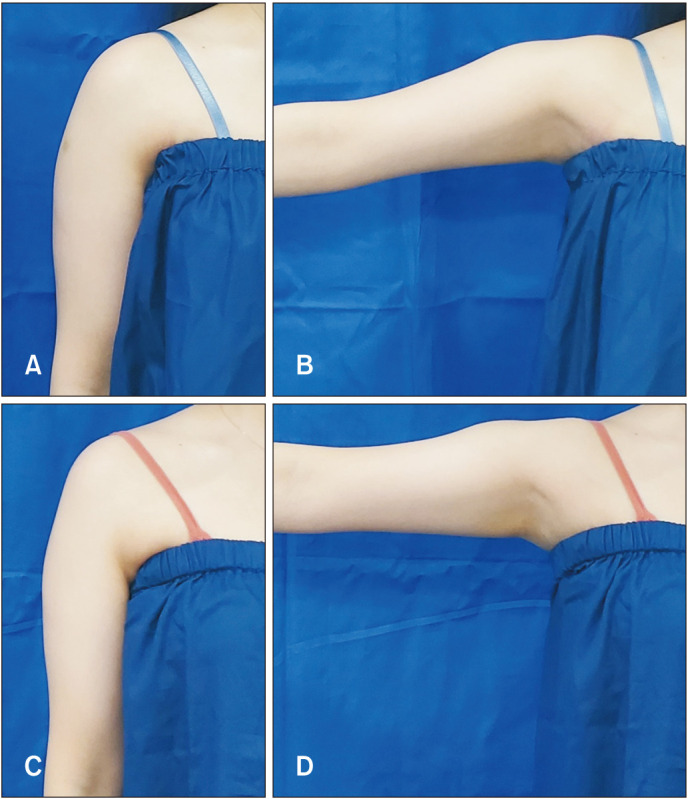
Fig. 3. Clinical photographs of the study participants. (A) Deltoid muscles in the relaxed state at baseline. (B) Deltoid muscles in the contracted state at baseline. (C) Deltoid muscles in the relaxed state with improved contour at week 12 following prabotulinum toxin A (PBoNT) (16 U) injection. (D) Deltoid muscles in the contracted state with improved contour at week 12 following PBoNT (16 U) injection.
Participants with a satisfaction score of 4 or higher were defined as the “satisfied group.” The satisfied group comprised 66.67%, 83.33%, and 66.67% of participants from the 32 U injection group and 16.67%, 16.67%, and 16.67% of participants from the 16 U injection group at weeks 2, 4, and 12, respectively. In clinical evaluation through photographs by two blinded dermatologists, the percentage of upper arms with a score of grade 2 or lower was defined as the “responder rate.” The responder rates of the 32 U group at weeks 2, 4, and 12 were 50.00%, 25.00%, and 16.67%, respectively, while those of the 16 U group were 25.00%, 66.67%, and 50.00%, respectively. Cohen’s kappa was calculated as 0.24, meaning fair agreement.
No major adverse events such as muscular dystrophy or moderate/severe muscle weakness were observed in any group. Several minor adverse events, including ecchymosis, pain, swelling, and mild weakness were observed in both groups. The incidence of adverse events was significantly less in the 16 U group (13 cases) compared to that in the 32 U group (26 cases) (p<0.05).
DISCUSSION
BTX-A is used widely for improving glabellar wrinkles, hyperhidrosis, body contouring, and other non-invasive facial aesthetic procedures8. Mechanistically, BTX inhibits presynaptic exocytosis of acetylcholine-containing vesicles at the neuromuscular junction, which occasionally leads to muscle weakness and paralysis9. Therefore, it is important to standardize BTX-A doses to prevent these complications10. In a previous pilot study, although objective measures indicated improvements, participants expressed low satisfaction, possibly because the aesthetic improvement did not meet their expectations7. Additionally, this study aims to determine whether the low aesthetic improvement observed in the previous study was caused by an inadequate BTX-A dosage or by the limited ability of BTX-A injection in correcting aesthetics of the upper arm.
Like the previous study, this study demonstrated improvements in objective measures after BTX-A injection, but subjective evaluations were not satisfactory. The disparity between the objective measurements and subjective evaluations may be attributable to confounding factors such as skin quality, fat distribution, and muscle shape, in addition to muscle bulk1. Although individuals with high BMI values were excluded, participants with low muscle mass and high fat mass may have been dissatisfied with the overall improvement. These variables likely acted as confounding factors and caused unexplained disparities. These findings suggest that BTX-A injection effectively improves deltoid muscle hypertrophy, but combining it with other procedures, such as liposuction, may increase overall cosmetic satisfaction.
This study has several limitations. Firstly, it was a pilot study with a small sample size and short-term follow-up. Secondly, the severity of deltoid hypertrophy and the dominant arm was not evaluated. In a previous trial for gastrocnemius hypertrophy, different doses of botulinum toxin were injected based on the disease severity11. It is worth noting that the dominant arm has more muscle mass than the non-dominant arm, which may require dose adjustments to achieve a symmetric shape and efficacy on both sides. Therefore, further studies are needed, including different dose settings, consideration of the dominant arm, larger sample sizes, and longer follow-up periods.
Ultrasound was used in this study to measure deltoid mucle thickness. Ultrasound is relatively inexpensive and easy to perform, it does require a skilled technician to operate the equipment12. Computed tomography, magnetic resonance imaging, or 3D mapping technology are also useful for measuring muscle thickness, but they have their own limitations, such as higher costs compared to sonography5,13,14.
In this study, the efficacy and safety of BTX-A injection at 16 U and 32 U doses for deltoid hypertrophy were evaluated. Both doses of BTX-A were found to be effective in reducing deltoid muscle volume. However, to achieve overall cosmetic satisfaction, it is recommended to combine BTX-A injections with other procedures for upper arm contouring.
ACKNOWLEDGMENT
The botulinum toxin used in this study, i.e., Prabotulinum toxin, Botulinum toxin Type A (NABOTA®), was supplied by Daewoong Pharmaceutical Co. Ltd., Seoul, Korea.
Footnotes
CONFLICTS OF INTEREST: K.Y. Park and W.W. Choi are consultants and speakers of Deawoong Pharmaceuticals Inc., Korea.
FUNDING SOURCE: None.
DATA SHARING STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Miotto G, Ortiz-Pomales Y. Arm contouring: review and current concepts. Aesthet Surg J. 2018;38:850–860. doi: 10.1093/asj/sjx218. [DOI] [PubMed] [Google Scholar]
- 2.Han KH, Joo YH, Moon SE, Kim KH. Botulinum toxin A treatment for contouring of the lower leg. J Dermatolog Treat. 2006;17:250–254. doi: 10.1080/09546630600899070. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Lee DW, Park YH, Cha MK, Kim HS, Ha SJ. Botulinum toxin A for aesthetic contouring of enlarged medial gastrocnemius muscle. Dermatol Surg. 2004;30:867–871. doi: 10.1111/j.1524-4725.2004.30255.x. [DOI] [PubMed] [Google Scholar]
- 4.Rauso R, Lo Giudice G, Tartaro G, Zerbinati N, Nicoletti GF, Fragola R. Botulinum toxin type A injections for masticatory muscles hypertrophy: a systematic review. J Craniomaxillofac Surg. 2022;50:7–18. doi: 10.1016/j.jcms.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Jeong SY, Park KY, Seok J, Ko EJ, Kim TY, Kim BJ. Botulinum toxin injection for contouring shoulder. J Eur Acad Dermatol Venereol. 2017;31:e46–e47. doi: 10.1111/jdv.13705. [DOI] [PubMed] [Google Scholar]
- 6.Zhou RR, Wu HL, Zhang XD, Ye LL, Shao HJ, Song XH, et al. Efficacy and safety of botulinum toxin type A injection in patients with bilateral trapezius hypertrophy. Aesthetic Plast Surg. 2018;42:1664–1671. doi: 10.1007/s00266-018-1201-3. [DOI] [PubMed] [Google Scholar]
- 7.Shin SH, Park SJ, Yeoum SH, Youn CS, Park KY. Efficacy and safety of botulinum toxin injection in reducing deltoid muscle hypertrophy. Dermatol Ther. 2021;34:e15168. doi: 10.1111/dth.15168. [DOI] [PubMed] [Google Scholar]
- 8.Park MY, Ahn KY. Scientific review of the aesthetic uses of botulinum toxin type A. Arch Craniofac Surg. 2021;22:1–10. doi: 10.7181/acfs.2021.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nestor MS, Arnold D, Fischer DL. The mechanisms of action and use of botulinum neurotoxin type A in aesthetics: Key Clinical Postulates II. J Cosmet Dermatol. 2020;19:2785–2804. doi: 10.1111/jocd.13702. Erratum in: J Cosmet Dermatol 2021;20:1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand PD, Couto RA, Isakov R, Yoo DB, Azizzadeh B, Guyuron B, et al. Botulinum toxin and muscle atrophy: a wanted or unwanted effect. Aesthet Surg J. 2016;36:482–487. doi: 10.1093/asj/sjv208. [DOI] [PubMed] [Google Scholar]
- 11.Seo KK. Botulinum toxin for Asians. Springer Singapore; 2017. pp. 52–58. [Google Scholar]
- 12.Dupont AC, Sauerbrei EE, Fenton PV, Shragge PC, Loeb GE, Richmond FJ. Real-time sonography to estimate muscle thickness: comparison with MRI and CT. J Clin Ultrasound. 2001;29:230–236. doi: 10.1002/jcu.1025. [DOI] [PubMed] [Google Scholar]
- 13.Bogari M, Tan A, Xin Y, Chai G, Lin L, Min P, et al. Treatment of gastrocnemius muscle hypertrophy with botulinum toxin injection followed by magnetic resonance imaging assessment and 3-dimensional evaluation. Aesthet Surg J. 2017;37:1146–1156. doi: 10.1093/asj/sjx070. [DOI] [PubMed] [Google Scholar]
- 14.Kim JH, Shin JH, Kim ST, Kim CY. Effects of two different units of botulinum toxin type A evaluated by computed tomography and electromyographic measurements of human masseter muscle. Plast Reconstr Surg. 2007;119:711–717. doi: 10.1097/01.prs.0000239453.67423.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.