Abstract
The sequence of the gene encoding a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-4) was determined. It was located in a plasmid harbored by a Salmonella typhimurium strain. CTX-M-4 was similar to the plasmidic cefotaxime-hydrolyzing β-lactamases CTX-M-2 and Toho-1 and related to the chromosomal β-lactamase of Klebsiella oxytoca. A Ser-237→Ala substitution, introduced by site-directed mutagenesis, caused minor alterations in the interaction of CTX-M-4 with β-lactams, reducing slightly the relative hydrolytic activity against cefotaxime and the susceptibility to inhibition by clavulanate.
Resistance to expanded-spectrum cephalosporins in enterobacteria is usually due to the expression of extended-spectrum TEM and SHV β-lactamases (14). The emergence of plasmid-mediated β-lactamases that are related to the chromosomal class A enzyme of Klebsiella oxytoca and that confer resistance to cephalosporins has recently been noted. The enzymes MEN-1 (3) and Toho-1 (11) were found in Escherichia coli clinical strains isolated in France and Japan, respectively; CTX-M-1, with an amino acid sequence identical to that of MEN-1, was found in an E. coli clinical isolate in Germany (5). β-Lactamase CTX-M-2 was from a Salmonella typhimurium strain isolated in Argentina (4). The β-lactamases described above have a distinct substrate profile in that they efficiently hydrolyze cefotaxime but spare ceftazidime (3–5, 11). It has been hypothesized that the extended-spectrum hydrolytic properties of the β-lactamases mentioned above are due, at least in part, to a serine residue located at position 237 (Ambler’s numbering scheme [1]) (3). In other studies, however, this hypothesis has been considered uncertain (11).
In a previous work, we described a plasmid-mediated β-lactamase, not belonging to either the TEM or SHV family or to AmpC cephalosporinases, that preferentially hydrolyzed cefotaxime. The plasmid encoding this enzyme was harbored by an outbreak strain of S. typhimurium isolated in St. Petersburg, Russia (8). In the present work, it is shown that the enzyme, designated CTX-M-4, is closely related to the above-mentioned plasmid-mediated cefotaxime-hydrolyzing β-lactamases. We also examined the role of the Ser-237 residue in determining the substrate specificity of CTX-M-4 β-lactamase.
The cefotaxime-resistant E. coli(pMSL) transconjugant was obtained after mating E. coli 14R525 (Nalr lac+) with an S. typhimurium clinical isolate (8). The E. coli strain C600 was used for the preparation of β-lactamase extracts. The E. coli strains JM109 and ES1301 mutS (Promega) were used for cloning and mutagenesis. The latter strain suppresses mismatch repair in vivo. The plasmid pBCSK(+) (Stratagene) was used for cloning and sequencing. The plasmid pAlter-Ex2 (Promega) was used for site-directed mutagenesis.
MICs of β-lactam antibiotics were determined by a microdilution method using Mueller-Hinton broth (15). Screening for cefotaxime-resistant clones was performed by using standard antibiograms.
A 2.7-kbp EcoRI-BamHI fragment from plasmid pMSL was ligated to pBCSK(+). The resulting plasmid, pMSL1, conferred cefotaxime resistance when used to transform E. coli C600 and JM109 cells. Several overlapping fragments of the EcoRI-BamHI insert were subcloned to pBCSK(+), and the DNA sequence was determined by the dideoxy chain termination method with the Sequenase 2.0 kit (United States Biochemicals—Amersham).
An intragenic PstI-BamHI fragment of 0.9 kbp from pMSL1 was subcloned into pAlter-Ex2, and the resulting plasmid (pMSL2) was used for site-specific mutagenesis with the pAlter-Ex2 mutagenesis kit (Promega) as recommended by the manufacturer. The mutagenic primer was 30 bp and contained two mismatches in the triplet corresponding to codon 237 (GCC [Ala] instead of AGC [Ser]; mismatches underlined). The mutagenized insert was reintroduced into PstI-BamHI-digested pMSL1, resulting in plasmid pMSL1-M. The mutant gene and the promoter region were entirely sequenced to confirm the desired exchanges in the nucleotide sequence.
For β-lactamase preparations, E. coli C600 strains transformed with pMSL1 and pMSL1-M were grown overnight in tryptone soy broth. β-Lactamases were released after ultrasonic treatment of cell suspensions. The extracts were clarified by ultracentrifugation, desalted, and concentrated. One unit of β-lactamase activity was defined as the amount of enzyme hydrolyzing 1 μmol of nitrocefin per min per mg of protein at 37°C and pH 7. Isoelectric focusing was performed on polyacrylamide gels containing ampholytes (pH range, 3.5 to 9.5) (Pharmacia-LKB).
Hydrolytic activity against β-lactams was evaluated by UV spectrophotometry as described previously (9). The maximum rate of hydrolysis (relative Vmax) and Km values were determined by Lineweaver-Burk plots. The Vmax values were expressed as hydrolysis rates relative to that of cephalothin, which was set at 100. Inhibition of β-lactamase activity by clavulanate, sulbactam, and tazobactam was estimated by using nitrocefin at a concentration of 50 μM (9). The 50% inhibitory concentrations (IC50s) were determined from plots of the inhibitor concentration versus percent inhibition.
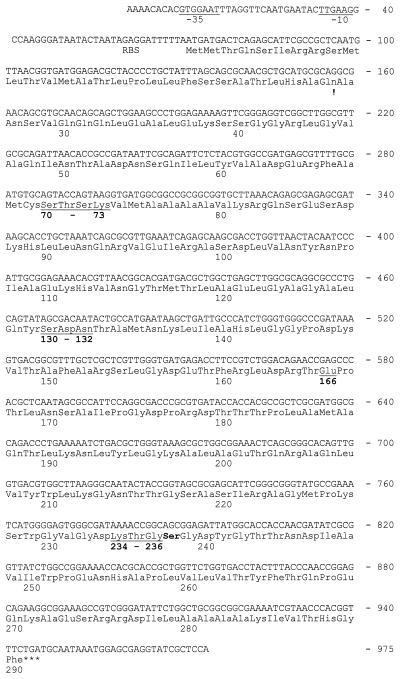
The nucleotide sequence of the blaCTX-M-4 gene and the deduced amino acid sequence are shown in Fig. 1. There was an open reading frame of 873 nucleotides corresponding to 291 amino acids. In the preceding sequence, putative −35 and −10 consensus sequences were located. A terminator hairpin loop was detected 10 nucleotides from the stop codon (TGA). The β-lactamase precursor showed >95% homology with CTX-M-2 (6) and Toho-1 (11). On the basis of this similarity and results from hydropathy plots, the signal peptide most likely consists of 29 amino acid residues. The mature β-lactamase comprised 262 amino acids with an isoelectric point equal to 8.4 (8) and an estimated molecular weight of 28,035. It included the 70SXXK73, 130SDN132, E-166, and 234KTG236 sequences that are typical of the class A enzymes (Fig. 1) (12). The mature β-lactamase differed in sequence by 8 amino acids from CTX-M-2 (96.9% homology) and 10 amino acids from Toho-1 (96.2% homology) (see Table 4); as for CTX-M-2 and Toho-1 β-lactamases, CTX-M-4 showed a lower degree of homology (80.9%) with CTX-M-1 (MEN-1) (3, 6).
FIG. 1.
Nucleotide and deduced amino acid sequences of CTX-M-4 β-lactamase. The conserved residues of the class A β-lactamases are underlined. The signal peptide is indicated by an exclamation mark. The Ambler numbering scheme was followed.
TABLE 4.
Amino acid differences between CTX-M-4 β-lactamase and the related cefotaxime-hydrolyzing enzymes Toho-1 and CTX-M-2a
| β-Lactamase | Amino acid residue at position:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 39 | 48 | 61 | 98 | 99 | 125 | 171 | 225 | 230 | 274 | |
| CTX-M-4 | Ser | Gln | Val | Arg | Ala | Gly | Ser | Met | Gly | Ser |
| CTX-M-2 | Ser | Leu | Arg | Lys | Lys | Ala | Thr | Leu | Val | Ser |
| Toho-1 | Arg | Leu | Arg | Lys | Lys | Ala | Thr | Leu | Val | Arg |
Only the differences in the mature peptides are indicated. The amino acid residues were numbered according to Ambler’s system (1).
The MICs of β-lactam antibiotics for E. coli JM109(pMSL1) and E. coli JM109(pMSL1-M) are given in Table 1. The MICs of the penicillins tested, piperacillin-tazobactam and ceftazidime, were essentially similar for the two strains. The combinations of amoxicillin with clavulanate and ampicillin with sulbactam, as well as aztreonam, were less active against the E. coli strain expressing the S237A mutant β-lactamase than the strain producing the wild-type CTX-M-4 enzyme. The S237A mutant β-lactamase, however, conferred lower levels of resistance to cefuroxime and cefotaxime than the wild-type CTX-M-4 did.
TABLE 1.
MICs of β-lactam antibiotics for strains producing CTX-M-4 wild-type and S237A mutant β-lactamases
| Antibiotic | MIC (μg/ml) for strain (enzyme)
|
||
|---|---|---|---|
| E. coli JM109(pMSL1) (CTX-M-4) | E. coli JM109(pMSL1-M) (S237A mutant) | E. coli JM109 | |
| Ampicillin | >512 | >512 | 4 |
| Amoxicillin-clavulanatea | 8 | 32 | 4 |
| Ampicillin-sulbactama | 32 | 128 | 4 |
| Ticarcillin | 128 | 256 | <1 |
| Piperacillin | 256 | 512 | <1 |
| Piperacillin-tazobactamb | 2 | 2 | <1 |
| Cefuroxime | 512 | 128 | |
| Cefotaxime | 512 | 64 | <1 |
| Ceftazidime | 2 | 4 | <1 |
| Aztreonam | 32 | 128 | <1 |
The ratio of penicillin/inhibitor was 2:1.
The inhibitor was used at a fixed concentration of 4 μg/ml.
The Ser237→Ala substitution caused a slight decrease in the relative hydrolytic efficiency against cefuroxime and cefotaxime by lowering both the relative hydrolysis rates and the affinity for the latter substrates. Although the level of the reduction was low, these differences were consistently observed in repeated assays. The relative rates of penicillin G and ampicillin hydrolysis by the mutant enzyme were two- to threefold higher than those observed with CTX-M-4 (Table 2). The S237A mutant β-lactamase appeared less susceptible to inhibition by clavulanic acid (by a factor of 2.5) than the wild-type CTX-M-4 did. The replacement of Ser-237 by alanine did not influence the susceptibility of the enzyme to inhibition by the penam sulfones sulbactam and tazobactam (Table 3).
TABLE 2.
Kinetic parameters of the CTX-M-4 wild-type β-lactamase and the S237A mutant enzymea
| Substrate | CTX-M-4
|
S237A mutant
|
||||
|---|---|---|---|---|---|---|
| Relative Vmaxb | Km (μM) | Relative Vmax/Km (%)c | Relative Vmaxb | Km (μM) | Relative Vmax/Km (%)c | |
| Cephalothin | 100 | 103 | 100 | 100 | 90 | 100 |
| Penicillin G | 14 | 22 | 66 | 42 | 18 | 259 |
| Ampicillin | 17 | 31 | 56.5 | 40 | 31 | 143 |
| Cefuroxime | 55 | 210 | 27 | 37 | 336 | 12.2 |
| Cefotaxime | 76 | 295 | 26.5 | 50 | 412 | 13.5 |
| Aztreonam | <0.5 | NDd | 1.8 | ND | ||
Each Km and relative Vmax is the mean value of three separate determinations not differing by more than 10%.
Values are percentages of the Vmax for cephalothin.
Values are percentages of the relative Vmax/Km ratio for cephalothin.
ND, not determined.
TABLE 3.
Inhibition profiles of CTX-M-4 wild-type and S237A mutant β-lactamases
| β-Lactamasea | IC50 (μM) of inhibitorb
|
||
|---|---|---|---|
| Clavulanate | Sulbactam | Tazobactam | |
| CTX-M-4 | 1.0 | 14 | 0.10 |
| S237A mutant | 2.5 | 17 | 0.12 |
Two hundred fifty units of β-lactamase was used.
IC50s were the mean values of three measurements not differing by more than 10%.
The DNA sequence data showed that CTX-M-4 belongs to the group of the recently emerged plasmid-mediated class A β-lactamases that preferentially hydrolyze cefotaxime. The amino acid substitutions in the CTX-M-4 β-lactamase sequence as compared with the sequences for Toho-1 and CTX-M-2 enzymes were located at positions that are not considered important for the hydrolytic activity of class A β-lactamases (Table 4). As can also be seen from the reported β-lactam resistance phenotypes and substrate profiles, substantial differences in their hydrolytic activity would not be expected. These enzymes are related to the species-specific β-lactamases of K. oxytoca (2, 18), Citrobacter diversus (17), and Proteus vulgaris (16), but the similarity does not exceed 80% and their chromosomal ancestor cannot be determined with certainty.
The process of hydrolysis of expanded-spectrum β-lactams by CTX-M-4 and the other plasmid-mediated class A cefotaxime-hydrolyzing β-lactamases may be different from that of the extended-spectrum derivatives of TEM-1, TEM-2, and SHV-1 penicillinases. Amino acid substitutions at positions 104, 164, 238, and 240, which have been associated with the expansion of the activity of TEM and SHV β-lactamases towards oxyimino-cephalosporins (13), are not present in CTX-M-4 and the similar enzymes. It has been observed that the hydrolytic activity of MEN-1 (CTX-M-1) against cephalosporins is greater than that of the chromosomally encoded β-lactamase of K. oxytoca (3). The reported data also suggest that the plasmid-mediated cefotaxime-hydrolyzing enzymes CTX-M-2, Toho-1, and CTX-M-4 hydrolyze cephalosporins more efficiently than the related chromosomal β-lactamases (2, 4, 5, 11, 16, 18). A serine residue at position 237 is present in all the former β-lactamases, whereas the same position is occupied by alanine in most of the class A enzymes, including the related chromosomal β-lactamases of K. oxytoca and C. diversus. The minimal changes in the kinetic parameters of cefotaxime hydrolysis caused by the substitution of Ser-237 by Ala, along with the moderate decrease in the MIC of the antibiotic, indicate that Ser-237 may be involved in the hydrolytic process but that its role is not critical. In class A β-lactamases, the backbone NH and CO groups of residue 237 participate in the hydrogen bond network that stabilizes binding of the substrate within the active site (13). Cefotaxime and cefuroxime may not be able to form the expected hydrogen bonds but interact, through the NH of the C-7 acylamido linkage, with the side chain OH group of threonine (as in TEM-5 and TEM-24) (13). Ser-237 in CTX-M-4, as well as in P. vulgaris β-lactamase (19), may act in an analogous mode. A similar interaction may account for the slight reduction of the inhibitory activity of clavulanate after replacement of Ser-237 by Ala. It has also been suggested that substitutions at position 237 in class A β-lactamases may alter the conformational flexibility of the B3 strand of the enzymes (7, 10). Such modifications may be responsible for the minor changes in the interaction of the S237A mutant enzyme with several β-lactam compounds.
Nucleotide sequence accession number.
The accession number of the nucleotide sequence of the blaCTX-M-4 gene in the EMBL database is Y14156.
REFERENCES
- 1.Ambler R P, Coulson A F W, Frère J-M, Ghuysen J-M, Joris B, Forsman M, Levesque R C, Tiraby G, Waley S G. A standard numbering scheme for the class A β-lactamases. Biochem J. 1991;276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother. 1989;33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthélémy M, Péduzzi J, Bernard H, Tancrède C, Labia R. Close amino acid sequence relationship between the new plasmid-mediated extended-spectrum β-lactamase MEN-1 and chromosomally encoded enzymes of Klebsiella oxytoca. Biochim Biophys Acta. 1992;1122:15–22. doi: 10.1016/0167-4838(92)90121-s. [DOI] [PubMed] [Google Scholar]
- 4.Bauernfeind A, Caselas J M, Goldberg M, Holley M, Jungwirth R, Mangold P, Röhnisch T, Schweighart S, Wilhelm R. A new plasmidic cefotaximase from patients infected with Salmonella typhimurium. Infection. 1992;20:158–163. doi: 10.1007/BF01704610. [DOI] [PubMed] [Google Scholar]
- 5.Bauernfeind A, Grimm H, Schweighart S. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection. 1990;18:294–298. doi: 10.1007/BF01647010. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind A, Stemplinger I, Jungwirth R, Ernst S, Casellas J M. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob Agents Chemother. 1996;40:509–513. doi: 10.1128/aac.40.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantu C, Huang W, Palzkill T. Selection and characterization of amino acid substitutions at residues 237–240 of TEM-1 β-lactamase with altered substrate specificity for aztreonam and ceftazidime. J Biol Chem. 1996;271:22538–22545. doi: 10.1074/jbc.271.37.22538. [DOI] [PubMed] [Google Scholar]
- 8.Gazouli M, Sidorenko S V, Tzelepi E, Kozlova N S, Gladin D P, Tzouvelekis L S. A plasmid-mediated β-lactamase conferring resistance to cefotaxime in a Salmonella typhimurium clone found in St. Petersburg, Russia. J Antimicrob Chemother. 1998;41:119–121. doi: 10.1093/jac/41.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Gazouli M, Tzouvelekis L S, Prinarakis E, Miriagou V, Tzelepi E. Transferable cefoxitin resistance in enterobacteria from Greek hospitals and characterization of a plasmid-mediated group 1 β-lactamase (LAT-2) Antimicrob Agents Chemother. 1996;40:1736–1740. doi: 10.1128/aac.40.7.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healey W J, Labgold M R, Richards J H. Substrate specificities in class A β-lactamases: preference for penams vs. cephems. The role of residue 237. Proteins. 1989;6:275–283. doi: 10.1002/prot.340060310. [DOI] [PubMed] [Google Scholar]
- 11.Ishii Y, Ohno A, Taguchi H, Imajo S, Ishiguro M, Matsuzawa H. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob Agents Chemother. 1995;39:2269–2275. doi: 10.1128/aac.39.10.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joris B, Ledent P, Dideberg O, Fonzé E, Lamotte-Brasseur J, Kelly J A, Ghuysen J M, Frère J M. Comparison of the sequences of class A β-lactamases and of the secondary structure elements of penicillin-recognizing proteins. Antimicrob Agents Chemother. 1991;35:2294–2301. doi: 10.1128/aac.35.11.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knox J R. Extended-spectrum and inhibitor-resistant TEM-type β-lactamases: mutations, specificity, and three-dimensional structure. Antimicrob Agents Chemother. 1995;39:2593–2601. doi: 10.1128/aac.39.12.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):19–45. [DOI] [PubMed]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard no. M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1995. [Google Scholar]
- 16.Péduzzi J, Reynaud A, Baron P, Barthélémy M, Labia R. Chromosomally encoded cephalosporin-hydrolyzing β-lactamase of Proteus vulgaris RO104 belongs to Ambler’s class A. Biochim Biophys Acta. 1994;1207:31–39. doi: 10.1016/0167-4838(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 17.Perilli M, Franceschini N, Segatore B, Amicosante G, Oratore A, Duez C, Joris B, Frère J-M. Cloning and nucleotide sequencing of the gene encoding the β-lactamase from Citrobacter diversus. FEMS Microbiol Lett. 1991;83:79–84. doi: 10.1016/0378-1097(91)90448-j. [DOI] [PubMed] [Google Scholar]
- 18.Reynaud A, Péduzzi J, Barthélémy M, Labia R. Cefotaxime-hydrolysing activity of the β-lactamase of Klebsiella oxytoca D488 could be related to a threonine residue at position 140. FEMS Microbiol Lett. 1991;81:185–192. doi: 10.1016/0378-1097(91)90301-p. [DOI] [PubMed] [Google Scholar]
- 19.Tamaki M, Nugaka M, Sawai T. Replacement of serine 237 in class A β-lactamase of Proteus vulgaris modifies its unique substrate specificity. Biochemistry. 1994;33:10200–10206. doi: 10.1021/bi00199a049. [DOI] [PubMed] [Google Scholar]