Abstract
Background
Lazertinib is a third generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) designed to overcome EGFR T790M mutation. Currently, lazertinib is approved for usage in the acquired EGFR T790M mutation population based on promising clinical and safety profiles. In this study, we evaluated the clinical outcomes of lazertinib in acquired EGFR T790M mutated non-small cell lung cancer (NSCLC) patients in a real-world clinical setting.
Methods
Clinical outcomes of 103 patients treated with lazertinib, from July 2021 to August 2022, for NSCLC with acquired EGFR T790M mutation were retrospectively analyzed. EGFR T790M mutation was confirmed using either a cell-free EGFR test (48.5%) or a tissue-based test (51.5%).
Results
The median follow-up duration was 11.4 months [95% confidence interval (CI): 10.4–13.1], and 58.3% of patients remained on treatment at the time point of data analysis. The objective response rate was 72.8%. The median progression-free survival (PFS) was not reached, and the PFS rates at 6 and 12 months were 81.7% and 65.1%, respectively. The median PFS in a subgroup treated with lazertinib based on T790M-positive tissue was significantly longer than that in a subgroup treated based on T790M-positive plasma (not reached vs. 14.5 months) (hazard ratio, 0.43; 95% CI: 0.21–0.87). Related to adverse events, there was no treatment-related mortality. However, the lazertinib dose was adjusted in 39 patients (37.9%), including 10 patients (9.7%) with permanent discontinuation. Peripheral sensory-motor related adverse events were observed in 65 patients (63.1%) (paresthesia: n=58; muscle cramping: n=24), leading to dose reduction and permanent discontinuation in 17 (16.5%) and 5 (4.9%) patients, respectively. There was no difference in PFS between the groups with and without dose-adjustment (P=0.40).
Conclusions
Our result demonstrates the real-world clinical efficacy of lazertinib in acquired EGFR T790M mutation. Although dose reduction due to adverse events was not uncommon, it did not impair clinical efficacy of lazertinib.
Keywords: Lazertinib, T790M, non-small cell lung cancer (NSCLC), epidermal growth factor receptor (EGFR)
Highlight box.
Key findings
• This study report the first clinical efficacy and safety profile of lazertinib in epidermal growth factor receptor (EGFR) T790M mutated non-small cell lung cancer patients who failed from prior 1st or 2nd generation EGFR tyrosine kinase inhibitors.
What is known and what is new?
• Real-world clinical efficacy of lazertinib was similar to the previous phase I/II clinical trial showing objective response rate of 72.8% and progression-free survival rates at 6 and 12 months of 81.7% and 65.1%, respectively.
• Although dose modification was observed in 37.9% of the patients, clinical efficacy was not impaired in these patients.
What is the implication, and what should change now?
• Lazertinib is a feasible option for acquired EGFR T790M mutated non-small cell lung cancer which reproduced promising clinical efficacy and tolerability in the real-world clinical setting.
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for up to 85% of all cases. Among the NSCLC-activating epidermal growth factor receptor (EGFR) mutations, exon 19 deletion and exon 21 L858R mutations are found at higher prevalence rates in the Asian population (40–60%) compared to the Western population (10–15%) (1). Patients with activating EGFR mutation who failed first- or second-generation EGFR tyrosine kinase inhibitor (TKI) are recommended to receive a second biopsy for identification of the acquired resistance mechanism (1). For those with acquired T790M mutation, found in approximately 60% of patients (2), a third-generation EGFR TKI, osimertinib, led to prolonged progression-free survival (PFS) and overall survival (OS) (3,4).
Recently, lazertinib, a third-generation EGFR TKI targeting both EGFR T790M and sensitizing mutations while sparing wild-type EGFR, was developed, and similar pre-clinical data to those of osimertinib were observed (5). In an open-label phase I/II dose escalation and expansion cohort (LASER201), the recommended dosage of lazertinib was determined to be 240 mg, which leads to a plasma concentration at a steady state higher than the IC50 level with no significant dose-limiting toxicity (6). In patients with T790M-positive tumor treated with 240 mg of lazertinib (n=76), the objective response rate was 55%, and the median PFS and OS were 11.1 and 38.9 months, respectively (7,8). In terms of the safety profile, treatment-related adverse events were mostly mild or moderate, and the frequent events were skin rash (37%), pruritus (35%), paresthesia (33%), headache (28%), and muscle spasm (28%), diarrhea (27%), and decreased appetite (26%) (7). Based on the LASER201 study, lazertinib was approved in Republic of Korea in January 2021 and is now clinically available for EGFR-mutated NSCLC patients with acquired T790M mutation after first- or second-generation EGFR TKI failure.
Given its recent clinical utility, lazertinib 240 mg was evaluated for clinical efficacy and safety profile in EGFR-mutated NSCLC patients with acquired T790M mutation in a real-world setting. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-160/rc).
Methods
Study design and participants
This study was conducted in a retrospective manner to evaluate the clinical efficacy and safety profile of 240 mg of lazertinib in a real-world setting. For the study, the medical records of patients treated with lazertinib at Samsung Medical Center from July 2021 to August 2022 were reviewed. All patients showed resistance to either first- or second-generation EGFR TKIs. EGFR T790M mutation was confirmed with a tissue-based or cell-free DNA-based test. Testing for T790M mutation from both tissue or cell-free DNA was conducted with cobas EGFR mutation Test V2 (Roche Diagnostic, USA).
Any patient who had received at least one dose of lazertinib was included in the analysis. To ensure a minimum follow-up duration of 6 months, patients who received lazertinib after September 2022 were not included in the analysis.
This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). Due to the retrospective design with no intervention, the need to collect informed consent was waived. The study was conducted under supervision of the Samsung Medical Center Institutional Review Board (No. SMC 2023-02-081).
Study objectives
The primary endpoint of this study was clinical efficacy and safety profile of 240 mg of lazertinib in a real-world setting.
Procedure and statistical analysis
The final data were collected on January 14, 2023. The clinical response was evaluated based on the response evaluation criteria in solid tumors (version 1.1). For those with at least one computed tomography scan available after treatment, data on response were collected as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). The safety profile was recorded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. PFS was calculated as the time interval between the start date of lazertinib and the date of disease progression or all-cause mortality. Overall survival was calculated as the time interval between the start date of lazertinib and the date of all-cause mortality. The Kaplan-Meier method was used to create survival curves. All data were analyzed using R version 4.2.2 (http://www.R-project.org/; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline demographics
A total of 103 patients was treated with lazertinib during the study period (Table 1). The median age of the study population was 65 years (range, 40–86 years). The proportion of female patients was higher (64.1%) than that of males, and most patients were never smokers (68.9%). Adenocarcinoma histology (99.0%) was predominant, with only one patient showing squamous cell carcinoma. As an initial EGFR mutation, exon 19 deletion was more frequent (66.0%), followed by L858R mutation (31.1%); three patients had uncommon EGFR mutation (2.9%) such as G719A and L861Q. All patients received either first- or second-generation EGFR TKI before lazertinib: afatinib in 44.7% of patients, followed by gefitinib (41.7%), erlotinib (7.8%), and dacomitinib (5.8%). Sixteen patients (15.5%) were exposed to previous cytotoxic chemotherapy. All patients were confirmed to have EGFR T790M mutation using either tissue-based (51.5%) or cell-free DNA-based (48.5%) testing.
Table 1. Baseline demographics.
| Characteristics | Median [range] or N (%) |
|---|---|
| Age, years | 65 [40–86] |
| Sex | |
| Male | 37 (35.9) |
| Female | 66 (64.1) |
| Smoking history | |
| Current or ex-smoker | 32 (31.1) |
| Never smoker | 71 (68.9) |
| ECOG performance score | |
| 0 | 16 (15.5) |
| 1 | 85 (82.5) |
| 2 | 2 (2.0) |
| Histology | |
| Adenocarcinoma | 102 (99.0) |
| Squamous cell carcinoma | 1 (1.0) |
| Initial EGFR mutation | |
| Exon 19 deletion | 68 (66.0) |
| L858R | 32 (31.1) |
| Other | 3 (2.9) |
| Previous EGFR TKI | |
| Gefitinib | 43 (41.7) |
| Erlotinib | 8 (7.8) |
| Afatinib | 46 (44.7) |
| Dacomitinib | 6 (5.8) |
| Previous cytotoxic treatment | 16 (15.5) |
| Line of lazertinib | |
| 2nd line | 87 (84.5) |
| ≥3rd line | 16 (15.5) |
| T790M mutation detection | |
| Cell-free based test | 50 (48.5) |
| Tissue-based test | 53 (51.5) |
Total number of patients (N=103). ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Clinical efficacy
The median follow-up duration of the study population was 11.4 months [95% confidence interval (CI): 10.4–13.1]. At the time of data analysis, 60 patients (58.3%) continued to receive treatment, and 43 patients (41.7%) had discontinued treatment due to disease progression (29.1%), adverse events (9.7%), or follow-up loss (2.9%). The best objective response rate was 72.8%, including 75 patients with PR (72.8%), 16 with SD (15.5%), 3 with PD (2.9%), and 9 patients not available for the evaluation (6.9%).
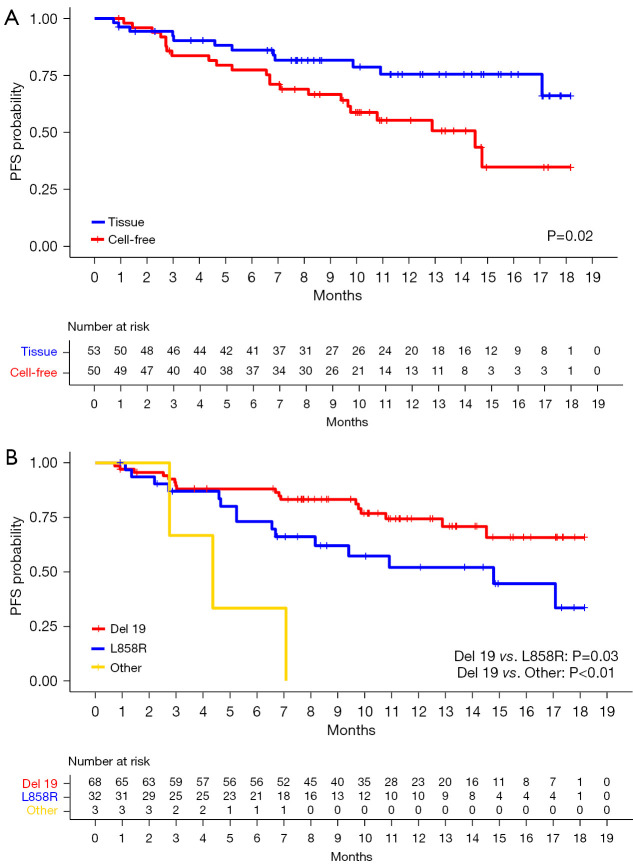
Median PFS was not achieved (95% CI: 14.8–NA), showing landmark PFS rates at 3, 6, 9, and 12 months of 88.0%, 81.7%, 74.1%, and 65.1%, respectively (Figure 1A). The landmark OS rates at 3, 6, 9, and 12 months were 98.1%, 96.1%, 94.0%, and 86.3%, respectively (Figure 1B). Subgroup analysis was conducted based on exposure to previous chemotherapy [hazard ratio (HR), 0.68; 95% CI: 0.26–1.77; P=0.44] and revealed no difference in PFS (Figure S1).
Figure 1.
PFS (A) and OS (B) of the study population. PFS, progression-free survival; OS, overall survival.
Patients whose T790M mutation was identified via the cell-free DNA-based method (n=50) after prior TKI therapy had a shorter median PFS (14.5 months; 95% CI: 9.67–NA) compared to patients whose T790M mutation was identified via tissue-based testing (n=53; median PFS, NA), revealing an HR that favored tissue-based testing (0.43; 95% CI: 0.21–0.87; P=0.02) (Figure 2A). Based on the initial EGFR mutation, patients with exon 19 deletion mutation (n=68) had a significantly longer median PFS (NA) compared to those with L858R (n=32; 14.8 months; 95% CI: 8.2–NA) or other mutations (n=3; 4.4 months; 95% CI: 2.8–NA). The HRs for L858R and other mutations compared to exon 19 deletion were 2.17 (95% CI: 1.08–4.35; P=0.03) and 8.54 (95% CI: 2.41–30.2; P<0.01), respectively (Figure 2B).
Figure 2.
PFS based on (A) the method of T790M mutation detection and (B) the underlying EGFR mutation. PFS, progression-free survival; EGFR, epidermal growth factor receptor; Del19, exon 19 deletion.
Safety profile
Peripheral sensory-motor related events, such as paresthesia (56.3%) and muscle cramping (23.3%), were frequently observed during the study period (Table 2). Though most events were grade 1 or 2, 2 (1.9%) patients experienced grade 3 paresthesia. As another notable adverse event, headache was not detected infrequently (12.6%). Additional noticeable adverse events (≥10%) included skin rash (26.2%), pruritus (17.5%), diarrhea (16.5%), and fatigue (11.6%) (Table 2).
Table 2. Adverse event.
| Adverse events | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) |
|---|---|---|---|
| Paresthesia | 37 (35.9) | 19 (18.4) | 2 (1.9) |
| Muscle cramping | 22 (21.4) | 2 (1.9) | – |
| Rash | 18 (17.5) | 9 (8.7) | 2 (1.9) |
| Bilirubin elevation | – | – | 1 (1.0) |
| Diarrhea | 15 (14.6) | 2 (1.9) | – |
| Pruritus | 13 (12.6) | 5 (4.9) | – |
| Headache | 10 (9.7) | 3 (2.9) | – |
| Fatigue | 6 (5.8) | 6 (5.8) | – |
| Anorexia | 4 (3.9) | 5 (4.9) | – |
| Nausea | 5 (4.9) | 2 (1.9) | – |
| Dizziness | 6 (5.8) | 1 (1.0) | – |
| Edema | 4 (3.9) | 1 (1.0) | – |
| Nail change | 3 (2.9) | 1 (1.0) | – |
| Mucositis | 3 (2.9) | 1 (1.0) | – |
| Pneumonitis | 1 (1.0) | 1 (1.0) | – |
| Myalgia | 7 (6.8) | – | – |
| AST/ALT elevation | 2 (1.9) | – | – |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Adverse events leading to dose reduction or treatment discontinuation during the study period are shown in Table 3. A total of 39 (37.9%) patients required dose adjustment or permanent discontinuation of lazertinib. A total of 29 patients (28.2%) was required to reduce lazertinib dose without permanent discontinuation, and lazertinib was reduced to 160 mg in 20 patients (19.4%) and to <160 mg in nine patients (8.7%) (Table 3). The most common adverse events leading to dose reduction without discontinuation were paresthesia (n=17), rash (n=3), fatigue (n=3), dizziness (n=2), weight loss (n=2), and anorexia (n=2). Other reasons for dose reduction were headache, urticaria, pneumonitis, and acneiform dermatitis observed in a single patient each.
Table 3. The pattern and causes of dose adjustment.
| Categories | Lazertinib (n=103), n (%) |
|---|---|
| No. of dose adjustment due to adverse events | 39 (37.9) |
| Last adjusted dose of lazertinib | |
| 160 mg per day | 20 (19.4) |
| ≥80 and <160 mg per day | 9 (8.7) |
| Final discontinuation following dose reduction | 6 (5.8) |
| Discontinuation without prior dose reduction | 4 (3.9) |
| Adverse events leading to dose reduction without discontinuation, n (%) | 29 (28.2) |
| Paresthesia | 17 (16.5) |
| Rash | 3 (2.9) |
| Fatigue | 3 (2.9) |
| Dizziness | 2 (1.9) |
| Weight loss | 2 (1.9) |
| Anorexia | 2 (1.9) |
| Headache | 1 (1.0) |
| Urticaria | 1 (1.0) |
| Pneumonitis | 1 (1.0) |
| Acneform dermatitis | 1 (1.0) |
| Adverse events leading to permanent discontinuation, n (%) | 10 (9.7) |
| Paresthesia | 5 (4.9) |
| Rash | 2 (1.9) |
| Fatigue | 2 (1.9) |
| Diarrhea | 2 (1.9) |
| Bilirubin elevation | 1 (1.0) |
| Pneumonitis | 1 (1.0) |
| Nausea | 1 (1.0) |
| Anorexia | 1 (1.0) |
| Dizziness | 1 (1.0) |
In addition, 10 patients were required to discontinue lazertinib therapy permanently due to adverse events; of these, 6 (5.8%) and 4 (3.9%) discontinued treatment with or without previous history of dose reduction of lazertinib, respectively (Table 3). The main reason for discontinuation was paresthesia (n=5, 1 patient with grade 3 and 4 patients with grade 2), grade 3 rash (n=1), grade 3 bilirubin elevation (n=1), and grade 2 pneumonitis (n=1). Two patients discontinued therapy due to combination of multiple grade 1 and grade 2 adverse events such as rash, nausea, fatigue, anorexia, and diarrhea. All patients were switched to osimertinib as a subsequent treatment.
The median interval from the point of starting lazertinib therapy to the first dose adjustment was 3.5 months (range, 0.5–16.5 months). Based on the demographics, the dose-adjustment rate in women was higher (40.9%) than that in men (32.4%) (P=0.72 by Chi-square test). However, the median body mass index showed no difference between patients with dose adjustment (22.8 kg/m2) and those without dose adjustment (23.0 kg/m2) (P=0.84 by t-test). When comparing the PFS between the group whose lazertinib dose was adjusted within 6 months after starting lazertinib therapy (n=31) and the others (n=72), who included eight patients where dose adjustment was performed 6 months or later after starting lazertinib, a trend of longer PFS (HR, 0.70; 95% CI: 0.31–1.61) was observed among the dose-adjusted patients (n=31) but was not statistically significant (P=0.40) (Figure S2).
Adverse events of specific interest
In this study, we observed several adverse events related to sensory changes. The most common adverse events observed in the study population were paresthesia of grade 3 (n=2, 1.9%), grade 2 (n=19, 18.4%), or grade 1 (n=37, 35.9%). Among these patients, sensory-motor polyneuropathy mimicking chemotherapy-induced peripheral neuropathy (CIPN) was documented by nerve conduction studies in all nine who were tested for abnormal sensation in their extremities. Muscle cramping was observed as grade 2 (1.9%) or grade 1 (21.4%). Combining all patients who reported at least one of the above symptoms, 65 (63.1%) experienced at least one of grade 1 or higher paresthesia, or muscle cramping, which led to dose reduction without permanent discontinuation in 17 (16.5%) and permanent discontinuation in 5 (4.9%) patients. The median time to the first report of sensory-motor related symptoms was 3.2 months (range, 0.5–14.7 months). Total 22 patients who experienced dose adjustment due to sensory-motor related symptoms were analyzed to determine whether their symptoms improve since dose adjustment of lazertinib. With the median follow-up time of 5.0 months (range, 0.3–13.0 months) since the first-dose adjustment, 5 (22.7%) improved in severity.
Cases of interest
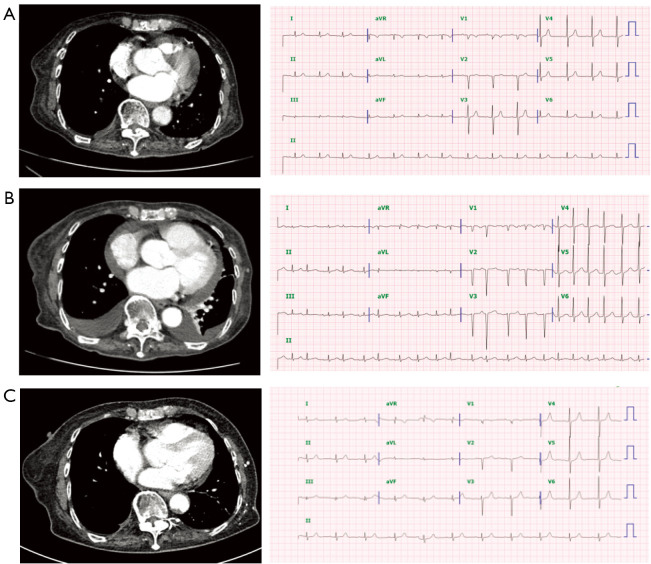
In our study population, there was a patient who switched to lazertinib from previous gefitinib (Figure 3). This patient was an 86-year-old female with an L858R mutation. After progressing on first-line gefitinib treatment following a response for 13 months, T790M mutation was confirmed, and osimertinib was initiated. This patient’s initial left ventricular ejection fraction (LVEF) was 68% and her QTcF was 383 ms. After 5 months of osimertinib treatment, increased bilateral pleural effusion and pericardial effusion were observed (Figure 3B). At this time point, echocardiography showed decreased left ventricular systolic function favoring stressed cardiomyopathy, LVEF was 30%, and prohormone B-type natriuretic peptide (proBNP) was 1,065 pg/mL. The electrocardiogram showed newly noted atrial fibrillation, ventricular premature complex, and increased QTcF to 455 ms. Osimertinib was switched to lazertinib and improvement in LV function (LVEF, 81%) was observed after 2 months, accompanied by decreased pericardial effusion and normalization of arrhythmia (Figure 3C).
Figure 3.
Computed tomography scans and electrocardiograms of a patient who switched from osimertinib to lazertinib due to cardiac toxicity (A) at initiation of osimertinib, (B) 8 months after the start of osimertinib therapy, and (C) 4 months after switching from osimertinib to lazertinib.
Discussion
To the best of our knowledge, this is the first and largest real-world study of the third-generation EGFR TKI lazertinib, focusing on the clinical efficacy and safety profile. The clinical efficacy observed in our real-world data is similar to or better than that reported by previous clinical trials; indeed, the PFS rates in our study at 6 and 12 months (81.7% and 65.1%, respectively) were numerically higher than those (59.3% and 48.0%) of a phase I/II study (7). In addition, the objective response rate of our study population was 72.8%, also numerically higher than that (55.3%) observed in the same previous trial (7). Although careful interpretation is warranted given the indirect comparison, these data also showed numerically better outcomes compared to real-world evidence observed in an EGFR T790M-mutated Korean population treated with another third-generation EGFR-TKI (osimertinib), which exhibited a 12-month PFS rate of 56.5% (9).
In a subgroup analysis of our data, we observed that patients with exon 19 deletion showed significantly better PFS than those with L858R mutation. This is consistent with previous data showing better clinical outcomes in an exon 19 deletion subset when treated with osimertinib (10). In addition, the same trend was observed in a treatment-naïve EGFR mutation population after treatment with lazertinib (11).
Another noticeable finding in our subgroup analysis is that clinical outcomes of lazertinib differed according to method of detecting the acquired T790M mutation. The PFS of lazertinib treatment based on T790M positivity in plasma was significantly inferior to that based on T790M positivity in tissue. Since the LASER201 population comprised mostly patients with T790M positivity in tissue (7), our findings cannot be compared with those of any prior lazertinib study. However, a prior osimertinib study showed that, in a population of patients with T790M-positive plasma, the PFS associated with osimertinib therapy was significantly inferior in T790M-negative tissue patients compared to that of T790M-positive tissue patients (12), compatible with the results of our subgroup analysis. Though these findings cannot be explained conclusively, T790M clones detected in plasma could be minor clones and may not represent a main component of tumors. Another minor reason could be that patients undergoing a cell-free DNA-based mutation test are more likely to have a greater tumor burden and a general condition unfit for repeat biopsy, it can be hypothetically assumed that these patients are more likely to show early resistance to the treatment.
Through the data collected from the real-world setting, we observed unique findings which had not been highlight in the previous clinical trials. Despite the promising clinical efficacy of lazertinib reproduced in real-world data, this study additionally observed unique categories of adverse events previously not observed in other EGFR TKIs. Patients complained of these symptoms in various ways, such as a decrease or change (pain, numbness, or tingling sense) in sensation or intermittent muscle cramping, although they all occurred in a glove- and-stocking–type distribution. The time of first symptom appearance also varied from 2 weeks to 15 months after the start of lazertinib therapy, and showed no correlation with other demographic factors such as gender, body weight, and body mass index. Among the study population, 63.1% of patients reported any sensory-motor related symptoms (paresthesia 56.3%, muscle cramping 23.3%), which is slightly higher than that 39% of paresthesia reported in the first-line lazertinib study (LASER301) (11). Regarding the recovery rate of sensory-motor related symptom, a small proportion (22.7%) of cases achieved symptom relief with dose reduction or permanent discontinuation of lazertinib combined with supportive medications, which rate is unusually low compared with other TKI-induced adverse event such as skin rash, stomatitis, and diarrhea. Considering that the results of nerve conduction studies in our populations are compatible with a diagnosis of CIPN, and that the pathophysiology of CIPN is related to damage or severance of long myelinated fibers of axons, requiring a relatively long-term period for repair (13), our documentation of a lower recovery rate even after dose reduction may be more persuasive in real practice. However, the follow-up time for the recovery of symptoms was relatively short (median 5.0 months), which needs longer follow-up to address this issue.
In addition, considering that CIPN is well known as a dose-dependent adverse event (13), the high incidence rate of sensory-motor related symptoms in our study should be compared with that of LASER201 (6). In LASER201, which study performed with various doses of lazertinib, the incidence rate of paresthesia increased as lazertinib dose increased from 10% (2/20) in an 80-mg group, 12% (3/25) in a 120-mg group, and 4% (1/23) in a 160-mg group to 25% (6/24) in a 240-mg group. In LASER301, the incidence of paresthesia was also reported high (39%) (11). Therefore, we could assume that lazertinib-induced sensory-motor related symptoms may also be a dose-dependent adverse event, like CIPN. Under this assumption, our investigators are now prospectively investigating the feasibility of a lazertinib starting dose of 160 mg/day in T790M-positive NSCLC (14), an initiative supported by the similar pharmacokinetics and PFS of a lazertinib 160-mg group to those of a lazertinib 240-mg group (6).
Although most adverse events were mild (grade 1) or moderate (grade 2) in our study, dose reduction rate was relatively higher (38%) than that (21%) reported in LASER301 (11). The one reason might be the innate nature of more generous dose reduction in real world practice than in prospective studies. This different reduction pattern was also found in other studies of EGFR TKIs: 42% of LUX-Lung7 population were required to reduce afatinib dose while 68% of a real-world practice population experienced afatinib dose reduction (15,16). Another reason for high reduction rate in our study might be explained by investigators’ belief that dose reduction performed to manage adverse events does not impair the efficacy of the target therapy. Our study showed no inferior outcome in a dose-adjusted group compared in the rest group, which is compatible with that from other TKI studies (16-18). In addition, the high discontinuation rate (10%) in our study may in part be affected by the existence of alternative treatment, and all discontinued patients received osimertinib as a subsequent therapy.
Another noticeable characteristic of lazertinib is a superior safety profile in terms of cardiac toxicity compared to osimertinib (19), though we present only a single episodic case in the current report. As an underlying mechanism of the cardiotoxicity of EGFR TKIs, decreased LVEF and prolonged QT interval may stem from inhibition of the HER2 and AMPK pathways (20). It is known that lazertinib exhibits 275-fold selectivity for exon 19 deletion or L858R mutation compared to HER2, resulting in minimal inhibition of HER2 (21). This might explain the superior cardiac safety profile to that of osimertinib, which showed 6.7-fold selectivity for HER2. In this study, we observed a case that switched to lazertinib due to a decrease in LVEF during treatment with osimertinib. As lazertinib becomes accessible in some regions in the clinical setting, this case provides clinical evidence that an alternative treatment strategy of switching to lazertinib is safe and tolerable in patients with specific occasions such as experience cardiotoxicity to osimertinib.
The efficacy of lazertinib in the central nervous system (CNS) was shown in a prior prospective trial (7). Due to our current study being designed in a real-world retrospective manner, however, it has limitations in interpreting clinical efficacy in the CNS. Most of our patients did not undergo CNS evaluation after failure of the first- or second-generation EGFR TKI, which limits the evaluation of intracranial efficacy. In addition, due to the long duration of response achieved by lazertinib, PFS and OS were not reached at the time of evaluation. However, we selected a study population with a follow-up duration of more than 6 months to minimize the potential bias observed with short durations of follow-up.
Conclusions
In conclusion, as the lazertinib become one of the standard options in EGFR T790M mutated patient, this study provides additional clinical evidence that lazertinib is an effective and viable option for subsequent treatment of patients with acquired T790M mutation after first- or second-generation EGFR TKI failure. At the same time, this study also highlighted there is clinical impact of lazertinib-induced sensory-related symptoms on patients, and the appropriate management for this adverse event needs to be considered during the treatment.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). This study was reviewed and approved by the Institutional Review Board (No. SMC 2023-02-081) at Samsung Medical Center, and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-160/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-160/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-160/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-160/coif). MJA is serving as an unpaid editorial board member of Translational Lung Cancer Research from October 2021 to September 2023. SHL is serving as an unpaid editorial board member of Translational Lung Cancer Research from October 2021 to September 2023. The other authors have no conflicts of interest to declare.
References
- 1.Tan AC, Tan DSW. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J Clin Oncol 2022;40:611-25. 10.1200/JCO.21.01626 [DOI] [PubMed] [Google Scholar]
- 2.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. 10.1056/NEJMoa1612674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 2020;31:1536-44. 10.1016/j.annonc.2020.08.2100 [DOI] [PubMed] [Google Scholar]
- 5.Yun J, Hong MH, Kim SY, et al. YH25448, an Irreversible EGFR-TKI with Potent Intracranial Activity in EGFR Mutant Non-Small Cell Lung Cancer. Clin Cancer Res 2019;25:2575-87. 10.1158/1078-0432.CCR-18-2906 [DOI] [PubMed] [Google Scholar]
- 6.Ahn MJ, Han JY, Lee KH, et al. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1-2 study. Lancet Oncol 2019;20:1681-90. 10.1016/S1470-2045(19)30504-2 [DOI] [PubMed] [Google Scholar]
- 7.Cho BC, Han JY, Kim SW, et al. A Phase 1/2 Study of Lazertinib 240 mg in Patients With Advanced EGFR T790M-Positive NSCLC After Previous EGFR Tyrosine Kinase Inhibitors. J Thorac Oncol 2022;17:558-67. 10.1016/j.jtho.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 8.Han J-Y. Overall survival in patients with EGFR T790M-positive advanced non-small cell lung cancer treated with lazertinib: Results from the Phase I/II study (LASER201). International Congress of Asian Oncology Society 2022. [Google Scholar]
- 9.Lee JH, Kim EY, Park CK, et al. Real-World Study of Osimertinib in Korean Patients with Epidermal Growth Factor Receptor T790M Mutation-Positive Non-Small Cell Lung Cancer. Cancer Res Treat 2023;55:112-22. 10.4143/crt.2022.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 11.Cho BC, Ahn M, Kang JH, et al. LBA7 A randomized, double-blind, multinational phase III study to assess the efficacy and safety of lazertinib versus gefitinib in the first-line treatment of patients with EGFR mutation (EGFRm), advanced NSCLC (LASER301; NCT04248829). Ann Oncol 2022;33:S1560-97. [Google Scholar]
- 12.Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. 10.1200/JCO.2016.66.7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starobova H, Vetter I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Front Mol Neurosci 2017;10:174. 10.3389/fnmol.2017.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.A Phase II Study for the Evaluation of the Feasibility of Lazertinib 160mg Per Day in Patients With EGFR T790M Mutant Non-small Cell Lung Cancer. NCT05701384.
- 15.Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 16.Kim Y, Lee SH, Ahn JS, et al. Efficacy and Safety of Afatinib for EGFR-mutant Non-small Cell Lung Cancer, Compared with Gefitinib or Erlotinib. Cancer Res Treat 2019;51:502-9. 10.4143/crt.2018.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CY, Wang CL, Li SH, et al. The efficacy of 40 mg versus dose de-escalation to less than 40 mg of afatinib (Giotrif) as the first-line therapy for patients with primary lung adenocarcinoma harboring favorable epidermal growth factor mutations. Oncotarget 2017;8:97602-12. 10.18632/oncotarget.18746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuler M, Tan EH, O'Byrne K, et al. First-line afatinib vs gefitinib for patients with EGFR mutation-positive NSCLC (LUX-Lung 7): impact of afatinib dose adjustment and analysis of mode of initial progression for patients who continued treatment beyond progression. J Cancer Res Clin Oncol 2019;145:1569-79. 10.1007/s00432-019-02862-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand K, Ensor J, Trachtenberg B, et al. Osimertinib-Induced Cardiotoxicity: A Retrospective Review of the FDA Adverse Events Reporting System (FAERS). JACC CardioOncol 2019;1:172-8. 10.1016/j.jaccao.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther 2018;11:6227-37. 10.2147/OTT.S170138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang SB, Kim KB, Sim S, et al. Cardiac Safety Assessment of Lazertinib: Findings From Patients With EGFR Mutation-Positive Advanced NSCLC and Preclinical Studies. JTO Clin Res Rep 2021;2:100224. 10.1016/j.jtocrr.2021.100224 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as