Abstract
Objectives
Endoscopic thoracic sympathectomy may be complicated by the onset of disabling compensatory sweating (CS). The objective of this case series is to report the 2-year outcomes after robotic sympathetic trunk reconstruction (STR) for the reversal of CS in patients who had undergone endoscopic thoracic sympathectomy.
Methods
We prospectively followed-up a total of 23 patients who had undergone robotic STR because of intolerable CS between October 2017 and January 2021. A visual analog scale ranging from 0 to 10 (with 10 indicating the highest degree) was used to assess the severity of CS at different anatomical locations, thermoregulatory alterations, and gustatory hyperhidrosis. Measurements were performed before STR and at 6-month and 2-year follow-up.
Results
The mean age of the study participants was 43.3 ± 7.8 years, and 20 (87%) were men. The reversal procedure was performed after a mean of 19.6 ± 7.8 years from endoscopic thoracic sympathectomy. In all patients, nerve defects were successfully bridged using sural nerves (mean length, 9.7 cm on the right and 9.8 cm on the left). No cases of Horner syndrome were noted. At 6 postoperative months, the severity of CS decreased significantly at all body surface areas. The observed improvements were effectively maintained at 24 post-STR months. There was no evidence of either recurrent hyperhidrosis at the primary site or transition of CS to other anatomical locations. Similar improvements were evident for thermoregulatory alterations and gustatory hyperhidrosis.
Conclusions
Robotic STR is safe and effective in reversing intolerable CS after endoscopic thoracic sympathectomy.
Key Words: endoscopic thoracic sympathectomy, compensatory sweating, sympathetic trunk reconstruction, robotic microsurgery

Thermograms obtained before and after 10 minutes in a sauna.
Central Message.
Robotic sympathetic trunk reconstruction is safe and effective in reversing intolerable compensatory sweating after thoracic sympathectomy.
Prospective.
At 6 postoperative months, robotic STR was effective in reducing the severity of CS at all sites. The observed improvements were effectively maintained at 24 post-STR months. There was no evidence of either recurrent hyperhidrosis at the primary site or transition of CS to other anatomical locations. Similar improvements were noted for thermoregulatory alterations and gustatory hyperhidrosis.
See Discussion on page 259.
See Discussion on page XXX.
Endoscopic thoracic sympathectomy (ETS) is a clinically feasible therapeutic option for patients with primary regional hyperhidrosis who are refractory to standard medical treatment.1,2 Unfortunately, this procedure can be beset by the onset of compensatory sweating (CS), thermoregulatory alterations, and gustatory hyperhidrosis.3 Of them, CS is the most commonly reported adverse effect—affecting up to 80% of patients undergoing operation.4,5 Although severe CS following ETS can compromise quality of life, current therapeutic options remain limited and poorly effective.6, 7, 8, 9
Theoretically, the restoration of nerve continuity through the reconstruction of the previously interrupted sympathetic trunk represents an ideal solution to reverse symptoms in patients who develop intolerable post-ETS CS. As of 2010, some authors have reported the clinical feasibility of sympathetic trunk reconstruction (STR) accomplished through thoracotomy or video-assisted thoracoscopy.10, 11, 12 However, there has been limited adoption of surgical approaches for the treatment of CS in daily practice. This can be attributed to the fact that clinical outcomes remain uncertain and adverse effects are still unpredictable.
In 2019, we described for the first time the safety and feasibility of robotic STR with sural nerve grafting with a pilot study that involved 7 patients.13 In the current case series, we sought to expand our previous findings by describing the 2-year outcomes of 23 patients who had undergone robotic STR for the reversal of CS. With this aim, a visual analog scale (VAS) was used to assess the severity of CS at different anatomical locations, thermoregulatory alterations, and gustatory hyperhidrosis. Measurements were performed before STR and at 6-month and 2-year follow-up.
Materials and Methods
Study Patients
Eligible participants for this study were patients who underwent robotic thoracic STR at Chang Gung Memorial Hospital between October 2017 and January 2021. Exclusions included subjects who received STR for reasons other than CS and those who failed to complete the outcome questionnaire. Ultimately, the final study sample comprised 23 patients. A study flowchart is provided in Figure 1. The research protocol complied with the tenets of the Helsinki Declaration and was granted ethical approval by the local institutional review board (approval No.: CGMH IRB-202100928B0, approval date: June 24, 2021). Patients provided informed written consent for publication of study data.
Figure 1.
Flow of patients through the study. STR, Sympathetic trunk reconstruction; CS, compensatory sweating.
Preoperative Survey
A detailed medical history—with a special focus on the clinical symptoms before and after ETS as well as the technique applied to interrupt the continuity of the sympathetic trunk—was collected from all participants. The physical examination was aimed at detecting the presence or absence of the Horner syndrome, the anatomical distribution of CS, and the relative temperature of the upper and lower extremities. An exercise-limited cardiac stress test was performed in patients with a history of exercise limitations. During the preoperative phase, all candidates for robotic STR met an experienced psychiatrist for a detailed psychological evaluation. Patients were deemed unsuitable for STR if they met the following criteria: inability to pass the psychological assessment, presence of unrealistic expectations on STR outcomes, and history of sympathectomy performed with nonsurgical techniques (including thermal ablation, stereotactic interventions, or chemical injections) that did not allow assessing the extent of the existing damage. After a detailed explanation of the potential risks inherent to STR, including the reappearance of symptoms that originally required reversal surgery and the potential occurrence of injuries to the sympathetic chain, all patients provided written informed consent. Patients were informed on the potential risk of direct or traction injuries to the stellate ganglion, which were expected to lead to transient or permanent symptoms typical of Horner syndrome.
Surgical Technique
The detailed STR procedure has been previously described. Following the induction of general anesthesia and intubation with a double-lumen endotracheal tube, the patient was placed in the left semiprone position. Carbon dioxide was subsequently insufflated (pressure: 6-8 mm Hg) to promote the formation of an artificial pneumothorax. After the creation of suitable thoracic ports, a 30° angled thoracoscope was positioned in the seventh intercostal space (ICS) along the posterior axillary line. The right robotic arm was placed in the fifth ICS along the posterior axillary line (medially to the scapula), whereas the left robotic arm was positioned in the ninth ICS along the posterior axillary line. The assistant port was placed either in the sixth or seventh ICS along the midaxillary line, and a da Vinci Xi robotic cart (Intuitive Surgical) was subsequently docked. Upon entering the thoracic cavity, pneumolysis was initially performed to expose the previous sympathectomy site, including the proximal and distal stumps as well as the affected intercostal nerve(s). While this procedure was being performed, a microsurgeon began harvesting the sural nerve according to the measured nerve defect. Coaptation was performed starting from the proximal sympathetic stump to the distal sural nerve using 8–0 nylon sutures. After the graft passed underneath the intercostal nerves, its redundant segments were cut and reserved for the contralateral side. The proximal sural nerve was coapted to the distal sympathetic stump, and the graft was fixed to the intercostal nerves in a side-to-side fashion (Figure 2 and Video 1). Upon completion of the entire procedure, hemostasis was carefully accomplished, and a 20Fr chest tube was inserted. The patient was then turned to the right semiprone position and prepared for an additional STR using the same technical approach. After all the procedure done, the patient turned back to the supine position and extubate carefully.
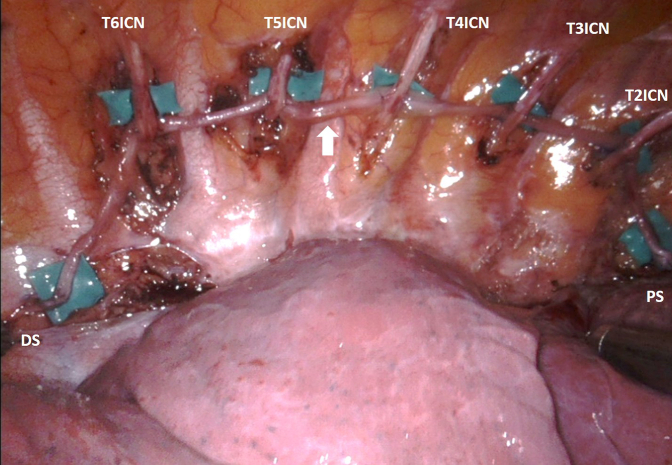
Figure 2.
The right sympathetic trunk was reconstructed using a sural nerve graft that was coapted to the involved intercostal nerves in a side to side fashion. The arrow indicates the sural nerve graft. T2ICN−T6ICN, T2−T6 intercostal nerves; PS, proximal stump; DS, distal stump.
Definition of Outcomes and Follow-up Schedule
Patients were followed-up on an outpatient basis or through telephone and Internet interviews every 6 months after robotic STR. The severity of CS at different anatomical locations—including chest, back, abdomen, buttocks, thighs, calf, and feet—was examined before and after reversal surgery using a VAS ranging from 0 to 10, with 10 indicating the highest degree. Thermoregulatory alterations, gustatory hyperhidrosis, and the presence of excessive hand dryness were investigated. We also invited patients to participate in a sauna test both before and after their reversal surgery. Each patient was instructed to wear a swimsuit and enter a dry sauna room heated to 70 °C for 10 minutes, with medical staff waiting nearby in case of an emergency. Thermographic images of both the anterior and posterior body surfaces were captured using an infrared camera (FLIR ONE Pro thermal camera for iOS; P/N 435-0006-01) before and immediately following the sauna challenge.
Statistical Analysis
Continuous data are expressed as mean ± SD, whereas categorical variables are given as counts and percentages. Differences in VAS values measured before STR and at 6-month and 2-year follow-up were analyzed with the Wilcoxon matched-pairs signed-rank test. Due to multiple comparisons, the Bonferroni adjustment was applied to control for type I error. All calculations were performed using SPSS version 22.0 (IBM-SPSS Inc), with all tests 2-sided at a 2.5% level of significance.
Results
Patient Demographic Characteristics
A total of 23 patients (20 men and 3 women; mean age: 42.7 ± 6.6 years) were included in the study (Table 1). Two participants underwent ETS for facial blushing, whereas the remaining 21 were treated for primary palmar hyperhidrosis. The levels at which ETS for primary hyperhidrosis was previously performed were as follows: left T2 (n = 3), T2−T3 (n = 10), T2−T4 (n = 3), T2−T5 (n = 1), T3 (n = 4), T3−T4 (n = 1); right T2 (n = 2), T2−T3 (n = 11), T2−T4 (n = 4), T2−T6 (n = 2), T3 (n = 2), and T3−T4 (n = 1). The remaining patient initially received T2 + T3 sympathectomy followed by secondary sympathectomy surgery (clipping on T5−T9 on the left side and T5−T6 on the right side). The mean interval from ETS to robotic STR was 19.6 ± 7.8 years.
Table 1.
General characteristics of the study patients
| Case No. | Sex | Age (y) | Smoking history | Indication for ETS | ETS level | ETS to STR (y) | Operating time (h) | Nerve defect (right, cm) | Nerve defect (left, cm) | Postop LOS (d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 59 | No | PPH | T2−T4 | 25 | 9.5 | 7 | 7 | 2 |
| 2 | M | 49 | Yes | PPH | T2−T3 | 24 | 13 | 6 | 7 | 2 |
| 3 | M | 37 | No | Facial blushing | T2-T3 | 2 | 10.5 | 8 | 10 | 1 |
| 4 | F | 44 | No | PPH | Right T2−T6 | 22 | 11.5 | 13 | 18 | 1 |
| Left T2−T9 | ||||||||||
| 5 | M | 41 | No | PPH | Right T2−T4 | 20 | 8 | 10 | 8 | 4 |
| Left T2−T3 | ||||||||||
| 6 | M | 31 | No | PPH | T2−T4 | 16 | 7 | 12 | 12 | 2 |
| 7 | M | 48 | No | Facial blushing | T2−T3 | 21 | 7 | 9 | 10 | 3 |
| 8 | M | 33 | Yes | PPH | T2−T4 | 4 | 7 | 10 | 14 | 4 |
| 9 | M | 45 | Yes | PPH | T2−T3 | 23 | 7.5 | 10 | 10 | 3 |
| 10 | M | 35 | No | PPH | T2−T3 | 14 | 8.5 | 6 | 10 | 5 |
| 11 | M | 38 | Yes | PPH | T2−T3 | 23 | 10.5 | 10 | 10 | 4 |
| 12 | M | 44 | No | PPH | T3 | 23 | 8.5 | 8 | 7 | 5 |
| 13 | M | 40 | No | PPH | Right T3–T4 | 27 | 6.5 | 9 | 9 | 4 |
| Left T3 | ||||||||||
| 14 | M | 40 | Yes | PPH | Right T2–T3 | 23 | 7.5 | 9 | 7 | 4 |
| Left T3 | ||||||||||
| 15 | M | 37 | No | PPH | Right T2 | 17 | 10 | 9 | 9 | 3 |
| Left T3–4 | ||||||||||
| 16 | M | 44 | No | PPH | Right T2–T3 | 22 | 10 | 9 | 3 | 5 |
| Left T2 | ||||||||||
| 17 | F | 44 | No | PPH | Right T2-T3 | 30 | 8 | 9 | 13 | 2 |
| Left T2–T3 | ||||||||||
| 18 | M | 41 | No | PPH | Right T2 | 19 | 8 | 7 | 7 | 3 |
| Left T2 | ||||||||||
| 19 | M | 57 | No | PPH | Right T2–T6 | 27 | 7.5 | 13 | 15 | 5 |
| Left T2–T5 | ||||||||||
| 20 | M | 43 | No | PPH | T2–T3 | 27 | 8 | 13 | 6 | 3 |
| 21 | M | 42 | No | PPH | T2–3 | 23 | 8 | 12 | 12 | 2 |
| 22 | M | 46 | No | PPH | Right T2–3 | 18 | 7.5 | 13 | 11 | 4 |
| Left T2 | ||||||||||
| 23 | M | 43 | No | PPH | T3 | 1.5 | 7 | 10 | 10 | 3 |
| Mean | – | 42.7 | – | – | 19.6 | 8.5 | 9.7 | 9.8 | 3.2 | |
| SD | – | 6.6 | – | – | 7.8 | 1.7 | 2.2 | 3.3 | 1.2 |
ETS, Endoscopic thoracic sympathectomy; STR, sympathetic trunk reconstruction; Postop, postoperative; LOS, length of stay; F, female; PPH, primary palmar hyperhidrosis; M, male.
Perioperative Results
The mean operating time was 8.5 ± 1.7 hours. No conversion to open technique was required, and intraoperative complications did not occur. No patient received a second surgery. Postoperative complications included pneumothorax (n = 1) and leg wound dehiscence (n = 1). The former was managed conservatively, whereas the latter was treated with wound debridement and primary closure under local anesthesia. Postoperative Horner syndrome was not observed in any patients. The mean length of postoperative hospital stay was 3.2 ± 1.2 days.
Postoperative Follow-up
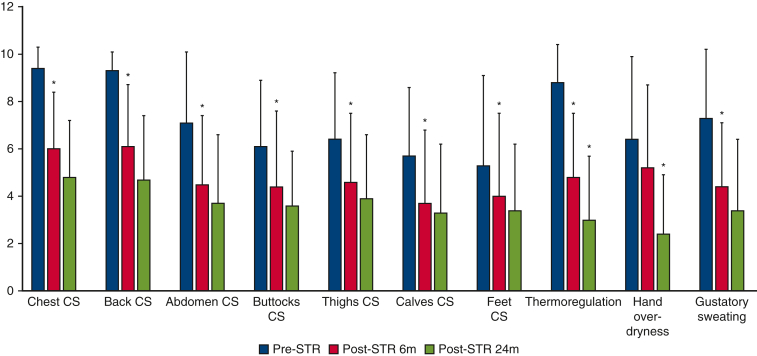
Table 2 and Figure 3 summarize the VAS scores measured before STR as well as at 6-month and 2-year follow-up for the following variables: site-specific CS, thermoregulatory alterations, hand dryness, and gustatory hyperhidrosis.
Table 2.
Visual analog scale scores (range, 0-10, with higher scores indicating greater severity) (n = 23)
| Site | Pre-STR | Post-STR, 6 mo | Post-STR, 24 mo | Statistically significant difference |
|---|---|---|---|---|
| Compensatory sweating | ||||
| Chest | 9.4 ± 0.9 | 6 ± 2.4 | 4.8 ± 2.4 | ∗ |
| Back | 9.3 ± 0.8 | 6.1 ± 2.6 | 4.7 ± 2.7 | ∗ |
| Abdomen | 7.1 ± 3 | 4.5 ± 2.9 | 3.7 ± 2.9 | ∗ |
| Buttocks | 6.1 ± 2.8 | 4.4 ± 3.2 | 3.6 ± 2.3 | ∗ |
| Thighs | 6.4 ± 2.8 | 4.6 ± 2.9 | 3.9 ± 2.7 | ∗ |
| Calves | 5.7 ± 2.9 | 3.7 ± 3.1 | 3.3 ± 2.9 | ∗ |
| Feet | 5.3 ± 3.8 | 4 ± 3.5 | 3.4 ± 2.8 | ∗ |
| Other ETS-related symptoms | ||||
| Thermoregulation | 8.8 ± 1.6 | 4.8 ± 2.7 | 3 ± 2.7 | ∗† |
| Hand dryness | 6.4 ± 3.5 | 5.2 ± 3.5 | 2.4 ± 2.5 | † |
| Gustatory sweating | 7.3 ± 2.9 | 4.4 ± 2.7 | 3.4 ± 3 | ∗ |
STR, Sympathetic trunk reconstruction; ETS, endoscopic thoracic sympathectomy.
Pre-STR vs post-STR at 6 months.
Post-STR at 6 months vs post-STR at 24 months (P < .025).
Figure 3.
Distribution of visual analog scale (range, 0-10, with higher scores indicating greater severity) scores for compensatory sweating at different anatomical sites and thermoregulatory alternations measured at 3 distinct time points (pre-sympathetic trunk reconstruction (STR), post-STR at 6 months, and post-STR at 24 months). CS, Compensatory sweating.
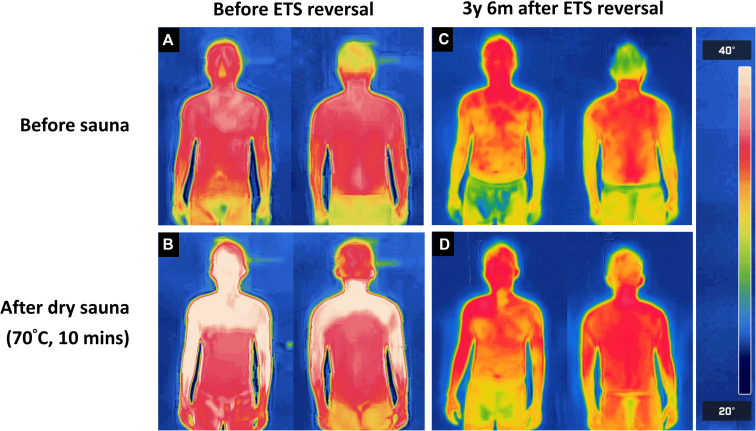
In general, 6-month assessments revealed that robotic STR was effective in reducing the severity of CS at all sites. The observed improvements were not only effectively sustained but also continued to progress up to 24 months post-STR, demonstrating the successful reinnervation of the sympathetic pathway. Similar trends were noted for thermoregulatory alterations and gustatory hyperhidrosis. Figure 4 shows the comparative images obtained from a patient who entered a thermal scanning room before (panels A and C) and after (panels B and D) spending 10 minutes in a sauna. Before STR, staying in a sauna produced 2 clearly distinct thermal regions (Figure 4, B). Conversely, the post-sauna image taken after STR (Figure 4, D) revealed an improved heat balance without any distinguishable thermal zone.
Figure 4.
Thermograms obtained before and after 10 minutes in a sauna. Before sympathetic trunk reconstruction (STR), a high skin temperature was evident over an anhidrotic area following the sauna test (A versus B). After STR, the skin temperature distribution was more uniform and did not change significantly before and after the sauna test (C versus D). ETS, Endoscopic thoracic sympathectomy.
Discussion
Robotic STR for CS reversal has been described for the first time in 2016 and successfully applied to 3 patients.14 To our knowledge, this is the largest case series to date reporting the clinical outcomes of robotic STR. By applying a thorough follow-up protocol, we were able to demonstrate that the benefits conferred by STR were durable and sustainable over time. Similar trends were evident for other ETS-related adverse effects, including thermoregulatory alterations and gustatory hyperhidrosis. Notably, none of the study patients developed recurrent primary hyperhidrosis. Collectively, our results demonstrate the robotic STR should be considered as a viable therapeutic option for patients who require reversal of thoracic sympathectomy.
In the realm of peripheral nerve reconstruction, nerve grafting has been a widely utilized surgical method for several decades.15 The primary function of the grafted nerve is to act as a conduit or scaffold, facilitating axonal regeneration and reestablishing connections between severed nerve segments. Theoretically, following successful nerve coaptation, nerve signals can traverse the graft, originating from healthy stumps and reinnervating receptors throughout the body, thereby restoring sympathetic function. Axonal regeneration typically occurs at an estimated rate of 1 mm/day.15 Consequently, it takes a significant amount of time for nerve signals to reach various end targets, making it unsurprising to observe consistent improvements at the 2-year mark compared with 6 months.
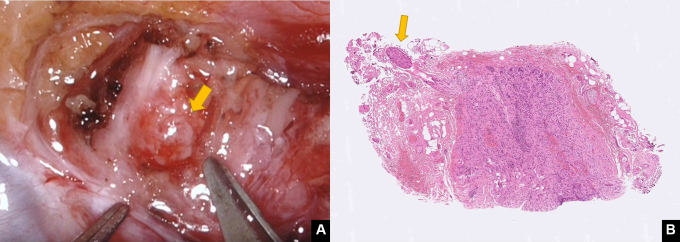
Prior studies published over the past 2 decades have shown that thoracoscopic STR is clinically feasible; however, our approach was found to outperform the results of this traditional technique. Several possible explanations may be advanced, including the quality of nerve coaptation. Due to inherent instrument limitations, nerve coaptation during thoracoscopy is performed using tissue glues (ie, without resorting to suturing).11,12 In this scenario, the quality of coaptation might vary widely and is largely unpredictable. Robot-assisted microsurgery offers an ideal solution to circumvent these issues through an unparalleled suturing ability, which can successfully be implemented even within limited anatomical spaces.16 This can be obtained by leveraging the benefits of tremor filtering and the use of articulated microinstrumentation to optimize the quality of nerve coaptation. Another important advantage lies in the accurate identification of healthy proximal stumps. Ideally, a nerve stump should be soft, pliable, fresh bleeding, and mushrooming over the fascicles and the ganglion (Figure 5). In our institution, robotic STR is accomplished through a collaborative multidisciplinary approach that involves 2 distinct teams specializing in thoracic surgery and plastic and reconstructive surgery (microsurgery subspecialty), respectively. Such a combination may have resulted in an increased attention to fine surgical details, which eventually offered sustained clinical benefits.
Figure 5.
Proximal sympathetic nerve stump. A, A healthy nerve stump (arrow) should be soft, pliable, fresh bleeding, and mushrooming over the fascicles. B, Histology findings of a specimen from the proximal stump, showing healthy ganglions and nerve fibers (arrow).
Although robotic STR appears safe and clinically feasible for the reversal of CS in patients who had previously undergone ETS, it is important to acknowledge certain limitations within our research. First, like many nonclinical trial studies, we encountered the issue of missing data. In our current report, approximately 15% (n = 4) of the patients were excluded from the analysis due to either complete (n = 2) or partial (n = 2) absence of questionnaire data. One patient, who initially demonstrated a positive 6-month outcome, unfortunately died 1.5 years after surgery due to a work-related injury. Another patient exhibited favorable results at the 2-year mark but lacked 6-month outcome data. As for the remaining 2 patients with entirely missing information, we received no updates from them since their discharge from the hospital. We cannot dismiss the possibility that these individuals experienced poor outcomes, so excluding them from the analysis may lead to an overestimation of our treatment results. Second, the sample size was relatively limited and did not allow performing subgroup analyses with the goal of identifying patients who were most likely to benefit from the robotic procedure. Third, this study was solely based on a subjective evaluation that relied on a simple VAS tool. Theoretically, it would have been interesting to include a validated instrument for assessing sweating–like the Hyperhidrosis Disease Severity Scale.17 However, per protocol, this scale has been designed for primary hyperhidrosis and it is not intended for use in patients with CS; therefore, most published studies in the field have resorted to nonstandardized questionnaires for outcome assessment.18, 19, 20 The future development and validation of an objective scale for CS should work to address this limitation.
Conclusions
Robotic STR is safe and effective in reversing intolerable CS after ETS. The results of STR are persistent and can be effectively maintained over time without any recurrence of CS at the primary sites.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://www.aats.org/resources/robotic-sympathetic-trunk-reconstruction-for-compensatory-sweating-after-thoracic-sympathectomy.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handing or reviewing manuscripts for which they have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Supported by a grant (CIRPG3M0021 & CIRPG3M0031) from the Chang Gung Memorial Hospital, Taiwan.
Supplementary Data
After docking, pneumolysis was carried out at the site of previous endoscopic thoracic sympathectomy, including the proximal and distal stumps as well as the affected intercostal nerves. The proximal and distal stumps were thoroughly examined and dissected until a healthy boundary was identified. We subsequently measured the defect length, starting from the proximal stump, through a passage underneath the involved intercostal nerve, until the distal stump was reached. Although this procedure was being performed, a microsurgeon began harvesting the sural nerve according to the measured defect. Coaptation was performed starting from the proximal sympathetic stump to the distal sural nerve using 8–0 nylon sutures. After the graft was passed underneath the intercostal nerves, its redundant segments were cut and reserved for the contralateral side. The proximal sural nerve was coapted to the distal sympathetic stump, and the graft was fixed to the intercostal nerves in a side to side fashion. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00201-8/fulltext.
References
- 1.Sang H.W., Li G.L., Xiong P., Zhu M.C., Zhu M. Optimal targeting of sympathetic chain levels for treatment of palmar hyperhidrosis: an updated systematic review. Surg Endosc. 2017;31:4357–4369. doi: 10.1007/s00464-017-5508-y. [DOI] [PubMed] [Google Scholar]
- 2.Cerfolio R.J., De Campos J.R., Bryant A.S., Connery C.P., Miller D.L., DeCamp M.M., et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg. 2011;91:1642–1648. doi: 10.1016/j.athoracsur.2011.01.105. [DOI] [PubMed] [Google Scholar]
- 3.Dumont P. Side effects and complications of surgery for hyperhidrosis. Thorac Surg Clin. 2008;18:193–207. doi: 10.1016/j.thorsurg.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Bryant A.S., Cerfolio R.J. Satisfaction and compensatory hyperhidrosis rates 5 years and longer after video-assisted thoracoscopic sympathotomy for hyperhidrosis. J Thorac Cardiovasc Surg. 2014;147:1160–1163.e1161. doi: 10.1016/j.jtcvs.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Chen J., Liu Y., Yang J., Hu J., Peng J., Gu L., et al. Endoscopic thoracic sympathicotomy for primary palmar hyperhidrosis: a retrospective multicenter study in China. Surgery. 2019;166:1092–1098. doi: 10.1016/j.surg.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Wolosker N., Milanez de Campos J.R., Fukuda J.M. Management of compensatory sweating after sympathetic surgery. Thorac Surg Clin. 2016;26:445–451. doi: 10.1016/j.thorsurg.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Loscertales J., Congregado M., Jimenez-Merchan R., Gallardo G., Trivino A., Moreno S., et al. Sympathetic chain clipping for hyperhidrosis is not a reversible procedure. Surg Endosc. 2012;26:1258–1263. doi: 10.1007/s00464-011-2023-4. [DOI] [PubMed] [Google Scholar]
- 8.Moon M.H., Hyun K., Park J.K., Lee J. Surgical treatment of compensatory hyperhidrosis: retrospective observational study. Medicine. 2020;99:e22466. doi: 10.1097/MD.0000000000022466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Currie A.C., Evans J.R., Thomas P.R. An analysis of the natural course of compensatory sweating following thoracoscopic sympathectomy. Int J Surg. 2011;9:437–439. doi: 10.1016/j.ijsu.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Haam S.J., Park S.Y., Paik H.C., Lee D.Y. Sympathetic nerve reconstruction for compensatory hyperhidrosis after sympathetic surgery for primary hyperhidrosis. J Korean Med Sci. 2010;25:597–601. doi: 10.3346/jkms.2010.25.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rantanen T., Telaranta T. Long-term effect of endoscopic sympathetic nerve reconstruction for side effects after endoscopic sympathectomy. Thorac Cardiovasc Surg. 2017;65:484–490. doi: 10.1055/s-0036-1582431. [DOI] [PubMed] [Google Scholar]
- 12.Wong R.H., Ng C.S., Wong J.K., Tsang S. Needlescopic video-assisted thoracic surgery for reversal of thoracic sympathectomy. Interact Cardiovasc Thorac Surg. 2012;14:350–352. doi: 10.1093/icvts/ivr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang T.N.-J., Chen L.W.-Y., Lee C.-P., Chang K.-H., Chuang D.C.-C., Chao Y.-K. Microsurgical robotic suturing of sural nerve graft for sympathetic nerve reconstruction: a technical feasibility study. J Thorac Dis. 2020;12:97. doi: 10.21037/jtd.2019.08.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connery C.P. Reconstruction of the sympathetic chain. Thorac Surg Clin. 2016;26:427–434. doi: 10.1016/j.thorsurg.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Grinsell D., Keating C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan Y.P.A., Liverneaux P., Wong J.K.F. Current limitations of surgical robotics in reconstructive plastic microsurgery. Front Surg. 2018;5:22. doi: 10.3389/fsurg.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wade R., Jones-Diette J., Wright K., Layton A.M., Woolacott N. Hyperhidrosis quality of life measures: review and patient perspective. J Dermatol Treat. 2019;30:303–308. doi: 10.1080/09546634.2018.1506080. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H., Okada M. The management of compensatory sweating after thoracic sympathectomy. J Thorac Cardiovasc Surg. 2019;158:1481–1488. doi: 10.1016/j.jtcvs.2019.05.062. [DOI] [PubMed] [Google Scholar]
- 19.Dumont P., Denoyer A., Robin P. Long-term results of thoracoscopic sympathectomy for hyperhidrosis. Ann Thorac Surg. 2004;78:1801–1807. doi: 10.1016/j.athoracsur.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Horslen L.C., Wilshire C.L., Louie B.E., Vallières E. Long-term impact of endoscopic thoracic sympathectomy for primary palmar hyperhidrosis. Ann Thorac Surg. 2018;106:1008–1012. doi: 10.1016/j.athoracsur.2018.04.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After docking, pneumolysis was carried out at the site of previous endoscopic thoracic sympathectomy, including the proximal and distal stumps as well as the affected intercostal nerves. The proximal and distal stumps were thoroughly examined and dissected until a healthy boundary was identified. We subsequently measured the defect length, starting from the proximal stump, through a passage underneath the involved intercostal nerve, until the distal stump was reached. Although this procedure was being performed, a microsurgeon began harvesting the sural nerve according to the measured defect. Coaptation was performed starting from the proximal sympathetic stump to the distal sural nerve using 8–0 nylon sutures. After the graft was passed underneath the intercostal nerves, its redundant segments were cut and reserved for the contralateral side. The proximal sural nerve was coapted to the distal sympathetic stump, and the graft was fixed to the intercostal nerves in a side to side fashion. Video available at: https://www.jtcvs.org/article/S2666-2507(23)00201-8/fulltext.