Abstract
To evaluate the effectiveness of antiseptic mouthwashes in reducing SARS-CoV-2 load clinically and in vitro. A systematic electronic search (MEDLINE/Scopus/Cochrane) was conducted to identify prospective clinical and in vitro studies published between 2019 included and 16 June 2023 assessing the effectiveness of mouthwashes in reducing SARS-CoV-2 load in saliva or surrogates. Data were summarized in tables and a network meta-analysis was performed for clinical trials. Thirty-five studies (14 RCTs, 21 in vitro) fulfilled the inclusion criteria. The risk of bias was judged to be high for 2 clinical and 7 in vitro studies. The most commonly test product was chlorhexidine alone or in combination with other active ingredients, followed by povidone-iodine, hydrogen peroxide and cetylpyridinium chloride. Overall, the descriptive analysis revealed the effectiveness of the mouthwashes in decreasing the salivary viral load both clinically and in vitro. Network meta-analysis demonstrated a high degree of heterogeneity. Among these studies, only chlorhexidine 0.20% was associated to a significant Ct increase in the saliva 5 min after rinsing compared to non-active control (p = 0.027). Data from clinical and in vitro studies suggested the antiviral efficacy of commonly used mouthwashes. Large well-balanced trials are needed to identify the best rinsing protocols.
Keywords: SARS-CoV-2, Viral load, Saliva, Mouthwash
1. Introduction
In early 2020, the World Health Organization (WHO) declared COVID-19, a disease caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), a pandemic. The oral cavity is one of the first gateways and a site of accumulation and replication for the virus, which has been found to reside in oral cavity epithelium, throat, nose and salivary gland cells [1]. SARS-CoV-2 virus can infect and replicate within salivary gland cells, especially minor ones, thus resulting in the presence of the virus in the saliva also in asymptomatic patients [2]. Furthermore, SARS-CoV-2 RNA was also detected in the saliva in the early phases of the infection before pulmonary clinical manifestations [3]. As saliva droplets seem to play a crucial role in SARS-CoV-2 transmission also from asymptomatic and mild symptomatic patients [4], [5], strategies to minimize SARS-CoV-2 spread through the saliva are pivotal in clinical procedures involving the oral cavity.
To protect both healthcare professionals and patients, several infection prevention and control measures have been introduced and implemented since the outbreak of COVID-19. In addition to personal protection equipment (PPE) for healthcare professionals, several European guidelines also recommend the use of preprocedural rinses with antiseptic mouthwashes in order to decrease the viral load contained in patients’ saliva [6]. The effectiveness of preprocedural rinsing to reduce bacterial contamination has been extensively demonstrated also prior to the pandemic, especially for professional oral hygiene and surgical procedures involving abundant aerosol production [7], [8], [9]. For instance, periprocedural rinsing with 0.12% and 0.20% chlorhexidine (CHX) was found to reduce bacterial, viral, and fungal load in the oral biofilm, thus decreasing the risk of cross infection [10]. As regards the SARS-CoV-2 virus, numerous preprocedural rinsing protocols have been proposed. SARS-CoV-2 is an enveloped virus, characterized by an outer lipid membrane that makes the virus highly sensitive to agents that disrupt it. One of these is ethanol at high concentrations of 60–70% (v/v), which has been found to be highly effective against several viral pathogens in vitro, including SARS-CoV-2, but at the same time to damage mammalian cells [11]. The clinical use of 1% hydrogen peroxide (H2O2) or 0.2% povidone iodine (PVP-I) mouthwashes has been suggested due to the vulnerability of the SARS-CoV-2 virus to oxidation [12]. CHX, essential oils, and cetylpyridinium chloride (CPC) alone or in combination with other compounds have also been recommended [6], [13].
Numerous national and international guidelines have been published so far, suggesting the use of different preprocedural mouth rinse protocols [6], [14]. Nevertheless, it can be affirmed that there is no universally accepted preprocedural rinsing protocol for the reduction of SARS-CoV-2 load in the oral cavity, most likely due to evidence showing the effectiveness of several compounds. Furthermore, it is not clear whether and to which extent these interventions are effective in the prevention of viral spread in dental setting. Despite numerous reviews have been published so far on the topic [15], [16], [17], to the best of the authors’ knowledge, this is the first systematic review and meta-analysis aiming to investigate, both in clinical and in vitro studies, the effectiveness of antiseptic mouthwashes and rinsing protocols in reducing SARS-CoV-2 load.
2. Materials and methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [18] was followed to design and write the present systematic review. The protocol was registered with the international prospective register of systematic reviews (PROSPERO) with registration number CRD42022318922.
2.1. PICOS question
The addressed PICOS (population, intervention, comparison, outcome, study) question was: “Are oral antiseptics able to reduce SARS-CoV-2 load in human oro-pharyngeal fluids and surrogates compared to control in human clinical and in vitro studies?” (Table 1).
Table 1.
Structured PICOS.
| P (Population) | Oropharyngeal fluids from COVID-19 positive patients or surrogates | COVID-19 positive patients |
|---|---|---|
| I (Intervention) | Exposure to oral antiseptics | Rinsing with oral antiseptics |
| C (Comparison) | Control solutions | Control solutions |
| O (Outcome) | SARS-CoV-2 load reduction in the samples | SARS-CoV-2 load reduction in the saliva |
| S (Study) | In vitro studies | Human clinical studies |
2.2. Search strategy, study selection, and data extraction
In human clinical and in vitro studies assessing the effectiveness of mouthwashes in reducing SARS-CoV-2 load were considered. To identify the studies to be included in this systematic review and meta-analysis, an electronical literature search was conducted on the databases MEDLINE (PubMed) online library, Scopus and Cochrane library on paper published between 2019 included and 15 June 2023. A specific search strategy was developed for each database, using a combination of the following MeSH and keywords: “mouthwash”, “mouthrinse”, “chlorhexidine”, “povidone iodine”, “cetylpyridinium chloride”, “hydrogen peroxide”, “delmopinol”, “listerine”, “essential oils”, “cyclodexstrin”, “citrox”, “SARS-CoV-2", ”coronavirus”, and “COVID-19″. Details on the search strategy are provided in Table 2. The included papers were selected by two independent reviewers (G.B. and L.Sc.) through 2 screening stages, i.e. abstract-title and full-text. To assess the level of inter-reviewer agreement at both stages, Kappa statistics were calculated with an online tool [19]. Any disagreement was resolved by discussion and, if necessary, a third reviewer (P.B. for in vitro and S.S. for clinical studies) was consulted.
Table 2.
Details on the electronic search strategy.
| Database | Search strings |
|---|---|
| MEDLINE (PubMed) |
(mouthwash OR mouthrinse OR chlorhexidine OR cetylpyridinium chloride OR "povidone iodine" OR "hydrogen peroxide" OR delmopinol OR listerine OR "essential oils" OR cyclodextrin OR citrox) AND (SARS-CoV-2 OR coronavirus OR COVID-19) Filters: from 2019 to 2023 (("mouthwashes"[Pharmacological Action] OR "mouthwashes"[MeSH Terms] OR "mouthwashes"[All Fields] OR "mouthwash"[All Fields] OR "mouthwashing"[All Fields] OR "mouthwashings"[All Fields] OR ("mouthrinse"[All Fields] OR "mouthrinsed"[All Fields] OR "mouthrinses"[All Fields] OR "mouthrinsing"[All Fields] OR "mouthrinsings"[All Fields]) OR ("chlorhexidine"[MeSH Terms] OR "chlorhexidine"[All Fields] OR "chlorhexidin"[All Fields]) OR ("cetylpyridinium"[MeSH Terms] OR "cetylpyridinium"[All Fields] OR ("cetylpyridinium"[All Fields] AND "chloride"[All Fields]) OR "cetylpyridinium chloride"[All Fields]) OR "povidone iodine"[All Fields] OR "hydrogen peroxide"[All Fields] OR ("delmopinol"[Supplementary Concept] OR "delmopinol"[All Fields]) OR ("listerine"[Supplementary Concept] OR "listerine"[All Fields] OR "listerine"[All Fields] OR "sodium fluoride"[MeSH Terms] OR ("sodium"[All Fields] AND "fluoride"[All Fields]) OR "sodium fluoride"[All Fields]) OR "essential oils"[All Fields] OR ("cyclodextrine"[All Fields] OR "cyclodextrines"[All Fields] OR "cyclodextrins"[MeSH Terms] OR "cyclodextrins"[All Fields] OR "cyclodextrin"[All Fields]) OR "citrox"[All Fields]) AND ("sars cov 2"[MeSH Terms] OR "sars cov 2"[All Fields] OR "sars cov 2"[All Fields] OR ("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields] OR "coronaviruses"[All Fields]) OR ("covid 19"[All Fields] OR "covid 19"[MeSH Terms] OR "covid 19 vaccines"[All Fields] OR "covid 19 vaccines"[MeSH Terms] OR "covid 19 serotherapy"[All Fields] OR "covid 19 nucleic acid testing"[All Fields] OR "covid 19 nucleic acid testing"[MeSH Terms] OR "covid 19 serological testing"[All Fields] OR "covid 19 serological testing"[MeSH Terms] OR "covid 19 testing"[All Fields] OR "covid 19 testing"[MeSH Terms] OR "sars cov 2"[All Fields] OR "sars cov 2"[MeSH Terms] OR "severe acute respiratory syndrome coronavirus 2"[All Fields] OR "ncov"[All Fields] OR "2019 ncov"[All Fields] OR (("coronavirus"[MeSH Terms] OR "coronavirus"[All Fields] OR "cov"[All Fields]) AND 2019/11/01:3000/12/31[Date - Publication])))) AND (2019:2023[pdat]) |
| Scopus | TITLE-ABS-KEY ((mouthwash OR mouthrinse OR chlorhexidine OR "cetylpyridinium chloride" OR "povidone iodine" OR "hydrogen peroxide" OR delmopinol OR listerine OR "essential oils" OR cyclodextrin OR citrox) AND (sars-cov-2 OR coronavirus OR covid-19)) AND PUBYEAR > 2018 AND PUBYEAR > 2018 |
| Cochrane | (mouthwash OR mouthrinse OR chlorhexidine OR cetylpyridinium chloride OR povidone iodine OR hydrogen peroxide OR delmopinol OR Listerine OR essential oils OR cyclodextrin OR citrox) AND (SARS-CoV-2 OR coronavirus OR COVID-19) in Title Abstract Keyword - with Publication Year from 2019 to 2023, with Cochrane Library publication date Between Dec 2019 and Jun 2023, in Trials |
Inclusion criteria were: randomized clinical trials (RCTs) and in vitro studies published in English, investigation/use of SARS-CoV-2, details on mouthwash formulation, concentration and application time, use of a control solution (active and/or non-active products), virus load quantification in oro-pharyngeal fluids and surrogates. For the quantitative analysis, it was also required that they adhered to one collection method (passive drool), utilized at least one of the most popular mouthwashes (i.e. CHX, CPC, H2O2, PVP-I), and a non-active control.
Exclusion criteria were: animal studies, review, case report and case series, investigation/use of other viruses (e.g. MERS, SARS-CoV-1, influenza virus) or pseudoviruses, less than 15 included patients (for clinical studies), oral/nasal spray and oral gel formulations. The same mouthwash at a different concentration or applied for a different exposure time was not alone considered as a control.
For each included study, relevant data were extracted and recorded on two previously outlined data collection tables for clinical and in vitro studies, respectively.
2.3. Risk of bias assessment
The risk of bias of the included studies was assessed independently and in duplicate by two reviewers (L.Sb. and L.Sc.). Disagreements between the reviewers were solved by discussion. The risk of bias of in vitro studies was assessed by using the Toxicological data Reliability assessment Tool (ToxRTool) [20], comprising 18 criteria.
For RCTs, the revised Cochrane risk-of-bias tool for randomised trials (RoB 2), structured in five bias domains was used [21]. An algorithm estimated the overall risk of the bias according to the results obtained for each domain, i.e. low risk, some concerns, or high risk.
2.4. Network meta-analysis
Network meta-analysis was performed on the included clinical trials reporting on ΔCt values from saliva samples calculated as the mean difference between immediate (T1; within 15 min post rinsing) follow-up and baseline (T0). Cycle threshold (Ct) values from quantitative reverse transcription polymerase chain reaction (RT-PCR) are commonly used to represent viral load [22]. In case that data were not available, standard errors were approximated using the R-package Metafor [23], starting from mean ΔCt or mean T0 and T1 and standard deviation values, if given.
Statistical heterogeneity among clinical trials was assessed using the Q test [24] as well as the I2 index [25] and tau2 [24]. Network meta-analysis was performed using the Netmeta-package [26] using a random effects model, and based on standardized mean differences (SMDs) and standard errors (SE). Non-active control groups were considered to be the reference. A random effects model was used [24] to account for methodological differences among the studies, and SMDs and 95% confidence intervals (CIs) were computed. Forest and Funnell Plots were created. Statistical significance was defined as a p value < 0.05.
3. Results
Details on the adherence to the updated PRISMA guidelines [18] are presented in Attachment 1.
3.1. Study selection process
From the electronic search, 2782 articles were retrieved. After the removal of the duplicates, 1957 titles and abstracts were reviewed. After the first screening phase, 95 studies were considered relevant for the present review (inter-examiner agreement k = 0.83). Following full-text reading, 14 clinical and 21 in vitro studies fulfilled the inclusion criteria (inter-examiner agreement k = 0.93). Out of these, three clinical studies were eligible for quantitative analysis [27], [28], [29]. The reasons for exclusion after full-text screening are reported in Table 3 [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87].
Table 3.
Details on the electronic search strategy.
| Reason | Main reason for exclusion | Number | References |
|---|---|---|---|
| 1. | Nasal spray(s) alone or in combination with mouthwash(es) | 8 | [30], [31], [33], [34], [40], [41], [59], [64] |
| 2. | Study type (in silico, animal studies, case report/series, non-randomized clinical trials, review, no experimental data) | 14 | [42], [46], [47], [48], [54], [60], [62], [63], [68], [71], [82], [85], [86], [125] |
| 3. | Off-topic | 4 | [32], [35], [45], [49] |
| 4. | No longitudinal data on SARS-CoV-2 viral load in human oropharyngeal fluids and surrogates | 11 | [54], [56], [57], [58], [66], [67], [68], [70], [80], [83], [87] |
| 5. | Investigation/use of viruses or pseudoviruses different from SARS-CoV-2 | 5 | [50], [51], [52], [77], [81] |
| 6. | Absence of a control group (as defined in the "Materials and methods" section) | 4 | [37], [38], [39], [65] |
| 7. | Missing details on mouthwash formulation, concentration and/or application time | 10 | [36], [44], [69], [72], [73], [74], [75], [76], [78], [85] |
| 8. | Language | 1 | [43] |
| 9. | Sample Size < 15 patients | 3 | [53], [79], [84] |
A flowchart summarizing the selection process is presented in Fig. 1.
Fig. 1.
Flowchart of the selection procedure according to PRISMA 2020 guidelines [18].
3.2. Main results from clinical studies
A summary of the main features of the included clinical studies is reported in Table 4. All the included studies were RCTs comparing the use of at least one mouthwash with a non-active control solution. The most frequently investigated mouthwash was found to be chlorhexidine (CHX) at concentration of 0.12% [27], [88], [89], [90], [91], [92], [93] or 0.20% [28], [29], [93]. In one study the sequential use of hydrogen peroxide (H2O2) 1.5% followed by CHX 0.12% was tested [88]. Mouthwashes containing as active ingredients H2O2 [88], [89], [90], [91], [92], [94], [95], povidone-iodine (PVP-I) [28], [29], [89], [90], [91], [92], [94], [95], cetylpyridinium chloride (CPC) [29], [90], [91], [92], [95], [96], [97], CPC in association with zinc lactate (Zn) [88], β-cyclodextrin and Citrox® (CDCM) [98], hypochlorous acid (HOCl) [95], Listerine® [90] and cymenol (Cym) + Zinc chloride (ZnCl2) [93] were also studied.
Table 4.
Main features of clinical studies.
| Ref. | Test (type, concentration, time, N patients) | Non-active control (N patients) | Collection time points | Diagnosis methods (PCR if not specified) | Included N. patient (recruited / virus detected in saliva) | Saliva sample and quantity (mL) | Quantitative analysis on viral reduction (RT-PCR) | Main results |
|---|---|---|---|---|---|---|---|---|
| Adl et al., 2023[94] |
|
10 mL saline (N = 40; 30 s) |
|
Nasal swab | 120/53 | Spitting (2 mL) |
Δ (T1-T0) viral load (copy/mL)
|
Among 53 positive patients, 50 were outpatient, while 3 hospitalized. All three mouthwashes reduced the viral load after gargling, but not in a statistically significant way (p > 0.05). No significant differences were observed between the three groups (p = 0.23) |
| Alemany et al., 2022[96] | 15 mL CPC 0.07% (N = 60; 60 s) | 15 mL distilled water with same colorant as test product (N = 58; 60 s) |
|
Not reported | 118/105 | Saliva passive drool (1–1.5 mL) |
Viral load (copy/mL) mean (SD)
|
No significant difference in viral load between the two groups at 1 h and at 3 h. The level of nucleocapsid protein were significantly higher in CPC group compared with placebo at 1 h and 3 h. |
| Alzahrani et al., 2023[95] |
|
|
|
Nasopharyngeal or throat swab | 241/55 | Saliva passive drool (≥2 mL) |
Mean (SD) viral load (copy/mL)
|
Salivary viral load reduction over time. Significant reduction at each timepoint (T1, T2, T3) only for H2O2, when compared to baseline (p = 0.0478, p = 0.0402; p = 0.0485, respectively). The effect of PVP-I, H2O2 and CPC mouth rinses on salivary viral load reduction was significant compared to the no-rinse group at 60 min. Distilled water also showed a significant decrease in viral load compared to the no-rinse group (P = 0.011) |
| Carrouel et al., 2021[98] | 30 mL β- cyclodextrin 0.1% and Citrox® 0.01% (CDCM group) (N = 88; 60 s 3 times a day for 6 days, i.e. at 9 am, 2 pm and 7 pm) | 30 mL placebo (N = 88; 60 s) | The first day:
|
Nasopharyngeal swab | 176/176 | Saliva passive drool (2 mL) |
Median SARS-CoV-2 IQR (Log10 copies/mL of saliva) Day 1 T0: CDCM: 4.05 (2.94–4.96); placebo: 3.85 (2.97–5.08) Day 1 T1: CDCM: 3.33 (2.29–4.23); placebo: 3.60 (2.07–4.83) Day 1 T2: CDCM: 3.08 (0–4.19); placebo: 3.31 (1.18–4.75) Day 7: CDCM: 0 (0–1.34); placebo: 1.62 (0–1.70) |
The first day:
|
| Chaudhary et al., 2021[89] |
|
15 mL saline (N = 10; 60 s) |
|
Not reported | 201/82§§ | Saliva passive drool |
Median reduction in viral load compared to T0 (%):
|
|
| Costa et al., 2021[27] |
|
15 mL Placebo (N = 50; 60 s)§ |
|
Nasopharyngeal swab (rapid antigen test) | 110/100 | Saliva passive drool (1.5 mL) |
ΔCt(T1-T0): test: 2.19 ± 4.30; control: − 0.40 ± 3.87 (p = 0.002) ΔCt(T2-T0): test: 2.45 ± 3.88; control: 0.76 ± 4.41 (p = 0.05) ΔCt(T2-T1): test: 0.26 ± 4.16; control: 1.16 ± 4.47 (p = 0.30) |
|
| Eduardo et al., 2021[88] |
|
20 mL distilled water (N = 9; 60 s) |
|
Nasal swab | 60/43 | Saliva passive drool |
Fold reduction relative to T0:
|
|
| Elzein et al., 2021[28] |
|
15 mL distilled water (N = 9; 30 s) |
|
Nasopharyngeal swab | 70/61 | Saliva caught out from throat (2 mL) |
ΔCtvalue T1-T0
|
|
| Farmaha et al., 2023[90] |
|
5 mL water (N = 6; 120 s) |
|
Nasopharyngeal swab or saliva sample | 410/32 | Spitting (∼1 mL) |
Mean Ct values
|
Compared to control, a significant increase in Ct values was observed immediately after rinsing with each of the mouthwashes testes (p < 0.05). The Ct values for the Listerine reached the statistical significance cut-off compared to H2O2 and PVP-I; a statistically significant difference in Ct values was also observed between CHX group and Listerine group compared to the water group one and two hours after rinse. |
| Ferrer et al., 2021[91] |
|
Distilled water (N = 13; 60 s) |
|
Nasopharyngeal swab | 98/67 | Saliva passive drool (≥0.5 mL) |
Median viral load (Log copies/mL)
|
None of the tested mouthwashes significantly reduced viral load at any timepoint compared with baseline. Compared to control, a significant increase in Ct values was observed immediately after rinsing with each of the tested mouthwashes (p < 0.05). The Ct values for the Listerine reached the statistical significance cut-off compared to H2O2 and PVP-I. |
| Sánchez Barrueco et al., 2022[92] |
|
15 mL distilled water (N = 10; 60 s) |
|
Nasopharyngeal swab | 75/44 | Saliva passive drool (≥2 mL) |
Median viral load (Log copies/mL)
|
None of the mouthwashes reduced the saliva viral load, either at 30 min after rinsing or at 1 h. Unexpectedly, a significant decrease in the mean values of viral load in saliva was detected 1 h after rinsing with water (p = 0.05). |
| Sánchez Barrueco et al., 2023[93] |
|
15 mL placebo (N = 12; 60 s) |
|
Nasopharyngeal swab | 48/43 | Saliva passive drool (≥2 mL) |
Median viral load (Log copies/mL)
|
Both in CHX 0.12% and in Cym+ZnCl2, a progressive decrease in salivary viral load was observed over time, being significant with respect to the baseline from 15 min after CHX 0.12% (p = 0.037) and with a trend towards significance in Cym+ZnCl2 (p = 0.054). In addition, the values were significantly different at 1 h after the mouthwash in both groups (CHX 0.12% group: p = 0.02; Cym+ZnCl2 group: p = 0.04). In contrast, in both the placebo and CHX 0.2% group, no differences were observed between time-points. |
| Seneviratne et al., 2021[29] |
|
15 mL water (N = 2: 30 s) |
|
Nasal swab | 36/16 | Saliva passive drool (3 mL) |
Fold reduction relative to T0:
|
|
| Tarrago-Gil et al., 2023[97] | 15 mL CPC 0.07% (N = 39; 60 s) | 15 mL placebo (N = 40; 60 s) |
|
Nasal swab (rapid antigen test) | 80/80 | Saliva passive drool |
Mean (SD) Ct values
|
RT-qPCR: Ct values demonstrated no statistically significant difference before and after 2 h. ELISA: The protein N concentration 2 h after rinsing was significantly higher in the test group than in the placebo group (p = 0.038). |
CHX: chlorhexidine; CPC: cetylpyridinium chloride; Cym: cymenol; H2O2: hydrogen peroxide; HOCl: hypochlorous acid; IQR: interquartile range; NA: not applicable; Zn: zinc lactate; pt: patient; PVP-I: povidone-iodine; § 15 mL gargling for 30 s + 15 mL rinsing for 30 s; §§ out of 82, only the 40 symptomatic patients were included (of these 1 dropped out).
In all the selected studies a rinsing time of 30 or 60 s was adopted. A longer rinsing time was reported only for the combination of H2O2 (60 s) and CHX (30 s) [88] and for all the mouthwashes investigated by Farmaha et al. (120 s) [90]. The study design always involved a single rinse except for one study, in which the mouthwash was utilized 3 times daily for 7 days [98]. Saliva samples were collected at least once within one hour in all studies but two [97], [98]. As reported in Table 4, the most used saliva collection method was passive drool. All the included studies evaluated the presence of SARS‐CoV‐2 by RT-PCR. Active viral replication assessment [92], [93] and enzyme-linked immunosorbent assay (ELISA) [96], [97] were also performed.
CHX 0.12% was found to be effective in reducing saliva viral load both after 5 and 60 min as compared to a placebo solution [27]. Similar results were observed in another study, at 60 and 120 min after rinsing [90], while CHX at the same concentration did not significantly reduced viral load in other investigations [91], [92]. In another RCT, in which multiple mouthwashes were tested, CHX 0.12% met minimum acceptance criteria of a ≥ 2-fold reduction compared to baseline, which was significant at 30 and 60 min, but not at the early time point [88]. However, the best performances were achieved with H2O2 1.5% as well as with CPC-Zn mouthwashes, especially within the first 30 min. The sequential use of H2O2 and CHX did not lead to a higher efficacy as compared with the two active ingredients alone [88]. In symptomatic patients no significant differences in saliva viral load reduction among the four different solutions (i.e. CHX 0.12%; H2O2 1%; PVP-I 0.5%; saline) were detected after both 15 and 45 min from the rinse [89]. CHX 0.2% and PVP-I 1% were found to be more effective than the non-active compound at 5 min, however no statistically significant difference was found between the two products [28]. After a single administration, PVP-I 0.5% as well as CPC 0.075% exhibited sustained effects in reducing viral load compared to the non-active control for up to six hours [29]. CPC at a similar concentration was found not to be effective in reducing saliva Ct values 2 h after rinsing. However, at the same timepoint, ELISA test showed a significantly higher N protein concentration in the test group compared to the non-active control, indicating an increase in lysed virus [97].
When viral culture was performed, mouthwashes showed modest capacity to reduce viral infectivity in vivo, with viral inactivation detected only in the CPC group one hour after rinsing [92]. Discording results were found concerning CHX 0.12%: while no significant viral inactivation activity was detected in Sánchez Barrueco et al. [92], in another study of the same group the remaining viruses were mostly viable, despite the high viral load reduction detected with RT-PCR [93].
3.3. Main results from in vitro studies
The main features of the included in vitro studies are presented in Table 5. The most commonly test product was CHX alone [99], [100], [101], [102], [103], [104], [105], [106] or in combination with other active ingredients, such as CPC [100], [101], CPC + F- [100] or ethanol [39], [105]. The in vitro behaviour of PVP-I at different dilutions was investigated in 10 articles [99], [104], [105], [106], [107], [108], [109], [110], [111], [112], followed by H2O2 in six articles [100], [104], [105], [106], [109], [113]. Other test solutions include: CPC [39], [100], [101], [112], [113], CPC + sodium fluoride (F-) [102], CPC + H2O2 [100], silver nanoparticles (AgNPs) [114], anionic phthalocyanine derivate (APD) [46], [115], octenidine dihydrochloride (OCT) [103], Listerine® Original [106], Listerine® Cool Mint [104], Dequonal® [104], Delmopinol hydrochloride [101], dipotassium oxalate [105], hypochlorous acid [105], thymol [102], Bactidol® [102], SP_T medical gargle [116], and hexadecyl pyridinium chloride [117].
Table 5.
Main features of in vitro studies.
| Ref. | Test (type, concentration, exposure time) | Control | SARS-CoV2 source | Assessment method | Initial viral concentration | Incubation temperature | Reduction in viral titer compared to non-active control | Main results |
|---|---|---|---|---|---|---|---|---|
| Almanza-Reyes et al., 2021[114] | AgNPs at concentrations of 0.5–0.0004% (24 h; 48 h; 72 h) | Virus + culture medium | SARS-CoV-2 NL/2020 strain (BetaCoV/Netherlands/01) | Plaque assay | Serial two-fold dilutions from 1/2–1/2048 | 37 °C | Percentages of infectivity: 0.03% AgNPs reduces infectivity by 80% | Although AgNPs did not totally abolish viral production, infection was clearly controlled to some extent with a viral load reduction of about 80% at a concentration of 0.03%. A 50% inhibitory concentration was determined by curve fitting (non-linear regression). |
| Anderson et al., 2022[126] |
|
|
SARS-CoV-2 variants:
|
Plaque assay | 1/10 dilution starting from unknown concentration | 37 °C |
Log10PFU/mL reduction
|
Mouthwashes containing CPC 0.07% effectively inactivated SARS-CoV-2 with greater than 4.0 Log10 PFU/mL reduction in viral titre. Virucidal activity of CPC was maintained in presence of human saliva. Both CPC 0.07% mouthwashes were as effective as ethanol 70% against four variants. The mouthwash containing CHX digluconate 0.2% did not have substantial action against SARS-CoV-2 in vitro. |
| Bidra et al., 2020[109] |
|
|
SARS-CoV-2, USA-WA1/2020 | Plaque assay | 5.0 Log10 CCID50/0.1 mL | 22 ± 2 °C |
Log10CCID50/0.1 mL
|
After 15 s all the three concentrations of PVP-I were equally effective in reducing the SARS-CoV-2 load compared to the water control·H2O2 both at 3% and 1.5% concentration showed minimal viricidal effect after 15 and 30 s. Ethanol inactivated the virus similarly to the PVP-I products. |
| Bidra et al., 2020[108] |
|
|
(SARS-CoV-2) USA-WA1/2020 | Plaque assay | 5.0 Log10 CCID50/0.1 mL | 22 ± 2 °C |
Log10CCID50/0.1 mL
|
After 15 s all the three concentrations of PVP-I were equally effective in reducing the SARS-CoV-2 load compared to the water control. EtOH 70% was unable to completely inactivate SARS-CoV-2 after 15 s but was able to inactivate the virus after 30 s. No cytotoxicity was observed with any of the test compounds. |
| Chen et al., 2022[117] | Hexadecyl pyridinium chloride (0.2, 0.1, 0.05, 0.025, or 0.0125 mg/mL) (30 s; 60 s; 120 s; 300 s) | Culture medium + test solution | SARS-CoV-2 (hCoV-19/Zhejiang/OS2/2020, GISAID, ID: 455692) isolated from a patient at the Zhejiang Provincial Center for Disease Control and Prevention |
|
3 × 103 U/mL | 35 °C |
Log TCID50/mL reduction
|
The disinfection effect of hexadecyl pyridinium chloride is time and concentration-dependent, with the strongest virus-elimination effect at a concentration of 0.1 mg/mL for 2 min |
| da Silva Santos et al., 2021[115] | APD 2.0 mg/mL (30 min) | Virus + culture medium | SARS.CoV2/SP02.2020. HIAE. Br |
|
1 × 102 TCID50/mL | 37 °C |
RT-PCR reduction in viral load at:
|
APD, when compared to the control, showed a significant reduction in SARS-CoV-2 load at 1:2, 1:4, 1:8 and 1:16 dilution, and partially inactivated SARS-CoV-2 at 1:32 and 1:64 dilution. Cytotoxic effect was detected only at the initial dilution 2 mg/mL (2:1). No virus neutralization was observed below the 1:128 titer. |
| Davies et al., 2021[105] |
|
Medium + test solution (60 s) | SARS-CoV-2 England 2 strain | Plaque assay | 1.7 × 106 TCID50/mL | 20 ± 2 °C |
Mean titre reduction (Log10TCID50/mL)
|
Dipotassium oxalate demonstrated effective inactivation of SARS-CoV-2 in vitro and by commercial mouthwashes containing 0.01–0.02% hypochlorous acid or 0.58% PVP-I, while both the CHX and H2O2 mouthwashes tested in this study resulted to be ineffective against SARS-CoV-2. |
| Jain et al., 2021[99] |
|
_ | SARS-CoV-2 strain isolated from an Indian patient and cultured using VeroE6 cells | RT-PCR | 2 × 106 PFU/mL | 37 °C |
Relative Ct change
|
CHX 0.2% inactivated more than 99.9% of SARS-CoV-2 virus after a contact time of 30 s, and was considered as more efficacious than PVP-I 1% utilized for 30 s and 60 s |
| Koch-Heier et al., 2021[100] |
|
|
SARS-CoV-2 strain FI-100 | Plaque assay | 6.25 × 106 PFU/mL | 37 °C |
Log10PFU/mL reduction
|
While a combination of CPC and CHX as well as CPC alone led to a significant reduction of infectious viral particles, H2O2 and CHX alone had no virucidal effect against SARS-CoV-2. At the crystal violet staining A reduction resulted from 0.05% CPC and the combination of 0.1% CHX with 0.05% CPC, while no virucidal effect was observed for 0.1% CHX and 1.5% H2O2. |
| Komine et al., 2021[101] |
|
|
SARS-CoV-2 (JPN/TY/WK-521 strain) | Plaque assay | 0.1 mL of virus suspension (viral titer 8.49 Log10 PFU/mL) | 25 °C |
|
No cytotoxic or interference effects at dilutions ranging from 1 to 1/100 for any of the tested solutions. All the mouthwashes containing 0.4–0.075% CPC inactivated SARS-CoV-2 with a reduction of 3.3 to > 4.4 Log10 PFU/mL regardless of dosage. Mouthwash containing 0.20% delmopinol hydrochloride inactivated SARS-CoV-2 with a > 5.4 Log10 PFU/mL reduction. However, the mouthwash containing only 0.12% CHX as antiseptic did not show a sufficient inactivation effect against SARS-CoV-2 in this study. |
| Meister et al., 2020[104] |
|
SARS-CoV-2 + medium |
|
Plaque assay | 5 × 105 TCID50/mL in 1 vol of organic load mimicking respiratory secretions | 37 °C |
TCID50/mL reduction
|
Medium control after 30 s exposure time did not reduce viral infectivity. All the three SARS-CoV-2 strains were highly susceptible to various oral rinses. All tested products showed virucidal activity against SARS-CoV-2. In particular, Dequonal®, PVP-I and Listerine® Cool Mint, significantly reduced viral infectivity to up to 3 orders of magnitude to background levels. Evidence that SARS-CoV-2 can be efficiently inactivated by commercially available oral rinses within 30 s was provided. |
| Okamoto et al., 2022[112] |
|
SARS-CoV-2 + medium | SARS-CoV-2/Hu/DP/Kng/19–027,LC528233: SARS-CoV-2 isolated from a patient who developed COVID-19 on the cruise ship Diamond Princess | Plaque assay | 10-fold dilution starting from unknown viral stock concentration | Not reported |
% reduction
|
CPC has dose- and time-dependent antiviral activity. In Western blotting after SDS-PAGE under reducing conditions, the molecular weights of four forms of the S protein were unchanged. |
| Pelletier et al., 2021[107] |
|
|
SARS-CoV-2, USA-WA1/2020 strain | Plaque assay | 5.3 Log10 CCID50/0.1 mL | 22 ± 2 °C |
Log10CCID50/0.1 mL reduction
|
All the oral antiseptics evaluated were effective at reducing > 4 Log10 CCID50 infectious virus, from 5.3 Log10 CCID50/0.1 mL to 1 Log10 CCID50/0.1 mL or less. No cytotoxicity, or cell death, was observed in any of the test wells. Positive control and neutralization controls performed as expected and did not cause cell death. |
| Ramji et al., 2022[113] |
|
Medium alone | SARS-CoV-2 USA-WA1/2020 (NR-52281, BEI Resources; Manassas, VA, USA) | Plaque assay | 1:100 dilution for the mouth rinse; 1:10 dilution for the dentifrice | 20 ± 2 °C |
|
Both tested mouthwashes demonstrated strong virucidal activity in the virucidal efficacy suspension test after a 30 s contact time. |
| Santos et al., 2021[115] | APD 0.1% (30 s, 60 s, 300 s) |
|
SARS-CoV-2 samples retrieved from oropharynx of patients diagnosed with the new COVID | Plaque assay | 5.5 ln TCDI50/mL | 37 °C |
SARS-CoV-2 inactivation (%)
|
Mouthwash 0.1% APD presents 1 reduction Log10, with 90% viral inactivation, with the same percentage of reduction for all the exposure times performed. |
| Shet et al., 2022[111] | PVP-I 0.5% (15 s; 30 s; 60 s; 300 s) |
|
SARS-CoV-2, strain USA-WA1/2020 | Plaque assay | 1:2 dilution from the highest viral suspension | 22 ± 2 °C |
Log10CCID50/0.1 mL reduction
|
PVP-I 0.5% demonstrated effective virucidal activity against SARS-CoV-2 at all the timepoints, with a reduction of viral titer up to 2.5 and < 0.67 log10 CCID50/0.1 mL at 15 s and 30 s, respectively. |
| Steinhauer et al., 2021[103] |
|
SARS-CoV-2 + medium | _ | Plaque assay | 0.7 × 106 PFU/mL | _ |
TCID50/mL reduction
|
The two formulations based on CHX were found to have only limited efficacy against SARS-CoV-2, while OCT demonstrated virucidal efficacy against SARS-CoV-2, meeting the > 4 Log10 requirement of EN 14476 within a contact time of only 15 s |
| Takeda et al., 2022[116] | SP_T medical gargle (Commercial name)
|
|
SARS-CoV-2 variants:
|
|
107 PFU/mL, 1:2 dilution | Room temperature |
SARS-CoV-2 inactivation
|
Plaque assay: CPC significantly suppressed the infectivity of all examined SARS-CoV-2 directly in a dose-dependent manner. CPC (50 μg/mL) treatment completely inactivated SARS-CoV-2 Wuhan strain similarly as Triton X-100 (1%). With saliva: CPC (25–40 μg/mL) significantly inactivate SARS-CoV-2 in saliva, in a dose-dependent manner. qRT-PCR: Viral RNA expression level in the cells was significantly reduced by CPC via dose-dependent manner at 24 h postinfection. Western blotting: PBS and CPC might have no effect on the structure of the SARS-CoV-2 virions, whereas Triton X-100 changed the structure. TEM analysis: spherical particle structure of SARS-CoV-2 treated with PBS remained unchanged. Most virus particles treated with 10 μg/mL CPC remained unchanged, whereas some disintegrated with 50 μg/mL CPC. In contrast, almost all virus particles treated with 250 μg/mL CPC were clearly disrupted (like 1% Triton X-100). |
| Tiong et al., 2021[102] |
|
|
SARS-CoV-2 virus isolated from NP/OP swab from a SARS-CoV-2-positive patient | Plaque assay | 5 × 104 TCID50/mL | 37 °C |
Log10TCID50/mL reduction
|
In this study, Bactidrol® and CPC + F- mouthwashes were the most effective against SARS-CoV-2, decreasing more than the 99.99% of SARS-CoV-2 load compared to the control after 30 and 60 s of exposure, under both clean and dirty conditions. The virucidal activity of the CHX containing mouthwash against SARS-CoV-2 was slightly lower than the virucidal activity of Bactidrol® and CPC based mouthwashes, with a viral inactivation percentage of 99.99% under both clean and dirty conditions. Salt water and thymol did not show any significant reduction in viral load. |
| Wang et al., 2021[110] |
|
Medium alone | SARS-CoV-2 (hCoV-19/Zhejiang/OS2/ 2020, GISAID, ID: 455692) isolated from a patient in Zhejiang Provincial Centre for Disease Control and Prevention |
|
1 × 106 IU/mL, diluted 1:1000 | 35 °C |
Log10TCID50/mL Medium ∼ 6 at all timepoints
|
Viral inhibitory effect at the same concentration was highest when the contact time was 1 min RT-PCR: PVP-I at a concentration of 1000 μg/mL had no viral inhibition (CC50 > 2.75 mM). Effect of PVP-I on virus inhibition rate was mainly concentration dependent. The viral titres for the same contact time but different PVP-I concentrations decreased as the concentration of PVP-I increased. At high concentrations, prolonging the contact time does not enhance PVP-I virus suppression capability. |
| Xu et al., 2021[106] |
|
SARS-CoV-2 + medium | Pseudotype SARS-CoV-2 (USA_WA1/2020 strain) expressing mNeonGreen | Fluorescent assay | MOI 1:5 | 37 °C |
|
All undiluted mouthwashes and both 1.5% (v/v) dilutions of HP and PVP-I were highly toxic to HeLa-hACE2 hACE2 and oral epithelial cells. All mouth washes at non-cytotoxic levels exhibited antiviral activity. Highly diluted PVP-I and H2O2 significantly inactivated viruses but their antiviral effects were associated with severe cytotoxicity at higher concentrations. Taken together, Listerine® Original and CHX may be better mouth rinse products for SARS-CoV-2 prevention since they did not show cytotoxic effects. |
AgNPs: silver nanoparticles; APD: anionic phthalocyanine derivate; CCID50: 50% cell culture infectious dose; CHX: chlorhexidine; CPC: cetylpyridinium chloride; EtOH: ethanol; F-: sodium fluoride; H2O2: hydrogen peroxide; NP: nasopharyngeal; OCT: Octenidine dihydrochloride; OP: oropharyngeal; PBS: phosphate buffered solution; PVP-I: povidone-iodine; PFU: plaque-forming unit; TCID50: 50% tissue culture infectious dose; TEM: transmission electron microscopy.
Mouthwash effectiveness was assessed by plaque assay in all studies but three [99], [106], [115]. The other reported assessment methods were fluorescent assay [106], [110], [115], [117], RT-PCR [99], [110], [115], [116], [117], western blotting [116], and transmission electron microscopy (TEM) [116]. Except for three studies [106], [114], [115], at least one of the investigated exposure time was comprised between 15 s and 1 min, which is compatible with preprocedural mouth rinsing in dental settings. Contact times above 5 min and up to 72 h were reported in a minority of the studies [103], [106], [114], [115].
A limited efficacy of CHX 0.12% for 30 s was reported in Komine et al. [101], while in combination with CPC presented a high reduction in viral titer comparable to other tested solutions. Even a longer exposure (up to 5 min) of a higher concentration of CHX (0.16%) was found to be of limited efficacy against SARS-CoV-2 [103]. In the same study, favorable results were obtained by OCT, which met the viral titer reduction of > 4 Log10, required by the European Standard 14476 [118], within a contact time of only 15 s [103]. These findings are in line with what reported by Meister et al. [104], in which three SARS-CoV-2 strains were not highly susceptible to two different commercially available CHX 0.2% mouthwashes, while a significantly reduced viral infectivity was noticed with Dequonal®, PVP-I and Listerine® Cool Mint [104]. By contrast, in Jain et al. [99], CHX 0.2% performed better than PVP-I at the same concentration tested in the former study [104]. As in other works [104], [105], a limited virucidal effect was reported for both CHX and H2O2, while CPC alone, or combined with other active ingredients (i.e·H2O2, CHX and CHX+F-) showed a higher inactivation of SARS-CoV-2 [100]. Also in Tiong et al. [102] CPC in combination with F- performed better than CHX in vitro. Indeed, in that study a decrease of SARS-CoV-2 load above 99.99% compared to the control was detected with both CPC + F- and Bactidrol® after a contact time of 30 s and 60 s. CPC 0.07% alone, after a contact time of 30 s, demonstrated strong virucidal activity in vitro [113]. When tested at different concentrations and timepoints, it showed a dose- and time-dependent antiviral activity [112]·H2O2 at both 1.5% and 3% exhibited minimal virucidal effect after a contact time of 15 s and 30 s. Whereas, at the same time points, complete SARS-CoV-2 inactivation was found with different concentrations of PVP-I, which was comparable to that obtained with ethanol 70% [109]. The in vitro effectiveness of PVP-I was confirmed by other studies using the same assessment method, i.e. plaque assay, and a maximum exposure time of one minute [105], [107], [108]. The antiviral efficacy of PVP-I was also confirmed by other studies [111], [112]. At fluorescent assay, PVP-I 1% and H2O2 1.5% showed high antiviral activity, which was however associated with high cytotoxicity also at a dilution of 0.1% and 0.05%, respectively [106]. Despite less effective, Listerine® Original and CHX 0.12% presented no cytotoxic effect [106].
APD was tested in two studies with encouraging results [46], [115]. It was found to induce a viral inactivation of 90% independently of the exposure time (i.e. 30 s, 1 min, 5 min) [46]. Moreover, when the viral load reduction was assessed by RT-PCR, a significant decrease was found with ADP 2 mg/mL at a dilution in the range between 1:2 and 1:16 as compared to the non-active control [115].
Other products worth mentioning are dipotassium oxalate and hypochlorous acid, which were effective at inactivating SARS-CoV-2 after a contact time of 1 min [105]. Finally, a long exposure to AgNPs was found to control viral infectivity in vitro, despite viral production was not completely abolished [114].
3.4. Study quality and risk of bias
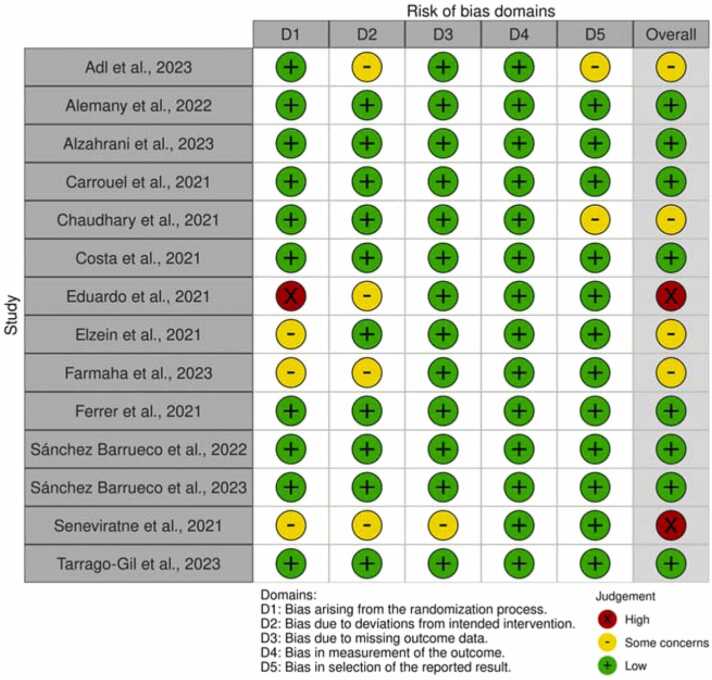
The quality assessment of the included RCTs is reported in Fig. 2 created using robvis [119]. Two clinical studies presented an overall high risk of bias [29], [88]. The main criticisms concerned the randomization process [28], [29], [88], [90] and the deviation from intended intervention [29], [88], [90], [94]. Eight clinical studies were considered at low risk of bias for every RoB2 parameter [27], [91], [92], [93], [95], [96], [97], [98].
Fig. 2.
Risk of bias traffic light plot of ROB 2 assessments created using robvis [119].
Risk of bias of the included in vitro studies is presented in Table 6. The toxRTool evaluation found 7 studies not meeting at least one “red criteria” [99], [106], [110], [113], [114], [115], [117], therefore they were considered not reliable. The remaining ten studies were considered reliable with scores ranging between fifteen and eighteen.
Table 6.
ToxRTool in vitro studies.
 |
ToxRTool criteria:
1. Was the test substance identified?
2. Is the purity of the substance given?
3. Is information on the source/origin of the substance given?
4. Is all information on the nature and/or physico-chemical properties of the test item given, which you deem indispensable for judging the data?
5. Is the test system described?
6. Is information given on the source/origin of the test system?
7. Are necessary information on test system properties, and on conditions of cultivation and maintenance given?
8. Is the method of administration given?
9. Are doses administered or concentrations in application media given?
10. Are frequency and duration of exposure as well as time-points of observations explained?
11. Were negative controls included?
12. Were positive controls included?
13. Is the number of replicates (or complete repetitions of experiment) given?
14. Are the study endpoint(s) and their method(s) of determination clearly described?
15. Is the description of the study results for all endpoints investigated transparent and complete?
16. Are the statistical methods for data analysis given and applied in a transparent manner?
17. Is the study design chosen appropriate for obtaining the substance-specific data aimed at?
18. Are the quantitative study results reliable?
3.5. Network meta-analysis
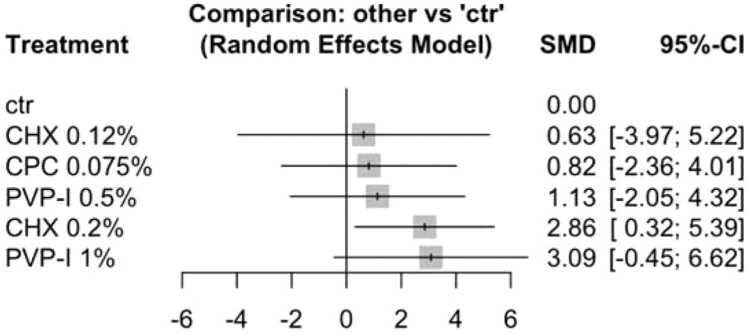
Network meta-analysis was performed (Fig. 3) estimating the effect of active ingredients from included studies at different concentrations against non-active controls [27], [28], [29]. The investigated active products were CHX 0.12% [27], CHX 0.2% [28], [29], CPC 0.075% [29], PVP-I 0.5% [29] and PVP-I 1% [28]. Rinsing time of studies included in the network meta-analysis amounted to either 30 s [28], [29] or 60 s [27], [29]. None of the studies included for quantitative analysis reported on the use of H2O2. As only studies reporting data at five minutes post rinsing were identified, the analysis was restricted to this time period. As none of the studies provided data on treatment effects and standard errors, these values were estimated using the Metafor package [23]. Additionally, two multi-arm studies had high variability among participants in the different groups, but lacked statistical comparison between the respective groups. Therefore, these values were estimated using the Metafor package [23].
Fig. 3.
Forest plot from random‐effects meta‐analysis of the included clinical studies on ΔCt (T1-T0).
The heterogeneity of the included studies was high. The I2 amounted to 94.9 [91.4%− 97.0%], and tau2 was 5.492. Q-statistics of heterogeneity (within design) amounted to 36.32 (p < 0.001) and inconsistency (between design) amounted to 62.19 (p < 0.001).
ΔCt (T1-T0) was found to be increased after rinsing with CHX 0.20% as compared to non-active controls (p = 0.027). Whereas, no significant difference was found between non-active controls and PVP-I 1% (p = 0.087), CHX 0.12% (p = 0.789), CPC 0.075% (p = 0.612), or PVP-I 0.5% (p = 0.485). A high confidence of interval was observed for PVP-I 1%, even though the SMD appeared promising. CPC alone at low concentration exhibited minor effect against non-active controls, while no data was available on the combination of CPC and CHX.
Funnel plot (Fig. 4) revealed no major asymmetry, giving no indication for publication bias. Egger’s test confirmed these trends of publication biases (p = 0.9702).
Fig. 4.
Funnel plot of the clinical studies included in the network meta-analysis.
4. Discussion
The present systematic review evaluated the effectiveness of preprocedural mouthwashes in reducing SARS-CoV-2 load. Clinical studies suggested the effectiveness of CHX 0.2% in reducing viral load after 5 min, while this evidence was not confirmed by in vitro studies. On the contrary, PVP-I 0.1% tended to reduce the saliva viral load after 5 min from rinsing as compared to non-active control, and these results are in line with in vitro observations [38], [120]. Another important difference between clinical and in vitro studies relates to the use of ethanol. As positive control, ethanol 70% proved to be effective against SARS-CoV-2 in vitro [121], [122]. As expected, no included clinical study used preprocedural mouthwashes with alcohol at this concentration. CPC demonstrated virucidal activity in vitro, while inconsistent results were observed in clinical studies regarding CPC-induced salivary viral load reduction. Nevertheless, increased detection of the SARS-CoV-2 nucleocapsid protein in the saliva by ELISA test indicating viral envelope disruption was observed after rinsing with CPC [92], [97]. The effectiveness of H2O2 mouthwashes remains uncertain, with conflicting results for both clinical and in vitro studies.
In the majority of the included clinical studies, the recruitment of COVID-19 positive patients was based on nasal or nasopharyngeal swab PCR tests and, in all trials, saliva viral load reduction after rinsing was evaluated by means of RT-PCR. Despite this test is rapid and highly sensitive, it allows to detect viral RNA, but does not provide any indication on the infectivity of the virus [123]. As described by Jefferson et al. [124], there is no clear correspondence between the presence of the active virus, which can only be assessed in cell culture systems. However, in a pilot study investigating the effectiveness of H2O2 1%, replicating virus could only be determined from one baseline saliva sample out of 5 positive COVID-19 patients with saliva viral load of at least 103 RNA copies per mL [53]. SARS-CoV-2 replication in Vero-E6 cell culture after rinsing has been reported only in two studies from the same group [92], [93]. Interestingly, the authors detected considerable viral infectivity also with high salivary Ct values [92], suggesting that low-sensitivity SARS-CoV-2 tests could fail to detect cases with infective potential.
Chaudhary et al. focused on a clinically relevant aspect, as they divided patients based on the presence or absence of symptoms [89]. The authors concluded that there are no statistically significant differences between symptomatic and asymptomatic patients and, therefore, preprocedural rinsing is always advisable. These results are confirmed by a recent work of Carrouel et al. [5], in which no differences in median viral load values between symptomatic and asymptomatic patients were found.
The maintenance of the virus inactivation effect over time represents another clinically relevant aspect. Only three of the considered clinical studies evaluated mouthwash efficacy over time after a single rinse [27], [88], [89]. Chaudary et al. reported the efficacy of CHX, PVP-I and H2O2 after forty-five minutes after the initial rinse, with an even increased effect of PVP-I at the latest time point [89]. The long-lasting effect (up to 60 min) of CHX 0.12% was demonstrated also in the other two studies [27], [88]· H2O2 resulted to be very effective in the short term, but it loses its effectiveness over time [88]. Furthermore, the sequential use of H2O2 followed by CHX did not increase the saliva viral load reduction, compared to CHX alone. Overall, these findings suggest that a single preprocedural rinse might be sufficient to achieve a viral load reduction that lasts for the entire time of a normal dental session and it is not necessary to have the patient perform multiple rinses during the session. The mechanical action of rinsing might be sufficient in reducing the salivary viral load, as observed with 30 s of rinsing with distilled water, that showed a significant decrease in viral load compared to the no-rinse group [95].
As regards in vitro studies, they should try to reproduce as much as possible the oral conditions. However, only five out of the fourteen included studies performed the experiments at 37 °C and used exposure times between 15 and 60 s, similarly to clinical settings [99], [100], [102], [104], [115]. The remaining studies performed the incubations at room temperature (22 °C) or used exposure times ranging from 30 min to 72 h. Moreover, viral load tested in in vitro studies is generally higher and difficult to compare with the variable concentrations of the virus retrieved in the human hosts [5]. Therefore, their results might not be translated to clinical situations.
Only two included clinical studies showed an overall high risk of bias [29], [88], including one eligible for quantitative analysis [29].
Due to the lack of data consistency and the small or unbalanced number of patients involved in the studies included in the quantitative analysis, network meta-analysis was challenging, and data had to be partially estimated. Hence, results must be interpreted with caution. Furthermore, owing to the high heterogeneity and lack of standard deviation values, no meta-analysis could be performed for in vitro studies. In a recent meta-analysis the effect of PVP-I, CHX and CPC was assessed [17]. Contrary to our quantitative evaluation, the analysis considered not only the immediate but also the long-lasting effect of the mouthwashes. However, the same active agent at different concentrations (e.g. CHX 0.20% and 0.12%) was considered as a unique product. PVP-I was confirmed to be effective against SARS-CoV-2 not only after 5 min from rinsing, but also after hours (i.e. 1 h, 2 h, 3 h and 6 h after rinsing) [17]. In the same meta-analysis, CHX and CPC were found not to be effective in reducing SARS-COV-2 viral load. The clinical efficacy of CHX and PVP-I in reducing the number of negative RT-PCR results in COVID-19 patients was reported in another systematic review, in which both agents gained only moderate effect size on reducing viral load in patients with COVID-19 compared to control [16].
Despite several national and international guidelines recommend preprocedural mouth rinsing [6], their use in dental practices is also related to increased costs and potential adverse reactions to the products. The included clinical studies were largely conducted before the high vaccination coverage of the population and when a high number of COVID-19 positive cases were daily reported. The costs and benefits of the rinsing protocols should be revised in light of the current epidemiological situation and taking into account the immunological and vaccination status of the patients. Finally, it has to be noted that the study was limited to the investigation of mouthwashes. Nonetheless, throat and nose sprays can be effective adjuvants in reducing patients’ viral load [49].
5. Conclusion
In conclusion, despite a huge heterogeneity among the studies, data suggested the antiviral efficacy of commonly used mouthwashes. Some included studies presented a high risk of bias and the results have to be considered with caution. Discrepancies between in vivo and in vitro studies were observed, which require attention in future investigations to confirm the transferability of the laboratory data to clinical settings. Clinical data are lacking on the concomitant use of CPC and CHX, which was a promising combination within in vitro experiments. In future studies, it would be interesting to compare the rinsing protocols (active compound, concentration, rinsing duration) that have obtained the most encouraging results so far. However, considering the reduced incidence of COVID-19 positive patients, the acquired immunity after infection, and the high vaccination coverage of the population, a highly relevant question is if these factors have an impact on the efficacy and utility of preprocedural rinsing in dental settings. Moreover, this could provide valuable information for potential future pandemics with similar enveloped viruses, since it is still not known in which patients and in which phases of a pandemic preprocedural rinsing could be beneficial.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
None.
Scientific field of dental science
Dentistry – miscellaneous.
Article type
Systematic review and network meta-analysis.
PROSPERO registration number
CRD42022318922.
Contributor Information
Luca Sbricoli, Email: luca.sbricoli@unipd.it.
Lucia Schiavon, Email: lucia.schiavon@unipd.it.
Giulia Brunello, Email: giulia.brunello@med.uni-duesseldorf.de.
Paola Brun, Email: paola.brun.1@unipd.it.
Kathrin Becker, Email: kathrin.becker@charite.de.
Stefano Sivolella, Email: stefano.sivolella@unipd.it.
Data Availability
Data will be provided upon reasonable request.
References
- 1.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P., et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. New Engl J Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang N., Pérez P., Kato T., Mikami Y., Okuda K., Gilmore R.C., et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matuck B.F., Dolhnikoff M., Duarte-Neto A.N., Maia G., Gomes S.C., Sendyk D.I., et al. Salivary glands are a target for SARS-CoV-2: a source for saliva contamination. J Pathol. 2021;254:239–243. doi: 10.1002/path.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99:989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 5.Carrouel F., Gadea E., Esparcieux A., Dimet J., Langlois M.E., Perrier H., et al. Saliva quantification of SARS-CoV-2 in real-time PCR from asymptomatic or mild COVID-19 adults. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.786042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker K., Gurzawska-Comis K., Brunello G., Klinge B. Summary of European guidelines on infection control and prevention during COVID-19 pandemic. Clin Oral Implants Res. 2021;32(21):353–381. doi: 10.1111/clr.13784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunello G., Gurzawska-Comis K., Becker K., Becker J., Sivolella S., Schwarz F., et al. Dental care during COVID-19 pandemic: Follow-up survey of experts' opinion. Clin Oral Implants Res. 2021;32(21):342–352. doi: 10.1111/clr.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marui V.C., Souto MLS, Rovai E.S., Romito G.A., Chambrone L., Pannuti C.M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. 2019;150:1015–1026. doi: 10.1016/j.adaj.2019.06.024. e1. [DOI] [PubMed] [Google Scholar]
- 9.Logothetis D.D., Martinez-Welles J.M. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126(1939):1634–1639. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 10.Villani F.A., Aiuto R., Paglia L., Re D. COVID-19 and Dentistry: Prevention in Dental Practice, a Literature Review. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17124609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell V.B., Thomas D., Stanton R., Maillard J.Y., Murphy R.C., Jones S.A., et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function. 2020;1 doi: 10.1093/function/zqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z.Y., Meng L.Y. The prevention and control of a new coronavirus infection in department of stomatology. Zhonghua kou qiang yi xue za zhi = Zhonghua kouqiang yixue zazhi = Chin J Stomatol. 2020;55 doi: 10.3760/cma.j.issn.1002-0098.2020.0001. [DOI] [PubMed] [Google Scholar]
- 13.Reis I.N.R., do Amaral G., Mendoza A.A.H., das Graças Y.T., Mendes-Correa M.C., Romito G.A., et al. Can preprocedural mouthrinses reduce SARS-CoV-2 load in dental aerosols? Med Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Australian Dental Association. ADA COVID-19 Risk Management Guidance. 2020.
- 15.Lim N.A., Teng O., Ng C.Y.H., Bao L.X.Y., Tambyah P.A., Quek A.M.L., et al. Repurposing povidone-iodine to reduce the risk of SARS-CoV-2 infection and transmission: a narrative review. Ann Med. 2022;54:1488–1499. doi: 10.1080/07853890.2022.2076902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasan F., Chiu H.Y., Salamanca E., Ridwan E.S., Wiratama B.S., Budi H.S. Effects of chlorhexidine and povidone-iodine on the SARS-CoV-2 load: a systematic review and meta-analysis. Eur J Dent. 2022 doi: 10.1055/s-0042-1753470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebrahimi T., Shamshiri A.R., Alebouyeh M., Mohebbi S.Z. Effectiveness of mouthwashes on reducing SARS-CoV-2 viral load in oral cavity: a systematic review and meta-analysis. BMC Oral Health. 2023;23 doi: 10.1186/s12903-023-03126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ Clin Res Ed. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Idostatistics. Cohen’s kappa free calculator. p. 〈https://idostatistics.com/cohen-kappa-free-calculator/#risultati〉.
- 20.Schneider K., Schwarz M., Burkholder I., Kopp-Schneider A., Edler L., Kinsner-Ovaskainen A., et al. ToxRTool", a new tool to assess the reliability of toxicological data. Toxicol Lett. 2009;189:138–144. doi: 10.1016/j.toxlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin Res Ed. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 22.Walker A.S., Pritchard E., House T., Robotham J.V., Birrell P.J., Bell I., et al. Ct threshold values, a proxy for viral load in community SARS-CoV-2 cases, demonstrate wide variation across populations and over time. eLife. 2021;10 doi: 10.7554/eLife.64683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viechtbauer W. Conducting Meta-Analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 24.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ Clin Res Ed. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rücker G., Krahn U., König J., Efthimiou O., Davies A., Papakonstantinou T., et al. Network meta-analysis using frequentist methods. R Package Version. 2022;0:25. [Google Scholar]
- 27.Costa D.D., Brites C., Vaz S.N., de Santana D.S., Dos Santos J.N., Cury P.R. Chlorhexidine mouthwash reduces the salivary viral load of SARS-CoV-2: a randomized clinical trial. Oral Dis. 2021 doi: 10.1111/odi.14086. [DOI] [PubMed] [Google Scholar]
- 28.Elzein R., Abdel-Sater F., Fakhreddine S., Hanna P.A., Feghali R., Hamad H., et al. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. J Evid-Based Dent Pract. 2021;21 doi: 10.1016/j.jebdp.2021.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seneviratne C.J., Balan P., Ko K.K.K., Udawatte N.S., Lai D., Ng D.H.L., et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2021;49:305–311. doi: 10.1007/s15010-020-01563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arefin M.K., Rumi S., Uddin A., Banu S.S., Khan M., Kaiser A., et al. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: an open-label randomized clinical trial. Indian J Otolaryngol Head neck Surg: Publ Assoc Otolaryngol India. 2021:1–5. doi: 10.1007/s12070-021-02616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capetti A.F., Borgonovo F., Morena V., Lupo A., Cossu M.V., Passerini M., et al. Short-term inhibition of SARS-CoV-2 by hydrogen peroxide in persistent nasopharyngeal carriers. J Med Virol. 2021;93:1766–1769. doi: 10.1002/jmv.26485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Izzotti A., Fracchia E., Au W., Colombo M., Pfeffer U., Emionite L., et al. Prevention of Covid-19 Infection and Related Complications by Ozonized Oils. J Pers Med. 2021;11 doi: 10.3390/jpm11030226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frank S., Brown S.M., Capriotti J.A., Westover J.B., Pelletier J.S., Tessema B. In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2. JAMA Otolaryngol-- Head Neck Surg. 2020;146:1054–1058. doi: 10.1001/jamaoto.2020.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Domênico M.B., Cesca H., Ponciano T.H.J., Dos Santos R.B., Lenz U., Antunes V.P., et al. Effectiveness of hydrogen peroxide as auxiliary treatment for hospitalized COVID-19 patients in Brazil: preliminary results of a randomized double-blind clinical trial. Epidemiol Health. 2021;43 doi: 10.4178/epih.e2021032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tulalamba W., Assawamakin A., Thayananuphat A., Viprakasit V. Evaluation of potassium peroxymonosulfate (MPS) efficacy against SARS-CoV-2 virus using RT-qPCR-based method. Int J Infect Dis: IJID: Publ Int Soc Infect Dis. 2021;110:162–164. doi: 10.1016/j.ijid.2021.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schürmann M., Aljubeh M., Tiemann C., Sudhoff H. Mouthrinses against SARS-CoV-2: anti-inflammatory effectivity and a clinical pilot study. Eur Arch Oto-Rhino-Laryngol: J Eur Fed Oto-Rhino-Laryngol Soc (EUFOS): Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2021;278:5059–5067. doi: 10.1007/s00405-021-06873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kariwa H., Sawa H., Kobayashi S. Inactivation of SARS-CoV-2 by povidone-iodine products: implications for effective mouth rinsing and gargling. Jpn J Vet Res. 2021;69:183–187. [Google Scholar]
- 38.Hassandarvish P., Tiong V., Mohamed N.A., Arumugam H., Ananthanarayanan A., Qasuri M., et al. In vitro virucidal activity of povidone iodine gargle and mouthwash against SARS-CoV-2: implications for dental practice. Br Dent J. 2020:1–4. doi: 10.1038/s41415-020-2402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson D.E., Sivalingam V., Kang A.E.Z., Ananthanarayanan A., Arumugam H., Jenkins T.M., et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect Dis Ther. 2020;9:669–675. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Köntös Z. Efficacy of "Essential Iodine Drops" against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) PLoS One. 2021;16 doi: 10.1371/journal.pone.0254341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guenezan J., Garcia M., Strasters D., Jousselin C., Lévêque N., Frasca D., et al. Povidone iodine mouthwash, gargle, and nasal spray to reduce nasopharyngeal viral load in patients with COVID-19: a randomized clinical trial. JAMA Otolaryngol-- Head Neck Surg. 2021;147:400–401. doi: 10.1001/jamaoto.2020.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadalam P.K., Varatharajan K., Rajapandian K., Chopra P., Arumuganainar D., Nagarathnam T., et al. Antiviral essential oil components against SARS-CoV-2 in pre-procedural mouth rinses for dental settings during COVID-19: a computational study. Front Chem. 2021;9 doi: 10.3389/fchem.2021.642026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin K.R., Kwak K., Cui C., Bae J.-Y., Hong W., Park M.-S. In vitro virucidal effect of povidone-iodine against SARS-CoV-2. J Bacteriol Virol. 2020;50:195–202. [Google Scholar]
- 44.Shewale J.G., Gelhaus H.C., Ratcliff J.L., Hernandez-Kapila Y.L. In vitro antiviral activity of stabilized chlorine dioxide containing oral care products. Oral Dis. 2021 doi: 10.1111/odi.14044. [DOI] [PubMed] [Google Scholar]
- 45.Senthil Kumar K.J., Gokila Vani M., Wang C.S., Chen C.C., Chen Y.C., Lu L.P., et al. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants. 2020;9 doi: 10.3390/plants9060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vilhena F.V., Brito Reia V.C., da Fonseca Orcina B., Santos C.A., Zangrando M., Cardoso de Oliveira R., et al. The use of antiviral Phthalocyanine mouthwash as a preventive measure against COVID-19. GMS Hyg Infect Control. 2021;16:Doc24. doi: 10.3205/dgkh000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Millones-Gómez P.A. Mouthwashes in COVID-19: Benefit or harm to the oral microbiome? Oral Dis. 2021 doi: 10.1111/odi.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez Lamas L., Diz Dios P., Pérez Rodríguez M.T., Del Campo Pérez V., Cabrera Alvargonzalez J.J., López Domínguez A.M., et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2022;28(S1):908–911. doi: 10.1111/odi.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seet R.C.S., Quek A.M.L., Ooi D.S.Q., Sengupta S., Lakshminarasappa S.R., Koo C.Y., et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: An open-label randomized trial. Int J Infect Dis: IJID: Publ Int Soc Infect Dis. 2021;106:314–322. doi: 10.1016/j.ijid.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tateyama-Makino R., Abe-Yutori M., Iwamoto T., Tsutsumi K., Tsuji M., Morishita S., et al. The inhibitory effects of toothpaste and mouthwash ingredients on the interaction between the SARS-CoV-2 spike protein and ACE2, and the protease activity of TMPRSS2 in vitro. PLoS One. 2021;16 doi: 10.1371/journal.pone.0257705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Orlando G., Chiavaroli A., Adorisio S., Delfino D.V., Brunetti L., Recinella L., et al. Unravelling the phytochemical composition and the pharmacological properties of an optimized extract from the fruit from Prunus mahaleb L.: from traditional liqueur market to the pharmacy shelf. Molecules. 2021;26 doi: 10.3390/molecules26154422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyers C., Robison R., Milici J., Alam S., Quillen D., Goldenberg D., et al. Lowering the transmission and spread of human coronavirus. J Med Virol. 2021;93:1605–1612. doi: 10.1002/jmv.26514. [DOI] [PubMed] [Google Scholar]
- 53.Gottsauner M.J., Michaelides I., Schmidt B., Scholz K.J., Buchalla W., Widbiller M., et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig. 2020;24:3707–3713. doi: 10.1007/s00784-020-03549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan M.M., Parab S.R., Paranjape M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan M.M., Parab S.R. 0.5% povidone iodine irrigation in otorhinolaryngology surgical practice during COVID 19 pandemic. Am J Otolaryngol. 2020;41 doi: 10.1016/j.amjoto.2020.102687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khan M.M., Parab S.R. Tolerability and usability of 0.5% PVP-I gargles and nasal drops in 6692 patients: Observational study. Am J Otolaryngol. 2021;42 doi: 10.1016/j.amjoto.2020.102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avhad S., Bhanushali M., Sachdev S., Save S., Kalra D., Kamala D. Comparison of effectiveness of chlorine dioxide mouthwash and chlorhexidine gluconate mouthwash in reduction of oral viral load in patients with covid-19. Indian J Public Health Res Dev. 2020:27–32. [Google Scholar]
- 58.Huang Y.H., Huang J.T. Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients. J Med Virol. 2021;93:4370–4373. doi: 10.1002/jmv.26954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Redmond S.N., Li D.F., Abou Ghaddara H., Haq M.F., Jones L.D., Nguyen A.M., et al. A pilot randomized trial to evaluate the efficacy of oral and nasal povidone iodine in reducing the burden of severe acute respiratory syndrome coronavirus 2 RNA in patients with coronavirus disease 2019. Infect Control Hosp Epidemiol. 2023;44:679–681. doi: 10.1017/ice.2022.257. [DOI] [PubMed] [Google Scholar]
- 60.Lippi G., Nocini R., Henry B.M., Plebani M. Virucidal effects of mouthwashes or mouth rinses: a world of caution for molecular detection of SARS-CoV-2 in saliva. Diagnosis. 2022;9:285–287. doi: 10.1515/dx-2022-0004. [DOI] [PubMed] [Google Scholar]
- 61.Gao J.C., Hood C., Safai B., Marmon S. Evidence for the use of mouthwash as a preprocedural preventive measure against COVID-19: Should we rinse and repeat? JAAD Int. 2022;6:109–110. doi: 10.1016/j.jdin.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nasiri K., Dimitrova A. The role of povidone-iodine in managing of SARS-CoV-2 pandemic. J Dent Sci. 2022;17:1437–1438. doi: 10.1016/j.jds.2022.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strub D.J., Talma M., Strub M., Rut W., Zmudzinski M., Brud W., et al. Evaluation of the anti-SARS-CoV-2 properties of essential oils and aromatic extracts. Sci Rep. 2022;12 doi: 10.1038/s41598-022-18676-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Di Domênico M.B., Collares K., Dos Santos R.B., Lenz U., Antunes V.P., Godinho V.W., et al. Hydrogen peroxide as an auxiliary treatment for COVID-19 in Brazil: a randomized double-blind clinical trial. Epidemiol Health. 2021;43 doi: 10.4178/epih.e2021051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuyama A., Okura H., Hashimoto S., Tanaka T. A prospective, randomized, open-label trial of early versus late povidone-iodine gargling in patients with COVID-19. Sci Rep. 2022;12 doi: 10.1038/s41598-022-24683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bonn E.L., Rohrhofer A., Audebert F.X., Lang H., Auer D.L., Scholz K.J., et al. Efficacy of a mouthwash containing CHX and CPC in SARS-CoV-2-positive patients: a randomized controlled clinical trial. J Dent Res. 2023;102:608–615. doi: 10.1177/00220345231156415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sulistyani L.D., Julia V., Soeprapto A., Swari R.P., Rosmanato F., Haryanto B., et al. The effects of mouth rinsing and gargling with mouthwash containing povidone-iodine and hydrogen peroxide on the cycle threshold value of Severe Acute Respiratory Syndrome Coronavirus 2: A randomized controlled trial of asymptomatic and mildly symptomatic patients. F1000Research. 2022;11:1238. doi: 10.12688/f1000research.110843.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meister T.L., Gottsauner J.M., Schmidt B., Heinen N., Todt D., Audebert F., et al. Mouthrinses against SARS-CoV-2 - High antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial. Virus Res. 2022;316 doi: 10.1016/j.virusres.2022.198791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fantozzi P.J., Pampena E., Pierangeli A., Oliveto G., Sorrentino L., Di Vanna D., et al. Efficacy of antiseptic mouthrinses against SARS-CoV-2: A prospective randomized placebo-controlled pilot study. Am J Otolaryngol. 2022;43 doi: 10.1016/j.amjoto.2022.103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ogun S.A., Erinoso O., Aina O.O., Ojo O.I., Adejumo O., Adeniran A., et al. Efficacy of Hexetidine, Thymol And Hydrogen Peroxide-containing Oral Antiseptics In Reducing Sars-Cov-2 Virus In The Oral Cavity: A Pilot Study. West Afr J Med. 2022;39:83–89. [PubMed] [Google Scholar]
- 71.Frank S., Ibrahim B., Feng R., Bidra A., Lafreniere D., Kuo C.L., et al. Tolerability of nasal and oral povidone-iodine antisepsis for in-office procedures. Clin Otolaryngol. 2023;48:696–699. doi: 10.1111/coa.14045. [DOI] [PubMed] [Google Scholar]
- 72.Natto Z.S., Bakhrebah M.A., Afeef M., Al-Harbi S., Nassar M.S., Alhetheel A.F., et al. The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study. Med (Baltim) 2022;101 doi: 10.1097/MD.0000000000028925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eggers M., Jungke P., Wolkinger V., Bauer R., Kessler U., Frank B. Antiviral activity of plant juices and green tea against SARS‐CoV‐2 and influenza virus. Phytother Res. 2022;36:2109–2115. doi: 10.1002/ptr.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teagle V., Clem D.S., Yoon T. Virucidal Properties of Molecular Iodine Oral Rinse Against SARS-CoV-2. Compend Contin Educ Dent. 2022;43:e13–e16. [PubMed] [Google Scholar]
- 75.Buonavoglia A., Lanave G., Marchi S., Lorusso P., Montomoli E., Martella V., et al. In vitro virucidal activity of mouthwashes on SARS-CoV-2. Oral Dis. 2022;28(Suppl 2):2509–2515. doi: 10.1111/odi.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghasemi S., Nadji S.A., Heidari A., James J.N., Karimi-Galougahi M., Raygani N., et al. Reduction in SARS-CoV-2 Oral Viral Load with Prophylactic Mouth Rinse. Eur J Gen Dent. 2022;11:094–101. [Google Scholar]
- 77.Refaey M.S., M A.A.F., Kutkat O., Moatasim Y., Sameh Tolba N., Anis A., et al. Bio-guided chemical characterization and nano-formulation studies of selected edible volatile oils with potentials antibacterial and anti-SARS-CoV-2 activities. Arab J Chem. 2023;16 doi: 10.1016/j.arabjc.2023.104813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bencze B., Temesfői V., Das S., Papp H., Kaltenecker P., Kuczmog A., et al. Development of a novel, entirely herbal-based mouthwash effective against common oral bacteria and SARS-CoV-2. BMC Complement Med Ther. 2023;23 doi: 10.1186/s12906-023-03956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seikai T., Takada A., Hasebe A., Kajihara M., Okuya K., Sekiguchi Yamada T., et al. Gargling with povidone iodine has a short-term inhibitory effect on SARS-CoV-2 in patients with COVID-19. J Hosp Infect. 2022;123:179–181. doi: 10.1016/j.jhin.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chalageri V.H., Bhushan S., Saraswathi S., Ranganath T.S., Rani V.D., Majgi S.M., et al. Impact of Steam Inhalation, Saline Gargling, and Povidone-Iodine Gargling on Clinical Outcome of COVID-19 Patients in Bengaluru, Karnataka: A Randomized Control Trial. Indian J Community Med. 2022;47:207–212. doi: 10.4103/ijcm.ijcm_804_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohamed M.E., Tawfeek N., Elbaramawi S.S., Fikry E. Agathis robusta Bark Essential Oil Effectiveness against COVID-19: Chemical Composition, In Silico and In Vitro Approaches. Plants (Basel, Switz) 2022;11 doi: 10.3390/plants11050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amoah G.B., Quakyi I.A., Sagoe K.W., Ayettey-Anie H.N.G., Ayettey-Adamafio M.N.B., Ayettey Brew R.N.A., et al. Re: Oral antiseptics against coronavirus: in-vitro and clinical evidence. J Hosp Infect. 2021;118:108–109. doi: 10.1016/j.jhin.2021.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohamed, Baharom N.A., Sulaiman WSW N., Rashid Z.Z., Ken W.K., Ali U.K., et al. Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils: a clinical trial. Int Med J. 2020;27:651–654. [Google Scholar]
- 84.Vilhena F.V., Orcina B.D.F., Lemos L., Less J.C.F., Pinto I., Santos P. Elimination of SARS-CoV-2 in nasopharynx and oropharynx after use of an adjuvant gargling and rinsing protocol with an antiseptic mouthwash. Einstein. 2022;19 doi: 10.31744/einstein_journal/2021CE6999. eCE6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Travis B.J., Elste J., Gao F., Joo B.Y., Cuevas-Nunez M., Kohlmeir E., et al. Significance of chlorine-dioxide-based oral rinses in preventing SARS-CoV-2 cell entry. Oral Dis. 2022;28(2):2481–2491. doi: 10.1111/odi.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodríguez-Casanovas H.J., la Rosa M., Bello-Lemus Y., Rasperini G., Acosta-Hoyos A.J. Virucidal activity of different mouthwashes using a novel biochemical assay. Healthcare. 2021;10 doi: 10.3390/healthcare10010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sevinç Gül S.N., Dilsiz A., Sağlık İ., Aydın N.N. Effect of oral antiseptics on the viral load of SARS-CoV-2: A randomized controlled trial. Dent Med Probl. 2022;59:357–363. doi: 10.17219/dmp/150831. [DOI] [PubMed] [Google Scholar]
- 88.Eduardo F.P., Corrêa L., Heller D., Daep C.A., Benitez C., Malheiros Z., et al. Salivary SARS-CoV-2 load reduction with mouthwash use: a randomized pilot clinical trial. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chaudhary P., Melkonyan A., Meethil A., Saraswat S., Hall D.L., Cottle J., et al. Estimating salivary carriage of severe acute respiratory syndrome coronavirus 2 in nonsymptomatic people and efficacy of mouthrinse in reducing viral load: A randomized controlled trial. J Am Dent Assoc. 2021;152(1939):903–908. doi: 10.1016/j.adaj.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farmaha J.K., James J.N., Frazier K., Sahajpal N.S., Mondal A.K., Bloomquist D.T., et al. Reduction of SARS-CoV-2 salivary viral load with pre-procedural mouth rinses: a randomised, controlled, clinical trial. Br Dent J. 2023;234:593–600. doi: 10.1038/s41415-023-5741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ferrer M.D., Barrueco Á.S., Martinez-Beneyto Y., Mateos-Moreno M.V., Ausina-Márquez V., García-Vázquez E., et al. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Sci Rep. 2021;11 doi: 10.1038/s41598-021-03461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sánchez Barrueco Á., Mateos-Moreno M.V., Martínez-Beneyto Y., García-Vázquez E., Campos González A., Zapardiel Ferrero J., et al. Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: evidence from a randomized double-blind clinical trial. Emerg Microbes Infect. 2022;11:1833–1842. doi: 10.1080/22221751.2022.2098059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sánchez Barrueco A., Mateos-Moreno M.V., Villacampa Aubá J.M., Campos González A., Bogoya Castaño A., Rubio Yanguas R., et al. In vivo effect of mouthwashes on viable viral load of SARS-CoV-2 in saliva: a pilot study. J Oral Microbiol. 2023;15 doi: 10.1080/20002297.2023.2198432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adl A., Sedigh-Shams M., Jamalidoust M., Rajabzadeh Z. Evaluating the effect of gargling with hydrogen peroxide and povidone-iodine on salivary viral load of SARS-CoV-2: a pilot randomized clinical trial. Niger J Clin Pr. 2023;26:391–396. doi: 10.4103/njcp.njcp_320_22. [DOI] [PubMed] [Google Scholar]
- 95.Alzahrani M.M., Bamashmous S., Alkharobi H., Alghamdi A., Alharbi R.H., Hassan A.M., et al. Mouth rinses efficacy on salivary SARS-CoV-2 viral load: a randomized clinical trial. J Med Virol. 2023;95 doi: 10.1002/jmv.28412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alemany A., Perez-Zsolt D., Raïch-Regué D., Muñoz-Basagoiti J., Ouchi D., Laporte-Villar C., et al. Cetylpyridinium chloride mouthwash to reduce shedding of infectious SARS-CoV-2: a double-blind randomized clinical trial. J Dent Res. 2022;101:1450–1456. doi: 10.1177/00220345221102310. [DOI] [PubMed] [Google Scholar]
- 97.Tarragó-Gil R., Gil-Mosteo M.J., Aza-Pascual-Salcedo M., Alvarez M.J.L., Ainaga R.R., Gimeno N.L., et al. Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load. J Clin Periodo. 2023;50:288–294. doi: 10.1111/jcpe.13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carrouel F., Valette M., Gadea E., Esparcieux A., Illes G., Langlois M.E., et al. Use of an antiviral mouthwash as a barrier measure in the SARS-CoV-2 transmission in adults with asymptomatic to mild COVID-19: a multicentre, randomized, double-blind controlled trial. Clin Microbiol Infect: Publ Eur Soc Clin Microbiol Infect Dis. 2021;27:1494–1501. doi: 10.1016/j.cmi.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jain A., Grover V., Singh C., Sharma A., Das D.K., Singh P., et al. Chlorhexidine: An effective anticovid mouth rinse. J Indian Soc Periodontol. 2021;25:86–88. doi: 10.4103/jisp.jisp_824_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koch-Heier J., Hoffmann H., Schindler M., Lussi A., Planz O. Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX(®) and BacterX(®) Pro. Microorganisms. 2021;9 doi: 10.3390/microorganisms9030521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Komine A., Yamaguchi E., Okamoto N., Yamamoto K. Virucidal activity of oral care products against SARS-CoV-2 in vitro. J Oral Maxillofac Surg, Med, Pathol. 2021;33:475–477. doi: 10.1016/j.ajoms.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiong V., Hassandarvish P., Bakar S.A., Mohamed N.A., Wan Sulaiman W.S., Baharom N., et al. The effectiveness of various gargle formulations and salt water against SARS-CoV-2. Sci Rep. 2021;11 doi: 10.1038/s41598-021-99866-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Steinhauer K., Meister T.L., Todt D., Krawczyk A., Paßvogel L., Becker B., et al. Comparison of the in-vitro efficacy of different mouthwash solutions targeting SARS-CoV-2 based on the European Standard EN 14476. J Hosp Infect. 2021;111:180–183. doi: 10.1016/j.jhin.2021.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies K., Buczkowski H., Welch S.R., Green N., Mawer D., Woodford N., et al. Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes. J Gen Virol. 2021;102 doi: 10.1099/jgv.0.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu C., Wang A., Hoskin E.R., Cugini C., Markowitz K., Chang T.L., et al. Differential effects of antiseptic mouth rinses on SARS-CoV-2 infectivity in vitro. Pathogens. 2021;10 doi: 10.3390/pathogens10030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pelletier J.S., Tessema B., Frank S., Westover J.B., Brown S.M., Capriotti J.A. Efficacy of povidone-iodine nasal and oral antiseptic preparations against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) Ear, Nose, Throat J. 2021;100 doi: 10.1177/0145561320957237. 192s-6s. [DOI] [PubMed] [Google Scholar]
- 108.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont: J Am Coll Prosthodont. 2020;29:529–533. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J Prosthodont: J Am Coll Prosthodont. 2020;29:599–603. doi: 10.1111/jopr.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y., Wu Y., Wang Q., Zhu J., Shi W., Han Z., et al. Virucidal effect of povidone-iodine against SARS-CoV-2 in vitro. J Int Med Res. 2021;49 doi: 10.1177/03000605211063695. 3000605211063695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shet M., Westover J., Hong R., Igo D., Cataldo M., Bhaskar S. In vitro inactivation of SARS-CoV-2 using a povidone-iodine oral rinse. BMC Oral Health. 2022;22 doi: 10.1186/s12903-022-02082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okamoto N., Saito A., Okabayashi T., Komine A. Virucidal activity and mechanism of action of cetylpyridinium chloride against SARS-CoV-2. J Oral Maxillofac Surg, Med, Pathol. 2022;34:800–804. doi: 10.1016/j.ajoms.2022.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramji N., Circello B., Winston J.L., Biesbrock A.R. Virucidal activity of over-the-counter oral care products against SARS-CoV-2. Oral Health Prev Dent. 2022;20:185–192. doi: 10.3290/j.ohpd.b2960525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Almanza-Reyes H., Moreno S., Plascencia-López I., Alvarado-Vera M., Patrón-Romero L., Borrego B., et al. Evaluation of silver nanoparticles for the prevention of SARS-CoV-2 infection in health workers: In vitro and in vivo. PloS One. 2021;16 doi: 10.1371/journal.pone.0256401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.da Silva Santos P.S., da Fonseca Orcina B., Machado R.R.G., Vilhena F.V., da Costa Alves L.M., Zangrando M.S.R., et al. Beneficial effects of a mouthwash containing an antiviral phthalocyanine derivative on the length of hospital stay for COVID-19: randomised trial. Sci Rep. 2021;11 doi: 10.1038/s41598-021-99013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeda R., Sawa H., Sasaki M., Orba Y., Maishi N., Tsumita T., et al. Antiviral effect of cetylpyridinium chloride in mouthwash on SARS-CoV-2. Sci Rep. 2022;12 doi: 10.1038/s41598-022-18367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen K.D., Ma F.K., Wang Q.J., Wang Y., Zhuang X.Y., Zhang X.N., et al. Disinfection effect of hexadecyl pyridinium chloride on SARS-CoV-2 in vitro. Intervirology. 2022 doi: 10.1159/000526241. [DOI] [PubMed] [Google Scholar]
- 118.CEN. EN 14476:2013+A2:2019. Chemical disinfectants and antiseptics - Quantitative suspension test for the evaluation of virucidal activity in the medical area - Test method and requirements (Phase 2/Step 1). 2019.
- 119.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 120.Chen M.H., Chang P.C. The effectiveness of mouthwash against SARS-CoV-2 infection: A review of scientific and clinical evidence. J Formos Med Assoc = Taiwan yi zhi. 2022;121:879–885. doi: 10.1016/j.jfma.2021.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nomura T., Nazmul T., Yoshimoto R., Higashiura A., Oda K., Sakaguchi T. Ethanol susceptibility of SARS-CoV-2 and other enveloped viruses. Biocontrol Sci. 2021;26:177–180. doi: 10.4265/bio.26.177. [DOI] [PubMed] [Google Scholar]
- 122.Takeda Y., Jamsransuren D., Makita Y., Kaneko A., Matsuda S., Ogawa H., et al. Inactivation activities of ozonated water, slightly acidic electrolyzed water and ethanol against SARS-CoV-2. Molecules. 2021;26 doi: 10.3390/molecules26185465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mostafa M., Barhoum A., Sehit E., Gewaid H., Mostafa E., Omran M.M., et al. Current trends in COVID-19 diagnosis and its new variants in physiological fluids: surface antigens, antibodies, nucleic acids, and RNA sequencing. Trends Anal Chem: TRAC. 2022;157 doi: 10.1016/j.trac.2022.116750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jefferson T., Spencer E.A., Brassey J., Heneghan C. Viral cultures for coronavirus disease 2019 infectivity assessment: a systematic review. Clin Infect Dis: Publ Infect Dis Soc Am. 2021;73:e3884–e3899. doi: 10.1093/cid/ciaa1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guimarães T.C., Marques B.B.F., Castro M.V., Secco D.A., Porto L., Tinoco J.M.M., et al. Reducing the viral load of SARS-CoV-2 in the saliva of patients with COVID-19. Oral Dis. 2022;28(Suppl 2):2474–2480. doi: 10.1111/odi.14118. [DOI] [PubMed] [Google Scholar]
- 126.Anderson E.R., Patterson E.I., Richards S., Pitol A.K., Edwards T., Wooding D., et al. CPC-containing oral rinses inactivate SARS-CoV-2 variants and are active in the presence of human saliva. J Med Microbiol. 2022;71 doi: 10.1099/jmm.0.001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided upon reasonable request.