Abstract
ADSCs are a large number of mesenchymal stem cells in Adipose tissue, which can be applied to tissue engineering. ADSCs have the potential of multi-directional differentiation, and can differentiate into bone tissue, cardiac tissue, urothelial cells, skin tissue, etc. Compared with other mesenchymal stem cells, ADSCs have a multitude of promising advantages, such as abundant number, accessibility in cell culture, stable function, and less immune rejection. There are two main methods to use ADSCs for tissue repair and regeneration. One is to implant the “ADSCs-scaffold composite” into the injured site to promote tissue regeneration. The other is cell-free therapy: using ADSC-exos or ADSC-CM alone to release a large number of miRNAs, cytokines and other bioactive substances to promote tissue regeneration. The tissue regeneration potential of ADSCs is regulated by a variety of cytokines, signaling molecules, and external environment. The differentiation of ADSCs into different tissues is also induced by growth factors, ions, hormones, scaffold materials, physical stimulation, and other factors. The specific mechanisms are complex, and most of the signaling pathways need to be further explored. This article reviews and summarizes the mechanism and clinical application of ADSCs in tissue injury repair so far, and puts forward further problems that need to be solved in this field, hoping to provide directions for further research in this field.
Keywords: ADSCs, Exosomes, Tissue repair, Mechanism
Graphical abstract
Highlights
-
•
Adipose-derived stem cells (ADSCs) are ideal seed cells of tissue engineering in future.
-
•
ADSCs have the potential of multi-directional differentiation, and can differentiate into bone tissue, cardiac tissue, urothelial cells, skin tissue, etc.
-
•
Advantages of ADSCs: abundant number, accessibility in cell culture, stable function, and less immune rejection.
-
•
Cell-free therapy: using ADSCs-derived exosomes (ADSC-exos) or ADSC-conditioned medium (ADSC-CM) alone to promote tissue regeneration.
1. Introduction
Tissue engineering is an emerging biomedical technology and an important part of regenerative medicine. Tissue engineering enables tissue regeneration by using living cells, biomaterials, and stimulating factors to produce viable biological substitutes to replace diseased tissues [1]. The selection of cells is an exceedingly significant issue in tissue engineering. Stem cells are widely used in tissue engineering due to their proliferation ability and multi-directional differentiation potential. Embryonic stem cells (ESCs) are ideal stem cells for tissue engineering with unlimited proliferation and multi-directional differentiation potential, which can differentiate into ectoderm, mesoderm, and endoderm to shape various tissues and organs. However, there are ethical issues due to the destruction of embryos to obtain embryonic stem cells. Shinya Yamanaka found that genes can be introduced into somatic cells to make induced pluripotent stem cells [2]. Although ethical issues have been solved and there is no immune rejection in this way, the use of induced pluripotent stem cells is also limited on account of the risk of teratogenesis, complex culture process and low transformation efficiency. Compared with embryonic stem cells, adult stem cells have the following advantages: firstly, there are no ethical problems, adult stem cells derived from the patient's own will not cause transplant rejection. Secondly, the differentiation of adult stem cells is relatively stable, and the use of adult stem cells has a lower risk of teratogenesis. The clinical application is safer [3]. Therefore, adult stem cells have become the main cells used in tissue engineering. Bone marrow mesenchymal stem cells (BM-MSCs) and umbilical cord mesenchymal stem cells (UC-MSCs) are adult stem cells that can meet the needs of tissue engineering. In particular, BM-MSCs have been widely used in the regeneration of bone tissue, cardiac tissue, nerve tissue and other tissues [4]. However, BM-MSCs have the problem of insufficient source, and the proliferation ability of BM-MSCs is also limited by age. The main source of BM-MSCs is bone marrow. With the increase of age, the content of bone marrow gradually decreases, so as to make it more difficult to obtain high purity and activity BM-MSCs [5]. Tissue engineering requires a large number of cells to form donor tissues, and the difficulties in obtaining and expanding mesenchymal stem cells in vitro have limited their clinical application.
In recent years, ADSCs have attracted more and more attention. ADSCs are stem cells isolated from stromal vascular cells of adipose tissue under the action of collagenase. Similar to other stem cells, ADSCs have the potential of multi-directional differentiation, which can repair traumatic tissues and differentiate into urothelium, bone, muscle, nerve, skin, etc. [[6], [7], [8]]. Compared with other mesenchymal stem cells(MSC), ADSCs have great advantages in skin tissue engineering, bone tissue engineering, urinary tract repair, cardiovascular disease, and nervous system disease treatment [9]. ADSCs are ideal seed cells of tissue engineering in future.
2. ADSCs and their functions
Derived from stromal vascular fraction (SVF), ADSCs are a kind of MSC abundantly present in adipose tissue [10]. In 1977, Van and Roncari reported that adipose tissue in adult rats contained cells with proliferative potential. In 2001, Zuk et al. isolated a sample of cells with multi-directional differentiation potential from adipose tissue for the first time and named it adipose-derived stem cells.
The extraction process of ADSCs is relatively simple. The extracted adipose tissue is washed with PBS to remove unrelated cells such as red blood cells, then type Ⅰ collagenase is added, red blood cells are further lysed with red blood cell lysates, and ADSCs are separated from mature adipocytes by centrifugation [7]. The ability of ADSCs to proliferate, differentiate and promote angiogenesis decreases with aging. Therefore, cryopreservation of ADSCs and reducing the number of passages can preserve the function of ADSCs to the greatest extent [11]. Moreover, ADSCs expressed CD13, CD44, CD90, CD49d, CD105, CD10 and so on. ADSCs expressed CD49d but not CD106, which is different from MSC. MSC expressed CD106 but not CD49d, which can be distinguished from ADSCs [12].
The morphology and function of ADSCs are similar to BM-MSC. ADSCs also have the potential of self-renewal and multi-directional differentiation. Derived from the mesoderm, ADSCs can promote the repair process of these tissues after injury and differentiate into bone tissue, cardiac tissue, urothelial cells, skin tissue, etc (Fig. 1). Furthermore, ADSCs can secrete a large number of cytokines to exert their effects. ADSCs can secrete cytokines such as IL-1α, IL-6, and IL-10 to play an anti-inflammatory role [13]. IL-1α binds to IL-1typeⅠreceptor and induces structural changes of IL-1R, interacts with IL-1RAcP, and initiates NF-κB signal transduction cascade [14]. IL-6 binds to mIL-6Rα and gp130 to form a hexamer complex, which induces the phosphorylation of Janus kinase (JAK), and then activates the dimerization of signal transducer and activator of transcription 3(STAT3) for downstream signal transduction [15]. Upon binding to IL-10 receptor, IL-10 activates Janus kinase1 (JAK1)/tyrosine kinase 2 (Tyk2)-signal transducer and activator of transcription 3 (STAT3) signaling pathway conducts downstream signal transduction and plays an anti-inflammatory role [16]. ADSCs secrete TGF-β, vascular endothelial growth factor (VEGF), placental growth factor (PGF), angiopoietin-1 (Ang-l) and so on to promote endothelial cell proliferation and angiogenesis [17]. After TGF-β binds to TGF-β type II receptor, TGF-β type II receptor induces the phosphorylation of type I receptor, and type I receptor promotes the phosphorylation of SMAD protein to conduct downstream signal transduction [18]. Upon binding to VEGF-R, VEGF regulates cell proliferation by activating the Ras/MAPK pathway, cell survival by activating the PI3K/AKT pathway, and new capillary formation by activating the RhoA/ROCK pathway [19]. PGF can directly bind to VEGFR-1 and activate PI3K/AKT signaling pathway. PGF can also replace vascular endothelial growth factor A (VEGFA) from transmembrane or soluble VEGFR-1, so that more VEGFA can activate VEGFR-2 to promote endothelial cell proliferation and neovascularization, which is the main mechanism of PGF [20]. Ang-1 binds to the tyrosine kinase receptor Tie-2 and plays an important role in vascular maturation, migration, adhesion and survival of endothelial cells [21]. These cytokines play an important role in tissue repair [22].
Fig. 1.
ADSCs have the potential of self-renewal and multidirectional differentiation. ADSCs originate from the mesoderm and can differentiate into osteoblasts, cardiomyocytes, urothelium, skin tissue, and nerve tissue.
MSC can exert paracrine effects through extracellular vesicles (EVs), which is one of the important forms of MSC action. Exosomes are the most common extracellular vesicles, which refer to membrane vesicles with a diameter of 30–100 nm containing bioactive substances such as proteins and RNA [23]. Similar to MSC, ADSCs can also play paracrine roles in tissue damage and repair through ADSC-exos. ADSC-exos is a lipid bilayer structure, its main components are proteins, nucleic acids and lipid molecules, which is the medium of information communication between cells [24]. ADSCs mainly regulate the surrounding environment through ADSC-exos [25]. ADSC-exos contain many angiogenic miRNAs, VEGFA, fibroblast growth factor (FGF), insulin-like growth factor (IGF), platelet-derived growth factor (PDGF) and other bioactive molecules [26]. After FGF binds to FGF receptor (FGFR), it phosphorylates FRS2, which recruits regulatory proteins GRB2 and SOS to further activate RAS and its downstream RAF and MAPK pathways to promote angiogenesis [27]. IGF can promote cell division, proliferation and repair by activating PI3K/AKT signaling pathway and Mek/Erk signaling pathway in cells [28]. PDGF activates MAPK, PI3K/Akt, JAK/STAT and NF-κB signaling pathways by binding to its receptors to promote cell growth and chemotaxis [29] (Fig. 2). Compared with ADSCs, cell-free therapy using ADSC-exos alone can reduce adverse reactions such as immune rejection, and is easier to store and transport than living cells.
Fig. 2.
Cytokines secreted by ADSCs and their effects. ADSCs expressed CD13, CD44, CD90, CD49d, CD105 and CD10 on their surface. ADSCs can secrete cytokines such as Il-1β, IL-6, and IL-10 to exert anti-inflammatory effects. ADSCs secrete TGF-β, VEGF, PGF and Ang-l to promote endothelial cell proliferation and angiogenesis. ADSC-exos is a lipid bilayer encapsulated structure, and its main components are proteins, nucleic acids, and lipid molecules. ADSC-exos contain many angiogenic miRNAs, VEGFA, FGF, IGF, PDGF and other bioactive molecules.
3. Advantages of ADSCs in tissue repair and regeneration
The number of ADSCs is abundant and acquisition of ADSCs is simple. Adipose tissue is widely distributed and abundant in the human body. The content of ADSCs in adipose tissue is up to 5000 colony-forming unit–fibroblastic (CFU-F), which is 500 times that of BM-MSCs [30]. Adipose tissue is obtained by liposuction. Only local anesthesia is needed to extract local adipose tissue to obtain ADSCs, which causes less trauma to patients and yields a large number of stem cells.
The in vitro culture conditions of ADSCs are simple. The survival rate of ADSCs is high, and the proliferation rate is faster. Human platelet lysate (HPL) can be used to promote cell expansion in vitro [31], so a large number of ADSCs can be obtained in a short period of time, and the problem of stem cell source can be effectively solved [32].
The safety of adipose-derived stem cells is higher. Since adipose tissue is abundant in the human body, ADSCs are mainly provided through their own body, avoiding the occurrence of immune rejection [33]. Compared with embryonic stem cells and induced pluripotent stem cells, ADSCs will not cause teratogenesis, tumor formation and other problems, and the safety is higher [34].
There are two main approaches to use ADSCs for tissue repair and regeneration: “ADSCs-scaffold composite” therapy and cell-free therapy. “ADSCs-scaffold composite” therapy refers to the method of transplanting “ADSCs-scaffold composite” into the injured site for tissue repair. Scaffold materials can guide tissue to grow into a specific shape, and have the role of mechanical support. In addition, scaffold materials contain a large number of molecules that promote tissue regeneration, such as cytokines, transcription factors, regulatory chemokines, etc., which are unstable and easy to be degraded under normal conditions. Encapsulation of these molecules in the scaffold material prevents degradation and gradual and slow release, ensuring that the molecular function can be maintained for a long time. With the support of scaffold materials, ADSCs can further proliferate and differentiate into target tissues and promote tissue regeneration [35]. Cell-free therapy refers to promoting cell proliferation, tissue regeneration, and neovascularization at the injured site by releasing a large number of miRNAs, cytokines, and other bioactive substances through ADSC-exos or ADSC-CM. ADSC-exos and ADSC-CM have the following advantages: Because stem cells have the risk of causing tumors after long-term culture, ADSC-exos and ADSC-CM do not have such a problem; Compared with ADSCs, ADSC-exos and ADSC-CM have lower immunogenicity and less chance of immune rejection. ADSC-exos and ADSC-CM are easier to preserve and transport than ADSCs [24].
4. ADSCs in bone tissue regeneration
4.1. Mechanism of ADSCs involved in bone tissue regeneration
In the process of bone tissue repair by ADSCs, the degree of differentiation of ADSCs into bone tissue and the degree of vascularization of new tissue are important factors affecting the repair effect [9] (Fig. 3A).
Fig. 3.
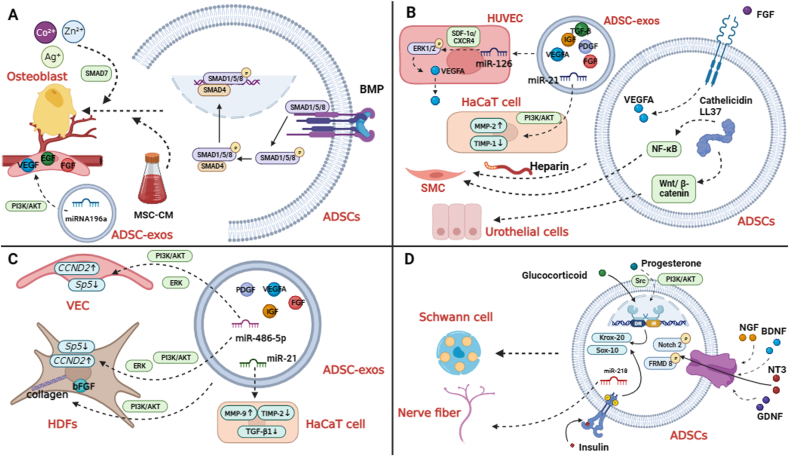
The mechanism of ADSCs and ADSC-exos in bone tissue regeneration, urinary tract repair, skin injury repair, and nerve regeneration. A, BMP is an important molecule that promotes the differentiation of ADSCs into bone tissue. Certain factors contained in MSC-CM can induce differentiation of ADSCs into bone tissue and promote cell growth and division. Metal ions (Co2+, Zn2+, Ag+) can promote the proliferation of osteoblasts, inhibit the activity of osteoclasts, and promote the regeneration of bone tissue. MiRNA196a can promote angiogenesis by increasing the secretion of VEGF, EGF, FGF, etc. B, Heparin is an inducer of SMC differentiation. Cathelicidin LL37 promotes the differentiation of ADSCs into urothelial cells and smooth muscle cells. ADSC-exos could up-regulate the expression of microRNA-126 to increase the secretion of VEGF and the expression of VEGFR2 in HUVEC. FGF-FGFR promotes tissue vascularization possibly by increasing the content of VEGFA in ADSC-exos. MiR-21 can affect the expression of MMP-2 and TIMP-1 proteins in HaCaT cells to increase the migration and proliferation of HaCaT cells. C, ADSC-exos can increase mRNA and protein levels of Col 1, Col 3, and bFGF in fibroblasts and increase the division rate of fibroblasts. ADSC-exos can promote proliferation of HUVEC and HDFs to achieve dermal regeneration and angiogenesis. MiR-486-5p can promote proliferation of HSFs and enhance activity of HMECs by down-regulating the expression of Sp5 and up-regulating the expression of CCND2. MiR-21 can enhance the division and migration of HaCaT cells and inhibit the apoptosis of HaCaT cells by enhancing the expression of MMP-9 and inhibiting the expression of TIMP2. D, NTFs (NGF, BDNF, NT3, GDNF) can enhance the nerve repair ability of ADSCs. The phosphorylation of FRMD 8 and Notch 2 is significantly increased in ADSCs stimulated with NT3. MiR-218 could promote nerve fiber growth. Insulin and glucocorticoids promote differentiation of ADSCs into SCLC by promoting the expression of myelin transcription factors (Krox-20 and Sox-10). Progesterone induces the differentiation of ADSCs into SCLC through Src and PI3K-Akt signaling pathways.
4.1.1. ADSCs differentiate into bone tissue
The main components of bone tissue include cells, bone glue fibers and extracellular matrix [36]. Osteogenic growth peptide (OGP) is a growth factor that can promote the proliferation of osteoblasts and fibroblasts, and plays an important role in the regeneration of bone tissue [37]. However, the “ADSCs-scaffold composite” implanted in bone tissue engineering cannot carry OGP. Therefore, finding the signaling molecules that stimulate the differentiation of ADSCs into bone tissue is an important problem in bone tissue engineering.
It has been found that the molecules and signaling pathways that play important roles in the process of bone development and maturation, such as bone morphogenetic protein (BMP), WNT protein, Notch signaling pathway and Hedgehog signaling pathway, also play an important role in the process of inducing osteogenic differentiation of ADSCs [38]. BMP is a member of the TGF-β superfamily, which can regulate bone formation and resorption by affecting the growth, division and activity of osteoblasts and osteoclasts, thus regulating bone homeostasis and playing an important role in bone remodeling [39]. BMP is also an important molecule that promotes the differentiation of ADSCs into bone tissue. BMP acts through two pathways: the SMAD-dependent pathway (also known as the typical SMAD pathway) and the p38 -MAPK pathway, of which the SMAD-dependent pathway is the main pathway through which BMP acts. After BMP binds to the receptor, the intracellular subunit of the receptor phosphorylates SMADs 1/5/8, after which the phosphorylated SMADs 1/5/8 binds to the Co SMAD (SMAD4) to form a complex, which is transported to the nucleus to affect the expression of osteogenic genes [40], further from promoting the differentiation of ADSCs to bone tissue.
Studies have shown that ADSCs cultured in the extracellular matrix produced by bone marrow cells have a higher proportion of bone tissue differentiation compared with other culture environments, and immunohistochemistry shows that the expression of osteogenic-related markers such as ALP and RUNX2 is increased [41], suggesting that BM-derived extracellular matrix has the function of inducing ADSCs to differentiate into bone tissue. Certain factors contained in mesenchymal stem cell (MSC)-derived conditioned medium (CM) can induce differentiation of ADSCs into bone tissue and promote cell growth and division [42].
In tissue engineering, scaffold materials are an important part, which are the environment for stem cells to survive. With the support of scaffold materials, ADSCs can differentiate into bone tissue, nerve tissue, skin, etc., and then complete tissue repair. Scaffolds are also involved in the induction of ADSCs. Recent studies have found that the growth and differentiation rate of ADSCs combined with polycaprolactone (PCL) scaffold materials is significantly increased, and the expression of osteogenic related markers such as ALP is also significantly increased. Therefore, PCL also has the potential to induce stem cells to differentiate into osteoblasts [43]. Fibroblast growth factor 2 (FGF2) is a cytokine that can promote bone and cartilage repair as well as tendon and ligament formation. Recent studies have shown that the combination of exogenous FGF2 and polyethylenimine nanocomposites can significantly improve the rate of bone formation in animal models of bone defects [44].
Various physical stimulation including mechanical stimulation such as stretch, compression, direct current and alternating current can promote the osteogenic differentiation of ADSCs by increasing the WNT signaling pathway [45]. In addition, the osteogenic differentiation potential of ADSCs is significantly increased when vibration stimulation is added in ADSCs culture dish compared with the control group [46]. Various ions such as Co2+、Zn2+、Ag+ and other metal ions can promote the proliferation of osteoblasts, inhibit the activity of osteoclasts, and promote the regeneration of bone tissue. Studies have shown that metal ions can promote the differentiation of ADSCs to bone tissue, and the specific mechanism is still unclear, probably through the cAMP signaling pathway [47]. Metal coating on scaffolds has been widely used to improve bone tissue repair [48].
4.1.2. ADSCs promote bone tissue vascularization
In bone tissue engineering, the degree of vascularization of new tissue affects the quality of tissue repair. It has been found that small vesicles or larger ADSC-exos secreted by ADSCs contain A large number of pro-angiogenic factors such as VEGFA, which can increase the degree of vascularization of new bone tissue and promote bone tissue repair [49]. There are many miRNAs that promote vascularization in ADSC-exos, and miRNA196a plays a major role in the process of bone tissue regeneration. MiRNA196a can promote angiogenesis by increasing the secretion of VEGF, epithelial growth factor (EGF), FGF, etc. This process may be achieved through the PI3K/AKT signaling pathway [50]. Studies in rabbits’ model of hip dysplasia have found that aggregation of ADSCs into 3D multicellular spheroids can increase the anti-inflammatory activity of stem cells after transplantation, avoid inflammatory damage caused by scaffold materials, and improve the survival rate after transplantation. In addition, the ability of ADSCs to promote angiogenesis in new tissues is further improved, which greatly improved the tissue repair ability of ADSCs [51].
4.2. Application of ADSCs in bone tissue regeneration
Most bone injuries are within the range of the body's compensation, and the body can repair through callus formation, intramembranous osteogenesis, and endochondral osteogenesis [52]. In the case of severe bone injury, due to the limited compensatory ability of the body, it may cause the formation of nonunion or deformity, so human intervention is needed [53].
Bone transplantation has been the main way to solve bone injury, but there are many problems such as difficult to survive, high degradation rate, and high risk of pathogen infection at the graft site. Recent studies have shown that when the “ADSCs-scaffold composite” is implanted into the animal model of skull defect, ADSCs can differentiate into bone tissue under the action of cytokines and growth stimulation signals, so that the skull defect can be repaired [54], and the degree of bone repair is higher than that of traditional bone transplantation. At present, PCL scaffolds have been successfully used in animal injury models. This kind of scaffold material has good biocompatibility and biodegradation rate, and less adverse reactions such as biological rejection and inflammation [55]. However, in a clinical study, Tuomo Thesleff et al. used cranioplasty using autologous ADSCs seeded on beta-tricalcium phosphate (betaTCP) granules to treat five patients with cranial injuries, and the results of the six-year follow-up showed that the outcome was were not superior to conventional repair of cranial injuries [56].
5. ADSCs in urinary tract repair
5.1. Mechanisms of ADSCs in urinary tract repair
In the process of urinary tract repair, the quality of new smooth muscle and the degree of early vascularization of new tissue are important factors affecting the repair effect [57] (Fig. 3B).
5.1.1. ADSCs differentiate into smooth muscle cells (SMC)
Studies have shown that the use of cell-free scaffolds to repair urinary tract trauma results in insufficient generation of smooth muscle in the reconstructed urinary tract, which makes it difficult to form a functional urinary tract [58]. ADSCs can differentiate into SMC, which has great application value in the treatment of ureteral trauma or stricture. When ADSCs are cultured in vitro, the levels of α-SMA, an early marker of SMC formation, and SM-MHC, a late marker of SMC formation, are increased by qPCR, indicating that ADSCs had the potential to differentiate into SMC. However, the expression of stem cell-related markers CD29, CD90 and CD105 is also detected by qPCR, indicating that ADSCs did not fully differentiate into SMC but are in an initiating state [59]. However, ADSCs transplanted in vivo can fully differentiate into SMC, indicating that the stimulation of the in vivo environment is essential. Cathelicidin is an important immune molecule in the innate immune system. Studies have shown that ADSCs overexpressing cathelicidin LL37 by lentiviral transfection, elevated levels of urothelial cell markers and key kinases of the Wnt/β-catenin pathway, smooth muscle cell markers and key kinases of the nuclear factor kappa B (NF-κB) pathway. Therefore, it is speculated that cathelicidin LL37 promotes the differentiation of ADSCs into urothelial cells and smooth muscle cells through Wnt/β-catenin and NF-κB pathways [60].
5.1.2. ADSCs promote angiogenesis
In the process of urinary tract reconstruction, if the degree of early vascularization of the new tissue is low, the source of tissue nutrition is insufficient, and there is a lack of cytokines transported through the blood, resulting in cell proliferation disorders, low tissue survival rate, and restenosis and scar formation [61]. Traditional biological materials for urinary tract repair usually have the problem of insufficient vascularization. Therefore, attempts to inject cytokines such as VEGF to stimulate neovascularization have been made, and the degree of vascularization has been improved to a certain extent, but still cannot meet the requirements [62]. It has been found that the degree of vascularization of the new tissue will be significantly increased when the scaffold material loaded with ADSCs is used for urinary tract repair [63]. Exosomes are a kind of vesicles secreted by cells, and their main role is to mediate intercellular information transmission. ADSCs mainly regulate the surrounding environment, including the degree of vascularization of tissues, through ADSC-exos [64].
What is the mechanism by which ADSCs and their secreted ADSC-exos enhance the vascularization of new tissues? Firstly, ADSC-exos contain a large number of cytokines such as VEGFA, FGF, IGF, PDGF, transforming growth factor-β (TGF-β) and so on [[65], [66], [67]]. VEGFA is the main molecule of ADSC-exos promoting neovascularization. Secondly, ADSC-exos could up-regulate the expression of microRNA-126 in human umbilical vein endothelial cells (HUVEC), further activate the SDF-1α/CXCR4 pathway and phosphorylate the downstream ERK1/2, ultimately increasing the secretion of VEGF and the expression of VEGFR2 in HUVEC. Thus, it promotes the formation of new blood vessels in the tissue [68]. NF-κB may be involved in this signaling pathway, and the use of NF-κB inhibitors can reduce VEGF secretion [69].
Studies have shown that the content of various cytokines in ADSCs increases under hypoxia to cope with hypoxia damage, among which the content of VEGFA increases most significantly. Therefore, hypoxia treatment of ADSCs can enhance the function of ADSCs in promoting angiogenesis [65].
Fibroblast growth factors and their receptors (FGFs/FGFR) play an important role in the regulation of urinary tract repair [70]. Studies in wild-type female mice have shown that increasing the expression of FGFR in ADSCs differentiated urothelial cells can accelerate tissue repair during the process of urinary tract repair. Conversely, inhibition of FGFR expression leads to impaired urothelial repair [71]. FGFs/FGFR also plays an important role in promoting tissue vascularization. Studies have found that overexpression of FGFR in ADSCs by lentivirus transfection can detect the increased expression of VEGFA in the culture medium supernatant [72], Therefore, it has been suggested that the mechanism by which FGFR promotes tissue vascularization is to increase the content of VEGFA in ADSC-exos.
5.1.3. ADSCs inhibit fibrosis
MiR-21 in ADSC-exos plays an important role in the prevention and treatment of urinary tract fibrosis. Studies have found that increasing the expression of miR-21 in ADSCs by lentivirus transfection, similar to FGFR, the expression of angiogenic cytokines such as VEGFA in ADSC-exos is also significantly increased [73]. MiR-21 in ADSC-exos can affect the expression of MMP-2 and TIMP-1 proteins in human immortalized epidermal cells (HaCaT cells) through the PI3K/AKT signaling pathway, increase the migration and proliferation of HaCaT cells, improve tissue vascularization and reduce fibrosis formation [74].
Ureteropelvic junction obstruction (UPJO) is prone to occur after pyeloplasty, and the occurrence of UPJO can lead to renal fibrosis. The mechanism may be that the high expression of transforming growth factor-β1 (TGF-β1) in the obstructed kidney promotes the accumulation of collagen [75]. In the UPJO Model of Wistar Rats, it is found that the level of TGF-β gradually decreased and the degree of renal fibrosis is less in the experimental group after tail vein injection of ADSCs [76]. The main mechanism is that ADSCs can inhibit the expression of pro-fibrotic genes such as COL 1A 1, TGF-β1, and connective tissue growth factor (CTGF) [77].
5.2. Application of ADSCs in urinary tract repair
Urinary tract dysfunction such as urinary tract stenosis is a common problem in urology. Common urinary tract stenosis includes ureteral stricture and urethral stricture. A variety of surgical operations and external trauma may cause urinary tract stenosis, which will have a great impact on the quality of life of individuals, and may even cause renal function damage. The traditional method for the treatment of urinary tract stricture is to perform urinary tract reconstruction, and the materials that can be used mainly include autologous tissue or allogeneic tissue such as skin, bladder mucosa, intestine, etc., but the effect of this method is not ideal. The incidence of restenosis after surgery is high, the sampling site is limited, and complications such as infection may occur [78].
The application of tissue engineering in the field of urinary tract reconstruction has been extensively studied and demonstrated. The selection of seed cells is the key. As mentioned above, ADSCs have the potential to differentiate into epithelial cells and smooth muscle cells, and their sources are rich. Compared with traditional surgery for urinary tract reconstruction, ADSCs have a better effect and a lower incidence of postoperative restenosis, so they have great application value in urinary tract repair. At present, there are two main methods for urinary tract repair by ADSCs: direct injection of ADSC-exos secreted by ADSCs to promote urinary tract repair or through the use of “ADSCs-scaffold composite”. For urinary tract repair, because the cell-free therapy is not mature at present, and the scaffold material has the effect of preventing restenosis, the second method is more satisfactory [79].
The choice of scaffold material is an important factor in determining the satisfaction of repair, including natural and artificial materials. Natural materials refer to decellularized scaffold materials obtained by decellularization of intestinal and urinary system tissues. The advantage of autologous natural materials is that there is no rejection reaction, but the disadvantage is that their biomechanical properties are poor, which can easily lead to scar formation and other complications. But it lacks smooth muscle cells [80]. Artificial materials have been widely used in tissue engineering. Silk fibroin is an excellent scaffold material due to its good biocompatibility, excellent mechanical structure, and controllable biodegradation rate [81]. Oxidized cellulose scaffold is also an excellent biological material. The biocompatibility of sulfonated bacterial cellulose materials obtained by sulfonation on the basis of oxidized cellulose scaffold has been greatly improved, and it is expected to become a new mainstream scaffold material in the future [82]. In order to increase the repair ability of synthetic materials, certain biological components such as hyaluronic acid or collagen are usually added to the material, and the effect of such synthetic materials in the process of ureteral and bladder reconstruction has been confirmed: ADSCs and SMC are seeded on polyglycolic acid (PGA) material and implanted into patients for ureteral reconstruction. The urothelium and smooth muscle grew well, and the effect is satisfactory, and there are no obvious complications [83]. In the latest study, bilayered ADSCs-gelatin film grafts are used to repair the ureter in New Zealand white rabbits after partial ureterectomy. Compared with the control group using bilayered acellular gelatin sheets, the smooth muscle of the new ureter in the experimental group is more abundant and arranged more regularly. There is no dilatation or curvature of the ureter [84]. Therefore, the bilayered ADSC-gelatin sheets have the potential to be a new graft for ureteral injury reconstruction.
6. ADSCs in skin injury
6.1. Mechanisms of ADSCs in skin injury
In the process of skin injury repair, the degree of vascularization, fibrosis and scar formation of new tissue are the main factors affecting the quality of repair (Fig. 3C).
6.1.1. Cell-free therapy: ADSC-exos and ADSC-CM promote skin wound repair
Studies in mouse models have shown that ADSC-exos can promote skin wound vascularization and accelerate skin wound repair in mice [85]. ADSC-exos contain a large number of cytokines such as VEGFA, FGF, IGF, and PDGF. ADSC-exos can promote skin wound healing by promoting collagen synthesis, fibroblast proliferation and migration, vascular endothelial cell proliferation, and angiogenesis [24].
ADSC-exos can be used in fibroblasts by being internalized by fibroblasts. It increases the gene expression of collagen type I (Col 1), collagen type III (Col 3) and the level of basic fibroblast growth factor (bFGF) in fibroblasts, and increases the rate of fibroblast division [86]. Ly294002 is an inhibitor of PI3K, and the function of ADSC-exos is reduced after treatment with Ly294002, so it is speculated that ADSC-exos may play a role through PI3K/Akt signaling pathway [86]. ADSC-exos can act on HUVEC and human dermal fibroblasts (HDFs) to promote their proliferation to achieve dermal regeneration and angiogenesis, which may be achieved by activating AKT and ERK signaling pathways [87].
MiRNAs in ADSC-exos are of great value in the process of skin injury repair. In a mouse model with full-layer skin injury, it is found that the content of miR-486-5p is very high in ADSC-exos, and the target cells of miR-486-5p are mainly human skin fibroblasts (HSFs) and human microvascular endothelial cells (HMECs). It can promote HSFs proliferation and enhance HMECs activity by down-regulating the expression of Sp5 and up-regulating the expression of CCND2 in target cells [88], thus promoting collagen synthesis and microangiogenesis to accelerate skin wound healing. If miR-486-5p antagonists are used, these effects are attenuated. MiR-21 in ADSC-exos can enhance the division and migration of HaCaT cells and inhibit the apoptosis of HaCaT cells by enhancing the expression of MMP-9 and inhibiting the expression of TIMP2, so as to promote the repair of skin injury [89]. TGF-β1 protein plays an important role in the formation of scar. High level of miR-21 can down-regulate the expression of TGF-β1 protein in HaCaT cells and inhibit the formation of scar [74].
In the rat model of diabetic foot ulcer, it is found that if ADSC-exos overexpressing nuclear erythrocyte 2-related factor 2 (NRF2) is used, the level of VEGF is increased, while the levels of inflammatory factors such as NOX1, IL-1β, and IL-6 are significantly decreased, and the effect of promoting ulcer healing is more significant [90].
ADSC-CM contains various active substances secreted by ADSC including ADSC-exos, such as EGF, VEGF, bFGF, DNA and RNA. Studies have shown that ADSC-CM can promote the proliferation and migration of fibroblasts and keratinocytes to promote the healing of skin injury, and bFGF plays a major role in the proliferation of fibroblasts [24].
6.1.2. “ADSCs-scaffold composite” promotes skin injury repair
In the sheep burn model study, the skin flap from autologous donor is transplanted to the burn site, and the local application of ADSCs in the experimental group showed better healing than that in the control group. It is speculated that ADSCs can increase the degree of tissue vascularization by increasing the level of VEGF, improve the blood supply of the affected area, and thus promote the wound healing [91]. If the effect of ADSCs alone is not satisfactory, integrin β1 and ADSCs can be used simultaneously: integrin β1 can act on the cell receptor and enhance the function of ADSCs through the PI3K/AKT pathway [92], thereby promoting skin injury repair. In the study of allogeneic ADSC treatment in dogs with different degrees of skin injury, it is found that the level of granulocyte-macrophage colony stimulating factor (GM-CSF) in the serum of dogs is increased after ADSC treatment, suggesting that ADSC can recruit cells related to wound repair by upregulating GM-CSF to promote wound healing [93].
6.2. Application of ADSCs in skin injury
Skin is the first barrier of the human body, and skin injury is a common clinical problem. If severe skin injury is not treated in time and the wound is not healed for a long time, it can lead to serious complications such as severe infection, dehydration, electrolyte disturbance, and even life-threatening [94]. Scarring can also occur if the healing time is too long or if the healing is poor [95]. At present, the treatment of skin injury is mainly to reduce pressure injury, surgical debridement, flap transplantation, and other measures. In many cases, these traditional methods cannot meet the needs of the skin, cannot regenerate the skin, and the effect is limited.
Tissue engineering has been proved to be of great value in the repair of skin injury. However, the lack of neovascularization in skin tissue differentiated from implanted stem cells is a major problem [96]. Many studies have shown that ADSCs can differentiate into fibroblasts, endothelial cells, and keratinocytes, and can promote the vascularization of new skin tissue through autocrine or paracrine pathways, so ADSCs have great application value in the field of skin repair [97]. A phase II clinical trial conducted by Kyung-Chul Moon et al. demonstrated positive efficacy of hydrogel sheet containing allogeneic ADSCs in diabetic foot ulcers, and no serious adverse effects were found [98]. After administration of ADSCs to 158 patients with skin injuries, the results showed significant improvement in granulation tissue coverage, healing rate and granulation tissue thickness at the site of injury, making ADSCs a safe and effective alternative therapy to promote wound healing [99]. A clinical study by Bing-rong Zhou et al. showed that ADSC-CM combined with fractional carbon dioxide laser resurfacing (FxCR) can effectively treat atrophic acne scars. The cytokines in ADSC-CM can promote the proliferation of dermal fibroblasts and the secretion of collagen, which play an important role in the treatment process [100].
For the repair of large skin defects, it is relatively more important to solve the problem of insufficient tissue vascularization. Endothelial progenitor cells (EPC) are the precursor cells of vascular endothelial cells. If ADSCs and EPC are co-cultured and applied to large area injury repair, better results can be obtained than ADSCs alone [101]. For refractory skin lesions, multiple injections of ADSCs can be used to accelerate wound healing [102].
In the process of skin injury repair, there are many choices of scaffold materials. Hydrogel is one of the commonly used scaffold materials [103]. Cellulose scaffolds are also used for their good biocompatibility. Compared with hydrogels, cellulose scaffolds have better biocompatibility, but their degradation rate is low [104]. It is necessary to further modify it to increase its degradation for better clinical application [105]. With the support of scaffold materials, ADSCs can differentiate into skin tissue cells to achieve skin repair.
In the process of using “ADSCs-scaffold composite” for skin injury repair, the survival time of ADSCs is also a major issue that needs to be solved. The 3D multicellular spheroids formed by aggregation of multiple ADSCs used in bone regeneration can also be applied to skin wound repair. Transplantation after the formation of cellular spheroids of ADSCs can increase the anti-inflammatory activity of stem cells to avoid inflammatory damage caused by scaffold materials, increase the survival time of ADSCs, and increase the degree of vascularization of the new tissue [106]. If the nanosheet scaffold material with high viscosity is used to increase the colonization ability of ADSCs, the survival time of ADSCs will be further increased [107], which may be applied to clinical practice in the future.
7. ADSCs in peripheral nerve injury
7.1. Mechanisms of ADSCs in peripheral nerve injury
ADSCs can promote peripheral nerve regeneration by secreting various cytokines and miRNAs, and can also differentiate into SC-like cells (SCLC) to enhance nerve repair function (Fig. 3D).
7.1.1. ADSCs promote peripheral nerve injury repair
Glial cells, also known as Schwann cells (SCs), play a key role in the regeneration of peripheral nerves [108]. In the repair process of peripheral nerve injury, SCs can down-regulate the expression of myelin-related genes and up-regulate the expression of growth-related genes, and further synthesize exogenous neurotrophic factors (NTFs), cell adhesion molecules NCAM and L1, and myelin-associated glycoprotein (MAG) to promote axonal regeneration and promote the myelination of regenerated axons [[109], [110], [111], [112]]. However, it is difficult to obtain and culture SCs [113], so it is difficult to apply SCs in clinical practice.
ADSC-exos can also release many growth factors such as nerve growth factor (NGF), IGF-1, and PDGF, which can promote axonal growth and repair [[114], [115], [116], [117], [118]]. In addition, miR-218 contained in ADSC-exos may have a role in promoting nerve fiber growth [119]. The advantages of using ADSCs to promote peripheral nerve regeneration are that ADSCs are simple to obtain, easy to culture, and abundant [120],Therefore, ADSCs are considered as an alternative to SCs for clinical nerve repair. However, the efficacy of ADSCs in nerve repair is worse than that of SCs [121].
Scaffold materials commonly used in the repair of peripheral nerve injury include artificial nerve conduits such as acellular allogeneic nerve grafts (ANAs), polycaprolactone (PCL) conduits, fibrin conduits, etc [[122], [123], [124]]. ANAs is a cell-free scaffold material that minimizes antigenicity [125]. Studies of sixty adult male Wistar rats have shown that ADSCs combined with ANAs implanted into the injured site of sciatic nerve can effectively promote the regeneration and repair of sciatic nerve. Compared with other cells combined with ANAs for nerve repair, ADSCs can secrete a large number of nerve growth factors to promote nerve regeneration, so it has better effects [124]. In the process of ADSCs + ANAs complex repairing sciatic nerve injury, the expression of NTFs increases, and the expression of JAK2 and STAT3 decreases. Therefore, it is speculated that nerve repair may be achieved by highly expressing NFs and inhibiting the JAK2∖STAT3 signaling pathway [126].
7.1.2. Methods to enhance the nerve repair ability of ADSCs
Studies have shown that, various NTFs receptors exist on the surface of ADSCs. NTFs, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin 3 (NT3), and glial cell line-derived neurotrophic factor (GDNF), can enhance the nerve repair ability of ADSCs [[127], [128], [129]]. The effect of NT3 is more significant: the ADSCs culture medium (containing ADSC-exos) supplemented with NT3 significantly promoted axon outgrowth [130]. Notch 2 promotes the proliferation and differentiation of neural precursor cells (NPCS) [131]. The phosphorylation of FRMD 8 and Notch 2 is significantly increased in ADSCs stimulated with NT3 [132]. The role of FRMD8 is to regulate the release of growth factors from cells [133]. Increased phosphorylation of FRMD8 indicates increased release of growth factors [134], which may be one of the mechanisms by which NT3 enhances the nerve repair capacity of ADSCs.
The external environment also has a certain effect on the nerve repair function of ADSCs. Studies have shown that low carbon dioxide environment can promote ADSCs to secrete smaller volume of concentrated ADSC-exos, and the concentration of active components in ADSC-exos is greater, so the function of ADSC-exos will also be improved, which can accelerate the repair of sciatic nerve injury in rat sciatic nerve injury model [135].
Human platelet lysate (HPL) is a medium supplement containing a large number of growth factors such as PDGF, IGF-1, BDNF, EGF, VEGF, etc. [136,137]. The axon growth of ADSCs treated with HPL is significantly increased compared with that of the blank control group in vitro axonal outgrowth experiments, indicating that HPL can significantly improve the nerve repair and neurotrophic ability of ADSCs [138].
7.1.3. ADSCs differentiate into SC-like cells (SCLC)
ADSCs can differentiate into SC-like cells (SCLC) when stimulated by a series of neurotrophic factors for a long time. SCLC has a better ability to promote axon growth than ADSCs [139]. However, the efficiency of ADSCs to differentiate into SCLC is very low, so it is very important to find ways to promote ADSCs to differentiate into SCLC.
Membrane progesterone receptors (mPRs) are receptors within ADSCs and have been shown to play an important role in the differentiation of ADSCs into SCLC: After binding to mPRs, progesterone induces the differentiation of ADSCs into SCLC through Src and PI3K-Akt signaling pathways, promotes the proliferation and migration of SCLC, and enhances the ability of SCLC to secrete cytokines such as BDNF [140].
MEG3/let-7a-5p/RBPJ axis plays an important role in the differentiation of ADSCs into SCLC [141]. S100, GFAP, SOX10, p75NTR are markers of SCLC [[142], [143], [144], [145]]. In the experiment, the levels of S100, GFAP, SOX10 and p75NTR in the culture medium of ADSCs treated with let-7a-5p analogues are significantly increased, indicating that let-7a-5p could promote the differentiation of ADSCs into SCLC. On the contrary, overexpression of MEG3 and RBPJ attenuated the effect of let-7a-5p and inhibited the differentiation of ADSCs into SCLC and the proliferation of SCLC [146,147].
Insulin and glucocorticoids can promote the differentiation of ADSCs into SCLC [148], and the mechanism may be to promote the proliferation of SCLC by promoting the expression of myelinating transcription factors such as Krox-20 and Sox-10 [149]. ALK5I is also found to induce the differentiation of ADSCs into SCLC through non-Smad signaling [150]. As a neurotrophic factor, folic acid can induce the differentiation of ADSCs into SCLC, enhance the secretion of nerve growth factor in SCLC, and enhance the nerve repair ability of SCLC [151].
In conclusion, it is possible to enhance the neural repair function of ADSCs by intervening in the molecular pathways that regulate the differentiation of ADSCs into SCLC in the future.
7.2. Application of ADSCs in the repair of peripheral nerve injury
Peripheral nerve injury (PNI) is a common complication of surgery, which can lead to the degeneration of the injured nerve and muscle, and the loss of sensory and motor function in the innervated area [152]. Nerve tissue can hardly regenerate spontaneously after injury, so it is usually necessary to treat PNI by autogenous nerve transplantation through microsurgery. However, this traditional treatment method has some problems, such as complex operation process, uncertain treatment effect, narrow application scope, and postoperative sensory loss at the donor site [153]. Studies have shown that neurons and glial cells in the peripheral nervous system have a certain ability to regenerate, but they need the stimulation of neurotrophic factors and the support of scaffold materials to achieve regeneration [154].
ADSCs can secrete a large number of neurotrophic factors and have great application value in the field of repairing peripheral nerve injury. There are several main ways to use ADSCs to treat peripheral nerve injury: The “ADSCs-scaffold composite” is implanted into the injured site. Local injection of ADSCs into the injured nerve for treatment: the drawback of local epineurium injection of ADSCs is that it may cause local injury. Intravenous injection of ADSCs: studies have shown that ADSCs have a large number of adhesion molecules on their surface and have the characteristics of strong adhesion. Therefore, intravenous injection of ADSCs can also be used to locate and reach the injured nerve site and achieve the same effect as local injection [155]. However, intravenous injection has the risk of adipose-derived stem cells retaining in other organs and causing damage and teratogenesis [[156], [157], [158]]. Clinical trials using ADSCs for peripheral nerve regeneration are limited and therefore require further evaluation prior to clinical application.
8. Applications of ADSCs in other fields
In recent years, a large number of studies on the tissue regeneration function of ADSCs have focused on bone regeneration, urinary tract repair, skin wound healing, peripheral nerve regeneration and other fields. In addition, due to the ability of ADSCs to differentiate into various lineages, ADSCs also have some application prospects in other fields. ADSCs can improve the wound healing of diabetic foot ulcer (DFU) [159] and treat inflammatory bowel disease [160] by activating VEGF-C/VEGFR-3 mediated signaling pathway to promote lymphangiogenesis and angiogenesis. ADSCs can inhibit podocyte apoptosis to alleviate diabetic nephropathy [161]. ADSCs can reduce hepatic ischemia-reperfusion injury (HIRI) by inhibiting oxidative stress and inflammation through paracrine effect [162]. ADSCs can reduce pulmonary fibrosis by increasing the number of anti-inflammatory pulmonary macrophages [163]. ADSCs can also be applied to myocardial infarction, immune diseases (including lupus, arthritis, multiple sclerosis, graft-versus-host disease), Alzheimer's disease, periodontal regeneration, spinal cord injury.
9. Summary and perspectives
ADSCs are ideal seed cells for future tissue engineering. Compared with conventional bone marrow mesenchymal stem cells and embryonic stem cells, the greatest advantages of ADSCs are their high concentration, easy availability, and low damage to the patient during sampling. ADSCs have the potential for multi-directional differentiation. Supported by non-biological scaffolds or cell-free biomaterials and induced by external signals such as cytokines and environmental stimuli, ADSCs can differentiate into bone tissue, urinary tract tissue, skin tissue, nerve tissue, etc. The main functional mode of ADSCs is the secretion of ADSC-exos. There are plenty of bioactive substances in ADSC-exos, such as miRNA and cytokines, which can promote the proliferation and migration of target tissue cells, promote tissue repair and regeneration, promote vascularization of new tissues, and prevent fibrosis and scar formation. ADSC-exos or ADSC-CM contains plenty of miRNAs and cytokines from ADSCs, so ADSC-exos or ADSC-CM alone can also promote tissue repair and regeneration, which is a new way to use stem cells for tissue repair and regeneration - cell-free therapy. In general, cell-free therapy has the following advantages: firstly, cell-free therapy does not have the risk of causing tumors. Secondly, ADSC-exos and ADSC-CM are less immunogenic and have a low probability of immune rejection. Lastly, ADSC-exos and ADSC-CM are easier to preserve and transport than ADSCs. Therefore, cell-free therapies may become main trend in the future, especially in urothelial tissue engineering and skin tissue engineering. The degree of vascularization of the neoplastic tissue is an important factor in determining the quality of tissue repair. Inadequate vascularization affects the repair outcome, which is a common problem in all tissue repair processes. ADSCs can secrete VEGFA, EGF, FGF and miRNAs to promote neovascularization.
At present, there are still some issues to be resolved for the application of ADSCs in tissue engineering. Although there are multiple studies on the tissue repair ability of ADSCs, no study has so far conclusively demonstrated the best way to administer ADSCs. Whether there are adverse effects of ADSCs being retained in other organs if the traditional intravenous administration is followed, and how to ensure the accurate targeting of ADSCs to the damaged site remain to be addressed. The main problem of local injection of ADSCs is that inflammation at the damaged site can cause low survival rate and short survival time of ADSCs, etc. Some studies have shown that aggregation of ADSCs into 3D multicellular spheroids can increase the anti-inflammatory activity of stem cells after transplantation, avoid inflammatory damage caused by scaffold materials, and improve the survival rate after transplantation [164]. Nevertheless, the safety and reliability of 3D multicellular spheroids and their therapeutic efficacy still need to be validated in further large-scale experiments. The optimal therapeutic dose, efficacy and safety of ADSCs and ADSC-exos still need to be explored in extensive clinical trials. Although numerous studies have shown that the likelihood of MSCs causing tumorigenesis is extremely low, whether ADSCs have the risk of causing tumors after long-term culture remains to be further validated.
For cell-free therapy using ADSC-exos, the main problems should be the high clearance rate, short half-life, and easy inactivation of free ADSC-exos. In this regard, these problems can be solved in two aspects in the future: 1. The survival time and biological activity of ADSC-exos can be increased by changing the treatment conditions, for which some studies have shown that hypoxic treatment of ADSC-exos can increase its ability to promote cellular value-added and neovascularization [165]. 2. By investigating new biomaterials combined with ADSC-exos to reduce its clearance, such as Nan Hu et al. developed hypoxia-pretreated ADSC-exo (ADSC-Hexo)-embedded GelMA hydrogels (GelMA-Hexo), a hydrogel that can slowly release ADSC-exos, increasing their half-life and more significant effect on diabetic wound healing [166]. This may become a new method of clinical treatment in the future. In addition, the amounts of exosomes produced and the active substances contained in them vary among different ADSCs and the same ADSCs under various physiological conditions. So the isolation, purification and activity assay of ADSC-exos are key measures for quality assurance. There is no efficient and accurate method for the isolation and detection of ADSC-exos, and how to monitor, control and regulate their biological activity and function is also a problem to be solved.
Tissue repair and regeneration using ADSCs and ADSC-exos is a complex process and most studies to date have focused on the cellular and animal levels, with many studies based on conclusions obtained from animal models, but the human in vivo environment is more complex and therefore more clinical reports are needed to provide evidence for the clinical application of ADSCs.
Although many exogenous molecules and stimuli have been found to promote the differentiation of ADSCs into new tissue cells and enhance the tissue repair function of ADSCs, such as various metal ions can promote the differentiation of ADSCs into bone tissue cells, the specific molecular mechanisms still need to be further explored. Various scaffold materials have disadvantages, such as immune rejection, low degradation rate, and poor differentiation induction of ADSCs. The development of scaffolds with higher biological efficacy remains a challenging task in the future. These gaps will be the focus of further research in this field, and addressing these issues is crucial for the wide application of ADSCs in the clinic and ensuring their safety in the future.
Funding
This study was supported by grants from the National Natural Science Foundation of China [82070704, 82270708].
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Lei Dong designed and wrote the manuscript. Xiaoyu Li, Wenyuan Leng, Zhenke Guo, Tianyu Cai, Chunru Xu, Xing Ji and Zhenpeng Zhu searched the literature and revised the manuscript. Jian Lin supervised the study. All authors contributed to the article and approved the submitted version.
Code availability
Not applicable.
Declaration of competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
Not applicable.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Z Y., S S., R X., et al. Supramolecular adhesive hydrogels for tissue engineering applications. Chem Rev. 2022;122:5604–5640. doi: 10.1021/acs.chemrev.1c00815. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. 2006/08/15. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez-Fuentes D.E., Fernández-Garza L.E., Samia-Meza J.A., et al. Mesenchymal stem cells current clinical applications: a systematic review. Arch Med Res. 2021;52:93–101. doi: 10.1016/j.arcmed.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 4.H Y., Z M., L Z., et al. Bone marrow mesenchymal stem cells in premature ovarian failure: mechanisms and prospects. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.997808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yh W., Cz Z., Ry W., et al. The crosstalk between macrophages and bone marrow mesenchymal stem cells in bone healing. Stem Cell Res Ther. 2022;13:511. doi: 10.1186/s13287-022-03199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazini L., Rochette L., Admou B., et al. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 2020 doi: 10.3390/ijms21041306. 21 2020/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C., Wei S., Xu Q., et al. Application of ADSCs and their exosomes in scar prevention. Stem Cell Rev Rep. 2022;18:952–967. doi: 10.1007/s12015-021-10252-5. 2021/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challapalli R.S., Dwyer R.M., McInerney N., et al. Effect of breast cancer and adjuvant therapy on adipose-derived stromal cells: implications for the role of ADSCs in regenerative strategies for breast reconstruction. Stem Cell Rev Rep. 2021;17:523–538. doi: 10.1007/s12015-020-10038-1. 2020/09/16. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J., Liu Y., Chen Y., et al. Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cell Int. 2020;2020 doi: 10.1155/2020/8810813. 2021/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulos S.P., Hausman D.B., Hausman G.J. The development and endocrine functions of adipose tissue. Mol Cell Endocrinol. 2010;323:20–34. doi: 10.1016/j.mce.2009.12.011. 2009/12/23. [DOI] [PubMed] [Google Scholar]
- 11.Li K., Shi G., Lei X., et al. Age-related alteration in characteristics, function, and transcription features of ADSCs. Stem Cell Res Ther. 2021;12:473. doi: 10.1186/s13287-021-02509-0. 2021/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ugarte D.A., Alfonso Z., Zuk P.A., et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol Lett. 2003;89:267–270. doi: 10.1016/S0165-2478(03)00108-1. Article. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q., Piao C., Xu J., et al. ADSCs-exo attenuates hepatic ischemia-reperfusion injury after hepatectomy by inhibiting endoplasmic reticulum stress and inflammation. J Cell Physiol. 2023;238:659–669. doi: 10.1002/jcp.30968. 2023/02/14. [DOI] [PubMed] [Google Scholar]
- 14.Sun R., Gao D.S., Shoush J., et al. The IL-1 family in tumorigenesis and antitumor immunity. Semin Cancer Biol. 2022;86:280–295. doi: 10.1016/j.semcancer.2022.05.002. 2022/05/18. [DOI] [PubMed] [Google Scholar]
- 15.Kang S., Narazaki M., Metwally H., et al. Historical overview of the interleukin-6 family cytokine. J Exp Med. 2020:217. doi: 10.1084/jem.20190347. 2020/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saraiva M., Vieira P., O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2020;217 doi: 10.1084/jem.20190418. 2019/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W., Zhang J., Zhang X., et al. VAP-PLGA microspheres (VAP-PLGA) promote adipose-derived stem cells (ADSCs)-induced wound healing in chronic skin ulcers in mice via PI3K/Akt/HIF-1α pathway. Bioengineered. 2021;12:10264–10284. doi: 10.1080/21655979.2021.1990193. 2021/11/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morikawa M., Derynck R., Miyazono K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology. Cold Spring Harbor Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021873. 2016/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apte R.S., Chen D.S., Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi: 10.1016/j.cell.2019.01.021. 2019/03/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molina E.R., Chim L.K., Lamhamedi-Cherradi S.E., et al. Correlation of nuclear pIGF-1R/IGF-1R and YAP/TAZ in a tissue microarray with outcomes in osteosarcoma patients. Oncotarget. 2022;13:521–533. doi: 10.18632/oncotarget.28215. 2022/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan H., Huang W., Wang Z., et al. The ACE2-ang-(1-7)-mas Axis modulates M1/M2 macrophage polarization to relieve CLP-induced inflammation via TLR4-mediated NF-кb and MAPK pathways. J Inflamm Res. 2021;14:2045–2060. doi: 10.2147/jir.S307801. 2021/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertolini F., Lohsiriwat V., Petit J.Y., et al. Adipose tissue cells, lipotransfer and cancer: a challenge for scientists, oncologists and surgeons. Biochim Biophys Acta. 2012;1826:209–214. doi: 10.1016/j.bbcan.2012.04.004. 2012/05/02. [DOI] [PubMed] [Google Scholar]
- 23.Arya S.B., Collie S.P., Parent C.A. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2023 doi: 10.1016/j.tcb.2023.06.006. 2023/07/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y., Li J., Jia C., et al. Therapeutic applications of adipose cell-free derivatives: a review. Stem Cell Res Ther. 2020;11 doi: 10.1186/s13287-020-01831-3. 312. 2020/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong P., Yang H., Wu Y., et al. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: a comprehensive review. Stem Cell Res Ther. 2019;10:242. doi: 10.1186/s13287-019-1358-y. 2019/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueroa V., Rodríguez M.S., Lanari C., et al. Nuclear action of FGF members in endocrine-related tissues and cancer: interplay with steroid receptor pathways. Steroids. 2019;152 doi: 10.1016/j.steroids.2019.108492. 2019/09/13. [DOI] [PubMed] [Google Scholar]
- 28.Forbes B.E., Blyth A.J., Wit J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol Cell Endocrinol. 2020;518 doi: 10.1016/j.mce.2020.111035. 2020/09/18. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos N., Lennartsson J. The PDGF/PDGFR pathway as a drug target. Mol Aspect Med. 2018;62:75–88. doi: 10.1016/j.mam.2017.11.007. 2017/11/16. [DOI] [PubMed] [Google Scholar]
- 30.Airuddin S.S., Halim A.S., Wan Sulaiman W.A., et al. Adipose-derived stem cell: "treat or trick". Biomedicines. 2021;9 doi: 10.3390/biomedicines9111624. 2021/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glovinski P.V., Herly M., Mathiasen A.B., et al. Overcoming the bottleneck of platelet lysate supply in large-scale clinical expansion of adipose-derived stem cells: a comparison of fresh versus three types of platelet lysates from outdated buffy coat-derived platelet concentrates. Cytotherapy. 2017;19:222–234. doi: 10.1016/j.jcyt.2016.10.014. 2016/11/27. [DOI] [PubMed] [Google Scholar]
- 32.Babu S., Krishnan M., Panneerselvam A., et al. A comprehensive review on therapeutic application of mesenchymal stem cells in neuroregeneration. Life Sci. 2023;327:121785. doi: 10.1016/j.lfs.2023.121785. 2023/05/18. [DOI] [PubMed] [Google Scholar]
- 33.Zhou R., Zheng B. [Research advance of on the support effect of adipose tissue-derived stem cell on hematopoietic stem/progenitor cell--review] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2021;29:301–305. doi: 10.19746/j.cnki.issn.1009-2137.2021.01.051. 2021/02/09. [DOI] [PubMed] [Google Scholar]
- 34.Zhou W., Lin J., Zhao K., et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med. 2019;47:1722–1733. doi: 10.1177/0363546519848678. 2019/05/18. [DOI] [PubMed] [Google Scholar]
- 35.Lee E.J., Kasper F.K., Mikos A.G. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42:323–337. doi: 10.1007/s10439-013-0859-6. 2013/07/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson R.A. Bone tissue: composition and function. Johns Hopkins Med J. 1979;145:10–24. 1979/07/01. [PubMed] [Google Scholar]
- 37.Pigossi S.C., Medeiros M.C., Saska S., et al. Role of osteogenic growth peptide (OGP) and OGP(10-14) in bone regeneration: a review. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111885. 2016/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hou H.W., Xue P., Wang Y., et al. Liraglutide regulates proliferation, differentiation, and apoptosis of preosteoblasts through a signaling network of Notch/Wnt/Hedgehog signaling pathways. Eur Rev Med Pharmacol Sci. 2020;24:12408–12422. doi: 10.26355/eurrev_202012_24037. 2020/12/19. [DOI] [PubMed] [Google Scholar]
- 39.Yao X.W., Liu H.D., Ren M.X., et al. Aloe polysaccharide promotes osteogenesis potential of adipose-derived stromal cells via BMP-2/Smads and prevents ovariectomized-induced osteoporosis. Mol Biol Rep. 2022 doi: 10.1007/s11033-022-08003-x. 2022/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamoto S., Yoshikawa H., Nakata K. Axial mechanical loading to ex vivo mouse long bone regulates endochondral ossification and endosteal mineralization through activation of the BMP-Smad pathway during postnatal growth. BoneKEy Rep. 2021;15 doi: 10.1016/j.bonr.2021.101088. 2021/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byun H., Jang G.N., Lee J., et al. Stem cell spheroid engineering with osteoinductive and ROS scavenging nanofibers for bone regeneration. Biofabrication. 2021 doi: 10.1088/1758-5090/abd56c. 13 2020/12/22. [DOI] [PubMed] [Google Scholar]
- 42.Soleimanifar F., Hosseini F.S., Atabati H., et al. Adipose-derived stem cells-conditioned medium improved osteogenic differentiation of induced pluripotent stem cells when grown on polycaprolactone nanofibers. J Cell Physiol. 2019;234:10315–10323. doi: 10.1002/jcp.27697. 2018/11/01. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y., Gu Z., Liu J., et al. Arginine based poly (ester amide)/hyaluronic acid hybrid hydrogels for bone tissue Engineering. Carbohydr Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115640. 2020/01/01. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J., Liu Z., Li Y., et al. FGF2: a key regulator augmenting tendon-to-bone healing and cartilage repair. Regen Med. 2020;15:2129–2142. doi: 10.2217/rme-2019-0080. 2020/11/18. [DOI] [PubMed] [Google Scholar]
- 45.Shafaei H., Kalarestaghi H. Adipose-derived stem cells: an appropriate selection for osteogenic differentiation. J Cell Physiol. 2020;235:8371–8386. doi: 10.1002/jcp.29681. 2020/04/03. [DOI] [PubMed] [Google Scholar]
- 46.Safavi A.S., Rouhi G., Haghighipour N., et al. Efficacy of mechanical vibration in regulating mesenchymal stem cells gene expression. Vitro Anim Cell Dev Biol. 2019;55:387–394. doi: 10.1007/s11626-019-00340-9. [DOI] [PubMed] [Google Scholar]
- 47.Glenske K., Donkiewicz P., Köwitsch A., et al. Applications of metals for bone regeneration. Int J Mol Sci. 2018;19:826. doi: 10.3390/ijms19030826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X., Yang S., Tong S., et al. Study on exosomes promoting the osteogenic differentiation of ADSCs in graphene porous titanium alloy scaffolds. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.905511. 2022/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Z., Sun W., Guo Z., et al. Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci. 2020;254 doi: 10.1016/j.lfs.2019.116900. 2019/12/02. [DOI] [PubMed] [Google Scholar]
- 50.Jeppesen D.K., Fenix A.M., Franklin J.L., et al. Reassessment of exosome composition. Cell. 2019;177:428–445. doi: 10.1016/j.cell.2019.02.029. e418. 2019/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petrenko Y., Syková E., Kubinová Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8:94. doi: 10.1186/s13287-017-0558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bei H.P., Hung P.M., Yeung H.L., et al. Bone-a-Petite: engineering exosomes towards bone, osteochondral, and cartilage repair. Small. 2021;17 doi: 10.1002/smll.202101741. 2021/07/22. [DOI] [PubMed] [Google Scholar]
- 53.Ho-Shui-Ling A., Bolander J., Rustom L.E., et al. Bone regeneration strategies: engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials. 2018;180:143–162. doi: 10.1016/j.biomaterials.2018.07.017. 2018/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu T., Zhang H., Yu W., et al. The combination of concentrated growth factor and adipose-derived stem cell sheet repairs skull defects in rats. Tissue Eng Regen Med. 2021;18:905–913. doi: 10.1007/s13770-021-00371-y. 2021/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao G., Dong H., Wu R., et al. 3D-printed regenerative polycaprolactone/silk fibroin osteogenic and chondrogenic implant for treatment of hip dysplasia. Biochem Biophys Res Commun. 2022;636:96–104. doi: 10.1016/j.bbrc.2022.10.046. 2022/11/05. [DOI] [PubMed] [Google Scholar]
- 56.Thesleff T., Lehtimäki K., Niskakangas T., et al. Cranioplasty with adipose-derived stem cells, beta-tricalcium phosphate granules and supporting mesh: six-year clinical follow-up results. Stem Cells Transl Med. 2017;6:1576–1582. doi: 10.1002/sctm.16-0410. 2017/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y., Huang L., Yuan W., et al. Sustained release of stromal cell-derived factor-1 alpha from silk fibroin microfiber promotes urethral reconstruction in rabbits. J Biomed Mater Res. 2020;108:1760–1773. doi: 10.1002/jbm.a.36943. 2020/04/11. [DOI] [PubMed] [Google Scholar]
- 58.Song B., Fang L., Mao X., et al. Gelatin-grafted tubular asymmetric scaffolds promote ureteral regeneration via activation of the integrin/Erk signaling pathway. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1092543. 2023/01/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Z., Yu H., Xiao F., et al. Differentiation of adipose-derived stem cells promotes regeneration of smooth muscle for ureteral tissue engineering. J Surg Res. 2012;178:55–62. doi: 10.1016/j.jss.2012.01.047. [DOI] [PubMed] [Google Scholar]
- 60.Li Y., Shan Z., Yang B., et al. Cathelicidin LL37 promotes epithelial and smooth-muscle-like differentiation of adipose-derived stem cells through the Wnt/β-catenin and NF-κB pathways. Biochemistry (Moscow) 2017;82:1336–1345. doi: 10.1134/S0006297917110116. [DOI] [PubMed] [Google Scholar]
- 61.Verla W., Oosterlinck W., Spinoit A.F., et al. A comprehensive review emphasizing anatomy, etiology, diagnosis, and treatment of male urethral stricture disease. BioMed Res Int. 2019;2019 doi: 10.1155/2019/9046430. 9046430. 2019/05/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y., Wang G., Hou X., et al. Urethral tissue reconstruction using the acellular dermal matrix patch modified with collagen-binding VEGF in beagle urethral injury models. BioMed Res Int. 2021;2021:5502740. doi: 10.1155/2021/5502740. 2021/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Nasrin A., Hassan M., Mirabet M.M., et al. 3D-printed bioresorbable poly(lactic-co-glycolic acid) and quantum-dot nanocomposites: scaffolds for enhanced bone mineralization and inbuilt co-monitoring. J Biomed Mater Res. 2022;110:916–927. doi: 10.1002/jbm.a.37340. 2021/12/10. [DOI] [PubMed] [Google Scholar]
- 64.Zhang J., Yi Y., Yang S., et al. [Effects of adipose-derived stem cell released exosomes on proliferation, migration, and tube-like differentiation of human umbilical vein endothelial cells] Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32:1351–1357. doi: 10.7507/1002-1892.201804016. 2019/01/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun Y., Xiong X., Wang X. The miR-590-3p/VEGFA axis modulates secretion of VEGFA from adipose-derived stem cells, which acts as a paracrine regulator of human dermal microvascular endothelial cell angiogenesis. Hum Cell. 2020;33:479–489. doi: 10.1007/s13577-019-00315-8. 2020/04/12. [DOI] [PubMed] [Google Scholar]
- 66.Kinnaird T., Stabile E., Burnett M.S., et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.Res.0000118601.37875.Ac. 2004/01/24. [DOI] [PubMed] [Google Scholar]
- 67.Rehman J., Traktuev D., Li J., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.Cir.0000121425.42966.F1. 2004/03/03. [DOI] [PubMed] [Google Scholar]
- 68.Xiao D., Yang M., Zhang M., et al. MicroRNA-126 from stem cell extracellular vesicles encapsulated in a tri-layer hydrogel scaffold promotes bladder angiogenesis by activating CXCR4/SDF-1α pathway. Chem Eng J. 2021;425 doi: 10.1016/j.cej.2021.131624. [DOI] [Google Scholar]
- 69.Mantsounga C.S., Lee C., Neverson J., et al. Macrophage IL-1β promotes arteriogenesis by autocrine STAT3- and NF-κB-mediated transcription of pro-angiogenic VEGF-A. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110309. 2022/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farooq M., Khan A.W., Kim M.S., et al. The role of fibroblast growth factor (FGF) signaling in tissue repair and regeneration. Cells. 2021;10 doi: 10.3390/cells10113242. 2021/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Narla S.T., Bushnell D.S., Schaefer C.M., et al. Loss of fibroblast growth factor receptor 2 (FGFR2) leads to defective bladder urothelial regeneration after cyclophosphamide injury. Am J Pathol. 2021;191:631–651. doi: 10.1016/j.ajpath.2020.12.011. 2021/01/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marofi F., Alexandrovna K.I., Margiana R., et al. MSCs and their exosomes: a rapidly evolving approach in the context of cutaneous wounds therapy. Stem Cell Res Ther. 2021;12:597. doi: 10.1186/s13287-021-02662-6. 2021/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.An Y., Zhao J., Nie F., et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9 doi: 10.1038/s41598-019-49339-y. 2019/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang C., Luo L., Bai X., et al. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch Biochem Biophys. 2020;681 doi: 10.1016/j.abb.2020.108259. 2020/01/12. [DOI] [PubMed] [Google Scholar]
- 75.Xiao M., Zheng L., Zhang X., et al. Renal-on-Chip microfluidic platform with a force-sensitive resistor (ROC-FS) for molecular pathogenesis analysis of hydronephrosis. Anal Chem. 2022;94:748–757. doi: 10.1021/acs.analchem.1c03155. 2021/12/25. [DOI] [PubMed] [Google Scholar]
- 76.Siregar S., Noegroho B.S., Karim M.I. The effect of intravenous human adipose-derived stem cells (hADSC) on transforming growth factor β1 (TGF-β1), collagen type 1, and kidney histopathological features in the unilateral ureteropelvic junction obstruction model of wistar rats. Turk J Urol. 2020;46:236–242. doi: 10.5152/tud.2020.20024. 2020/05/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gharbia S., Nazarie S.R., Dinescu S., et al. Adipose-derived stem cells (ADSCs) supplemented with hepatocyte growth factor (HGF) attenuate hepatic stellate cell activation and liver fibrosis by inhibiting the TGF-β/smad signaling pathway in chemical-induced liver fibrosis associated with diabetes. Cells. 2022;11 doi: 10.3390/cells11213338. 2022/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tran C., Damaser M.S. The potential role of stem cells in the treatment of urinary incontinence. Ther Adv Urol. 2015;7:22–40. doi: 10.1177/1756287214553968. 2015/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nyambat B., Manga Y.B., Chen C.H., et al. New insight into natural extracellular matrix: genipin cross-linked adipose-derived stem cell extracellular matrix gel for tissue engineering. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21144864. 2020/2107/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xuan Z., Zachar V., Pennisi C.P. Sources, selection, and microenvironmental preconditioning of cells for urethral tissue engineering. Int J Mol Sci. 2022;23 doi: 10.3390/ijms232214074. 2022/11/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gholipourmalekabadi M., Sapru S., Samadikuchaksaraei A., et al. Silk fibroin for skin injury repair: where do things stand? Adv Drug Deliv Rev. 2020;153:28–53. doi: 10.1016/j.addr.2019.09.003. 2019/11/05. [DOI] [PubMed] [Google Scholar]
- 82.da Silva L.C.E., Gonçalves M.D.C., de Oliveira M.G. Nitric oxide-releasing supramolecular cellulose nanocrystal/silsesquioxane foams. Macromol Rapid Commun. 2022;43 doi: 10.1002/marc.202100930. 2022/03/11. [DOI] [PubMed] [Google Scholar]
- 83.Raya-Rivera A., Esquiliano D.R., Yoo J.J., et al. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377:1175–1182. doi: 10.1016/s0140-6736(10)62354-9. 2011/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogawa N., Imamura T., Minagawa T., et al. Autologous bilayered adipose-derived mesenchymal cell-gelatin sheets reconstruct ureters in rabbits. Tissue Eng. 2022;28:855–866. doi: 10.1089/ten.TEA.2022.0087. 2022/07/20. [DOI] [PubMed] [Google Scholar]
- 85.Qian L., Pi L., Fang B.R., et al. Adipose mesenchymal stem cell-derived exosomes accelerate skin wound healing via the lncRNA H19/miR-19b/SOX9 axis. Lab Invest. 2021;101:1254–1266. doi: 10.1038/s41374-021-00611-8. 2021/05/29. [DOI] [PubMed] [Google Scholar]
- 86.Zhang W., Bai X., Zhao B., et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370:333–342. doi: 10.1016/j.yexcr.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 87.Qiu H., Liu S., Wu K., et al. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: a review. J Cosmet Dermatol. 2020;19:574–581. doi: 10.1111/jocd.13215. [DOI] [PubMed] [Google Scholar]
- 88.Lu Y., Wen H., Huang J., et al. Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J Cell Mol Med. 2020;24:9590–9604. doi: 10.1111/jcmm.15387. 2020/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma T., Fu B., Yang X., et al. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J Cell Biochem. 2019;120:10847–10854. doi: 10.1002/jcb.28376. 2019/01/27. [DOI] [PubMed] [Google Scholar]
- 90.Li X., Xie X., Lian W., et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1–14. doi: 10.1038/s12276-018-0058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fujiwara O., Prasai A., Perez-Bello D., et al. Adipose-derived stem cells improve grafted burn wound healing by promoting wound bed blood flow. Burns & Trauma. 2020;8 doi: 10.1093/burnst/tkaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Roles of exosomes from mesenchymal stem cells in treating osteoarthritis. Cell Reprogr. 2020;22:107–117. doi: 10.1089/cell.2019.0098. [DOI] [PubMed] [Google Scholar]
- 93.Enciso N., Avedillo L., Fermín M.L., et al. Cutaneous wound healing: canine allogeneic ASC therapy. Stem Cell Res Ther. 2020;11:261. doi: 10.1186/s13287-020-01778-5. 2020/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.LeBlanc K., Langemo D., Woo K., et al. Skin tears: prevention and management. Br J Community Nurs. 2019;24 doi: 10.12968/bjcn.2019.24.Sup9.S1. S12–s18. 2019/09/04. [DOI] [PubMed] [Google Scholar]
- 95.Yannas I.V., Tzeranis D.S., So P.T.C. Regeneration of injured skin and peripheral nerves requires control of wound contraction, not scar formation. Wound Repair Regen. 2017;25:177–191. doi: 10.1111/wrr.12516. 2017/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zarei F., Abbaszadeh A. Application of cell therapy for anti-aging facial skin. Curr Stem Cell Res Ther. 2019;14:244–248. doi: 10.2174/1574888x13666181113113415. 2018/11/14. [DOI] [PubMed] [Google Scholar]
- 97.Stachura A., Paskal W., Pawlik W., et al. The use of adipose-derived stem cells (ADSCs) and stromal vascular fraction (SVF) in skin scar treatment-A systematic review of clinical studies. J Clin Med. 2021;10 doi: 10.3390/jcm10163637. 2021/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moon K.C., Suh H.S., Kim K.B., et al. Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes. 2019;68:837–846. doi: 10.2337/db18-0699. 2019/01/27. [DOI] [PubMed] [Google Scholar]
- 99.Zhou L., Wang H., Yao S., et al. Efficacy of human adipose derived mesenchymal stem cells in promoting skin wound healing. J Healthc Eng. 2022;2022:6590025. doi: 10.1155/2022/6590025. 6592022/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100.Zhou B.R., Zhang T., Bin Jameel A.A., et al. The efficacy of conditioned media of adipose-derived stem cells combined with ablative carbon dioxide fractional resurfacing for atrophic acne scars and skin rejuvenation. J Cosmet Laser Ther. 2016;18:138–148. doi: 10.3109/14764172.2015.1114638. 2016/01/07. [DOI] [PubMed] [Google Scholar]
- 101.Lin S., He X., He Y. Co-culture of ASCs/EPCs and dermal extracellular matrix hydrogel enhances the repair of full-thickness skin wound by promoting angiogenesis. Stem Cell Res Ther. 2021;12:129. doi: 10.1186/s13287-021-02203-1. 2021/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou X., Ning K., Ling B., et al. Multiple injections of autologous adipose-derived stem cells accelerate the burn wound healing process and promote blood vessel regeneration in a rat model. Stem Cell Dev. 2019;28:1463–1472. doi: 10.1089/scd.2019.0113. 2019/09/19. [DOI] [PubMed] [Google Scholar]
- 103.Vriend L., van Dongen J.A., Sinkunas V., et al. Limited efficacy of adipose stromal cell secretome-loaded skin-derived hydrogels to augment skin flap regeneration in rats. Stem Cell Dev. 2022;31:630–640. doi: 10.1089/scd.2022.0003. 2022/05/19. [DOI] [PubMed] [Google Scholar]
- 104.Spicer C.D. Hydrogel scaffolds for tissue engineering: the importance of polymer choice. Polym Chem. 2020;11:184–219. doi: 10.1039/C9PY01021A. 10.1039/C9PY01021A. [DOI] [Google Scholar]
- 105.Hickey R.J., Pelling A.E. Cellulose biomaterials for tissue engineering. Front Bioeng Biotechnol. 2019;7:45. doi: 10.3389/fbioe.2019.00045. 2019/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iwai R., Nemoto Y., Nakayama Y. Preparation and characterization of directed, one-day-self-assembled millimeter-size spheroids of adipose-derived mesenchymal stem cells. J Biomed Mater Res. 2016;104:305–312. doi: 10.1002/jbm.a.35568. 2015/09/20. [DOI] [PubMed] [Google Scholar]
- 107.Nagano H., Suematsu Y., Takuma M., et al. Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds. Sci Rep. 2021;11 doi: 10.1038/s41598-021-93642-6. 2021/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nocera G., Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci. 2020;77:3977–3989. doi: 10.1007/s00018-020-03516-9. 2020/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li R., Li D., Wu C., et al. Nerve growth factor activates autophagy in Schwann cells to enhance myelin debris clearance and to expedite nerve regeneration. Theranostics. 2020;10:1649–1677. doi: 10.7150/thno.40919. 2020/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bolívar S., Navarro X., Udina E. Schwann cell role in selectivity of nerve regeneration. Cells. 2020;9 doi: 10.3390/cells9092131. 2020/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Askar P., Xu J., Hu J., et al. The Etv1/Er81 transcription factor coordinates myelination-related genes to regulate Schwann cell differentiation and myelination. Ann Transl Med. 2022;10:875. doi: 10.21037/atm-22-3489. 2022/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gordon T. Peripheral nerve regeneration and muscle reinnervation. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21228652. 2020/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mikhailova M.M., Sydoruk K.V., Davydova L.I., et al. Nonwoven spidroin materials as scaffolds for ex vivo cultivation of aortic fragments and dorsal root ganglia. J Biomater Sci Polym Ed. 2022;33:1685–1703. doi: 10.1080/09205063.2022.2073426. 2022/05/03. [DOI] [PubMed] [Google Scholar]
- 114.Fang Y., Zhang Y., Zhou J., et al. Adipose-derived mesenchymal stem cell exosomes: a novel pathway for tissues repair. Cell Tissue Bank. 2019;20:153–161. doi: 10.1007/s10561-019-09761-y. [DOI] [PubMed] [Google Scholar]
- 115.Wu H., Peng Z., Xu Y., et al. Engineered adipose-derived stem cells with IGF-1-modified mRNA ameliorates osteoarthritis development. Stem Cell Res Ther. 2022;13:19. doi: 10.1186/s13287-021-02695-x. 2022/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pak C.S., Heo C.Y., Shin J., et al. Effects of a catechol-functionalized hyaluronic acid patch combined with human adipose-derived stem cells in diabetic wound healing. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22052632. 2021/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chiang B.J., Liao C.H., Mao S.H., et al. Adipose-derived stem cells and their derived microvesicles ameliorate detrusor overactivity secondary to bilateral partial iliac arterial occlusion-induced bladder ischemia. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22137000. 2021/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo S., Wang T., Zhang S., et al. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol Cell Biochem. 2020;463:67–78. doi: 10.1007/s11010-019-03630-8. 2019/10/12. [DOI] [PubMed] [Google Scholar]
- 119.Melnik S., Gabler J., Dreher S.I., et al. MiR-218 affects hypertrophic differentiation of human mesenchymal stromal cells during chondrogenesis via targeting RUNX2, MEF2C, and COL10A1. Stem Cell Res Ther. 2020;11:532. doi: 10.1186/s13287-020-02026-6. 2020/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ko Y.G., Kim Y.J., Park W.H., et al. Surface modification of PHBV nanofiber mats for rapid cell cultivation and harvesting. J Biomater Sci Polym Ed. 2018;29:1026–1041. doi: 10.1080/09205063.2017.1414481. 2017/12/08. [DOI] [PubMed] [Google Scholar]
- 121.Liu Y., Dong R., Zhang C., et al. Therapeutic effects of nerve leachate-treated adipose-derived mesenchymal stem cells on rat sciatic nerve injury. Exp Ther Med. 2020;19:223–231. doi: 10.3892/etm.2019.8203. 2019/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Erdal A.I., Findikçioğlu K., Karasu O., et al. Use of erythropoietin and fibrin glue mixture for peripheral nerve repair. Plast Reconstr Surg. 2022;149:395–403. doi: 10.1097/prs.0000000000008796. 2021/12/14. [DOI] [PubMed] [Google Scholar]
- 123.Entekhabi E., Haghbin Nazarpak M., Shafieian M., et al. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J Biomed Mater Res. 2021;109:300–312. doi: 10.1002/jbm.a.37023. 2020/06/04. [DOI] [PubMed] [Google Scholar]
- 124.Pedrini F.A., Boriani F., Bolognesi F., et al. Cell-Enhanced acellular nerve allografts for peripheral nerve reconstruction: a systematic review and a meta-analysis of the literature. Neurosurgery. 2019;85:575–604. doi: 10.1093/neuros/nyy374. 2018/09/25. [DOI] [PubMed] [Google Scholar]
- 125.Pan D., Hunter D.A., Schellhardt L., et al. The accumulation of T cells within acellular nerve allografts is length-dependent and critical for nerve regeneration. Exp Neurol. 2019;318:216–231. doi: 10.1016/j.expneurol.2019.05.009. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fu X.M., Wang Y., Fu W.L., et al. The combination of adipose-derived schwann-like cells and acellular nerve allografts promotes sciatic nerve regeneration and repair through the JAK2/STAT3 signaling pathway in rats. Neuroscience. 2019;422:134–145. doi: 10.1016/j.neuroscience.2019.10.018. 2019/11/05. [DOI] [PubMed] [Google Scholar]
- 127.Razavi S., Jahromi M., Vatankhah E., et al. Differential effects of rat ADSCs encapsulation in fibrin matrix and combination delivery of BDNF and Gold nanoparticles on peripheral nerve regeneration. BMC Neurosci. 2021;22:50. doi: 10.1186/s12868-021-00655-y. 2021/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang L., Zhao Y., Pan X., et al. Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Res. 2021;1750 doi: 10.1016/j.brainres.2020.147121. 2020/09/14. [DOI] [PubMed] [Google Scholar]
- 129.Zheng T., Zhang T., Zhang W., et al. Icariside II facilitates the differentiation of ADSCs to schwann cells and restores erectile dysfunction through regulation of miR-33/GDNF axis. Biomed Pharmacother. 2020;125 doi: 10.1016/j.biopha.2020.109888. 2020/02/18. [DOI] [PubMed] [Google Scholar]
- 130.Prautsch K.M., Schmidt A., Paradiso V., et al. Modulation of human adipose stem cells' neurotrophic capacity using a variety of growth factors for neural tissue engineering applications: axonal growth, transcriptional, and phosphoproteomic analyses in vitro. Cells. 2020;9 doi: 10.3390/cells9091939. 2020/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang R., Boareto M., Engler A., et al. Id4 downstream of Notch2 maintains neural stem cell quiescence in the adult Hippocampus. Cell Rep. 2019;28:1485–1498.e1486. doi: 10.1016/j.celrep.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 132.Yang Z., Yang Y., Xu Y., et al. Biomimetic nerve guidance conduit containing engineered exosomes of adipose-derived stem cells promotes peripheral nerve regeneration. Stem Cell Res Ther. 2021;12:442. doi: 10.1186/s13287-021-02528-x. 2021/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Künzel U., Grieve A.G., Meng Y., et al. FRMD8 promotes inflammatory and growth factor signalling by stabilising the iRhom/ADAM17 sheddase complex. Elife. 2018;7 doi: 10.7554/eLife.35012. 2018/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Künzel U., Grieve A.G., Meng Y., et al. FRMD8 promotes inflammatory and growth factor signalling by stabilising the iRhom/ADAM17 sheddase complex. Elife. 2018;7 doi: 10.7554/eLife.35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y., Yu T., Hu F. Hypocapnia stimuli-responsive engineered exosomes delivering miR-218 facilitate sciatic nerve regeneration. Front Bioeng Biotechnol. 2022;10:825146. doi: 10.3389/fbioe.2022.825146. 2022/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petsoglou C., Wen L., Hoque M., et al. Effects of human platelet lysate on the growth of cultured human corneal endothelial cells. Exp Eye Res. 2021;208 doi: 10.1016/j.exer.2021.108613. 2021/05/14. [DOI] [PubMed] [Google Scholar]
- 137.Li T., Lu H., Zhou L., et al. Growth factors-based platelet lysate rejuvenates skin against ageing through NF-κB signalling pathway: in vitro and in vivo mechanistic and clinical studies. Cell Prolif. 2022;55 doi: 10.1111/cpr.13212. 2022/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lischer M., di Summa P.G., Oranges C.M., et al. Human platelet lysate stimulated adipose stem cells exhibit strong neurotrophic potency for nerve tissue engineering applications. Regen Med. 2020;15:1399–1408. doi: 10.2217/rme-2020-0031. 2020/04/21. [DOI] [PubMed] [Google Scholar]
- 139.Prautsch K.M., Degrugillier L., Schaefer D.J., et al. Ex-vivo stimulation of adipose stem cells by growth factors and fibrin-hydrogel assisted delivery strategies for treating nerve gap-injuries. Bioengineering. 2020;7 doi: 10.3390/bioengineering7020042. 2020/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Castelnovo L.F., Thomas P. Membrane progesterone receptor α (mPRα/PAQR7) promotes migration, proliferation and BDNF release in human Schwann cell-like differentiated adipose stem cells. Mol Cell Endocrinol. 2021;531 doi: 10.1016/j.mce.2021.111298. 2021/05/01. [DOI] [PubMed] [Google Scholar]
- 141.Wang W., Gu M.F., Wang Z.F., et al. Let-7a-5p regulated by lncRNA-MEG3 promotes functional differentiation to Schwann cells from adipose derived stem cells via directly inhibiting RBPJ-mediating Notch pathway. Apoptosis. 2021;26:548–560. doi: 10.1007/s10495-021-01685-x. 2021/08/20. [DOI] [PubMed] [Google Scholar]
- 142.Couve E., Lovera M., Suzuki K., et al. Schwann cell phenotype changes in aging human dental pulp. J Dent Res. 2018;97:347–355. doi: 10.1177/0022034517733967. 2017/10/04. [DOI] [PubMed] [Google Scholar]
- 143.Zhang H., Wu J., Shen F.F., et al. Activated Schwann cells and increased inflammatory cytokines IL-1β, IL-6, and TNF-α in patients' sural nerve are lack of tight relationship with specific sensory disturbances in Parkinson's disease. CNS Ne-urosci Ther. 2020;26:518–526. doi: 10.1111/cns.13282. 2019/12/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Masuda H., Nemoto M., Okonogi S., et al. Utility of Schwann/2E and Sox10 in distinguishing CD57-negative olfactory groove schwannoma from olfactory ensheathing cell tumor: a case report and review of the literature. Neuropathology. 2020;40:373–378. doi: 10.1111/neup.12650. 2020/04/18. [DOI] [PubMed] [Google Scholar]
- 145.Gonçalves N.P., Jager S.E., Richner M., et al. Schwann cell p75 neurotrophin receptor modulates small fiber degeneration in diabetic neuropathy. Glia. 2020;68:2725–2743. doi: 10.1002/glia.23881. 2020/07/14. [DOI] [PubMed] [Google Scholar]
- 146.Ma Y., Zhai D., Zhang W., et al. Down-regulation of long non-coding RNA MEG3 promotes Schwann cell proliferation and migration and repairs sciatic nerve injury in rats. J Cell Mol Med. 2020;24:7460–7469. doi: 10.1111/jcmm.15368. 2020/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miller S.R., Benito C., Mirsky R., et al. Neural crest Notch/Rbpj signaling regulates olfactory gliogenesis and neuronal migration. Genesis. 2018;56 doi: 10.1002/dvg.23215. 2018/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kang Y., Liu Y., Liu Z., et al. Differentiated human adipose-derived stromal cells exhibit the phenotypic and functional characteristics of mature Schwann cells through a modified approach. Cytotherapy. 2019;21:987–1003. doi: 10.1016/j.jcyt.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 149.Guennoun R., Benmessahel Y., Delespierre B., et al. Progesterone stimulates Krox-20 gene expression in Schwann cells. Mol Brain Res. 2001;90:75–82. doi: 10.1016/S0169-328X(01)00094-8. Article. [DOI] [PubMed] [Google Scholar]
- 150.Sawai S., Kishida T., Kotani S.I., et al. ALK5 i II accelerates induction of adipose-derived stem cells toward schwann cells through a non-smad signaling pathway. Stem Cell Int. 2021;2021:8307797. doi: 10.1155/2021/8307797. 2021/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen S., Ikemoto T., Tokunaga T., et al. Effective in vitro differentiation of adipose-derived stem cells into Schwann-like cells with folic acid supplementation. J Med Invest. 2021;68:347–353. doi: 10.2152/jmi.68.347. 2021/11/12. [DOI] [PubMed] [Google Scholar]
- 152.Modrak M., Talukder M.A.H., Gurgenashvili K., et al. Peripheral nerve injury and myelination: potential therapeutic strategies. J Neurosci Res. 2020;98:780–795. doi: 10.1002/jnr.24538. 2019/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lopes B., Sousa P., Alvites R., et al. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. nt J Mol Sci. 2022;23 doi: 10.3390/ijms23020918. 2022/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lischer M., Summa PGd, Oranges C.M., et al. Human platelet lysate stimulated adipose stem cells exhibit strong neurotrophic potency for nerve tissue engineering applications. Regen Med. 2020;15:1399–1408. doi: 10.2217/rme-2020-0031. [DOI] [PubMed] [Google Scholar]
- 155.Schweizer R., Schnider J.T., Fanzio P.M., et al. Effect of systemic adipose-derived stem cell therapy on functional nerve regeneration in a rodent model. Plast Reconstr Surg Glob Open. 2020;8:e2953. doi: 10.1097/gox.0000000000002953. 2020/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Podsednik A., Cabrejo R., Rosen J. Adipose tissue uses in peripheral nerve surgery. Int J Mol Sci. 2022;23 doi: 10.3390/ijms23020644. 2022/01/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Syu W.Z., Hueng D.Y., Chen W.L., et al. Adipose-derived neural stem cells combined with acellular dermal matrix as a neural conduit enhances peripheral nerve repair. Cell Transplant. 2019;28:1220–1230. doi: 10.1177/0963689719853512. 2019/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen S., Ikemoto T., Tokunaga T., et al. Newly generated 3D schwann-like cell spheroids from human adipose-derived stem cells using a modified protocol. Cell Transplant. 2022;31 doi: 10.1177/09636897221093312. 9636897221093312. 2022/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhou J., Wei T., He Z. ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C m(6)A modification to improve wound healing of diabetic foot ulcers. Mol Med. 2021;27:146. doi: 10.1186/s10020-021-00406-z. 2021/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang L., Ye C., Li P., et al. ADSCs stimulated by VEGF-C alleviate intestinal inflammation via dual mechanisms of enhancing lymphatic drainage by a VEGF-C/VEGFR-3-dependent mechanism and inhibiting the NF-κB pathway by the secretome. Stem Cell Res Ther. 2022;13:448. doi: 10.1186/s13287-022-03132-3. 2022/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jin J., Shi Y., Gong J., et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res Ther. 2019;10:95. doi: 10.1186/s13287-019-1177-1. 2019/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Piao C., Zhang Q., Xu J., et al. Optimal intervention time of ADSCs for hepatic ischemia-reperfusion combined with partial resection injury in rats. Life Sci. 2021;285 doi: 10.1016/j.lfs.2021.119986. 2021/10/01. [DOI] [PubMed] [Google Scholar]
- 163.Rahman M., Wang Z.Y., Li J.X., et al. Single-cell RNA sequencing reveals the interaction of injected ADSCs with lung-originated cells in mouse pulmonary fibrosis. Stem Cell Int. 2022;2022:9483166. doi: 10.1155/2022/9483166. 2022/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Petrenko Y., Syková E., Kubinová Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res Ther. 2017;8:94. doi: 10.1186/s13287-017-0558-6. 2017/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li W., Chen X., Zou F., et al. Extracellular vesicles derived from hypoxia treated human adipose stem cells increase proliferation and angiogenic differentiation in human adipose stem cells. Aesthetic Surg J. 2023 doi: 10.1093/asj/sjad139. 2023/05/09. [DOI] [PubMed] [Google Scholar]
- 166.Hu N., Cai Z., Jiang X., et al. Hypoxia-pretreated ADSC-derived exosome-embedded hydrogels promote angiogenesis and accelerate diabetic wound healing. Acta Biomater. 2023;157:175–186. doi: 10.1016/j.actbio.2022.11.057. 2022/12/13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.