Abstract
Objective
Surgeons shy away from using the Cabrol fistula (perigraft space to right atrium [RA] shunt) due to unfamiliarity, concern for persistent left-to-right shunting, and fear of “painting-over” anastomotic defects that will result in later problems. We review nearly 2 decades of experience with application of the Cabrol fistula in a large thoracic aortic practice, with emphasis on precise surgical techniques, early and late outcomes, and detailed radiographic analysis.
Methods
Operative records of all procedures in which the Cabrol fistula was used were retrieved and analyzed, with precise review of the details of construction of each Cabrol fistula and clinical and radiologic (echocardiographic and computed tomographic) patient follow-up.
Results
The Cabrol fistula successfully controlled the bleeding in all cases. There were no late false aneurysms at any anastomotic sites. There was no continued flow through any fistula. Good perioperative and long-term survival was achieved in these complex cases that found themselves at a very dangerous crux before application of the Cabrol fistula.
Conclusions
The Cabrol fistula is an important tool for the thoracic aortic surgeon to have in the toolbox. We found the Cabrol fistula to be extremely effective at controlling bleeding, with no late persistent fistula flow and no late false aneurysm formation. Without the fistula, outcome in these patients would likely have been lethal. We recommend the Cabrol fistula technique strongly for life-saving application in rare cases of bleeding uncontrollable by conventional methods.
Key Words: intractable bleeding, Cabrol fistula, aortic root surgery, ascending aortic surgery, aortic arch surgery, hemorrhage
Graphical abstract

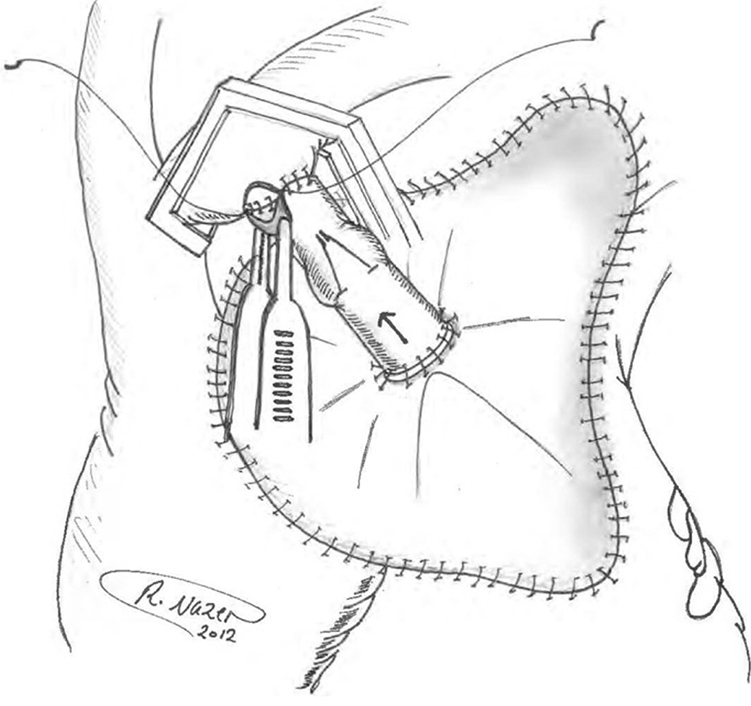
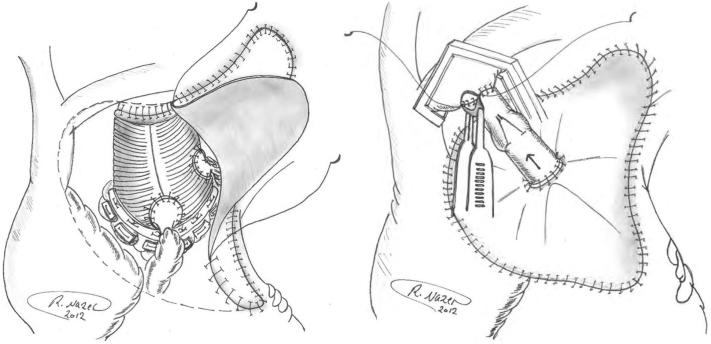
Pericardial patch sewn in place with the inflow end of valved graft anastomosed to patch.
Central Message.
The Cabrol fistula provides a safe and effective way to control intractable bleeding following aortic root, ascending aorta and/or arch operations.
Perspective.
Surgeons have previously been reluctant to use the Cabrol fistula procedure as a last-resort mechanism to control intractable bleeding following aortic root, ascending aorta, or aortic arch operations. We review 2 decades of favorable experiences using this procedure and find no long-term adverse complications.
Construction of an aorta-to-right atrium (RA) fistula, initially described by Cabrol in his 1981 report of the aortic root replacement procedure itself,1 has since been supported by other authors as well for control of intractable bleeding at the time of aortic root, ascending aortic, and aortic arch surgery.2, 3, 4, 5, 6, 7, 8, 9, 10 In these cases, the Cabrol fistula was applied for its often-remarkable potential to correct an unsustainable bleeding situation.1 The technique involves capping the bleeding area with a patch (usually pericardium) and funneling the shed arterial blood from the perigraft space to the venous system (RA or innominate vein). The principle underlying this technique is to capture and return the shed blood automatically, anticipating spontaneous resolution of the bleeding site over time. The Cabrol fistula is applied only when full, careful, exhaustive conventional methods have been applied. Often, it is inaccessibility of the bleeding site (for inspection and suturing) that necessitates the Cabrol technique.
The original procedure, outlined by Cabrol and colleagues, calls for a patch of pericardium or prosthetic material to be sewn over the anterior perigraft space to collect any blood oozing from suture lines.1,3 A fistula (traditionally a Dacron graft) is then anastomosed to the patch in order to drain this periprosthetic space into a venous outlet. Thus, there is no longer any external blood loss, as the blood is instantaneously returned to the circulation. In most cases, these 2 steps are sufficient to achieve hemostasis, and the fistula is left to close spontaneously. Late follow-up to confirm fistula closure is poorly documented in the literature. In cases in which the heart is struggling, high right-sided pressures may actually exacerbate bleeding, retrogtradely into the perigraft space.
In rare cases in which bleeding does not cease to flow, the fistula can, in principle, result in a significant left-to-right shunt. Late follow-up of use of the Cabrol fistula, examining for such complications, is scant. In order to avoid retrograde (right-to left) flow, we described a valved-graft modification of the original Cabrol fistula technique to prevent this complication.3 We have applied a variety of valving techniques to assure and maintain unidirectional flow toward the RA. We have used bovine jugular vein grafts, with their contained native valves, and the Contegra Graft (Medtronic Inc, off-label use).3
In this study, we retrieved cases of Cabrol fistula use from our aortic surgery database. We evaluate and categorize the precise methods of construction. We evaluate for any undesired long-term patency of the fistula. We examine for evidence of persistent left-to-right shunting or right heart failure. We determine long-term clinical follow-up. We perform late radiographic follow-up by echocardiography (ECHO) and computed tomography (CT).
Methods
This investigation was approved by the Human Investigation Committee of the Yale University School of Medicine (institutional review board #1609018416; date of approval: August 25, 2020). Patients' informed consent was waived due to the study being a medical record review.
From among ∼1500 ascending, root, and arch open surgical procedures by a single surgeon between 2004 and 2020, we identified 17 surgical patients in whom a Cabrol fistula was used to control recalcitrant, life-threatening bleeding after aortic repair. Before fistula creation, all patients required continuous blood transfusion and could not otherwise be closed or leave the operating room. Exhaustive conventional methods were applied before resorting to a Cabrol fistula (exposure, suturing, packing, glue application, restoration of blood coagulation parameters). We reviewed operative notes and postoperative ECHO/CT scans in detail. We examined specifically for effectiveness in controlling bleeding, persistent left-to-right shunt, anastomotic pseudoaneurysm, and operative, hospital, and long-term survival.
The Cabrol fistulas were constructed with a pericardial patch sewn to the surrounding tissues in a rectangular configuration around the ascending aorta, using any connective tissues available (clockwise from the top: fatty tissues below innominate vein, fibrotic tissue at medial edge of main pulmonary artery, right ventricular fatty tissue, and superior vena cava adventitia [delicate bites], as well as pericardium, where applicable; Figure 1). Although this technique is difficult to describe in specific terms, the surgeon uses any safe connective tissue that allows completion of the suturing of the roughly rectangular Cabrol patch to cardiac tissues in its perimeter. The goal is to form a seal that confines all excess bleeding under the patch. A small-caliber graft (Dacron, homograft vein, bovine vein, or Contegra valved graft) is then used to connect the center of the pericardial patch to the RA (or innominate vein). In addition to the conduits that we have used, the patient's own saphenous vein can also be effectively used for this purpose. Please see Table 1 for a step-by-step description of our general construction of the Cabrol fistula.
Figure 1.
Left, a pericardial patch being sewn in place over the composite graft repair site. Right, the pericardial patch has been sewn in place. The outflow end of the Contegra valved graft has been anastomosed to the innominate vein under site biting-control. Reproduced with permission from Elefteriades and colleagues.3
Table 1.
Step-by-step construction of the Cabrol fistula
| Step | Description |
|---|---|
| Pre-Cabrol | The aortic root, ascending, or arch procedure has been completed; protamine and coagulation factors have been given; and bleeding continues despite all conventional measures. |
| Pre-Cabrol | Often we wrap the distal anastomosis with a small-caliber graft to seal any bleeding from that area. (This wrap then provides secure “tissue” at the upper margin of a subsequent Cabrol fistula.) |
| Cabrol | A pericardial patch is sewn to cover the entire site of the aortic replacement. We use running 4-0 PROLENE.
|
| Temp vent | To decompress the patch at this point, before construction of the shunt, we make a tiny incision in the periphery of the patch and place a small vent catheter under the patch, which we then attach to strong suction from the cell saver. This pulls the patch into tight approximation to the tissues, like a “shrink wrap,” while simultaneously collecting the shed blood for retransfusion. |
| Shunt prox anast | We now construct the proximal anastomosis of the graft that will carry the blood to the RA. See text for details of conduit choices. We prefer a valved-conduit of some type, for unidirectional flow. We use 4-0 PROLENE. |
| Shunt distal anast | We now perform the distal anastomosis of the graft, to either the innominate vein (preferred) or the RA proper. (4-0 PROLENE). Note: The innominate vein is preferred, as raising the head of the bed can minimize the back pressure in the vein as needed. |
| Open shunt | The shunt is now opened and allowed to channel blood from the bleeding site under the patch back into the venous circulation. |
| Bleed conrol | Near-immediate control of hemorrhage is usually achieved. |
| Close Chest | We close the chest, in standard fashion. |
(1) In some cases, the shrink wrap during construction of the Cabrol fistula leads to cessation of bleeding on its own, and there is no need at that point to complete the shunt. In such a case, we leave the large patch in place over the previous bleeding zone. (2) The Cabrol fistula is more easily accomplished effectively in reoperative cases, as the scarred tissues provide better substrate for suturing and there are intrinsically fewer degrees of freedom for shed blood to disperse. (3) We do not routinely re-explore patients the day after operation to decompress clot. We do so only if there is a pressure effect (rarely). PA, Pulmonary artery; RV, right ventricle; SVC, superior vena cava; RA, right atrium.
In occasional cases, especially in patients with acutely high right-sided filling pressures, there occurred an initial large volume backflow from the RA to the perigraft space. This problem led to our modification of using a valved graft (homograft, bovine graft, or Contegra graft), which eliminated the problem of retrograde bleeding into the Cabrol patch zone.3
In a number of cases, we were able simply to create a stab-wound in the surface of the RA under the patch, allowing the flow of bleeding to enter the atrium directly, without an interposed graft. This technical modification was especially applicable in cases in which the RA pressure was low.
Results
Patient Substrate
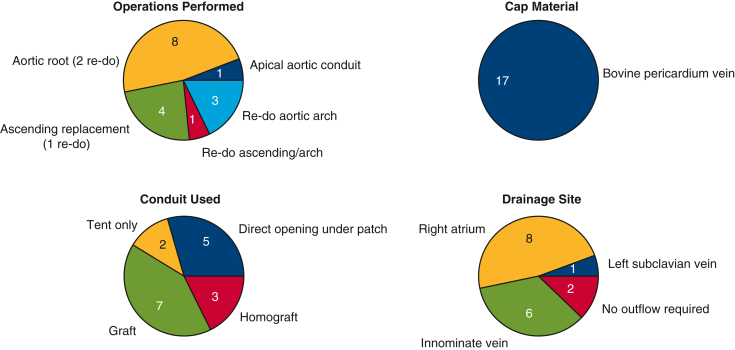
See Table 2 and Figure 2. The procedures in which the Cabrol fistula was applied included aortic root replacement (n = 8), ascending/arch replacement (n = 8), and apical–aortic conduit (n = 1). In total, 7 of 17 (41.2%) were reoperations. Mean patient age was 68 years. In total, 10 of 17 (59%) of patients underwent surgery under deep hypothermic circulatory arrest, and 3 of 17 (17.6%) of the procedures were done for acute type A dissection. (The patients who required a Cabrol procedure were often reoperation cases [42%]. This is a much greater rate of reoperations than in our general ascending aortic patient pool.)
Table 2.
Surgical details for Cabrol procedures
| Patient number | Year | Operation | Cap material | Conduit used | Drainage site |
|---|---|---|---|---|---|
| 1 | 2004 | Apical aortic conduit | Bovine pericardium | Apical-aortic conduit (22 conduit, 21 CE valve) 10-mm Hemashield graft |
Left subclavian vein |
| 2 | 2006 | Redo aortic root | Bovine pericardium | Direct opening under patch | Right atrium |
| 3 | 2008 | Redo aortic arch | Bovine pericardium | Tent only | No outflow required—patch provided adequate hemostasis |
| 4 | 2008 | Redo aortic arch | Bovine pericardium | Tent only | No outflow required—patch provided adequate hemostasis |
| 5 | 2008 | Redo aortic arch | Bovine pericardium | 8-mm Dacron graft | Innominate vein |
| 6 | 2010 | Redo ascending replacement | Bovine pericardium | Direct opening under patch | Right atrium |
| 7 | 2011 | Aortic root | Bovine pericardium | Valved Contegra graft | Right atrium |
| 8 | 2011 | Aortic root | Bovine pericardium | Valved Contegra graft | Innominate vein |
| 9 | 2013 | Aortic root | Bovine pericardium | Valved Contegra graft | Innominate vein |
| 10 | 2013 | Redo aortic root | Bovine pericardium | Direct opening under patch | Right atrium |
| 11 | 2013 | Ascending replacement | Bovine pericardium | Cryopreserved homograft vein | Right atrium |
| 12 | 2013 | Aortic root | Bovine pericardium | Direct opening under patch | Right atrium |
| 13 | 2016 | Aortic root | Bovine pericardium | Cryopreserved homograft vein | Right atrium |
| 14 | 2016 | Aortic root | Bovine pericardium | 6-mm Gore-Tex graft | Innominate vein |
| 15 | 2016 | Ascending replacement | Bovine pericardium | Direct opening under patch | Right atrium |
| 16 | 2016 | Redo ascending arch | Bovine pericardium | 8-mm Hemashield graft | Innominate vein |
| 17 | 2020 | Ascending replacement | Bovine pericardium | Femoral vein homograft | Innominate vein |
Figure 2.
Surgical details for Cabrol procedures. Upper Left, main operative procedure; lower left, conduit used; upper right, cap material; lower right, drainage site (to which Cabrol fistula shunts blood).
Surgical Techniques
Bovine pericardium was used for the cap material in all 17 patients. A variety of options were used for the conduit itself: prosthetic Dacron graft in 7 and homograft in 3. In 7 patients, no conduit was required: in 5 of these, a simple direct opening (stab wound) into the RA sufficed, and in the other 2 bleeding stopped just from the tamponade effect of patch application, so no chamber drainage was required. Outflow drainage from the Cabrol fistula was directed to the RA in 8 cases (graft or direct puncture), the innominate vein in 6, and the left subclavian vein in 1. Two patients required no drainage site.
Outcomes
Recalcitrant bleeding was successfully controlled by the Cabrol fistula in all patients. In terms of major complications, 2 patients required re-exploration for clot evacuation, 1 patient (root replacement) was returned to the operating room for a supplementary saphenous vein graft to the right coronary artery, and 1 patient developed sepsis. These patients all survived to discharge. The average total number of units of blood transfused in these Cabrol patients was 3.6 units of packed red blood cells intraoperatively and 1.2 units postoperatively.
Three patients did not survive the hospitalization. Causes of death in the 3 patients who died were multiple organ failure in 2 and intraoperative technical difficulties in 1; all 3 were very complex cases—2 were reoperative and the third was an acute aortic dissection. The Cabrol fistula itself could not be implicated in any of those cases. Nine of the other 14 patients remain alive currently (6.76 mean postoperative years). In no case did ECHO identify sustained left-to-right shunting or any patent shunt beyond a few days postoperatively. In no case did later CT scan identify continued flow via the Cabrol shunt or any evidence of anastomotic pseudoaneurysm.
Discussion
The Cabrol fistula procedure is an infrequently used option for intractable bleeding after ascending aorta or aortic arch operations. Historically, leaving the chest packed and open has been a popular option (hoping for slow resolution of bleeding over time); however, this option allows for the grafts to be exposed to the environment, increasing the risk of infection. Some recent series have reported mortalities of 25% to 50% or greater when the chest is left open after open-heart surgery.11, 12, 13, 14
In this current report, we review our extremely favorable experience with the Cabrol fistula for control of intractable intraoperative bleeding in ascending surgery. These patients, after often-complex, extensive, or emergent procedures, manifested bleeding that would have proved lethal without some method of control.
In our directed review of 17 patients, we found that we had employed a variety of specific techniques (in a variety of clinical settings) in the construction of the Cabrol fistula. Also, after having been “burned” by opposite direction flow (backwards into the perigraft space from the RA) in patients with high RA pressure, we implemented “valving” of the conduit to obligate flow only in the desired direction.
Although we were pleased with our short-term outcomes with the rare use of the Cabrol fistula out of many thousands of cases, we had never rigorously examined our patients' long-term experiences. This left us with 2 lingering concerns: (1) are we leaving the patient with a persistent left-to-right shunt? and (2) are we “painting over” an anastomotic defect that may result in a pseudoaneurysm over the long term?
Through our directed long-term analyses, we found that the Cabrol fistula was extremely effective at controlling intraoperative bleeding (100% success rate), there was no evidence of persistent left-to-right shunting from the aorta to RA on either ECHO or CT, and there was no evidence of the development of a pseudoaneurysm on any of the anastomotic sites in any of these patients.
We have found the Cabrol fistula to be extremely effective, safe, and free of any significant long-term complications (Figure 3). In the high-jeopardy settings, the use of a Cabrol fistula facilitated survival in both the short and long term. No Cabrol fistula–related late complications (persistent fistula, pseudoaneurysm) were identified.
Figure 3.
The Cabrol fistula for control of intractable intraoperative bleeding is a lifesaving procedure without adverse long-term consequences. CT, Computed tomography.
Although persistent fistula, congestive heart failure, and pseudoaneurysms have been noted by others,15, 16, 17 we identified no such cases in our own experience. Other centers as well have found their Cabrol experience to be extremely positive.18 We strongly recommend this procedure for control of intractable intraoperative bleeding when compared with the conventional method of packing an open chest.
Conclusions
We conclude that (1) The Cabrol fistula can be truly life-saving. (2) The valved modification of the Cabrol fistula completely eliminates the problem of retrograde filling of the perigraft space. (3) There were no late anastomotic problems that had been “obscured” or “covered over” by the Cabrol patch. (4) There were no sustained fistulae in the long-term. (5) Satisfactory short- and long-term survival were achieved, thanks to the Cabrol fistula, in these otherwise-desperate situations.
The Cabrol fistula, although so effective, should not be seen as a substitute for proper suture control of bleeding. The Cabrol fistula is most applicable for bleeding sites inaccessible to repair suturing. The brilliance of Dr Cabrol is reflected in the true life-saving ability of this technique.
Conflict of Interest Statement
Dr Elefteriades reported Principal of Coolspine. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Cabrol C., Pavie A., Gandjbakhch I., Villemot J.P., Guiraudon G., Laughlin L., et al. Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg. 1981;81:309–315. [PubMed] [Google Scholar]
- 2.Rosero H., Nathan P.E., Rodney E., Vasavada B., Sacchi T.J. Aorta to right atrium fistula with congestive heart failure resulting from a patent Cabrol shunt after repair of aortic dissection. Am Heart J. 1994;128:608–609. doi: 10.1016/0002-8703(94)90637-8. [DOI] [PubMed] [Google Scholar]
- 3.Elefteriades J.A., Youssef S., Rousou L., Nazer R. Novel valved graft modification of Cabrol fistula for bleeding after aortic root surgery. Ann Thorac Surg. 2012;94:1741–1743. doi: 10.1016/j.athoracsur.2012.05.114. [DOI] [PubMed] [Google Scholar]
- 4.Elefteriades J.A. Mechanism to valve a Cabrol fistula for bleeding control. Aorta (Stamford) 2013;1:65–66. doi: 10.12945/j.aorta.2013.13.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoover E.L., Hsu H.K., Ergin A., Ketosugbo A., Webb H., Kharma B., et al. Left-to-right shunt in control of bleeding following surgery for aneurysms of the ascending aorta. Chest. 1987;91:844–849. doi: 10.1378/chest.91.6.844. [DOI] [PubMed] [Google Scholar]
- 6.Estrera A.L., Miller C.C., Kaneko T., Lee T.Y., Walkes J.C., Kaiser L.R., et al. Outcomes of acute type A aortic dissection after previous cardiac surgery. Ann Thorac Surg. 2010;89:1467–1474. doi: 10.1016/j.athoracsur.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Vogt P.R., Akinturk H., Bettex D.A., Schmidlin D., Lachat M.L., Turina M.I. Modification of surgical aortoatrial shunts for inaccessible bleeding in aortic surgery: modification of the Cabrol-shunt technique. Thorac Cardiovasc Surg. 2001;49:240–242. doi: 10.1055/s-2001-16102. [DOI] [PubMed] [Google Scholar]
- 8.Nielson D.H., Sutter F.P., Goldman S.M. Use of venous fistula technique for intraoperative cardiac hemorrhage. J Card Surg. 1993;8:558–561. doi: 10.1111/j.1540-8191.1993.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 9.Maisano F., Lorusso R., Alfieri O. The periprosthetic sac innominate vein shunt: an effective way to control bleeding after aortic root operations. J Thorac Cardiovasc Surg. 1995;109:396–397. doi: 10.1016/S0022-5223(95)70406-X. [DOI] [PubMed] [Google Scholar]
- 10.Mehta I.D., Elefteriades J.A. Funnel graft to innominate vein to control epicardial bleeding. Ann Thorac Surg. 1998;66:1413–1414. doi: 10.1016/s0003-4975(98)00786-3. [DOI] [PubMed] [Google Scholar]
- 11.Salerno T.A., Carvalho E.M.F., Panos A.L., Ricci M. Modified Cabrol shunt after complex aortic surgery. Ann Thorac Surg. 2008;86:669–670. doi: 10.1016/j.athoracsur.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 12.Bakaeen F.G., Haddad O., Ibrahim M., Pasadyn S.R., Germano E., Mok S., et al. Advances in managing the noninfected open chest after cardiac surgery: negative-pressure wound therapy. J Thorac Cardiovasc Surg. 2019;157:1891–1903.e9. doi: 10.1016/j.jtcvs.2018.10.152. [DOI] [PubMed] [Google Scholar]
- 13.Kurazumi H., Suzuki R., Nawata R., Yokoyama T., Tsubone S., Matsuno Y., et al. Feasibility of open chest management with modified negative pressure wound therapy immediately after cardiac surgery. Interact Cardiovasc Thorac Surg. 2022;35 doi: 10.1093/icvts/ivac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boeken U., Assmann A., Mehdiani A., Akhyari P., Lichtenberg A. Open chest management after cardiac operations: outcome and timing of delayed sternal closure. Eur J Cardiothorac Surg. 2011;40:1146–1150. doi: 10.1016/j.ejcts.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Savic V., Schmiady M.O., Khargi K., Maisano F., Mestres C.A. How does a Cabrol fistula look at reoperation? Ann Thorac Surg. 2019;108:e277. doi: 10.1016/j.athoracsur.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Goossens D., Brutel de la Rivière A., Ernst S., Vermeulen F. Reoperative aortic root surgery late after use of histo-acryl. Eur J Cardiothorac Surg. 1997;11:194–195. doi: 10.1016/s1010-7940(96)01086-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B., Liu Y., Dun Y., Sun X. Early obliterated Cabrol shunt: culprit of aortopulmonary fistula in large pseudoaneurysm after Bentall procedure. J Cardiovasc Dev Dis. 2022;9:449. doi: 10.3390/jcdd9120449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H., Wu X., Fang G., Qiu Z., Chen L.W. Is it justified to apply a modified Cabrol fistula in surgical repair of acute type A aortic dissection? J Thorac Cardiovasc Surg. 2019;158:1307–1314.e2. doi: 10.1016/j.jtcvs.2018.12.082. [DOI] [PubMed] [Google Scholar]