Abstract
Introduction
The impact of COVID-19 pandemic represents a serious challenge for ‘frail’ patients' populations with inflammatory autoimmune systemic diseases such as systemic sclerosis (SSc). We investigated the prevalence and severity of COVID-19, as well the effects of COVID-19 vaccination campaign in a large series of SSc patients followed for the entire period (first 38 months) of pandemic.
Patients and method
This prospective survey study included 1755 unselected SSc patients (186 M, 1,569F; mean age 58.7 ± 13.4SD years, mean disease duration 8.8 ± 7.3SD years) recruited in part by telephone survey at 37 referral centers from February 2020 to April 2023. The following parameters were carefully evaluated: i. demographic, clinical, serological, and therapeutical features; ii. prevalence and severity of COVID-19; and iii. safety, immunogenicity, and efficacy of COVID-19 vaccines.
Results
The prevalence of COVID-19 recorded during the whole pandemic was significantly higher compared to Italian general population (47.3 % vs 43.3 %, p < 0.000), as well the COVID-19-related mortality (1.91 % vs 0.72 %, p < 0.001). As regards the putative prognostic factors of worse outcome, COVID-19 positive patients with SSc-related interstitial lung involvement showed significantly higher percentage of COVID-19-related hospitalization compared to those without (5.85 % vs 1.73 %; p < 0.0001), as well as of mortality rate (2.01 % vs 0.4 %; p = 0.002). Over half of patients (56.3 %) received the first two plus one booster dose of vaccine; while a fourth dose was administered to 35.6 %, and only few of them (1.99 %) had five or more doses of vaccine. Of note, an impaired seroconversion was recorded in 25.6 % of individuals after the first 2 doses of vaccine, and in 8.4 % of patients also after the booster dose. Furthermore, the absence of T-cell immunoreactivity was observed in 3/7 patients tested by QuantiFERON® SARSCoV-2 Starter Set (Qiagen). The efficacy of vaccines, evaluated by comparing the COVID-19-related death rate recorded during pre- and post-vaccination pandemic periods, revealed a quite stable outcome in SSc patients (death rate from 2.54 % to 1.76 %; p = ns), despite the significant drop of mortality observed in the Italian general population (from 2.95 % to 0.29 %; p < 0.0001).
Conclusions
An increased COVID-19 prevalence and mortality rate was recorded in SSc patients; moreover, the efficacy of vaccines in term of improved outcomes was less evident in SSc compared to Italian general population. This discrepancy might be explained by concomitant adverse prognostic factors: increased rate of non-responders to vaccine in SSc series, low percentage of individuals with four or more doses of vaccine, ongoing immunomodulating treatments, disease-related interstitial lung disease, and/or reduced preventive measures in the second half of pandemic. A careful monitoring of response to COVID-19 vaccines together with adequate preventive/therapeutical strategies are highly recommendable in the near course of pandemic in this frail patients’ population.
Keywords: COVID-19, SARS-CoV-2, Systemic sclerosis, Scleroderma, Interstitial lung disease, COVID-19 vaccine
Highlights
-
•
Scleroderma patients showed higher COVID-19 prevalence and mortality rate than general population.
-
•
Mortality remained stable comparing pre-/post-vaccine periods, despite net drop in general population.
-
•
It might be due to impaired vaccine immunogenicity, immunomodulating drugs, scleroderma lung fibrosis.
-
•
Monitoring of vaccine response is highly recommendable in the next pandemic phase.
-
•
Harmonization of immunomodulator cycles and vaccine booster is very advisable.
1. Introduction
Since the outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic in the early 2020, an increasing number of survey studies, often based on telemedicine [[1], [2], [3], [4], [5], [6], [7], [8], [9]], have been conducted in order to evaluate its impact on patients with different autoimmune systemic diseases (ASD). The latter represent a heterogeneous group of disorders, including chronic inflammatory arthritis, connective tissue diseases, systemic vasculitides and thyroid autoimmunity disorders [10], characterized by an increased susceptibility to infections, because of either immune system alterations and/or concurrent immunomodulating therapies. Therefore, ASD may be regarded as one of predisposing conditions to be infected by SARS-CoV-2 and to develop overt coronavirus disease 2019 (COVID-19), such as the diabetes, cardiovascular or neoplastic disorders [11,12]. Systemic sclerosis (SSc) can be regarded as one of most severe ASD [[13], [14], [15]]; it is due to in-depth immunological, endothelial, and fibroblast alterations leading to microangiopatic and fibrotic multiple organ involvement, such as lung fibrosis and cardiovascular manifestations, which may severely affect the overall disease outcome in a significant proportion of patients [[13], [14], [15]]. Therefore, SSc may represent one of the most frail patients’ populations towards the COVID-19 pandemic.
At the first months of pandemic, a strict lockdown was decided in order to limit the SARS-CoV-2 diffusion. Consequently, to overcome the severe restrictions on regular outpatient visits at rheumatic clinics, the COVID-19 & ASD Italian Study Group organized a telemedicine network for a remote monitoring and management of ASD, including SSc patients [16].
The present multicenter survey study focused on the overall impact pandemic as regards the COVID-19 prevalence, severity, and outcomes, as well on the long-term efficacy of vaccination campaign in a very large SSc patients’ series.
2. Patients and methods
The present multicenter, prospective survey study included 1755 SSc patients (186 M, 1569F; mean age 58.7 ± 13.4SD years, mean disease duration 8.8 ± 7.3SD years; Table 1) consecutively recruited since the first month of pandemic (February 2020) at the 37 referral centers of the COVID-19 & ASD Italian Study Group, equally distributed throughout different Italian macro-areas. This patient population was surveyed for all subsequent 38-month period of pandemic years until April 2023 by means of telemedicine as a useful tool for overcoming the major limits imposed by the tight lockdown, especially in the first year of the pandemic.
Table 1.
Main demographic, clinical, and serological features of 1755 SSc patients.
| Demographic | N | % |
|---|---|---|
| Sex Males | 186 | 10.6 |
| Age mean (SD) | 58.7 ± 13.4 | |
| Dis dur yrs mean (SD) | 8.8 ± 7.3 | |
| Clinical | ||
| Limited cutaneous SSc | 1139 | 64.9 |
| Diffuse cutaneous SSc | 616 | 35.1 |
| Teleangectasias | 1037 | 59.1 |
| Calcinosis | 207 | 11.8 |
| Digital ulcers | 399 | 19.3 |
| Esophageal inv. | 834 | 47.5 |
| Sicca syndrome | 573 | 32.6 |
| Lung inv. | 547 | 31.2 |
| Heart inv. | 473 | 26.9 |
| Renal inv. | 311 | 17.7 |
| Serological | ||
| ANA test - no (%) | 1699 | 96.8 |
| anti-Scl70 - no (%) | 711 | 40.5 |
| ACA - n (%) | 875 | 49.9 |
Legend: SSc: systemic sclerosis; Dis Dur: disease duration: ANA: anti-nuclear antibodies; ACA: anti-centromere antibodies.
In all patients the SSc was classified according to current criteria [17]. The main demographic, clinical, and laboratory assessment was carried out in all cases following the previously described methodologies [18]; the SSc-related cutaneous and internal organ involvement, the main comorbidities, such as hypertension and diabetes, and the ongoing treatments were obtained from individual patient's records (Table 1). In particular, the large majority of recruited patients (96.1 %), underwent one or more of the following drugs: low dosage steroids, conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD), rituximab, mycophenolate mofetil, vasoactive drugs (monthly infusion of iloprost, less frequently other prostanoids, bosentan, and/or Ca-channel blockers), and/or low dosage aspirin; while biological-DMARD were administered in only small number of individuals.
In order to evaluate the efficacy of anti-COVID-19 vaccines, we compared the prevalence and severity of COVID-19 between the two main pandemic phases, i.e. the first one (February 2020–April 2021) and the second period (May 2022–April 2023) following the introduction of COVID-19 vaccination campaign in Italy, which includes the administration of one or more booster doses of vaccine.
The study was performed in accordance with the principles of the Declaration of Helsinki; moreover, it was approved by the Ethics Committee CEAVNO (Comitato Etico Area Vasta Nord Ovest; registration n° 23872); all patients gave their informed consent before participation.
The occurrence of COVID-19 manifestations during the long observation period was recorded by means of telephone survey using a standardized symptom-assessment questionnaire that included updated ASD clinical information, and possible COVID-19 manifestations (fever, fatigue, headache, anosmia, dysgeusia, cough, dyspnea, nausea, diarrhea, vomiting, arthralgia, myalgia, coryza). In addition, a sudden worsening of pre-existing manifestations, such as respiratory symptoms (dyspnea, dry cough) possibly due to SSc-related lung fibrosis, as well as the results of oral/nasopharyngeal swab for the detection of SARS-CoV-2 by polymerase-chain-reaction (PCR) testing, were carefully recorded.
The observed findings of COVID- 19 prevalence and outcomes observed in our SSc patients were compared to that found in the Italian general population reported in the Italian Superior Institute of Health (https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_26-aprile-2023.pdf.).
The immunogenicity of COVID-19 vaccines was evaluated by measuring the titer of neutralizing antibodies (NAb) against SARS-CoV-2 trimeric spike S1/S2 glycoproteins on serum samples obtained within 2–4 weeks and 6 months after completion of the first vaccination cycle, and after the booster vaccine dose [[19], [20], [21]]. The NAb levels were measured by means of SARS- CoV-2 IgG II Quant antibody test kit (Abbott Laboratories, Chicago, IL). The NAb titers are expressed as Binding Antibody Units (BAU)/ml, with a cut-off for positive testing of 7 BAU/ml, as recommended by the World Health Organization (WHO). A serum titer of NAb 10 × normal upper limit (≤70 BAU/ml) was classified as suboptimal response.
In few patients, the T cell response to vaccines was assessed by measuring interferon-gamma (IFN-γ) production by peripheral blood lymphocytes upon SARS-CoV-2 glycoprotein stimulation using the QuantiFERON® SARSCoV-2 Starter Set (Qiagen), an interferon gamma release assay (IGRA), following the previously described methodology [21].
3. Statistical analysis
Data are expressed as mean ± standard deviation (SD), median (Interquartile-IQ-range: 25th-75th percentile) or numbers (percentage) as appropriate. The one-way analysis of variance (ANOVA) and the Kruskal–Wallis H test were used for comparing three or more groups, as appropriate. Nonparametric tests as Mann-Whitney or Wilcoxon were used for comparing continuous variables between two groups as appropriate. Fisher's exact test was used to compare categorical variables. All tests were two tailed. Analyses were performed using GraphPad Prism v 9 Software.
4. Results
The main demographic, clinical, and serological features of the 1755 SSc patients recruited for the present study are summarized in Table 1. On the whole, the clinical and laboratory features of this patients’ population are in keeping with that observed in recent studies on large Italian SSc series [18].
As regards the impact of pandemic on SSc patients our survey study focused on two main aspects; from one side, the prevalence and severity of COVID-19, as well as the presence of possible predisposing/prognostic factors; on the other side, the effects of COVID-19 vaccination, including vaccine safety, immunogenicity, and efficacy. The Table 2 and Fig. 1, Fig. 2, Fig. 3 describe the main findings recorded during our long-term observational study.
Table 2.
SARS-CoV-2 infection and SARS-CoV-2 infection and SARS-CoV-2 infection and SARS-CoV-2 infection and COVID-19 vaccination in 1755 Italian SSc patients.
| Prevalence of COVID-19 | ||||
|---|---|---|---|---|
| Total COVID19+ | Pre- vaccine period (a) | Post-vaccine period (b) | P (a Vs b) | |
| SSc COVID-19+ Vs Italian general population |
838/1755 (47.7 %) Vs 43379/100000 (43.3 %) p < 0.0001 (OR 1.193; 1.085–1.311) |
157/1755 (8.9 %) Vs 6492/100000 (6.49 %) p < 0.0001 (OR 1.415; 1.199–1.670) |
681/1755 (38.8 %) Vs 36887/100000 (36.8 %) p = 0.1 (OR 1.085; 0.985–1.195) |
p < 0.0001 OR 0.155 (0.128–0.187) p < 0.0001 (OR 0.119; 0.115–0.122) |
| Severity of COVID-19 | ||||
| Total COVID-19+ | Mild-moderate | Severe | Death | |
| 838 | 785/838 (93.67 %) | 53/838 (6.33 %) | 16/838 (1.91 %) | |
| Death rate due to COVID-19 | ||||
| Total deaths | Pre-vaccine period (a) | Post-vaccine period (b) | P (a Vs b) | |
| SSc Vs Italian general population |
16/838 (1.91 %) 187403/25892918 (0.72 %) p < 0.001 (OR 2.670; 1.628–4.379) |
4/157 (2.54 %) 123276/4051401 (2.95 %) P = 0.130( OR 0.833; 0.309–2.248) |
12/681 (1.76 %) 64127/21841517 (0.29 %) p < 0.0001 (OR 6.091; 3.442–10.781) |
p = 0.517 OR 1.458 (0.464–4.581) p < 0.0001 (OR 10.658; 10.556–10.760) |
| SSc-related interstitial lung involvement (ILD) & severe COVID-19 + | ||||
| SSc-ILD + (n. 547) Vs SSc-ILD -(n. 1208) |
Hospitalized+ 32/547 (5.85 %) Hospitalized+ 21/1208 (1.73 %) |
p < 0.0001(OR 3.512; 2.006- 6.149) |
Deaths + 11/547 (2.01 %) Deaths + 5/1208 (0.4 %) |
p < 0.001(OR 4.938; 1.707- 14.281) |
| Doses of COVID-19 Vaccine (1755 SSc patients) | ||||
| 0 | First 2 | 3 (1 booster) | 4 | >4 |
| 67 (3.8 %) | 133 (7.6 %) | 896 (51.0 %) | 624 (35.6 %) | 35 (1.99 %) |
| 88.6 % of SSc patients received at least three doses of vaccine, only 35.6 % four doses | ||||
Legend: SSc: systemic sclerosis; ILD: interstitial lung disease.
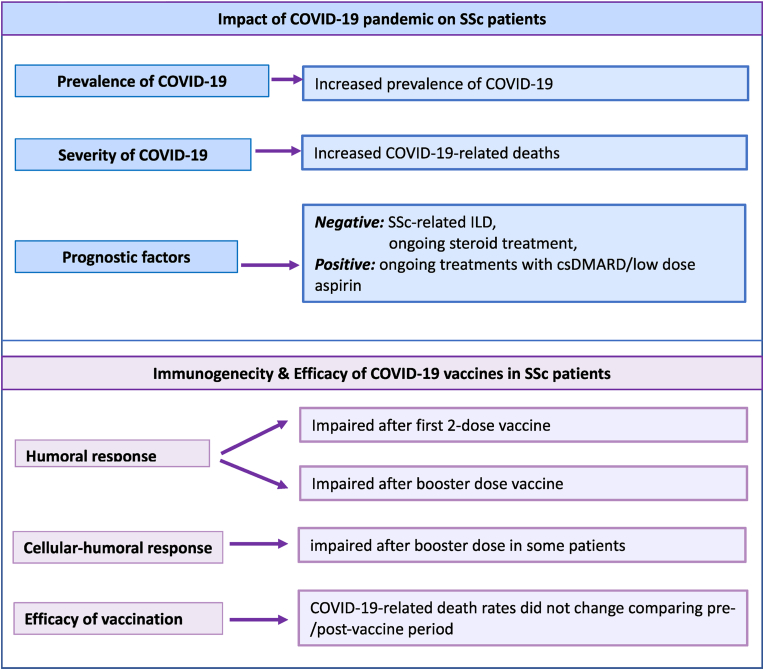
Fig. 1.
The figure describes the main findings of the impact of COVID-19 in systemic sclerosis (SSc) patients; namely, prevalence and death rate recorded during the whole pandemic period and before/after the vaccination campaign.
Upper panel: the prevalence of COVID-19 was significantly higher in SSc compared to Italian general population; it significantly increased in both patients and controls during the second phase of pandemic. In contrast, the death rate remained quite stable in SSc patients comparing the pre-/post vaccination campaign, while markedly decreased in Italian general population (panel below).
Fig. 2.
The figure describes two SSc patients with impaired immunoreactivity to COVID-19 vaccines, both undergoing immunomodulating treatments with mycophenolate mofetil (2 g/day). A 42-year-old woman (panel A) showed the absence of seroconversion after the first two doses of COVID-19 vaccine, possibly due to a late effect of rituximab administered 8 months before the first dose of COVID-19 vaccine. In this patient, the booster dose of vaccine was followed by a valuable humoral response. In the second case, a 66-year-old woman (panel B), the administration of the first two doses plus a booster dose of vaccine was unable to stimulate neither humoral nor cellular response. Based on this recent finding, the patient was advised to follow the necessary precautions in her daily life and to attempt a fourth dose of vaccine after adequate period of discontinuation of mycophenolate mofetil treatment.
Fig. 3.
The upper panel summarizes the impact of COVID-19 pandemic on our series of 1755 Italian SSc patients observed during the entire pandemic period, from February 2020 to April 2023.
Both prevalence and severity (hospitalization and mortality rates) are significantly higher in SSc patients than Italian general population. Possible adverse prognostic factors were the pre-existing interstitial lung disease (ILD) and long-term steroid treatment. In contrast, ongoing treatments with both conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; see reference n.16) and/or low dose aspirin appeared to be able to prevent the worst COVID-19 outcomes.
The panel below summarizes the findings regarding the COVID-19 vaccination; namely, the humoral and cellular response evaluated after the first two and the booster doses of vaccine in SSc patients consecutively investigated, as well as the efficacy of vaccines evaluated by death rate variation before/after the introduction of vaccination.
4.1. COVID-19 in SSc: prevalence, severity, and outcome
The overall prevalence of COVID-19 in our SSc series observed during the entire survey period was statistically higher if compared to the Italian general population (47.3 % vs 43.3 %, p < 0.0001; Table 2). COVID-19 was observed in the 43 % (80/186) of males and in a comparable percentage of females (48 %, 758/1,569; p: ns). Moreover, severe COVID-19 manifestations, namely respiratory symptoms requiring hospitalization, were observed in 6.32 % (53/838) of infected patients; among them, a significant higher death rate was recorded if compared to Italian general population (1.91 % vs 0.72 %, p < 0.001; Table 2, Fig. 1). With regards the relationship between the worse prognosis (death recorded in 16 patients) and the main demographic factors, i.e. patient age and gender, only two SSc patients were below 60 years, while the majority was over 70; moreover, a slightly higher percentage of COVID-19-related deaths was recorded in males compared to females [2.5 % (2/80) vs 1.8 % (14/758), p: ns]. These findings were in agreement with those observed in the general Italian population as reported by the Italian Superior Institute of Health (https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_26-aprile-2023.pdf).
As regards the putative prognostic factors of worse outcome, COVID-19 positive patients with SSc-related interstitial lung involvement (ILD) showed significantly higher percentage of hospitalization compared to those without ILD (5.85 % vs 1.73 %; p < 0.0001), as well as of COVID-19-related mortality rate recorded during the whole pandemic period (2.01 % vs 0.4 %; p = 0.002).
Finally, the presence of COVID-19 did not correlate neither with other SSc clinical symptoms nor specific serological markers; no relationships were also observed between ongoing treatments and the appearance of symptomatic COVID-19, with the exception of significantly lower rate of COVID-19 in patients treated with chronic low dosage aspirin compared to those without (1.96 %, 10/499 vs 4.89 %, 61/1185; p = 0.005 (OR = 0.389; CI = 0.198–0.766).
4.2. Impact of COVID-19 vaccination: safety and immunogenicity
From the early 2021, our SSc patients underwent to COVID-19 vaccination with a variable number of cumulative doses administered at the end of the survey (Table 2); in particular 7.57 % (133/1755) of individuals received only the first two doses, while over half cases (51 %, 896/1755) had the first two plus a booster dose. Of note, a fourth dose of vaccine was administered to little more than a third of patients (35.6 %, 624/1755), but only few of them (1.99 %, 35/1755) had five or more doses of vaccine. Finally, a very small percentage of individuals did not get the anti-COVID-19 vaccination (3.8 %, 66/1755; Table 2). Overall, the great majority of SSc patients (88.6 %, 1555/1755) received at least three doses of vaccine, more frequently a mRNA vaccine. In the large majority of patients, the vaccine administration revealed an individual good tolerability; only mild and reversible symptoms such as malaise, arthralgias, low-grade fever were recorded within the first four weeks from vaccination.
With regards the immunogenicity of COVID-19 vaccines administered during the second phase of pandemic, the serum levels of NAb were investigated in 495 unselected SSc patients; an impaired seroconversion was recorded in 25.6 % (127/495) of individuals after the first 2 doses of vaccine. More interesting, a deficient response was still observed in 8.4 % (22/260) of patients retested randomly after the booster dose of vaccine. The cellular response to booster dose was also investigated in 7 patients showing the absence of T-cell reactivity in individual cases (3/7). In this respect, the Fig. 2 describes two SSc patients, both undergoing immunomodulating treatments with mycophenolate mofetil (2 g/day) but with different patterns of altered immunoreactivity to COVID-19 vaccine. In a 42-year-old woman the seroconversion was totally absent after the first two doses of COVID-19 vaccine, possibly due to a late effect of previous rituximab cycle; in this patient, the administration of a booster dose was followed by valuable NAb production. In the second case observed very recently, a 64-year-old woman, the administration of the first two doses of COVID-19 vaccine plus a booster dose was unable to stimulate neither humoral nor cellular response. Therefore, the patient was advised to follow the necessary preventive measures in her daily life and possibly to take a fourth dose of vaccine after adequate discontinuation of ongoing mycophenolate mofetil treatment. In this respect, the Fig. 4 summarizes the different management strategies that could be proposed, according to the SSc activity, in patients undergoing immunomodulating treatments and impaired anti-COVID-19 immunoreactivity.
Fig. 4.
The experience of the first three years of the pandemic suggests that in the new pandemic phase we still have to manage a number of ‘frail’ scleroderma patients needing immunomodulating treatments and who at the same time have an impaired protection against SARS-CoV-2 infection possibly due to the negative effects of immunomodulating drugs themselves on vaccine immunoreactivity and/or an insufficient number of vaccine booster doses. Therefore, a periodic check of patients' immune status is recommended in order to avoid a dangerous appearance of COVID-19 manifestations in a frequently frail patient because of multi-organ scleroderma involvement, possible comorbidities, and often undergoing immunomodulatory drugs.
The upper panel represents a SSc patient with absent/low disease activity and impaired immune response to COVID-19 vaccine; in this case it is possible to discontinue the ongoing therapy and proceed with vaccination or booster dose administration.
The panel below concerns a patient with active-progressive disease, eg interstitial lung disease, requiring immunomodulating therapies but without immune protection against SARS-CoV-2 infection; in this case, it is possible to start/continue the immunomodulating therapy and postpone vaccination or booster vaccine doses. In addition, tight clinical control and anti-SARS-CoV-2 infection protection measures are highly advisable, as well the timely administration of specific antiviral drugs in the event of COVID-19 manifestations.
4.3. Efficacy of COVID-19 vaccination
To evaluate the expected efficacy of COVID-19 vaccination in SSc patients we compared the cumulative prevalence of COVID-19, as well as the COVID-19-related hospitalization and mortality rates, recorded during the initial phase of the pandemic (February 2020–February 2021) with those of the second phase following the introduction of vaccination campaign (March 2021–April 2023). The prevalence of COVID-19 was significantly higher in SSc patients than Italian general population during the initial phase of pandemic (8.9 % vs 6.49 %, p < 0.0001). Moreover, a significant increase of the COVID-19 prevalence was recorded in both SSc patients (from 8.9 % to 38.8 %; p < 0.0001) and Italian general population (from 6.49 % to 36.8 %; p < 0.0001) during the second phase of pandemic (Table 2, Fig. 1). Of note, the percentage of both COVID-19-related hospitalization (from 5.7 % to 6.4 %; p = ns) and deaths (from 2.54 % to 1.76 %; p = ns) remained quite stable in the SSc patients’ series, while a significant decline of mortality was observed in the Italian general population comparing the first with second phase of pandemic (from 2.95 % to 0.29 %; p < 0.0001).
5. Discussion
The results of the present long-term survey further expanded our knowledge acquired during the first pandemic phase as regards the impact of COVID-19 on SSc patients; moreover, it allowed to first evaluate the long-term efficacy of anti-COVID-19 vaccine in this particularly frail patients’ population. Considering the entire 38-month pandemic period, the SSc patients showed a significantly higher prevalence of COVID-19 and mortality rate when compared to Italian general population. The presence of pre-existing SSc-related interstitial lung involvement was correlated with worst COVID-19 outcomes, namely increased percentages of both hospitalized and deceased patients.
Noteworthy, the introduction of anti-COVID-19 vaccines since the early 2021 did not affect significantly the COVID-19-related mortality in the SSc patients, despite the improved COVID-19 outcomes observed in the Italian general population in the same period of pandemic. This somewhat unexpected observation could be tentatively explained by different considerations. First of all, the second pandemic phase represents a longer observational period with higher availability of SARS-Cov2 testing and therefore improved diagnostic possibilities also for mild COVID-19 variants. Furthermore, the lack of decline of both hospitalization and mortality rates in SSc patients in this phase coincided with the reopening of health care activities at the referral centers and the progressive reduction of social distancing measures; the latter may have penalized above all the subgroups of 'frail' patients compared to general population. Probably most important, the initial period of good compliance towards COVID-19 vaccines, during which the majority of scleroderma patients have taken the first three doses, was followed by lower adherence to the vaccination campaign either by the general population and by the patients themselves. To date, just over a third of patients received the fourth dose of vaccine; this finding in combination with the impaired immunoreactivity to vaccines [19,20,[22], [23], [24]], could be responsible for the overall toll of vaccination campaign in SSc patients. The resulting scenario, especially in the last 6–12 months of pandemic, was most likely characterized by an increasing number of patients with a peculiar combination of some adverse factors, namely SSc multiple organ involvement, mainly ILD, ongoing immune suppressor treatments, and impaired humoral/cellular anti-COVID-19 response, all in the context of weakened pandemic containment measures.
An updated review of the world literature on the impact of COVID-19 in scleroderma patients revealed the presence of several reports quite heterogeneous as regards i. the entity of the SSc series investigated, frequently only anecdotal observations or small case series [25,26]; ii. the observational period, more often limited to first pandemic phase; iii. the possible ethnic/genetic and/or environmental differences; iv. the social context and healthcare organization systems of different countries, including the COVID-19 vaccination campaign and therapeutic strategies employed. There are very few reports including large patients’ series, generally observational retrospective studies focusing on the first 1–2 years of pandemic [15].
Taken together the above considerations may explain some contrasting data on the prevalence and outcomes of COVID-19 among different SSc series [15,27,28]. Regarding the presence of negative prognostic factors, besides the disease-related ILD present in a relevant number of patients, other symptoms such as pulmonary arterial hypertension, cardiac involvement, as well the use of RTX might affect the overall outcome of COVID-19 in SSc [28].
The increased prevalence and worse outcomes of COVID-19, particularly in patients with pre-existing ILD, was also observed in our previous studies during the first pandemic phase as regards either SSc and other autoimmune systemic diseases [29] and confirmed by other authors [28,30].
The effects of anti-COVID-19 vaccination are reported in small patients' series [[31], [32], [33], [34], [35], [36]], showing a generally good tolerability to different anti-COVID-19 vaccines, mostly mRNA types [[19], [20], [21],35,37]. The percentage of impaired seroconversion largely varied among different patients’ series [19,31,34,37,38]. It was frequently evaluated after the first two doses of vaccine [31,32,34,39] and in only two reports after the booster dose [19,21]; one of them included the detection of the T-cell immunoreactivity [21].
In the majority of previous investigations, the ongoing or recent administration of immunomodulating drugs, mainly anti-CD20 cycles, seems to exert a negative role on the humoral immunogenecity of anti-COVID-19 vaccines [19,20,38]. In this context, an increasing number of studies suggested a possible role of genetic factors on the impaired response to vaccines [40,41]. Given the persistent risk of SARS-CoV2 infection in the near future, the overall management of different frail patients’ populations could represent a particularly challenging issue; both therapeutical decisions and protection measures should be individually tailored on the basis of disease activity/severity and patients' lifestyle. In this respect, the vaccine administration and the timing of booster dose(s) should be constantly decided on the basis of close monitoring of humoral/cellular response, as well as disease activity (Fig. 4). As suggested by the clinical history of individual patients here described and by other observations [31,37,38], not infrequently we will have to manage patients with only the first 2–3 doses of COVID-19 vaccine but needing long-term treatment with immunomodulatory drugs; in these instances, it would be highly recommendable to verify their actual anti-COVID-19 immunogenic condition starting with the serum NAb level detection (Fig. 4).
The experience of COVID-19 pandemic evidenced some important contrasting effects of immunomodulating treatments in the management of different frail patients' populations such as scleroderma patients and other autoimmune systemic disorders [16,28,42]. From one side, ongoing immunomodulating treatments at the time of SARS-CoV2 infection, such as conventional disease-modifying antirheumatic drugs, seemed to be able to contrast the strong immunoreactivity to virus, the so-called ‘cytokine storm’, observed in those patients who developed rapidly severe COVID-19 inflammatory manifestations and worst outcomes [16]. From the other side, these drugs may weaken the immunogenicity of COVID-19 vaccines, especially if used closely to vaccine administration [19,20,38]. Given the divergent effects of immunomodulating drugs, their balanced use may be decisive in immunocompromised patients during the pandemic.
The immunoreactivity to COVID-19 vaccines is quite unpredictable in individual patients; an impaired response could be correlated to possible genetic factors [43,44], as well to ongoing immunomodulating treatments. The latter can be decisive for the management of the most severe disease manifestations; therefore, in the clinical practice a careful monitoring of the patient's disease activity, immunomodulating treatments, and mostly anti-COVID-19 immune protection is highly recommendable (Fig. 4).
6. Conclusions
Our long-term survey study provides a number of useful information regarding the impact of COVID-19 pandemic on scleroderma patients that may be also applied to other subgroups of ‘frail’ patients with autoimmune systemic diseases. The main world health organizations have declared the first dramatic period of the pandemic concluded (https://news.un.org/en/story/2023/05/1136367#:∼:text=WHO%20chief%20declares%20end%20to%20COVID%2D19%20as%20a%20global%20health%20emergency,5%20May%202023&text=The%20head%20of%20the%20UN,no%20longer%20a%20global%20threat), even if a trend of alternating phases of remission and exacerbation is expected due to the possible appearance of new viral variants with high infectivity and/or morbidity. We can reasonably assume that in the new pandemic phase we still have to deal with an increased risk for many SSc patients; this was suggested by the persistence of high COVID-19-related death rate recorded in the post-vaccine pandemic period, possibly due to multiple adverse factors above mentioned. Therefore, a careful monitoring of individual patients should take into account the SSc activity and progression, the ongoing therapies, and the immune status towards SARS-CoV2 infection. All together these elements can usefully direct the disease treatment strategies and the timing of vaccine booster doses, as well as the correct management of a possible emergence of COVID-19 manifestations.
Author contribution
Clodoveo Ferri MD: Conceptualization; Data curation; Methodology; Formal analysis; Supervision; Visualization; Validation; Writing original draft; Vincenzo Raimondo MD: Methodology; Investigation; Data curation; Validation; Formal analysis; Visualization; Dilia Giuggioli MD: Investigation; Data curation; Validation; critical revision; Laura Gragnani PhD: Data curation; Validation; Formal analysis; Serena Lorini PhD: Formal analysis; Resources; Software; Lorenzo Dagna MD: Investigation; Data curation; Silvia Laura Bosello MD: Investigation; critical revision; Rosario Foti MD: Investigation; Data curation; Valeria Riccieri MD: Investigation; Validation; Serena Guiducci MD: Investigation; Data curation; Giovanna Cuomo MD: Investigation; Validation; Antonio Tavoni MD: Investigation; Data curation; critical revision; Rossella De Angelis MD: Investigation; Data curation; Fabio Cacciapaglia MD: Investigation; Data curation; Elisabetta Zanatta MD: Investigation; Data curation; Franco Cozzi MD: Investigation; Data curation; Giuseppe Murdaca MD: Investigation; Data curation; Ilaria Cavazzana MD: Investigation; Data curation; Nicoletta Romeo MD: Investigation; Data curation; Veronica Codullo MD: Investigation; Data curation; Roberta Pellegrini MD: Investigation; Data curation; Giuseppe Varcasia MD: Investigation; Validation; Maria De Santis MD: Investigation; Data curation; Carlo Selmi: Investigation; Validation; Giuseppina Abignano: Investigation; Data curation; Maurizio Caminiti MD: Investigation; Validation; Massimo L'Andolina MD: Investigation; Data curation; Domenico Olivo MD: Investigation; Data curation; Ennio Lubrano MD: Investigation; Data curation; Amelia Spinella MD: Investigation; Data curation; Federica Lumetti MD: Investigation; Data curation; Giacomo De Luca MD: Investigation; Data curation; Piero Ruscitti MD: Investigation; Validation; Teresa Urraro MD: Investigation; Data curation; Marcella Visentini: Investigation; Data curation; Silvia Bellando-Randone MD: Investigation; Data curation; Elisa Visalli MD: Investigation; Data curation; Davide Testa MD: Investigation; Data curation; Gabriella Sciascia MD: Investigation; Data curation; Francesco Masini MD: Investigation; Data curation; Greta Pellegrino MD: Investigation; Validation; Data curation; Francesca Saccon MD: Investigation; Data curation; Eugenia Balestri MSc: Investigation; Data curation; Giusy Elia MSc: Investigation; Formal analysis; Data curation; review & editing. Silvia Martina Ferrari MSc: Investigation; Data curation; Antonio Tonutti MD: Investigation; Data curation; Francesca Dall’Ara MD: Investigation; Data curation; Giuseppa Pagano Mariano MD: Investigation; Data curation; Giorgio Pettiti MD: Investigation; Data curation; Giovanni Zanframundo MD: Investigation; Data curation; Raffaele Brittelli MD: Investigation; Data curation; Vincenzo Aiello MD: Investigation; Data curation; Ylenia Dal Bosco, MD: Investigation; Data curation; Roberta Foti, MD: Investigation; Data curation; Ilenia Di Cola MD: Investigation; Data curation; Daniela Scorpiniti MD: Investigation; Data curation; Enrico Fusaro MD: Investigation; Data curation; Tommaso Ferrari MD: Investigation; Data curation; Pietro Gigliotti MD: Investigation; Data curation; Corrado Campochiaro MD: Investigation; Data curation; Francesca Francioso MD: Investigation; Data curation; Carlo Iandoli MD: Investigation; Data curation; Virginia Caira MD: Investigation; Data curation; Anna Linda Zignego MD: Investigation; critical revision; Salvatore D'Angelo MD: Investigation; critical revision; Franco Franceschini MD: Investigation; Validation; critical revision; Marco Matucci-Cerinic MD: Investigation; critical revision; Roberto Giacomelli MD: Investigation; critical revision; Andrea Doria MD: Validation; critical revision; Stefano Angelo Santini MD: Validation; critical revision; Poupak Fallahi MD: Investigation; Data curation; critical revision; Florenzo Iannone MD: Investigation; Data curation; critical revision; Alessandro Antonelli MD: Conceptualization; Data curation; Methodology; Formal analysis; Resources; critical revision.
Consent for publication
All patients gave us their consent obviously.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Alessandro Antonelli reports financial support was provided by Italian Ministry of Health, Ricerca Finalizzata (RF-2021-12374986) Destinatario istituzionale: Regione Toscana. Unità Operative:U.O.1: Azienda Ospedaliero-Universitaria Pisana; U.O.2: Azienda Ospedaliero-Universitaria Aldo Moro Bari; U.O.3: Azienda Ospedaliero-Universitaria Modena, CUP Master: D55E22000670001.
Acknowledgements
This research has been granted by Italian Ministry of Health, Ricerca Finalizzata (RF-2021- 12374986). Destinatario istituzionale: Regione Toscana. Unità Operative:U.O.1: Azienda Ospedaliero-Universitaria Pisana; U.O.2: Azienda Ospedaliero-Universitaria Aldo Moro Bari; U.O.3: Azienda Ospedaliero-Universitaria Modena, CUP Master: D55E22000670001.
Handling editor: Y Renaudineau
Data availability
Data will be made available on request.
References
- 1.Costa L., Tasso M., Scotti N., Mostacciuolo E., Girolimetto N., Foglia F., et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort. Ann. Rheum. Dis. 2020;80:e46. doi: 10.1136/annrheumdis-2020-217806. [DOI] [PubMed] [Google Scholar]
- 2.Ferri C., Giuggioli D., Raimondo V., L'Andolina M., Tavoni A., Cecchetti R., et al. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. Clin. Rheumatol. 2020;39:3195–3204. doi: 10.1007/s10067-020-05334-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haberman R., Axelrad J., Chen A., Castillo R., Yan D., Izmirly P., et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N. Engl. J. Med. 2020;383:85–88. doi: 10.1056/NEJMc2009567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., et al. COVID-19 and kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 5.Bozzalla Cassione E., Zanframundo G., Biglia A., Codullo V., Montecucco C., Cavagna L. COVID-19 infection in a northern-Italian cohort of systemic lupus erythematosus assessed by telemedicine. Ann. Rheum. Dis. 2020;79:1382–1383. doi: 10.1136/annrheumdis-2020-217717. [DOI] [PubMed] [Google Scholar]
- 6.Ferri C., Giuggioli D., Raimondo V., Fallahi P., Antonelli A., Group C.-A.I.S. COVID-19 in Italian patients with rheumatic autoimmune systemic diseases. Ann. Rheum. Dis. 2020;82:e211. doi: 10.1136/annrheumdis-2020-219113. [DOI] [PubMed] [Google Scholar]
- 7.Monti S., Balduzzi S., Delvino P., Bellis E., Quadrelli V.S., Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann. Rheum. Dis. 2020;79:667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikuls T.R., Johnson S.R., Fraenkel L., Arasaratnam R.J., Baden L.R., Bermas B.L., et al. American college of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol. 2020;72:1241–1251. doi: 10.1002/art.41301. [DOI] [PubMed] [Google Scholar]
- 9.Gianfrancesco M.A., Hyrich K.L., Gossec L., Strangfeld A., Carmona L., Mateus E.F., et al. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallahi P., Ferrari S.M., Elia G., Paparo S.R., Patrizio A., Balestri E., et al. Thyroid autoimmunity and SARS-CoV-2 infection: report of a large Italian series. Autoimmun. Rev. 2022;21 doi: 10.1016/j.autrev.2022.103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin. Med. 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonelli A., Fallahi P., Elia G., Ragusa F., Paparo S.R., Mazzi V., et al. Effect of the COVID-19 pandemic on patients with systemic rheumatic diseases. Lancet Rheumatol. 2021;3:e675–e676. doi: 10.1016/S2665-9913(21)00243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denton C.P., Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 14.Ferri C., Valentini G., Cozzi F., Sebastiani M., Michelassi C., La Montagna G., et al. Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltim.) 2002;81:139–153. doi: 10.1097/00005792-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann-Vold A.M., Distler O., Bruni C., Denton C.P., de Vries-Bouwstra J., Matucci Cerinic M., et al. Systemic sclerosis in the time of COVID-19. Lancet Rheumatol. 2022;4:e566–e575. doi: 10.1016/S2665-9913(22)00130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferri C., Raimondo V., Gragnani L., Giuggioli D., Dagna L., Tavoni A., et al. Prevalence and death rate of COVID-19 in systemic autoimmune diseases in the first three pandemic waves. Relationship to disease subgroups and ongoing therapies. Curr. Pharmaceut. Des. 2022;28:2022–2028. doi: 10.2174/1381612828666220614151732. [DOI] [PubMed] [Google Scholar]
- 17.Bijlsma J.W., editor. EULAR Compendium on Rheumatic Diseases. New Edition ed. BMJ Publishing Group Ltd; London: 2018. [Google Scholar]
- 18.Ferri C., Giuggioli D., Guiducci S., Lumetti F., Bajocchi G., Magnani L., et al. Systemic sclerosis Progression INvestiGation (SPRING) Italian registry: demographic and clinico-serological features of the scleroderma spectrum. Clin. Exp. Rheumatol. 2020;38(Suppl 125):40–47. [PubMed] [Google Scholar]
- 19.Ferri C., Ursini F., Gragnani L., Raimondo V., Giuggioli D., Foti R., et al. Impaired immunogenicity to COVID-19 vaccines in autoimmune systemic diseases. High prevalence of non-response in different patients' subgroups. J. Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferri C., Gragnani L., Raimondo V., Visentini M., Giuggioli D., Lorini S., et al. Absent or suboptimal response to booster dose of COVID-19 vaccine in patients with autoimmune systemic diseases. J. Autoimmun. 2022;131 doi: 10.1016/j.jaut.2022.102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gragnani L., Visentini M., Lorini S., La Gualana F., Santini S.A., Cacciapaglia F., et al. COVID-19 vaccine immunogenicity in 16 patients with autoimmune systemic diseases. Lack of both humoral and cellular response to booster dose and ongoing disease modifying therapies. J. Transl. Autoimmun. 2022;5 doi: 10.1016/j.jtauto.2022.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Connolly C.M., Teles M., Frey S., Boyarsky B.J., Alejo J.L., Werbel W.A., et al. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann. Rheum. Dis. 2021;81:291–293. doi: 10.1136/annrheumdis-2021-221206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connolly C.M., Chiang T.P., Teles M., Frey S., Alejo J.L., Massie A., et al. Factors associated with poor antibody response to third-dose SARS-CoV-2 vaccination in patients with rheumatic and musculoskeletal diseases. Lancet Rheumatol. 2022;4:e382–e384. doi: 10.1016/S2665-9913(22)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renaudineau Y., Sailler L., Abravanel F., Izopet J., Delourme A., Biotti D., et al. Glucocorticoid use as a cause of non-cellular immune response to SARS-Cov2 Spike in patients with immune system diseases. J. Autoimmun. 2022;133 doi: 10.1016/j.jaut.2022.102912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moiseev S., Avdeev S., Brovko M., Yavorovskiy A., Novikov P.I., Umbetova K., et al. Rheumatic diseases in intensive care unit patients with COVID-19. Ann. Rheum. Dis. 2021;80:e16. doi: 10.1136/annrheumdis-2020-217676. [DOI] [PubMed] [Google Scholar]
- 27.Del Papa N., Sambataro G., Minniti A., Maglione W., Pignataro F., Caminati A., et al. Impact of COVID-19 outbreak in an Italian cohort of patients with systemic sclerosis. Ther. Adv. Musculoskelet. Dis. 2020;12 doi: 10.1177/1759720X20953356. 1759720X20953356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira S.M., Martins L.V.O., Lupino-Assad A.P., Medeiros-Ribeiro A.C., de Moraes D.A., Del-Rio A.P.T., et al. Severity and mortality of COVID-19 in patients with systemic sclerosis: a Brazilian multicenter study. Semin. Arthritis Rheum. 2022;55 doi: 10.1016/j.semarthrit.2022.151987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferri C., Giuggioli D., Raimondo V., L’Andolina M., Dagna L., Tavoni A., et al. Covid-19 and rheumatic autoimmune systemic diseases: role of pre-existing lung involvement and ongoing treatments. Curr. Pharmaceut. Des. 2021;27:4245–4252. doi: 10.2174/1381612827666210903103935. [DOI] [PubMed] [Google Scholar]
- 30.Del Papa N., Sambataro G., Minniti A., Pignataro F., Caporali R. Novel COronaVirus Disease 2019 (COVID-19) epidemic: what are the risks for systemic sclerosis patients? Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haidar G., Agha M., Bilderback A., Lukanski A., Linstrum K., Troyan R., et al. Prospective evaluation of coronavirus disease 2019 (COVID-19) vaccine responses across a broad spectrum of immunocompromising conditions: the COVID-19 vaccination in the immunocompromised study (COVICS) Clin. Infect. Dis. 2022;75:e630–e644. doi: 10.1093/cid/ciac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oyaert M., De Scheerder M.A., Van Herrewege S., Laureys G., Van Assche S., Cambron M., et al. Evaluation of humoral and cellular responses in SARS-CoV-2 mRNA vaccinated immunocompromised patients. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.858399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szebeni G.J., Gémes N., Honfi D., Szabó E., Neuperger P., Balog J., et al. Humoral and cellular immunogenicity and safety of five different SARS-CoV-2 vaccines in patients with autoimmune rheumatic and musculoskeletal diseases in remission or with low disease activity and in healthy controls: a single center study. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.846248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Santis M., Motta F., Isailovic N., Clementi M., Criscuolo E., Clementi N., et al. vol. 10. Vaccines; Basel): 2022. (Dose-Dependent Impairment of the Immune Response to the Moderna-1273 mRNA Vaccine by Mycophenolate Mofetil in Patients with Rheumatic and Autoimmune Liver Diseases). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pri-Paz Basson Y., Tayer-Shifman O.E., Naser R., Tartakover Matalon S., Kimhi O., Gepstein R., et al. Correction to: immunogenicity and safety of the mRNA-based BNT162b2 vaccine in systemic autoimmune rheumatic diseases patients. Clin. Rheumatol. 2022;41:3925. doi: 10.1007/s10067-022-06412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naveen R., Thakare D.R., Kuwana M., Pauling J.D., Day J., Joshi M., et al. Systemic sclerosis and COVID-19 vaccine safety: short-term insights from the global COVID-19 vaccination in autoimmune disease (COVAD) survey. Rheumatol. Int. 2023;43:1265–1275. doi: 10.1007/s00296-023-05310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pellicano C., Campagna R., Oliva A., Leodori G., Miglionico M., Colalillo A., et al. Antibody response to BNT162b2 SARS-CoV-2 mRNA vaccine in adult patients with systemic sclerosis. Clin. Rheumatol. 2022;41:2755–2763. doi: 10.1007/s10067-022-06219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampaio-Barros P.D., Medeiros-Ribeiro A.C., Luppino-Assad A.P., Miossi R., da Silva H.C., Yuki E.F.V.N., et al. SARS-CoV-2 vaccine in patients with systemic sclerosis: impact of disease subtype and therapy. Rheumatology. 2022;61:SI169–S174. doi: 10.1093/rheumatology/keab886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sieiro Santos C., Calleja Antolin S., Moriano Morales C., Garcia Herrero J., Diez Alvarez E., Ramos Ortega F., et al. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open. 2022;8 doi: 10.1136/rmdopen-2021-001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mentzer A.J., O'Connor D., Bibi S., Chelysheva I., Clutterbuck E.A., Demissie T., et al. Human leukocyte antigen alleles associate with COVID-19 vaccine immunogenicity and risk of breakthrough infection. Nat. Med. 2023;29:147–157. doi: 10.1038/s41591-022-02078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemmati D., Longo G., Gallo I., Silva J.A., Secchiero P., Zauli G., et al. Host genetics impact on SARS-CoV-2 vaccine-induced immunoglobulin levels and dynamics: the role of. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1028081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marques C.D.L., Kakehasi A.M., Pinheiro M.M., Mota L.M.H., Albuquerque C.P., Silva C.R., et al. High levels of immunosuppression are related to unfavourable outcomes in hospitalised patients with rheumatic diseases and COVID-19: first results of ReumaCoV Brasil registry. RMD Open. 2021;7 doi: 10.1136/rmdopen-2020-001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niemi M.E.K., Daly M.J., Ganna A. The human genetic epidemiology of COVID-19. Nat. Rev. Genet. 2022;23:533–546. doi: 10.1038/s41576-022-00478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Initiative C.-H.G. A first update on mapping the human genetic architecture of COVID-19. Nature. 2022;608:E1–E10. doi: 10.1038/s41586-022-04826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.