Abstract
It has been proposed that the Plasmodium falciparum cysteine protease falcipain and aspartic proteases plasmepsin I and plasmepsin II act cooperatively to hydrolyze hemoglobin as a source of amino acids for erythrocytic parasites. Inhibitors of each of these proteases have potent antimalarial effects. We have now evaluated the antimalarial effects of combinations of cysteine and aspartic protease inhibitors. When incubated with cultured P. falciparum parasites, cysteine and aspartic protease inhibitors exhibited synergistic effects in blocking parasite metabolism and development. The inhibitors also demonstrated apparent synergistic inhibition of plasmodial hemoglobin degradation both in culture and in a murine malaria model. When evaluated for the treatment of murine malaria, a combination of cysteine and aspartic protease inhibitors was much more effective than higher concentrations of either compound used alone. These results support a model whereby plasmodial cysteine and aspartic proteases participate in the degradation of hemoglobin, and they suggest that combination antimalarial therapy with inhibitors of the two classes of proteases is worthy of further study.
Malaria is one of the most important infectious diseases in the world. Infections with Plasmodium falciparum, the most virulent human malaria parasite, are responsible for hundreds of millions of illnesses and over a million deaths per year (22). A major reason for the continued severity of the worldwide malaria problem is the increasing resistance of malaria parasites to available drugs (12). Thus, it is important to identify new targets for antimalarial therapy and to evaluate new modes of therapy directed against these targets.
Potential new targets for antimalarial chemotherapy include parasite enzymes required for the degradation of hemoglobin. Erythrocytic malaria parasites degrade hemoglobin in an acidic food vacuole to provide amino acids for parasite protein synthesis (reviewed in references 5 and 16). The food vacuole of P. falciparum contains the cysteine protease falcipain and the aspartic proteases plasmepsin I and plasmepsin II (7, 8, 15). Each of these proteases degrades hemoglobin in vitro, and it has been proposed that the enzymes act in a concerted manner to hydrolyze globin to small peptides or free amino acids (5, 16). In a number of in vitro studies, inhibitors of both cysteine and aspartic proteases had potent effects against cultured malaria parasites (1, 4, 11, 14, 15, 17, 18, 20). In an in vivo study utilizing a murine malaria model, a peptidyl cysteine protease inhibitor cured Plasmodium vinckei-infected mice (14). However, high doses of this inhibitor (200 to 400 mg/kg of body weight/day) were required for a pronounced antimalarial effect.
As cysteine and aspartic proteases appear to act cooperatively to degrade hemoglobin, and as inhibitors of both classes of proteases have antimalarial effects, it may be appropriate to use combinations of inhibitors to treat malaria. Such combination therapy might improve efficacy and also slow the development of resistance to new agents. We now report an evaluation of the in vitro and in vivo antimalarial effects of combinations of peptidyl cysteine and aspartic protease inhibitors. These combinations had strong, apparently synergistic inhibitory effects on plasmodial development and hemoglobin degradation in both cultured parasites and in a murine malaria model.
MATERIALS AND METHODS
Protease inhibitors.
l-Transepoxy-succinyl-leucylamido-(4-guanidino)-butane (E-64) and pepstatin were from Sigma. The vinyl sulfone cysteine protease inhibitors morpholine urea leucine-homophenylalanine-phenyl vinyl sulfone (Mu-Leu-Hph-VSPh) and N-methyl piperazine urea-leucine-homophenylalanine-phenyl vinyl sulfone (N-Me-pipu-Leu-Hph-VSPh) were kindly provided by James Palmer, Axys Pharmaceuticals. Protease inhibitors were solubilized as 100X stocks in dimethyl sulfoxide (DMSO). The inhibition of falcipain and its P. vinckei analogue by protease inhibitors was assessed as previously described by using the fluorogenic substrate benzyloxycarbonyl-Phe-Arg-7-amino-4-methyl-coumarin (14, 17). The 50% inhibitory concentrations (IC50s) were determined from curves plotting the inhibition of the cysteine proteases (each at 30 nM) at multiple concentrations of each inhibitor.
Evaluations of cultured malaria parasites.
P. falciparum parasites (It strain except when otherwise noted) were cultured by standard methods (21) in RPMI culture medium supplemented with 10% serum or AlbuMAX I serum substitute (Gibco BRL) and a 2% hematocrit of human erythrocytes (17). Parasite synchrony was maintained by serial treatments with sorbitol (10). Parasite metabolism was assessed by using a minor modification, as previously described (17), of a standard assay of the uptake of [3H]hypoxanthine by cultured parasites (3). Parasite development was assessed by incubating P. falciparum cultures with inhibitors for 48 h, beginning at the ring stage, and then counting new ring-stage parasites on Giemsa-stained smears. For both assays, inhibitors were added to 1-ml cultures from 100X stocks in DMSO, and the results were compared with those from control cultures containing an equal concentration of DMSO. Potential synergy was evaluated by determining the IC50 for the inhibition of parasite metabolism or development for each inhibitor and then evaluating the effects of multiple combinations of cysteine and aspartic protease inhibitors. Concentrations of the two inhibitors that yielded 50% inhibition in activity were plotted on isobolograms.
To evaluate the effects of protease inhibitors on hemoglobin degradation by cultured parasites, cultures were incubated with inhibitors for 4 h, and soluble parasite extracts were then prepared by freeze-thaw and hypotonic lysis as previously described (14). The hydrolysis of [14C]hemoglobin by extracts was then quantitated by scintillation counting of supernatants after treatment with trichloroacetic acid (TCA), also as previously described (15). The presence of radioactive counts in supernatants indicated the hydrolysis of hemoglobin to peptides or individual amino acids, as proteins and large polypeptides are precipitated by TCA.
Evaluations of murine malaria.
Swiss Webster mice were infected with P. vinckei by intraperitoneal injection of parasites from a previously infected mouse. To evaluate the in vivo effects of protease inhibitors on hemoglobin degradation, mice infected with 20 to 40% parasitemias were treated with a single intraperitoneal injection of protease inhibitors in DMSO or, as a control, DMSO alone. After 4 h the mice were sacrificed, their blood was collected, soluble parasite extracts were prepared as previously described (14), and the hydrolysis of [14C]hemoglobin by extracts from treated and control animals was determined as discussed above for cultured parasites. Results were standardized for the parasitemias and blood volume of each animal.
To evaluate the antimalarial efficacy of treatment with protease inhibitors, mice were infected by intraperitoneal injection of 1 × 105 to 5 × 105 parasites (each mouse received the same number of parasites in a given experiment), and after 3 days treatment was initiated with protease inhibitors or, as a control, DMSO, each administered intraperitoneally every 12 h for 4 to 7 days. Mice were evaluated daily for toxicity and for parasitemia by evaluation of Giemsa-stained blood smears. Animals were sacrificed when parasitemias topped 50%.
RESULTS
In vitro antimalarial effects of cysteine and aspartic protease inhibitors.
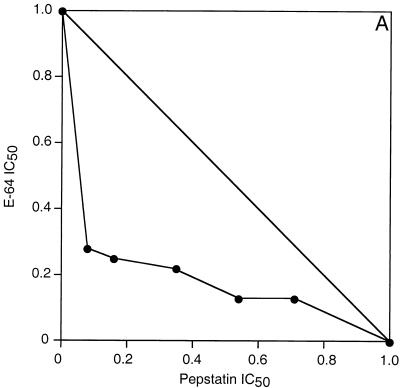
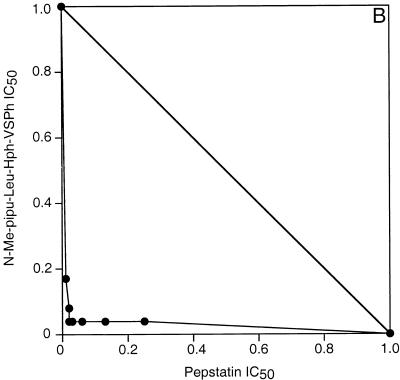
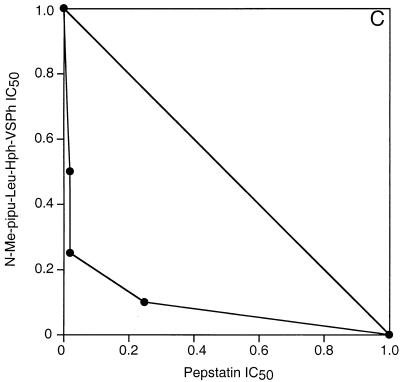
Cultured It strain (chloroquine-resistant) P. falciparum parasites were treated with E-64, which inhibits falcipain and many other cysteine proteases, and pepstatin, which inhibits plasmepsins I and II and many other aspartic proteases (8, 15, 19). As has been previously described (1, 13), each of these compounds inhibited the metabolism and development of cultured parasites, and the combination demonstrated strong antimalarial synergy. An isobologram describing the cooperative inhibition of parasite uptake of [3H]hypoxanthine by E-64 and pepstatin showed a concave slope, indicating synergy (Fig. 1A). Similar studies of E-64 and a peptidomimetic aspartic protease inhibitor also demonstrated synergistic inhibition of parasite hypoxanthine uptake (7). However, all of these protease inhibitors required fairly high concentrations for their antimalarial effects. In our studies the IC50s for the inhibition of [3H]hypoxanthine uptake were 8 μM for E-64 and 4 μM for pepstatin (mean values from nine experiments). In order to develop a combination regimen with a potential for in vivo efficacy, we evaluated much more potent vinyl sulfone inhibitors of falcipain. Morpholine urea leucine-homophenylalanine-phenyl vinyl sulfone (Mu-Leu-Hph-VSPh) was previously shown to inhibit falcipain and block the hemoglobin degradation, metabolism, and development of cultured parasites at low to mid-nanomolar concentrations (17). Combinations of Mu-Leu-Hph-VSPh and pepstatin were strongly synergistic in inhibiting the metabolism ([3H]hypoxanthine uptake) (Fig. 1B) and development (formation of new ring-stage parasites) (Fig. 1C) of cultured parasites. Similar isobolograms demonstrating synergy between Mu-Leu-Hph-VSPh and pepstatin were also generated for two other P. falciparum strains, D6, which is chloroquine sensitive, and W2, which is chloroquine resistant, showing that the effects of the protease inhibitors were independent of resistance to quinoline antimalarials (9).
FIG. 1.
In vitro synergy of cysteine and aspartic protease inhibitors. Parasites grown in microwell cultures were incubated with different concentrations of protease inhibitors, and the effects of combinations of inhibitors were compared to the effects of each inhibitor used alone. Results were plotted by using an isobologram analysis based on the IC50 for each inhibitor. Assays measured the metabolism (uptake of [3H]hypoxanthine) (A and B) and development (formation of new ring-stage parasites) (C) of cultured parasites. Inhibitor concentrations yielding 50% inhibition of control values with each assay were plotted. The concave slopes of the isobologram plots indicate synergistic interactions of the inhibitors.
In vitro inhibition of hemoglobin degradation by cysteine and aspartic protease inhibitors.
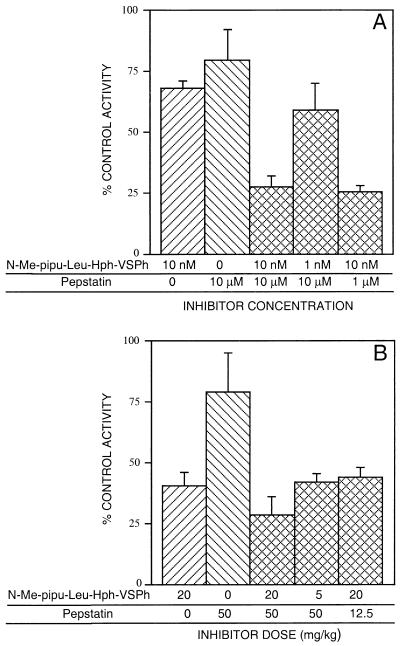
Cysteine protease inhibitors block hemoglobin degradation by P. falciparum, causing the accumulation of undegraded hemoglobin in the parasite food vacuole (1, 6, 13, 15, 17, 18). Pepstatin and other aspartic protease inhibitors also have marked antimalarial effects (1, 4, 11), although a direct effect on hemoglobin degradation by cultured parasites has not been demonstrated. To better characterize the roles of cysteine and aspartic proteases in parasite hemoglobin degradation, we evaluated the effects of combinations of protease inhibitors on this process. These studies utilized pepstatin and N-Me-pipu-Leu-Hph-VSPh, a compound that offers improved solubility over Mu-Leu-Hph-VSPh and has very similar antimalarial effects (the IC50s for 30 nM falcipain were 7 nM for Mu-Leu-Hph-VSPh and 5 nM for N-Me-pipu-Leu-Hph-VSPh). Hemoglobin degradation was measured as the hydrolysis by parasite extracts of [14C]hemoglobin to TCA-soluble peptides or free amino acids (15). The relatively low concentrations of N-Me-pipu-Leu-Hph-VSPh and pepstatin chosen for study caused only modest inhibitions of hemoglobin degradation after a 4-h incubation (Fig. 2A). A combination of the same concentrations of these inhibitors caused much greater inhibition than either agent used alone. A combination including a 10-fold lower concentration of pepstatin was also strongly inhibitory, while a 10-fold reduction in the concentration of N-Me-pipu-Leu-Hph-VSPh provided an intermediate level of inhibition (Fig. 2A).
FIG. 2.
Synergistic inhibition of hemoglobin degradation by cysteine and aspartic protease inhibitors. (A) Cultured P. falciparum trophozoites at 18% parasitemia were incubated with the indicated concentrations of protease inhibitors. (B) Mice infected with 20 to 30% parasitemias of P. vinckei were treated with a single dose, as indicated, of the protease inhibitors by intraperitoneal injection. In each case, after 4 h, parasites were collected, soluble extracts of parasite proteins were prepared, and the abilities of the extracts to degrade [14C]hemoglobin were evaluated. The hemoglobin-degrading activities of protease inhibitor-treated samples are plotted as percentages of the activity of parasites not treated with protease inhibitors. Results shown are mean values from three independent assays of cultured parasites (A) or from two separate analyses of mice, each involving a duplicate assessment of hemoglobin degradation (B). Error bars represent standard deviations of the results.
In vivo inhibition of hemoglobin degradation by cysteine and aspartic protease inhibitors.
We next evaluated the in vivo effects of combinations of N-Me-pipu-Leu-Hph-VSPh and pepstatin on parasite hemoglobin degradation. Mice were infected with the murine malaria parasite P. vinckei, which expresses a cysteine protease very similar to falcipain (14). The P. vinckei cysteine protease, however, is less sensitive to N-Me-pipu-Leu-Hph-VSPh than is falcipain (the IC50 for inhibition of 30 nM enzyme was 200 nM). The expression of aspartic proteases by P. vinckei has not been studied. Infected mice received a single dose of protease inhibitors, and after 4 h their parasites were collected and evaluated for their ability to degrade [14C]hemoglobin. Hemoglobin degradation by parasite extracts was inhibited 59.8% by a 20-mg/kg dose of N-Me-pipu-Leu-Hph-VSPh and 21.0% by a 50-mg/kg dose of pepstatin (Fig. 2B). Combination therapy at the same doses yielded 71.6% inhibition of hemoglobin degradation, and combinations including one-fourth of the full dose of either inhibitor provided about 60% inhibition (Fig. 2B).
In vivo antimalarial effects of cysteine and aspartic protease inhibitors.
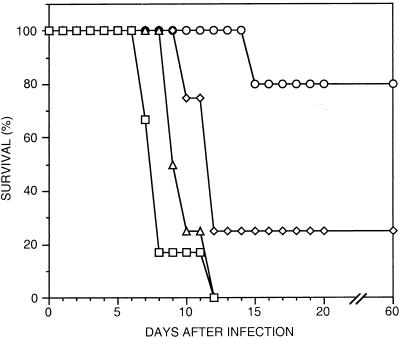
To begin to study whether the apparent synergistic effects of cysteine and aspartic protease inhibitors might be exploited in the therapy of malaria, we treated P. vinckei-infected mice with N-Me-pipu-Leu-Hph-VSPh and pepstatin. Mice were infected with 105 parasites, a quantity which generally causes fatal disease, and after 3 days they were treated with relatively low doses of either protease inhibitor alone or a combination of even lower doses of each inhibitor twice a day for 7 days (Fig. 3). In a representative experiment, N-Me-pipu-Leu-Hph-VSPh (20 mg/kg/dose) offered minimal benefit, and pepstatin (50 mg/kg/dose) cured only 25% of animals. However, a combination of lower doses of N-Me-pipu-Leu-Hph-VSPh (10 mg/kg) and pepstatin (20 mg/kg) was very effective, providing cures in 80% of animals treated for 7 days. Two other experiments incorporating minor alterations in length of treatment (4 days) and inoculum size (1 × 105 to 5 × 105 parasites) also demonstrated improved survival and, in nonsurvivors, prolonged time to lethal parasitemia in animals treated with combinations of N-Me-pipu-Leu-Hph-VSPh and pepstatin.
FIG. 3.
In vivo antimalarial synergy of cysteine and aspartic protease inhibitors. Mice were infected with 105 P. vinckei-infected erythrocytes, and after 3 days therapy was initiated with injections of protease inhibitors in DMSO every 12 h for 7 days. Survival over time is plotted for mice treated with DMSO alone (squares; n = 6), N-Me-pipu-Leu-Hph-VSPh (20 mg/kg/dose) (n = 4; triangles), pepstatin (50 mg/kg/dose, (n = 4; diamonds), or a combination of N-Me-pipu-Leu-Hph-VSPh (10 mg/kg) and pepstatin (20 mg/kg) (n = 5; circles). One mouse treated with the combination, which died of apparent drug toxicity early in the experiment, was excluded from this analysis. Animals treated with the combination of protease inhibitors had markedly improved survival compared to those treated with higher doses of either protease inhibitor administered alone.
Both vinyl sulfone cysteine protease inhibitors and pepstatin are broad-spectrum inhibitors of their respective protease classes that also inhibit many host proteases (2, 19). It was thus not unexpected that the combination of inhibitors engendered some toxicity. Animals treated with higher dose combinations than those discussed above (20 mg/kg/dose of N-Me-pipu-Leu-Hph-VSPh and 50 mg/kg/dose of pepstatin) developed lethal toxicity, although malaria infections were well controlled. Of 10 mice treated in two experiments with the combination dose shown in Fig. 3, 3 animals died without demonstrable parasitemia, indicating probable combination drug toxicity due to the inhibition of host cysteine and aspartic proteases. In additional experiments, lower doses of the two inhibitors caused less toxicity but also yielded diminished antimalarial efficacy, indicating dose responses of combination therapy for both toxicity and antimalarial effects.
DISCUSSION
Our results indicate that combinations of cysteine and aspartic protease inhibitors have strong antimalarial effects, both against cultured P. falciparum parasites and against murine P. vinckei infections. The in vitro effects of these inhibitors on parasite metabolism and development were clearly synergistic, as indicated by markedly concave slopes on isobolograms. We hypothesized that the two classes of protease inhibitors were synergistic due to the inhibition of two classes of proteases that cooperatively degrade hemoglobin. In order to test this hypothesis, we evaluated the effects of cysteine and aspartic protease inhibitors on plasmodial hemoglobin degradation. In in vitro studies with P. falciparum, a combination of cysteine and aspartic protease inhibitors exerted greater inhibition of hemoglobin degradation than would be predicted for a simple additive effect. These studies likely understated the true effects of the inhibitors on hemoglobin degradation, as the assay only identified hydrolysis of hemoglobin to small TCA-soluble peptides. Pepstatin had a relatively small effect on hemoglobin degradation when used alone, suggesting that, as has been previously proposed (5), plasmepsins I and II may be responsible primarily for early cleavages of hemoglobin that do not yield TCA-soluble peptides. In addition, pepstatin is a hydrophobic peptide that may not be transported efficiently to the food vacuole, and so vacuolar concentrations of the inhibitor may not have been high enough to maximally inhibit plasmepsins I and II. Thus, the maximal inhibitory effect of cysteine and aspartic protease inhibitors on plasmodial hemoglobin degradation is likely to be greater than that suggested by our studies with pepstatin and the [14C]hemoglobin degradation assay. In any event, our results suggest that cysteine and aspartic protease inhibitors exert their synergistic antimalarial effects via a synergistic inhibition of hemoglobin degradation.
In vivo studies utilized a P. vinckei murine malaria model. As shown in vitro, combinations of cysteine and aspartic protease inhibitors caused a more marked inhibition of hemoglobin degradation by murine parasites than would be predicted for an additive inhibitory effect. We next evaluated the ability of protease inhibitor combinations to treat murine malaria. The combinations demonstrated potent antimalarial efficacy. At doses that were ineffective when either compound was used alone, the combined protease inhibitors cured the majority of infected animals. These results suggest that combinations of cysteine and aspartic protease inhibitors are synergistic in the inhibition of plasmodial hemoglobin degradation in vivo and in the treatment of murine malaria.
The apparent synergistic antimalarial effects of cysteine and aspartic protease inhibitors were accompanied by an apparent synergistic toxicity. This increased toxicity was likely due to the inhibition of host cysteine and aspartic proteases. Clearly, therapy with broadly active cysteine and aspartic protease inhibitors will have limitations, and our current results do not yet offer specific new compounds for therapeutic trials. Optimal antimalarial therapy will utilize highly specific inhibitors of falcipain and plasmepsins I and II that should prevent toxicity due to the inhibition of host enzymes. Efforts to develop such compounds are currently under way.
Our results offer strong support to models suggesting that plasmodial cysteine and aspartic proteases act cooperatively to degrade hemoglobin. Combination therapy with cysteine and aspartic protease inhibitors is therefore rational, and our present results suggest that it is likely to offer potent antimalarial efficacy. In addition to improved efficacy, the use of protease inhibitor combinations should decrease the rate of development of parasite resistance to new compounds. As new selective inhibitors of falcipain and plasmepsins I and II are identified, the evaluation of their combined in vitro and in vivo antimalarial effects is warranted.
ACKNOWLEDGMENTS
We thank James Palmer, Axys Pharmaceuticals, for generously providing vinyl sulfone protease inhibitors and Dennis Kyle, Walter Reed Army Institute of Research, for kindly performing in vitro assays on additional parasite strains.
This work was supported by grants from the National Institutes of Health, the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and the American Heart Association. P.J.R. is an Established Investigator of the American Heart Association.
REFERENCES
- 1.Bailly E, Jambou R, Savel J, Jaureguiberry G. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and pepstatin A (aspartyl protease inhibitor) J Protozool. 1992;39:593–599. doi: 10.1111/j.1550-7408.1992.tb04856.x. [DOI] [PubMed] [Google Scholar]
- 2.Bromme D, Klaus J L, Okamoto K, Rasnick D, Palmer J T. Peptidyl vinyl sulphones: a new class of potent and selective cysteine protease inhibitors: S2P2 specificity of human cathepsin O2 in comparison with cathepsins S and L. Biochem J. 1996;315:85–89. doi: 10.1042/bj3150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis S E, Gluzman I Y, Oksman A, Knickerbocker A, Mueller R, Bryant M L, Sherman D R, Russell D G, Goldberg D E. Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 1994;13:306–317. doi: 10.1002/j.1460-2075.1994.tb06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Francis S E, Sullivan D J, Goldberg D E. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 6.Gamboa de Domínguez N D, Rosenthal P J. Cysteine proteinase inhibitors block early steps in hemoglobin degradation by cultured malaria parasites. Blood. 1996;87:4448–4454. [PubMed] [Google Scholar]
- 7.Gluzman I Y, Francis S E, Oksman A, Smith C E, Duffin K L, Goldberg D E. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J Clin Investig. 1994;93:1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg D E, Slater A F G, Beavis R, Chait B, Cerami A, Henderson G B. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J Exp Med. 1991;173:961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle, D. E. Personal communication.
- 10.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 11.Moon R P, Tyas L, Certa U, Rupp K, Bur D, Jacquet C, Matile H, Loetscher H, Grueninger-Leitch F, Kay J, Dunn B M, Berry C, Ridley R G. Expression and characterisation of plasmepsin I from Plasmodium falciparum. Eur J Biochem. 1997;244:552–560. doi: 10.1111/j.1432-1033.1997.00552.x. [DOI] [PubMed] [Google Scholar]
- 12.Olliaro P, Cattani J, Wirth D. Malaria, the submerged disease. JAMA. 1996;275:230–233. [PubMed] [Google Scholar]
- 13.Rosenthal P J. Plasmodium falciparum: effects of proteinase inhibitors on globin hydrolysis by cultured malaria parasites. Exp Parasitol. 1995;80:272–281. doi: 10.1006/expr.1995.1033. [DOI] [PubMed] [Google Scholar]
- 14.Rosenthal P J, Lee G K, Smith R E. Inhibition of a Plasmodium vinckei cysteine proteinase cures murine malaria. J Clin Investig. 1993;91:1052–1056. doi: 10.1172/JCI116262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal P J, McKerrow J H, Aikawa M, Nagasawa H, Leech J H. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J Clin Investig. 1988;82:1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal P J, Meshnick S R. Hemoglobin catabolism and iron utilization by malaria parasites. Mol Biochem Parasitol. 1996;83:131–139. doi: 10.1016/s0166-6851(96)02763-6. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal P J, Olson J E, Lee G K, Palmer J T, Klaus J L, Rasnick D. Antimalarial effects of vinyl sulfone cysteine proteinase inhibitors. Antimicrob Agents Chemother. 1996;40:1600–1603. doi: 10.1128/aac.40.7.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal P J, Wollish W S, Palmer J T, Rasnick D. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. J Clin Investig. 1991;88:1467–1472. doi: 10.1172/JCI115456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvesen G, Nagase H. Inhibition of proteolytic enzymes. In: Beynon R J, Bond J S, editors. Proteolytic enzymes. Oxford, United Kingdom: IRL Press; 1989. pp. 83–104. [Google Scholar]
- 20.Silva A M, Lee A Y, Gulnik S V, Majer P, Collins J, Bhat T N, Collins P J, Cachau R E, Luker K E, Gluzman I Y, Francis S E, Oksman A, Goldberg D E, Erickson J W. Structure and inhibition of plasmepsin II, a hemoglobin-degrading enzyme from Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:10034–10039. doi: 10.1073/pnas.93.19.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 22.Walsh J A. Disease problems in the Third World. Ann N Y Acad Sci. 1989;569:1–16. doi: 10.1111/j.1749-6632.1989.tb27354.x. [DOI] [PubMed] [Google Scholar]