Abstract
Background
Urinary tract infection (UTI) is one of the most common bacterial infections in infants. The most severe form of UTI is acute pyelonephritis, which results in significant acute morbidity and may cause permanent kidney damage. There remains uncertainty regarding the optimum antibiotic regimen, route of administration and duration of treatment. This is an update of a review that was first published in 2003 and updated in 2005 and 2007.
Objectives
To evaluate the benefits and harms of antibiotics used to treat children with acute pyelonephritis. The aspects of therapy considered were 1) different antibiotics, 2) different dosing regimens of the same antibiotic, 3) different duration of treatment, and 4) different routes of administration.
Search methods
We searched the Cochrane Renal Group's Specialised Register, CENTRAL, MEDLINE, EMBASE, reference lists of articles and conference proceedings without language restriction to 10 April 2014.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing different antibiotic agents, routes, frequencies or durations of therapy in children aged 0 to 18 years with proven UTI and acute pyelonephritis were selected.
Data collection and analysis
Four authors independently assessed study quality and extracted data. Statistical analyses were performed using the random‐effects model and the results expressed as risk ratio (RR) for dichotomous outcomes or mean difference (MD) for continuous data with 95% confidence intervals (CI).
Main results
This updated review included 27 studies (4452 children). This update included evidence from three new studies, and following re‐evaluation, a previously excluded study was included because it now met our inclusion criteria.
Risk of bias was assessed as low for sequence generation (12 studies), allocation concealment (six studies), blinding of outcome assessors (17 studies), incomplete outcome reporting (19 studies) and selective outcome reporting (13 studies). No study was blinded for participants or investigators. The 27 included studies evaluated 12 different comparisons. No significant differences were found in duration of fever (2 studies, 808 children: MD 2.05 hours, 95% CI ‐0.84 to 4.94), persistent UTI at 72 hours after commencing therapy (2 studies, 542 children: RR 1.10, 95% CI 0.07 to 17.41) or persistent kidney damage at six to 12 months (4 studies, 943 children: RR 0.82, 95% CI 0.59 to 1.12) between oral antibiotic therapy (10 to 14 days) and intravenous (IV) therapy (3 days) followed by oral therapy (10 days). Similarly, no significant differences in persistent bacteriuria at the end of treatment (4 studies, 305 children: RR 0.78, 95% CI 0.24 to 2.55) or persistent kidney damage (4 studies, 726 children: RR 1.01, 95% CI 0.80 to 1.29) were found between IV therapy (three to four days) followed by oral therapy and IV therapy (seven to 14 days). No significant differences in efficacy were found between daily and thrice daily administration of aminoglycosides (1 study, 179 children, persistent clinical symptoms at three days: RR 1.98, 95% CI 0.37 to 10.53). Adverse events were mild and uncommon and rarely resulted in discontinuation of treatment.
Authors' conclusions
This updated review increases the body of evidence that oral antibiotics alone are as effective as a short course (three to four days) of IV antibiotics followed by oral therapy for a total treatment duration of 10 to 14 days for the treatment of acute pyelonephritis in children. When IV antibiotics are given, a short course (two to four days) of IV therapy followed by oral therapy is as effective as a longer course (seven to 10 days) of IV therapy. If IV therapy with aminoglycosides is chosen, single daily dosing is safe and effective. Insufficient data are available to extrapolate these findings to children aged less than one month of age or to children with dilating vesicoureteric reflux (grades III‐V). Further studies are required to determine the optimal total duration of antibiotic therapy required for acute pyelonephritis.
Plain language summary
Are oral antibiotics as effective as a combination of injected and oral antibiotics for kidney infections in children?
Acute pyelonephritis refers to infection of the kidneys and is the most severe form of urinary tract infection (UTI). Acute pyelonephritis causes high fever, vomiting, stomach pain, irritability and poor feeding in infants.
We wanted to find out if oral antibiotics were as effective as combined oral and injected antibiotics to treat children for kidney infection. This review updates our previous investigations published in 2003, 2005 and 2007. This review included evidence from 27 studies that involved 4452 children. The last literature search date was April 2014. This update included evidence from three new studies and from one study that was previously excluded.
Review results suggested that children aged over one month with acute pyelonephritis can be treated effectively with oral antibiotics (cefixime, ceftibuten or amoxicillin/clavulanic acid) or with short courses (two to four days) of intravenous (IV) therapy followed by oral therapy. If IV therapy with aminoglycosides is needed, single daily dosing is safe and effective.
Summary of findings
Summary of findings for the main comparison. Oral versus IV followed by oral (11 days) therapy for acute pyelonephritis in children.
| Oral versus IV followed by oral (11 days) therapy for acute pyelonephritis in children | ||||||
| Patient or population: children with acute pyelonephritis Intervention: oral versus IV followed by oral (11 days) therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral | IV followed by oral (11 days) therapy | |||||
| Time to fever resolution (hours) | The mean time to fever resolution (hours) in the intervention groups was 2.05 higher (0.84 lower to 4.94 higher) | 808 (2) | ⊕⊕⊕⊝ moderate1 | |||
| Renal parenchymal damage at 6 to 12 months: all included children with acute pyelonephritis DMSA scans Follow‐up: 6 to 12 months | Study population | RR 0.82 (0.59 to 1.12) | 943 (4) | ⊕⊕⊕⊝ moderate2 | ||
| 224 per 1000 | 184 per 1000 (132 to 251) | |||||
| Moderate | ||||||
| 313 per 1000 | 257 per 1000 (185 to 351) | |||||
| Renal parenchymal damage at 6 to 12 months: children with renal parenchymal damage on initial DMSA Follow‐up: 6 to 12 months | Study population | RR 0.79 (0.61 to 1.03) | 681 (4) | ⊕⊕⊕⊝ moderate3 | ||
| 320 per 1000 | 253 per 1000 (195 to 330) | |||||
| Moderate | ||||||
| 382 per 1000 | 302 per 1000 (233 to 393) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence intervals due to large standard deviations around the mean durations of fever 2 Large number of patients excluded because of lack of follow‐up DMSA scans 3 No explanation was provided
Summary of findings 2. Short duration (3 to 4 days) versus long duration (7 to 14 days) IV therapy for acute pyelonephritis in children.
| Short duration (3 to 4 days) versus long duration (7 to 14 days) IV therapy for acute pyelonephritis in children | ||||||
| Patient or population: children with acute pyelonephritis Intervention: short duration (3 to 4 days) versus long duration (7 to 14 days) IV therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short duration (3 to 4 days) | Long duration (7 to 14 days) IV therapy | |||||
| Persistent bacteriuria after treatment | Study population | RR 0.78 (0.24 to 2.55) | 305 (4) | ⊕⊕⊝⊝ low1,2 | ||
| 38 per 1000 | 30 per 1000 (9 to 98) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Recurrent UTI within 6 months | Study population | RR 0.97 (0.58 to 1.62) | 993 (5) | ⊕⊕⊕⊝ moderate1 | ||
| 59 per 1000 | 57 per 1000 (34 to 95) | |||||
| Moderate | ||||||
| 56 per 1000 | 54 per 1000 (32 to 91) | |||||
| Persistent renal damage at 3 to 6 months: all included children with acute pyelonephritis | Study population | RR 1.01 (0.8 to 1.29) | 726 (4) | ⊕⊕⊕⊝ moderate1,3 | ||
| 246 per 1000 | 249 per 1000 (197 to 318) | |||||
| Moderate | ||||||
| 257 per 1000 | 260 per 1000 (206 to 332) | |||||
| Persistent renal damage at 3 to 6 months: children with initial renal parenchymal damage on initial DMSA scan Follow‐up: 6‐12 months | Study population | RR 1.1 (0.84 to 1.45) | 315 (3) | ⊕⊕⊕⊝ moderate1,3 | ||
| 357 per 1000 | 393 per 1000 (300 to 518) | |||||
| Moderate | ||||||
| 327 per 1000 | 360 per 1000 (275 to 474) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear or inadequate allocation concealment 2 Small number of patients and events leading to wide confidence intervals 3 In several studies, more than 10% patients lost to follow‐up or did not have follow‐up DMSA scans
Summary of findings 3. Different dosing regimens of aminoglycosides (daily versus 8 hourly) for acute pyelonephritis in children.
| Different dosing regimens of aminoglycosides (daily versus 8 hourly) for acute pyelonephritis in children | ||||||
| Patient or population: children with acute pyelonephritis Intervention: different dosing regimens of aminoglycosides (daily versus 8 hourly) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Daily dose | 8 hourly dose | |||||
| Persistent bacteriuria after 1 to 3 days of treatment | Study population | RR 1.05 (0.15 to 7.27) | 435 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 9 per 1000 | 10 per 1000 (1 to 67) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Hearing impairment following treatment | Study population | RR 2.83 (0.33 to 24.56) | 271 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Increase in serum creatinine during treatment | Study population | RR 0.75 (0.2 to 2.82) | 419 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 25 per 1000 | 19 per 1000 (5 to 70) | |||||
| Moderate | ||||||
| 25 per 1000 | 19 per 1000 (5 to 70) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear allocation concealment in two of three studies 2 Few events resulting in wide confidence intervals
Summary of findings 4. Agent: Third generation cephalosporin versus other antibiotic for acute pyelonephritis in children.
| Agent: Third generation cephalosporin versus other antibiotic for acute pyelonephritis in children | ||||||
| Patient or population: children with acute pyelonephritis Intervention: third generation cephalosporin versus other antibiotic | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antibiotic | Third generation cephalosporin | |||||
| Persistent bacteriuria | Study population | RR 2.41 (0.98 to 5.93) | 439 (3) | ⊕⊕⊝⊝ low1,2 | ||
| 34 per 1000 | 81 per 1000 (33 to 199) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Recurrent UTI after end of therapy | Study population | RR 1.23 (0.32 to 4.74) | 491 (4) | ⊕⊕⊝⊝ low1,2 | ||
| 18 per 1000 | 22 per 1000 (6 to 87) | |||||
| Moderate | ||||||
| 8 per 1000 | 10 per 1000 (3 to 38) | |||||
| Persistent symptoms after end of treatment | Study population | RR 0.28 (0.13 to 0.62) | 471 (3) | ⊕⊕⊝⊝ low1,3 | ||
| 104 per 1000 | 29 per 1000 (14 to 64) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear allocation in several studies 2 Few events leading to imprecision 3 Meta‐analysis dominated by single trial and results inconsistent with bacteriologic results
Background
Description of the condition
The urinary tract is a common site of bacterial infection in infants and young children. Pooled prevalence data demonstrate that approximately 7% of girls and boys are diagnosed with at least one urinary tract infection (UTI) by the age of 19 years (Shaikh 2008). Girls are more susceptible to UTIs than boys after the first six months of life with a prevalence of 11% in girls and 4% in boys (Brkic 2010). UTI is defined by the presence of bacteria in urine (bacteriuria), which when cultured is measured in colony forming units/mL (CFU/mL) of uncentrifuged urine. The diagnosis of UTI in children is generally confirmed by the pure growth of a bacteria of greater than 10³ CFU/mL from a suprapubic aspirate, 10⁴ CFU/mL from a bladder catheter specimen and 10⁵ CFU/mL from non‐invasive collection methods (clean catch and urinary bag specimens) (Bhat 2011).
UTIs can be clinically grouped into asymptomatic bacteriuria, cystitis and acute pyelonephritis.
Asymptomatic bacteriuria is the presence of bacteriuria without clinical signs and symptoms.
Cystitis is a UTI limited to the urethra and bladder and is seen most commonly in girls over two years of age. It presents with localising symptoms of dysuria (pain when passing urine), frequency, urgency, cloudy urine and lower abdominal discomfort. Pyuria (white cells in the urine) and haematuria (blood in the urine) may also be found.
Acute pyelonephritis refers to infection of the kidneys, and is the most severe form of UTI in children. Clinically, this is associated with systemic features such as high fever, malaise, vomiting, abdominal and loin pain and tenderness, poor feeding and irritability in infants. Together with urine culture, diagnosis may be assisted by imaging using technetium 99m labelled dimercaptosuccinic acid (DMSA) renal scan and markers of inflammation in the blood such as erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP).
Acute pyelonephritis is associated with significant short‐term morbidity, including shock and septicaemia, especially in infants. Acute kidney parenchymal injury has been demonstrated on DMSA scan in about 60% of children shortly after UTI diagnosis (Shaikh 2010). Permanent kidney damage may occur following acute pyelonephritis and is more frequent in children who have multiple episodes or who have vesicoureteric reflux (VUR) (Shaikh 2010; Smellie 1985). Serial DMSA scans of children after a first episode of acute pyelonephritis show that 15% of children with acute changes on DMSA scans have permanent kidney scarring at follow up (Shaikh 2010). However, the long term significance of kidney damage following acute pyelonephritis is debatable. In children with previously normal kidneys, the amount of damage is small and unlikely to cause disease (Salo 2011; Toffolo 2012).
Description of the intervention
A wide variety of antibiotic agents have been used to treat acute pyelonephritis in children. Antibiotics that have been used include aminoglycosides, cephalosporins, penicillins and trimethoprim/sulphamethoxazole (TMP/SMX). Historically, children who are judged by clinicians to be in poor general condition are given parenteral antibiotics and those who appear less sick have been given oral antibiotics, without clarity whether one route of administration is superior. An antibiotic course of seven to 14 days is generally recommended although the optimal duration of therapy is not known. Shorter courses may be associated with treatment failure while longer courses may unnecessarily expose children to the adverse effects of treatment. This review evaluated antibiotic therapies used to treat acute pyelonephritis, with consideration of different antibiotic agents, different dosing regimens of the same antibiotic, different durations of treatment and different routes of administration.
How the intervention might work
Antibiotics work in the treatment of acute pyelonephritis by eliminating bacterial infection in the urinary tract. The purpose of antibiotic therapy is to eradicate and prevent progressive infection and its consequences, including shock and septicaemia, reduce acute kidney injury and resolve the acute clinical symptoms of infection. The efficacy of treatment depends on using the antibiotic(s) to which the bacteria is sensitive. While the results of antibiotic sensitivity testing are pending, initial treatment is chosen on an empiric basis to cover the most likely cause of infection.
Why it is important to do this review
Acute pyelonephritis is a common serious infection in children. Nonetheless, there remains no consensus on the most effective antibiotic regimen for the treatment of acute pyelonephritis. There is also uncertainty regarding the optimal route of administration of antibiotic therapy. Previously, most authorities recommended commencing antibiotic therapy by the parenteral route. The most recent guidelines however recommend initial treatment with oral antibiotics for children older than two months (AAP 2011) or three months of age (NICE 2007) unless children are considered to be too unwell or unable to take oral antibiotics. This is advantageous because oral therapy is more convenient and does not require hospital admission, thereby reducing costs. There is further uncertainty about the optimal duration of antibiotic therapy. Currently, the recommended duration of therapy varies from seven to 14 days.
This review update was necessary to provide additional information about the optimum antibiotic regimens, route of administration and duration of treatment for acute pyelonephritis in children and about adverse effects of treatment.
Objectives
To evaluate the benefits and harms of antibiotics used to treat children with acute pyelonephritis. The aspects of therapy considered were:
different antibiotics
different dosing regimens of the same antibiotic
different duration of treatment
different routes of administration.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) in which antibiotics were used in the treatment of children (birth to 18 years) with acute pyelonephritis were included. Where studies included both children with acute pyelonephritis and those with cystitis, these were included if data for participants with acute pyelonephritis could be extracted separately; otherwise, these studies were excluded.
Types of participants
Inclusion criteria
Children from birth to 18 years with acute pyelonephritis treated either in hospital or as outpatients with antibiotics were included. For this review, the diagnosis of acute pyelonephritis required UTI (as specified in the included studies but generally requiring a bacterial growth on urine culture of more than 10⁵ CFU/mL or 10⁸ CFU/L) with at least one symptom or sign of systemic illness such as fever, loin pain or toxicity and additional diagnostic criteria as defined by the authors of the included studies. Children with previously diagnosed urinary tract abnormalities including VUR or previous UTI could be included.
Exclusion criteria
Patients considered to have asymptomatic bacteriuria or cystitis (UTI as defined in Inclusions with no symptom or sign of systemic illness) were excluded.
Types of interventions
Different antibiotic agents
IV antibiotic versus oral antibiotic
Different doses or duration or both of the same antibiotic
Antibiotic versus placebo, no therapy or alternative non‐antibiotic therapy.
Types of outcome measures
Primary outcomes
Short‐term outcome measures.
Duration of fever
Persistent symptoms (e.g. UTI at 72 hours; inflammatory markers at 72 hours (ESR, WCC, CRP)
Acute kidney parenchymal damage on DMSA scan
Length of hospital stay for inpatients
Persistent bacteriuria after completion of antibiotics
Recurrent UTI
Adverse effects of treatment including minor (e.g. vomiting, discomfort from IV cannula) and major (e.g. anaphylaxis, hearing impairment)
Economic costs of treatment (if data available).
Secondary outcomes
Long‐term outcome measures.
Persistent kidney damage (as defined by authors of included studies)
Hypertension
Chronic kidney disease.
Search methods for identification of studies
Electronic searches
For the 2014 update, we searched the Cochrane Renal Group's Specialised Register through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of the Cochrane Renal Group. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group.
See Appendix 1 for search terms used in strategies for this review update.
For previous search strategies please refer to our earlier reviews (Bloomfield 2003; Bloomfield 2005; Hodson 2007).
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies relevant to the review. Titles and abstracts were screened independently by four authors, who discarded studies that were not applicable. However, studies and reviews that included relevant data or information on studies were retained initially. Four authors independently assessed retrieved abstracts, and if necessary the full text, to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by four authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions those data were used. Any discrepancy between published versions was highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by four authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (persistent bacteriuria, recurrent UTI, persistent clinical symptoms, presence of kidney parenchymal damage, adverse effects) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (duration of fever, inflammatory markers, extent of kidney parenchymal damage), the mean difference (MD) was used.
Unit of analysis issues
The unit of analysis was the study participant and not events, that is, the number of children with acute pyelonephritis rather than the number of episodes of acute pyelonephritis per child.
Dealing with missing data
Where data were missing or unclear, we contacted the original authors of studies to request additional data. An attempt to obtain the preliminary results of the terminated study (NCT00724256) was made by contacting the lead investigator. We did not impute missing data.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi squared test on N‐1 degrees of freedom with an alpha of 0.1 used for statistical significance and the I² statistic (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We planned to assess publication bias by constructing funnel plots; however, there were insufficient data in each meta‐analysis to enable this analysis to be conducted.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned to explore possible sources of heterogeneity (participants, treatments and study quality) but could not be undertaken because of the small number of studies for each comparison. Heterogeneity among participants could be related to age (infants versus adolescents) and pre‐existing renal tract pathology. Heterogeneity in treatment could be related to inpatient versus outpatient management, prior antibiotics used and the antibiotic, dose, duration and route of administration of therapy.
Sensitivity analysis
Sensitivity analysis was planned to identify individual studies that were contributing to significant heterogeneity (I² value greater than 75%) but the I² values of all meta‐analyses were less than 50% so sensitivity analysis was not undertaken.
Results
Description of studies
Results of the search
First version published 2003
The initial search in September 2002 identified 1520 titles and abstracts of which 51 were screened. We found that 16 parallel RCTs (16 reports) involving 1872 children fulfilled the eligibility criteria and were included in the review. We excluded 11 studies (11 reports) (Bloomfield 2003).
Review updates published 2005 and 2007
A search in June 2004 identified two additional studies (three reports) (Chong 2003; Montini 2007). A total of 18 studies (19 reports) involving 2612 children were included in our 2005 review update (Bloomfield 2005).
A search from 2004 to July 2007 identified 26 reports of which 16 were excluded (not randomised, mixed populations or wrong interventions). We included five new studies (nine reports) (Banfi 1993; Cheng 2006; Fujii 1987; Neuhaus 2008; Noorbakhsh 2004). The final results of the multicentre study by Montini 2007 were published and included in this update. We included 23 studies (29 reports) that involved 3407 children in the 2007 update (Hodson 2007).
Review update 2014
A search in 10 April 2014 identified 32 reports. In addition, a previously excluded study was re‐evaluated and included because it had been excluded incorrectly based on how outcomes were reported. Khan 1981 had been previously excluded because results were reported as episodes of acute pyelonephritis rather than number of patients with an episode of acute pyelonephritis. We excluded 17 studies (17 reports) and identified one eligible study that was terminated (see Characteristics of ongoing studies); contact with the triallists confirmed that no results were available (NCT00724256). We found that nine new records were further reports of four previously included studies (Benador 2001; Cheng 2006; Montini 2007; Neuhaus 2008). Reports relating to Benador 2001, Cheng 2006 and Montini 2007 did not provide any new data; however, the final results of Neuhaus 2008 (two new reports) were published in September 2008 and results were included in this review update. Four reports were of three newly identified studies (Bocquet 2012; Bouissou 2008; Marild 2009). This update included 27 studies (42 reports) that involved 4452 children.
See Figure 1.
1.

Study flow diagram.
Included studies
The characteristics of the 27 included studies are summarised in Characteristics of included studies.
Participants
Studies recruited participants from the ages of two weeks to 16 years. Three studies did not specify age range (Bakkaloglu 1996; Levtchenko 2001; Pylkkänen 1981).
Healthcare settings
Children received treatment while inpatients in seven studies (Bakkaloglu 1996; Carapetis 2001; Chong 2003; Fujii 1987; Kafetzis 2000; Montini 2007; Vigano 1992).
Children were treated as outpatients only in four studies (Baker 2001; Khan 1981; Pylkkänen 1981; Repetto 1984).
Children received treatment in both in‐ and outpatient settings in 16 studies (Banfi 1993; Benador 2001; Bocquet 2012; Bouissou 2008; Cheng 2006; Fischbach 1989; Francois 1997; Hoberman 1999; Grimwood 1988; Levtchenko 2001; Marild 2009; Neuhaus 2008; Noorbakhsh 2004; Schaad 1998; Toporovski 1992; Vilaichone 2001).
Urine collection
All urine specimens were collected by suprapubic aspiration, catheter or midstream specimens in 14 studies (Banfi 1993; Baker 2001; Bouissou 2008; Carapetis 2001;Chong 2003; Grimwood 1988; Hoberman 1999; Kafetzis 2000; Khan 1981; Neuhaus 2008; Pylkkänen 1981; Repetto 1984; Toporovski 1992; Vigano 1992).
Specimens were obtained by strap‐on bag collection in nine studies (Benador 2001; Bocquet 2012; Cheng 2006; Levtchenko 2001; Marild 2009; Montini 2007; Noorbakhsh 2004; Schaad 1998; Vilaichone 2001).
The method of urine collection was not specified in four studies (Bakkaloglu 1996; Fischbach 1989; Francois 1997; Fujii 1987).
Diagnosis
All participants had acute pyelonephritis in 22 studies (Baker 2001; Bakkaloglu 1996; Benador 2001; Bocquet 2012; Bouissou 2008; Carapetis 2001; Cheng 2006; Chong 2003; Fischbach 1989; Francois 1997; Fujii 1987; Hoberman 1999; Kafetzis 2000; Levtchenko 2001; Marild 2009; Montini 2007; Neuhaus 2008; Noorbakhsh 2004; Schaad 1998; Toporovski 1992; Vigano 1992; Vilaichone 2001).
Five studies enrolled children with both acute pyelonephritis and lower UTI (Banfi 1993; Grimwood 1988; Khan 1981; Pylkkänen 1981; Repetto 1984); data from children with acute pyelonephritis, which could be separated, were included in this review.
Definition of acute pyelonephritis
All studies required positive urine culture. Additional criteria required for diagnosis of acute pyelonephritis in children with UTI varied among studies:
Four studies required fever > 38°C (Baker 2001; Bocquet 2012; Hoberman 1999; Khan 1981).
Eight required fever and at least one additional clinical feature (Bakkaloglu 1996; Carapetis 2001; Chong 2003; Grimwood 1988; Noorbakhsh 2004; Repetto 1984; Schaad 1998; Toporovski 1992).
Nine required fever, clinical features and/or laboratory abnormalities (CRP, ESR, white blood count) (Bouissou 2008; Fischbach 1989; Francois 1997; Kafetzis 2000; Levtchenko 2001; Marild 2009; Montini 2007; Pylkkänen 1981; Vigano 1992).
Three required fever, clinical features and acute kidney parenchymal injury on DMSA scan (Benador 2001; Neuhaus 2008; Vilaichone 2001).
Five other studies (Bocquet 2012; Chong 2003; Hoberman 1999; Levtchenko 2001; Montini 2007) provided information on the number of children with acute pyelonephritis based on clinical characteristics, who had DMSA abnormalities at study entry.
One study required fever with computer tomography scan evidence of acute lobular nephronia (Cheng 2006).
Two studies did not report the definition used for acute pyelonephritis (Banfi 1993; Fujii 1987).
Commonly reported exclusion criteria
Impaired kidney function (12 studies: Carapetis 2001; Chong 2003; Fischbach 1989; Francois 1997; Kafetzis 2000; Khan 1981; Noorbakhsh 2004; Repetto 1984; Schaad 1998; Toporovski 1992; Vigano 1992; Vilaichone 2001).
Known severe urinary tract abnormality (14 studies: Baker 2001; Benador 2001; Bocquet 2012; Bouissou 2008; Francois 1997; Hoberman 1999; Khan 1981; Levtchenko 2001; Marild 2009; Neuhaus 2008; Noorbakhsh 2004; Repetto 1984; Vigano 1992; Vilaichone 2001).
Known sensitivity to study medications (17 studies: Banfi 1993; Baker 2001; Benador 2001;Bocquet 2012; Carapetis 2001; Chong 2003; Fischbach 1989; Francois 1997; Hoberman 1999; Kafetzis 2000; Marild 2009; Noorbakhsh 2004; Repetto 1984; Schaad 1998; Toporovski 1992; Vigano 1992; Vilaichone 2001).
Other exclusion criteria
Recent antibiotic use (10 studies: Banfi 1993; Baker 2001; Bocquet 2012; Chong 2003; Fischbach 1989; Kafetzis 2000; Marild 2009; Montini 2007; Noorbakhsh 2004; Vilaichone 2001).
Previous UTI (seven studies: Bouissou 2008; Fischbach 1989; Francois 1997; Hoberman 1999; Marild 2009; Montini 2007; Vilaichone 2001).
Clinical signs of shock at presentation (six studies: Baker 2001; Bocquet 2012; Francois 1997; Hoberman 1999; Montini 2007; Neuhaus 2008).
Immune compromise (six studies: Banfi 1993; Bouissou 2008; Carapetis 2001; Francois 1997; Noorbakhsh 2004; Schaad 1998).
Known hearing impairment (four studies: Carapetis 2001; Chong 2003; Kafetzis 2000; Vigano 1992).
Uncomplicated acute pyelonephritis (APN) (one study: Cheng 2006).
Four studies did not specify any exclusion criteria (Bakkaloglu 1996; Fujii 1987; Grimwood 1988; Pylkkänen 1981).
Study comparisons
The 27 included studies evaluated eight different comparisons.
Four studies compared oral therapy with short duration IV therapy followed by oral therapy (Bocquet 2012; Hoberman 1999; Montini 2007; Neuhaus 2008).
In six studies, short duration IV therapy (three to four days) followed by oral therapy was compared with long duration IV therapy (seven to 14 days) (Benador 2001; Bouissou 2008; Francois 1997; Levtchenko 2001; Noorbakhsh 2004; Vilaichone 2001).
A single dose of parenteral antibiotic added to oral therapy was compared to oral therapy alone in one study (Baker 2001).
Three studies compared different dosing frequencies of the same antibiotic agents (Carapetis 2001; Chong 2003; Vigano 1992).
Seven studies compared different antibiotics (Banfi 1993; Bakkaloglu 1996; Fischbach 1989; Kafetzis 2000; Marild 2009; Schaad 1998; Toporovski 1992). Toporovski 1992 included two experimental groups who received different doses of antibiotic. Because treatment response did not differ, experimental group data were combined.
Three studies compared different durations of antibiotics (Cheng 2006; Khan 1981; Pylkkänen 1981).
Two studies assessed single dose parenteral therapy against seven to 10 days of oral antibiotic therapy (Grimwood 1988; Repetto 1984).
One study compared ampicillin suppositories with oral ampicillin (Fujii 1987).
Reported outcomes
Not all studies reported the same outcomes. Table 5shows the outcomes reported for each study comparison.
1. Reported outcomes of included studies.
|
Study/ comparisons |
Reported outcomes | ||||||
| Persistent bacteriuria at 48 to 72 hours | Bacteriuria at the end or ≥ 5 days of treatment | UTI at follow‐up | Resolution of clinical symptoms | Symptomatic recurrence of UTI | Parenchymal renal damage on DSMA scan | Adverse effects | |
| Oral therapy versus sequential short duration IV therapy and oral therapy | |||||||
| Bocquet 2012 | • | • | • | ||||
| Hoberman 1999 | • | • | • | ||||
| Montini 2007 | • | • | • | • | |||
| Neuhaus 2008 | • | • | |||||
| Sequential short duration (3 to 4 days) IV therapy and oral therapy versus long duration (7to 14 days) IV therapy | |||||||
| Benador 2001 | • | • | |||||
| Bouissou 2008 | • | ||||||
| Francois 1997 | • | • | • | ||||
| Levtchenko 2001 | • | • | • | ||||
| Noorbakhsh 2004 | • | ||||||
| Vilaichone 2001 | • | • | • | • | |||
| Single dose parenteral therapy and oral therapy versus oral therapy alone | |||||||
| Baker 2001 | • | • | • | • | |||
| Different dosing regimens of aminoglycoside therapy | |||||||
| Carapetis 2001 | • | • | • | ||||
| Chong 2003 | • | • | • | ||||
| Vigano 1992 | • | • | • | ||||
| Third generation cephalosporins versus other antibiotics | |||||||
| Banfi 1993 | • | • | • | • | • | ||
| Fischbach 1989 | • | • | • | • | |||
| Marild 2009 | • | • | • | ||||
| Toporovski 1992 | • | • | • | ||||
| Third generation cephalosporins versus fourth generation cephalosporins | |||||||
| Schaad 1998 | • | • | • | • | • | ||
| Ceftriaxone versus cefotaxime | |||||||
| Bakkaloglu 1996 | • | • | • | • | |||
| Aminoglycosides versus aminoglycosides | |||||||
| Kafetzis 2000 | • | • | • | ||||
| Different durations of the same oral antibiotic | |||||||
| Pylkkänen 1981 | • | ||||||
| Single dose parenteral therapy versus oral therapy alone | |||||||
| Grimwood 1988 | • | • | |||||
| Repetto 1984 | • | • | |||||
| Different durations of different antibiotics | |||||||
| Cheng 2006 | • | • | |||||
| Different routes of antibiotic administration | |||||||
| Fujii 1987 | • | • | |||||
| Three days versus 10 days of oral therapy | |||||||
| Khan 1981 | • | ||||||
DMSA ‐ Tc99m‐dimercaptosuccinic acid nuclear scan; UTI ‐ urinary tract infection
Excluded studies
We excluded a total of 43 studies because: data from children with acute pyelonephritis could not be separated from those with lower UTI (18), children had lower UTI only (8), antibiotics were studied as prophylactic agents (5), the studies involved ineligible interventions or populations (5) or the study was not randomised (7).
Risk of bias in included studies
The assessment of risk of bias is shown in Figure 2 and Figure 3. Figure 2 shows the proportion of studies assessed as low, high or unclear risk of bias for each risk of bias indicator. Figure 3 shows the risk of bias indicators for individual studies.
2.
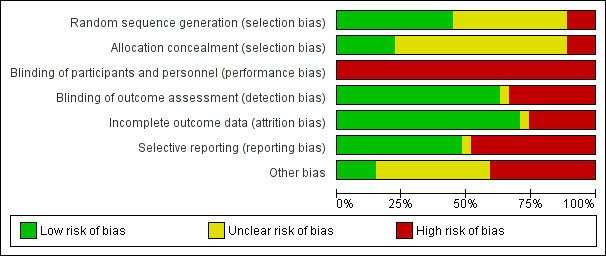
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation was considered to be at low risk of bias in 12 studies (Baker 2001; Benador 2001; Bocquet 2012; Bouissou 2008; Carapetis 2001; Fischbach 1989; Francois 1997; Grimwood 1988; Marild 2009; Montini 2007; Neuhaus 2008; Toporovski 1992) and assessed at high risk in three studies (Cheng 2006; Khan 1981; Noorbakhsh 2004). Randomisation methods were not reported in 12 studies.
Allocation concealment was considered to be at low risk of bias in six studies (Baker 2001; Benador 2001; Bouissou 2008; Marild 2009; Montini 2007; Neuhaus 2008) and high risk in three studies (Cheng 2006; Khan 1981;Noorbakhsh 2004). Allocation concealment was assessed as unclear in 18 studies.
Blinding
Participants and investigators were not blinded in any of the included studies. The absence of blinding was considered to be a high risk of bias because symptom reporting and clinical management could be influenced by knowledge of the treatment group (performance bias). Bakkaloglu 1996 was reported to be double‐blinded but antibiotics were administered at different frequencies and no placebos were given.
Outcome assessors (detection bias) were blinded in 17 studies (Baker 2001; Benador 2001; Bocquet 2012; Bouissou 2008; Chong 2003; Francois 1997; Grimwood 1988; Hoberman 1999; Kafetzis 2000; Khan 1981; Levtchenko 2001; Montini 2007; Neuhaus 2008; Pylkkänen 1981; Schaad 1998; Vigano 1992; Vilaichone 2001). Blinding of outcome assessors was not carried out in nine studies (Bakkaloglu 1996; Banfi 1993; Carapetis 2001; Cheng 2006; Fischbach 1989; Marild 2009; Noorbakhsh 2004; Repetto 1984; Toporovski 1992). There was no reporting of outcome assessment blinding in Fujii 1987 (abstract only available).
Incomplete outcome data
Not all studies reported all outcomes. The reported outcomes from each of the included studies are summarised in Table 5. Incomplete outcome data was considered to be at low risk of bias in 19 studies because they reported outcomes in more than 90% of participants (Baker 2001; Bakkaloglu 1996; Benador 2001; Carapetis 2001; Cheng 2006; Chong 2003; Fischbach 1989; Francois 1997; Fujii 1987; Grimwood 1988; Kafetzis 2000; Levtchenko 2001; Marild 2009; Noorbakhsh 2004; Repetto 1984; Schaad 1998; Toporovski 1992; Vigano 1992; Vilaichone 2001). Attrition and exclusion of participants after the randomisation process was considered to be at a high risk of bias in seven studies (Banfi 1993; Bocquet 2012; Bouissou 2008; Hoberman 1999; Montini 2007; Neuhaus 2008; Pylkkänen 1981) and unclear in Khan 1981.
Selective reporting
There were 13 studies that reported bacteriological, clinical and adverse outcomes and were considered at low risk of bias (Baker 2001; Bakkaloglu 1996; Banfi 1993; Benador 2001; Carapetis 2001; Chong 2003; Fischbach 1989; Francois 1997; Kafetzis 2000; Marild 2009; Montini 2007; Schaad 1998; Toporovski 1992). Fujii 1987 was an abstract and did not clearly indicate outcomes investigated. The remaining 13 studies did not report all three types of outcomes and were considered at high risk of bias.
Other potential sources of bias
We assessed that 11 studies were at high risk of bias because they reported receiving funding from pharmaceutical companies (Baker 2001; Banfi 1993; Bouissou 2008; Hoberman 1999; Kafetzis 2000; Marild 2009; Neuhaus 2008; Noorbakhsh 2004; Pylkkänen 1981; Schaad 1998; Toporovski 1992). Four received funding from hospital grants (Bocquet 2012; Chong 2003; Grimwood 1988) or a government grant (Montini 2007) and were considered at low risk of bias. The source of funding in the remaining 12 studies was unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Because results from random and fixed‐effect models did not differ, only results from the random‐effects model were reported. Few studies were available for pooling in meta‐analyses. No pre‐planned subgroup analyses for outcomes according to patient age (infant, child, adolescent) were possible from the available data. Post hoc subgroup analyses were reported for age (less than or greater than one year of age, Benador 2001) and VUR (Benador 2001; Hoberman 1999; Vilaichone 2001) and delay in treatment (less than or greater than seven days, Levtchenko 2001).
Oral therapy versus sequential IV therapy and oral therapy
We found four studies (Bocquet 2012; Hoberman 1999; Montini 2007; Neuhaus 2008) involving 1131 children compared oral antibiotics (cefixime, ceftibuten or amoxicillin/clavulanic acid) for 10 to 14 days with IV cefotaxime (Hoberman 1999) or ceftriaxone (Bocquet 2012; Montini 2007; Neuhaus 2008) for three to four days or until resolution of fever followed by oral antibiotics to complete the course of therapy.
Time to resolution of fever did not differ significantly between groups (Analysis 1.1 (2 studies, 808 children): MD 2.05 hours, 95% CI ‐0.84 to 4.94; I² = 0%). Neuhaus 2008 reported the number of children with fever on day three did not differ significantly between groups (Analysis 1.2 (1 study, 152 children): RR 0.79, 95% CI 0.30 to 2.06).
The number of children with persistent UTI at 72 hours after commencing therapy did not differ significantly between groups (Analysis 1.3 (2 studies, 542 children): RR 1.10, 95% CI 0.07 to 17.41).
Montini 2007 reported no significant difference between groups in the mean levels of inflammatory markers: WCC (Analysis 1.4.1 (1 study, 473 children): MD 0.30 x 10⁹/L, 95% CI ‐0.30 to 0.90), ESR (Analysis 1.4.2 (1 study, 338 children): MD ‐1.80 mm/60 min, 95% CI‐8.20 to 4.60]) or CRP (Analysis 1.4 (1 study, 486 children): MD 1.10 mg/L, 95% CI ‐2.18 to 4.38).
Hoberman 1999 reported no significant difference between groups in the rate of recurrences of bacteriuria (Analysis 1.5.1 (1 study, 287 children): RR 0.65, 95% CI 0.28 to 1.51) or symptomatic UTI within six months (Analysis 1.5.2 (1 study, 287 children): RR 0.67, 95% CI 0.27 to 1.67).
There were no significant differences among treatment groups in the rate of persistent kidney parenchymal defects on DMSA scan whether considered in relation to the total number of children with acute pyelonephritis (Analysis 1.6.1 (4 studies, 943 children): RR 0.82, 95% CI 0.59 to 1.12; I² = 41%) or only those with defects on the initial DMSA scan (Analysis 1.6.2 (4 studies, 681 children): RR 0.79, 95% CI 0.31 to 1.03; I² = 19%). Hoberman 1999 reported no significant difference between groups in the size of persistent kidney parenchymal defects on DMSA scan (Analysis 1.7 (1 study, 272 children): MD ‐0.70, 95% CI ‐1.74 to 0.34).
Post hoc subgroup analysis (Analysis 1.8) by Hoberman 1999 found no difference in the number of kidney parenchymal defects on DMSA scan at six months between children with VUR (Analysis 1.8.1 (1 study, 107 children): RR 1.88, 95% CI 0.83 to 4.24) and those without VUR (Analysis 1.8.2 (1 study, 107 children): RR 0.80, 95% CI 0.23 to 2.73). However, post hoc analysis (Analysis 1.8.4) raised the possibility that among children with VUR (grades III‐V), persistent kidney parenchymal defects on DMSA scan at six months occurred more frequently after oral than IV therapy (RR 7.33, 95% CI 1.00 to 54.01).
The average cost of treatment for each patient was USD 3630 and USD 7382 for oral and IV groups respectively (Hoberman 1999).
Adverse effects were reported in three studies (Bocquet 2012; Montini 2007; Neuhaus 2008). No children experienced therapy‐related adverse effects in Neuhaus 2008. In Bocquet 2012 two children experienced vomiting with oral cefixime and required change to parenteral therapy. In Montini 2007 15 children experienced diarrhoea or vomiting (13), erythema (1) and leucopenia (1) with oral amoxicillin and clavulanic acid; 10 required a change of antibiotics. In the same study three children experienced diarrhoea (1), erythema (1) and candida (1) with ceftriaxone; none required change of treatment. Hoberman 1999 did not report on adverse effects.
1.1. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 1 Time to fever resolution.
1.2. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 2 Fever on Day 3.
1.3. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 3 Number with persistent UTI at 72 hours.
1.4. Analysis.
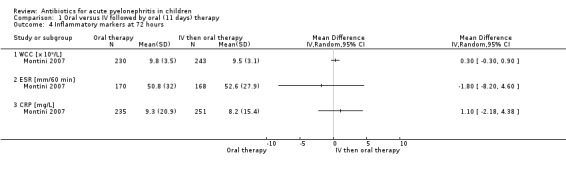
Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 4 Inflammatory markers at 72 hours.
1.5. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 5 Recurrent UTI within 6 months.
1.6. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 6 Persistent kidney damage at 6‐12 months.
1.7. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 7 Proportion of kidney parenchyma with damage at 6 months.
1.8. Analysis.

Comparison 1 Oral versus IV followed by oral (11 days) therapy, Outcome 8 Kidney damage at 6 months (post hoc subgroup analysis).
Sequential short duration (three to four days) IV therapy and oral therapy versus long duration (seven to 14 days) IV therapy
There were six studies (Benador 2001; Bouissou 2008; Francois 1997; Levtchenko 2001; Noorbakhsh 2004; Vilaichone 2001) involving 917 children that compared oral therapy after an initial three to four days of IV therapy with a long duration of IV therapy alone. Two studies compared IV ceftriaxone (three to four days) followed by oral cefixime (Benador 2001) or ceftibuten (Vilaichone 2001) with IV ceftriaxone (10 days). Levtchenko 2001 compared IV temocillin (three days) followed by oral amoxicillin or amoxicillin/clavulanic acid with IV temocillin (seven days). Noorbakhsh 2004 compared IV ceftriaxone (two to three days) followed by oral ceftibuten with IV amikacin or gentamicin with IV ampicillin (14 days). Francois 1997 compared IV cefotaxime (four days) followed by oral amoxicillin/clavulanic acid with IV cefotaxime (14 days). Bouissou 2008 compared IV netilmicin (two days) and ceftriaxone (three days) followed by oral antibiotics (cefixime, amoxicillin/clavulanic acid, TMP/SMX) chosen according to sensitivity with IV netilmicin (two days) and ceftriaxone (eight days). Benador 2001 and Levtchenko 2001 also converted the IV group to oral therapy after seven to 10 days to complete 15 to 21 days of treatment.
There was no significant difference between the risk of persistent bacteriuria at the end of treatment (Analysis 2.1 (4 studies, 305 children): RR 0.78, 95% CI 0.24 to 2.55; I² = 0%).
There was no significant difference between groups for recurrent UTI within six months (Analysis 2.2 (5 studies, 993 children): RR 0.97, 95% CI 0.58 to 1.62; I² = 0%).
The number of persisting kidney parenchymal defects seen on DMSA scan at three to six months did not differ significantly between treatment groups when considered in relation to the total number of children with acute pyelonephritis (Analysis 2.3.1 (4 studies, 726 children): RR 1.01, 95% CI 0.80 to 1.29; I² = 0%) or only those with defects on the initial DMSA scan (Analysis 2.3.2 (3 studies, 315 children): RR 1.10, 95% CI 0.84 to 1.45; I² = 0%).
Post hoc subgroup analysis showed that the number of children with persisting kidney parenchymal defects on DMSA scan did not differ between those with VUR (Analysis 2.4.1 (2 studies, 81 children): RR 0.99, 95% CI 0.69 to 1.43; I² = 0%) and without VUR (Analysis 2.4.2 (2 studies, 173 children): RR 1.19, 95% CI 0.81 to 1.76; I² = 0%), those aged under one year (Analysis 2.4.3 (1 study, 22 children): RR 1.46, 95% CI 0.71 to 3.01) and aged one year and over (Analysis 2.4.4 (1 study, 54 children): RR 0.89, 95% CI 0.59 to 1.34), and those who had a delay of treatment of less than seven days (Analysis 2.4.5 (1 study, 13 children): RR 1.52, 95% CI 0.59 to 3.92) or more than seven days (Analysis 2.4.6 (1 study, 8 children): RR 2.10, 95% CI 0.92 to 4.77).
Adverse effects were reported in Francois 1997 and Vilaichone 2001; both related to gastrointestinal upsets, and frequency did not differ between therapy routes (Analysis 2.5.1 (2 studies, 175 children): RR 1.29, 95% CI 0.55 to 3.05; I² = 0%). Four studies did not report on adverse effects (Benador 2001; Bouissou 2008; Levtchenko 2001; Noorbakhsh 2004).
Duration of hospitalisation was 4.9 days for the IV and oral group compared with 9.8 days for the IV group (Vilaichone 2001).
Costs of treatment for four days of IV therapy followed by six days of oral therapy were 513 French Francs (range 176 to 896) compared with 3545 French Francs (range 2478 to 4673) for 10 days of IV therapy (Francois 1997).
2.1. Analysis.

Comparison 2 Short duration (3‐4 days) versus long duration (7‐14 days) IV therapy, Outcome 1 Persistent bacteriuria after treatment.
2.2. Analysis.

Comparison 2 Short duration (3‐4 days) versus long duration (7‐14 days) IV therapy, Outcome 2 Recurrent UTI within 6 months.
2.3. Analysis.

Comparison 2 Short duration (3‐4 days) versus long duration (7‐14 days) IV therapy, Outcome 3 Persistent kidney damage at 3‐6 months.
2.4. Analysis.

Comparison 2 Short duration (3‐4 days) versus long duration (7‐14 days) IV therapy, Outcome 4 Persistent kidney damage at 3‐6 months (post hoc subgroup analysis).
2.5. Analysis.

Comparison 2 Short duration (3‐4 days) versus long duration (7‐14 days) IV therapy, Outcome 5 Adverse effects.
Single dose parenteral therapy and oral treatment versus oral therapy alone
Baker 2001 (69 children) compared the addition of a single intramuscular dose of the third generation cephalosporin, ceftriaxone, to an oral course of TMP/SMX. There was no significant difference in:
persistence of bacteriuria after 48 hours (Analysis 3.1: RR 0.77, 95% CI 0.19 to 3.20)
persistence of clinical symptoms (Analysis 3.2: RR 0.82, 95% CI 0.24 to 2.81), or
total adverse events (Analysis 3.4.1: RR 1.37, 95% CI 0.33 to 5.68) between groups.
3.1. Analysis.

Comparison 3 Single dose parenteral therapy and oral therapy versus oral therapy alone, Outcome 1 Persistent bacteriuria at 48 hours.
3.2. Analysis.

Comparison 3 Single dose parenteral therapy and oral therapy versus oral therapy alone, Outcome 2 Treatment failure after 48 hours of therapy.
3.4. Analysis.

Comparison 3 Single dose parenteral therapy and oral therapy versus oral therapy alone, Outcome 4 Adverse events.
No child developed symptomatic UTI during one month after treatment.
Different dosing regimens of aminoglycoside therapy
Three studies that involved 495 children compared daily parenteral administration of gentamicin (Carapetis 2001; Chong 2003) or netilmicin (Vigano 1992) to eight‐hourly administration of aminoglycosides.
There was no significant difference in the risk for persisting bacteriuria one to three days after commencing treatment with either dose frequency (Analysis 4.1 (3 studies, 435 children): RR 1.05, 95% CI 0.15 to 7.27).
Carapetis 2001 reported no difference in numbers of children with persisting clinical symptoms after three days of gentamicin (Analysis 4.2 (1 study, 179 children): RR 1.98, 95% CI 0.37 to 10.53).
Vigano 1992 reported persisting bacteriuria one week after (Analysis 4.3 (1 study, 144 children): RR 2.84, 95% CI 0.12 to 68.57) and recurrent UTI within one month (Analysis 4.4 (1 study, 144 children): RR 1.18, 95% CI 0.33 to 4.23) after completing netilmicin treatment did not differ between treatment groups.
There was no significant difference in numbers of children with hearing impairment (Analysis 4.5 (3 studies, 271 children): RR 2.83, 95% CI 0.33 to 24.56; I² = 0%) or kidney dysfunction (Analysis 4.6 (3 studies, 419 children): RR 0.75, 95% CI 0.20 to 2.82; I² = 0%).
Chong 2003 reported mean time to resolution of fever with gentamicin did not differ between groups (Analysis 4.7 (1 study, 172 children): MD 2.40 hours, 95% CI ‐7.90 to 12.70). Median time to resolution of fever was 27 hours (interquartile range 15 to 48 hours) with daily dosing and 33 hours (interquartile range 12 to 48 hours) with eight‐hourly dosing in a second study (Carapetis 2001).
4.1. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 1 Persistent bacteriuria after 1‐3 days of treatment.
4.2. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 2 Persistent symptoms at end of 3 days of IV therapy.
4.3. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 3 Persistent bacteriuria at 1 week after treatment.
4.4. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 4 Reinfection at 1 month after completing treatment.
4.5. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 5 Hearing impairment following treatment.
4.6. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 6 Increase in serum creatinine during treatment.
4.7. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 7 Time to resolution of fever.
Different antibiotic agents
Six studies compared different antibiotics (Bakkaloglu 1996; Carapetis 2001; Chong 2003; Kafetzis 2000; Schaad 1998; Vigano 1992).
Third generation cephalosporins versus other antibiotics
In four studies involving 491 children treatment with third generation cephalosporins (IV cefotaxime (Fischbach 1989), oral cefetamet (Toporovski 1992) or oral ceftibuten (Banfi 1993; Marild 2009) were compared with amoxicillin/clavulanic acid (Fischbach 1989; Toporovski 1992) or TMP/SMX (Banfi 1993; Marild 2009).
There was no significant difference in the number of children with persistent bacteriuria after 48 hours of therapy (Analysis 5.1 (3 studies, 433 children): RR 2.41, 95% CI 0.98 to 5.93; I² = 0%).
There was no significant difference in numbers of children who had recurrent UTI at 4 to 10 days after treatment (Analysis 5.2 (4 studies, 419 children): RR 1.23, 95% CI 0.32 to 4.74; I² = 0%).
A significantly greater number of children treated with TMP/SMX had persistent clinical symptoms at four to 10 days after treatment compared with those treated with a third generation cephalosporin (Analysis 5.3 (3 studies, 471 children): RR 0.28, 95% CI 0.13 to 0.62; I² = 0%). The study by Marild 2009 contributed to 94% of the weight of this result.
Fischbach 1989 reported no significant difference in numbers of children with persistent fever for more than 48 hours (Analysis 5.4 (1 study, 20 children): RR 5.00, 95% CI 0.27 to 92.62).
Banfi 1993 reported no significant difference between groups in the rate of recurrences of bacteriuria (Analysis 5.5 (1 study, 28 children): RR 2.14, 95% CI 0.11 to 40.30) or symptomatic UTI at four to six weeks (Analysis 5.6; no symptomatic UTIs in either group).
All four studies reported adverse effects. There was no significant difference in numbers of children who experienced gastrointestinal adverse effects (Analysis 5.7 (4 studies, 591 children): RR 0.93, 95% CI 0.34 to 2.58; I² = 0%). Marild 2009 reported that four children in each group discontinued treatment because of adverse reactions (Analysis 5.8 (1 study, 461 children): RR 0.49, 95% CI 0.12 to 1.94).
5.1. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 1 Persistent bacteriuria.
5.2. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 2 Recurrent UTI after end of therapy.
5.3. Analysis.
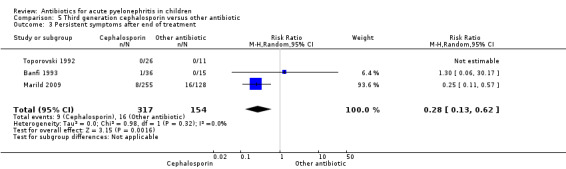
Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 3 Persistent symptoms after end of treatment.
5.4. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 4 Number with fever for more than 48 hours.
5.5. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 5 Recurrent bacteriuria at 4‐6 weeks.
5.6. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 6 Recurrent symptomatic UTI at 4‐6 weeks.
5.7. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 7 Gastrointestinal adverse events.
5.8. Analysis.

Comparison 5 Third generation cephalosporin versus other antibiotic, Outcome 8 Number discontinuing treatment for adverse effect.
Third generation cephalosporins versus fourth generation cephalosporins (Analysis 6)
In Schaad 1998, which included 299 children, IV cefepime (a fourth generation cephalosporin) was compared to IV ceftazidime (a third generation cephalosporin).
No significant differences between groups were detected in numbers of children with persistent or recurrent bacteriuria with the same pathogen at different time points after therapy (Analysis 6.1).
Recurrent UTI with a different pathogenic organism at four to six weeks did not differ between groups (Analysis 6.2: RR 1.19, 95% CI 0.45 to 3.18).
There were no significant differences in the occurrence of an unsatisfactory clinical response at different time points after therapy (Analysis 6.3).
The frequency of adverse effects did not differ between treatment groups (Analysis 6.4).
6.1. Analysis.

Comparison 6 Cefepime versus ceftazidime, Outcome 1 Persistence or recurrence of initial pathogen.
6.2. Analysis.

Comparison 6 Cefepime versus ceftazidime, Outcome 2 Infection with new pathogen at 4‐6 weeks.
6.3. Analysis.

Comparison 6 Cefepime versus ceftazidime, Outcome 3 Unsatisfactory clinical response.
6.4. Analysis.

Comparison 6 Cefepime versus ceftazidime, Outcome 4 Adverse effects.
Ceftriaxone versus cefotaxime
Bakkaloglu 1996 compared ceftriaxone and cefotaxime in 100 children aged over 24 months.
No child had persistent bacteriuria at 48 hours (Analysis 7.1).
There were no significant differences between groups for bacteriuria at the end of treatment (Analysis 7.2.1: RR 0.87, 95% CI 0.37 to 2.03), for recurrent infection at one month after therapy (Analysis 7.3.1: RR 0.68, 95% CI 0.30 to 1.50), or for total adverse events (Analysis 7.4.1: RR 0.67, 95% CI 0.12 to 3.82).
Post hoc subgroup analysis (Analysis 7.2.2 and Analysis 7.2.3) revealed no differences in outcomes for bacteriuria at the end of treatment or recurrent UTI at one month after therapy between children with and without abnormalities on imaging studies of the urinary tract.
7.1. Analysis.

Comparison 7 Ceftriaxone versus cefotaxime, Outcome 1 Persistent bacteriuria at 48 hours.
7.2. Analysis.

Comparison 7 Ceftriaxone versus cefotaxime, Outcome 2 Bacteriuria 10 days after end of treatment.
7.3. Analysis.

Comparison 7 Ceftriaxone versus cefotaxime, Outcome 3 UTI at 1 month after therapy.
7.4. Analysis.

Comparison 7 Ceftriaxone versus cefotaxime, Outcome 4 Adverse events.
Aminoglycosides
Kafetzis 2000 compared the aminoglycosides isepamicin and amikacin in 16 children.
No child in either group had persistent bacteriuria after 48 hours of treatment, or seven days or 30 days after treatment (Analysis 8.1).
Mean time to resolution of fever in each group was identical (24 hours).
No child in either treatment group developed hearing impairment on testing.
8.1. Analysis.

Comparison 8 Isepamicin versus amikacin, Outcome 1 Persistent bacteriuria.
Duration of antibiotic administration
Four studies compared different durations of antibiotic administration (Cheng 2006; Grimwood 1988; Pylkkänen 1981; Repetto 1984).
Ten days versus 42 days of oral sulphafurazole
The study by Pylkkänen 1981 involved 149 children and compared 10 days with 42 days of oral sulphafurazole.
Recurrence of UTI within one month of ceasing therapy was significantly higher in children treated for 10 days compared with children treated for 42 days (Analysis 9.1: RR 17.70, 95% CI 2.42 to 129.61).
The number of children with recurrent UTI from one to 12 months after ceasing therapy did not differ between groups (Analysis 9.2: RR 0.87, 95% CI 0.40 to 1.88).
9.1. Analysis.

Comparison 9 10 days versus 42 days of oral sulfafurazole, Outcome 1 Recurrent UTI within 1 month after ceasing therapy.
9.2. Analysis.

Comparison 9 10 days versus 42 days of oral sulfafurazole, Outcome 2 Recurrent UTI at 1‐12 months after completing therapy.
Single dose parenteral antibiotic therapy versus seven to 10 days of oral therapy
Grimwood 1988 and Repetto 1984 (involving a total of 61 children) compared single dose parenteral antibiotic therapy with seven to 10 days of oral therapy.
There were no significant differences in the number of children with persistent bacteriuria after treatment (Analysis 10.1 (2 studies, 35 children): RR 1.73, 95% CI 0.18 to 16.30; I² = 15%) or with recurrent UTI within six weeks (Analysis 10.2 (2 studies, 35 children): RR 0.24, 95% CI 0.03 to 1.97).
10.1. Analysis.

Comparison 10 Single dose of parenteral antibiotic versus 7‐10 days oral therapy, Outcome 1 Persistent bacteriuria 1‐2 days after treatment.
10.2. Analysis.

Comparison 10 Single dose of parenteral antibiotic versus 7‐10 days oral therapy, Outcome 2 UTI relapse or reinfection within 6 weeks.
Three weeks with two weeks of antibiotics
Cheng 2006, which involved 80 children, compared three weeks with two weeks of antibiotics for children with acute lobar nephronia. Antibiotics were chosen according to sensitivities.
Seven children treated for two weeks had persistent or recurrent bacteriuria; this was not significantly different (Analysis 11.1: RR 0.07, 95% CI 0.00 to 1.19).
Two children had recurrence of clinical symptoms with bacteriuria; this was not significantly different (Analysis 11.2: RR 0.21, 95% CI 0.01 to 4.24).
11.1. Analysis.

Comparison 11 3 weeks versus 2 weeks, Outcome 1 Persistence/recurrence of bacteriuria.
11.2. Analysis.

Comparison 11 3 weeks versus 2 weeks, Outcome 2 Recurrence of clinical UTI.
Three days with 10 days of antibiotics
Khan 1981 (54 children) compared three and 10 days of oral antibiotics. Data were reported as episodes of UTI (asymptomatic, lower tract, APN) and could not be included in a meta‐analysis. Of 31 episodes of UTI, 23 were cured in 27 children in the three day treatment group and 25 of 31 episodes were cured in 27 children in the 10 day treatment group. Of episodes of acute pyelonephritis, four were cured in five episodes in the three day treatment group and five were cured in six episodes in the 10 day treatment group.
Different routes of antibiotic administration
Fujii 1987, which reported on 105 children, compared ampicillin administered by suppository with oral administration.
There was no significant difference between treatments in the risk of persistent clinical symptoms (Analysis 12.1: RR 0.89, 95% CI 0.51 to 1.56) or bacteriuria (Analysis 12.2: RR 0.89, 95% CI 0.53 to 1.50).
12.1. Analysis.

Comparison 12 Suppositories versus oral ampicillin, Outcome 1 Persistence of clinical symptoms.
12.2. Analysis.

Comparison 12 Suppositories versus oral ampicillin, Outcome 2 Persistence of bacteriuria.
Discussion
Summary of main results
This review was designed to include all RCTs addressing all aspects of antibiotic treatment for children with acute pyelonephritis. Identified studies formed a heterogeneous group with few studies addressing the same or similar comparisons to enable assessment in meta‐analyses. The 27 included studies addressed a variety of different questions related to the therapy of children with acute pyelonephritis.
Oral therapy versus IV therapy
Four studies compared an oral antibiotic (ceftibuten, cefixime or amoxicillin/clavulanic acid) alone with IV therapy (cefotaxime or ceftriaxone) followed by oral therapy. These studies found:
No significant difference in bacteriological outcomes between groups.
The number of children with kidney parenchymal damage on DMSA scan at follow‐up whether expressed as a proportion of the total number considered to have acute pyelonephritis or as a proportion of those with DMSA changes at entry did not differ significantly between groups.
Thus, there were no significant differences in efficacy between treatment with oral ceftibuten, cefixime and amoxicillin/clavulanic acid and IV therapy followed by oral therapy. Studies that support these findings enrolled children older than one month of age and hence the finding cannot be extrapolated to children aged less than one month.
Short duration versus long duration IV therapy
A meta‐analysis of six studies showed:
No significant differences in clinical or bacteriological outcomes between IV antibiotic therapy given for three to four days followed by oral therapy and IV therapy for seven to 14 days.
That the prevalence of kidney parenchymal injury on DMSA scan at three to six months after UTI therapy did not differ significantly between treatment groups.
These data show that short duration IV therapy (three to four days) can be used instead of longer courses of IV therapy to treat childhood acute pyelonephritis. The findings cannot be extrapolated to children less than one month of age as such children were excluded from the studies.
Single daily dosing with aminoglycosides
If IV therapy is required, three studies provide data to support the safety and efficacy of daily dosing with aminoglycosides (gentamicin and netilmicin) compared with eight‐hourly dosing in children with acute pyelonephritis. Once daily dosing has been studied extensively in adults and is preferred due to improved efficacy, similar or reduced toxicity, convenience and lower costs. These findings have also been supported in children justifying the use of single daily dosing of aminoglycosides (Contopoulos‐Ioannidis 2004; Jenh 2011), although aminoglycoside pharmacokinetics and toxicity differ in children from adults.
Efficacy of different antibiotics
The seven studies that compared different antibiotics did not demonstrate any advantage of one agent over another. Four studies compared a cephalosporin with amoxicillin/clavulanic acid or TMP/SMX. A meta‐analysis of three studies demonstrated that children treated with oral ceftibuten had a higher clinical cure rate than TMP/SMX. However one large study (Marild 2009) contributed most of the weight of the analysis. The study defined clinical cure as the resolution of all symptoms related to the infection within 10 days. It is possible that there was some cross‐over of symptoms related to the infection and those due to adverse effects of medication such as vomiting. Furthermore, the study did not demonstrate any difference in bacteriological elimination rates despite 15% of the pathogens responsible for the infection being resistant to TMP/SMX compared to 2% that were resistant to ceftibuten.
One study demonstrated that a single dose of parenteral medication added to oral therapy did not improve efficacy compared with oral therapy alone.
Adverse events
Adverse events resulting from antibiotics were reported in 16 studies. Events were uncommon and rarely resulted in treatment discontinuation or significant alteration.
Overall completeness and applicability of evidence
In this review a comprehensive and extensive literature review was performed to identify studies that assessed the benefits and harms of antibiotics to treat children with acute pyelonephritis. We found that oral antibiotic therapy alone is as effective as IV therapy followed by oral therapy, and similarly short IV therapy is as effective as longer courses of IV therapy to treat childhood acute pyelonephritis. It is unknown whether these findings apply to children less than one month of age since children aged below one month of age were excluded from studies. The exclusion criteria for participation in the included studies mean that our findings may not be generalizable to all children with acute pyelonephritis. There were 10 studies that excluded children who were severely ill or clinically unstable. Four studies did not specify any exclusion criteria. The applicability of the findings in children with uropathy may also be limited since 10 studies excluded children with known uropathy. Hoberman 1999 performed a post hoc subgroup analysis to analyse differences in efficacy between children with or without VUR but the study was not designed and had no power to detect differences between small subgroups. Further data are required to determine whether treatment efficacy differs in children with non‐dilating VUR (grades I‐II) and dilating VUR (grades III‐V).
Most of the studies examined the bacteriological efficacy of antibiotic therapy. Few studies compared the efficacy of antibiotic therapies on the resolution of clinical symptoms other than fever.
None of the 27 included studies analysed the optimal duration of antibiotic therapy for childhood acute pyelonephritis. Our review evaluated oral antibiotic regimens in which oral antibiotics were used either alone or following IV antibiotics for a total duration of eight to 42 days of therapy. Inadequate data are available on the benefits and harms of shorter duration therapies (e.g. seven days or less). Three studies compared single dose parenteral antibiotic therapy or short course oral therapy with seven to 10 days of oral therapy and showed no significant differences but the studies were small. This is unlike the evidence supporting the use of short‐course therapies for the treatment of lower urinary tract infections in children (Michael 2002; Michael 2003).
From the low reported incidence of adverse events, we were only able to detect common adverse effects e.g. gastrointestinal upsets. Generally, RCTs are not powered to detect rare but serious side effects e.g. Stevens–Johnson syndrome, so our findings of adverse effects may not be generalizable to larger groups of children.
Most of the included studies reported on short‐term outcomes. Nine studies analysed kidney parenchymal damage on DSMA scan at three to 12 months following an episode of acute pyelonephritis. Five of the nine studies had a high loss to follow up because of refusal to do a DMSA scan among well children and one study (not included) was terminated without any results for this reason (NCT00724256). The loss to follow up of well children may lead to an underestimate of the effect of treatment. As the longest follow up of children was 12 months, this review cannot provide data on the likelihood of long term kidney scarring following antibiotic therapy.
Although several studies potentially included adolescents aged to 16 years, none of the studies reported results for different age groups. Thus we could not determine whether there was any difference in results according to the patients' ages.
Quality of the evidence
The quality of the included studies was quite variable. The main limitations in the quality of the studies were concealment of allocation, blinding of participants and personnel and sponsorship from pharmaceutical companies. Of the 27 included studies, 12 reported adequate sequence generation and six demonstrated adequate allocation concealment. The lack of adequate sequence generation and allocation concealment can lead to biased estimates of treatment effects in the original study and therefore the results of a systematic review (Hollis 1999; Juni 1999; Moher 1998; Schulz 1995). All of the studies were unblinded to participants and personnel primarily because antibiotics were delivered by the parenteral route compared with oral or used different dosing regimens. This was considered a high risk of bias because clinicians’ management could be influenced by knowledge of the treatment group. For blinding of outcome assessors, blinding was adequate in 17 studies where the primary outcome was bacteriological or radiological and considered unlikely to be influenced by lack of blinding. We found that 19 studies provided complete data reporting and 13 reported on all reasonably expected outcomes (bacteriological eradication, clinical cure and adverse effects). The authors of 11 studies reported receiving sponsorship from pharmaceutical companies.
The quality of the evidence was assessed according to the GRADE approach and is displayed in summary of findings tables.
The evidence that oral therapy alone is as effective as IV therapy followed by oral therapy in children with acute pyelonephritis is considered to be of moderate quality. The quality of evidence was downgraded because of imprecision regarding the time to resolution of fever and the loss to follow‐up for DMSA scans (Table 1).
The evidence that short duration IV therapy followed by oral therapy is as effective as long duration IV therapy is considered to be of moderate quality. The quality of evidence was downgraded because of unclear or inadequate allocation concealment in the included studies which may increase the risk of selection bias, the studies being too small and the loss to follow‐up for DMSA scans (Table 2).
The evidence that daily dosing of aminoglycosides is as safe and effective as thrice daily dosing of aminoglycosides is considered to be of low quality. The quality of evidence was downgraded because of unclear allocation concealment in two of three studies and the small number of participants combined with the low frequency of events that made the analysis significantly underpowered to detect a difference (Table 3).
The evidence that third generation cephalosporins are no more effective than other antibiotics (amoxicillin/clavulanic acid and TMP/SMX) is low. The quality of evidence was downgraded because of unclear allocation, imprecision from sparse data, and inconsistent bacteriologic results from one study that provided most of the weight of the meta‐analysis (Table 4).
Potential biases in the review process
We attempted to reduce publication bias by searching multiple databases and the grey literature without language restriction. Although the Cochrane Renal Group's Specialised Register contains the handsearched reports of studies, it is possible that we missed unpublished data presented at smaller conferences or studies published in foreign language journals and low impact journals. Studies may have been added since our last search of the register. No data were available from the terminated study (NCT00724256) after personal communication with the lead author. Not all included studies reported all outcomes. Some outcomes that would be expected to be known (e.g. resolution of clinical symptoms) were not reported which may have affected the results of meta‐analyses.
Agreements and disagreements with other studies or reviews
A systematic review published in 2008 evaluated an early switch to oral antibiotics after at least one day of initial IV antibiotics with IV therapy alone for hospitalised patients with acute pyelonephritis (Vouloumanou 2008). The meta‐analysis included both children and adults with acute pyelonephritis but the data from the two populations could be separated. It identified all six RCTs in children that we included in our review for this comparison. The authors found there was no difference in the incidence of kidney scars, microbiological eradication, clinical cure, reinfection, persistence of acute pyelonephritis, or adverse events between the two treatment regimens. This is consistent with our finding that short duration IV therapy followed by oral therapy is as effective as longer courses of IV therapy for the treatment of acute pyelonephritis in children.
No other systematic reviews were found for the 11 other comparisons in our review.
This review agrees with recently published guidelines (AAP 2011; Ammenti 2012; NICE 2007) for the treatment of childhood UTI, which recommend oral antibiotics for the initial treatment of children with acute pyelonephritis unless the child is seriously ill and/or unable to tolerate oral antibiotics. Our findings can be applied to children aged over one month. The NICE 2007 guidelines apply to children aged three months or older, the AAP 2011 guidelines apply to children aged 2 to 24 months and the Ammenti 2012 recommendations apply to children aged two months to three years. The guidelines also suggest seven or more days of antibiotic treatment but recognise that this is not based on best evidence because there are no data on the optimal duration of antibiotic therapy, particularly shorter courses.
Authors' conclusions
Implications for practice.
The following implications for practice in the treatment of children with acute pyelonephritis have been identified:
Oral antibiotics (cefixime, ceftibuten or amoxicillin/clavulanic acid) given alone for 10 to 14 days are as effective as sequential IV therapy given for three days followed by oral therapy for a total duration of 10 to 14 days suggesting that children with acute pyelonephritis can be treated effectively with oral antibiotics.
If IV antibiotic therapy is given, a short course of IV therapy given for two to four days followed by oral therapy with total therapy duration of 10 to 21 days is as effective as a longer duration of IV antibiotic therapy given for seven to 10 days with total duration of therapy of 10 to 21 days.
Studies comparing oral therapy alone with IV then oral antibiotics or IV then oral with IV therapy involved children greater than one month of age and were biased towards children who were less sick and so findings cannot be extrapolated to children less than one month of age or who are severely ill. The studies were also not stratified according to the grade of VUR so it remains unclear whether results differ according to the presence or absence of dilating VUR (grades III‐V).
Adequate data from RCTs are not available to determine the optimal total duration of antibiotic therapy required for acute pyelonephritis.
Implications for research.
Further RCTs are required to determine the benefits and harms in children of different ages with acute pyelonephritis of:
Treatment for shorter periods (seven days or less) compared with 10 to 14 days.
Initial treatment with oral antibiotics compared with parenteral therapy or IV then oral therapy compared with IV therapy in children with dilating VUR or other major urinary tract malformation.
Treatment with aminoglycosides alone or in combination with other antibiotics compared with other antibiotics including third generation cephalosporins in initial parenteral treatment.
Treatment with cheaper and more widely available oral antibiotics e.g. cephalexin.
What's new
| Date | Event | Description |
|---|---|---|
| 10 July 2014 | New search has been performed | New search, new studies included |
| 10 July 2014 | New citation required and conclusions have changed | New studies included |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 1 February 2010 | Amended | Minor correction of data for Analysis 1.3.2 ‐ no change in summary estimate of effect |
| 13 May 2009 | Amended | Contact details updated. |
| 17 June 2008 | Amended | Converted to new review format. |
| 20 October 2007 | Amended | Full study data for Monitini 2003 added |
| 14 August 2007 | New citation required and conclusions have changed | Substantive amendment |
| 5 February 2007 | Amended | Five new studies added |
| 15 October 2004 | Amended | Two new studies added |
Notes
Issue 3, 2010. Correction of data entered for Hoberman 1999 in Analysis 1.6.2 (Patients with kidney parenchymal damage on initial DMSA). Data published in Issue 4, 2007 were 17/100 for oral therapy and 12/87 for IV then oral therapy. The correct numbers are 15/100 for oral therapy and 11/87 for IV then oral therapy. There is no significant change in the summary estimate (Issue 4, 2007: RR 0.79, 95% CI 0.53 to 1.16; Issue 3, 2010: RR 0.77, 95% CI 0.53 to 1.11).
The authors wish to thank Dr Bodil Als‐Nielsen for notifying us of this error.
Acknowledgements
We are grateful to Dr Paul Bloomfield, who contributed to the original iterations of this review (Bloomfield 2003; Bloomfield 2005), contributing to the design, quality assessment, data collection, entry, analysis and interpretation, and writing. The authors would like to thank Professors Neuhaus and Lari and Drs Carapetis, Cheng, Hoberman, Lazzerini and Schaad who provided additional information about their studies We wish to thank the referees for their comments and feedback during the preparation of this review and its updates. We would also like to thank Ruth Mitchell, Trials Search Co‐ordinator, Cochrane Renal Group, for her assistance with this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL | #1 PYELONEPHRITIS* explode all trees #2 pyelonephritis #3 URINARY TRACT INFECTIONS explode all trees #4 urinary next tract next infection* #5 kidney next infection* #6 #1 or #2 or #3 or #4 or #5 #7 CHILD explode all trees #8 #6 and #7 #9 ADULT explode all trees #10 #8 not #9 |
| MEDLINE | 1. pyelonephritis/ 2. urinary tract infections/ 3. UTI.tw. 4. urinary tract infection$.tw. 5. pyelonephritis.tw. 6. or/1‐5 7. exp antibiotics/ 8. antibiotic treatment.tw. 9. antibiotic therap$.tw. 10. antibiotic$.tw. 11. or/7‐10 12. 6 and 11 13. limit 12 to all child <0 to 18 years> |
| EMBASE | 1. exp pyelonephritis/ 2. urinary tract infection/ 3. UTI.tw. 4. urinary tract infection$.tw. 5. pyelonephritis.tw. 6. or/1‐5 7. exp antibiotic agent/ 8. antibiotic therapy/ 9. antibiotic treatment.tw. 10. antibiotic therap$.tw. 11. antibiotic$.tw. 12. or/7‐11 13. 6 and 12 14. exp child/ 15. exp adolescent/ 16. 14 or 15 17. 13 and 16 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Oral versus IV followed by oral (11 days) therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to fever resolution | 2 | 808 | Mean Difference (IV, Random, 95% CI) | 2.05 [‐0.84, 4.94] |
| 2 Fever on Day 3 | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Number with persistent UTI at 72 hours | 2 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 17.41] |
| 4 Inflammatory markers at 72 hours | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 WCC [×10⁹/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 ESR [mm/60 min] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 CRP [mg/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Recurrent UTI within 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Total UTIs | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Symptomatic UTIs | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Persistent kidney damage at 6‐12 months | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 All included patients with acute pyelonephritis | 4 | 943 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.59, 1.12] |
| 6.2 Patients with kidney parenchymal damage on initial DMSA | 4 | 681 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.61, 1.03] |
| 7 Proportion of kidney parenchyma with damage at 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Kidney damage at 6 months (post hoc subgroup analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Persistent damage in children with VUR | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Persistent damage in children without VUR | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Persistent kidney damage with VUR grades 1‐2 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Persistent damage with VUR grades 3‐5 | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Short duration (3‐4 days) versus long duration (7‐14 days) IV therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria after treatment | 4 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.24, 2.55] |
| 2 Recurrent UTI within 6 months | 5 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.58, 1.62] |
| 3 Persistent kidney damage at 3‐6 months | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 All included patients with acute pyelonephritis | 4 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.29] |
| 3.2 Patients with renal parenchymal damage on initial DMSA scan | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.84, 1.45] |
| 4 Persistent kidney damage at 3‐6 months (post hoc subgroup analysis) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 VUR present | 2 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.69, 1.43] |
| 4.2 No VUR | 2 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.76] |
| 4.3 Age less than 1 year | 1 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.71, 3.01] |
| 4.4 Age 1 year or over | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.34] |
| 4.5 Delay in treatment less than 7 days in individual kidneys | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.59, 3.92] |
| 4.6 Delay in treatment of 7 days or more in individual kidneys | 1 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.92, 4.77] |
| 5 Adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Gastrointestinal effects | 2 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.55, 3.05] |
Comparison 3. Single dose parenteral therapy and oral therapy versus oral therapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria at 48 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Treatment failure after 48 hours of therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Recurrent UTI within 1 month | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Total adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Gastrointestinal adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.3. Analysis.

Comparison 3 Single dose parenteral therapy and oral therapy versus oral therapy alone, Outcome 3 Recurrent UTI within 1 month.
Comparison 4. Different dosing regimens of aminoglycosides (daily versus 8 hourly).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria after 1‐3 days of treatment | 3 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.15, 7.27] |
| 2 Persistent symptoms at end of 3 days of IV therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Persistent bacteriuria at 1 week after treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Reinfection at 1 month after completing treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Hearing impairment following treatment | 3 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [0.33, 24.56] |
| 6 Increase in serum creatinine during treatment | 3 | 419 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.82] |
| 7 Time to resolution of fever | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Kidney parenchymal damage at 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.8. Analysis.

Comparison 4 Different dosing regimens of aminoglycosides (daily versus 8 hourly), Outcome 8 Kidney parenchymal damage at 3 months.
Comparison 5. Third generation cephalosporin versus other antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 3 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [0.98, 5.93] |
| 2 Recurrent UTI after end of therapy | 4 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.32, 4.74] |
| 3 Persistent symptoms after end of treatment | 3 | 471 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.62] |
| 4 Number with fever for more than 48 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Recurrent bacteriuria at 4‐6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Recurrent symptomatic UTI at 4‐6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Gastrointestinal adverse events | 4 | 591 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.34, 2.58] |
| 8 Number discontinuing treatment for adverse effect | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Cefepime versus ceftazidime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistence or recurrence of initial pathogen | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 At end of IV therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At the end of IV and oral therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 5‐9 days after treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 4‐6 weeks after treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Infection with new pathogen at 4‐6 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Unsatisfactory clinical response | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 At end of IV therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At end of IV and oral therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 At 5‐9 days after treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 At 4‐6 weeks after treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Total adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Drug‐related adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Gastrointestinal adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Cutaneous adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Discontinuation due to drug related adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Ceftriaxone versus cefotaxime.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria at 48 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Bacteriuria 10 days after end of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 All patients | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Normal renal tract imaging (post hoc analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Abnormal renal tract imaging (post hoc analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 UTI at 1 month after therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 All patients | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Normal renal tract imaging | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Abnormal renal tract imaging | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 All adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Skin eruptions | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Gastrointestinal adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Isepamicin versus amikacin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 After 2‐3 days of therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 7 days after completing therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 30 days after completing therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. 10 days versus 42 days of oral sulfafurazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent UTI within 1 month after ceasing therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Recurrent UTI at 1‐12 months after completing therapy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Single dose of parenteral antibiotic versus 7‐10 days oral therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistent bacteriuria 1‐2 days after treatment | 2 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.18, 16.30] |
| 2 UTI relapse or reinfection within 6 weeks | 2 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 1.97] |
Comparison 11. 3 weeks versus 2 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistence/recurrence of bacteriuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Recurrence of clinical UTI | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. Suppositories versus oral ampicillin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Persistence of clinical symptoms | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Persistence of bacteriuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baker 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patient's nurse blindly selected opaque envelopes containing group assignment from a bin" |
| Allocation concealment (selection bias) | Low risk | "The patient's nurse blindly selected opaque envelopes containing group assignment from a bin" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injections so participants aware of assignment. "Physicians caring for the patients were unaware of study group assignment". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians caring for the children were unaware of the assignment at follow up. "Physician caring for patient at follow‐up usually was not the physician who cared for the patient at first visit". All children had bandage on thigh (IM injection) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four (5.5%) lost to FU and excluded. Unlikely to influence results as balanced across groups. |
| Selective reporting (reporting bias) | Low risk | Reported all expected outcomes |
| Other bias | High risk | Study grant from Roche Pharmaceuticals, Denver, Colorado |
Bakkaloglu 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Said to be "double‐blind, randomized clinical trial" but no placebo injection given to ceftriaxone group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Lack of blinding could influence assessment of clinical response |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in follow‐up |
| Selective reporting (reporting bias) | Low risk | Reports expected outcomes (clinical and bacteriological response, adverse effects) |
| Other bias | High risk | Grant from Hoffmann La Roche Ltd |
Banfi 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomly assigned. 2:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and lack of blinding could influence clinical outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16% of total group excluded from analysis for reasons other than not meeting entry criteria and this could influence results |
| Selective reporting (reporting bias) | Low risk | Data reported on clinical & bacteriologic response & adverse effects |
| Other bias | Unclear risk | No information provided |
Benador 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of 20 sealed opaque envelopes with equal numbers of treatment assignments, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome (DMSA scans) assessed by radiologists unaware of patient assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 (8 in 10 day group, 1 in 3 day group) of 229 (4.4%) were excluded from results. Unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes + DMSA outcome |
| Other bias | Unclear risk | No information provided |
Bocquet 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated code (Clean Web)". Blocked and stratified by centre and age (≤ 1 year/> 1 year) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Clinical management could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome (DMSA scans) assessed without knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18.5% (27/146) excluded for reasons other than no APN on acute DMSA |
| Selective reporting (reporting bias) | High risk | No report on bacteriologic resolution of UTI |
| Other bias | Low risk | Ministry of Health via Unit of Clinical Research, Necker Hospital, grant PHRC no. AOM 04 105 |
Bouissou 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation (random tables) was centralised and stratified by centre by blocks of 20 numbered sealed opaque envelopes with equal numbers of treatment assignments" |
| Allocation concealment (selection bias) | Low risk | Allocation was done by local investigator by opening a numbered sealed envelope 48 hours after admission and after informed consent by the parents" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome (DMSA scan) assessed by 4 independent physicians without knowledge of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 117/300 (23%) were lost to follow‐up or refused DMSA after secondary exclusions made. Could influence results |
| Selective reporting (reporting bias) | High risk | No information on clinical or bacteriologic cure or adverse effects |
| Other bias | High risk | Supported by grants from Roche Laboratory and French Ministry of Health |
Carapetis 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation stratified for age < 2 years and ≥ 2 years |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label study. Knowledge of treatment group could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label study. Primary outcome of clinical response could be influenced by knowledge of treatment group. "Audiology, bacteriology and biochemistry personnel were blinded to treatment status" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None of 179 excluded from primary outcome of clinical response |
| Selective reporting (reporting bias) | Low risk | Reported expected outcomes (clinical and bacteriologic eradication, adverse effects) |
| Other bias | Unclear risk | No information provided |
Cheng 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomly allocated with serial entry" |
| Allocation concealment (selection bias) | High risk | Patients allocated alternately to each group (information from the authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical assessment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Lack of blinding could influence outcome assessment of clinical symptoms |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. Information confirmed by authors |
| Selective reporting (reporting bias) | High risk | No reporting of adverse effects. Incomplete data on clinical symptom resolution |
| Other bias | Unclear risk | No information provided |
Chong 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but primary outcome was a laboratory result (negative urine culture) and unlikely to influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 (8%, excluding patients without UTI) were excluded from analysis (not including patients without UTI). This is unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Funded by KK Women's and Children's Hospital RAU Grant 029/1999 |
Fischbach 1989.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table used |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and clinical management could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding and primary clinical outcome assessment could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes are included |
| Other bias | Unclear risk | No information provided |
Francois 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random list |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Clinical management and assessment could be influenced by blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but outcomes evaluated by a scientific committee so unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients with positive urine cultures evaluated for efficacy |
| Selective reporting (reporting bias) | Low risk | Expected outcomes included |
| Other bias | Unclear risk | No information provided |
Fujii 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding and unclear whether clinical or laboratory outcomes were primary |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Expected outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | No information provided |
Grimwood 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each child was randomly allocated by random numbers to two treatment groups". Not stratified by clinical presentation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but primary outcome was laboratory based and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed study |
| Selective reporting (reporting bias) | High risk | No results provided on clinical resolution or adverse events |
| Other bias | Low risk | National Children's Health Research Foundation |
Hoberman 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomized at each site based on age and duration of fever" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Clinical management could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of radiologists, who assessed DMSA scans. Short term outcome (urine culture) was laboratory based and unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 34/306 (11%) no follow‐up DMSA scans |
| Selective reporting (reporting bias) | High risk | No information on adverse effects |
| Other bias | High risk | Supported by Lederle/Wyeth‐Ayerst Laboratories and by NIH grants |
Kafetzis 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes | 1. Persistent bacteriuria 7 days after end of therapy. 2. UTI 30 days after end of therapy. 3. Adverse events. | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. 2:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary efficacy outcome was laboratory based and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed study |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Sponsored by Schering‐Plough Research |
Khan 1981.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Episodes of UTI "treated prospectively on a random basis alternately" |
| Allocation concealment (selection bias) | High risk | Episodes of UTI "treated prospectively on a random basis alternately" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical assessment. Patients retrospectively divided into APN, lower UTI or asymptomatic |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary efficacy outcome was laboratory based and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all patients completed the study |
| Selective reporting (reporting bias) | High risk | No information on adverse effects. Data only available as the number of episodes of APN |
| Other bias | Unclear risk | No information provided |
Levtchenko 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Both groups given temocillin IV 3 days and then randomised Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Clinical assessment could be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding Primary outcome of kidney scarring; DMSA scans reviewed without knowledge of treatment assignment. Primary outcome of urine culture: laboratory based and unlikely to affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 (5.4%) of patients did not complete follow‐up. Unlikely to influence outcome |
| Selective reporting (reporting bias) | High risk | No detailed information on clinical response or adverse effects |
| Other bias | Unclear risk | No information provided |
Marild 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation stratified by gender. 2:1 allocation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing assigned treatment and randomisation number were opened in numerical order for eligible study patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label study. Lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical outcome assessment could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% of 420 patients excluded. Unlikely to influence results |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | High risk | Supported by grant from Schering‐Plough |
Montini 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated block randomisation with stratification by hospital, sex, age (< 2 years; ≥ 2years) at coordinating centre |
| Allocation concealment (selection bias) | Low risk | "Each participating centre received 4 series of 10 allocation codes in sealed and sequentially numbers opaque envelopes. The sequence was concealed until interventions assigned. Each participating centre allocated the children following a numeric order" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical assessment. "Could not blind group assignment because of the different routes of administration of the drug" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was scarring on DMSA at 12 months. "Two nuclear physicians blinded to test results interpreted the scans independently" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up was 20.3% and could influence results |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported |
| Other bias | Low risk | Region of Veneto (research project 40/01) and Association Il Sogno di Stephano |
Neuhaus 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated code. Independent clerk sealed and bundled blocks of 24 opaque sealed envelopes containing an equal number of assignments provided to centres |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes "so that people enrolling the patient into the study would not have known patient's assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | DMSA scans read by investigators without knowledge of assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 67/219 (30%) excluded from analysis as had no FU DMSA. This could influence results |
| Selective reporting (reporting bias) | High risk | No report of adverse effects |
| Other bias | High risk | Financial support from the Essex Company |
Noorbakhsh 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Children were allocated alternately to each group (information from authors) |
| Allocation concealment (selection bias) | High risk | Children were allocated alternately to each group (information from authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management. "After 2‐3 days of parenteral study therapy, investigators had the option to switch to oral cefixime if the patients had clinically improved". Unclear whether this referred to both treatment groups or whether patients in Group 1 could be continued on IV therapy for a longer duration. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Primary outcome of clinical response could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (7%) patients did not complete follow‐up and were excluded from the study |
| Selective reporting (reporting bias) | High risk | No report on adverse effects |
| Other bias | High risk | Pharmacist employed by Exir Pharmaceutical Co is study author |
Pylkkänen 1981.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Not stratified for APN. "Patients were randomly divided.." |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding. Primary outcome was laboratory based and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36/271 (13%) excluded. |
| Selective reporting (reporting bias) | High risk | No report of clinical resolution or adverse effects |
| Other bias | High risk | Supported by Foundation for Pediatric Research, Sigrid Juselius Foundation, Orion Pharmaceutical Co. |
Repetto 1984.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. "Patients..were treated randomly with either...). Not stratified for APN |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Primary outcomes were clinical and laboratory based. Clinical outcomes could be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | High risk | No clinical outcomes reported |
| Other bias | Unclear risk | No information provided |
Schaad 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by age (1 month to 2 years; > 2 years) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management. "Study drugs were administered in an open label manner" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Individual results were evaluated by blinded committee of experts" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 40/299 excluded for no pathogen; 24/259 (9%) excluded for other reasons but should have been included in analysis. 9% exclusions unlikely to influence outcomes. All patients included in safety analysis |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Biostatistics and data management by Bristol‐Myers Squibb. Grant from Bristol‐Myers Squibb |
Toporovski 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Supported by F. Hoffman‐La Roche Ltd |
Vigano 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that patients were randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | Stated that patients were randomly allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but primary outcome was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6/150 (4%) excluded from analysis. This is unlikely to influence results |
| Selective reporting (reporting bias) | High risk | No clinical outcomes reported |
| Other bias | Unclear risk | No information provided |
Vilaichone 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized by blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Only information provided is "prospective randomized trial" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence clinical management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was DMSA scan results. "The site and number of lesions for each kidney were independently reported by 2 experienced nuclear medicine physicians" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed follow‐up |
| Selective reporting (reporting bias) | High risk | Incomplete reporting of adverse effects |
| Other bias | Unclear risk | No information provided |
Amox/clav ‐ amoxicillin/clavulanic acid; APN ‐ acute pyelonephritis; CKD ‐ chronic kidney disease; CRP ‐ C reactive protein; CT ‐ computer tomography; DMSA ‐ Tc99m‐dimercaptosuccinic acid nuclear scan; ED ‐ emergency department; ESR ‐ erythrocyte sedimentation rate; IM ‐ intramuscular; IP ‐ inpatient; IQR ‐ interquartile range; MSU ‐ midstream urine specimen; NS ‐ not stated; OAE ‐ otoacoustic emission; OPD ‐ outpatient department; SPA ‐ suprapubic bladder aspiration; TMP/SMX ‐ trimethoprim/sulphamethoxazole; US ‐ ultrasound; UTI ‐ urinary tract infection; VUR ‐ vesicoureteric reflux; WBC ‐ white blood count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1982 | RCT; lower UTI |
| Avner 1983 | RCT; lower UTI |
| Belet 2004 | RCT; prophylaxis study |
| Bose 1974 | Quasi‐RCT; cannot separate data on children with pyelonephritis from those with lower UTI |
| Clemente 1994 | RCT; immunomodulating agents not antibiotics in APN |
| Cox 1985 | Adult data |
| Dagan 1992 | RCT; cannot separate data on children with APN from those with lower UTI |
| Ellerstein 1977 | RCT; unclear as to whether patients with APN included |
| Elo 1975 | RCT; cannot separate data on children with APN from those with lower UTI |
| Fine 1985 | RCT; lower UTI |
| Francois 1995 | RCT; cannot separate data on children with APN from those with lower UTI |
| Garin 2006 | RCT; prophylaxis study |
| Ginsburg 1982 | RCT; cannot separate data on children with APN from those with lower UTI |
| Godard 1980 | Not RCT |
| Gok 2001 | RCT; cannot separate data on children with APN from those with lower UTI |
| Goldberg 1977 | RCT; cannot separate data on children with APN from those with lower UTI |
| Helin 1978 | Not RCT |
| Howard 1971 | Not RCT |
| Howard 1978 | RCT; cannot separate data on children with APN from those with lower UTI |
| Huang 2011 | RCT comparing effect of methylprednisolone vs placebo on kidney scarring with same antibiotic regimen in each group |
| Iravani 1992 | RCT; lower UTI |
| Ivanov 1999 | RCT; does not compare antibiotic therapies |
| Kenda 1995 | RCT; cannot separate data on children with APN from those with lower UTI |
| Kontiokari 2005 | RCT; prophylaxis study |
| Kornberg 1994 | RCT; lower UTI |
| Lake 1971 | RCT; UTI but cannot separate data for febrile children from adult data |
| Lubitz 1984 | RCT; cannot separate data on children with APN from those with lower UTI |
| Madrigal 1988 | RCT; cannot separate data on children with APN from those with lower UTI |
| Moe 1977 | RCT; cannot separate data on children with APN from those with lower UTI |
| Olbing 1970 | RCT; prophylaxis study |
| Orekhova 2009 | Study of non‐antibiotic (immunological stimulating agent) as prophylaxis against UTI |
| Palcoux 1986 | Not RCT |
| Petersen 1991 | RCT; lower UTI |
| Piekkala 1985 | Not RCT |
| Pitt 1982 | RCT; cannot separate data on children with APN from those with lower UTI |
| Ray 1970 | RCT; prophylaxis |
| Russo 1977 | RCT; cannot separate data on children with APN from those with lower UTI |
| Sember 1985 | RCT; cannot separate data on children with APN from those with lower UTI |
| Shapiro 1981 | RCT; lower UTI |
| Thomas 1972 | Not RCT |
| Vlatković 1972 | Not RCT |
| Wallen 1983 | RCT; lower UTI |
| Weber 1982 | RCT; cannot separate data on children with APN from those with lower UTI |
APN ‐ acute pyelonephritis; RCT ‐ randomised controlled trial; UTI ‐ urinary tract infection
Characteristics of ongoing studies [ordered by study ID]
NCT00724256.
| Trial name or title | Short‐term antibiotic therapy for pyelonephritis in childhood (STUTI) |
| Methods | Country: Italy, USA Tertiary hospital ED |
| Participants | Inclusion: children aged 1 month to 5 years with first episode of acute pyelonephritis Exclusions: children with vomiting/sepsis or other condition where oral antibiotics could not be given. Pyelonephritis with abscess. Allergy to ceftibuten. Antibiotic prophylaxis with antibiotic of same class |
| Interventions | Group1: oral ceftibuten 9 mg/kg once daily for 7 days Group 2: oral ceftibuten 9 mg/kg once daily for 10 days |
| Outcomes | 1. Rate of kidney parenchymal damage at 6 to 12 months post UTI 2. Relapses of UTI 3. Adverse effect of therapy |
| Starting date | Start date: July 2006 |
| Contact information | Maria Lazzerini, IRCCS Burlo Garofolo |
| Notes | Trial terminated because of patients' refusal of DMSA scans on follow‐up |
DMSA ‐ Tc99m‐dimercaptosuccinic acid nuclear scan; ED ‐ emergency department; UTI ‐ urinary tract infection
Differences between protocol and review
Risk of bias assessment has replaced the quality assessment checklist; methodology has been updated to be in line with current Cochrane Collaboration guidelines.
Contributions of authors
Designing the review: PB, EH with Cochrane Renal Group guidelines Coordinating the review; PB, EH Data collection: PB, EH, NW, YS Entering data into RevMan; PB, EH, YS Analysis of data; PB, EH, YS Interpretation of data: PB, EH, NW, YS, AW Writing the review; PB, EH, NW, YS, AW Providing general advice on the review; EH, JC, AW
Declarations of interest
Yvonne Strohmeier: nothing to declare Elisabeth Hodson: nothing to declare Narelle Willis: nothing to declare Angela Webster: nothing to declare Jonathan Craig: nothing to declare
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Baker 2001 {published data only}
- Baker PC, Nelson DS, Schunk JE. The addition of ceftriaxone to oral therapy does not improve outcome in febrile children with urinary tract infections. Archives of Pediatrics & Adolescent Medicine 2001;155(2):135‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakkaloglu 1996 {published data only}
- Bakkaloglu A, Saatci U, Soylemezoglu O, Ozen S, Topaloglu R, Besbas N, et al. Comparison of ceftriaxone versus cefotaxime for childhood upper urinary tract infections. Journal of Chemotherapy 1996;8(1):59‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Banfi 1993 {published data only}
- Banfi AG, Hill‐Juarez JM, Kaufman A, Moens E. Multinational comparative trial of ceftibuten and trimethoprim‐sulfamethoxazole in the treatment of children with complicated or recurrent urinary tract infections. Members of the ceftibuten urinary tract infection international study group. Pediatric Infectious Disease Journal 1993;12(6 Suppl):S84‐S91. [CENTRAL: CN‐00334829] [Google Scholar]
Benador 2001 {published data only}
- Benador D, Neuhaus TJ, Papazyan J‐P, Willi UV, Engel‐Bicik I, Nadal D, et al. Randomised controlled trial of three day versus 10 day intravenous antibiotics in acute pyelonephritis: effect on renal scarring. Archives of Disease in Childhood 2001;84(3):241‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin E, Neuhaus T, Papazyan J, Willi UV, Bicik I, Nadal D, et al. Acute pyelonephritis: comparison of 3 vs 10 days IV antibiotic treatment [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):592A. [CENTRAL: CN‐00583905] [Google Scholar]
Bocquet 2012 {published data only}
- Bocquet N, Sergent Alaoui A, Jais JP, Gajdos V, Guigonis V, Lacour B, et al. Randomized trial of oral versus sequential IV/oral antibiotic for acute pyelonephritis in children. Pediatrics 2012;129(2):e269‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouissou 2008 {published data only}
- Bouissou F, Munzer C, Decramer S, Roussel B, Novo R, Morin D, et al. Prospective, randomized trial comparing short and long intravenous antibiotic treatment of acute pyelonephritis in children: dimercaptosuccinic acid scintigraphic evaluation at 9 months. Pediatrics 2008;121(3):e553‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carapetis 2001 {published and unpublished data}
- Carapetis J, Jaquiery A, Buttery J, Starr M. A randomised controlled trial of once‐daily gentamicin in children with urinary tract infections [abstract]. Australian & New Zealand Journal of Medicine 1999;29:608. [CENTRAL: CN‐00583423] [Google Scholar]
- Carapetis J, Jaquiery A, Buttery J, Starr M, Cranswick N, Kohn S, et al. Randomized, controlled trial comparing once daily and three times daily gentamicin in children with urinary tract infections. Pediatric Infectious Disease Journal 2001;20(3):240‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 2006 {published data only}
- Cheng CH, Tsau YK, Chang CJ, Chang YC, Kuo CY, Tsai IJ, et al. Acute lobar nephronia is associated with a high incidence of renal scarring in childhood urinary tract infections. Pediatric Infectious Disease Journal 2010;29(7):624‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cheng CH, Tsau YK, Lin TY. Effective duration of antimicrobial therapy for the treatment of acute lobar nephronia. Pediatrics 2006;117(1):e84‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chong 2003 {published data only}
- Chao SM, Chong CY, Tan‐Hendrick A, Tan AS, Ng WY. Efficacy and safety of once‐a‐day gentamicin in the treatment of childhood acute pyelonephritis [abstract]. Pediatric Nephrology 2001;16(8):C105. [CENTRAL: CN‐00444755] [Google Scholar]
- Chong CY, Tan AS, Ng W, Tan‐Kendrick A, Balakrishnan A, Chao SM. Treatment of urinary tract infection with gentamicin once or three times daily. Acta Paediatrica 2003;92(3):291‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Fischbach 1989 {published data only}
- Fischbach M, Simeoni U, Mengus L, Jehl F, Monteil H, Geisert J, et al. Urinary tract infections with tissue penetration in children: cefotaxime compared with amoxycillin/clavulanate. Journal of Antimicrobial Chemotherapy 1989;24(Suppl B):177‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Francois 1997 {published data only}
- Francois P, Bensman A, Begue P, Artaz M‐A, Coudeville L, Lebrun T, et al. Assessment of the efficacy and cost efficiency of two strategies in the treatment of acute pyelonephritis in children: oral cefixime or parenteral ceftriaxone after an initial IV combination therapy [Evaluation de l'efficacité et du coût de deux stratégies thérapeutiques dans les pyélonéphrites de l'enfant: céfixime per os versus ceftriaxone parentérale en relais d'une bithérapie intraveineuse]. Medecine et Maladies Infectieuses 1997;27(Spec Iss June):667‐73. [EMBASE: 1997230388] [Google Scholar]
Fujii 1987 {published data only}
- Fujii R, Shinozaki T, Meguro H, Arimasu O, Izumi K, Osano M, et al. Comparative, controlled study on an ampicillin suppository (KS‐R 1) with an oral form of ampicillin in urinary tract infections. Japanese Journal of Antibiotics 1987;40(3):476‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Grimwood 1988 {published data only}
- Grimwood K, Abbott GD, Fergusson DM. Single dose gentamicin treatment of urinary infections in children. New Zealand Medical Journal 1988;101(852):539‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Hoberman 1999 {published and unpublished data}
- Hoberman A, Wald ER. Intravenous versus oral antibiotics for urinary tract infections. Pediatrics 2000;106(4):865. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hoberman A, Wald ER, Hickey RW, Baskin M, Charron M, Majd M, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics 1999;104(1 Pt 1):79‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kafetzis 2000 {published data only}
- Kafetzis DA, Maltezou HC, Mavrikou M, Siafas C, Paraskakis I, Delis D, et al. Isepamicin versus amikacin for the treatment of acute pyelonephritis in children. International Journal of Antimicrobial Agents 2000;14(1):51‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khan 1981 {published data only}
- Kahn AJ, Kumar K, Evans HE. Three‐day antimicrobial therapy of urinary tract infection. Journal of Pediatrics 1981;99(6):992‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levtchenko 2001 {published data only}
- Levtchenko E, Lahy C, Levy J, Ham H, Piepsz A. Treatment of children with acute pyelonephritis: a prospective randomized study. Pediatric Nephrology 2001;16(11):878‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marild 2009 {published data only}
- Marild S, Esbjorner E, Jakobsson B, Linne T, Lubeck P, Sjogren I, et al. Randomised study of ceftibuten vs trimethoprim‐sulfamethoxazole in pyelonephritis in children [abstract no: P466]. Pediatric Nephrology 2004;19(9):C208. [Google Scholar]
- Marild S, Jodal U, Sandberg T. Ceftibuten versus trimethoprim‐sulfamethoxazole for oral treatment of febrile urinary tract infection in children. Pediatric Nephrology 2009;24(3):521‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Montini 2007 {published data only}
- Hewitt IK, Tomasi L, Pavanello L, Maschio F, Molinari PP, Toffolo A, et al. Early treatment of acute pyelonephritis in children fails to reduce renal scarring [abstract no: SA‐PO1100]. Journal of the American Society of Nephrology 2006;17(Abstracts):804A. [CENTRAL: CN‐00653791] [Google Scholar]
- Hewitt IK, Zucchetta P, Rigon L, Maschio F, Molinari PP, Tomasi L, et al. Early treatment of acute pyelonephritis in children fails to reduce renal scarring: data from the Italian Renal Infection Study Trials. Pediatrics 2008;122(3):486‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scola C, Mutiis C, Hewitt IK, Puccio G, Toffolo A, Zucchetta P, et al. Different guidelines for imaging after first UTI in febrile infants: yield, cost, and radiation. Pediatrics 2013;131(3):e665‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Montini G, Murer L, Gobber D, Commacchio S, Toffolo A, Dall'Amico R, et al. Oral vs initial intravenous antibiotic treatment of urinary tract infections in children: a multicentre study [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):816. [CENTRAL: CN‐00446817] [Google Scholar]
- Montini G, Toffolo A, Zucchetta P, Dall'Amico R, Gobber D, Calderon A, et al. Antibiotic treatment for pyelonephritis in children: multicentre randomised controlled non‐inferiority trial. BMJ 2007;335(7616):386‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini G, Zucchetta P, Gobber D, Murer L, Rigon L, Toffolo A, et al. Usefulness of renal radiology and scintigraphy in children with a first episode of upper urinary tract infection [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):813. [CENTRAL: CN‐00446818] [Google Scholar]
- Montini G, Zucchetta P, Tomasi L, Talenti E, Rigamonti W, Picco G, et al. Value of imaging studies after a first febrile urinary tract infection in young children: data from Italian renal infection study 1. Pediatrics 2009;123(2):e239‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neuhaus 2008 {published data only}
- Buechner K, Girardin F, Berger C, Willi U, Nadaf D, Neuhaus T for the Swiss Pyelonephritis Study Group. Randomised controlled trial of oral versus sequential intravenous/oral cephalosporins in DMSA scintigraphy‐documented acute pyelonephritis [abstract no: COD. OP 18]. Pediatric Nephrology 2006;21(10):1511. [CENTRAL: CN‐00601934] [Google Scholar]
- Neuhaus TJ, Berger C, Buechner K, Parvex P, Bischoff G, Goetschel P, et al. Randomised trial of oral versus sequential intravenous/oral cephalosporins in children with pyelonephritis. European Journal of Pediatrics 2008;167(9):1037‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Neuhaus TJ, Büchner K, Girardin E, Willi U, Berger C, Nadal D. Oral versus sequential intravenous/oral cephalosporin‐therapy in DMSA‐scintigraphy documented acute pyelonephritis: randomized Swiss pyelonephritis‐study [abstract] [Orale versus sequentiell intravenös/orale Cephalosporin‐Therapie bei DMSA‐Szintigraphie dokumentierter akuter Pyelonephritis: Randomisierte Schweizer Pyelonephritis‐Studie]. Nieren‐ und Hochdruckkrankheiten 2007;36(2):43. [CENTRAL: CN‐00615897] [Google Scholar]
Noorbakhsh 2004 {published data only}
- Noorbakhsh S, Lari AR, Masjedian F, Mostafavi H, Alaghehbandan R. Comparison of intravenous aminoglycoside therapy with switch therapy to cefixime in urinary tract infections. Saudi Medical Journal 2004;25(10):1513‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Pylkkänen 1981 {published data only}
- Pylkkänen J, Vilska J, Koskimies O. The length of antimicrobial therapy in upper vs. lower urinary tract infection of childhood. Acta Paediatrica Scandinavica 1981;70(6):885‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pylkkänen J, Vilska J, Koskimies O. The value of level diagnosis of childhood urinary tract infection in predicting renal injury. Acta Paediatrica Scandinavica 1981;70(6):879‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Repetto 1984 {published data only}
- Repetto HA, MacLoughlin GJ. Single‐dose cefotaxime in the treatment of urinary tract infections in children: a randomized clinical trial. Journal of Antimicrobial Chemotherapy 1984;14(Suppl B):307‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaad 1998 {published and unpublished data}
- Schaad UB, Eskola J, Kafetzis D, Fischbach M, Ashekenazi S, Syriopoulou V, et al. Cefepine vs. ceftazidime treatment of pyelonephritis: a European, randomized, controlled study of 300 pediatric cases. European Society for Paediatric Infectious Diseases (ESPID) Pyelonephritis Study Group. Pediatric Infectious Disease Journal 1998;17(7):639‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Toporovski 1992 {published data only}
- Toporovski J, Steffens L, Noack M, Kranz A, Burdeska A, Kissling M. Effectiveness of cefetamet pivoxil in the treatment of pyelonephritis in children. Journal of International Medical Research 1992;20(1):87‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vigano 1992 {published data only}
- Vigano A, Principi N, Brivio L, Tommasi P, Stasi P, Villa AD. Comparison of 5 milligrams of netilmicin per kilogram of body weight once daily versus 2 milligrams per kilogram thrice daily for treatment of gram‐negative pyelonephritis in children. Antimicrobial Agents & Chemotherapy 1992;36(7):1499‐503. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vilaichone 2001 {published data only}
- Vilaichone A, Watana D, Chaiwatanarat T. Oral ceftibuten switch therapy for acute pyelonephritis in children. Journal of the Medical Association of Thailand 2001;84 Suppl(1):61‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1982 {published data only}
- Adam D, Hager C, Dorn G, Bamberg P. A comparison of co‐trimazine once daily and co‐trimoxazole twice daily in treatment of urinary tract infections in children. Journal of Antimicrobial Chemotherapy 1982;10(5):453‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Avner 1983 {published data only}
- Avner ED, Ingelfinger JR, Herrin JT, Link DA, Marcus E, Tolkoff‐Rubin NE, et al. Single‐dose amoxicillin therapy of uncomplicated pediatric urinary tract infections. Journal of Pediatrics 1983;102(4):623‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Belet 2004 {published data only}
- Belet N, Islek I, Belet U, Sunter AT, Kucukoduk S. Comparison of trimethoprim‐sulfamethoxazole, cephadroxil and cefprozil as prophylaxis for recurrent urinary tract infections in children. Journal of Chemotherapy 2004;16(1):77‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bose 1974 {published data only}
- Bose W, Karama A, Linzenmeier G, Olbing H, Wellman P. Controlled trial of co‐trimoxazole in children with urinary tract infection. Lancet 1974;2(7881):614‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clemente 1994 {published data only}
- Clemente E, Solli R, Mei V, Cera R, Caramia G, Carnelli V, et al. Therapeutic efficacy and safety of pidotimod in the treatment of urinary tract infections in children. Arzneimittel‐Forschung 1994;44(12A):1490‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Cox 1985 {published data only}
- Cox CE. Comparative study of ticarcillin plus clavulanate potassium versus piperacillin in the treatment of hospitalized patients with urinary tract infections. The American Journal of Medicine 1985;79(5B):88‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dagan 1992 {published data only}
- Dagan R, Einhorn M, Lang R, Pomeranz A, Wolach B, Miron D, et al. Once daily cefixime compared with twice daily trimethoprim/sulfamethoxazole for the treatment of urinary tract infection in infants and children. Pediatric Infectious Disease Journal 1992;11(3):198‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ellerstein 1977 {published data only}
- Ellerstein NS, Sullivan TD, Baliah T, Neter E. Trimethoprim/sulfamethoxazole and ampicillin in the treatment of acute urinary tract infections in children: a double‐blind study. Pediatrics 1977;60(2):245‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Elo 1975 {published data only}
- Elo J, Ahava K. Cephalexin compared with ampicillin in urinary tract infections in children. Journal of Antimicrobial Chemotherapy 1975;1(3 Suppl):85‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fine 1985 {published data only}
- Fine JS, Jacobson MS. Single‐dose versus conventional therapy of urinary tract infections in female adolescents. Pediatrics 1985;75(5):916‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Francois 1995 {published data only}
- Francois P, Croizé J, Bost C, Wollschlager K. Comparative study of cefixime versus amoxycillin‐clavulanate for oral treatment of urinary tract infections in children [Étude comparant le céfixime à l'association amoxicilline‐acide clavulanique dans le traitement par voie orale des infections urinaires de l'enfant]. Archives de Pediatrie 1995;2(2):136‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garin 2006 {published data only}
- Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, Young L. Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics 2006;117(3):626‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ginsburg 1982 {published data only}
- Ginsburg CM, McCracken GH, Petruska M. Once‐daily cefadroxil versus twice‐daily cefaclor for treatment of acute urinary tract infections in children. Journal of Antimicrobial Chemotherapy 1982;10(Suppl B):53‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Godard 1980 {published data only}
- Godard C, Girardet P, Frutiger P, Hynek R, Delarue C, Christen JP. Short treatment of urinary tract infections in children. Paediatrician 1980;9(5‐6):309‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Gok 2001 {published data only}
- Gok F, Duzova A, Baskin E, Ozen S, Besbas N, Bakkaloglu A. Comparative study of cefixime alone versus intramuscular ceftizoxime followed by cefixime in the treatment of urinary tract infections in children. Journal of Chemotherapy 2001;13(3):277‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldberg 1977 {published data only}
- Goldberg IL, Weidman ML, Caplan AH, Williams TW, Stebbing N. Sulfacytine compared with sulfisoxazole in children with acute uncomplicated urinary tract infections. Current Therapeutic Research ‐ Clinical & Experimental 1977;21(4):468. [EMBASE: 0978092013] [Google Scholar]
Helin 1978 {published data only}
- Helin I, Jodal U. Treatment of childhood pyelonephritis with two daily doses of pivampicillin. Lakartidningen 1978;75(18):1825‐6. [EMBASE: 1978316975] [PubMed] [Google Scholar]
Howard 1971 {published data only}
- Howard JE, Donoso E, Mimica I, Zilleruelo G. Gentamicin for urinary‐tract infections in infants. Journal of Infectious Diseases 1971;124 Suppl:S234‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Howard 1978 {published data only}
- Howard JB, Howard JE Sr. Trimethoprim‐sulfamethoxazole vs sulfamethoxazole for acute urinary tract infections in children. American Journal of Diseases of Children 1978;132(11):1085‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang YY, Chen MJ, Chiu NT, Chou HH, Lin KY, Chiou YY. Adjunctive oral methylprednisolone in pediatric acute pyelonephritis alleviates renal scarring. Pediatrics 2011;128(3):e496‐504. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Iravani 1992 {published data only}
- Iravani A, Doyle CA, Durham SJ, Wilber RB. Cefprozil versus cefaclor in the treatment of acute and uncomplicated urinary tract infections. Cefprozil Multicenter Study Group. Clinical Therapeutics 1992;14(2):314‐26. [MEDLINE: ] [PubMed] [Google Scholar]
Ivanov 1999 {published data only}
- Ivanov DD, Kyshirenko SV, Stefanov AV, Steczko VA. Phosphotydilcholine liposomes usage in pyelonephritis with sirs [abstract]. 36th Congress ‐ European Renal Association, European Dialysis and Transplantation Association; 1999 Sep 5‐8; Madrid (Spain). 1999:130. [CENTRAL: CN‐00484470]
Kenda 1995 {published data only}
- Kenda R, Kaplar VM, Jeler D, Kunstelj T, Truden E, Smole A. The treatment of bacterial infection of the urinary tract in infants and small children using Amoksiklav. Paediatria Croatica 1995;39(3):155‐8. [EMBASE: 1996020626] [Google Scholar]
Kontiokari 2005 {published data only}
- Kontiokari T, Salo J, Eerola E, Uhari M. Cranberry juice and bacterial colonization in children‐‐a placebo‐controlled randomized trial. Clinical Nutrition 2005;24(6):1065‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kornberg 1994 {published data only}
- Kornberg AE, Sherin K, Veiga P, Mydlow PK, Collins JJ, Feld LG. Two‐day therapy with cefuroxime axetil is effective for urinary tract infections in children. American Journal of Nephrology 1994;14(3):169‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lake 1971 {published data only}
- Lake B. Ampicillin in acute infections of general practice: a controlled trial. Medical Journal of Australia 1971;1(12):636‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Lubitz 1984 {published data only}
- Lubitz L, Cullen J, Nolan T, Oberklaid F. A prospective study comparing single dose trimethoprim with a 7 day course of co‐trimoxazole in the treatment of urinary tract infection in children [abstract]. Australian Paediatric Journal 1984;20(3):236‐7. [CENTRAL: CN‐00282528] [Google Scholar]
Madrigal 1988 {published data only}
- Madrigal G, Odio CM, Mohs E, Guevara J, McCracken GH. Single dose antibiotic therapy is not as effective as conventional regimens for management of acute urinary tract infections in children. Pediatric Infectious Disease Journal 1988;7(5):316‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moe 1977 {published data only}
- Moe OJ, Meberg A, Eng J. Ampicillin and pivampicillin in the treatment of urinary tract infection in children. Scandinavian Journal of Infectious Diseases 1977;9(1):31‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Olbing 1970 {published data only}
- Olbing H, Reischauer HC, Kovacs I. Prospective comparison between nitrofurantoin and sulphamethoxydiazine in the long‐term therapy of children suffering from severe chronic recurrent pyelonephritis. Deutsche Medizinische Wochenschrift 1970;95(49):2469‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Orekhova 2009 {published data only}
- Orekhova SB, Botvin'ev OK, Romantsov MG. Type 1 and type 2 interferon inductor (cycloferon) in therapy of children with pyelonephritis associated with herpes viruses. Antibiotiki i Khimioterapiia 2009;54(5‐6):48‐53. [MEDLINE: ] [PubMed] [Google Scholar]
Palcoux 1986 {published data only}
- Palcoux JB, Raynaud EJ, Borderon JC, Dalous A, Geisert J, Pennaforte F, et al. Clinical trial of clavulanic acid‐amoxicillin combination in child urinary infections [Essai clinique de l'association acide clavulanique‐amoxicilline dans les infections urinaires de l'enfant]. Annales de Pediatrie 1986;33(8):761‐76. [MEDLINE: ] [PubMed] [Google Scholar]
Petersen 1991 {published data only}
- Petersen S, Brendstrup L, Petersen KE, Hansen A, Hvorslev V, Braendholt JV, et al. Short‐term treatment of acute urinary tract infection in girls. Scandinavian Journal of Infectious Diseases 1991;23(2):213‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piekkala 1985 {published data only}
- Piekkala P, Huovinen P, Valimaki I. Comparative study of cefuroxime vs. amoxycillin in the parenteral treatment of children with upper urinary tract infection. Current Therapeutic Research, Clinical & Experimental 1985;37(6):1152‐9. [EMBASE: 1985140197] [Google Scholar]
Pitt 1982 {published data only}
- Pitt WR, Dyer SA, McNee JL, Burke JR. Single dose trimethoprim‐sulphamethoxazole treatment of symptomatic urinary infection. Archives of Disease in Childhood 1982;57(3):229‐39. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 1970 {published data only}
- Ray CG, Ching YC, Shurtleff DB, Hill ML, Ansell JS, Wedgwood RJ. Chronic urinary tract infections in children: a double‐blind study of medical management. Pediatrics 1970;45(3):456‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Russo 1977 {published data only}
- Russo RM, Gururaj VJ, Laude TA, Rajkumar SV, Allen JE. The comparative efficacy of cephalexin and sulfisoxazole in acute urinary tract infection in children. Clinical Pediatrics 1977;16(1):83‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sember 1985 {published data only}
- Sember ME, Mosso F, Meregalli MA. Randomized bicentric study carried out with kelfiprim and cotrimoxazole in children with acute or chronic urinary infections [Estudio randomizado bicentrico realizado con kelfiprim y cotrimoxazol en ninos portadores de infecciones urinarias agudas o cronicas]. Prensa Medica Argentina 1985;72(9):301‐5. [EMBASE: 1986006721] [Google Scholar]
Shapiro 1981 {published data only}
- Shapiro ED, Wald ER. Single‐dose amoxicillin treatment of urinary tract infections. Journal of Pediatrics 1981;99(6):989‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomas 1972 {published data only}
- Thomas M, Hopkins JM. Co‐trimoxazole and cephalexin. A clinical trial in urinary tract infections in children with spina bifida cystica. Developmental Medicine & Child Neurology 1972;14(3):342‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vlatković 1972 {published data only}
- Vlatković G, Babić I. Treatment of urinary tract infection in the child using Ceporex (cephalexin) [Lijecenje infekcija mokraćnog trakta djece Ceporexom (cefaleksin)]. Lijecnicki Vjesnik 1972;94(10):518‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Wallen 1983 {published data only}
- Wallen L, Zeller WP, Goessler M, Connor E, Yogev R. Single‐dose amikacin treatment of first childhood E. coli lower urinary tract infections. The Journal of Pediatrics 1983;103(2):316‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weber 1982 {published data only}
- Weber HP, Aberfeld U, Hildenbrand G, Knopfle G. Treatment of first urinary tract infection in children with cotrifamole and cotrimoxazole. A double‐blind study. Deutsche Medizinische Wochenschrift 1982;107(24):937‐41. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
NCT00724256 {published data only}
- NCT00724256. Randomised controlled trial on efficacy and safety of short term versus long term antibiotic therapy for pyelonephritis in childhood. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/results?term=NCT00724256; Vol. (accessed 4 July 2014).
Additional references
AAP 2011
- American Academy of Pediatrics. Subcommittee On Urinary Tract Infection, Steering Committee On Quality Improvement and Management. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 2011;128(3):595‐610. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ammenti 2012
Bhat 2011
- Bhat RG, Katy TA, Place FC. Pediatric urinary tract infections. Emergency Medicine Clinics of North America 2011;29(3):637‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brkic 2010
- Brkic S, Mustafic S, Nuhbegovic S, Ljuca F, Gavran L. Clinical and epidemiology characteristics of urinary tract infections in childhood. Medicinski Arhiv 2010;64(3):135‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Contopoulos‐Ioannidis 2004
- Contopoulos‐Ioannidis DG, Giotis ND, Baliatsa DV, Ioannidis JP. Extended‐interval aminoglycoside administration for children: a meta‐analysis. Pediatrics 2004;114(1):e111‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Altman D, Gotzsche P, Juni P, Moher D, Oxman A, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; Vol. 343:d5928. [MEDLINE: ] [DOI] [PMC free article] [PubMed]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7221):670‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenh 2011
- Jenh AM, Tamma PD, Milstone AM. Extended‐interval aminoglycoside dosing in pediatrics. Pediatric Infectious Disease Journal 2011;30(4):338‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Juni 1999
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Michael 2002
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short compared with standard duration of antibiotic treatment for urinary tract infection: a systematic review of randomised controlled trials. Archives of Disease in Childhood 2002;87(2):118‐23. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michael 2003
- Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short versus standard duration oral antibiotic therapy for acute urinary tract infection in children. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003966] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does the quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NICE 2007
- National Institute for Health and Clinical Excellence. Urinary tract infection in children: diagnosis, treatment and long‐term management CG54. 2007. http://www.nice.org.uk/guidance/CG54. London: RCOG Press, (accessed 4 July 2014). [PubMed]
Salo 2011
- Salo J, Ikäheimo R, Tapiainen T, Uhari M. Childhood urinary tract infections as a cause of chronic kidney disease. Pediatrics 2011;128(5):840‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaikh 2008
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta‐analysis. Pediatric Infectious Disease Journal 2008;27(4):302‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaikh 2010
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 2010;126(6):1084‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smellie 1985
- Smellie JM, Ransley PG, Normand ICS, Prescod N, Edwards D. Development of renal scars: a collaborative study. BMJ 1985;290(6486):1957‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toffolo 2012
- Toffolo A, Ammenti A, Montini G. Long‐term clinical consequences of urinary tract infections during childhood: a review. Acta Paediatrica 2012;101(10):1018‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vouloumanou 2008
- Vouloumanou EK, Rafailidis PI, Kazantzi MS, Athanasiou S, Falagas ME. Early switch to oral versus intravenous antimicrobial treatment for hospitalized patients with acute pyelonephritis: a systematic review of randomized controlled trials. Current Medical Research & Opinion 2008;24(12):3423‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bloomfield 2002
- Bloomfield P, Hodson E, Craig J. Antibiotics for acute pyelonephritis in children. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003772] [DOI] [PubMed] [Google Scholar]
Bloomfield 2003
- Bloomfield P, Hodson EM, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003772] [DOI] [PubMed] [Google Scholar]
Bloomfield 2005
- Bloomfield P, Hodson EM, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD003772.pub2] [DOI] [PubMed] [Google Scholar]
Hodson 2007
- Hodson EM, Willis NS, Craig JC. Antibiotics for acute pyelonephritis in children. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003772.pub3] [DOI] [PubMed] [Google Scholar]


